Abstract
Immune responses to factor IX (F.IX), a major concern in gene therapy for hemophilia, were analyzed for adeno-associated viral (AAV-2) gene transfer to skeletal muscle and liver as a function of the F9 underlying mutation. Vectors identical to those recently used in clinical trials were administered to four lines of hemophilia B mice on a defined genetic background [C3H/HeJ with deletion of endogenous F9 and transgenic for a range of nonfunctional human F.IX (hF.IX) variants]. The strength of the immune response to AAV-encoded F.IX inversely correlated with the degree of conservation of endogenous coding information and levels of endogenous antigen. Null mutation animals developed T- and B-cell responses in both protocols. However, inhibitor titers were considerably higher upon muscle gene transfer (or protein therapy). Transduced muscles of Null mice had strong infiltrates with CD8+ cells, which were much more limited in the liver and not seen for the other mutations. Sustained expression was achieved with liver transduction in mice with crm− nonsense and missense mutations, although they still formed antibodies upon muscle gene transfer. Therefore, endogenous expression prevented T-cell responses more effectively than antibody formation, and immune responses varied substantially depending on the protocol and the underlying mutation.
Introduction
Adeno-associated viral (AAV) transfer of a functional coagulation factor IX gene (F9) for treatment of hemophilia B resulted in long-term partial to complete correction of this X-linked bleeding disorder in small and large animal models.1,2 Treatment was based on intramuscular (IM) injection at multiple sites for transduction of skeletal muscle fibers or by infusion of vector into the hepatic circulation (via hepatic artery or portal vein) for transduction of hepatocytes.3,4 Both protocols were tested in phase I/II clinical trials using single-stranded AAV serotype 2 vectors.5,6,7
Skeletal muscle was chosen as the initial site of gene transfer because one was able to test this novel approach using a noninvasive procedure while not targeting a major organ and while minimizing biodistribution of vector.8 Although factor IX protein (F.IX) is normally synthesized in the liver, muscle fibers are capable of producing biologically active F.IX. However, a comparison between experiments in different animal models showed that sustained systemic expression, as initially observed in dogs with a F.IX missense mutation, was blocked by an antibody response against F.IX in animals with more severe loss of coding information (such as mice with F9 gene deletion or canines with nonsense mutation/unstable mRNA).9,10 Additionally, induction of T cells and formation of inhibitory antibodies against F.IX (“inhibitors”) were found at high vector doses in the missense mutation animals, in particular if high doses were delivered to a single injection site.11 A local immune response resulted in activation of F.IX-specific B and T cells in the lymph nodes draining the transduced muscle unless transient immune suppression was applied.12,13 We failed to prevent inhibitor formation using a muscle-specific promoter, which may reflect activation of T cells by cross-presentation or leaky transgene expression in antigen presenting cells.14,15 As a consequence of these findings, only subjects with F9 missense mutations were enrolled for muscle-directed AAV-2 gene transfer, and the vector dose per injection site was capped at 1.5 × 1012 vector genomes (vg).6 The safety profile of the trial was excellent, and no inhibitor formation was observed. Sustained local F9 gene expression was demonstrated on muscle biopsies.16 However, systemic expression was not consistently demonstrated and was at best ~1% of normal.
The hepatic gene transfer protocol showed higher efficacy in animal models and resulted in a phenotypic change from severe to mild disease in hemophilia B dogs, which has been sustained for >8 years.17 In a clinical trial based on administration of AAV-2 vector to the hepatic artery of patients with severe hemophilia B, a subject with low pre-existing neutralizing antibodies to AAV-2 gained therapeutic levels of F.IX expression (11% of normal) after treatment with 2 × 1012 vg/kg.7 Expression was transient and declined to pregene transfer levels by 2 months. Subsequent studies strongly suggested that CD8+ T cells against viral capsid caused transaminitis and elimination of transduced hepatocytes.18,19 No evidence for an immune response against the F.IX transgene product was found even in subjects with nonsense mutations.7
Hepatic AAV-2 gene transfer in mice with a F9 gene deletion demonstrated induction of immune tolerance to the F.IX transgene product in several strains.20 Hepatic expression induces transgene product-specific regulatory CD4+CD25+FoxP3+ T cells, which suppress humoral and cellular immune responses against the transgene product.21,22,23 The importance of this regulatory T-cell population in maintaining tolerance to the F.IX transgene product has also been demonstrated in nonhuman primates.24 Tolerance to F.IX, established by hepatic gene transfer, is maintained after subsequent supplementary gene transfer to other organs.25 Tolerance induction with this method was highly effective in several, but not all, strains of mice with targeted F9 gene deletion, suggesting that additional genetic factors influence the immune response.20,26,27
Hemophilia B patients display a large variety of F9 mutations. Those subjects who develop inhibitors during traditional protein replacement therapy typically have a gene deletion, early stop codon, or other mutation that results in extensive loss of coding information.28 Past assessments of the effects of the underlying F9 mutation and the route of vector administration on immune responses in gene therapy have relied on comparisons between different strains of mice and dogs, or have addressed only B-cell responses and a single target tissue.29 The high number of variables between experiments, including genetic effects, limited conclusions. This new study for the first time provides a comprehensive assessment of B- and T-cell responses upon liver- or muscle-directed AAV-2 gene transfer in animals with identical genetic background but distinct F9 mutations.
Results
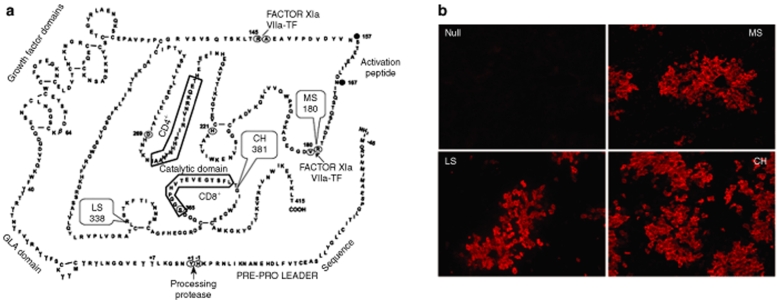
The objective of this investigation was to compare human F.IX (hF.IX)–specific immune responses upon muscle- and liver-directed AAV-2-mediated gene transfer as a function of the underlying genetic F9 defect. C3H/HeJ mice were chosen as a deliberatively provocative model, because mice on this genetic background, unlike C57BL/6 or BALB/c mice, develop antibodies to hF.IX upon hepatic AAV-2-mediated gene transfer.20,30 Mice transgenic for a liver-specific human F9 mini gene were backcrossed from a C57BL/6 onto a C3H/HeJ background and finally crossed with hemophilia B C3H/HeJ mice that carry a targeted deletion of the endogenous murine F9 gene. We obtained four lines of hemophilia B C3H/HeJ mice with <1% systemic F.IX activity. These included gene deletion mice without additional transgene (“Null” mutation), mice expressing hF.IX with a late stop codon at amino acid residue 338 (“LS”; crm− mutation, i.e., no circulating antigen), with the crm− G381E missense mutation (“CH”, equivalent mutation to hemophilia B dogs of University of North Carolina –Chapel Hill strain), or the crm+ R180W missense mutation (“MS”; see Figure 1a). LS and MS mutations were selected from the hemophilia B database of F9 mutations in humans.31,32 All lines, except Null mice, showed expression of hF.IX in the liver (Figure 1b). Antigen levels were 0, 1.7 ± 1.2, 4.7 ± 1.3, and 103 ± 10 ng hF.IX/mg liver protein for Null, LS, CH, and MS mice, respectively (n = 3 male mice per line, data not shown).
Figure 1.
Lines of hemophilia B mice. (a) Primary amino acid sequence of hF.IX and locations of F9 mutations expressed in transgenic lines of hemophilia B mice: late stop codon at amino acid residue 338 (“LS”, crm−, i.e., no circulating antigen); crm− G381E missense mutation (“CH”, identical mutation as in hemophilia B dogs of University of North Carolina–Chapel Hill strain); crm+ R180W missense mutation. Boxed sequences contain CD4+ and CD8+ T-cell epitopes in C3H/HeJ mice. (b) Immunostain of hF.IX in livers of the four lines. Original magnification ×100.
Hemophilia B mice received IM injections of AAV-2-CMV-hF.IX vector or hepatic gene transfer with AAV-2-ApoE/hAAT-hF.IX vector at a dose of 1 × 1011 vg/mouse (or ~4 × 1012 vg/kg). We would like to point out that the identical vectors have been used previously in clinical trials at doses up to 2 × 1012 vg/kg.6,7 Transduced animals were followed for 3 months.
Crm+ missene mutation provides most robust protection from antibody response to hF.IX in muscle gene transfer
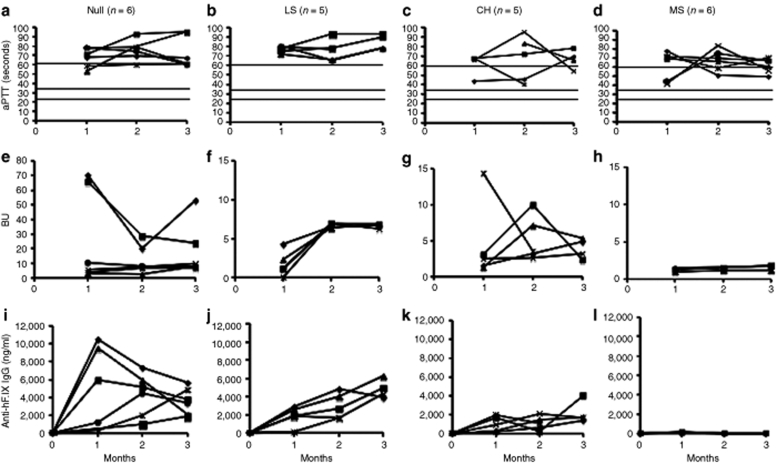
Following muscle-directed gene transfer, inhibitors were formed rapidly in all Null mice (Figure 2e). Inhibitor titers reached 7–11 Bethesda units (BU) in 4/6 animals, whereas 2/6 mice produced 20–70 BU during the 3 months of observation (comparable to protein therapy, see below). LS mice had lower-titer inhibitors at 1 month, which increased to 6–7 BU by 2 months and remained unchanged by 3 months (Figure 2f). Inhibitor formation was variable for CH mice with peak titers of 3–14 BU (Figure 2g). Although Null and LS mice showed no correction of the coagulation times [activated partial thromboplastin times (aPTTs) were >60 seconds, Figure 2a,b], intermittent shortening of the aPTT was seen in 3/5 CH mutation mice (Figure 2c). Immunoglobin G (IgG) titers against hF.IX were highest in Null mice at 1–2 months, while at 3 months, Null and LS mice had similar IgG titers (Figure 2i,j). CH mice formed comparatively low-titer IgGs (Figure 2k). Antibodies reported in Figure 2 for muscle gene transfer as well as in liver-directed gene transfer (Figure 3) were predominantly of the IgG1 isotype indicating that help was mediated by Th2 cells.
Figure 2.
Systemic expression of and humoral immune responses to hF.IX as a function of time after muscle-directed gene transfer in hemophilia B mice. (a–d) Coagulation times (aPTT in seconds). (e–h) Inhibitory antibody titers against hF.IX in BU. (i–l) IgG1 titers against hF.IX in ng/ml. (a,e,i) Mice with F9 gene deletion (“Null” mutation, n = 6). (b,f,j) Mice expressing F9 with late stop codon at amino acid residue 338 (“LS” mice, n = 5). (c,g,k) Mice expressing F9 with crm− missense mutation G381E as found in the hemophilia B dogs of the Chapel Hill strain (“CH” mutation, n = 5). (d,h,l) Mice expressing F9 with crm+ missense mutation R180W (“MS” mutation, n = 6). Each line represents an individual animal. Horizontal lines for aPTT indicate range of normal mouse plasma (25–35 seconds) and of untreated hemophilia B plasma (>60 seconds). aPTT, activated partial thromboplastin times; BU, Bethesda unit; hF.IX, human factor IX, IgG1, immunoglobin G-1.
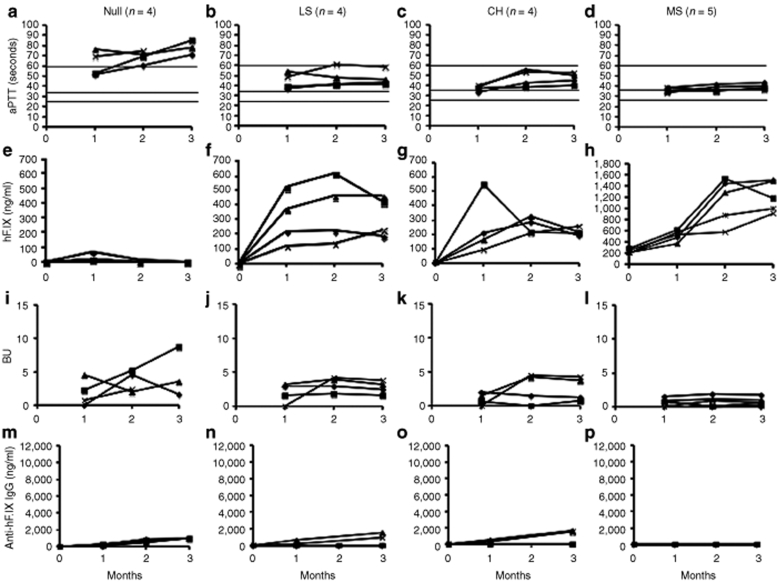
Figure 3.
Systemic expression of and humoral immune responses to hF.IX as a function of time after liver-directed gene transfer in hemophilia B mice. (a–d) Coagulation times (aPTT in seconds). (e–h) Plasma levels of hF.IX (in ng/ml). (i–l) Inhibitory antibody titers against hF.IX in BU. (m–p) IgG1 titers against hF.IX in ng/ml. (a,e,i,m) Null mice (with F9 gene deletion, n = 4). (b,f,j,n) Mice expressing F9 with late stop codon at amino acid residue 338 (“LS” mice, n = 4). (c,g,k,o) Mice expressing F9 with crm− missense mutation G381E as found in the hemophilia B dogs of the Chapel Hill strain (“CH” mutation, n = 4). (d,h,l,p) Mice expressing F9 with crm+ missense mutation R180W (“MS” mutation, n = 5). Each line represents an individual animal. Horizontal lines for aPTT indicate range of normal mouse plasma (25–35 seconds) and of untreated hemophilia B plasma (>60 seconds). aPTT, activated partial thromboplastin times; BU, Bethesda unit; hF.IX, human factor IX.
MS mice had no detectable antibodies by enzyme-linked immunosorbent assay and no or at best very low-titer inhibitors of <2 BU (Figure 2h,l), and 3/6 mice showed marginal correction of coagulation (Figure 2d). Because of the high levels of endogenous circulating hF.IX antigen in MS mutation mice, we were not able to quantify a presumably modest increase of systemic hF.IX expression following muscle gene transfer. In the crm− Null, LS, and CH mutations, where antibodies formed, no circulating hF.IX was detected by enzyme-linked immunosorbent assay (data not shown).
Hepatic gene transfer results in sustained correction in all mice except for those with Null mutation
Consistent with our previously published study, hepatic gene transfer to the Null mice resulted in only transient systemic expression, which was lost by 2 months (Figure 3a,e).30 Inhibitors (3–5 BU) and IgG titers were detected in all animals, but were substantially lower compared to muscle gene transfer (Figures 2e,i and 3i,m). Importantly, all mice with endogenous hF.IX expression showed stable expression and modest to substantial correction of coagulation (Figure 3b–d,f–h). Among LS mice, only two of these mice had detectable IgG titers, and Bethesda titers were low at 2–4 BU (Figure 3j,n). Those mice without detectable IgG formation showed more substantial correction of the aPTT and had higher circulating hF.IX antigen levels (Figure 3b,f). Similarly, 2/4 mice with CH mutation had low-titer IgG (and 4–5 BU) and less correction of the aPTT compared to 2/4 mice without IgG formation and <2 BU (Figure 3c,k,o).
Hepatic protocol is more efficacious and causes less antibody formation
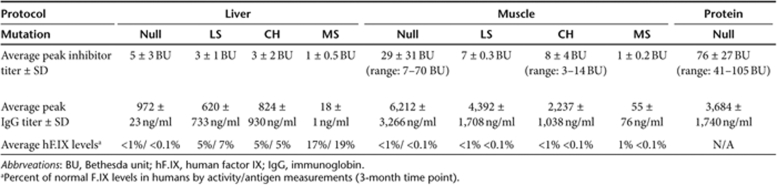
As summarized in Table 1, the hepatic protocol did not result in correction of hemophilia in Null mice, but was substantially more efficacious than muscle gene transfer in all other mouse lines. In LS and CH mice, hF.IX activity levels were on average 5% of normal (11% in those animals without antibody formation). MS mice produced an average hF.IX activity of 17%. Average vector-derived hF.IX antigen levels at 3 months were 5, 7, and 19% of normal for CH, LS, and MS mice, respectively. In muscle gene transfer, no correction was achieved for Null and LS mutations. MS mice showed an average hF.IX activity of ~1% of normal at 3 months, and CH mice had an average activity slightly <1%. Overall, antibody formation was substantially less following hepatic compared to muscle gene transfer (Table 1). In muscle gene transfer, the Null mutation is associated with the highest and most rapid antibody response followed by LS, CH, and MS mutations (Figure 2 and Table 1). These data inversely correlate with average systemic hF.IX activity, and also illustrate the high humoral immune response in the muscle compared to liver protocol (Table 1).
Table 1.
Summary of peak antibody responses to hF.IX and systemic hF.IX expression following muscle or liver gene transfer or protein therapy
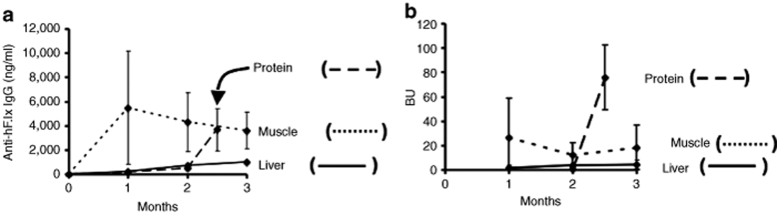
In order to obtain a comparison to treatment with recombinant hF.IX protein, the current standard therapy for hemophilia B, four Null mice received weekly intravenous injections of BeneFix (Wyeth Pharmaceuticals, Philadelphia, PA; 1 IU to restore ~70% of coagulation activity). After 10 weeks of treatment, the mice consistently formed high-titer inhibitors of 41–105 BU, similar to a subset of Null mice that received muscle gene transfer and 10 to 20-times higher than upon liver gene transfer (Figure 4a and Table 1). IgG titers were similar to muscle gene transfer at that time point (Figure 4b).
Figure 4.
Summary of humoral immune responses to hF.IX after liver- or muscle-directed gene transfer or protein therapy in Null mice. (a) IgG anti-hF.IX and (b) inhibitor titers upon protein therapy in Null mice (intravenous injection of 1 IU recombinant hF.IX protein once per week starting at week 0, n = 4, dashed line) in comparison to muscle (dotted line) and liver (solid line) gene transfer to Null mice. hF.IX, human factor IX; IgG, immunoglobin G.
Muscle gene transfer causes CD8+ cellular infiltrates in Null mutation mice
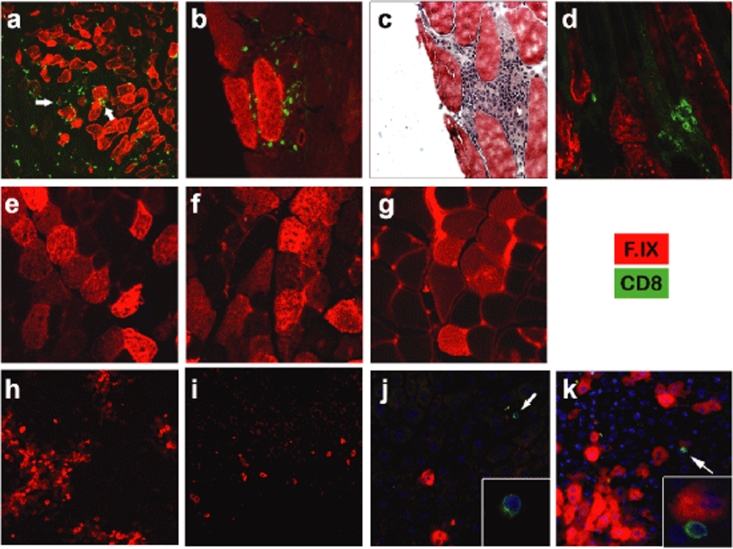
Transduced skeletal muscles of Null mice displayed large inflammatory infiltrates at 1 and 3 months after gene transfer, which contained CD8+ cells (Figure 5a–d). Despite these infiltrates, which were seen at all sites of transgene expression, hF.IX expressing fibers persisted without an obvious decline between 1 and 3 months, which is similar to our published results in outbred CD-1 mice using the identical vector.12 No CD8+ cellular infiltrates were seen for any of the other mutations at either time point (Figure 5e–g and data not shown).
Figure 5.
Immunofluorescent staining of hF.IX (red) and CD8 (green) in muscle and liver cross-sections after gene transfer to hemophilia B mice. (a–d) Muscle gene transfer to Null mice, (e) LS mice, (f) CH mice, and (g) MS mice. (c) Hematoxylin and eosin stain. Muscles were collected 30 days after gene transfer except for d (90 days). Two examples of the large number of CD8+ cells visible in a are indicated by arrows. (h–k) Hepatic gene transfer to Null mice (h, 1 month after gene transfer; i, 3 months, j–k, 2 months). DAPI stain of nuclei is shown in blue. Inserts: enlargement of CD8+ cell. Original magnification: a, ×40; b–g, ×200; h–k, ×100. DAPI, 4′-6-diamidino-2-phenylindole; hF.IX, human factor IX.
Hepatic gene transfer results in low grade CD8+ cell infiltrate and in gradual reduction of hF.IX expressing hepatocytes in Null mutation mice
Systemic hF.IX expression was transient upon hepatic gene transfer to Null mice. Livers of these mice showed hF.IX transgene expression in a large number of hepatocytes at 1 month after gene transfer (>5% as determined by image analysis), but substantially fewer expressing cells at 3.5 months (<1%, Figure 5h,i). However, no CD8+ cells or obvious inflammation or liver damage was found at either time point (Figure 5h,i, and data not shown). Therefore, additional animals were analyzed 2.5 months after gene transfer. Numbers of transgene expressing hepatocytes were similar to the 1-month time point. However, single CD8+ cells were found throughout liver parenchyma near areas of transgene expression, and, in some instances, adjacent to a hF.IX expressing hepatocyte (Figure 5j,k). Quantitative PCR showed similar vector copy numbers for 1- and 2.5-month time points followed by a approximately threefold decline by 3.5 months (data not shown).
Because systemic hF.IX expression levels were stable following hepatic gene transfer to LS, CH, and MS mice, and because we cannot differentiate between endogenous and AAV vector-derived hF.IX expression by hepatocytes by means of immunostaining, livers of these mice were not further investigated.
Mapping of hF.IX-specific CD8+ T-cell epitope in C3H/HeJ mice
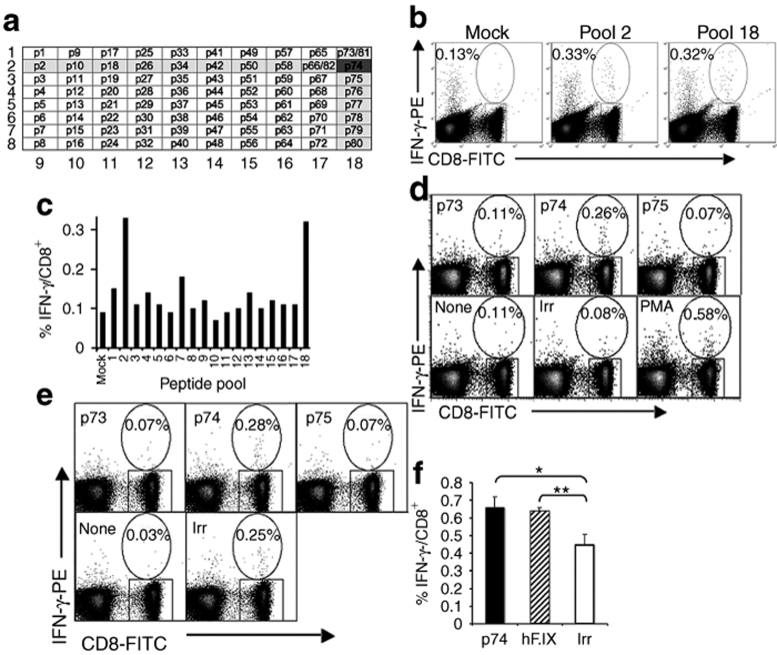
Previously, we had identified a dominant CD4+ T-cell epitope of hF.IX in C3H/HeJ mice (H-2k major histocompatibility complex haplotype).30 In order to be able to measure frequencies of hF.IX-specific CD8+ T-cell responses, we first determined CD8+ T-cell epitopes. Initially, wild-type C3H/HeJ mice received IM injections of adenoviral vector expressing hF.IX. Figure 6a–c shows the result of one of three independent experiments, in which splenocytes of immunized mice were stimulated in vitro with pools of 15-mer peptides spanning the entire mature hF.IX sequence. In all experiments, only pools containing peptide p74 (SGGPHVTEVEGTSFL) gave a CD8+IFN-γ+ response 2.5- to 3-fold above mock stimulation. When individual peptides of pools 2 and 18 were analyzed, only peptide p74 caused a CD8+IFN-γ+ response (~0.3% of CD8+ cells, fourfold above mock stimulation) in wild type or F9−/− (Null mutation) C3H/HeJ mice (Figure 6d,e and data not shown), thereby confirming that p74 contains a CD8+ T-cell epitope. Similar to the CD4+ T-cell epitope, the CD8+ T-cell epitope is located in the catalytic domain of hF.IX (Figure 1). CD8+IFN-γ+ T-cell responses to hF.IX were also detectable in Null mice upon AAV gene transfer, albeit at low frequency (~0.15% above background, Figure 6f).
Figure 6.
Identification of CD8+ T-cell epitope in C3H/HeJ mice by immunization using IM injection with Ad-hF.IX vector. (a) Matrix of pools of eight 15-mer peptides spanning the entire mature hF.IX amino acid sequence. Highlighted are pools 2 and 18, which yielded an IFN-γ+ CD8+ response, pointing toward peptide p74 to contain a CD8+ T-cell epitope. (b) Splenocytes showed an increased frequency of IFN-γ+ CD8+ when stimulated in vitro with peptide pools 2 and 18 and analyzed by flow cytometry. (c) Summary of IFN-γ+ CD8+ frequencies after stimulation with different peptide pools. (d–e) IFN-γ+ CD8+ T cell frequencies after stimulation with peptide p74, flanking peptides p73 or p75, mock media, an irrelevant peptide (ova), or nonspecifically stimulated with PMA or SEB. Experiments were performed in (b–d) wild-type or (e) F9−/− (F9 gene deletion) C3H/HeJ mice. (f) IFN-γ+ CD8+ T cell frequencies after IM administration of AAV-2-CMV-hF.IX vector in F9−/− C3H/HeJ mice and in vitro stimulation with irrelevant peptide (Ova), peptide p74, or F.IX protein. Shown are average frequencies ± SEM for three animals (experiments in b–d were performed using splenocytes pooled from three to four mice, while splenocytes from individual animals were assayed separately in the experiment in f; *P < 0.05, **P < 0.01 by unpaired Student's t-test). AAV-2, adeno-associated viral; CMV, cytomegalovirus; hF.IX, human factor IX; IFN-γ, interferon-γ.
T-cell responses against hF.IX are only detectable in Null mutation mice
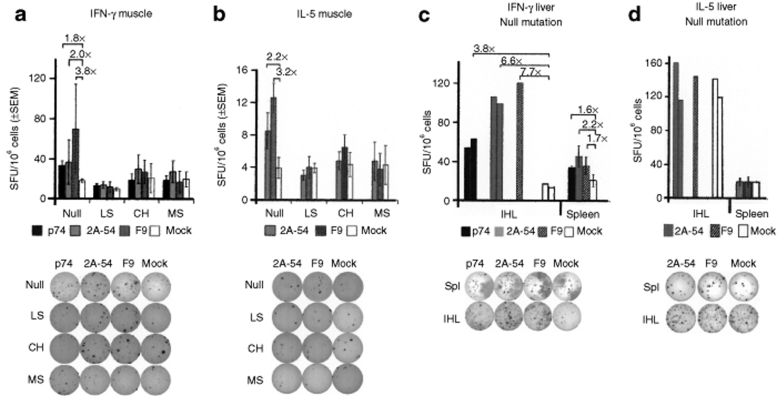
Because our previous studies showed that T-cell responses to a transgene product expressed from an AAV vector were detectable in the spleen only by 4 weeks after gene transfer, splenocytes were harvested at 30 days in experiments shown in Figures 6f and 7.12,33 Using the more sensitive enzyme-linked immunospot (ELISpot) assay, Th1, Th2, and CD8+ T-cell activation was detectable upon muscle gene transfer in Null mice, but not for the other mutations (Figure 7a,b). Hepatic gene transfer to Null mice resulted in similar frequencies of Th1 and CD8+ T cells in the spleen (Figure 7a,c). Intrahepatic lymphocytes contained higher frequencies of such cells compared to the splenocytes (Figure 7c). Lack of detectable Th2 response correlated with reduced IgG1 formation compared to the muscle protocol (Figures 2i and 7d, and Table 1).
Figure 7.
ELISpot assays to determine frequencies of hF.IX-specific cytokine secreting cells in hemophilia B C3H/HeJ containing different F9 mutations. (a) IFN-γ producing splenocytes following muscle-directed gene transfer and in vitro stimulation with peptide p74 (CD8+ T-cell epitope, black bars), peptide 2A-54 (CD4+ T-cell epitope, gray bars), or hF.IX protein (hatched bars), or mock (white bars). Mice were assayed individually; stimulations were performed in triplicate for each antigen and mouse. Results are average ± SEM as SFU per million splenocytes (n = 3–4 per line; Null: F9 gene deletion, LS: R338stop, CH: G381E, MS: R180W). (b) IL-5 producing splenocytes following muscle-directed gene transfer and in vitro stimulation with peptide 2A-54 (CD4+ T-cell epitope), or hF.IX protein, or mock media (n = 3–4 mice per line). (c) IFN-γ producing IHL or splenocytes following liver-directed gene transfer in Null mice and in vitro stimulation with peptide p74 (CD8+ T-cell epitope), peptide 2A-54 (CD4+ T-cell epitope), or hF.IX protein, or mock media (n = 3). In contrast to splenocytes, IHL were pooled prior to culture, and data are reported for individual wells. (d) IL-5 producing IHL or splenocytes following liver-directed gene transfer in Null mice and in vitro stimulation with peptide peptide 2A-54 (CD4+ T-cell epitope), or hF.IX protein, or mock media. Examples of wells are shown below each graph. hF.IX, human factor IX; IFN-γ, interferon-γ; IHL, intrahepatic lymphocytes; SFU, spot-forming units.
Discussion
The results of this study support the hypothesis that expression of endogenous protein, even a mutant one, such as in the setting of a late stop codon or a missense mutation, reduces the risk and the magnitude of immune responses against a therapeutic protein in gene replacement therapy. In addition, the specific design of the protocol, specifically the choice of target tissue, influences transgene product-specific immunity.
Expression levels achieved in the experiments detailed here in mice were comparable to those reported at the highest vector dose administered in clinical trials with the same vectors, i.e., ~1% of normal for muscle-directed gene transfer and 11–17% of normal in hepatic gene transfer (up to 1% for muscle and 11% for liver transduction were reported in the respective human studies).6,7 Furthermore, transgenic mice expressed F.IX variants found in humans or analogous to a canine mutation, so that results can be compared to clinical and large animal studies (albeit T-cell responses in canine skeletal muscle may be more marked than in mice).34,35
Antibody formation against hF.IX is modulated as a function of the mutation and is substantially reduced upon hepatic gene transfer
Mechanisms have evolved that allow the immune system to eliminate or regulate self-reactive lymphocytes. Consistent with this concept, the strength of the immune response to hF.IX inversely correlated with the degree of conservation of endogenous coding information and the level of hepatic hF.IX antigen expressed in the hemophilic mice. No evidence for cellular or humoral immune responses to hF.IX were detected upon gene transfer in MS mice, which have circulating nonfunctional antigen and high hepatic expression. Interestingly, Zhang et al. found that hemophilia B mice expressing crm+ hF.IX variant R333Q also failed to form inhibitors after muscle gene transfer with AAV vectors (but still produced noninhibitory IgG).36
All three mutations with endogenous hF.IX antigen (LS, CH, and MS) express the dominant CD4+ T-cell epitope and, in contrast to Null mice, had undetectable T-cell responses by ELISpot. Yet, animals expressing crm− hF.IX variants (LS and CH) were not entirely protected from antibody formation. This problem remained significant in muscle-directed gene transfer. The muscle protocol consistently induced an inhibitor response of >5 BU in LS mice, and more variable responses in CH mice. IgG titers against hF.IX in LS were intermediate between Null and CH mice. Although we have documented previously that canines with the CH mutation may form inhibitors after IM injection at high vector doses (or if a different viral capsid was used), the sum of all data indicate that the LS mutation is overall less protective against antibody formation than the CH mutation.11,37,38 Hemophilic C57BL/6 mice formed inhibitors only in Null and LS mice (but not in CH or MS mice) upon muscle gene transfer with identical vector and dose, albeit at lower titers. The stronger response to hF.IX in C3H/HeJ mice allowed further differentiation between these mutations. In spite of this, antibody titers that developed in LS and CH mice were low to undetectable and did not interfere with a stable increase in coagulation activity using the hepatic route.
In all experiments, antibodies against hF.IX were almost exclusively of the Th2-dependent IgG1 isotype. Muscle gene transfer to Null mice generated the most potent antibody response, and only in this combination of mutation and route, were we able to detect a Th2 response by ELISpot.
Hepatic gene transfer results in weaker antibody formation compared to protein therapy
It is noteworthy that human subjects with Null mutations often develop inhibitors during protein therapy, and therefore would not have been eligible for enrollment in the gene therapy trials.28 Interestingly, hepatic gene transfer elicits a substantially weaker antibody response compared to protein therapy. Antibody titers upon muscle gene transfer were more similar to protein therapy. In humans, the MS and LS mutations are not associated with inhibitor formation in protein treatment, which is also the case for the MS mutation in treatment of murine hemophilia B by gene therapy. Missense mutations in general are very rarely associated with inhibitor formation in humans receiving protein therapy. Additional experiments are required to study inhibitor formation in protein therapy for LS and CH mice. Of note, IgG titers in liver gene transfer in Null mice were below those measured for muscle gene transfer in LS and CH mice.
Endogenous hF.IX expression prevents CD8+ T-cell responses and inflammation in skeletal muscle
All mutations with endogenous expression failed to develop an hF.IX-specific CD8+ T-cell response. This was also observed for the nonsense mutation at amino acid residue 338 (LS), although the resulting hF.IX molecule, in contrast to the missense mutation, lacked the identified CD8+ T-cell epitope. Because the LS mutation retained the CD4+ T-cell epitope, it is possible that there was insufficient T help (albeit CD4+ T-cell responses to the viral capsid should have provided help) or that endogenous regulatory T cells controlled the response.
Despite activation of hF.IX-specific CD8+ T cells after liver- or muscle-directed gene transfer in the Null mice, the outcome of these responses were different. Skeletal muscle showed a robust cellular infiltrate at all sites of transgene expression, which was part of a prolonged inflammatory response. Because this response was not seen for mice with other F9 mutations, and consistent with findings by several other labs, we can surmise that CD8+ T-cell infiltrates were directed against hF.IX rather than capsid antigen.12,13,39 Similar to our published study in other strains, the infiltrate lasted for at least 3 months without noticeable loss of transduced fibers.12 We previously documented that CD8+ T cells activated by AAV-mediated gene transfer to muscle are not fully functional in the presence of persistent antigen expression.33 These cells retain their lytic activity, but proliferate less and upregulate molecules associated with T-cell suppression (such as CTLA4) and exhaustion (such as PD-1).34 Despite the resulting lack of destruction of transduced fibers, the prolonged inflammatory response in the muscle remains a safety concern.
Liver gene transfer also resulted in CD8+ T-cell activation in the Null mice. In this case, we observed the expected level of hepatocyte transduction at 1 month. A substantial but not complete loss of expression was seen by 3.5 months without clear evidence for inflammation or liver damage. However, intrahepatic lymphocytes contained hF.IX-specific interferon-γ (IFN-γ) producing CD4+ and CD8+ T cells, and low numbers of CD8+ T cells were transiently detected in transduced liver parenchyma. LS/CH/MS mutation mice had stable expression during the same time period. These results imply that hF.IX-specific CD8+ T cell–mediated lysis of transduced hepatocytes contributes to the decline in vector copy numbers and loss of F.IX expression.
In summary, our study documents that on an otherwise genetically identical background, the underlying F9 mutation has a substantial effect on the immune response to the transgene product in gene therapy for hemophilia B. Endogenous expression of a nonfunctional protein modifies the immune response. Incidence and titers of antibodies against hF.IX were highest for mice with a Null mutation (gene deletion), reduced for a late stop codon, more reduced for a crm− missense mutation, and absent for a crm+ missense mutation. CD8+ T-cell responses were only observed for the Null mutation, suggesting that endogenous expression more easily abrogates CTL responses than antibody formation. The degree, to which the immune response is reduced by endogenous expression, is not only a function of the specific mutation but also of the gene transfer protocol. Hepatic gene transfer as performed in an ongoing clinical trial showed better efficacy and substantially reduced immune responses compared to the muscle protocol. Sustained expression was documented for all except the Null mutation. Even in Null mice, inflammatory responses to the hF.IX transduced livers were limited, and antibody titers against hF.IX were minimal compared to muscle-directed or protein therapy. Further improvement in vector development could additionally reduce such responses upon hepatic gene transfer.40
Materials and Methods
Vectors. Expression cassettes for AAV-2-CMV-hF.IX and AAV-2-ApoE/hAAT-hF.IX vectors were identical to those used in clinical trials.6,7 The cytomegalovirus (CMV) immediate early enhancer/promoter was used for expression of hF.IX in muscle, whereas the human α1-antitrypsin promoter combined with the hepatocyte control region and enhancer of the apolipoprotein A were used for liver gene transfer. Both cassettes contain a 1.4-kb portion of intron I of the human F9 gene and SV40 or bovine growth hormone polyadenylation signals. AAV vectors (serotype 2) were produced by triple transfection of human embryonic kidney-293 cells in roller bottles, and were purified from lysate by polyethylene glycol precipitation followed by cesium chloride density gradient centrifugation.6,7,41 Vectors were filter-sterilized, and titers and purity were determined by slot-blot hybridization and silver staining.
Animal strains and experiments. Hemophilia B mice with targeted deletion of endogenous F9 (“Null mutation”) were bred on C3H/HeJ background for >10 generations. Mice transgenic for hF.IX variants (human F9 complementary DNA including a 0.3-kb portion of intron I expressed from liver-specific transthyretin promoter) were as published.38 These animals express hF.IX with late stop codon at amino acid residue 338 (“LS”, crm−); crm− G381E missense mutation (“CH”, identical mutation as in hemophilia B dogs at University of North Carolina–Chapel Hill); or crm+ R180W missense mutation. These lines were originally numbered as LS-37, cCH-6, and MS-12, and contain 6, 10, and 1 copy of the human F9 gene, respectively.38 The lines were repeatedly backcrossed onto C3H/HeJ background (>10 generation), and finally crossed with Null mice in order to eliminate endogenous murine F.IX expression. All resulting lines of hemophilia B mice (hemizygous for hF.IX transgene) had aPTTs of >60 seconds, consistent with a coagulation activity of <1%. Null, LS, and CH mice have no detectable circulating hF.IX antigen (<3 ng/ml), whereas MS mice express ~250 ng hF.IX/ml plasma. At each breeding step, genomic DNA isolated from blood cells served as a template for three separate PCRs using primers specific to murine F9 wild type and knock out alleles and to the human F9 transgene as published.38,42 To determine hF.IX antigen levels in the liver, hepatic protein was extracted from mice each line using IPP-150 buffer (50 mmol/l Tris–Cl, pH 7.4, and 150 mmol/l NaCl; supplemented with 0.1% Igepal and protease inhibitor mix) and sonication. Total protein was measured with the Pierce bicinchoninic acid protein assay kit (Thermo Scientific, Waltham, MA).
Viral vectors were administered IM into two sites (quadriceps and tibialis anterior of one hind limb) or into the portal circulation via the splenic capsule as published.9,20 For the latter procedure, hemostasis was temporarily restored with wild-type C3H/HeJ plasma. For protein therapy, recombinant hF.IX (BeneFix; Wyeth Pharmaceuticals) was injected weekly into the tail vein at 1 IU/dose. Blood samples were collected from retro-orbital plexus using heparinized capillary tubes or from the tail into citrate buffer as published.9,20
Analyses of plasma samples. Enzyme-linked immunosorbent assay–based measurements of hF.IX antigen and anti-hF.IX IgG levels in murine plasma samples were as published.20 The enzyme-linked immunosorbent assay for anti-hF.IX IgG was sensitive to ~200 ng/ml. Measurements of the aPTT using a fibrometer also as described.9 For the Bethesda assay, mouse plasma samples were used for twofold serial dilutions in imidazole buffer (BioMerieux, Durham, NC). Each dilution (50 µl) was mixed with an equal volume of Verify 1 human plasma (Organon Technika, Durham, NC), and incubated at 37 °C for 2 hours followed by addition of F.IX-deficient human plasma and aPTT reagent (50 µl each, Organon Technika) at 37 °C for 3 minutes. After addition of CaCl2 (50 µl), time-to-clot formation was measured with a fibrometer (Fibrosystem; BBL, Cockeysville, MD). Percent residual activity was calculated from a standard curve that was obtained by aPTT on twofold serial dilutions of Verify 1 human plasma (diluted in imidazole buffer and mixed with F.IX deficient plasma and aPTT reagent in identical fashion; aPTTs of twofold serial dilutions, up to 1:4, of Verify 1, were also determined after 2 hours at 37 °C to adjust for incubation-related loss of coagulation activity).
Immunohistochemistry. Liver tissue was frozen in optimal cutting temperature in a dry ice-isopentene bath. Muscles were snap-frozen in liquid nitrogen–cooled isopentene.6,43 Cryosections of tissue were fixed with 3% paraformaldehyde for 10 minutes at room temperature, followed by permeabilization with methanol for 10 minutes. Sections were blocked with 2% donkey serum in phosphate buffered saline for 30 minutes. Rat anti-CD8α (Pharmingen, San Diego, CA) in a dilution of 1:20 and goat anti-hF.IX (Affinity Biologicals, Ontario, Canada; 1:400) were applied in 2% donkey serum for 90 minutes. After washing, tissue sections were incubated with secondary antibody Alex Fluor-488 donkey anti-rat IgG for CD8 stain and Alex Fluor-568 donkey anti-goat IgG for hF.IX (1:100 dilution; Invitrogen, Oregon, WA). Fluorescence microscopy was performed with a Nikon E800 microscope (Nikon, Tokyo, Japan). Images were captured with a Cool Snap-Pro camera and analyzed with Image Pro-Plus software (Media Cybernetics, Silver Spring, MD).
CD8+ T-cell epitope mapping. An library of 82 peptides spanning the entire mature hF.IX sequence was generated as 15 mers overlapping by 10 amino acids (Mimotopes, Victoria, Australia). Peptide stocks were resuspended at 5 mg/ml in 50% acetonitrile/0.1% acetic acid. A matrix was designed, so that each peptide was represented in 2 of 18 pools, each containing 8–11 peptides. C3H/HeJ mice received IM injections of E1/E3-deleted adenoviral vector expressing hF.IX from the CMV immediate early enhancer/promoter. Spleens were harvested 9 days later for in vitro stimulation. Mouse IFN-γ secretion assay-detection kit (phycoerythrin) (Miltenyi Biotech, Auburn, CA) was used as per the manufacturer's recommendations. Briefly, spleens were harvested into 1× Liebowitz's-15 (Cellgro; Mediatech, Herndon, VA) at room temperature, homogenized, filtered through a 70-µm cell strainer, and centrifuged for 10 minutes at 300 g at room temperature. Cells were incubated with ACK lysing buffer (BD Bioscience, San Jose, CA) for 5 minutes and washed twice with 5-mMLC medium (Dulbecco's modified Eagle's medium, 5% mouse serum, 1% Pen/Strep, 10 mmol/l Hepes, 1 mmol/l sodium pyruvate, 0.1 mmol/l nonessential amino acids, 10−6 mol/l 2-mercaptoethanol). The cells were resuspended in 5-mMLC medium at 1 × 107 cells/ml and added to a round-bottom 96-well plate (1 × 106/well in triplicate) for in vitro stimulation and cytokine secretion assay. Peptide was added to a final concentration of 2 µg/ml of each peptide for the pools or 10 µg/ml for a single peptide. Media also contained 10 U/ml rmIL-2. PMA/ionomycin (at 0.05 µg/ml and 1 µg/ml, respectively) or staphylococcal enterotoxin B was used as a positive control (Sigma, St Louis, MO). Cells were incubated for 5–6 hours at 10% CO2 at 37 °C. Subsequently, cells were transferred to 5 ml × 75 mm tubes and incubated with “Catch Reagent” for 5 minutes at 4 °C. Prewarmed media was added, and samples were incubated under slow continuous rotation at 37 °C for 45 minutes. Following washes, cells were incubated with phycoerythrin-conjugated anti-IFN-γ detection antibody (Miltenyi Biotech), allophycocyanin -conjugated anti-CD45/B220 (BD Bioscience) (for B-cell exclusion), and fluorescein isothiocyanate-conjugated anti-CD8 (BD Bioscience). Just prior to analysis, triplicates were combined, and 7-amino-actinomycin D was added. Samples were analyzed using an EPICS XL (Beckman–Coulter, Miami, FL) or LSR-II (BD Bioscience) flow cytometer.
ELISpot assays. ELISpot kits for mouse IFN-γ and interleukin (IL)-5 cytokines from R&D Systems (Minneapolis, MN) were used as per the manufacturer's recommendations. Briefly, splenocytes from individual animals were applied in triplicate at 106 cells/well (coated with cytokine-specific antibody) in 5-MLC (containing 5% FCS, otherwise identical to 5-mMLC). Wells additionally contained 10 U/ml rmIL-2 and either 10 µg/ml hF.IX-derived peptide containing an immunodominant CD4+ T-cell epitope (2A-54), CD8+ T-cell epitope (p74) (Anaspec, San Jose, CA), or rhF.IX (BeneFix). Plates were incubated in 10% CO2 at 37 °C for 18 hours for IFN-γ or 48 hours for IL-5. Plates were washed and incubated with cytokine-specific biotinylated detection antibodies. Spots were detected using ELISpot Blue Color Module (R&D Systems) and counted with the ImmunoSpot Analyzer (Cellular Technology, Shaker Heights, OH). Results were calculated as spot-forming units per 106 total cells and compared to mock-stimulated cultures.
DNA analysis. Total genomic DNA was extracted from liver tissue of Null mice 1 or 3 months after hepatic gene transfer (n = 3 per time point). Quantitative PCR for glyceraldehyde-3-phosphate dehydrogenase (endogenous gene) and hF.IX (vector-encoded transgene) was performed with the MiQ cycler system (Bio-Rad, Hercules, CA). Amplification was compared to a standard curve of plasmid DNA mixed with genomic DNA from an untreated Null mouse.
Acknowledgments
This work was supported by NIH grants P01 HL078810 to H.C.J.E., K.A.H., and R.W.H. (Projects 1, 2, and 3), R01 AI/HL51390 to R.W.H., and by T32DK074367 (support for S.N.); and by the Howard Hughes Medical Institute (K.A.H.). B.E.H. and O.C. were supported by a fellowship and a Scientist Development Grant from the American Heart Association. The authors thank Ryan Fiske and David Miller for assistance with the hemophilia B mouse colony. R.W.H. has been receiving royalty payments from Genzyme Corp., for license of AAV-FIX gene transfer technology. O.C. and B.E.H. performed most experiments and wrote parts of the manuscript; B.M. contributed experimental data; S.N. and M.C. assisted with animal experiments; S.Z. produced viral vectors; H.C.J.E. contributed experimental strategies, provided protocols, and edited the manuscript; K.A.H. directed parts of the study, provided critical reagents, and edited the manuscript; R.W.H. directed most of the study and wrote parts of the manuscript.
REFERENCES
- Wang L., and , Herzog RW. AAV-mediated gene transfer for treatment of hemophilia. Curr Gene Ther. 2005;5:349–360. doi: 10.2174/1566523054065048. [DOI] [PubMed] [Google Scholar]
- Nienhuis AW. Development of gene therapy for blood disorders. Blood. 2008;111:4431–4444. doi: 10.1182/blood-2007-11-078121. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Yang EY, Couto LB, Hagstrom JN, Elwell D, Fields PA, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- Snyder RO, Miao C, Meuse L, Tubb J, Donahue BA, Lin HF, et al. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Arruda VR, Fields PA, Milner R, Wainwright L, De Miguel MP, Donovan PJ, et al. Lack of germline transmission of vector sequences following systemic administration of recombinant AAV-2 vector in males. Mol Ther. 2001;4:586–592. doi: 10.1006/mthe.2001.0491. [DOI] [PubMed] [Google Scholar]
- Fields PA, Arruda VR, Armstrong E, Chu K, Mingozzi F, Hagstrom JN, et al. Risk and prevention of anti-factor IX formation in AAV-mediated gene transfer in the context of a large deletion of F9. Mol Ther. 2001;4:201–210. doi: 10.1006/mthe.2001.0441. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Mount JD, Arruda VR, High KA., and , Lothrop CD. Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001;4:192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Fields PA, Arruda VR, Brubaker JO, Armstrong E, McClintock D, et al. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum Gene Ther. 2002;13:1281–1291. doi: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- Wang L, Cao O, Swalm B, Dobrzynski E, Mingozzi F., and , Herzog RW. Major role of local immune responses in antibody formation to factor IX in AAV gene transfer. Gene Ther. 2005;12:1453–1464. doi: 10.1038/sj.gt.3302539. [DOI] [PubMed] [Google Scholar]
- Wang L, Dobrzynski E, Schlachterman A, Cao O., and , Herzog RW. Systemic protein delivery by muscle-gene transfer is limited by a local immune response. Blood. 2005;105:4226–4234. doi: 10.1182/blood-2004-03-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom JN, Couto LB, Scallan C, Burton M, McCleland ML, Fields PA, et al. Improved muscle-derived expression of human coagulation factor IX from a skeletal actin/CMV hybrid enhancer/promoter. Blood. 2000;95:2536–2542. [PubMed] [Google Scholar]
- Liu YL, Mingozzi F, Rodriguéz-Colôn SM, Joseph S, Dobrzynski E, Suzuki T, et al. Therapeutic levels of factor IX expression using a muscle-specific promoter and adeno-associated virus serotype 1 vector. Hum Gene Ther. 2004;15:783–792. doi: 10.1089/1043034041648453. [DOI] [PubMed] [Google Scholar]
- Jiang H, Pierce GF, Ozelo MC, de Paula EV, Vargas JA, Smith P, et al. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol Ther. 2006;14:452–455. doi: 10.1016/j.ymthe.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Niemeyer GP, Herzog RW, Mount J, Arruda VR, Tillson DM, Hathcock J, et al. Long-term correction of inhibitor-prone hemophilia B dogs treated with liver-directed AAV2-mediated factor IX gene therapy. Blood. 2009;113:797–806. doi: 10.1182/blood-2008-10-181479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F., and , High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7:316–324. doi: 10.2174/156652307782151425. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C, et al. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao O, Furlan-Freguia C, Arruda VR., and , Herzog RW. Emerging role of regulatory T cells in gene transfer. Curr Gene Ther. 2007;7:381–390. doi: 10.2174/156652307782151506. [DOI] [PubMed] [Google Scholar]
- Lüth S, Huber S, Schramm C, Buch T, Zander S, Stadelmann C, et al. Ectopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific Tregs. J Clin Invest. 2008;118:3403–3410. doi: 10.1172/JCI32132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE, et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110:2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman BE, Dobrzynski E, Wang L, Hirao L, Mingozzi F, Cao O, et al. Muscle as a target for supplementary factor IX gene transfer. Hum Gene Ther. 2007;18:603–613. doi: 10.1089/hum.2007.042. [DOI] [PubMed] [Google Scholar]
- Lozier J.Genetics of the immuneresponse to proteins expressed after gene transfer Gene Therapy Immunology 2009Wiley-Blackwell: Hoboken, NJ; 289–310.In: Herzog, RW (ed.) [Google Scholar]
- Zhang HG, High KA, Wu Q, Yang P, Schlachterman A, Yu S, et al. Genetic analysis of the antibody response to AAV2 and factor IX. Mol Ther. 2005;11:866–874. doi: 10.1016/j.ymthe.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Ljung RC.Gene mutations and inhibitor formation in patients with hemophilia B Acta Haematol 19959449–52.suppl 1 [DOI] [PubMed] [Google Scholar]
- Herzog RW., and , Dobrzynski E. Immune implications of gene therapy for hemophilia. Semin Thromb Hemost. 2004;30:215–226. doi: 10.1055/s-2004-825635. [DOI] [PubMed] [Google Scholar]
- Cao O, Armstrong E, Schlachterman A, Wang L, Okita DK, Conti-Fine B, et al. Immune deviation by mucosal antigen administration suppresses gene-transfer-induced inhibitor formation to factor IX. Blood. 2006;108:480–486. doi: 10.1182/blood-2005-11-4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannelli F, Green PM, Sommer SS, Poon M, Ludwig M, Schwaab R, et al. Haemophilia B: database of point mutations and short additions and deletions--eighth edition. Nucleic Acids Res. 1998;26:265–268. doi: 10.1093/nar/26.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillicrap D. The molecular basis of haemophilia B. Haemophilia. 1998;4:350–357. doi: 10.1046/j.1365-2516.1998.440350.x. [DOI] [PubMed] [Google Scholar]
- Lin SW, Hensley SE, Tatsis N, Lasaro MO., and , Ertl HC. Recombinant adeno-associated virus vectors induce functionally impaired transgene product-specific CD8+ T cells in mice. J Clin Invest. 2007;117:3958–3970. doi: 10.1172/JCI33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog RW., and , Arruda VR. Substantial immune suppression required in gene therapy for muscular dystrophy. Neuromuscul Disord. 2008;18:83–84. doi: 10.1016/j.nmd.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Yuasa K, Yoshimura M, Urasawa N, Ohshima S, Howell JM, Nakamura A, et al. Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products. Gene Ther. 2007;14:1249–1260. doi: 10.1038/sj.gt.3302984. [DOI] [PubMed] [Google Scholar]
- Zhang TP, Jin DY, Wardrop RM, Gui T, Maile R, Frelinger JA, et al. Transgene expression levels and kinetics determine risk of humoral immune response modeled in factor IX knockout and missense mutant mice. Gene Ther. 2007;14:429–440. doi: 10.1038/sj.gt.3302881. [DOI] [PubMed] [Google Scholar]
- Arruda VR, Schuettrumpf J, Herzog RW, Nichols TC, Robinson N, Lotfi Y, et al. Safety and efficacy of factor IX gene transfer to skeletal muscle in murine and canine hemophilia B models by adeno-associated viral vector serotype 1. Blood. 2004;103:85–92. doi: 10.1182/blood-2003-05-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino DE, Armstrong E, Edmonson S, Liu YL, Pleimes M, Schuettrumpf J, et al. Novel hemophilia B mouse models exhibiting a range of mutations in the Factor IX gene. Blood. 2004;104:2767–2774. doi: 10.1182/blood-2004-03-1028. [DOI] [PubMed] [Google Scholar]
- Siders W, Shields J, Kaplan J, Lukason M, Woodworth L, Wadsworth S, et al. Hum Gene Ther. epub ahead of print; 2008. Cytotoxic T-Lymphocyte (CTL) responses to the transgene product and not AAV capsid protein limit transgene expression in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M, Nayak S, Hoffman BE, Terhorst C, Cao O., and , Herzog RW. Hum Gene Ther 2009. Improved induction of immune tolerance to factor IX by hepatic AAV-8 gene transferepub ahead of print [DOI] [PMC free article] [PubMed]
- Liu YL, Wagner K, Robinson N, Sabatino D, Margaritis P, Xiao W, et al. Optimized production of high-titer recombinant adeno-associated virus in roller bottles. BioTechniques. 2003;34:184–189. doi: 10.2144/03341dd07. [DOI] [PubMed] [Google Scholar]
- Fields PA, Kowalczyk DW, Arruda VR, McCleland ML, Hagstrom JN, Pasi KJ, et al. Choice of vector determines T cell subsets involved in immune responses against the secreted transgene product factor IX. Mol Ther. 2000;1:225–235. doi: 10.1006/mthe.2000.0032. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Hagstrom JN, Kung SH, Tai SJ, Wilson JM, Fisher KJ, et al. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]