Abstract
The human telomerase reverse transcriptase (hTERT) is an attractive target for human cancer vaccination because its expression is reactivated in most human tumors. We have evaluated the ability of DNA electroporation (DNA-EP) and adenovirus serotype 6 (Ad6) to induce immune responses against hTERT in nonhuman primates (NHPs) (Macaca mulatta). Vaccination was effective in all treated animals, and the adaptive immune response remained detectable and long lasting without side effects. To further enhance the efficacy of the hTERT vaccine, we evaluated the combination of hTERT vaccine and a novel TLR9 agonist, referred to as immunomodulatory oligonucleotide (IMO). Monkeys were dosed weekly with IMO concurrently with the vaccine regimen and showed increases in cytokine secretion and activation of natural killer (NK) cells compared with the group that received vaccine alone. Using a peptide array, a specific profile of B-cell reactive epitopes was identified when hTERT vaccine was combined with IMO. The combination of IMO with hTERT genetic vaccine did not impact vaccine-induced TERT-specific cell-mediated immunity. Our results show that appropriate combination of a DNA-EP/Ad6-based cancer vaccine against hTERT with IMO induces multiple effects on innate and adaptive immune responses in NHPs.
Introduction
Telomerase is a ribonucleoprotein comprising an RNA component and a catalytic protein component (telomerase reverse transcriptase, TERT).1,2 As telomerase confers immortality to cells, telomerase activity has been detected in cancerous cell lines and in a variety of tumor types.3 Conversely, telomerase is inactive or only transiently expressed at low levels in normal human tissues and somatic cell cultures. The combination of telomerase overexpression in most tumor types, and low or absent expression in normal cells makes TERT a tumor-associated antigen and a suitable target for cancer immunotherapy.
Genetic vaccines represent promising methods to elicit immune responses against a wide variety of antigens, including tumor-associated antigens.4 Among these, in vivo electroporation of plasmid DNA (DNA-EP) and replication-defective recombinant adenoviruses (Ads) have been proven very efficacious to induce strong antibody and cellular antigen–specific immune responses.5,6,7,8 Combinations of heterologous modalities of immunization induce superior immune reactions as compared to single modality vaccines.9,10
Toll-like receptors (TLRs) constitute the first line of immune defense receptors that recognize microbial pathogens, leading to the activation of protective immune responses.11 One of the most promising targets for therapeutic immune activation is TLR9, which detects unmethylated CpG dinucleotides present in viral and prokaryotic genomes.12 TLR9 stimulates innate immunity with a predominantly Th1-type cytokine and chemokine secretion by B cells, plasmacytoid dendritic cells, and by other immune cells. TLR9 agonists have been shown to induce strong CD4+ and CD8+ T-cell responses and rapid production of antigen-specific Th1-type antibody responses when used as adjuvants with vaccines.13,14,15,16 Agonists of TLR9-containing novel DNA structures and synthetic immune stimulatory motifs, referred to as immunomodulatory oligonucleotides (IMOs), have been reported.17,18,19,20,21,22 IMOs have been shown to induce potent and distinct cytokine profiles in vitro and in vivo and exhibit higher metabolic stability.17,18,19,20,21,22 Previous studies have demonstrated potent antitumor activity of IMOs as monotherapies or in combination with chemotherapeutic agents and monoclonal antibodies.23,24,25 Recently, we showed that the treatment of BALB/neuT mice with a combination of an IMO and HER-2/neu DNA-EP/Ad vaccine results in tumor stabilization/regression and durable protection against spontaneous mammary carcinoma.26 The antitumor activity of the combination was associated with antibody isotype switch, antibody-dependent cell-mediated cytotoxicity, and cell-mediated immune responses. Currently, an IMO referred to as IMO-2055 is under clinical evaluation, in combination with chemotherapy and other agents in cancer patients.27,28
We have studied DNA-EP and Ad type 6 (Ad6) vaccine targeting TERT in combination with a novel TLR9 agonist in tumor models in mice (A. Conforti, B. Cipriani, D. Peruzzi, S. Dharmapuri, F. Mori, E.R. Kandimalla et al., unpublished results). The combination of TERT vaccine and IMO showed significant antitumor activity compared with vaccine or IMO alone. The tumor-bearing mice vaccinated with combination showed increased levels of TERT-specific antibodies, natural killer (NK) cell activation, and TERT-specific CD8+ T cells within the tumor mass following peritumoral administration of IMO (A. Conforti, B. Cipriani, D. Peruzzi, S. Dharmapuri, F. Mori, E.R. Kandimalla et al., unpublished results). Based on these encouraging results in mouse models, in the present study, for the first time, a DNA-EP and Ad6 vaccine targeting human TERT (hTERT) in combination with a novel TLR9 agonist, IMO, was evaluated for innate humoral and cell-mediated immune responses in rhesus monkeys. The results show that DNA-EP and Ad6 vaccine targeting hTERT can induce strong immune response to the antigen. Moreover, the combination of IMO with hTERT vaccine showed a significant impact on innate and antibody immunity. The associated immunological biomarkers have been characterized.
Results
hTERT vaccine induces strong immune response in nonhuman primates
The human and rhesus (Macaca mulatta) TERT protein sequences share 96% identity. Therefore, hTERT immunization in rhesus monkeys is similar to syngeneic vaccination in a tolerant model. To assess whether vaccination was capable of eliciting an immune response against hTERT antigen, two delivery vectors were constructed: (i) a DNA plasmid encoding a human codon-optimized, catalytically inactive hTERT protein fused to a TPA (human tissue plasminogen activator) leader sequence at the N-terminus and to the β-subunit of Escherichia coli heat labile enterotoxin (LTB) at the C-terminus; (ii) an Ad6 vector expressing hTERT with wild-type codon usage. The nucleotide sequence was mutated in the region corresponding to the catalytic site. For both vectors, transcription was controlled by the human cytomegalovirus (CMV) major immediate-early enhancer/promoter and was terminated by the bovine growth hormone polyadenylation signal.
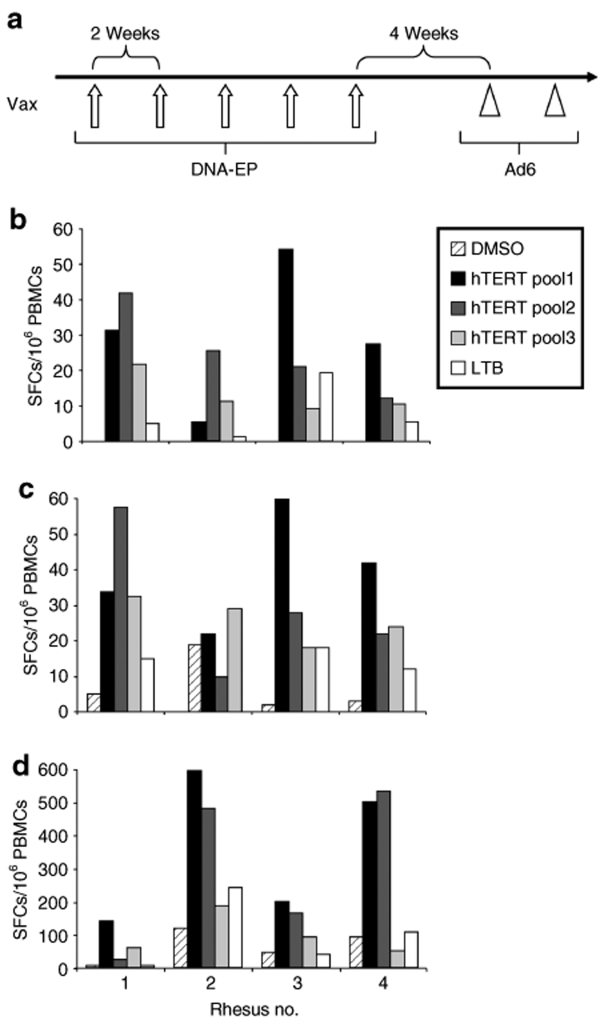
The immunogenicity of these vectors was initially studied in mice (data not shown). Macaques were vaccinated five times by DNA-EP (5 mg/injection) every 2 weeks and then after four additional weeks, they were boosted twice with Ad6 (1011 viral particles) with a two-week interval between doses (see scheme in Figure 1a). Heterologous prime–boost immunization regimens like this have been shown capable of generating higher amplitude and more durable immune responses and to exert better prophylactic and therapeutic efficacy in a variety of preclinical disease models. The induced immune response was monitored by enzyme-linked immunosorbent spot (ELISPOT) using peptide pools covering hTERT and LTB sequence. No T-cell reactivity was observed in the negative control group immunized with phosphate-buffered saline (PBS) (data not shown) or against dimethyl sulfoxide. Responses were scored positive when they were at least four times higher than background reactivity (dimethyl sulfoxide). A detectable and multi-epitope response was found in all four vaccinated monkeys after the first DNA-EP vaccinations (Figure 1b). A significant reactivity was measured against all hTERT and LTB peptide pools. A slight increase in the response was observed after the fifth dose of DNA-EP (Figure 1c). An additional measure of the immune response was performed at the end of the immunization, 1 week after the Ad6 boost. The Ad6 injections induced a consistent increase in the amplitude of cell-mediated immune response in three monkeys (20–30-fold, Figure 1d). No treatment-related effects on body weight and clinical signs, such as local inflammation at the injection site or other symptoms, were observed in animals during the course of experiment. These data indicate that DNA-EP/Ad6 hTERT vaccine is highly immunogenic in monkeys and further confirm the power of the heterologous prime–boost modality.
Figure 1.
Induction of cell-mediated immune response to hTERT in rhesus monkeys by DNA-EP and Ad regimen. (a) Schematic representation of the vaccination schedule. (b) Enzyme-linked immunosorbent spot (ELISPOT) was performed on PBMCs from hTERT-immunized rhesus monkeys after the second DNA-EP. Three pools of 15-mer peptides covering hTERT (pool 1, 2, and 3) and one pool covering LTB were utilized. (c) Analysis was performed after the fifth DNA-EP. (d) ELISPOT was performed at the end of the immunization program based on five DNA-EP and two Ad (Ad6-hTERT) injections. The assay was run in duplicate and the average value from the replicates is shown. Ad6, adenovirus serotype 6; DMSO, dimethyl sulfoxide; DNA-EP, DNA electroporation; hTERT, human telomerase reverse transcriptase; LTB, β-subunit of Escherichia coli heat labile enterotoxin; PBMC, peripheral blood mononuclear cell; SFC, spot-forming cell; Vax, vaccination.
TLR9 agonist induces innate immunity
To enhance the potency of hTERT vaccine, IMO and DNA-EP/Ad6 were tested in a combination treatment. Groups of rhesus monkeys were vaccinated with five DNA-EP, followed by two Ad6 injections. IMO was administered subcutaneously every week for a total of 15 times to synchronize with DNA vaccine expression at 1, 2.5, or 10 mg per injection (Figure 2). A weekly administration schedule and dosage ranging from 0.04 to 0.64 mg/kg/week have been used of a TLR9 agonist, referred to as IMO-2055 in clinical studies, in refractory cancer patients.27,28
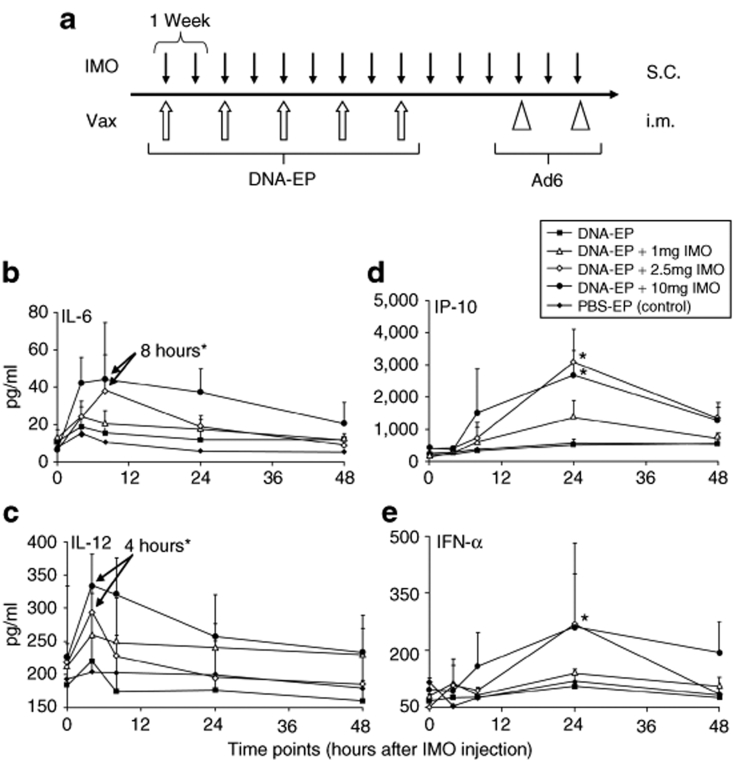
Figure 2.
Time course of cytokine levels detected in rhesus serum as early biomarkers. (a) Groups of four macaques were treated as described in the text and are represented schematically. Cytokine concentrations of (in pg/ml) (b) IL-6, (c) IL-12, (d) IP-10, and (e) IFN-α were measured after the first administration of IMO as specified in Materials and Methods. All the treatment groups received the first DNA-EP injection just before the IMO. Average values and standard deviations are shown. Group symbols are indicated in the figure legend. Asterisks indicate time points where differences are statistically significant (P < 0.05; Student's t-test). Ad6, adenovirus serotype 6; DNA-EP, DNA electroporation; IFN-α, interferon-α; IL, interleukin; i.m., intramuscular; IMO, immunomodulatory oligonucleotide; IP-10, interferon-γ-induced protein-10; PBS, phosphate-buffered saline; s.c., subcutaneous; Vax, vaccination.
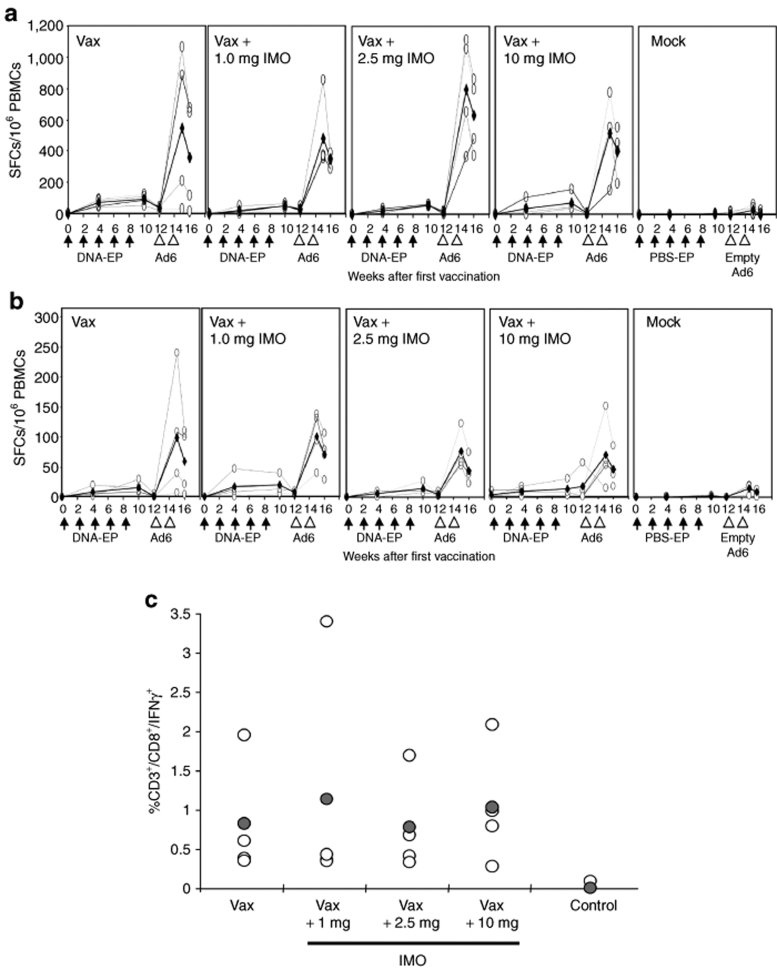
To study the cytokine induction29 and NK cell activation13 by IMO, sera were collected just prior to DNA-EP/IMO administration (t = 0, 4, 8, 24, and 48 hours after the first injection). The kinetics of cytokine induction were measured by enzyme-linked immunosorbent assay (ELISA) in response to the first DNA-EP/IMO treatment. Interleukin-6 (IL-6) levels peaked by 8 hours after vaccination and declined to basal levels within 24–48 hours (Figure 2b). IL-12 peaked at 4 hours (Figure 2c). Maximal levels of interferon-γ-induced protein-10 and interferon-α (IFN-α) were found 24 hours after treatment and declined rapidly thereafter (Figure 2d,e). The extent of cytokine induction was dependent on the dose of IMO. No changes in cytokine levels were detected in monkeys treated with DNA-EP alone or PBS.
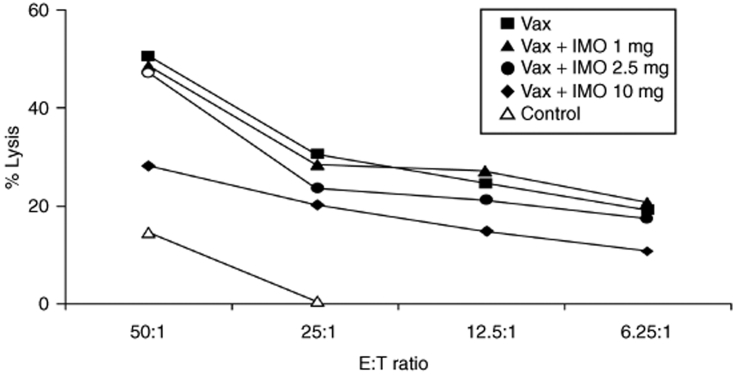
The impact of DNA-EP/Ad6 and IMO on NK cells was analyzed ex vivo at the end of vaccination regimen by surface marker staining and lytic activity. Surface staining and fluorescence-activated cell sorting (FACS) analysis were conducted for markers of NK expansion (CD3−/CD16+) and activation (CD3−/CD16+/CD69+). A dose-dependent increase in the number of circulating activated NK cells was observed in monkeys treated with the vaccine and IMO (data not shown). The cytotoxic activity of NK cells was measured in a standard 4-hour 51Cr release assay against K562 target cells. Fresh peripheral blood mononuclear cells (PBMCs) isolated from blood 2 weeks after the final Ad6 vaccination were used to lyse 51Cr-labeled cells. DNA-EP/Ad6 regimen stimulated significant NK lytic activity (20% lysis) and that DNA-EP/Ad6 + IMO combination treatment elicited the highest cytolytic activity (up to 50% lysis) (Figure 3). Thus, IMO induces cytokine secretion and promotes NK activation and lytic activity.
Figure 3.
Activity of natural killer (NK) cells. Fresh peripheral blood mononuclear cells were collected 2 weeks after the 2nd Ad6 injection and were used in a standard NK lysis assay using the K562 cell line as target. The assays have been executed twice with similar results. E:T, effector: target; IMO, immunomodulatory oligonucleotide; Vax, vaccination.
IMO induces antibody response
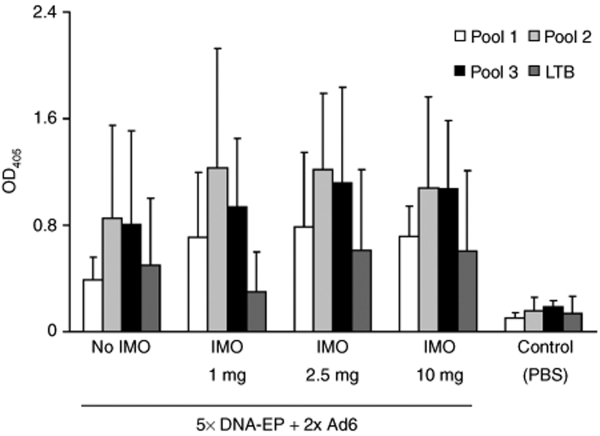
To assess whether B cells are activated in nonhuman primate (NHP) following IMO treatment, total antibody secretion [immunoglobulin G (IgG) and IgM] was measured at different time points by ELISA.14 IMO coadministration had a distinct positive effect on both isotypes (data not shown), thus confirming published data. We next measured the hTERT-specific antibody response in treated macaques. A coarse but robust ELISA method based on hTERT and LTB peptide pools was set up to detect antibodies to hTERT-LTB. A clear response against each peptide pool was detected in all vaccinated groups. No statistically significant difference was noted between the two vaccination regimens (Figure 4).
Figure 4.
Immunomodulatory oligonucleotide treatment increases hTERT antibody response. Antigen-specific humoral response in nonhuman primate was measured by enzyme-linked immunosorbent assay by overlaying sera on hTERT and LTB peptide pools. Data indicate IgG seroconversion as indicated by OD measurement at 405 nm. Sera were collected 2 weeks after the 2nd Ad6 injection. Ad6, adenovirus serotype 6; DNA-EP, DNA electroporation; hTERT, human telomerase reverse transcriptase; LTB, β-subunit of Escherichia coli heat labile enterotoxin; OD, optical density.
Identification of hTERT B-cell epitopes by peptide array technology
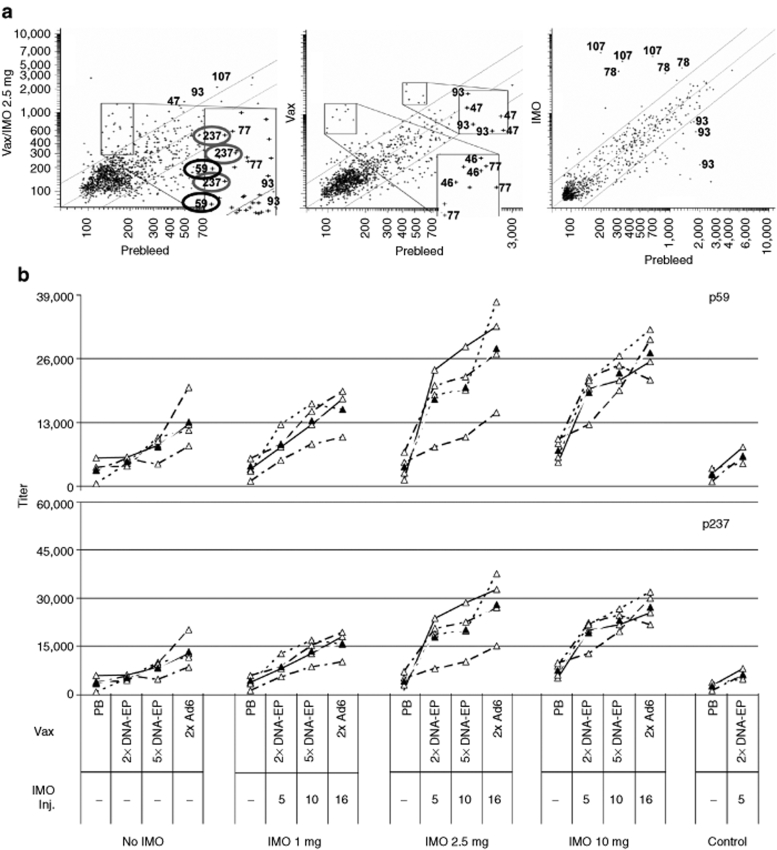
A peptide array composed of 15-mer peptides overlapping by 11 residues and encompassing the entire hTERT and LTB proteins was set up and the antibody profile in each serum sample was examined. Differential analysis identified multiple hTERT peptides that were recognized by specific antibodies in the vaccinated monkeys. A mock-treated group that received only IMO injections was used as control. Five hTERT peptides (p47, p59, p77, p93, and p237) were recognized preferentially in hTERT vaccine/IMO-treated monkeys (Figure 5a). No binding to the LTB peptides was detected. Among hTERT epitopes, the reactivities against p107, p77, and p93 were differentially displayed as a consequence of IMO monotherapy and could be considered antigen-unspecific signals. Sequence analysis of p46 and p47 showed five amino-acid residue mismatches compared with rhesus TERT sequence (181ATQA(G)RPPP(AA)HASGP(T)RRR(G)LGC199, human-specific residue in parentheses). Thus, the signal can be referred to as a nonself specificity and was not further analyzed.
Figure 5.
Identification of hTERT B-cell epitopes. (a) Peptide array. Two hundred and forty peptides of hTERT and 25 peptides of LTB were spotted on glass slides. Data are generated as fluorescent spots, and the distribution of reactivity as intensities in a scatter plot format is shown for three representative monkeys. The x- axis indicates the reactivity of the prebleed (control); the y- axis shows the response after DNA-EP/Ad6 regimen (Vax) plus 2.5 mg IMO (left panel), Vax only (center), or IMO only (right). Relevant identified epitopes are shown. The assay was run in triplicates. (b) Antibody titers were measured by enzyme-linked immunosorbent assay (ELISA) against the identified hTERT peptides. Antibody titers across four time points against hTERT peptides 59 and 237 were measured by ELISA. The assay was run in duplicates. Open triangles indicate titers of individual monkeys and filled triangles denote the group average over time. Ad6, adenovirus serotype 6; DNA-EP, DNA electroporation; hTERT, human telomerase reverse transcriptase; IMO, immunomodulatory oligonucleotide; Inj., injection; PB, prebleed; Vax, vaccination.
An ELISA assay was performed using single peptides and sera collected from the monkeys at various time points of the vaccine regimen, i.e., prior to the start of the experiment (prebleed), after 2 DNA-EP (5 IMO injections), after 5 DNA-EP (10 IMO injections), and after 2 Ad6 injections (16 IMO injections). p59 and p237 reactivities showed a clear correlation with the IMO dose (Figure 5b). In case of p59, a spike in the antibody titer was observed after 5 IMO injections at 2.5 and 10 mg doses. This increase with respect to vaccination alone (or vaccination + 1 mg IMO) was maintained throughout the experiment, suggesting that antibodies against this epitope were induced at the highest IMO doses. Similar data were obtained for p237. Animals mock-treated with only five IMO injections did not show anti-p59 and anti-p237 antibodies.
These data suggest that the antibody response against p59 and p237 is linked to IMO administration and that anti-p59 and anti p237 titers could represent potential antigen-specific biomarkers of the hTERT vaccine/IMO treatment.
Effect of IMO on hTERT-specific T-cell response and memory phenotype
The T-cell immune response to hTERT and LTB was measured over time in monkeys that received DNA-EP/Ad6 treatment alone or in combination with each of the three doses of IMO. The total reactivity to hTERT peptides after five DNA-EP injections showed a mean of 90 spot-forming cells (SFCs)/106 PBMCs. No statistically significant differences were observed in animals that received DNA-EP and IMO (mean varying from 37–73 SFCs/106 PBMCs, Figure 6a) nor different distribution of hTERT immunogenic epitopes was detected (data not shown). The reactivity to LTB was consistently lower across all groups (10–29 SFCs/106 PBMCs, Figure 6b). One week following Ad6 injections, there was a striking 20–30-fold increase in T-cell response (Figure 6a): the reactivity of the group immunized with DNA-EP alone increased from a mean of 90 SFCs/106 PBMCs to 550. A similar increase was observed against LTB. The differences among the groups were not statistically significant, indicating that IMO did not impact the T-cell response to hTERT. The kinetics of T-cell reactivity was also monitored 2 weeks after the second Ad6 injection (Figure 6a,b). The average T-cell response in monkeys peaked 7 days after the final injection and then began to decline: 15 days after the second Ad6 injection, the response to hTERT was reduced by ~30%. The reactivity to LTB peptides also declined over this period. To characterize the quality of the immune response, an intracellular staining for IFN-γ was performed at this time point. The reactivity was exclusively attributable to CD8+ T cells, and no significant differences could be observed among vaccinated groups independently from the IMO combination group (Figure 6c).
Figure 6.
Kinetics of the hTERT-specific T-cell response in nonhuman primate. PBMCs from treated monkeys were purified at the indicated time points and the response was measured by enzyme-linked immunosorbent spot with hTERT and LTB peptide pools. Open circles indicate individual animals, whereas filled circles are the group averages. The total reactivity for (a) hTERT peptide pools is calculated as follows: total reactivity = σ(Tert1 + Tert2 + Tert3 pools) − 3(DMSO). Reactivity to (b) LTB peptides is also shown. Vax indicates 5× DNA-EP and 2× Ad6 injections. The measurements were made 2 weeks after 2× and 5× DNA-EP injections and 1 and 2 weeks after the second Ad6 injection. (c) Immune response was measured by intracellular staining for IFN-γ. The reactivity was exclusively CD8+. No significant differences were observed among vaccinated groups. Ad6, adenovirus serotype 6; DMSO, dimethyl sulfoxide; DNA-EP, DNA electroporation; hTERT, human telomerase reverse transcriptase; IFN-γ, interferon-γ; IMO, immunomodulatory oligonucleotide; Inj., injection; LTB, β-subunit of Escherichia coli heat labile enterotoxin; PBMC, peripheral blood mononuclear cell; PBS, phosphate-buffered saline; SFC, spot-forming cell; Vax, vaccination.
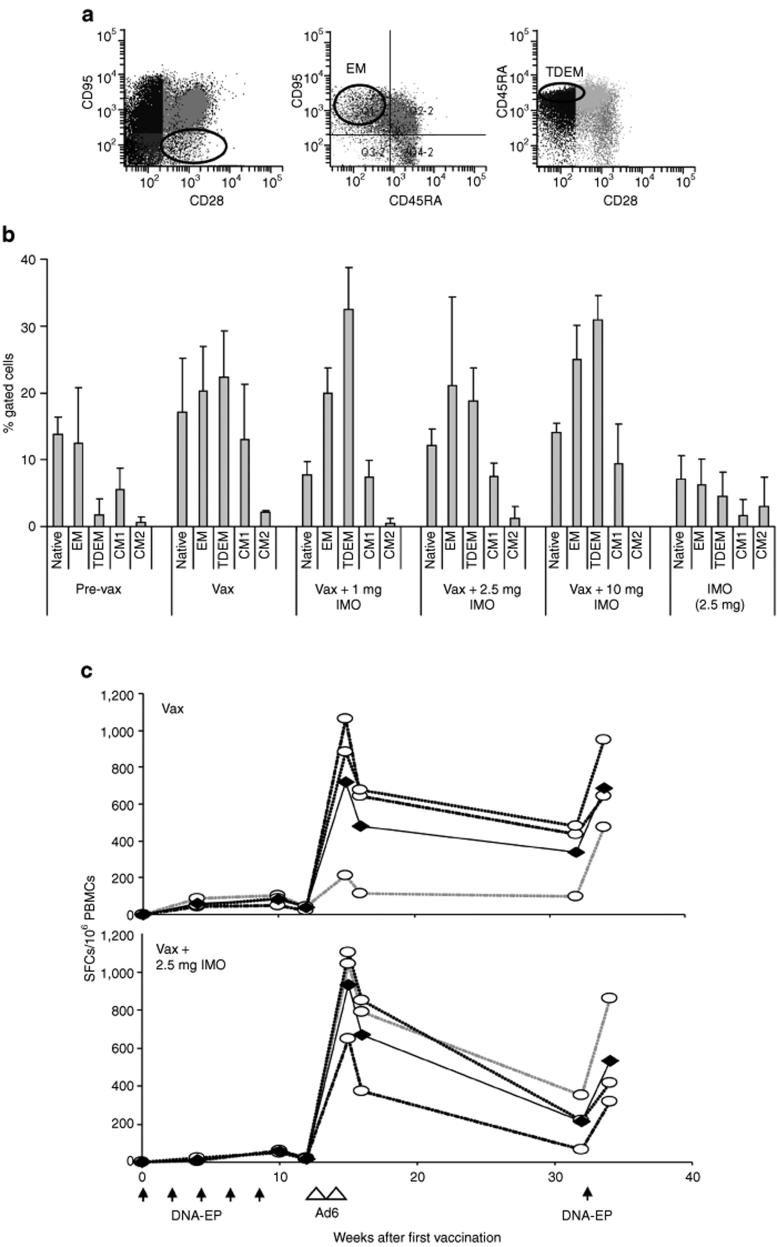
To verify whether IMO could impact the T-cell memory compartment, memory surface markers were analyzed 4 months after the last Ad6 injection. The initial values were set from the prebleeds of the group that received vaccination alone. The analysis was performed on the basis of data available in the literature.30 The phenotypes that were identified by flow cytometry (see Figure 7a) were CD95− (naive); CD95+, CD28−, and CD45RA− [effector memory (EM)]; CD95+, CD28−, and CD45RA+ [terminally differentiated EM (TDEM)]; CD95+, CD28+, and CD45RA− (central memory 1); CD95+, CD28+, and CD45RA+ (central memory 2). Figure 7b shows the proportion of these populations. Vaccination resulted in a significant increase in EM and TDEM compartments. The increase in EM and TDEM cells is almost twofold and tenfold, respectively, after immunization compared with the values prior to vaccination (pre-vax). A similar effect was observed in all the other groups. The dosing of IMO had little or no effect on the various memory T-cell populations: however, an increase in TDEM, but not other memory cells, was noted with IMO coadministration at 1 and 10 mg dose. The proportion of central memory cells was largely unaffected by either vaccine alone or the combination with IMO.
Figure 7.
IMO does not alter T-cell memory populations and long-term response. (a) Memory T-cell analysis. Gating scheme was used in flow cytometry analysis. Cell numbers were characterized by FACS analysis of markers as described in the text. (b) Two monkeys per group were analyzed 4 months after the last Ad6 injection. The bars indicate CD95− (naive); CD95+, CD28−, and CD45RA− (effector memory, EM); CD95+, CD28−, and CD45RA+ (terminally differentiated effector memory, TDEM); CD95+, CD28+, and CD45RA− (central memory 1, CM1); CD95+, CD28+, and CD45RA+ (central memory 2, CM2). Asterisks indicate time points where differences are statistically significant (P < 0.05; Student's t-test). (c) A single DNA-EP boost 4 months after the second Ad6 injection restores the T-cell response. Groups of four monkeys were treated with vaccine alone (Vax) or combined with IMO (Vax + 2.5 mg IMO) were treated by DNA-EP at the indicated time point. Open circles indicate individual animals, whereas filled circles are the group averages. The total reactivity for human telomerase reverse transcriptase peptide pools was calculated as before. Ad6, adenovirus serotype 6; CM, central memory; DNA-EP, DNA electroporation; IMO, immunomodulatory oligonucleotide; PBMC, peripheral blood mononuclear cell; SFC, spot-forming cell; Vax, vaccination.
To verify whether hTERT-specific immune response could be maintained over time and whether IMO could influence long-term responses, the groups previously treated with vaccine alone and vaccine + 2.5 mg IMO were boosted with a single injection of DNA-EP 4 months after the last Ad6 immunization. PBMCs were isolated before the DNA injection and again 2 weeks later. The T-cell response detected by IFN-γ ELISPOT further decreased in the 4-month period after the second Ad6 injection. For both groups, the response fell by 25–30%, although a substantial response (100–300 SFCs/106 PBMCs) was still detected. When injected again with DNA-EP, the T-cell response in the first group increased almost to its previous highest level (Figure 7c): 1 week after Ad6 boost, average total reactivity was 720 SFCs that reduced to 339 after 4 months and then rose to 689 after the additional DNA-EP. A similar response was observed in 2.5 mg IMO combination group and the values were 930, 213, and 531 SFCs, respectively. This suggested that even after 4 months, a stable memory T-cell response was maintained in these monkeys that was stimulated by DNA-EP. No T-cell response was observed in macaques dosed only with IMO (data not shown).
Discussion
Overexpression of hTERT is associated with the development and progression in 85% of cancer types in a variety of species.31 When telomerase is reactivated in tumor cells, TERT is processed and presented in the context of major histocompatibility complex class I, and tumors are recognized by TERT-specific T lymphocytes.32,33 These findings have justified clinical trials based either on autologous dendritic cells transfected or loaded with human TERT–derived peptides.34,35
DNA-EP/Ad6 regimens have elicited significant immune responses against mouse TERT and therapeutic effects in two different models of spontaneous carcinogenesis.36 In this study, we demonstrate the elicitation of a strong T-cell and antibody response against the hTERT in NHPs with this platform. The human and rhesus TERT protein sequence share 96% identity. Therefore, tolerance is expected to play a major role in determining vaccination efficacy. Two DNA-EP injections appeared to be sufficient to break immune tolerance (Figures 1 and 6) in immunized animals, as T-cell response against hTERT was much higher than what has been previously observed for other tumor-associated antigens, such as carcinoembryonic antigen6 or HER-2/neu.37 The response was polyspecific and epitopes were distributed throughout the entire antigen (Figure 1). Two Ad6 injections dramatically increased the T-cell response (Figures 1 and 6). These data, together with the observation that strong immune response against the telomerase has been achieved also in mice36 indicate that hTERT is a strong immunogen per se and further demonstrate the efficacy of the DNA-EP/Ad heterologous vaccination.
The T-cell reactivity fell by 30% a week later but remarkably held steady (250–300 SFCs/106 PBMCs) when measured after 4 months (Figure 7c). A single DNA-EP injection restored the T-cell reactivity to almost that obtained a week after the second Ad6 injection. This observation suggests that a vaccination regimen with multiple DNA-EP injections at appropriate intervals can maintain an optimal level of antigen-specific T-cell activity. This is further supported by the data from T-cell memory analysis (Figure 7b). Based on a flow cytometric study used for detection of degranulating cells in humans that was adapted to rhesus macaques, memory T-cell populations were identified. The increase in EM and TDEM cells strongly suggested that the DNA-EP/Ad6 vaccination regimen can stimulate durable immune responses.
The combination of DNA-EP/Ad-based cancer vaccine against HER2/neu and a TLR9 agonist activates innate and adaptive immune responses and exerts strong antitumor effects in a spontaneous murine mammary tumor model.26 Here, we have evaluated the impact of IMO on DNA-EP/Ad6 vaccine for TERT. NHPs are the most suitable preclinical model for these studies because human and rhesus share similar TLR9 expression and tissue distribution. Animals were dosed weekly with IMO subcutaneously at three different doses: this schedule and route of administration were chosen on the basis of current clinical trials to achieve systemic activation of the immune system. Cytokine secretion was monitored as an early marker of TLR9 agonist–induced immune response and found to be dose-dependent. IMO induced IL-6, IL-12, interferon-γ-induced protein-10, and IFN-α secretion with kinetics that differed for each cytokine (Figure 2). Expansion and phenotype of NK cells were analyzed as late biomarkers of innate immunity. Activation of plasmacytoid dendritic cell and melanoma-specific CD8+ T-cell reactivity are reported to be associated with increased frequencies of NK cells,38 and most importantly, NK activity correlates with antitumoral effect and clinical benefit in a Phase 2 melanoma trial.39 Analysis of CD16+ and CD69+ populations during the course of immunization showed that activated NK cells increased with increasing number of IMO doses administered (data not shown). The amplification and the expression of activation markers were also associated with an enhanced lytic activity in IMO combination groups (Figure 3).
Addition of TLR9 agonists to DNA vaccines has been shown to enhance antigen-specific T-cell responses in a number of studies.26,40,41 Administration of IMO had little or no impact on the hTERT-specific T-cell response elicited by DNA-EP/Ad6 (Figure 6). As shown above, five DNA-EP elicited T-cell responses in all groups and two Ad6 injections augmented this response by 20–30-fold. The treatment with IMO did not alter the kinetics of antigen-specific immune response and the composition of memory T-cell compartments (Figure 7). A possible explanation is that IMO subcutaneous administration and systemic effects are not able to exert immunostimulatory activity on local antigen-presenting cells following vaccination and antigen expression in the muscle. Indeed, co-delivery of proteins or peptides with TLR9 agonists have been shown very efficacious in eliciting immune responses in NHP42 and in cancer patients.43,44 Nevertheless, coadministration of Ad6 and IMO in the muscle or in the area of the same draining lymph node was equally ineffective in enhancing T-cell adaptive immunity (data not shown).
Human naive B cells express low levels of TLRs and the expression of TLR9 rapidly increases following B-cell receptor triggering.45 In contrast, memory B cells constitutively express several TLRs. The differential expression of TLR9 correlates with responsiveness to its agonist. Thus, human memory B cells proliferate and differentiate to Ig-secreting cells in response to TLR9 agonists, whereas naive B cells do so only if simultaneously triggered through the B-cell receptor. The B-cell receptor–induced expression of TLRs in human naive B cells prevents polyclonal activation in a primary response because it restricts stimulation to antigen-specific B cells. In contrast, the constitutive expression of TLRs in memory B cells allows polyclonal activation of the entire memory pool.46 In fact, in our macaques, the effect of TLR triggering by IMO led to secretion of Ig such as IgG and IgM (data not shown). We hypothesized that this in turn should selectively enhance the development of antigen-specific antibodies through secretion of the engineered form TPA-hTERT-LTB and/or the change of subcellular localization and degradation process induced by overexpression. In the absence of a purified hTERT protein, we addressed this question using a peptide array technique to identify B-cell epitopes.26 The drawback of this method is that it identifies only linear immunogenic epitopes. Five peptides were recognized as being differentially recognized among the IMO, the vaccine and vaccine + IMO groups (Figure 5a). Notably, IMO alone administration resulted in perturbation of antibody fingerprint. This effect could be due to the general activation of memory B-cell pool and secretion of hTERT-unrelated but crossreactive antibodies by plasma cells. Nevertheless, the antibody titer against two of these epitopes, p59 and p237, increased as a function of hTERT vaccine and IMO dosage (Figure 5b). Antibodies against TERT are not expected to play a biological/therapeutic role because TERT is a nuclear protein and it is not naturally present on cell surface or secreted. However, recent evidences suggest that the induction of autoantibodies against intracellular components may be relevant to cancer. In particular, anti-hTERT autoantibodies in sera are elevated during progression of malignancies, such as hepatocellular carcinoma, and have been described as a possible novel early tumor marker.47
These data indicate that DNA-EP/Ad6 can break immune tolerance to TERT in NHP and achieve a durable immune response. We have also combined hTERT vaccine with an agonist of TLR9 and characterized the immunological parameters associated with the treatment. IMO induces potent innate immune responses, increases antigen-specific antibody, but not T-cell-mediated immunity to hTERT.
Materials and Methods
Vaccine vectors. hTERT.LTB gene was synthesized at GENEART (Regensburg, Germany) and encodes the codon-optimized, catalytically inactive (A2135C, G2137A, and G2139C), full-length human telomerase fused to codon-optimized LTB.48 The complementary DNA was cloned as BglII/SalI fragment in pV1J-nsA expression vector, generating pV1J-hTERT.LTB. For Ad generation, pV1J plasmid vector containing the wild-type sequence of hTERT was mutated by site-directed mutagenesis, using Stratagene Quikchange multi site-directed mutagenesis kit (Stratagene, La Jolla, CA) and an oligonucleotide (5′-CTGTACTTTGTCAAGGTGGCTATCACGGGCGCGTACG-3′) to introduce the above reported mutations of the hTERT ORF sequence. The final product, pV1J/hTERT plasmid, was digested with BglII and SalI and cloned into the plasmid pNEBAd6-CMVpA shuttle vector with the same restriction sites. To construct preAd pMRKAd6hTERT, the plasmid pNEBAd6-CMVpA was digested with PacI/PmeI restriction enzymes and the hTERT transgene–containing fragment was gel-purified. The purified fragment was then co-transformed into E. coli strain BJ5183 with linearized (ClaI-digested) adenoviral backbone plasmid, pAd6MRKΔE1ΔE3. The plasmid was cut with PmeI to release the Ad ITRs and transfected in PerC-6 cells (Crucell, Leiden, the Netherlands) by Lipofectamine 2000 (Life Technologies, Carlsbad, CA). The vector was amplified through serial passages and purified through standard CsCl purification protocol, then extensively dialyzed against A105 buffer (5 mmol/l Tris pH 8.0, 1 mmol/l MgCl2, 75 mmol/l NaCl, 5% sucrose, and 0.005% Tween 20).
TLR9 agonist. IMO, 5′-TCTGTCRTTCT-X-TCTTRCTGTCT-5′, (molecular weight = 7,146; X and R are glycerol linker and 2′-deoxy-7-deazaguanosine, respectively) was synthesized with phosphorothioate backbone, purified and characterized as described previously.20,22
Rhesus monkeys and immunizations. Rhesus monkey experimentation was performed at IRBM animal house. The IRBM Animal Program conforms with AAALAC International standards as set forth in the European Council Directive 86/609/EEC on the approximation of the laws, regulations, and administrative provisions of the Member States regarding the protection of animal used for experimental and other scientific purposes, Italian implementing regulations as well as the Guide for the Care and Use of Laboratory Animals, NRC 1996. Monkey immunization studies were performed on groups of four rhesus macaques (Macaca mulatta) as described earlier.49 The DNA injection was performed in quadriceps muscle and consisted of a 1 ml solution (split over two injection sites with 0.5 ml/site) containing 5 mg pV1J-hTERT.LTB. Coupled hypodermic needles soldered on a printed circuit board and connected to the electrical field generator were used to perform the injection via two independent insulin syringes. Electrical conditions for electroporation consisted of 2 trains of 100 square bipolar pulses (1 second each) delivered every other second for a total treatment time of 3 seconds. The pulse length was 2 ms/phase with a frequency and amplitude of 100 Hz and 100 mA, respectively (constant current mode). Rhesus monkeys were injected in deltoid muscle with a dose of 1011 Ad viral particles. Blood was collected at specific time points, and PBMCs were isolated by density gradient centrifugation columns (Accuspin; Sigma, St Louis, MO). Fresh or frozen PBMC samples were analyzed for immunologic assays.
IFN-γ ELISPOT. A standard kit was used to perform the assay according to the manufacturer's instructions (U-CyTech, Utrecht, the Netherlands). Briefly, standard 96-well plates were coated with anti-rhesus IFN-γ antibody diluted 1:200 in sterile PBS (final concentration 10 µg/ml). PBMCs were plated at 4 × 105 and 2 × 105 cells/well, in duplicate, with three pools of peptides covering the entire sequence of human TERT and one pool covering LTB (all at 8 µg/ml). After overnight stimulation at 37 °C, plates were washed and incubated with biotinylated anti-rhesus IFN-γ antibody. The next day, plates were washed and incubated for 2 hours at room temperature (RT) with streptavidin–alkaline phosphatase-conjugated antibody. After extensive washing, plates were developed by adding 50 µl/well of the substrate (provided by the manufacturer). The reaction was stopped by washing plates thoroughly with distilled water. Plates were allowed to air-dry completely and spots counted using an automated ELISPOT reader (AID ELISPOT v3.0; AID, Strassberg, Germany).
Intracellular cytokine staining. Intracellular staining for IFN-γ was performed as described previously.49 PBMCs (fresh or frozen) were resuspended at 2 × 106 cells/ml in R10 medium. Frozen samples were incubated at 37 °C, in R10 medium for at least 5–6 hours before proceeding with the assay. Co-stimulatory monoclonal antibodies, against CD28 and CD49d, were added to each tube for a final concentration of 1 µg/ml. Individual peptides or peptide pools were added to samples for a final concentration of 6 µg/ml. Tubes were incubated overnight at 37 °C with brefeldin A. Cells were washed with FACS buffer and incubated with the surface antibodies CD4-phycoerythrin, CD8 PerCP, and CD3-allophycocyanin for 30 minutes at RT, washed and permeabilized with FACS Perm buffer (10 minutes at RT). Cells were washed with FACS buffer and incubated for 30 minutes at RT with IFN-γ-fluorescein isothiocyanate (all reagents and antibodies from Becton Dickinson, San Diego, CA). Samples were resuspended in 1% formaldehyde/PBS and analyzed on a FACSCanto (BD Biosciences, San Jose, CA).
Staining for NK and memory markers. Staining for NK cell markers was done with CD3-allophycocyanin, CD16-fluorescein isothiocyanate, or CD69-fluorescein isothiocyanate antibodies against human antigens. Memory cell analysis was performed on the basis of published data.30 CD95 phycoerythrin, CD45RA-PerCP/Cy5, and CD28-fluorescein isothiocyanate were used in this study.
NK cytotoxic assay. Activity of NK cells was determined with a classic NK assay against K562 cells. Briefly, 1 × 106 K562 cells were labeled with 100 µCi of Na51CrO4 for 1 hour at 37 °C. After washing, rhesus PBMCs were added at the indicated concentrations and incubation was carried out for 4 hours at 37 °C. Cell-free supernatant was collected and the release of chromium by lysed cells measured using a scintillation counter. Percent lysis = 100 × (experimental − spontaneous lysis)/(maximum lysis − spontaneous lysis).
Measurement of cytokine and humoral responses. Cytokines and global antibody response were measured by standard kits for human sera according to the manufacturer's instructions: human IL-6 and IL-12 (Cell Sciences, Canton, MA), IFN-α (PBL, Piscataway, NJ), and interferon-γ-induced protein-10 (R&D Systems, Minneapolis, MN). Total IgM and IgG titers were measured using reagents from Alpha Diagnostics (San Antonio, TX). Anti-dsDNA antibodies were measured using kits from Bio-Rad (Hercules, CA). Antigen-specific responses were detected by coating 96-well monkeys with the peptide pools and hybridizing with sera from monkeys. An alkaline phosphatase–linked antibody (Sigma) against total IgG was used to measure the titer using conventional ELISA quantitation software (v3.5; Synergy Software, Reading, PA).
Peptide array technology. The hTERT.LTB peptide collection has been used to print on CodeLink Activated Slides (cat. no. 300011 00; GE Healthcare, Milan, Italy) using Piezorray (PerkinElmer, Waltham, MA). Each peptide was prepared in PBS 1×, 10% dimethyl sulfoxide at a concentration of 3 mg/ml. Every spot of the array was made using one drop of <1 nl, and the diameter was of around 200 µm. A peptide array incorporating 240 peptides of hTERT and 25 peptides of LTB (about 60 peptides were omitted for reasons of uneven solubility in dimethyl sulfoxide) was spotted on glass slides.
Slides were incubated with 1.5% rabbit serum (cat. no. R9133; Sigma) in PBS for 30 minutes at RT. After a quick rinse in PBS, the arrays were incubated for 2 hours at RT with 1/50 diluted rhesus serum in binding buffer (0.1 mol/l Tris/HCl pH 8.0, 0.1 mol/l NaCl, 0.02% wt/vol Tween 20, and 1% wt/vol BSA). The slides were washed in PBS 1×, 0.02% Tween 20, and then incubated with 1/250 diluted fluorescein isothiocyanate–rabbit anti-monkey IgG (cat. no. F3893; Sigma). After two washings in PBS 1×, 0.02% Tween 20, slides were rinsed in distilled water, dried and scanned with ScanArray Express (PerkinElmer). Sera from the three control monkeys (immunized with PBS) were hybridized to these slides, and the resultant analysis indicated peptides that were recognized by sera of nonimmunized animals. These peptides were, therefore, set as the background against which sera from immunized monkeys were analyzed. Sera from treated animals were then hybridized to the array, and analytical software was used to develop the peptide hybridization profile for each serum sample. Peptides (and their sequences) were identified for each signal, and these were short-listed as potential epitopes for further experiments. These peptides were then coated on 96-well plates and hybridized to sera at various dilutions. The titer was measured as described above.
Acknowledgments
We thank IRBM-LAR personnel for excellent technical support in conducting animal experiments and Jose Lebron for safety studies. We also thank Raffaele Cerino for lab assistance. We are grateful to Janet Clench for editorial assistance and Manuela Emili for graphics. No competing financial and commercial interests exist.
REFERENCES
- Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–795. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, et al. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955–959. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Prud'homme GJ. DNA vaccination against tumors. J Gene Med. 2005;7:3–17. doi: 10.1002/jgm.669. [DOI] [PubMed] [Google Scholar]
- Babiuk S, Baca-Estrada ME, Foldvari M, Storms M, Rabussay D, Widera G, et al. Electroporation improves the efficacy of DNA vaccines in large animals. Vaccine. 2002;20:3399–3408. doi: 10.1016/s0264-410x(02)00269-4. [DOI] [PubMed] [Google Scholar]
- Aurisicchio L, Mennuni C, Giannetti P, Calvaruso F, Nuzzo M, Cipriani B, et al. Immunogenicity and safety of a DNA prime/adenovirus boost vaccine against rhesus CEA in nonhuman primates. Int J Cancer. 2007;120:2290–2300. doi: 10.1002/ijc.22555. [DOI] [PubMed] [Google Scholar]
- Scheerlinck JP, Karlis J, Tjelle TE, Presidente PJ, Mathiesen I., and , Newton SE. In vivo electroporation improves immune responses to DNA vaccination in sheep. Vaccine. 2004;22:1820–1825. doi: 10.1016/j.vaccine.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415:331–335. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- Yang ZY, Wyatt LS, Kong WP, Moodie Z, Moss B., and , Nabel GJ. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J Virol. 2003;77:799–803. doi: 10.1128/JVI.77.1.799-803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro DR, Bett AJ, Fu TM, Davies ME, Tang A, Wilson KA, et al. Heterologous human immunodeficiency virus type 1 priming-boosting immunization strategies involving replication-defective adenovirus and poxvirus vaccine vectors. J Virol. 2004;78:11434–11438. doi: 10.1128/JVI.78.20.11434-11438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A., and , Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Krieg AM. Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene. 2008;27:161–167. doi: 10.1038/sj.onc.1210911. [DOI] [PubMed] [Google Scholar]
- Ballas ZK. Modulation of NK cell activity by CpG oligodeoxynucleotides. Immunol Res. 2007;39:15–21. doi: 10.1007/s12026-007-0066-3. [DOI] [PubMed] [Google Scholar]
- He B, Qiao X., and , Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol. 2004;173:4479–4491. doi: 10.4049/jimmunol.173.7.4479. [DOI] [PubMed] [Google Scholar]
- Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- Yi AK, Chang M, Peckham DW, Krieg AM., and , Ashman RF. CpG oligodeoxyribonucleotides rescue mature spleen B cells from spontaneous apoptosis and promote cell cycle entry. J Immunol. 1998;160:5898–5906. [PubMed] [Google Scholar]
- Kandimalla ER, Bhagat L, Cong YP, Pandey RK, Yu D, Zhao Q, et al. Secondary structures in CpG oligonucleotides affect immunostimulatory activity. Biochem Biophys Res Commun. 2003;306:948–953. doi: 10.1016/s0006-291x(03)01080-5. [DOI] [PubMed] [Google Scholar]
- Kandimalla ER, Bhagat L, Li Y, Yu D, Wang D, Cong YP, et al. Immunomodulatory oligonucleotides containing a cytosine-phosphate-2'-deoxy-7-deazaguanosine motif as potent toll-like receptor 9 agonists. Proc Natl Acad Sci USA. 2005;102:6925–6930. doi: 10.1073/pnas.0501729102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandimalla ER, Bhagat L, Yu D, Cong Y, Tang J., and , Agrawal S. Conjugation of ligands at the 5'-end of CpG DNA affects immunostimulatory activity. Bioconjug Chem. 2002;13:966–974. doi: 10.1021/bc0200374. [DOI] [PubMed] [Google Scholar]
- Kandimalla ER, Bhagat L, Zhu FG, Yu D, Cong YP, Wang D, et al. A dinucleotide motif in oligonucleotides shows potent immunomodulatory activity and overrides species-specific recognition observed with CpG motif. Proc Natl Acad Sci USA. 2003;100:14303–14308. doi: 10.1073/pnas.2335947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Kandimalla ER, Yu D, Tang JX., and , Agrawal S. Oral administration of second-generation immunomodulatory oligonucleotides induces mucosal Th1 immune responses and adjuvant activity. Vaccine. 2005;23:2614–2622. doi: 10.1016/j.vaccine.2004.11.028. [DOI] [PubMed] [Google Scholar]
- Yu D, Kandimalla ER, Bhagat L, Tang JY, Cong Y, Tang J, et al. ‘Immunomers'—novel 3'-3'-linked CpG oligodeoxyribonucleotides as potent immunomodulatory agents. Nucleic Acids Res. 2002;30:4460–4469. doi: 10.1093/nar/gkf582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Rayburn ER, Wang W, Kandimalla ER, Agrawal S., and , Zhang R. Immunomodulatory oligonucleotides as novel therapy for breast cancer: pharmacokinetics, in vitro and in vivo anticancer activity, and potentiation of antibody therapy. Mol Cancer Ther. 2006;5:2106–2114. doi: 10.1158/1535-7163.MCT-06-0158. [DOI] [PubMed] [Google Scholar]
- Wang H, Rayburn ER, Wang W, Kandimalla ER, Agrawal S., and , Zhang R. Chemotherapy and chemosensitization of non-small cell lung cancer with a novel immunomodulatory oligonucleotide targeting Toll-like receptor 9. Mol Cancer Ther. 2006;5:1585–1592. doi: 10.1158/1535-7163.MCT-06-0094. [DOI] [PubMed] [Google Scholar]
- Damiano V, Caputo R, Garofalo S, Bianco R, Rosa R, Merola G, et al. TLR9 agonist acts by different mechanisms synergizing with bevacizumab in sensitive and cetuximab-resistant colon cancer xenografts. Proc Natl Acad Sci USA. 2007;104:12468–12473. doi: 10.1073/pnas.0705226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurisicchio L, Peruzzi D, Conforti A, Dharmapuri S, Biondo A, Giampaoli S, et al. Treatment of mammary carcinomas in HER-2 transgenic mice through combination of genetic vaccine and an agonist of Toll-like receptor 9. Clin Cancer Res. 2009;15:1575–1584. doi: 10.1158/1078-0432.CCR-08-2628. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Hwang J, McGreivy J, Park S, Malik S, Martin RR, et al. Phase I trial of escalating doses of the TLR9 agonist HYB2055 in patients with advanced solid tumors J Clin Oncol 2005232503suppl [Google Scholar]
- Hwang JJ, Park S, Amin A, Martin RR, Sullivan T, Burns T, et al. A phase I study of HYB2055 in patients (pts) with advanced solid malignancies J Clin Oncol 2004223111suppl [Google Scholar]
- Krieg AM, Efler SM, Wittpoth M, Al Adhami MJ., and , Davis HL. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J Immunother. 2004;27:460–471. doi: 10.1097/00002371-200411000-00006. [DOI] [PubMed] [Google Scholar]
- Chan KS., and , Kaur A. Flow cytometric detection of degranulation reveals phenotypic heterogeneity of degranulating CMV-specific CD8+ T lymphocytes in rhesus macaques. J Immunol Methods. 2007;325:20–34. doi: 10.1016/j.jim.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;8:167–179. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- Vonderheide RH. Universal tumor antigens for cancer vaccination: targeting telomerase for immunoprevention. Discov Med. 2007;7:103–108. [PubMed] [Google Scholar]
- Chen DY, Vance BA, Thompson LB, Domchek SM., and , Vonderheide RH. Differential lysis of tumors by polyclonal T cell lines and T cell clones specific for hTERT. Cancer Biol Ther. 2007;6:1991–1996. doi: 10.4161/cbt.6.12.5078. [DOI] [PubMed] [Google Scholar]
- Su Z, Dannull J, Yang BK, Dahm P, Coleman D, Yancey D, et al. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol. 2005;174:3798–3807. doi: 10.4049/jimmunol.174.6.3798. [DOI] [PubMed] [Google Scholar]
- Domchek SM, Recio A, Mick R, Clark CE, Carpenter EL, Fox KR, et al. Telomerase-specific T-cell immunity in breast cancer: effect of vaccination on tumor immunosurveillance. Cancer Res. 2007;67:10546–10555. doi: 10.1158/0008-5472.CAN-07-2765. [DOI] [PubMed] [Google Scholar]
- Mennuni C, Ugel S, Mori F, Cipriani B, Iezzi M, Pannellini T, et al. Preventive vaccination with telomerase controls tumor growth in genetically engineered and carcinogen-induced mouse models of cancer. Cancer Res. 2008;68:9865–9874. doi: 10.1158/0008-5472.CAN-08-1603. [DOI] [PubMed] [Google Scholar]
- Fattori E, Aurisicchio L, Zampaglione I, Arcuri M, Cappelletti M, Cipriani B, et al. Her2/neu genetic cancer vaccine in non human primates: relevance of single nucleotide polymorphisms. Hum Gene Ther. 2009;20:253–265. doi: 10.1089/hum.2008.153. [DOI] [PubMed] [Google Scholar]
- Molenkamp BG, Sluijter BJ, van Leeuwen PA, Santegoets SJ, Meijer S, Wijnands PG, et al. Local administration of PF-3512676 CpG-B instigates tumor-specific CD8+ T-cell reactivity in melanoma patients. Clin Cancer Res. 2008;14:4532–4542. doi: 10.1158/1078-0432.CCR-07-4711. [DOI] [PubMed] [Google Scholar]
- Pashenkov M, Goëss G, Wagner C, Hörmann M, Jandl T, Moser A, et al. Phase II trial of a toll-like receptor 9-activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol. 2006;24:5716–5724. doi: 10.1200/JCO.2006.07.9129. [DOI] [PubMed] [Google Scholar]
- Kwissa M, Amara RR, Robinson HL, Moss B, Alkan S, Jabbar A, et al. Adjuvanting a DNA vaccine with a TLR9 ligand plus Flt3 ligand results in enhanced cellular immunity against the simian immunodeficiency virus. J Exp Med. 2007;204:2733–2746. doi: 10.1084/jem.20071211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HI, Niu G, Bradley N., and , Celis E. Optimized DNA vaccines to specifically induce therapeutic CD8 T cell responses against autochthonous breast tumors. Cancer Immunol Immunother. 2008;57:1695–1703. doi: 10.1007/s00262-008-0465-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille-Reece U, Flynn BJ, Loré K, Koup RA, Miles AP, Saul A, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203:1249–1258. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser DE, Liénard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest. 2005;115:739–746. doi: 10.1172/JCI23373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcade J, Kudela P, Andrade Filho PA, Janjic B, Land SR, Sander C, et al. Immunization with analog peptide in combination with CpG and montanide expands tumor antigen-specific CD8+ T cells in melanoma patients. J Immunother. 2008;31:781–791. doi: 10.1097/CJI.0b013e318183af0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccellini MB, Busconi L, Green NM, Busto P, Christensen SR, Shlomchik MJ, et al. Autoreactive B cells discriminate CpG-rich and CpG-poor DNA and this response is modulated by IFN-alpha. J Immunol. 2008;181:5875–5884. doi: 10.4049/jimmunol.181.9.5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernasconi NL, Onai N., and , Lanzavecchia A. A role for toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003;101:4500–4504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- Masutomi K, Kaneko S, Yasukawa M, Arai K, Murakami S., and , Kobayashi K. Identification of serum anti-human telomerase reverse transcriptase (hTERT) auto-antibodies during progression to hepatocellular carcinoma. Oncogene. 2002;21:5946–5950. doi: 10.1038/sj.onc.1205788. [DOI] [PubMed] [Google Scholar]
- Facciabene A, Aurisicchio L, Elia L, Palombo F, Mennuni C, Ciliberto G, et al. Vectors encoding carcinoembryonic antigen fused to the B subunit of heat-labile enterotoxin elicit antigen-specific immune responses and antitumor effects. Vaccine. 2007;26:47–58. doi: 10.1016/j.vaccine.2007.10.060. [DOI] [PubMed] [Google Scholar]
- Mennuni C, Calvaruso F, Facciabene A, Aurisicchio L, Storto M, Scarselli E, et al. Efficient induction of T-cell responses to carcinoembryonic antigen by a heterologous prime-boost regimen using DNA and adenovirus vectors carrying a codon usage optimized cDNA. Int J Cancer. 2005;117:444–455. doi: 10.1002/ijc.21188. [DOI] [PubMed] [Google Scholar]