Abstract
Ex vivo gene therapy is an interesting alternative to orthotopic liver transplantation (OLT) for treating metabolic liver diseases. In this study, we investigated its efficacy and biosafety in nonhuman primates. Hepatocytes isolated from liver lobectomy were transduced in suspension with a bicistronic liver-specific lentiviral vector and immediately autotransplanted (SLIT) into three cynomolgus monkeys. The vector encoded cynomolgus erythropoietin (EPO) and the conditional suicide gene herpes simplex virus-thymidine kinase (HSV-TK). Survival of transduced hepatocytes and vector dissemination were evaluated by detecting transgene expression and vector DNA. SLIT was safely performed within a day in all three subjects. Serum EPO and hematocrit rapidly increased post-SLIT and their values returned to baseline within about 1 month. Isoforms of EPO detected in monkeys' sera differed from the physiological renal EPO. In liver biopsies at months 8 and 15, we detected EPO protein, vector mRNA and DNA, demonstrating long-term survival and functionality of transplanted lentivirally transduced hepatocytes. Valganciclovir administration resulted in complete ablation of the transduced hepatocytes. We demonstrated the feasibility and biosafety of SLIT, and the long term (>1 year) functionality of lentivirally transduced hepatocytes in nonhuman primates. The HSV-TK/valganciclovir suicide strategy can increase the biosafety of liver gene therapy protocols by safely and completely ablating transduced hepatocytes on demand.
Introduction
Although results of orthotopic liver transplantation (OLT) in children are nowadays very good, with one-year survival rates exceeding 90% (ref. 1) and close to normal quality of life in most transplanted children, OLT remains a delicate operation, and may have numerous surgical and medical complications. OLT is limited by the scarcity of transplantable organs, and lifelong immunosuppression is required. Uncertainties remain about the long-term outcome of these children, with several long-term problems such as liver graft fibrosis and possible need for retransplantation in adulthood, renal failure due to toxicity of medications, and increased risk of malignancies due to immunosuppression.2 These limitations prompted the search for alternate therapies. Promising results have been reported with hepatocyte transplantation for inborn errors of metabolism,3 resulting in a less invasive surgical approach. Allogeneic hepatocyte transplantation is a promising approach,4,5 but is hampered by the scarcity of transplantable allogeneic hepatocytes, their variable engraftment rate and the difficulty to monitor allograft rejection, leading to the loss of transplanted hepatocytes and related metabolic function.6 Nevertheless, it may be useful as a bridge to OLT. To overcome limitations of allogeneic hepatocyte transplantation, autotransplantation of genetically modified hepatocytes, or so-called ex vivo liver gene therapy, seems an interesting approach to provide long-lasting treatment of metabolic liver diseases. A landmark pilot study in patients with familial hypercholesterolemia,7 using oncoretroviral vectors and cultured hepatocytes, showed some important obstacles: (i) low transduction efficiency of the oncoretroviral vector, which is limited by the low in vitro proliferative ability of adult hepatocytes even in the presence of growth factors, and (ii) low recovery of cell (30%) due to in vitro damage to cultured hepatocytes7 and diminished survival after reimplantation. The advent of vectors derived from human immunodeficiency virus and other lentiviruses could overcome these obstacles. Indeed, lentiviral vectors have a large cloning capacity, are easy to generate, and can lead to an efficient delivery, integration, and long-term expression of transgenes into nondividing differentiated cells.8 Human primary hepatocytes are highly susceptible to lentiviral vectors, almost all cells being transduced after a single and short exposure to those vectors.9 Here, we describe the upscaling to macaques liver of our ex vivo approach, in which isolated hepatocytes are transduced in suspension with a lentiviral vector and immediately transplanted (SLIT).10 SLIT alleviates the need for plating and primary culture of isolated hepatocytes. It preserves full engraftment and functionality of transduced hepatocytes, which can participate in liver regeneration.9,11 It should be noted that the easy manipulation of cell suspensions greatly facilitates large-scale ex vivo transduction of those billions of hepatocytes isolated from a patient, and consequently, almost all isolated hepatocytes can be transplanted. We recently demonstrated the therapeutic proof-of-principle of SLIT for treating inherited metabolic liver diseases in the Gunn rat, the animal model of Crigler-Najjar syndrome type 1 (ref. 10)
In the present study, we aim to (i) demonstrate the feasibility of SLIT in the cynomolgus monkey, an animal model close to human infants, (ii) demonstrate the long-term transgene expression in transplanted animals, and (iii) assess the biosafety of this procedure, which is a prerequisite before conducting clinical trials.
Results
Vector design and functionality in vitro and in mice
To easily assess the long-term viability and functionality of lentivirally transduced hepatocytes following SLIT, we designed a bicistronic lentiviral vector encoding the cynomolgus erythropoietin (EPO) and herpes simplex virus-thymidine kinase (HSV-TK) under the control of the liver-specific murine transthyretin (mTTR) promoter.11 We verified the in vitro functionality of the mTTR-cmEPO-TK vector by transducing hepatoma HuH7 cells, and we evaluated the release of EPO in cell culture medium by enzyme-linked immunosorbent assay and the integrated vector copy number per cell by quantitative PCR (qPCR). Inclusion of the hepatic locus region from apolipoprotein E gene in vector backbone increased EPO secretion by fivefold in comparison with a similar vector without (data not shown). The functionality of HSV-TK conditional cell killing system was then tested 4 days postinfection by cultivating transduced HuH7 cells in the presence of 2 µmol/l ganciclovir (GCV). GCV-treated cells displayed a dose–response decrease in cell viability in culture by analyzing both the cell shape and cell viability using colorimetric cell viability assay (Supplementary Figures S1 and S2). Finally, we confirmed the in vivo functionality of mTTR-cmEPO-TK vector in mice. One-day-old mice were injected via the temporal vein with mTTR-cmEPO-TK, vector dose of 2 × 108 transducing units. Serum EPO level increased in injected mice and, as a consequence, an increase in hematocrit by 69% was observed, as measured at day 30 postinjection (Supplementary Figure S3). EPO was not detected in the sera of control noninjected mice.
In conclusion, these results demonstrate both the in vitro and in vivo functionality of the mTTR-cmEPO-TK lentiviral vector, which is able to stably transduce hepatocytes with secretion of EPO, to increase hematocrit and to selectively ablate cells expressing HSV-TK upon GCV treatment of transduced cells.
Follow-up of serum EPO in transplanted macaques
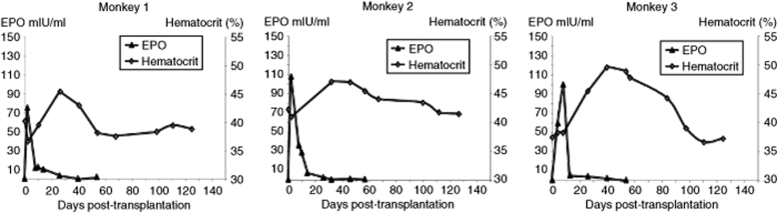
The complete SLIT was safely performed in all animals. Serum level of alanine aminotransferase significantly increased by 10-fold and returned to normal level by 10 days post-SLIT as a normal physiological response after liver surgery. Serum levels of other established markers of liver injury (aspartate aminotransferase, γ-glutamyltransferase, and bilirubin) did not change significantly (data not shown). Following autologous transplantation of lentivirally transduced hepatocytes, a rapid increase in serum EPO in all three animals was observed (Figure 1). However, serum EPO concentration returned to baseline within 1 month with a peak at day 2–4 post-transplantation. This increase in EPO was translated physiologically in a subsequent increase in hematocrit (Figure 1).
Figure 1.
Serum EPO and hematocrit follow-up in the three monkeys after SLIT using the mTTR-cmEPO-TK lentiviral vector. EPO, erythropoietin; SLIT, suspension with a lentiviral vector and immediately transplanted.
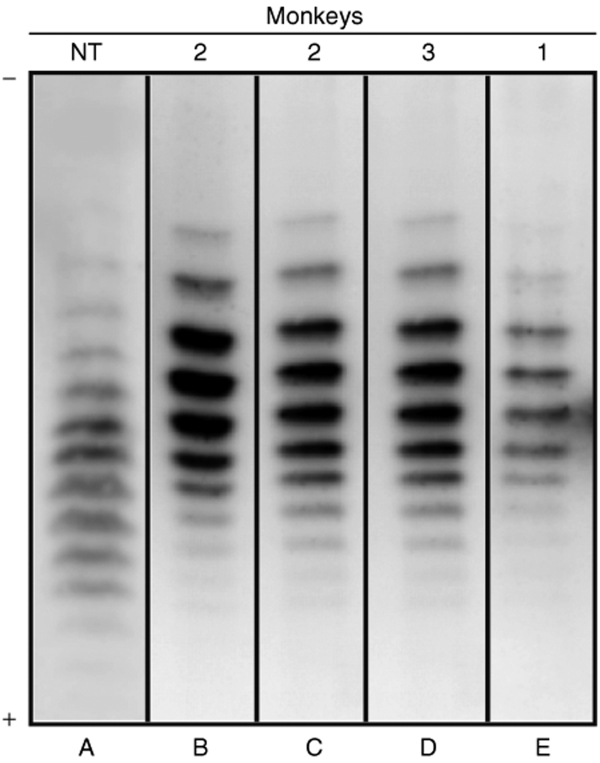
To demonstrate that the increase in EPO was not a response to surgical bleeding, serum EPO was analyzed for its isoelectric profile. Indeed, it had been previously shown that transgene-derived EPOs following adeno-associated virus–cmEPO vector injection in macaques presented different isoelectric patterns as compared to endogenous EPO.12,13 Serum EPO patterns observed after SLIT were clearly different (more basic isoforms) from that of the physiological hormone (Figure 2). This demonstrates the in vivo functionality of transduced hepatocytes.
Figure 2.
Isoelectric patterns of serum erythropoietin (EPO). Physiological serum EPO from (A) nontransplanted monkey. Serum EPO at (B) day 2 and (C) day 8 for one monkey and (D, E) day 8 for the two other monkeys after SLIT. Cathode (−) is at the top. The number of the monkey is indicated on top of each lane. NT, nontransplanted; SLIT, suspension with a lentiviral vector and immediately transplanted.
Long-term survival of transduced hepatocytes and vector dissemination
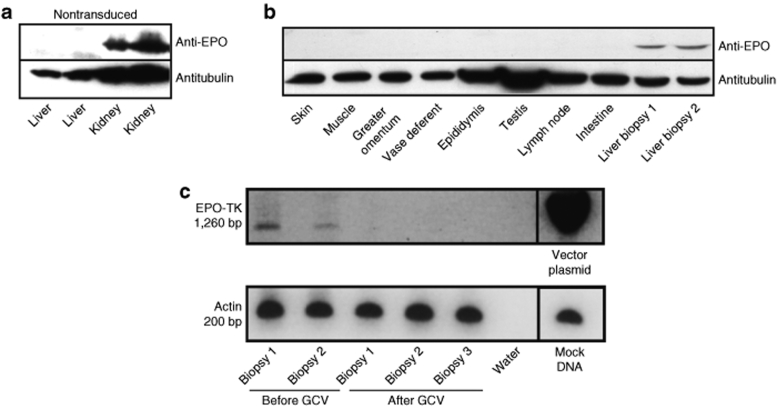
To determine whether transduced hepatocytes still expressed EPO in the long-term, western blot analyses were performed on liver biopsies harvested at 8 months and >1 year (day 487, day 446, and day 412, respectively) post-transplantation. EPO was detected in all liver biopsies (Figure 3b). No EPO protein expression was detected in other tissues of transplanted macaques and in the liver of a nontransplanted macaque (Figure 3a,b). As a positive control, EPO could be detected in the kidney of nontransplanted macaques (Figure 3a). Finally, reverse transcription–PCR analysis amplifying a region in EPO/HSV-TK expression cassette demonstrated that transgene transcription did occur in these liver biopsies (Figure 3c, lanes 1 and 2). Altogether, the data show that SLIT achieved long-term functionality of transduced hepatocytes in the liver. They also show that the designed lentiviral vector achieved a stable transduction and long-term liver-specific transgene expression in hepatocytes of macaques.
Figure 3.
Representative western blot and RT-PCR analysis of biopsies from macaques transplanted with transduced hepatocytes. Western blot analysis using antibodies against EPO or tubulin, on protein extracts of (a) control nontransduced monkey liver and kidney. (b) Proteins were extracted from biopsies at 1 month before death (before GCV treatment) and (c) RNA was extracted from liver biopsies from different lobes before and at death (after GCV treatment). For RT analysis, the mTTR-cmEPO-TK vector plasmid was used as positive control. bp, base pair; EPO, erythropoietin; GCV, ganciclovir; TK, thymidine kinase.
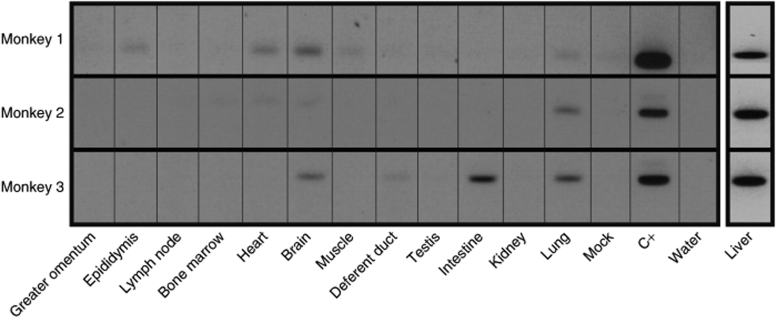
To evaluate the vector biodistribution, we performed a nested Alu-long terminal repeat PCR analysis to detect lentiviral vector in extrahepatic tissues. Vector DNA was detected in very few extrahepatic biopsies (Figure 4).
Figure 4.
Detection of integrated vector provirus. Genomic DNA isolated from several tissues was subjected to nested Alu-long terminal repeat PCR. The 2% agarose gels show the second rounds of PCR. Limit of detection of the PCR second rounds is one vector plasmid copy on 107 cells (C+).
Valganciclovir “cell suicide” treatment of macaques
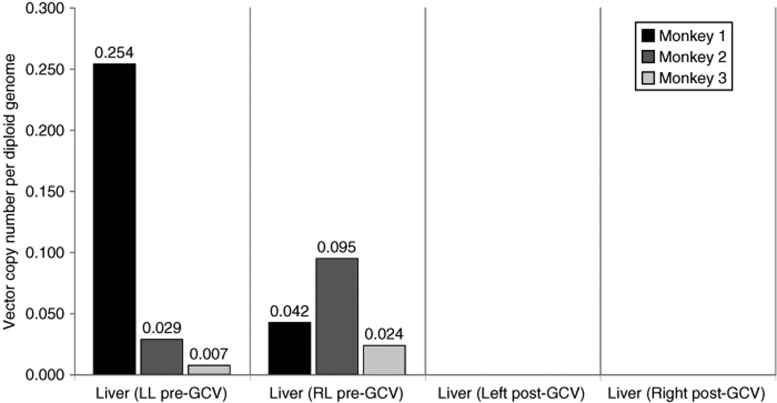
To address whether transduced hepatocytes could be eliminated from the liver of transplanted animals, macaques were treated with valganciclovir, the “prodrug” of GCV with improved bioavailability after oral administration. Liver biopsies before and 1 month after valganciclovir administration were compared for their vector copy numbers. No vector copy was detected by qPCR after treatment with valganciclovir (Figure 5 and Supplementary Figure S4) as suggested by transcription analysis (Figure 3c). Standard liver histology at death (1 month post-GCV treatment) was normal without inflammation or fibrosis. However, some apoptotic cells were detected, although they had not been in liver histology specimens before GCV treatment (data not shown).
Figure 5.
Real-time quantitative PCR on genomic liver left and right lobe DNA from the three monkeys before and after GCV administration. GCV, ganciclovir; LL, left lobe; RL, right lobe.
Discussion
These experiments clearly demonstrate the feasibility of SLIT in Macaca fascicularis, an animal model very close to human children for both size and physiology. All macaques tolerated SLIT, accomplished within 8 hours, without complications. We autotransplanted an amount of hepatocytes corresponding to an average of 3% of the recipient liver mass. We showed that lentivirally modified hepatocytes engrafted and were functional in the long term, expressing EPO and HSV-TK transgenes for up to 487 days. Furthermore, we successfully eliminated transduced hepatocytes from the liver upon administration of valganciclovir to transplanted macaques. To our knowledge, this is the first demonstration of the successful utilization of the HSV-TK/GCV suicide gene strategy to control the fate of transduced hepatocytes in a gene therapy protocol in nonhuman primates.
The use of EPO as a transgene allowed a noninvasive quantitative evaluation of viability of transduced hepatocytes. We also chose the cynomolgus EPO to avoid a specific immune response against a noncynomolgus protein, as previously shown in other studies.14,15,16 Early experiments confirmed that the designed lentiviral vector was capable of promoting the secretion of EPO and increasing hematocrit, in vitro and in vivo in mice. In macaques, unequivocal evidence that serum EPO originated from transduced hepatocytes was provided by the analysis of its isoelectric pattern, as previously reported after adeno-associated virus–mediated in vivo gene transfer.12,13 The ectopic expression of EPO in transduced hepatocytes led to secretion of EPO isoforms different from those of endogenous EPO (renal). Interestingly, these isoforms were different from those of transduced muscle and retina,12,13 thus confirming the importance of cell type on the characteristics of recombinant EPO. As soon as 2 days post-transplantation, we detected serum transgene–derived EPO showing that transduced hepatocytes were functional and rapidly produced EPO. Serum EPO peaked at 2–4 days post-transplantation and then fell to baseline within 1 month post-transplantation. We observed a subsequent and transient increase in hematocrit, showing that serum transgene–derived EPO isoforms were biologically active. Sustained expression of EPO by the transduced hepatocytes was proven both by protein detection and reverse transcription–PCR in the liver biopsies up to 16 months after SLIT, suggesting the absence of cytotoxic immune response, as observed in previous experiments where transduced simian hepatocytes were eliminated within 5 weeks,16 and showing that mTTR promoter was not silenced. The decrease of serum EPO levels to baseline after 1 month may be explained by the following two hypotheses:
1. Low yield of engraftment of the transduced hepatocytes and subsequent low production of EPO. Transplantation studies in rodents have shown that most transplanted hepatocytes (70–80%) are entrapped in the portal system and are destroyed by phagocytic responses within 48 hours.17,18 In macaques, clearance of transplanted hepatocytes might be a slower process as compared to that observed in rodents because the peak of EPO was at 2–4 days post-transplantation. Assuming a 70% transduction efficiency at the multiplicity of infection of 30 (ref. 19) and a 20–30% engraftment efficacy,17 transduced and transplanted hepatocytes would amount to <1% of the total liver mass. In the long-term liver biopsies collected between 412 and 487 days post-transplantation, we detected vector DNA, with an average of 0.15 copy number/diploid genome, confirming a low level of liver repopulation.
2. Humoral immune response against the isoforms of EPO produced by the transduced hepatocytes, which might specifically inactivate circulating exogenous EPO. We did not detect the presence of circulating immune complexes 15 days, 1 month, and 3 months after the procedure (data not shown), which seems to refute this hypothesis.
We previously showed that the Gunn rat, an animal model of Crigler-Najjar syndrome type 1, was completely treated with <0.1 retroviral vector copies per diploid genome.20 Therefore, SLIT may provide enough functional cells in some inherited liver diseases like Crigler-Najjar syndrome type 1. For other liver diseases requiring a higher liver repopulation rate, SLIT has to be combined with strategies either to improve hepatocyte engraftment or to confer a selective proliferation of transduced hepatocytes. Interestingly, a recent study showed that a partial portal embolization before hepatocyte transplantation allowed repopulating 10% of the liver mass with transplanted Hoechst-labeled hepatocytes.21 Liver stem-like cells redifferentiation techniques, combined with ex situ lentiviral gene therapy, might represent another interesting approach to overcome the low rate of liver repopulation by one single injection of the transduced hepatocytes.22
Insertional mutagenesis represents a major concern for gene therapy applications. Such a safety concern has dramatically risen after serious events in some of the patients with X-linked severe combined immunodeficiency, who received gene therapy treatment with murine oncoretroviral vectors.23,24 So far, in contrast to oncoretroviral vectors, no adverse events have been reported after gene transfer with human immunodeficiency virus-1–derived lentiviral vector, and tumorigenesis was not stimulated in tumor-prone mice after human immunodeficiency virus-1– derived lentiviral vector integration.25,26 However, preclinical animal and clinical studies may underscore vector genotoxicity because of the short animal lifespan as compared to humans, and because some observed adverse events might be due to negative synergy with underlying disease and other treatments. Therefore, it is important to allow conditional ablation of transduced hepatocytes when required. The HSV-TK/GCV suicide strategy has been proven to be safe in a clinical trial of cancer gene therapy27 or modulation of graft-versus-host disease.28 The combined expression of the suicide HSV-TK gene with a therapeutic gene (in our study, the marker gene EPO) has, therefore, a clinically relevant application in liver gene therapy. HSV-TK–expressing cells could be selectively eliminated by GCV treatment, a drug commonly used in clinical medicine for viral infections, which in itself is not toxic, but is converted to a toxic drug by viral thymidine kinase phosphorylation. Cells expressing HSV-TK are producing highly toxic GCV-triphosphates that lead to cell death by inhibiting cellular DNA polymerase activity.29 Human, or simian in our study, thymidine kinase has narrow nucleotide specificity and is unable to activate the drug. We demonstrated HSV-TK functionality of the mTTR-cmEPO-TK vector both in human hepatic cell lines and in vivo in macaques upon GCV treatment. It should be noted that we waited for up to 16 months before complete ablation of transduced hepatocytes in macaques with GCV treatment, as assessed by qPCR. This data gave additional evidence that transduced hepatocytes were present and functional in the long term and that the mTTR promoter was not silenced. This could be interpreted as an absence of immune response against the transgenes, but we did not perform assay to directly evaluate the presence of a cellular immune response against HSV-TK (or cmEPO), or this could be explained by a selection of low expressing EPO/HSV-TK hepatocytes over time, and these cells could be responsible for the residual EPO expression or HSV-TK in the long term.
This finding demonstrates the possibility to selectively eliminate the transplanted cells, and suggest they could be similarly killed if they displayed uncontrolled proliferation. Thus, the inclusion of the HSV-TK gene in the lentiviral vectors constitutes an additional guarantee for biosafety. Noteworthy, GCV administration results in depletion of transduced hepatocytes without inducing significant histological liver lesions and with a good clinical tolerance. Hepatocytes are known to be in a quiescent status, and the toxic triphosphate processes by the suicide gene HSV-TK after GCV administration theoretically need cell replication to induce apoptosis. The fact that administration of GCV results in depletion of EPO/TK–expressing hepatocytes is probably due to the incorporation of the toxic triphosphates into mitochondrial DNA.30 Braun and colleagues also reported that HSV-TK–expressing hepatocytes were ablated in adult animals in HSV-TK transgenic mice upon GCV treatment.31
A low systemic dissemination of the vector in extrahepatic tissues was pointed out by Alu-long terminal repeat PCR. Alu-long terminal repeat PCR was specifically used to distinguish the integrated provirus from the plasmidic contamination (data not shown). Dissemination is probably due to noninternalized lentiviral vectors that adhere to hepatocyte membranes resulting in in vivo lentiviral vector spreading.32 Because brain biopsies were taken at the time of killing, i.e., after GCV-mediated ablation of transduced hepatocytes, the brain-detected vector DNA may be due to monocytes/macrophages trafficked into brain after having phagocyted apoptotic transduced hepatocytes and/or being lentivirally transduced.33,34,35 We observed that the vector integration in nonhepatic tissues does not lead to protein expression. This demonstrates the liver specificity of mTTR promoter in nonhuman primates and suggests inactivity of mTTR enhancers in these tissues. This inactivity may be important considering that the in vivo genotoxicity side effects reported for oncoretroviral vectors were mostly related to retroviral enhancer activity.36 No gross macroscopic or histological anomalies were detected in any organ at the killing of macaques. Nevertheless, because it is important to avoid vector dissemination, we performed preliminary experiments to completely remove noninternalized infectious virus after transduction. Our preliminary results show that the sequential incubation of transduced hepatocytes with a protease and human serum efficiently eliminated those infectious virus particles without impairing viability of transduced hepatocytes (J. Birraux, B.E. Wildhaber, C. Jond, D.C. Belli, and O. Menzel, personal communication). Protease treatment would release cell surface–bound viruses and human serum was shown to inactivate vesicular stomatitis virus glycoprotein–pseudotyped lentiviral vectors.37
In conclusion, this study demonstrates the feasibility of SLIT in the Macaca fascicularis, an animal model very close to human infants, with long-term (more than a year) survival and functionality of the autotransplanted lentivirally transduced hepatocytes. Transduced hepatocytes were successfully eliminated after oral administration of valganciclovir, indicating the efficacy of the HSV-TK/GCV suicide gene strategy. These findings confirm the possibility to increase the biosafety of gene therapy using lentiviral vectors for the treatment of liver-based inborn errors of metabolism, which remains a prerequisite before clinical application.
Materials and Methods
Animals. Nonhuman primates were male captive-bred Macaca fascicularis (n = 3), aged between 2 and 3 years, weighing 2.5 ± 0.5 kg, and purchased from Bioprim (Baziège, France). Experiments were performed in the animal facilities of the “Centre of Boisbonne” (Nantes, France). Animals were maintained on a standard diet and kept in 12-hour light-dark cycles. All animal studies were performed according to institutional guidelines set forth by the Swiss National Animal Care Committee and by the French Institutional Animal Care and Use Committee of the University of Nantes.
Surgical procedures. Induction and maintenance of anesthesia were achieved with isoflurane in a mixture of 50% O2 in air. Following tracheal intubation, remifentanil was infused continuously in order to ensure adequate analgesia throughout the surgical procedure. After a median laparotomy, a left lobectomy was performed. Hepatocytes were isolated from the resected left lateral lobe (weighed about 8 g) and rapidly transduced in suspension (see below). Transduced hepatocytes (1–2 × 108) were infused back to the macaques via a branch of the mesenteric vein at a rate of 2 ml/minute using a 25G catheter. No thrombosis of the portal venous system was observed. Animals were maintained under general anesthesia during the whole time of the ex vivo cell manipulation. Postoperative analgesia (buprenorphine) was provided, and alimentation was reintroduced in the evening on the same day. For viral vector dissemination analysis, multiple surgical biopsies (testis, epididymis, deferent duct, muscle, intestine, lymph nodes, greater omentum, and bone marrow) were performed under general anesthesia 8 and 15 months after transplantation. At the end of the follow-up period, valganciclovir (Valcyte; Roche, Basel, Switzerland) (GCV) was administered orally for 1 week (50 mg/kg once a day). One month after GCV treatment, animals were euthanized and the same multiple biopsies were done as well as kidney, pulmonary, cardiac, and brain biopsies.
Hepatocyte isolation and cell culture. HeLa and 293T cells were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 10 mmol/l 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 2 mmol/l glutamine, and antibiotics (Invitrogen, Carlsbad, CA). Primary hepatocytes were isolated from the left liver lobe of the macaques by a three-step collagenase perfusion as previously described.38 The liver capsule was then disrupted and cells were dispersed in cold Dulbecco's modified Eagle's medium/F12 medium (Invitrogen) without serum or antibiotics. Hepatocytes were purified from nonparenchymal cells by sedimentation on 30% Percoll isodensity solution (GE Healthcare, Buckinghamshire, England). Final cell viability was >90%, as determined by Trypan blue exclusion test performed respectively after Percoll purification and before transplantation in all three monkeys.
Description and production of lentiviral vectors. High-titer lentiviral vector stocks were generated as previously described by calcium phosphate–mediated transient transfection of three plasmids and production in serum-free medium: the transfer vector plasmid, the packaging plasmid psPAX2, and the vesicular stomatitis virus glycoprotein envelope protein-coding plasmid pMD2G.39 The self-inactivating transfer vector, plox-mTTR-cmEPO-TK, harbors the cynomolgus EPO complementary DNA (kindly provided by P. Moullier, Nantes, France) under the control of a liver-specific promoter, the mTTR promoter fused to a synthetic hepatocyte-specific enhancer (mTTR).11 It harbors a second cistron, which is the thymidine kinase of HSV type 1, downstream of the internal ribosomal entry site of Encephalomyocarditis virus. Finally, a 775 base pair of the hepatic locus region from apolipoprotein E gene (HCR-APOE) (kindly provided by L. Chan, Baylor College of Medicine, Houston, TX) was inserted upstream of the mTTR promoter to improve EPO transgene expression in the liver.
Viral titer was determined on HeLa cells by real-time qPCR, using primers and probes specific for 5′-untranslated lentiviral vectors as previously described.10 A total of four vector batches, corresponding to a total of 6 × 1010 HeLa transducing units, were produced. Absence of replication-competent recombinant lentivirus was verified for two batches (3.55 × 1010 HeLa transducing units total) by GenoSafe (Evry, France) using a cell-based assay developed by Cell Genesys (South San Francisco, CA).40
In suspension transduction. Isolated hepatocytes (1–2 × 108) were resuspended at a cell density of 1 × 107 cells/ml in University of Wisconsin solution (ViaSpan; DuPont Pharmaceuticals, Geneva, Switzerland) solution containing 50 µmol/l vitamin E succinate (Sigma, Buchs, Switzerland) with the mTTR-cmEPO-TK vectors at a multiplicity of infection of 30 in ultra-low attachment 10 cm plates (Corning, Lowell, MA). Two hours later, cells were carefully washed three times in cold 400 ml serum-free Dulbecco's modified Eagle's medium/F12. For cell infusion, transduced hepatocytes were resuspended in normal saline containing heparin (5 U/ml) at a cell concentration of 1 × 107 cells/ml.
Protein analyses. For western blot analyses, tissues were lysed in radioimmunoprecipitation assay buffer (1% nonylphenoxypolyethoxyethanol, 0.1% sodium dodecyl sulfate, and 0.5% sodium deoxycholate in phosphate-buffered saline 1×). After 30-minute lysis on ice, lysates were centrifuged (10,000g at 4 °C for 30 minutes) to pellet the nuclei. Protein extracts were resolved on a NuPAGE 10% Novex Bis-Tris Gel (Invitrogen). The proteins were transferred to nitrocellulose membrane. After blocking in 5% skim milk, immunoblots were sequentially incubated with rabbit polyclonal human anti-EPO antibody (R&D Systems, Minneapolis, MN) diluted 1:400 and horseradish peroxidase–conjugated goat anti-rabbit IgG (Pierce, Rockford, IL) diluted 1:2500. Detection was performed with SuperSignal West Dura Extended Duration Substrate (Pierce).
Serum concentration of EPO was determined by enzyme-linked immunosorbent assay (Quantikine IVD; R&D Systems) according to the instructions provided by the manufacturer.
Isoelectric profiles of serum EPO were analyzed by double-blotting following isoelectric focusing as previously described.12,41
qPCR and qualitative PCR. DNA of harvested organs was isolated using the DNeasy kit (Qiagen, Huntsville, AL). Genomic DNA was subjected to TaqMan PCR as described above for vector detection. For normalization of amount of genomic DNA, primers and probe specific for simian β-actin gene were used: SB2-F, 5′-TCTGTGTGGATCGGCGGCTCCA-3′; SB2-R, 5′-CTGCTTGCTGATCCACATCTG-3′; SB2-P, 5′-(Yakima Yellow)-CCTGGCCTCGCTGTCCACCTTCCA-(Eclipse Dark Quencher)-3′. Reactions were performed using Rotor Gene RG-3000 (Corbett Research, Sydney, Australia). A standard curve was generated by using dilutions of the mTTR-cmEPO-TK vector plasmid in genomic DNA extract from mock-transduced macaque liver, simulating the presence of 25 to 0.025 vector copies per haploid genome. Vector copy numbers were calculated by interpolating Ct(GAG)-Ct(β-actin) sample values to standard curve values.
Qualitative PCR for determining vector biodistribution was performed using a nested Alu-PCR on 200 ng genomic DNA, as previously described.42
Analysis of mRNA. Total RNA was purified from homogenized tissue biopsy specimens (25 mg) using TRIzol (Invitrogen) and DNase I (Promega, Madison, WI). Reverse transcriptions with a specific primer (HSV1-TKr, 5′-ATGCTGCCCATAAGGTATCG-3′) and random primers were performed at 37 °C for 50 minutes using M-MLV Reverse Transcriptase (Invitrogen) for detecting vector and β-actin mRNA, respectively. PCR analyses were then performed using primers designed to amplify a 1260 base pair region of the cmEPO-HSV-TK region (HSV1-TKf, 5′-CAACAAAAAGCCACGGAAGT-3′; HSV1-TKr1, 5′-ACACCC GCCAGTAAGTCATC-3′), or a 200 base pair region of β-actin (5′-GGCGGCACCACCATGT-3′ and 5′-AGGGGCCGGACTCGTC-3′). PCR amplification conditions were as follows: 95 °C for 5 minutes, 40 cycles of 94 °C for 1 minute, 52 °C for 1 minute, 72 °C for 2 minutes, and finally, 72 °C for 10 minutes.
SUPPLEMENTARY MATERIALFigure S1. Tetrazolium (MTS) was used for monitoring cell viability in culture. Low confluence Hepatoma HuH7 cells were transduced with 500μl (MOI of 10) (a) or 10μl (MOI of 0.2) (b) of crude non-concentrated supernatant. The functionality of HSV-TK conditional cell killing system was then tested 4 days post-infection on cultured transduced-HuH7 cells in the presence of 2 μM GCV. GCV-treated cells displayed a clear decrease in cell viability in culture by analyzing the cell viability using colorimetric MTS assay.Figure S2. Hepatoma HuH7 cells were transduced with 500 µl (MOI of 10) of crude non-concentrated supernatant. The functionality of HSV-TK conditional cell killing system was then tested 4 days post-infection on cultured transduced-HuH7 cells in the presence of 2 μM GCV. GCV-treated cells showed a clear decrease in cell viability in culture by analyzing the cell shape.Figure S3. An increase in hematocrit was observed in EPO-transduced mice as a consequence of serum EPO level increased (not shown), measured at day 30 post-injection. EPO was not detected in the sera of control non-injected mice.Figure S4. Amplification plots of real-time Taqman quantitative PCR of monkey genome DNA showed expression of lentiviral vector before and after GCV treatment of a monkey transplanted with EPO-TK vector hepatocytes (a representative plot is depicted for all monkeys). Calculation of the deltaCT values (CT vector minus CT beta-actin) allowed comparison of the relative level of vector expression between animal groups (as showed in figure 5).
Supplementary Material
Tetrazolium (MTS) was used for monitoring cell viability in culture. Low confluence Hepatoma HuH7 cells were transduced with 500μl (MOI of 10) (a) or 10μl (MOI of 0.2) (b) of crude non-concentrated supernatant. The functionality of HSV-TK conditional cell killing system was then tested 4 days post-infection on cultured transduced-HuH7 cells in the presence of 2 μM GCV. GCV-treated cells displayed a clear decrease in cell viability in culture by analyzing the cell viability using colorimetric MTS assay.
Hepatoma HuH7 cells were transduced with 500 µl (MOI of 10) of crude non-concentrated supernatant. The functionality of HSV-TK conditional cell killing system was then tested 4 days post-infection on cultured transduced-HuH7 cells in the presence of 2 μM GCV. GCV-treated cells showed a clear decrease in cell viability in culture by analyzing the cell shape.
An increase in hematocrit was observed in EPO-transduced mice as a consequence of serum EPO level increased (not shown), measured at day 30 post-injection. EPO was not detected in the sera of control non-injected mice.
Amplification plots of real-time Taqman quantitative PCR of monkey genome DNA showed expression of lentiviral vector before and after GCV treatment of a monkey transplanted with EPO-TK vector hepatocytes (a representative plot is depicted for all monkeys). Calculation of the deltaCT values (CT vector minus CT beta-actin) allowed comparison of the relative level of vector expression between animal groups (as showed in figure 5).
Acknowledgments
This project was supported by a grant of the Swiss National Science Foundation (no. 3100A0-109814), and the Gabriella Giorgi-Cavaglieri Foundation and the Institut Clayton de la Recherche (to D.T.). We are grateful to the Ecole Nationale Vétérinaire de Nantes and the Centre de Thérapie Génique et Cellulaire de Boisbonne directed by Philippe Moullier for the kind disposition of the macaque facilities and the cmEPO cDNA. We thank Anne-Laure Rougemont [Pathology Department, University of Geneva Medical School (CMU), Geneva, Switzerland] for histological analysis, Catherine Fux for technical support, and Oliver Sanchez to revise this manuscript. We are grateful to I. Voria, Ethicon Endo-Surgery, at Johnson and Johnson Medical for the Ultracision.
REFERENCES
- Reding R, Gennari F, Janssen M, Jamart J, de Ville de Goyet J, Lerut J, et al. The pediatric liver transplant program at the Université Catholique de Louvain, Cliniques Saint-Luc, Brussels: overall results in 444 children (1984-1997) Acta Gastroenterol Belg. 1999;62:285–289. [PubMed] [Google Scholar]
- Kelly DA. Current issues in pediatric transplantation. Pediatr Transplant. 2006;10:712–720. doi: 10.1111/j.1399-3046.2006.00567.x. [DOI] [PubMed] [Google Scholar]
- Burlina AB. Hepatocyte transplantation for inborn errors of metabolism. J Inherit Metab Dis. 2004;27:373–383. doi: 10.1023/B:BOLI.0000031095.57411.8d. [DOI] [PubMed] [Google Scholar]
- Fisher RA., and , Strom SC. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82:441–449. doi: 10.1097/01.tp.0000231689.44266.ac. [DOI] [PubMed] [Google Scholar]
- Fox IJ., and , Roy-Chowdhury J. Hepatocyte transplantation. J Hepatol. 2004;40:878–886. doi: 10.1016/j.jhep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Dhawan A, Mitry RR., and , Hughes RD. Hepatocyte transplantation for metabolic disorders, experience at King's College hospital and review of literature. Acta Gastroenterol Belg. 2005;68:457–460. [PubMed] [Google Scholar]
- Grossman M, Rader DJ, Muller DW, Kolansky DM, Kozarsky K, Clark BJ, 3rd, et al. A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat Med. 1995;1:1148–1154. doi: 10.1038/nm1195-1148. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Oberholzer J, Birraux J, Majno P, Morel P., and , Trono D. Highly efficient lentiviral vector-mediated transduction of nondividing, fully reimplantable primary hepatocytes. Mol Ther. 2002;6:199–209. doi: 10.1006/mthe.2002.0653. [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Birraux J, Wildhaber B, Myara A, Trivin F, Le Coultre C, et al. Ex vivo lentivirus transduction and immediate transplantation of uncultured hepatocytes for treating hyperbilirubinemic Gunn rat. Transplantation. 2006;82:794–803. doi: 10.1097/01.tp.0000234675.56598.35. [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Khakhoulina T, Simmons A, Morel P., and , Trono D. A simple and highly effective method for the stable transduction of uncultured porcine hepatocytes using lentiviral vector. Cell Transplant. 2005;14:489–496. doi: 10.3727/000000005783982828. [DOI] [PubMed] [Google Scholar]
- Lasne F, Martin L, de Ceaurriz J, Larcher T, Moullier P., and , Chenuaud P. “Genetic Doping” with erythropoietin cDNA in primate muscle is detectable. Mol Ther. 2004;10:409–410. doi: 10.1016/j.ymthe.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Stieger K, Le Meur G, Lasne F, Weber M, Deschamps JY, Nivard D, et al. Long-term doxycycline-regulated transgene expression in the retina of nonhuman primates following subretinal injection of recombinant AAV vectors. Mol Ther. 2006;13:967–975. doi: 10.1016/j.ymthe.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Ghivizzani SC, Lechman ER, Tio C, Mulé KM, Chada S, McCormack JE, et al. Direct retrovirus-mediated gene transfer to the synovium of the rabbit knee: implications for arthritis gene therapy. Gene Ther. 1997;4:977–982. doi: 10.1038/sj.gt.3300486. [DOI] [PubMed] [Google Scholar]
- Xu L, Gao C, Sands MS, Cai SR, Nichols TC, Bellinger DA, et al. Neonatal or hepatocyte growth factor-potentiated adult gene therapy with a retroviral vector results in therapeutic levels of canine factor IX for hemophilia B. Blood. 2003;101:3924–3932. doi: 10.1182/blood-2002-10-3050. [DOI] [PubMed] [Google Scholar]
- Andreoletti M, Loux N, Vons C, Nguyen TH, Lorand I, Mahieu D, et al. Engraftment of autologous retrovirally transduced hepatocytes after intraportal transplantation into nonhuman primates: implication for ex vivo gene therapy. Hum Gene Ther. 2001;12:169–179. doi: 10.1089/104303401750061230. [DOI] [PubMed] [Google Scholar]
- Gupta S, Rajvanshi P, Sokhi R, Slehria S, Yam A, Kerr A, et al. Entry and integration of transplanted hepatocytes in rat liver plates occur by disruption of hepatic sinusoidal endothelium. Hepatology. 1999;29:509–519. doi: 10.1002/hep.510290213. [DOI] [PubMed] [Google Scholar]
- Malhi H, Irani AN, Volenberg I, Schilsky ML., and , Gupta S. Early cell transplantation in LEC rats modeling Wilson's disease eliminates hepatic copper with reversal of liver disease. Gastroenterology. 2002;122:438–447. doi: 10.1053/gast.2002.31086. [DOI] [PubMed] [Google Scholar]
- Parouchev A, Nguyen TH, Dagher I, Mainot S, Groyer-Picard MT, Branger J, et al. Efficient ex vivo gene transfer into non-human primate hepatocytes using HIV-1 derived lentiviral vectors. J Hepatol. 2006;45:99–107. doi: 10.1016/j.jhep.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Nguyen TH, Aubert D, Bellodi-Privato M, Flageul M, Pichard V, Jaidane-Abdelghani Z, et al. Critical assessment of lifelong phenotype correction in hyperbilirubinemic Gunn rats after retroviral mediated gene transfer. Gene Ther. 2007;14:1270–1277. doi: 10.1038/sj.gt.3302993. [DOI] [PubMed] [Google Scholar]
- Dagher I, Boudechiche L, Branger J, Coulomb-Lhermine A, Parouchev A, Sentilhes L, et al. Efficient hepatocyte engraftment in a nonhuman primate model after partial portal vein embolization. Transplantation. 2006;82:1067–1073. doi: 10.1097/01.tp.0000236103.99456.8f. [DOI] [PubMed] [Google Scholar]
- Najimi M, Khuu DN, Lysy PA, Jazouli N, Abarca J, Sempoux C, et al. Adult-derived human liver mesenchymal-like cells as a potential progenitor reservoir of hepatocytes. Cell Transplant. 2007;16:717–728. doi: 10.3727/000000007783465154. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- Board of the European Society of Gene and Cell Therapy Case of leukaemia associated with X-linked severe combined immunodeficiency gene therapy trial in London. Hum Gene Ther. 2008;19:3–4. doi: 10.1089/hum.2007.1221. [DOI] [PubMed] [Google Scholar]
- Bauer G, Dao MA, Case SS, Meyerrose T, Wirthlin L, Zhou P, et al. In vivo biosafety model to assess the risk of adverse events from retroviral and lentiviral vectors. Mol Ther. 2008;16:1308–1315. doi: 10.1038/mt.2008.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- Nasu Y, Saika T, Ebara S, Kusaka N, Kaku H, Abarzua F, et al. Suicide gene therapy with adenoviral delivery of HSV-tK gene for patients with local recurrence of prostate cancer after hormonal therapy. Mol Ther. 2007;15:834–840. doi: 10.1038/sj.mt.6300096. [DOI] [PubMed] [Google Scholar]
- Traversari C, Marktel S, Magnani Z, Mangia P, Russo V, Ciceri F, et al. The potential immunogenicity of the TK suicide gene does not prevent full clinical benefit associated with the use of TK-transduced donor lymphocytes in HSCT for hematologic malignancies. Blood. 2007;109:4708–4715. doi: 10.1182/blood-2006-04-015230. [DOI] [PubMed] [Google Scholar]
- van Dillen IJ, Mulder NH, Vaalburg W, de Vries EF., and , Hospers GA. Influence of the bystander effect on HSV-tk/GCV gene therapy. A review. Curr Gene Ther. 2002;2:307–322. doi: 10.2174/1566523023347733. [DOI] [PubMed] [Google Scholar]
- Herraiz M, Beraza N, Solano A, Sangro B, Montoya J, Qian C, et al. Liver failure caused by herpes simplex virus thymidine kinase plus ganciclovir therapy is associated with mitochondrial dysfunction and mitochondrial DNA depletion. Hum Gene Ther. 2003;14:463–472. doi: 10.1089/104303403321467225. [DOI] [PubMed] [Google Scholar]
- Braun KM, Degen JL., and , Sandgren EP. Hepatocyte transplantation in a model of toxin-induced liver disease: variable therapeutic effect during replacement of damaged parenchyma by donor cells. Nat Med. 2000;6:320–326. doi: 10.1038/73179. [DOI] [PubMed] [Google Scholar]
- Pan YW, Scarlett JM, Luoh TT., and , Kurre P. Prolonged adherence of human immunodeficiency virus-derived vector particles to hematopoietic target cells leads to secondary transduction in vitro and in vivo. J Virol. 2007;81:639–649. doi: 10.1128/JVI.01089-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, et al. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- Clay CC, Rodrigues DS, Ho YS, Fallert BA, Janatpour K, Reinhart TA, et al. Neuroinvasion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection. J Virol. 2007;81:12040–12048. doi: 10.1128/JVI.00133-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyoda T, Nagata K, Akashi M., and , Kobayashi Y. Neutrophils accelerate macrophage-mediated digestion of apoptotic cells in vivo as well as in vitro. J Immunol. 2005;175:3475–3483. doi: 10.4049/jimmunol.175.6.3475. [DOI] [PubMed] [Google Scholar]
- Nienhuis AW, Dunbar CE., and , Sorrentino BP. Genotoxicity of retroviral integration in hematopoietic cells. Mol Ther. 2006;13:1031–1049. doi: 10.1016/j.ymthe.2006.03.001. [DOI] [PubMed] [Google Scholar]
- DePolo NJ, Reed JD, Sheridan PL, Townsend K, Sauter SL, Jolly DJ, et al. VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol Ther. 2000;2:218–222. doi: 10.1006/mthe.2000.0116. [DOI] [PubMed] [Google Scholar]
- Seppen J, van der Rijt R, Looije N, van Til NP, Lamers WH., and , Oude Elferink RP. Long-term correction of bilirubin UDPglucuronyltransferase deficiency in rats by in utero lentiviral gene transfer. Mol Ther. 2003;8:593–599. doi: 10.1016/s1525-0016(03)00234-x. [DOI] [PubMed] [Google Scholar]
- Bovia F, Salmon P, Matthes T, Kvell K, Nguyen TH, Werner-Favre C, et al. Efficient transduction of primary human B lymphocytes and nondividing myeloma B cells with HIV-1-derived lentiviral vectors. Blood. 2003;101:1727–1733. doi: 10.1182/blood-2001-12-0249. [DOI] [PubMed] [Google Scholar]
- Escarpe P, Zayek N, Chin P, Borellini F, Zufferey R, Veres G, et al. Development of a sensitive assay for detection of replication-competent recombinant lentivirus in large-scale HIV-based vector preparations. Mol Ther. 2003;8:332–341. doi: 10.1016/s1525-0016(03)00167-9. [DOI] [PubMed] [Google Scholar]
- Lasne F, Martin L, Martin JA., and , de Ceaurriz J. Isoelectric profiles of human erythropoietin are different in serum and urine. Int J Biol Macromol. 2007;41:354–357. doi: 10.1016/j.ijbiomac.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tetrazolium (MTS) was used for monitoring cell viability in culture. Low confluence Hepatoma HuH7 cells were transduced with 500μl (MOI of 10) (a) or 10μl (MOI of 0.2) (b) of crude non-concentrated supernatant. The functionality of HSV-TK conditional cell killing system was then tested 4 days post-infection on cultured transduced-HuH7 cells in the presence of 2 μM GCV. GCV-treated cells displayed a clear decrease in cell viability in culture by analyzing the cell viability using colorimetric MTS assay.
Hepatoma HuH7 cells were transduced with 500 µl (MOI of 10) of crude non-concentrated supernatant. The functionality of HSV-TK conditional cell killing system was then tested 4 days post-infection on cultured transduced-HuH7 cells in the presence of 2 μM GCV. GCV-treated cells showed a clear decrease in cell viability in culture by analyzing the cell shape.
An increase in hematocrit was observed in EPO-transduced mice as a consequence of serum EPO level increased (not shown), measured at day 30 post-injection. EPO was not detected in the sera of control non-injected mice.
Amplification plots of real-time Taqman quantitative PCR of monkey genome DNA showed expression of lentiviral vector before and after GCV treatment of a monkey transplanted with EPO-TK vector hepatocytes (a representative plot is depicted for all monkeys). Calculation of the deltaCT values (CT vector minus CT beta-actin) allowed comparison of the relative level of vector expression between animal groups (as showed in figure 5).