Abstract
In recent years, numerous reports have identified in mouse different sources of myogenic cells distinct from satellite cells that exhibited a variable myogenic potential in vivo. Myogenic stem cells have also been described in humans, although their regenerative potential has rarely been quantified. In this study, we have investigated the myogenic potential of human muscle–derived cells based on the expression of the stem cell marker CD133 as compared to bona fide satellite cells already used in clinical trials. The efficiency of these cells to participate in muscle regeneration and contribute to the renewal of the satellite cell pool, when injected intramuscularly, has been evaluated in the Rag2−/− γC−/− C5−/− mouse in which muscle degeneration is induced by cryoinjury. We demonstrate that human muscle–derived CD133+ cells showed a much greater regenerative capacity when compared to human myoblasts. The number of fibers expressing human proteins and the number of human cells in a satellite cell position are all dramatically increased when compared to those observed after injection of human myoblasts. In addition, CD133+/CD34+ cells exhibited a better dispersion in the host muscle when compared to human myoblasts. We propose that muscle-derived CD133+ cells could be an attractive candidate for cellular therapy.
Introduction
Adult human skeletal muscle is composed of multinucleated terminally differentiated myofibers with a very low rate of cellular turnover under normal conditions.1 However, it has a remarkable capacity to respond rapidly to modifications in physiological stimuli such as growth and exercise and to regenerate in response to injury or disease due to a small population of quiescent mononucleated cells. These cells, located beneath the basal lamina of muscle fibers, are called satellite cells,2 and they can be identified by the expression of a number of molecular markers such as Pax7 (ref. 3), M-cadherin,4 neural cell adhesion molecule (CD56) (ref. 5), CD34 (ref. 6), and myf5 (ref. 6) (also reviewed in ref. 7). After activation, satellite cells will proliferate as myoblasts and fuse to form new multinucleated myofibers. A small percentage of these cells will, however, escape the terminal differentiation pathway to return to quiescence and will restore the reserve pool of satellite cells under the basal lamina. In the group of muscular dystrophies, such as Duchenne muscular dystrophy (DMD), this scenario has dramatically changed. The absence or modification of cytoskeletal proteins, such as dystrophin, leads to a permanent fragility and leakiness of the sarcolemma, and disruption of the muscle fibers that culminates in continuous cycles of degeneration/regeneration that finally depletes the pool of satellite cells.8,9 Gene therapy can be envisaged for genes that can be inserted into a viral vector, but still poses the problem of the immune reaction against the vector after the first injection.10 Exon-skipping allows the elimination of an exon bearing a mutation, thus producing a truncated protein if the reading frame is conserved through the exon-skipping. This strategy is applicable for proteins, such as dystrophin, that can still be functional even when missing a complete exon. Oligonucleotides triggering exon-skipping can either be directly administered in a stabilized form,11,12 or can be transferred using a viral vector such as adeno-associated virus.13 Cell therapy for muscular dystrophies has been mainly developed using allogeneic muscle progenitors, i.e., satellite cells from healthy donors, but this requires major immune suppression.14 Autologous satellite cells from the patients cannot be used because they are rapidly exhausted through the dystrophic process.9 Therefore, cell therapy based on an alternative source of myogenic cells could provide a solution to treat some of these diseases either by direct intramuscular injections if the target is limited, or by systemic delivery if applicable. New assessment should thus be established as preclinical requirements for these new types of myogenic cells, such as the characterization of their proliferative potential by telomere length determination, or a quantitative assay of their myogenic potential in vivo. This article describes such an assay. Recently, it was demonstrated that a fraction of the mononucleated cells present in the adult peripheral blood expressing the stem cell marker, CD133, presents a myogenic potential.15 The present study describes the quantification of the regenerative potential of CD133+ human muscle–derived stem cells in vivo, as well as their ability to repopulate the satellite cell niche, as compared to myoblasts. A small proportion of the CD133+ cells, normally localized in the interstitial space between muscle fibers,16 also express CD34, a surface glyco-phosphoprotein expressed by hematopoietic stem cells, small-vessel endothelial cells, and embryonic fibroblasts (reviewed in ref. 17), as well as by satellite cells.6 Whether the expression of CD34 is linked to a myogenic potential is still unknown. In order to investigate whether the expression of CD34 could be involved in defining subpopulations of stem cells having a different behavior in muscle regeneration, we have assessed in vivo the regenerative potential of both subfractions CD133+/CD34+ and CD133+/CD34−, using implantation into regenerating tibialis anterior (TA) muscle of Rag2−/− γC−/− C5−/− mice.18 We demonstrate that human muscle–derived CD133+ cells, when injected intramuscularly, are significantly more efficient at regenerating skeletal muscle than human myoblasts derived from bona fide satellite cells, currently used in several clinical trials. CD133+ cells migrate extensively throughout the length of the injected muscle, and repopulate the satellite cell niche. We propose that these stem cells represent a very attractive new candidate for cell transplantation therapy in skeletal muscle.
Results
Initial characterization of muscle-derived CD133+ subpopulations
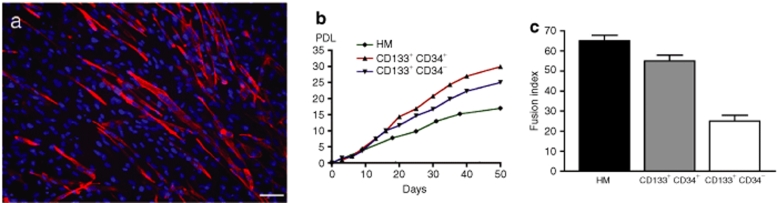
Expression of CD56, a satellite cell marker,5 was analyzed by fluorescence-activated cell sorting in all the cells. More than 80% of human myoblasts expressed CD56 confirming their myogenic purity. In contrast, only 19% of the CD133+/CD34+ cells and 23% of the CD133+/CD34− cells were CD56+. In proliferation conditions, the muscle-derived CD133+CD34+ and CD133+CD34− cells are able to make 29.95 and 25.05 population doubling levels (PDLs), respectively, in 50 days of culture (versus 17.00 PDL for the human myoblasts) (Figure 1b), demonstrating a high proliferation rate for these cells. To test the myogenic potential of the muscle-derived CD133+CD34+ and CD133+CD34− subpopulations, we analyzed their myogenic differentiation, i.e., myotube formation, after 14 days of culture in low serum “fusion-promoting” condition. The muscle-derived CD133+CD34+ cells formed more multinucleated myotubes expressing myosin heavy chain (Figure 1a) in comparison to the CD133+CD34− population. The fusion index obtained in the CD133+CD34+ fraction was higher (55%) than that obtained from the CD133+CD34− fraction (25%), whereas primary cultured human myoblasts used as control presented a fusion index of 65% as illustrated in Figure 1c. Telomere lengths of the implanted cells were measured just prior to implantation. Telomeres of CD133+CD34+ cells had an average value of 10.9 kb, whereas those of CD133+CD34− cells had an average value of 12.3 kb. Telomeres of control satellite cells had an average value of 10.5 kb at 25 PDL when they were injected.
Figure 1.
In vitro myogenic differentiation of muscle-derived CD133+ cells. (a) After 14 days in low serum “fusion-promoting” condition, muscle-derived CD133+CD34+ cells fuse into multinucleated myotubes expressing myosin heavy chain. (b) Proliferation capacity (population doubling level, PDL) of muscle-derived CD133+CD34+ (red line), CD133+CD34− (blue line), and human myoblasts (HM) (green line). (c) Fusion index of muscle derived CD133+CD34+, CD133+CD34−, and HM. Bar = 100 µm.
Engrafted muscle-derived CD133+ cells form more human muscle fibers than human myoblasts
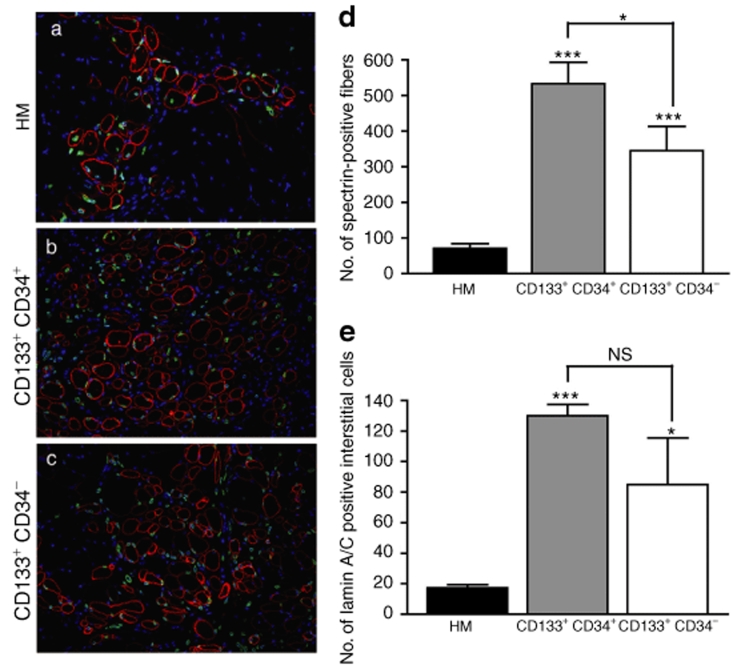
To investigate their in vivo regenerative capacity, 2.5 × 105 cells from each subpopulation (CD133+/CD34+ and CD133+/CD34−) were grafted into the right TA of Rag2−/− γC−/− C5−/− mice (n = 4 and n = 5, respectively), whereas the contralateral TAs were injected with 2.5 × 105 human myoblasts (n = 8). Prior to engrafting, the TA muscles were injured with a cryolesion to provoke a degeneration/regeneration cycle. Grafted muscles were analyzed 4 weeks after transplantation. The participation of the injected cells in muscle regeneration was quantified by counting the number of human spectrin–positive fibers along the entire muscle length. As shown in Figure 2, the injected cells showed very different regenerative potentials. The largest number of spectrin-positive fibers (532.50 ± 60.93) were detected in muscles grafted with CD133+/CD34+ cells when compared to the CD133+/CD34− cells (344.60 ± 68.57, P < 0.05) and to the control human myoblasts (70.75 ± 12.93, P < 0.001). A significant difference was also observed between CD133+/CD34− cells and human myoblasts (P < 0.01) (Figure 2d). We also quantified the number of human cells that had not been incorporated into the muscle fibers but remained located in an interstitial position outside the basal lamina of the fibers. This number was significantly larger for the CD133+/CD34+ (129.90 ± 7.54) and CD133+/CD34− (85.05 ± 30.53) cells compared to control human myoblasts (17.25 ± 2.09, P < 0.001 and P < 0.01). In contrast, no difference was observed between CD133+/CD34+ and CD133+/CD34− cells (P > 0.05) (Figure 2e). To compare the regenerative potential of the CD133+ cell with a satellite cell population that match with the age range of the CD133+ cell donors, we also assessed the regenerative potential of the 15-year-old donor primary culture. The number of fibers expressing human proteins and therefore containing human nuclei was even lower (18.60 ± 2.40, P < 0.01) than that of the control satellite cells isolated from a 5-day-old infant.
Figure 2.
Quantification of the regenerative potential of human muscle–derived CD133+ cells in regenerating TAs of Rag2−/− γC−/− C5−/− mice. (a–c) Representative transverse sections showing the myogenic potential of human myoblasts (HM), CD133+CD34+, and CD133+CD34− cells. Human nuclei are revealed using a human-specific antilamin A/C antibody (green), and fibers are visualized using an antihuman spectrin–specific antibody (red). Nuclei are counterstained with Hoechst. (d) Quantification of the participation in the mouse muscle regeneration using the antispectrin antibody. (e) Quantification of the number of human lamin A/C+ nuclei interstitially located outside the basal lamina of the fibers. Results are means of the different TAs analyzed ± SEM. NS, nonsignificant, *P < 0.05, P < 0.01, ***P < 0.001. Original magnification ×200. TA, tibialis anterior.
Dispersion of muscle-derived CD133+ cells after engraftment
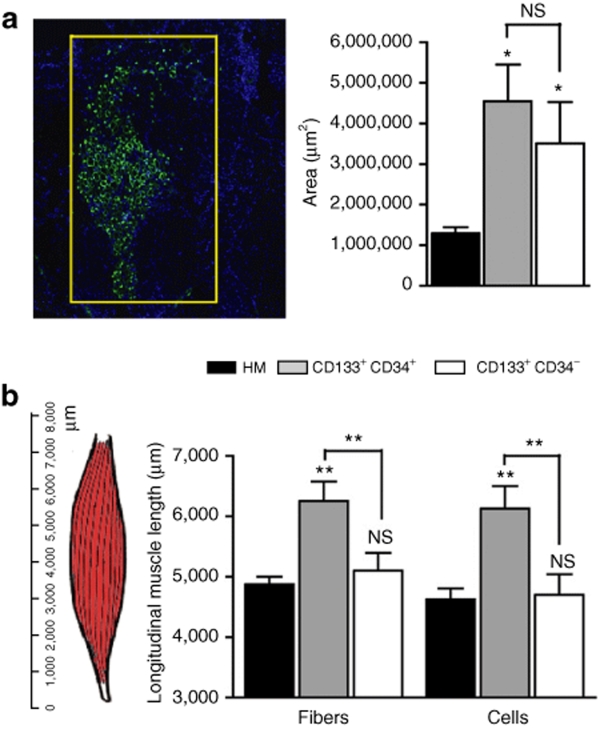
For each muscle, representative sections showing the maximum number of spectrin-positive fibers were used to assess the transversal dispersion of human engrafted cells. CD133+CD34+ cells occupied an area of 4.50 ± 0.90 mm2; CD133+CD34−, an area of 3.51 ± 1.02 mm2; and human myoblasts, an area of 1.30 ± 0.15 mm2. A statistical difference was observed between CD133+CD34+ cells and human myoblasts (P < 0.05), and CD133+CD34− cells and human myoblasts (P < 0.05), whereas no statistical difference was found between CD133+CD34+ and CD133+CD34− cells (P > 0.05) (Figure 3a). To assess the longitudinal dispersion of these different cell types within the host muscle, we counted the distance (i.e., number of sections) along which we detected the presence of lamin A/C positive nuclei and/or human spectrin–positive fibers along the longitudinal length of the muscle (Figure 3b). In the TA muscles injected with CD133+/CD34+ cells, fibers (6,125 ± 375 µm) and cells (6,250 ± 323 µm) occupied a much larger region when compared to CD133+/CD34− [fibers: 4,700 ± 339 µm (P < 0.01); cells: 5,100 ± 291 µm (P < 0.01)]; and human myoblasts [fibers: 4,625 ± 183 µm (P < 0.01); cells: 4,875 ± 125 µm (P < 0.01)]. No statistical difference was observed between CD133+/CD34− and human myoblasts (Figure 3b).
Figure 3.
Dispersion of injected human cells in Rag2−/− γC−/− C5−/− TAs. (a) Figure on the left shows a representative section with the area occupied by the injected cells used to measure the transversal dispersion. Sections are stained with an antibody directed against human lamin A/C to identify human cells and against spectrin to identify human fibers. For both antibodies (IgG2b), an Alexa Fluor 488 goat anti-mouse IgG2b was used. The graph on the right shows the quantification of the transversal dispersion. (b) A schematic representation of TA and its longitudinal length in µm is shown on the left. The maximal longitudinal distance between sections showing the expression of lamin A/C or human spectrin was measured and quantified (graph on the right). Results are means of the different TAs analyzed ± SEM. (NS, nonsignificant, *P < 0.05, **P < 0.01, ***P < 0.001). HM, human myoblasts; TA, tibialis anterior.
Muscle-derived CD133+ cells can enter the satellite cell compartment after in vivo transplantation
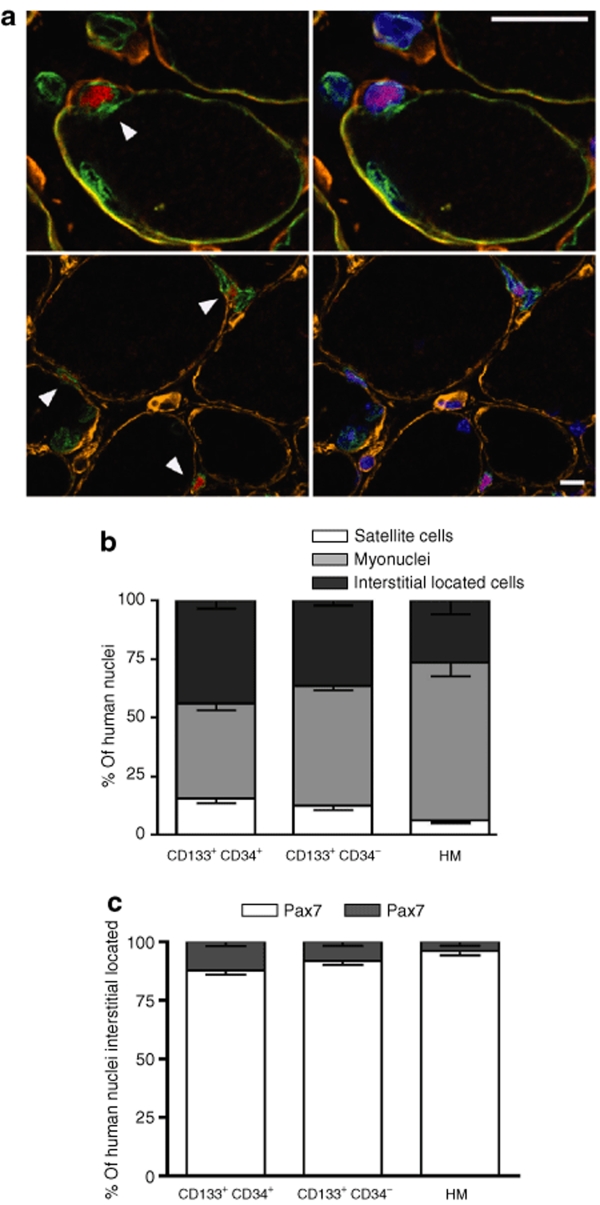
We investigated whether the engrafted CD133+ muscle-derived human cells were able to occupy the satellite cell niche within the host muscle using antibodies specific for the basal lamina protein laminin, the satellite cell marker Pax7, lamin A/C (human nuclei), and spectrin (specific to the human protein) to identify fiber sarcolemma (Figure 4). The number of Pax7/lamin A/C double-positive cells localized at the periphery of the muscle fiber and beneath the basal lamina (i.e., satellite cells from human origin) was calculated as a percentage of the total number of lamin A/C positive cells. Of the CD133+/CD34+ injected cells, 15.50% were identified as satellite cells as compared to 12.61% for CD133+/CD34− and only 6.19% for human myoblasts (Figure 4b). Interestingly, some Pax7-expressing human cells were also localized in the interstitial position between fibers [CD133+/CD34+: 12.20%, CD133+/CD34−: 8.20%, and 3.95% for human myoblasts control (Figure 4c)]. Quantification of human myonuclei that had been incorporated into the regenerated fibers showed the opposite tendency: the largest fraction of human myonuclei was found in TAs injected with human myoblasts (67.18%), as compared to 40.38% and 50.96% for the CD133+/CD34+ and CD133+/CD34− cells, respectively (Figure 4b).
Figure 4.
In vivo localization of human CD133/CD34+ and CD133+/CD34− cells. (a) Immunofluorescence analyses using antibodies directed against human lamin A/C and spectrin (green), Pax7 (red), and laminin (orange). Nuclei are counterstained with Hoechst (right panels). Human lamin A/C+ Pax7+ satellite cell localized between spectrin and basal lamina (arrowheads). Lamin A/C+ human myonuclei and human interstitial cells are also observed. (lower panels) Interstitially located lamin A/C+ human cells that can be Pax7+ (arrowhead) or Pax7−. (b) In vivo quantification of the repartition of muscle-derived CD133+CD34+, CD133+CD34−, and human myoblasts (HM). All lamin A/C+ cells on representative sections are counted and classed according to their position and the marker they express. (c) In vivo quantification of lamin A/C+ Pax7+ or Pax7− cells within the interstitial space. Each histogram represents the relative percentage for each of the injected populations (CD133+CD34+, CD133+CD34−, and HM) of Pax7+ and Pax7− cells. Bar = 10 µm.
Discussion
Over the past years, several reports have identified in mice different types of stem cells, distinct from satellite cells. Muscle-derived stem cells,19 side population cells,20,21,22 muscle interstitial cells,23,24,25 or vasculature-associated mesoangioblasts26 have been isolated, and have exhibited variable myogenic potentials in vivo. Mesoangioblasts have been extensively characterized both in mouse where they are able to rescue the dystrophic phenotype of the α-sarcoglycan-null mice,27 and in golden retriever muscular dystrophic dogs28 where they have been shown to restore dystrophin expression. A “pericyte-like” stem cell population has also been isolated from microvascular walls of adult human skeletal muscle, phenotypically distinct from satellite cells, and able, after systemic injection, to generate many human dystrophin–expressing fibers in scid-mdx mice muscles.29 Other reports have provided evidence of pericytes30 and of several other possible sources of human stem cells isolated from human peripheral blood,31 adult liver,32 or multipotent adult progenitor cells isolated from bone marrow,33 mesenchymal cells derived from synovial membrane,34 bone marrow stromal cells,35 or cells from human adipose tissue.36 Most of these cell types were demonstrated to have a myogenic potential, but this potential has been even less characterized than that of human pericytes. The aim of this study was to define a preclinical test to quantify the regenerative capacity of human myogenic stem cells, taking CD133+ cells as compared to human myoblasts already used in clinical trials as a proof of principle. The antigen surface marker CD133 (AC133 or Prominin-1) was first described in the mouse neuroepithelium,37 and is expressed by a subset of human hematopoietic stem cells.38 Although the structure of this new stem cell marker is known,38 its ligands, interactions, and biological role are still unclear (reviewed in ref. 39). In a recent paper, Torrente et al. have combined cell therapy using CD133+ cells isolated from DMD patients and gene therapy based on exon-skipping correction to deliver a functional dystrophin into scid/mdx mice muscles. The skipped DMD blood-derived and muscle-derived CD133+ cells were able to fuse in vivo with regenerating fibers, express functional human dystrophin and restore the dystrophin-associated protein complex.40 However, their myogenic potential in vivo as compared to muscle progenitors, such as bona fide satellite cells already used in clinical trials, was not quantified. To evaluate and quantify the myogenic regenerative capacity of CD133+ muscle-derived cells, we used the immunodeficient Rag2−/− γC−/− C5−/− mouse model as recipient. As demonstrated previously,18 these mice represent an ideal recipient model for cell xenotransplantation because they lack all lymphocytes (B, T) and natural killer cells, and in addition have a defect in the innate immunity, due to the lack of the C5 component cascade, which results in a compromised immune response.18 To ensure a complete and synchronized degeneration of the host TA and trigger the process of regeneration, a severe cryolesion was performed immediately prior to the injection of human cells (74% of the fibers have on average nuclei centrally located 4 weeks after a cryolesion). This procedure has the advantage of killing a majority of the endogenous satellite cells, favoring the formation of muscle tissue by the implanted human cells.41 Moreover, we have shown previously that cryolesion provides an optimal microenvironment for long-term in vivo transplantation with less infiltration of inflammatory cell as compared to toxin-treated muscles.18 In the present study, we carried out a single injection in each muscle, in order to maximize the quantitative information that can be drawn out of these experiments: multiple injections are more difficult to interpret because variations could be due to a discrete number of failed injections within one single muscle. Although the capacity of CD133+ cells isolated from healthy donors to form fibers after injection in scid/mdx mice has been described,15,40 their regenerative potential has not yet been quantified. In this study, the quantification of the fiber profiles expressing human spectrin, and thus containing human nuclei, 1 month after transplantation with adult CD133+ muscle-derived cells clearly shows an impressive myogenic potential for these cells. The number of human fiber profiles is higher than that obtained with bona fide human satellite cells presenting already a high regenerative capacity.42 However, these satellite cells were isolated from a very young donor and have been extensively expanded after their isolation. Therefore, we also assessed the regenerative potential of CD133+ cells as compared to satellite cells freshly isolated from a 15-year-old donor, which would match the age range of the CD133+ cell donors: the regenerative potential of the 15–year-old donor primary culture was even lower than that of the control satellite cells isolated from the 5-day-old infant muscle, and therefore much lower than that of CD133+ cells. A significant difference was also found in the number of humanized fibers formed after injection of the CD133+/CD34+ and CD133+/CD34− cells (1.5-fold augmentation in favor of CD133+CD34+), suggesting a different behavior of these two subpopulation regarding their incorporation into regenerating muscle fibers of the host. It has been shown that expression of CD34 could be related to the state of stem cells in the adult hematopoietic system,43 indicating activated cells (CD34+) or quiescent (CD34−) stem cells. The presence of the CD34 marker could identify cells more prone to proliferation and differentiation among the CD133+ population, whereas its absence would identify cells nearer the stem compartment. The values for telomere lengths, which decreases during proliferation in vitro and in vivo and have been proposed as a marker of the remaining proliferative capacity (reviewed in ref. 44), are in agreement with our proposition: the CD34− cells have longer telomeres (12.3 kb) than CD34+ cells (10.9 kb). However, it does not correlate with the regenerative capacity because CD34+ cells have shorter telomeres than CD34−, and their telomere length is similar to that of the control satellite cells used in this study (10.5 kb), thus further suggesting that the state of committed stem cells characteristic of CD133+CD34+ cells is enhancing their regenerative capacity. It should be noticed that both populations of CD133+ cells (either CD34+ or CD34−) represent most probably a heterogeneous population, as suggested by the variable level of expression of CD56 in these populations (19% of the CD34+ cells are CD56+, whereas 23% of the CD34− cells express this marker). However, this minority of cells expressing CD56, a marker of muscle progenitors, but also expressed by other types of cells, cannot explain that they have a myogenic potential higher than that of bona fide satellite cells, which are over 80% positive for CD56.
The difference in commitment between CD34+ and CD34− cells could explain that the number of CD133+CD34+ cells is increased in DMD muscle biopsies (79%) where mobilization and activation of myogenic precursors also occur45 as compared to healthy biopsies. In conclusion, CD133+/CD34+ cells could represent the fraction of CD133+ progenitors that have already been activated and are able to react more rapidly and efficiently to the signals and/or stimuli for cell proliferation and differentiation induced by cryoinjury. Poor migration is one of the major problems in myoblast transplantation, and it has been frequently demonstrated that injected cells migrate only a few micrometers from the site of implantation.46 In addition to their increased ability to participate in muscle regeneration, analysis of the transversal and longitudinal dispersion of the human markers in regenerating fibers confirms the increased migratory potential of the CD133+CD34+ cells as compared to human myoblasts. CD133+ cells in general are less committed to myogenesis, being in an earlier stage of myogenic conversion as compared to already committed myoblasts, as evidenced by their limited and delayed differentiation in vitro: although CD133+CD34+ cells can reach in vitro a percentage of fusion of 55%, this level of fusion is reached only after 14 days of differentiation, whereas satellite cells have already reached their maximum level of fusion after 5 days of differentiation. Because differentiated muscle cells cannot migrate, we propose that these CD133+ cells may commit gradually to terminal myogenesis, and thus differentiate with a delay as compared to myoblasts, which leaves them more time to migrate within the host's tissue. CD133+/CD34+ cells also disperse better within the host's muscle than CD133+/CD34− cells. Therefore, the expression of the CD34 marker that is normally associated with human hematopoietic stem cells47 defines within the CD133+ population a subset of precursors with a better myogenic potential and/or improved migratory properties. A subpopulation of the injected cells do not fuse to form multinucleated fibers, and either stay in the intercellular space between the fibers or adopt a satellite cell position and fate. Although the population of cells that adopt a position of satellite cells may participate in further cycles of regeneration, the fate of the interstitial population is still unknown: it has been shown in the mouse that some subpopulations of interstitial cells such as side population cells22 can participate in muscle regeneration, but with a much lower efficiency than myoblasts. In this report, we show that more human mononuclear cells remained in the interstitial space in the muscle injected with the CD133+/CD34+ cells when compared to the human myoblasts and/or the CD133+/CD34−, although this last difference is not significative. It has already been observed, using the same experimental model,42 that a large number of implanted cells persist after injection as mononucleated cells located outside the basal lamina of the fibers. In the case of myoblasts, these interstitial cells may result from a contamination of the population by other cell types, such as fibroblasts, which therefore did not enter the myogenic program. In the case of CD133+ cells, these cells are purified on the basis of the CD133 marker, absent from fibroblasts. This high percentage of nondifferentiated interstitial cells probably reveals that all CD133+ cells are not at the same stage of myogenic commitment, and that some of these cells never reach terminal differentiation. This is reinforced by the fact that 12.20% of CD133+/CD34+ cells and 8.20% of CD133+/CD34− cells situated in the interstitial space express Pax7 (Figure 4c), a muscle lineage marker. This suggests that each of these two populations is heterogeneous, and contains a fraction already committed to the myogenic pathway, that gives rise to myonuclei or satellite cells, and another characterized by slower differentiation kinetics that requires much more time or stronger signals to be converted to the myogenic lineage. This is further confirmed by the heterogeneity in CD56 expression because about 20% of the CD133+ cells express this marker. Although this marker is not specific of satellite cells in skeletal muscle (it is also expressed by a small myoendothelial population localized between muscle fibers48), its expression could indicate that a fraction of CD133+ cells is already committed enough to express CD56. In contrast, only 3.95% of interstitially located human myoblasts express Pax7 (Figure 4c), indicating that almost all myogenic cells participate in muscle regeneration.
Another important property of myogenic precursors is their capacity to restore the pool of satellite cells. This can be crucial in cell therapy because a contribution to the satellite cell pool represents the possibility for these cells to participate in successive events of regeneration, and thus to further amplify their therapeutic role during the dystrophic process. The quantification of satellite cells, using a Pax7 antibody, shows that a much higher fraction of cells derived from the CD133+ population are able to occupy the satellite cell niches as compared to human myoblasts. In contrast, human myoblasts seem to be more readily incorporated into myofibers during the regeneration process because the number of myonuclei originating from human satellite cells is considerably higher (1.5 increase) when compared to the injected CD133+ cells. Although we cannot conclude from this study that CD133+ cells will be as efficient in a dystrophic human muscle as we have measured it in a cryodamaged mouse muscle, a situation that is not comparable to that of DMD muscles, the fact that CD133+ cells participate with a higher efficiency in the satellite cell pool represents another clinical advantage over committed myoblasts derived from bona fide satellite cells. Clinicians should also consider a combination of different therapeutic approaches: cell therapy using stem cells with myogenic potential could be used to complement gene therapy approaches such as exon-skipping with adeno-associated virus where it would be difficult to treat a second time with the viral vector, due to the immune response to the adeno-associated virus. In conclusion, in this article, we propose a new preclinical test adapted to evaluate the myogenic potential of human stem cells, as compared to cells already used in clinical trial, i.e., bona fide satellite cells. Using this novel quantitative approach, we demonstrate that CD133+ human muscle–derived cells participate in muscle regeneration with a greater efficiency as compared to myoblasts. Therefore, we propose that CD133+ muscle-derived cells, and particularly the subfraction expressing both CD133 and CD34, represent novel candidates for cell therapy, and their behavior in a dystrophic environment should be investigated in an appropriate animal model.
Materials and Methods
Animals. 2- to 3-month-old immunodeficient Rag2−/− γC−/− C5−/− mice were used as recipients for human cell implantation. Mice were anesthetized with an intraperitoneal injection of ketamine hydrochloride (80 mg/kg) and xylazine (10 mg/kg) (Sigma-Aldrich, St Louis, MO) as described previously,18 in accordance with the French legislation.
Human myoblast cultures. Satellite cell populations were isolated from the quadriceps muscle of a 5-day-old infant in accordance with the French legislation on ethical rules. These cells were expanded in F10 medium (Invitrogen, Carlsbad, CA) supplemented with 20% fetal bovine serum (Invitrogen), and their PDL was determined at each passage as described previously.49 All experiments were performed on cells between 20 and 30 PDLs. Myogenic purity was determined by counting the number of desmin-positive cells as a percentage of the total number of nuclei. A total of 500 nuclei were counted. All of the human myoblast preparations used in this study displayed a myogenicity of at least 80%. Immunocytochemistry was performed as already described.49 For the in vivo regenerative capacity experiment, a second satellite cell population, isolated from a 15-year-old subject and expanded in the same conditions as those described above, was implanted at 5.5 PDLs.
In vitro characterization of human muscle–derived CD133+/CD34+ and CD133+/CD34− cells. Muscle-derived CD133+ cells were collected from muscle biopsies (40–300 mg) of healthy subjects after informed consent according to the guidelines of the Committee on the Use of Human Subjects in Research of the Policlinico Hospital of Milan (Milan, Italy). Muscle samples were weighed and washed several times in phosphate-buffered saline (PBS), finely minced with scissors, and incubated at 37 °C for 45 minutes with 1 mg/ml collagenase type IA, 1 mg/ml collagenase type II, and 1 mg/ml collagenase type IV (all from Sigma-Aldrich), in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 20% fetal bovine serum. Most of the skeletal muscle stem cells were released from the tissue after this step. The cell extract was filtered with a 70-µm nylon mesh (BD Biosciences, Immunocytometry Systems, Mountain View, CA) and plated in noncoated wells for 24 hours in the presence of proliferation medium composed of Dulbecco's modified Eagle's medium/F-12 (1:1), 20% fetal bovine serum, including 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer (5 mmol/l), glucose (0.6%), sodium bicarbonate (3 mmol/l), glutamine (2 mmol/l), stem cell factor (100 ng/ml; TEBU, Frankfurt, Germany), vascular endothelial growth factor (50 ng/ml; TEBU), and leukemia inhibitory factor (20 ng/ml; R&D Systems, Minneapolis, MN). Nonadherent cells were resuspended in PBS, incubated with CD133-conjugated superparamagnetic microbeads (CD133 Isolation Kit; Miltenyi Biotec, Bergisch Gladbach, Germany), washed and sorted using a magnetic-activated cell sorting device (Miltenyi Biotec). A second column run was used to obtain CD133+ cells of higher purity. CD133+/CD34+ and CD133+/CD34− were isolated by flow cytometry cell sorting (CD34-APC; BD Biosciences, San Diego, CA). After selection, an aliquot of each cell fraction was analyzed by flow cytometry to assess purity, which was on average 95% for both subpopulations (range 92–97%). All these experiments were performed using a FACSVantage flow cytometer (BD Biosciences, San Diego, CA). To investigate myogenicity, cells (human myoblasts, CD133+/CD34+, and CD133+/CD34−) were also incubated with antihuman CD56-PE monoclonal antibody (clone AF12-7H3; Miltenyi Biotec) according to the manufacturer's recommendations. Cell analysis was performed on at least 10,000 events for each sample using a FACSCalibur cytometer and CellQuest software (BD Biosciences, San Diego, CA). In order to assess differentiation in vitro, muscle-derived CD133+ subpopulations were plated in 48-well plates coated with 0.2% gelatin, and when they reached 60–70% confluence, serum concentration was decreased to 2%. After 14 days of culture in this differentiation medium, the ratio between the number of nuclei per multinucleated myotube and the total number of nuclei (i.e., fusion index) was calculated. Differentiated myotubes were detected by immunostaining with antibody reactive to myosin heavy chain (MF20, mouse monoclonal IgG2b, 1:20; Developmental Studies Hybridoma Bank, Iowa City, IA). Before human cells were injected into the TA muscle, their mean telomere lengths were measured by telomeric restriction fragment length analysis, as described previously.50
Induction of host muscle regeneration and implantation of human cells. Recipient TAs were exposed and subjected to three cycles of muscle freezing–thawing for 10 seconds each to induce severe muscle damage and trigger regeneration.42 Immediately after cryodamage, cells (15 µl cell suspension containing 2.5 × 105 cells in PBS) were injected, using a 25-µl Hamilton syringe, in a single midpoint site along the longitudinal axis of the TA. The skin was then closed with a fine suture. At 4 weeks after transplantation, the mice were killed and TA muscles were dissected, mounted in gum tragacanth (6% in water; Sigma-Aldrich), and frozen in isopentane precooled in liquid nitrogen. Surgical procedures were performed in accordance with the legal regulations in France and European Union ethical guidelines for animal research.
Immunofluorescence. 5-µm transverse cryostat sections were incubated with human-specific antibodies: lamin A/C (NCL-Lam-A/C, mouse monoclonal IgG2b, 1:400) and spectrin (NCL-Spec1, mouse monoclonal IgG2b, 1:50) from Novocastra (Newcastle-upon-Tyne, UK), detecting human nuclei (antilamin A/C) and fibers expressing human proteins (antispectrin). A second lamin A/C antibody (clone JOL2, mouse monoclonal IgG1, 1:300; Abcam, Cambridge, UK) was used in combination with the spectrin antibody. Other antibodies used were directed against Pax7 (mouse monoclonal, IgG1, 1:20; Developmental Studies Hybridoma Bank) and laminin (1:400, rabbit polyclonal; Dako, Trappes, France). The secondary antibodies used in this study were Alexa Fluor 488 goat anti-mouse IgG2b (1:300; Molecular Probes, Montluçon, France) for lamin A/C (purchased from Novocastra) and spectrin, Cy3-conjugated goat anti-mouse IgG1 (1:500; Jackson ImmunoResearch, West Grove, PA) for lamin A/C (clone JOL2), Cy5-conjugated streptavidin goat anti-rabbit (1:500; Jackson ImmunoResearch) for laminin, and biotin-conjugated goat anti-mouse IgG1 antibody (1:1000) followed by Cy3-conjugated streptavidin (1/400), both from Jackson ImmunoResearch, for Pax7 staining. The specificity of these antibodies was tested on control mouse and human sections (data not shown).
Briefly, 5 µm transverse cryostat sections were used either unfixed (for lamin A/C and spectrin) or fixed with 4% PFA for 5 minutes at room temperature, washed twice in PBS and blocked with 4% bovine serum albumin/PBS. Sections were incubated with primary antibody for 1 hour at room temperature, then washed in PBS and stained with appropriate secondary antibodies for 45 minutes at room temperature. To visualize nuclei, the sections were mounted in medium (DakoCytomation fluorescent mounting medium, S3023; Dako) containing Hoechst (bisbenzimide, 0.0001% wt/vol, no. 33258; Sigma-Aldrich). Images were visualized using an Olympus BX60 microscope (Olympus Optical, Hamburg, Germany), digitalized using a CCD camera (Photometrics CoolSNAP fx; Roper Scientific, Tucson, AZ) and analyzed using MetaView image analysis system (Universal Imaging, Downington, PA), except images in Figure 4 that were captured using a Leica confocal TCS SPE microscope (Leica, Wetzlar, Germany).
Analyses of muscle samples. TA muscles were entirely cut into 5 µm sections. For every 450 µm along the complete length of the muscle, 10 sections corresponding to a 50 µm length were used for quantitative analyses. The number of spectrin-positive profiles in each section examined was counted and the maximum value was determined for each TA investigated. To quantify mononucleated interstitial lamin A/C positive cells, three of the 10 sections analyzed, each separated by at least 15 µm, were assessed and the mean value of lamin A/C positive cells was calculated. Finally, the mean value of lamin A/C positive cells was calculated for all of the slides examined covering the entire length of the TA muscle. In order to evaluate the longitudinal dispersion of the cells, the presence of lamin A/C positive cells and human spectrin–positive fibers was analyzed, and the results were plotted as a function of the longitudinal length of the muscle. An average of the entire region occupied by lamin A/C positive cells or human spectrin fibers for all of the TA muscles of each group was calculated. To analyze the transversal dispersion of the myogenic injected cells, representative sections of the muscle of each group bearing the larger number of spectrin-positive fibers along the entire muscle were chosen. The area of the smallest rectangle containing inside all the human fibers formed was calculated using MetaView image analysis system software.
Statistical analysis. Data are presented as the mean of the different animals ± SEM. All statistical analyses were carried out using GraphPad Prism (version 4.0b; GraphPad Software, San Diego, CA). Statistical significance of the differences between mean values was assessed by one-way analysis of variance followed by the Newman-Keuls post-test. A difference was considered to be significant at P < 0.05 (*), P < 0.01 (**), or P < 0.001 (***).
Acknowledgments
The authors wish to acknowledge their financial support: the AFM (Association Française contre les Myopathies), INSERM, Université Pierre et Marie Curie, the MYORES Network of Excellence (contract 511978) and MYOAMP STREP (contract 037479), both from the European Commission 6th Framework Programme, the CAPES/COFECUB and INSERM/FIOCRUZ French/Brazilian conjoint programs, and the Parents Project Monaco. The authors would like to thank Anne Bigot, Denis Furling, Daniella Arêas Mendes da Cruz, and Luc Pettavino for fruitful discussions; Kathryn Mitchell and Vincent Gache for microscopy; Lidia Dollé, Maximilien Bencze-Rovez, and Annie Wolff for technical assistance.
REFERENCES
- Decary S, Mouly V, Hamida CB, Sautet A, Barbet JP., and , Butler-Browne GS. Replicative potential and telomere length in human skeletal muscle: implications for satellite cell-mediated gene therapy. Hum Gene Ther. 1997;8:1429–1438. doi: 10.1089/hum.1997.8.12-1429. [DOI] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P., and , Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Irintchev A, Zeschnigk M, Starzinski-Powitz A., and , Wernig A. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dyn. 1994;199:326–337. doi: 10.1002/aja.1001990407. [DOI] [PubMed] [Google Scholar]
- Renault V, Rolland E, Thornell LE, Mouly V., and , Butler-Browne G. Distribution of satellite cells in the human vastus lateralis muscle during aging. Exp Gerontol. 2002;37:1513–1514. doi: 10.1016/s0531-5565(02)00095-5. [DOI] [PubMed] [Google Scholar]
- Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, et al. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit PS, Partridge TA., and , Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- Blau HM, Webster C., and , Pavlath GK. Defective myoblasts identified in Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 1983;80:4856–4860. doi: 10.1073/pnas.80.15.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decary S, Hamida CB, Mouly V, Barbet JP, Hentati F., and , Butler-Browne GS. Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscul Disord. 2000;10:113–120. doi: 10.1016/s0960-8966(99)00093-0. [DOI] [PubMed] [Google Scholar]
- Wang Z, Allen JM, Riddell SR, Gregorevic P, Storb R, Tapscott SJ, et al. Immunity to adeno-associated virus-mediated gene transfer in a random-bred canine model of Duchenne muscular dystrophy. Hum Gene Ther. 2007;18:18–26. doi: 10.1089/hum.2006.093. [DOI] [PubMed] [Google Scholar]
- Alter J, Lou F, Rabinowitz A, Yin H, Rosenfeld J, Wilton SD, et al. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med. 2006;12:175–177. doi: 10.1038/nm1345. [DOI] [PubMed] [Google Scholar]
- van Deutekom JC, Janson AA, Ginjaar IB, Frankhuizen WS, Aartsma-Rus A, Bremmer-Bout M, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. [DOI] [PubMed] [Google Scholar]
- Goyenvalle A, Vulin A, Fougerousse F, Leturcq F, Kaplan JC, Garcia L, et al. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science. 2004;306:1796–1799. doi: 10.1126/science.1104297. [DOI] [PubMed] [Google Scholar]
- Skuk D, Goulet M, Roy B, Chapdelaine P, Bouchard JP, Roy R, et al. Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J Neuropathol Exp Neurol. 2006;65:371–386. doi: 10.1097/01.jnen.0000218443.45782.81. [DOI] [PubMed] [Google Scholar]
- Torrente Y, Belicchi M, Sampaolesi M, Pisati F, Meregalli M, D'Antona G, et al. Human circulating AC133(+) stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J Clin Invest. 2004;114:182–195. doi: 10.1172/JCI20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollemann D, Budka H, Löscher WN, Yanagida G, Fischer MB., and , Wanschitz JV. Endothelial and myogenic differentiation of hematopoietic progenitor cells in inflammatory myopathies. J Neuropathol Exp Neurol. 2008;67:711–719. doi: 10.1097/NEN.0b013e31817d8064. [DOI] [PubMed] [Google Scholar]
- Krause DS, Fackler MJ, Civin CI., and , May WS. CD34: structure, biology, and clinical utility. Blood. 1996;87:1–13. [PubMed] [Google Scholar]
- Silva-Barbosa SD, Butler-Browne GS, Di Santo JP., and , Mouly V. Comparative analysis of genetically engineered immunodeficient mouse strains as recipients for human myoblast transplantation. Cell Transplant. 2005;14:457–467. doi: 10.3727/000000005783982837. [DOI] [PubMed] [Google Scholar]
- Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodell MA, Brose K, Paradis G, Conner AS., and , Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- Asakura A, Seale P, Girgis-Gabardo A., and , Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesskaya A, Seale P., and , Rudnicki MA. Wnt signaling induces the myogenic specification of resident CD45+ adult stem cells during muscle regeneration. Cell. 2003;113:841–852. doi: 10.1016/s0092-8674(03)00437-9. [DOI] [PubMed] [Google Scholar]
- Kuang S, Chargé SB, Seale P, Huh M., and , Rudnicki MA. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172:103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki T, Akatsuka A, Ando K, Nakamura Y, Matsuzawa H, Hotta T, et al. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J Cell Biol. 2002;157:571–577. doi: 10.1083/jcb.200112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M, Torrente Y, Innocenzi A, Tonlorenzi R, D'Antona G, Pellegrino MA, et al. Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science. 2003;301:487–492. doi: 10.1126/science.1082254. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M, Blot S, D'Antona G, Granger N, Tonlorenzi R, Innocenzi A, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Herrera MB, Bruno S, Buttiglieri S, Tetta C, Gatti S, Deregibus MC, et al. Isolation and characterization of a stem cell population from adult human liver. Stem Cells. 2006;24:2840–2850. doi: 10.1634/stemcells.2006-0114. [DOI] [PubMed] [Google Scholar]
- Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L., and , Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–2625. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- De Bari C, Dell'Accio F, Vandenabeele F, Vermeesch JR, Raymackers JM., and , Luyten FP. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol. 2003;160:909–918. doi: 10.1083/jcb.200212064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S, et al. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309:314–317. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- Rodriguez AM, Pisani D, Dechesne CA, Turc-Carel C, Kurzenne JY, Wdziekonski B, et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J Exp Med. 2005;201:1397–1405. doi: 10.1084/jem.20042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigmann A, Corbeil D, Hellwig A., and , Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci USA. 1997;94:12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, et al. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- Shmelkov SV, St Clair R, Lyden D., and , Rafii S. AC133/CD133/Prominin-1. Int J Biochem Cell Biol. 2005;37:715–719. doi: 10.1016/j.biocel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Benchaouir R, Meregalli M, Farini A, D'Antona G, Belicchi M, Goyenvalle A, et al. Restoration of human dystrophin following transplantation of exon-skipping-engineered DMD patient stem cells into dystrophic mice. Cell Stem Cell. 2007;1:646–657. doi: 10.1016/j.stem.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Irintchev A, Langer M, Zweyer M, Theisen R., and , Wernig A.Functional improvement of damaged adult mouse muscle by implantation of primary myoblasts J Physiol (Lond) 1997500775–785.Pt 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RN, Thiesson D, Furling D, Di Santo JP, Butler-Browne GS., and , Mouly V. Extended amplification in vitro and replicative senescence: key factors implicated in the success of human myoblast transplantation. Hum Gene Ther. 2003;14:1169–1179. doi: 10.1089/104303403322168000. [DOI] [PubMed] [Google Scholar]
- Sato T, Laver JH., and , Ogawa M. Reversible expression of CD34 by murine hematopoietic stem cells. Blood. 1999;94:2548–2554. [PubMed] [Google Scholar]
- Mouly V, Aamiri A, Bigot A, Cooper RN, Di Donna S, Furling D, et al. The mitotic clock in skeletal muscle regeneration, disease and cell mediated gene therapy. Acta Physiol Scand. 2005;184:3–15. doi: 10.1111/j.1365-201X.2005.01417.x. [DOI] [PubMed] [Google Scholar]
- Torrente Y, Belicchi M, Marchesi C, Dantona G, Cogiamanian F, Pisati F, et al. Autologous transplantation of muscle-derived CD133+ stem cells in Duchenne muscle patients. Cell Transplant. 2007;16:563–577. doi: 10.3727/000000007783465064. [DOI] [PubMed] [Google Scholar]
- Satoh A, Huard J, Labrecque C., and , Tremblay JP. Use of fluorescent latex microspheres (FLMs) to follow the fate of transplanted myoblasts. J Histochem Cytochem. 1993;41:1579–1582. doi: 10.1177/41.10.8245416. [DOI] [PubMed] [Google Scholar]
- Civin CI, Strauss LC, Brovall C, Fackler MJ, Schwartz JF., and , Shaper JH. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984;133:157–165. [PubMed] [Google Scholar]
- Zheng B, Cao B, Crisan M, Sun B, Li G, Logar A, et al. Prospective identification of myogenic endothelial cells in human skeletal muscle. Nat Biotechnol. 2007;25:1025–1034. doi: 10.1038/nbt1334. [DOI] [PubMed] [Google Scholar]
- Edom F, Mouly V, Barbet JP, Fiszman MY., and , Butler-Browne GS. Clones of human satellite cells can express in vitro both fast and slow myosin heavy chains. Dev Biol. 1994;164:219–229. doi: 10.1006/dbio.1994.1193. [DOI] [PubMed] [Google Scholar]
- Di Donna S, Mamchaoui K, Cooper RN, Seigneurin-Venin S, Tremblay J, Butler-Browne GS, et al. Telomerase can extend the proliferative capacity of human myoblasts, but does not lead to their immortalization. Mol Cancer Res. 2003;1:643–653. [PubMed] [Google Scholar]