Abstract
Glial cell line-derived neurotrophic factor (GDNF) gene transfer is being developed as a treatment for Parkinson's disease (PD). Due to the potential for side effects, external transgene regulation should enhance this strategy's safety profile. Here, we demonstrate dynamic control during long-term expression of GDNF using a recombinant adeno-associated virus (rAAV)-based bicistronic tetracycline (tet)-off construct. Nigrostriatal GDNF overexpression induces body weight alterations in rodents, enabling longitudinal in vivo tracking of GDNF expression after nigral vector delivery. Regulated GDNF expression was highly sensitive to dietary doxycycline (DOX), displaying undetectable striatal GDNF levels at serum DOX levels below those required for antimicrobial activity. However, in the absence of DOX, striatal GDNF levels exceeded levels required for efficacy in PD models. We also demonstrate the absence of a series of known GDNF-associated side effects when using direct intrastriatal vector delivery. Therefore, this single rAAV vector system meets most of the requirements for an experimental reagent for treatment of PD.
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disease and results in significant motor dysfunction. PD is characterized by the progressive loss of the dopaminergic innervation of the striatum and the subsequent dopaminergic neuron death in the substantia nigra (SN) pars compacta.1 The etiology of PD is unknown, and probably multifactorial; thus, to date, the main therapeutic approach has been palliative L-dihydroxyphenylalanine (Sinimet or Madopar) administration as a neurotransmitter replacement strategy. In contrast, neurotrophic factors, such as glial cell–line derived neurotrophic factor (GDNF) have been shown to greatly improve nigral dopaminergic cell survival and terminal fiber density resulting in functional recovery in a diverse assortment of PD animal models.2
GDNF is a member of the transforming growth factor-β family of growth factors, and its main receptor dimers, RET and GFRα1, are expressed during, and participate in, dopaminergic nigrostriatal prenatal development.3 GDNF signaling has also been shown to be an absolute requirement for dopaminergic cell survival in adults.4,5 Consequently, although GDNF expression is greatly reduced in the adult, the receptor expression continues even in the aging and the PD brain.3,6 Receptor binding by GDNF initiates an intracellular signal cascade leading to the activation of the P13K/akt neurite outgrowth pathway as well as the extracellular signal-regulated kinase prosurvival pathway.3 Taken together, the properties of GDNF have led to PD clinical trials where the recombinant protein was delivered either into the cerebral ventricles or into the putamen via mechanical pumps, with disappointing results.2,7
Similar to the protein-injection strategy, direct gene delivery of GDNF has also been shown to be efficacious in PD animal models,8 however, continuous vector-mediated GDNF delivery requires much lower GDNF tissue levels for neuroprotection.9 Unfortunately, the striatal GDNF tissue levels required to preserve the human nigrostriatal tract are not known, and existing animal models cannot fully predict potential intracerebral vector delivered GDNF-induced side effects.7,10 For these reasons, the safest PD clinical trials using vector-mediated GDNF delivery would ideally include some ability to regulate transgene expression externally, although the absolute need for regulation is controversial.11,12 The most developed regulation system in the context of viral vectors is the tetracycline (tet)-mediated transcriptional regulation system.13 This system uses the tet repressor protein from Escherichia coli combined with a small portion of the herpes simplex virus VP-16 protein. This fusion protein then acts as a transcriptional activator. In the absence of tet or its analogs, the tet/VP-16 transactivator is able to bind to a promoter containing regulatory elements from the E. coli tet-operon and initiate transcription. However, in the presence of a tet analog, the regulatory molecule will bind to the transactivator with high affinity and inhibit any interaction with the promoter elements, effectively silencing the gene expression.
In its array of configurations, the tet-regulation system has displayed a range of basal GDNF expression levels (leakiness) in vivo even in the presence of a silencing mechanism.14,15,16 A GDNF expression system that provides repeated reversible control of expression in vivo for extended periods of time has never been reported. Although low levels of background expression may be tolerable in other gene-therapy strategies for neurological disorders, given the relatively long in vivo half-life of GDNF, any in vivo leakiness will lead to unpredictable GDNF accumulation.
Here we report the use of a single recombinant adeno-associated viral (rAAV) vector tet-regulated expression system allowing for complete and reversible shutdown of GDNF gene expression in the nigrostriatal tract over a 6-month period as measured in vivo by significant fluctuations in body weight corresponding to doxycycline (DOX) dosing. We have also tested potential GDNF-mediated side effects and found this system to display a favorable profile of gene expression and safety. The single vector design was the end result of an arduous period of testing several permutations of promoters, transactivators, and rAAV genomic components. However, all these earlier constructs displayed a variety of unacceptable properties such as low inducibility or detectable basal expression (Supplementary Figure S1).
Results
ON–OFF experiment
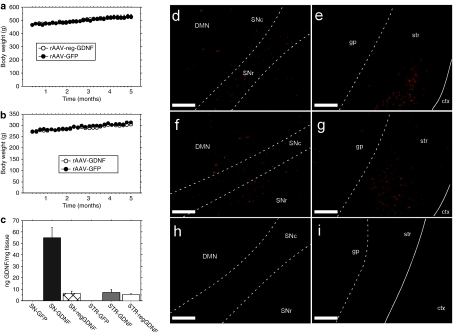
To evaluate the ability of DOX to control GDNF expression in vivo, adult male middle-aged rats received bilateral SN pars compacta injections of rAAV-encoding GDNF under the control of the strong constitutive cytomegalovirus/chicken β-actin (CBA) promoter (CBA-GDNF, Figure 1a) as a positive control for weight loss or the regulated expression cassette (SN-regGDNF1 and SN-regGDNF2, Figure 1c; DOX− = GDNF expression ON: DOX+= GDNF expression OFF). The SN-GDNF1 and SN-GDNF2 groups received identical injections of the regulated GDNF construct but followed different DOX treatment schedules as indicated in Figures 1d and 2a,b. Finally, a subset of animals was injected with the green fluorescent protein (GFP) reporter virus, CBA-GFP (Figure 1b) as a control for rAAV2/5 transduction.
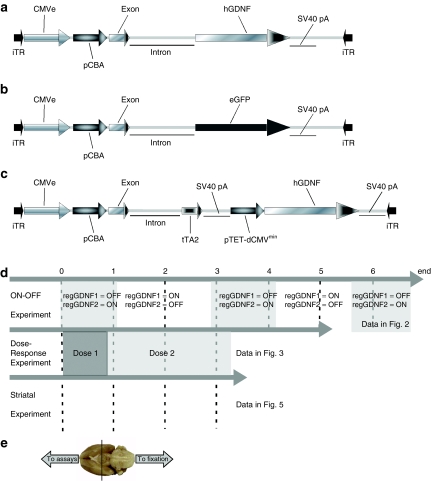
Figure 1.
Experimental design and vector schematics. rAAV genomic maps of vectors used in all three experiments. (a) CBA-GDNF, (b) CBA-GFP, and (c) regGDNF. (d) Experimental design and timeline of the three experiments performed in this study. The alternating white gray areas indicate the crossover of feeding DOX or normal food in the ON–OFF experiment. At the end of the ON–OFF study, SN-regGDNF1 was in the OFF state (expressing GDNF) and SN-regGDNF2 was in the ON state. The different shades of gray in the dose–response experiment denote the fact that the DOX diet doses were changed during that experiment as indicated. No DOX was administered during the striatum experiment. (e) Photograph of the ventral surface of the rat brain displaying how the brain was divided for the assays and histology as described. CMVe, cytomegalovirus enhancer element; DOX, doxycycline; eGFP, enhanced green fluorescent protein; hGDNF, human glial cell line-derived neurotrophic factor; iTR, inverted terminal repeat; pCBA, chicken β-actin promoter; pTET-dCMVminimal, tetracycline-responsive delta-CMV minimal promoter; rAAV, recombinant adeno-associated virus; SV40 pA, SV40 poly-A sequence; tTA2, transactivator containing VP-16 sequence.
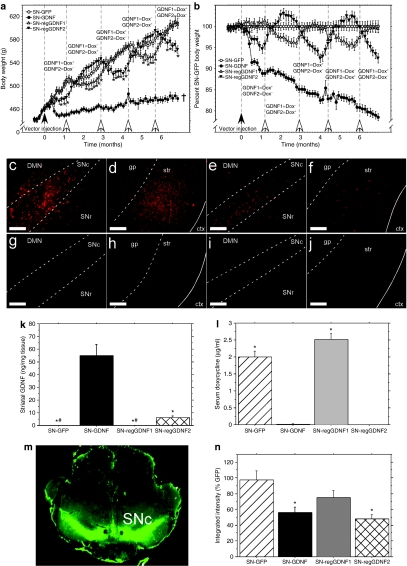
Figure 2.
DOX administration repeatedly and reversibly inhibits nigrostriatal GDNF expression. (a) Bilateral overexpression of GDNF protein in the nigrostriatal tract leads to significant weight loss during constitutive expression [CBA-GDNF, closed circles (n = 12), †P < 0.001 for the entire curve compared to all other groups]. Regulation of GDNF expression via DOX administration [SN-regGDNF1 open triangles (n = 14), SN-regGDNF2 closed triangles (n = 13)] causes significant weight loss in the ON state (DOX−) when compared to the OFF state (DOX+) and the GFP control (open circles; n = 14); however, this weight loss is fully reversible and can be repeated several times through crossover of DOX diet (open arrows = DOX diet crossover; *P <0.001 versus GFP controls). (b) Body weight in (a) normalized to the GFP control to more clearly demonstrate the ON–OFF effect of DOX diet. (c–j) Photomicrographs of GDNF immunoreactivity from each group. Abundant GDNF expression was observed in the (c) SN and surrounding areas and the (d) striatum and GP for the CBA-GDNF group and to a lesser extent in the SN-regGDNF2 (ON) group where the (e) midbrain and (f) striatal spread was reduced. No GDNF expression was detected in (g,h) CBA-GFP treated animals or (i,j) SN-regGDNF1 (OFF) group. Striatal ELISA measurements of (k) GDNF confirmed the histological observations (*P < 0.0001 versus SN-GDNF; #P < 0.0001 versus SN-regGDNF2). (l) Diet containing 3 g/kg DOX resulted in serum levels of 2–2.5 µg/ml DOX (SN-GFP, SN-regGDNF1); however, 3 weeks following DOX withdrawal serum levels were below the limits of detection (SN-GDNF, SN-regGDNF2; *P < 0.0001 versus SN-GDNF and SN-regGDNF2). S, solid white line, external capsule. Bar = 0.5 mm. Tyrosine hydroxylase densitometry. (m) Representative near infrared immunohistochemistry as scanned on the LI-COR Odyssey. The hole was purposely produced to indicate the left side of the brain in each animal. (n) Quantification of nigrostriatal TH levels, groups receiving GDNF had reduced TH levels (P < 0.01) as reported previously.15,17 CBA, chicken β-actin; ctx, cortex; DMN, deep mesencephalic nucleus; DOX, doxycycline; ELISA, enzyme-linked immunosorbent assay; GDNF, glial cell line-derived neurotrophic factor; gp, globus pallidus; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata; str, striatum.
Body weight
Immediately after the vector injection, the experimental groups received their respective DOX diet (3 g/kg of diet) as outlined in Figures 1d and 2a, and body weight was recorded twice per week thereafter. Approximately 1 week after the vector injection, the CBA-GDNF positive control group displayed a significant loss of body weight. Although this rapid weight loss waned after ~1-month post-injection, this group showed an extremely slow rate of weight gain until the end of the experiment when compared to the GFP control (Figure 2a–b). Similarly, the SN-regGDNF2 group which received normal food immediately after the vector administration initially lost a small amount of body weight and then began gaining weight at a rate that was significantly different from that of the SN-regGDNF1 group receiving 3 g/kg DOX diet and the GFP control (P < 0.01).
One month post-injection, the DOX diet treatment was crossed-over for both regulated GDNF groups as well as in the GFP group to control for an effect of DOX diet on body weight. Immediately after the DOX diet crossover, the SN-regGDNF2 group (DOX+) experienced a rapid increase in the rate of body weight gain and the group now receiving regular food (SN-regGDNF1, DOX−) immediately reduced their rate of weight gain. This pattern was repeated throughout three additional food switches. At the apex of each cycle, the body weight of the group receiving DOX was significantly higher than that of the ON group, but not different from the GFP negative control (P < 0.001). In addition, the ON group displayed a 5–10% decrease in body weight at the apex of each cycle when normalized against GFP control (Figure 2b).
Transgene levels
Approximately 3 weeks after the last food switch, the animals were euthanized and striatal transgene levels were analyzed by means of enzyme-linked immunosorbent assay (ELISA). Striatal levels of GDNF in the SN-regGDNF2 (ON) group were approximately ninefold lower than that of the CBA-GDNF group (6.2 and 54.9 ng GDNF/mg tissue, respectively), but significantly higher than that of the GFP control and the SN-regGDNF1 (OFF) which displayed only background levels of GDNF (Figure 2k).
In addition, to verify the relative amounts of transgene levels obtained from the GDNF ELISA, transgene expression was also evaluated using immunohistochemistry. GDNF expression in the CBA-GDNF group mirrored that seen in previous studies, with a widespread diffuse spread of GDNF throughout the midbrain and ultimately released in striatum via the medial forebrain bundle (Figure 2c,d). The ON group displayed a similar, but relatively weaker, transduction pattern in the midbrain extending dorsally from the SN into the deep mesencephalic nucleus and ventrally to the SN pars reticulata. Likewise, a substantial amount of GDNF was also observed in the striatum (Figure 2e,f). No transgene was observed in the OFF (Figure 2i,j) or GFP (Figure 2g,h) control groups by immunohistochemical detection in either anatomical area.
Tyrosine hydroxylase
Several reports have demonstrated that high level overexpression of GDNF in the nigrostriatal tract results in a downregulation of TH in the rodent.17,18,19,20 Nigral TH immunohistochemical densitometric measurements using secondary antibodies tagged with near-infrared chromophores, allowing for a wider linear range of signal intensity than conventional densitometric measurements, indeed indicated a significant 50% reduction in TH immunoreactivity in the CBA-GDNF and ON animals when compared to the GFP control and OFF animals (Figure 2m,n). No significant difference was observed between the CBA-GDNF and ON groups.
Serum DOX
High-performance liquid chromatography (HPLC) -based measurements using fluorometric detection of serum DOX levels confirmed that no detectable DOX was present in the ON animals (DOX−) post mortem. In contrast, the GFP group, whose diet had mirrored that of the SN-regGDNF1 group (DOX+), and the OFF animals (DOX+) contained 2.0 and 2.5 µg/ml serum, respectively (Figure 2l).
Dose–response experiment: body weight
In order to determine whether the regulated GDNF construct would respond to submaximal DOX doses with a range of in vivo GDNF levels, a different set of young male rats were fed increasing doses of DOX (0, 40 mg, 100 mg, 1 g, and 3 g/kg of DOX diet) immediately after the vector injection. However, upon monitoring the body weight for 1 month after the injection, no difference in weight was seen among the DOX-treated animals, but, as expected, the normal food control animals displayed a significant reduction in the ability to gain weight (Figure 3a).
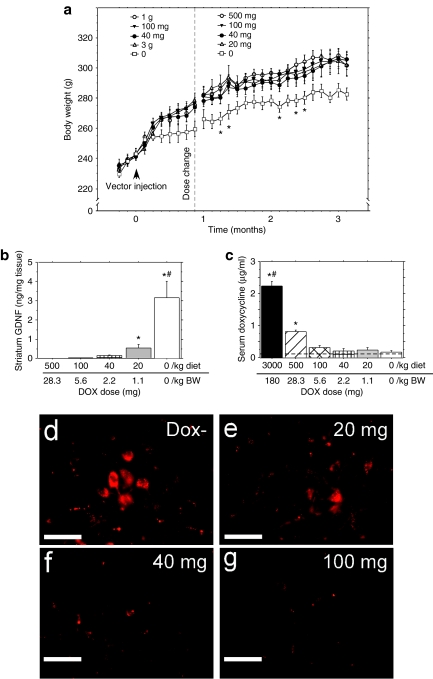
Figure 3.
DOX diet dose–response experiment. Administration of dietary doses of DOX. (a) Before dose change (left of dotted line): 40 mg/kg of diet (closed circles, n = 6), 100 mg/kg of diet (closed triangles, n = 8), 1 g/kg of diet (open circles, n = 8), and 3 g/kg diet (open triangles, n = 8) did not result in weight loss when compared to a normal food (ON) control (open squares, n = 5, P > 0.05). After changing the two indicated DOX doses to lower dietary doses (right of dotted line): 20 mg/kg diet (open triangles, n = 8) and 500 mg/kg diet (open circles; n = 8) there still was not any reduction in body weight compared to other DOX treated groups (P > 0.05). However, the rAAV-regGDNF treated rats fed normal diet (ON) did display significantly lower body weights compared to all other DOX fed groups on several occasions as indicated (*P < 0.05). (b) Striatal GDNF levels as measured via ELISA. The top dose scale on the x axis refers to the DOX dose per kg of diet, the bottom dose scale refers to the approximate dose per kg of BW as calculated for a 300 g rat (approximate average weight at time of assay). Zero (0) refers to DOX−-treated group. #P < 0.0001 versus all groups, *P < 0.0001 versus 100 and 500 mg/kg. (c) Serum levels in DOX-treated animals were detected in animals receiving doses between 100 mg/kg and 3 g/kg, but were below the limits of detection at lower doses; #P < 0.0001 versus all groups, *P < 0.05 versus 100 mg/kg diet, 40 mg/kg diet, 20 mg/kg diet, and normal diet. (d,e) Color-enhanced immuno-micrographs of GDNF staining in SN (d) DOX− (ON) GDNF expression (e) 20 mg/kg DOX diet (f) 40 mg/kg showing consistent but near the limit of detectable GDNF. (f) 100 mg/kg DOX diet showing GDNF staining consistent with the staining seen in GFP-treated animals shown in Figure 1g–j. Bar = 0.1 mm. The lack of GDNF protein expression was confirmed by sensitive infrared densitometric analysis of GDNF staining via the LiCor Odyssey imaging system (data not shown). AAV, adeno-associated virus; BW, body weight; DOX, doxycycline; ELISA, enzyme-linked immunosorbent assay; GDNF, glial cell line-derived neurotrophic factor.
At this point in the experiment, we had not euthanized any animals to determine striatal GDNF levels and therefore it was impossible to know whether all the DOX doses were completely blocking GDNF protein expression and thereby not inducing any weight loss. Another possibility was that some of the doses partially blocked striatal GDNF expression to a level below that required for weight loss. We therefore decided to implement some lower DOX diet doses in a conservative manner so that we would not miss the dietary DOX dose that completely turned off striatal GDNF. Therefore, we changed the two highest dietary DOX doses (1 g and 3 g) to lower doses (500 mg and 20 mg, respectively) resulting in the following DOX diet doses: 20, 40, 100, and 500 mg/kg of diet as shown in Figure 3a (right side).
Following the dosing switch, the animals were monitored for changes in body weight for an additional 2 months. No difference in body weight was seen between the animals receiving DOX, and all groups weighed significantly more than the normal (DOX−) food control group (Figure 3a), although, the weight-loss effect was not as robust as in the older, heavier rats (Figure 2a).
Dose–response experiment: transgene levels
The striatal GDNF dose-response for DOX treatment is shown in Figure 3b. The apparent maintenance of a normal body weight gain in rAAV2/5-regGDNF treated rats did not necessarily indicate a complete shutdown in GDNF expression. The ON–OFF experiment indicated that there was a relationship between the levels of GDNF and the severity of the weight loss when comparing the ON group with the CBA-GDNF group producing significantly higher levels of GDNF. As there is no weight loss in rAAAV2/5 regGDNF treated rats receiving 20 mg/kg DOX diet, these data suggest that there is a threshold for nigrostriatal GDNF-induced weight loss which is within the interval between 0.35 ng/mg striatal tissue (20 mg/kg DOX diet) and 3.3 ng/mg striatal tissue (ON) which induces weight loss (Figure 3b).
Immunohistochemical evaluation of GDNF expression in the SN revealed detectable expression in the ON group and 20 mg/kg animals (Figure 3d,e), but this expression was largely localized to the SN pars compacta and was cell specific. No GDNF expression above background levels was detected in the 40 mg/kg DOX diet (Figure 2f) and 100 mg/kg DOX diet animals (Figure 3g), confirming the observations made from the striatal ELISA samples.
Serum DOX levels
Blood samples were taken from each animal at the time of euthanasia in order to determine the serum levels of DOX required for gene silencing (Figure 3c). HPLC measurements indicated that rats that ate 100 mg/kg DOX diet which was the lowest DOX diet dose to abrogate striatal GDNF expression had serum DOX levels of 0.6 µg/ul. However, DOX levels from the animals in the 500 mg/kg group (0.8 µg/ml) was significantly lower than those of the in the 3 g/kg DOX diet animals from the OFF group in experiment 1 (P < 0.01).
VP-16 expression
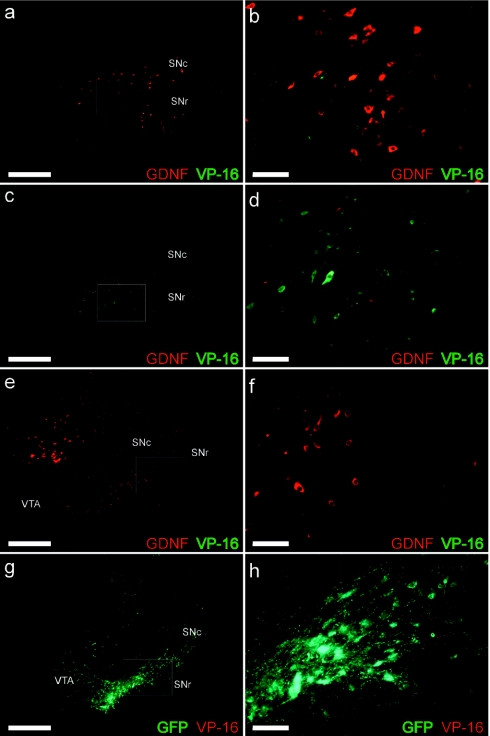
One concern with the overexpression of the VP-16 motif is the potential for this transcription factor to escape the infected cell and potentially affect endogenous transcription in surrounding cells. However, immunohistological double labeling of GDNF and VP-16 suggested no such spread (Figure 4). VP-16 expression in the midbrain (Figure 4a–d) was virtually identical to that typically seen with a nonsecreted transgene such as GFP (Figure 4g–h). VP-16 immunoreactivity was seen largely in the SN pars compacta and SN pars reticulata (Figure 4a–d), and, in the ON group, GDNF transgene was observed in an area vastly surpassing that of VP-16 (Figure 4a,b). Again, this area is virtually identical to that seen when overexpressing GDNF in the same vector as GFP.21 Again, no GDNF was observed in the OFF group (Figure 4c,d), and no VP-16 immunoreactivity was seen in either the CBA-GDNF (Figure 4e,f) or CBA-GFP (Figure 4g,h) animals.
Figure 4.
GDNF and VP-16 expression patterns. The GDNF expression pattern of the final ON group (a,b) was similar to that previously observed where the GDNF transgene (red) is observed distally from that of the transduced cells and VP-16 (green). (b) Increased magnification of area outlined in a. VP-16 expression in the OFF group (c,d) was similar to that seen in the ON group; however, no GDNF protein expression was observed. GDNF protein expression in the CBA-GDNF group (e,f) extended in a diffuse pattern into the VTA and the DMN indicating that GDNF protein was distributed away from the transduced cells in contrast to the pattern of expression observed from an intracellular transgene such as the SN-GFP group (g,h). As expected, no immunoreactivity was observed for VP-16 in either the CBA-GDNF group (e,f, green) or the SN-GFP group (g,h, red). (f and h) Higher magnifications of areas outlined in e and g, respectively. Bar: a,c,e,g = 1.0 mm, bar: b,d,f,h = 0.1 mm. CBA, chicken β-actin; DMN, deep mesencephalic nucleus; GDNF, glial cell line-derived neurotrophic factor; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulate; VTA, ventral tegmental area.
Striatal rAAV injections
Injections of rAAV2/5 directly into the striatum typically results in lower local transgene levels as well as lower levels in the nigrostriatal tract than that seen from anterograde transport due to injections targeting the SN. Furthermore, delivery approaches in the clinic have largely been aimed at increasing levels of GDNF in the caudate/putamen. Thus, we wanted to explore whether striatal protein overexpression of GDNF would lead to weight loss similar to that seen in nigral protein overexpression. Animals were injected bilaterally in striatum with either rAAV2/5-regGDNF (Figure 5a) or CBA-GDNF (Figure 5b) and their weight was monitored thereafter for 5 months. Neither striatal vector treatment resulted in any weight loss when compared to the GFP-injected controls.
Figure 5.
Striatal targeting of rAAV2/5-GDNF does not lead to weight loss. Direct injections into the striatum of (a) rAAV2/5− regGDNF [open circles (n = 8)] or (b) CBA-GDNF [open circles, n = 8)] did not result in weight loss when compared to the GFP control (a,b closed circles, n = 8 per group). (c) Striatal GDNF protein levels determined by ELISA. The histograms from the three SN groups (GFP, GDNF, regGDNF) where taken from Figure 2i. for the sake of comparison and were not included in the statistical analysis. Robust striatal GDNF expression was observed histologically in the striatum and the SN for both the (d,e) CBA-GDNF and (f,g) regGDNF groups, no GDNF expression was observed in the (h) striatum or the (i) SN in the GFP control animals. (i) Striatal levels of GDNF were similar in both GDNF groups. Bar = 0.5 mm. Solid white line, external capsule; CBA, chicken β-actin; ctx, cortex; DMN, deep mesencephalic nucleus; GDNF, glial cell line-derived neurotrophic factor; GFP, green fluorescent protein; gp, globus pallidus; rAAV, recombinant adeno-associated virus; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulate; str, striatum.
Transgene levels
Striatal GDNF content for either striatally injected GDNF virus was similar, but significantly lower than that observed in the SN injected CBA-GDNF animals when measured by ELISA (striatal regGDNF = 5.3 ng/mg tissue, striatal CBA-GDNF = 7.1 ng/mg tissue, nigral CBA-GDNF = 55 ng/mg tissue). In addition, no significant differences were seen when comparing the direct striatal injections with that of the SN-regGDNF virus (6.2 ng/mg tissue; Figure 5c) indicating that this striatal GDNF level does not affect body weight (cf. Figure 3a versus 5b).
Striatal transgene expression
Significant transgene expression was observed in the striatum for either striatally targeted GDNF virus. However, the transgene was largely observed in a bolus pattern presumably representing the direct spread of the virus at the injection site (Figure 5e,g). The midbrain expression pattern mimicked that seen in the ON group in Figure 1e albeit at a lower level (Figure 5d,f). Striatal injection with the CBA-GFP control virus yielded no detectable GDNF expression (Figure 5h,i).
Cerebellar purkinje cells
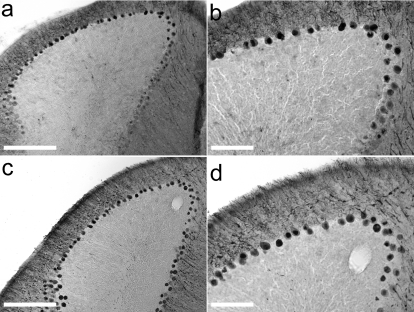
Observations from a preclinical trial in nonhuman primates have suggested that high doses of GDNF over a 6-month period resulted in purkinje cell pathology.22 Evaluation of purkinje cell-layer integrity by calbindin immunohistochemistry yielded no difference between the GFP (Figure 6a,b) and GDNF (Figure 6c,d) striatal groups.
Figure 6.
Five months of striatal protein overexpression of GDNF has no effect on cerebellar purkinje cells. The integrity of cerebellar purkinje cells was assessed using calbindin immunohistochemistry from the same CBA-GDNF animals shown in Figure 5. (a) Cerebellar calbindin immunoreactivity in striatally injected CBA-GFP animal highlighting survival of purkinje cells (large dark cell bodies). (b) Increased magnification of a. (c) Striatal GDNF protein overexpression does not lead to cell loss, or abnormal cells, of the purkinje cell layer. (d) Increased magnification of c. Bar: a,c = 1 mm, bar: b,d = 200 µm.
Discussion
The absolute requirement for external transgene regulation for trophic factor treatment of PD has recently been debated.11,12 One perspective is that regulated vectors are needed as a safety measure against unexpected side effects that could not be predicted by preclinical toxicology experiments.12 Moreover, in the event that individual patients might respond to different doses of the gene product, regulated vectors would be needed.12 In contrast, an opposing viewpoint argued: (i) no clinically relevant regulatable system is currently available, (ii) transcriptional regulation systems represent a clinical complication on their own because the transactivator is not regulated, and (iii) as long as extremely careful preclinical toxicology shows that unregulated gene expression is safe, human trials should be attempted, especially because no treatment is available to affect the progressive nature of PD.11
While both competing viewpoints have merit, a major experimental design advantage of external transgene regulation was overlooked. For example, with the use of a regulated vector, clinical dose-escalation studies would be simplified because equal amounts of vector can be delivered to each patient, while only the amount of the regulating compound is varied. In addition, highly efficient dose-escalation designs such as a balanced Latin-square design wherein each patient receives all doses and carryover effects of each dose are controlled can be used.23 Finally, the use of a regulated vector would remove the need for the ethically controversial surgical placebo control group in a double-blinded study. However, in the end, Kordower and Olanow11 were correct that, at present, there is no transcriptional regulation system that has been used clinically. Indeed, PD clinical trials using rAAV delivered neurturin have been allowed to go forward without gene regulation.24
In contrast, our laboratory has chosen to attempt to develop a clinically relevant rAAV based regulatable system to express GDNF for the potential treatment of PD. The features of a successful functional gene regulatory system are: (i) the vector derived regulatory elements are nontoxic, (ii) the systemically delivered controlling molecule has an acceptable safety profile (preferably an already US Food and Drug Administration approved drug), (iii) there is no basal expression in the off state, (iv) there is biologically relevant gene expression in the ON state, (v) the ability to regulate the gene product is permanent (i.e., the gene product can be turned on and off repeatedly), and (vi) there is a demonstrable dynamic dose response to the external control agent. To date, no vector-mediated GDNF-regulation system has been demonstrated to meet all these criteria when delivered to the brain in vivo.
Several reports have been published where various versions of the tet-regulatory system has been used to overexpress GDNF or reporter genes in vivo.14,15,25,26 Although, some approaches have been successful in terms of inducibility and control, dose–response or long-term control were not demonstrated in these earlier studies. In a different regulatory setup, researchers used the Krüppel associated box domain, a human zinc finger transcriptional repression domain, to control GDNF expression with similar results.25 Most in vivo studies attempting to use the tet-regulatory system have suffered from leaky basal expression.14,15,26,27 Other reports highlight the problem that the repeated ability to regulate the transgene in a single rAAV vector has been problematic.28,29 For example, when using a single vector tet-off system to overexpress human aromatic amino acid decarboxylase in the rodent brain, high inducibility was seen in the initial absence of the regulatory agent minocycline.29 However, following a period with minocycline administration effectively turning the expression off, withdrawal of the drug failed to reinduce expression, demonstrating the lack of a dynamic ability to regulate the transgene.29
In this study, we demonstrate a single rAAV construct that meets most of the defined criteria for a successful GDNF regulation system. An enabling discovery was the finding that nigral GDNF overexpression leads to weight loss in aged leptin-resistant rats.21 This property of rAAV-GDNF allowed us to use body weight as a means to track real-time in vivo GDNF levels. Thus, we have demonstrated that rAAV2/5-regGDNF can be turned on and off four times over a 6-month period without any alteration in the ability to regulate body weight. We have shown that there is no detectable intracerebral GDNF in the OFF state and that GDNF induces weight loss and reduces nigrostriatal TH expression in the ON state indicating clear biological effects at the striatal GDNF protein levels achieved here.
Furthermore, at each expression crossover, the animals displayed a remarkably rapid recovery of their body weight trajectory. However, we do not believe that GDNF protein expression is reversed immediately after a DOX diet crossover. We interpret the rapid change in body weight, in response to DOX diet crossover as indicative of the in vivo GDNF levels in the ON state being close to the threshold required for the observed weight-loss phenomenon. This GDNF threshold allows small alterations in GDNF levels to rapidly affect body weight and this contention is supported by the dose–response data in Figure 3a. However, rapid but small changes in GDNF levels (and body weight) also suggest that the DOX-mediated effect on transcription did initiate rapidly following removal or introduction of DOX.
The tet transactivator contains a minimal portion of the herpes simplex virus gene VP-16 consisting of a triplet repeat of 30 base pairs out of 1,450 base pairs in the full-length complementary DNA that was originally developed to reduce the potential for nonspecific DNA binding and potential immune response.30 Regardless, the unregulated expression of the transactivator remains a concern due to the possibility of reactivation of an anti-herpes simplex virus immune response. Antigen presentation via major histocompatibility complex I in neurons is inefficient in brain.31 For example, the tet transactivator does not elicit a striatal immune response even when rats are preimmunized against the VP-16 containing transactivator.32 However, given that the rodent immune system is not identical to the human and the vast majority of the human population has been exposed to some form of herpes simplex virus, the potential for a VP-16-induced immune response cannot be ruled out. In addition, the phenomenon of “squelching” of endogenous expression has been raised as a concern with unregulated overexpression of the VP-16 domain.33 However, previous studies have demonstrated that the squelching phenomenon is only observed in developing cells and absent, despite very high levels of expression, in differentiated cells.34,35
Although DOX, the external control agent used in this study, is US Food and Drug Administration approved and has a well characterized favorable toxicology profile, long-term treatment is not entirely benign. Long-term DOX treatment is associated with increased photosensitivity among other low incidence side effects.36 However, long-term treatment with sub-antimicrobial doses significantly lowers limiting side effects.37 In this study, the DOX serum levels needed to completely abrogate nigrostriatal GDNF expression are at least eightfold lower than levels required for antimicrobial activity36 and similar to those required for anti-rosacea activity.38
A PD clinical trial where GDNF was delivered directly to the putamen via an implanted cannula and mechanical pump system was halted early because circulating anti-GDNF antibodies were detected.39 In addition, concurrent primate toxicology studies indicated that high doses of intraputamenal GDNF could cause significant cerebellar purkinje cell death.22 Moreover, there have also been reports of weight loss in intracerebroventricular PD trials and in preclinical primate studies.10,22 Therefore, we attempted to investigate these possible rAAV-GDNF side effects by intrastriatal injection of regulated rAAV2/5 vectors and vectors constitutively expressing striatal GDNF. Striatal GDNF expression from the rAAV2/5 vector using a regulated promoter or from the constitutive promoter produced no weight loss. Indeed, in the constitutive striatal GDNF group, striatal GDNF levels were equivalent to a regulated nigral GDNF group that lost weight, indicating that there is a higher threshold for weight loss when rAAV-GDNF is delivered directly to the striatum. Moreover, we were unable to detect any cerebellar purkinje cell damage in either the regulated or constitutive striatal GDNF groups even after 5 months of continuous striatal GDNF expression. Additionally, we have previously reported that, in the rat, the main striatal immune response after rAAV2 transduction is to the AAV capsid, not to the transgene, including human GDNF.40 Moreover, we have not been able to immunize rats using rAAV injections in the brain even after readministration.40 Therefore, as suggested previously, it is likely that GDNF somehow escaped into the periphery for antigen presentation in the clinical trial.7
It is evident from the current and previous studies that the weight loss observed in nigral GDNF protein overexpression may preclude this anatomical target in the clinic.21 In our previous study focused on high constitutive nigrostriatal GDNF overexpression, we used obese aged rats and found highly significant weight loss.21 Thus, it could be asserted that bilateral nigral rAAV-mediated GDNF (or neurturin) might only be a concern with pre-existing obesity. However, in this study, we have shown that bilateral nigral rAAV-mediated GDNF overexpression abrogates normal weight gain in middle-aged rats (Figure 1a,b) and also in young rats (Figure 2a) even though neither of these populations of rats had become obese yet. These data further highlight potential problems with the SN as the target of unregulated GDNF in PD.
On the other hand, current clinical trials have been focused on direct injections into the striatum, and although some retrograde transport via the nigrostriatal tract would be expected when using most rAAV serotypes,41,42 the extent of striatal dopamine innervation in PD patients is markedly reduced thereby mitigating the probability that nigral GDNF protein expression after striatal rAAV injections will induce weight loss in PD. Moreover, our dose–response data indicate that there is a threshold level of nigrostriatal GDNF needed for weight loss to occur that would further mitigate any possibility of weight loss from a striatal rAAV-GDNF injection in PD. Finally, striatal GDNF protein overexpression at levels equal to that seen after nigral rAAV2/5-regGDNF injection did not induce weight loss, indicating either that striatal GDNF protein overexpression is less efficient than nigrostriatal GDNF protein overexpression, or striatal GDNF protein overexpression cannot induce weight loss. Nonetheless, the striatal GDNF levels obtained after striatal administration of rAAV2/5-regGDNF were ~25-fold higher than those needed for a therapeutic response in the rat9 and ~20,000-fold lower than toxic infusion doses in the primate.22
Materials and Methods
Animals. Twelve-month-old male Sprague–Dawley rats (Harlan, Indianapolis, IN) were used for the ON–OFF and regulated GDNF striatal studies. Two-month-old Sprague–Dawley rats were used for the dose–response and striatal CBA-GDNF experiments. Upon arrival, animals were quarantined for 1 week prior to any testing. Animals were cared for in accordance with the principles of the Guide to the Care and Use of Experimental Animals. Rats were housed individually with a 12:12 hour light:dark cycle (0700–1900 hours). Food and water were available ad libitum throughout the study. Prior to the start of the study, animals were weighed and placed in experimental groups in a fashion that yielded equal average body weights among the groups. DOX was administered in the diet using standardized diet containing a quality-controlled dose of DOX (Bio-Serv, Frenchtown, NJ). All doses are expressed as the amount of DOX in each kg food (except on the x axis of Figure 3 where both food dosage and approximate mg/kg body weight was given). Following vector administration, animals were placed on DOX diets reflecting the various groups: ON/OFF animals were cycled between 3 g/kg DOX and regular food. We have shown that 3 g/kg dietary DOX produces DOX serum levels equivalent to that of 1 mg/ml DOX in drinking water (S.L. Semple-Rowland, J.E. Coleman, and R.J. Mandel, unpublished data). The diet schedule for the SN-GFP group was yoked to that of the SN-regGDNF1 group. All striatal injection groups received normal food. Dose–response animals were placed on normal, 20 mg/kg, 40 mg/kg, 100 mg/kg, 500 mg/kg, 1 g, or 3 g DOX diet (Bio-Serv). Body weights were measured twice weekly.
rAAV2/5 production. The AAV backbone for the control viruses was identical to that previously reported.43,44 Briefly, the GDNF complementary DNA was under the control of the cytomegalovirus/CBA promoter hybrid, followed directly downstream by the wood-chuck hepatitis post-transcriptional regulatory element. The complete transcription cassette was flanked by AAV2 terminal repeats. The GFP vector consisted of the humanized enhanced GFP complementary DNA downstream of the same promoter elements as in the GDNF plasmid. The bicistronic-regulated GDNF vector contained the ptTA2 tet-off transactivator expressed by the cytomegalovirus-CBA hybrid promoter followed by a downstream expression cassette containing a modified tet response element (TRE) containing seven repeats of the 19 base pairs tet operator sequence (Figure 1). A minimal cytomegalovirus promoter was situated immediately downstream of the TRE driving the expression of the same GDNF complementary DNA used in the CBA-GDNF plasmid. This construct is similar to that reported previously.45 The viruses were produced via the triple transduction method and purified as described previously.46 The final vector titers were rAAV2/5 CBA-GDNF 3.8 × 1012 genome copies/ml, rAAV2/5 CBA-GFP 4.5 × 1013 genome copies/ml, and rAAV2/5 regGDNF 7.3 × 1013 genome copies/ml. The virus stock purity was determined to be >99% as judge by silver-stained SDS acrylamide gel fractionation.
Intracerebral administration of rAAV2/5. All surgical procedures were performed as previously described.44 Each animal in the nigral injection groups received bilateral injections of the virus in the SN. The coordinates were anterior–posterior −5.4 mm, ±2.0 mm medial–lateral from bregma, and −7.2 mm dorsoventral from the dura. The total volume injected was 2 µl at a rate of 0.5 µl/minute. To allow for viral diffusion, the micropipette was allowed to remain for an additional 5 minutes after the injection and thereafter slowly removed from the brain. Animals in the striatal injection groups received intraperitoneal injections of mannitol (3 ml sterile 25% mannitol in 0.9% saline/100 g body weight) 15 minutes prior to receiving one rAAV injections of 3 µl in each hemisphere at a rate of 0.5 µl/minute.43 The coordinates were anterior–posterior ± 0.0 mm and medial–lateral ± 2.7 mm from bregma, dorsoventral −4.0 mm from dura. One minute after the completion of each injection the micropipette was retracted 1 mm and left in place for an additional 4 minutes before being slowly removed from the brain.
Tissue harvesting and preparation. At the termination of the study, the animals were deeply anesthetized with pentobarbital, blood samples were collected by heart puncture, and serum was isolated as described previously.47,48 The animals were thereafter decapitated and brains were rapidly removed and placed in a block and sectioned coronally at the level of the mid-hypothalamus in order to separate the anterior and posterior parts of the striatum (Figure 1). The portion of the brain posterior to the cut was immediately placed in ice-cold 4% paraformaldehyde in 0.01 mol/l phosphate-buffered saline and postfixed for 24 hours. Following fixation, the brains were treated with 30% sucrose until saturation was achieved and subsequently cut into 40 µm thick sections using a freezing stage sliding microtome. The striatum was dissected from the portion of the brain anterior to the cut by visual guidance. The dissected sample was weighed and then immediately frozen in dry ice and thereafter stored in −80 °C until further use.
GDNF ELISA. Striatal samples from the dissections were analyzed with a GDNF Emaxkit (Promega, Madison, WI). Lysis buffer, as described in the kit protocol, was added to the tissue to achieve a final concentration of 100 mg tissue/ml lysis solution. The tissue was thereafter disrupted using a model-100 sonic dismembrator (Fisher Scientific, Waltham, MA). Samples were subsequently assayed at various concentrations until raw plate values were in the middle range of the standard curve. Absorbance values were obtained with a VersaMax microplate reader (Molecular Devices, Sunnyvale, CA). Raw values were then adjusted for tissue weight.
Immunohistochemistry. Immuno-staining procedures were identical to those previously described.44 Primary antibodies used were mouse anti-TH (1:2,000), rabbit anti-GFP (1:2,000), mouse anti-calbindin (1:200), rabbit anti-VP-16 (1:50) (Chemicon, Temecula, CA), goat anti-GDNF (1:1,000) (R&D Systems, Minneapolis, MN). The secondary antibody for calbindin brightfield microscopy was biotinylated anti-rabbit immunoglobin G and the sections were developed with NovaRed (Vector Laboratories, Burlingame, CA). Fluorescently labeled secondary antibodies were Alexa Fluor 594 donkey anti-goat and anti-rabbit, and Alexa Fluor 488 donkey anti-rabbit (Invitrogen, Carlsbad, CA). The near-infrared secondary antibody for the TH densitometry was IRdye 800 (LI-COR Biosciences, Lincoln, NE).
Densitometry. Serial TH-labeled sections were scanned at a resolution of 21 µm and a 5.5 (arbitrary unit) sensitivity setting on a LI-COR Odyssey scanner (LI-COR Biosciences). The SN was thereafter outlined and the value for the integrated intensity was obtained from each section on a normalized slide. The average for the raw integrated intensity was subsequently obtained for each animal in order to normalize for variations in the number of sampling sites between different animals.
Statistical analysis. All statistical differences between groups were tested using analysis of variance (ANOVA). For the ON–OFF experiment weight measures, four epochs corresponding to the crossover of the DOX diet were analyzed separately by repeated-measures ANOVA. Statistical significance for individual contrasts were undertaken via simple-main effects analysis49 followed by a Fisher's paired least significant difference post hoc test after obtaining a significant GROUP × TIME interaction (P < 0.0001 for all epochs). We accepted an α level of P < 0.001 for these contrasts to adjust for 42 individual contrasts. The weight dose–response experiment was also analyzed using repeated-measures ANOVA in two separate epochs (pre- and postdose change). The second epoch displayed a significant GROUP × TIME interaction (P < 0.01) enabling the post hoc testing as described above. All ELISA data were analyzed via ANOVA after a log10 transformation of the data. All other data were analyzed via ANOVA followed by paired least significant difference post hoc test.
DOX HPLC. The content of DOX in serum samples was determined via a slightly modified HPLC method.50 Briefly, DOX was extracted from serum samples using a HLB Extraction cartridge (Waters, Milford, MA), 1 mg/10 mg. Each sample was diluted with an equal volume of acidified water containing 2% phosphoric acid to disrupt protein binding, then vortexed and applied to a preconditioned extraction cartridge. This was followed by washing with 5% methanol in water in order to wash off unbound materials. The DOX was finally eluted off the cartridge using mobile phase. Tet was added as an internal standard in a final concentration of 1 µg/ml then filtered through 0.2-µm nylon syringe filter, and collected into an HPLC vial. An HPLC-DAD HP1030 system (Agilent Technologies, Santa Clara, CA) was used to carry out the analysis. Separation was carried out at 35 °C on a Hydro-RP, reverse-phase column (Phenomenex Synergy, Torrance, CA; 250 × 4.6 mm internal diameter, and 4 µm particle size). This column was preceded by a reversed-phase AQ-C18, (40 × 3 mm) guard column. The detector wavelength was set at 350 nm. The mobile phase consisted of 60% water containing 0.15% trifluoroacetic acid and 40% acetonitrile (pH 2.3). The mobile phase was filtered through 0.22-µm filter membrane and degassed with helium for 15 minutes before used. The flow rate was set at 0.7 ml/minute with a 25 µl injection volume.
SUPPLEMENTARY MATERIALFigure S1. Early vector designs. Prior to the construction of the single vector tet-off construct used in the main study several permutations of tet-on vectors were tested (a-b). The dual vector system (a) consisted of a vector containing the rtTA2 reverse transactivator and the tTS trans-silencer under the control of the constitutive CBA promoter separated by an internal ribosome entry site. The second vector consisted of the GDNF transgene behind the same tet responsive promoter used in the main study. (c). During the initial injection paradigm the vectors were injected at a 1:1 ratio. However, inducibility was remarkably low yielding only a roughly two-fold increase over background levels. Furthermore, injection of the tet-GDNF alone resulted in significantly higher levels of GDNF indicating a basal promoter activity, perhaps due to an endogenous promoter function of the ITRs which is actively repressed when tTS is present (*, p < 0.05 vs. white bar vs. black bar). Consequently, increasing the ratio of the rtTA/tTS vector to 2:1 resulted in background GDNF levels comparable to that of control (c, right portion). (d). A dose-response evaluation of the double vector injected at a 2:1 ratio indicated maximum inducibility at 400 mg/kg DOX (administered by time-release subcutaneous pellets; *, p < 0.05 vs. uninjected control, dagger, p <0.05 vs. all other DOX doses). (e). Basal expression (no DOX) of GDNF in striatum injected with tet-GDNF only. (f) Basal GDNF protein expression (no DOX) after addition of the rtTA/tTS vector in a 2:1 ratio that yielded background GDNF protein measurements. This demonstrates that even when ELISA measurements of striatal GDNF indicate background levels, there is still leaky expression from this regulated vector combination. Furthermore, the bi-directional construct shown in (b) displayed no inducibility and GDNF levels in the absence of DOX were similar to that seen with expression of GDNF shown in (e, data not shown). In addition, replacing the AAV2 ITRs with AAV5 ITRs only reduced background expression levels slightly (data not shown).
Supplementary Material
Early vector designs. Prior to the construction of the single vector tet-off construct used in the main study several permutations of tet-on vectors were tested (a-b). The dual vector system (a) consisted of a vector containing the rtTA2 reverse transactivator and the tTS trans-silencer under the control of the constitutive CBA promoter separated by an internal ribosome entry site. The second vector consisted of the GDNF transgene behind the same tet responsive promoter used in the main study. (c). During the initial injection paradigm the vectors were injected at a 1:1 ratio. However, inducibility was remarkably low yielding only a roughly two-fold increase over background levels. Furthermore, injection of the tet-GDNF alone resulted in significantly higher levels of GDNF indicating a basal promoter activity, perhaps due to an endogenous promoter function of the ITRs which is actively repressed when tTS is present (*, p < 0.05 vs. white bar vs. black bar). Consequently, increasing the ratio of the rtTA/tTS vector to 2:1 resulted in background GDNF levels comparable to that of control (c, right portion). (d). A dose-response evaluation of the double vector injected at a 2:1 ratio indicated maximum inducibility at 400 mg/kg DOX (administered by time-release subcutaneous pellets; *, p < 0.05 vs. uninjected control, dagger, p <0.05 vs. all other DOX doses). (e). Basal expression (no DOX) of GDNF in striatum injected with tet-GDNF only. (f) Basal GDNF protein expression (no DOX) after addition of the rtTA/tTS vector in a 2:1 ratio that yielded background GDNF protein measurements. This demonstrates that even when ELISA measurements of striatal GDNF indicate background levels, there is still leaky expression from this regulated vector combination. Furthermore, the bi-directional construct shown in (b) displayed no inducibility and GDNF levels in the absence of DOX were similar to that seen with expression of GDNF shown in (e, data not shown). In addition, replacing the AAV2 ITRs with AAV5 ITRs only reduced background expression levels slightly (data not shown).
Acknowledgments
This work was supported by NINDS NS P01 36302 to N.M., A.S.L., and R.J.M. We thank Isabelle Williams and Bobbi Johnson for technical assistance. N.M. is an inventor of patents related to recombinant AAV technology and owns equity in a gene therapy company that is commercializing AAV for gene therapy applications.
REFERENCES
- Fearnley JM., and , Lees AJ.1991Ageing and Parkinson's disease: substantia nigra regional selectivity Brain 114Pt 5): 2283–2301. [DOI] [PubMed] [Google Scholar]
- Peterson AL., and , Nutt JG. Treatment of Parkinson's disease with trophic factors. Neurotherapeutics. 2008;5:270–280. doi: 10.1016/j.nurt.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov MM., and , Saarma M. GDNF family receptor complexes are emerging drug targets. Trends Pharmacol Sci. 2007;28:68–74. doi: 10.1016/j.tips.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gómez-Díaz R., and , López-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Ibanez CF. Catecholaminergic neuron survival: getting hooked on GDNF. Nat Neurosci. 2008;11:735–736. doi: 10.1038/nn0708-735. [DOI] [PubMed] [Google Scholar]
- Walker DG, Beach TG, Xu R, Lile J, Beck KD, McGeer EG, et al. Expression of the proto-oncogene Ret, a component of the GDNF receptor complex, persists in human substantia nigra neurons in Parkinson's disease. Brain Res. 1998;792:207–217. doi: 10.1016/s0006-8993(98)00131-0. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Herzog CD, Bishop K, Ostrove JM, Tuszynski M, Kordower JH, et al. 2007Issues regarding gene therapy products for Parkinson's disease: the development of CERE-120 (AAV-NTN) as one reference point Parkinsonism Relat Disord 13suppl. 3): S469–S477. [DOI] [PubMed] [Google Scholar]
- Mandel RJ, Manfredsson FP, Foust KD, Rising A, Reimsnider S, Nash K, et al. Recombinant adeno-associated viral vectors as therapeutic agents to treat neurological disorders. Mol Ther. 2006;13:463–483. doi: 10.1016/j.ymthe.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Björklund A, Kirik D, Rosenblad C, Georgievska B, Lundberg C., and , Mandel RJ. Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res. 2000;886:82–98. doi: 10.1016/s0006-8993(00)02915-2. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Palfi S, Chen EY, Ma SY, Sendera T, Cochran EJ, et al. Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson's disease. Ann Neurol. 1999;46:419–424. doi: 10.1002/1531-8249(199909)46:3<419::aid-ana21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Kordower JH., and , Olanow CW. Regulatable promoters and gene therapy for Parkinson's disease: is the only thing to fear, fear itself. Exp Neurol. 2008;209:34–40. doi: 10.1016/j.expneurol.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cress DE. The need for regulatable vectors for gene therapy for Parkinson's disease. Exp Neurol. 2008;209:30–33. doi: 10.1016/j.expneurol.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Gossen M., and , Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgievska B, Jakobsson J, Persson E, Ericson C, Kirik D., and , Lundberg C. Regulated delivery of glial cell line-derived neurotrophic factor into rat striatum, using a tetracycline-dependent lentiviral vector. Hum Gene Ther. 2004;15:934–944. doi: 10.1089/hum.2004.15.934. [DOI] [PubMed] [Google Scholar]
- Chtarto A, Bender HU, Hanemann CO, Kemp T, Lehtonen E, Levivier M, et al. Tetracycline-inducible transgene expression mediated by a single AAV vector. Gene Ther. 2003;10:84–94. doi: 10.1038/sj.gt.3301838. [DOI] [PubMed] [Google Scholar]
- Szulc J., and , Aebischer P. Conditional gene expression and knockdown using lentivirus vectors encoding shRNA. Methods Mol Biol. 2008;434:291–309. doi: 10.1007/978-1-60327-248-3_18. [DOI] [PubMed] [Google Scholar]
- Georgievska B, Kirik D., and , Björklund A. Aberrant sprouting and downregulation of tyrosine hydroxylase in lesioned nigrostriatal dopamine neurons induced by long-lasting overexpression of glial cell line derived neurotrophic factor in the striatum by lentiviral gene transfer. Exp Neurol. 2002;177:461–474. doi: 10.1006/exnr.2002.8006. [DOI] [PubMed] [Google Scholar]
- Rosenblad C, Georgievska B., and , Kirik D. Long-term striatal overexpression of GDNF selectively downregulates tyrosine hydroxylase in the intact nigrostriatal dopamine system. Eur J Neurosci. 2003;17:260–270. doi: 10.1046/j.1460-9568.2003.02456.x. [DOI] [PubMed] [Google Scholar]
- Georgievska B, Kirik D., and , Björklund A. Overexpression of glial cell line-derived neurotrophic factor using a lentiviral vector induces time- and dose-dependent downregulation of tyrosine hydroxylase in the intact nigrostriatal dopamine system. J Neurosci. 2004;24:6437–6445. doi: 10.1523/JNEUROSCI.1122-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajadi A, Bauer M, Thöny B., and , Aebischer P. Long-term glial cell line-derived neurotrophic factor overexpression in the intact nigrostriatal system in rats leads to a decrease of dopamine and increase of tetrahydrobiopterin production. J Neurochem. 2005;93:1482–1486. doi: 10.1111/j.1471-4159.2005.03139.x. [DOI] [PubMed] [Google Scholar]
- Manfredsson FP, Tumer N, Erdos B, Landa T, Broxson CS, Sullivan LF, et al. Nigrostriatal rAAV-mediated GDNF overexpression induces robust weight loss in a rat model of age-related obesity. Mol Ther. 2009;17:980–991. doi: 10.1038/mt.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovland DN, Boyd RB, Butt MT, Engelhardt JA, Moxness MS, Ma MH, et al. Six-month continuous intraputamenal infusion toxicity study of recombinant methionyl human glial cell line-derived neurotrophic factor (r-metHuGDNF in rhesus monkeys. Toxicol Pathol. 2007;35:1013–1029. doi: 10.1177/01926230701481899. [DOI] [PubMed] [Google Scholar]
- Cochran WG., and , Cox GM.1957Experimental Design5th edn, New York: Wiley [Google Scholar]
- Marks WJ, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- Szulc J, Wiznerowicz M, Sauvain MO, Trono D., and , Aebischer P. A versatile tool for conditional gene expression and knockdown. Nat Methods. 2006;3:109–116. doi: 10.1038/nmeth846. [DOI] [PubMed] [Google Scholar]
- Fitzsimons HL, Bland RJ., and , During MJ. Promoters and regulatory elements that improve adeno-associated virus transgene expression in the brain. Methods. 2002;28:227–236. doi: 10.1016/s1046-2023(02)00227-x. [DOI] [PubMed] [Google Scholar]
- Goverdhana S, Puntel M, Xiong W, Zirger JM, Barcia C, Curtin JF, et al. Regulatable gene expression systems for gene therapy applications: progress and future challenges. Mol Ther. 2005;12:189–211. doi: 10.1016/j.ymthe.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Rivera VM, Zoltick P, Cerasoli F, Schnell MA, Gao G, et al. Regulated delivery of therapeutic proteins after in vivo somatic cell gene transfer. Science. 1999;283:88–91. doi: 10.1126/science.283.5398.88. [DOI] [PubMed] [Google Scholar]
- Chang Q, Virag T, Rampalli S, Vanin EF., and , Bohn MC. Molecular, cellular, and behavioral characterization of a tetracycline (Tet)-regulated rAAV vector for human aromatic amino acid decarboxylase (hAADC) in a rat model of Parkinson's disease (PD) Mol Ther. 2008;16:S264. [Google Scholar]
- Baron U, Gossen M., and , Bujard H. Tetracycline-controlled transcription in eukaryotes: novel transactivators with graded transactivation potential. Nucleic Acids Res. 1997;25:2723–2729. doi: 10.1093/nar/25.14.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein PR. Immunology of viral-vector-mediated gene transfer into the brain: an evolutionary and developmental perspective. Trends Immunol. 2002;23:23–30. doi: 10.1016/s1471-4906(01)02063-4. [DOI] [PubMed] [Google Scholar]
- Xiong W, Candolfi M, Kroeger KM, Puntel M, Mondkar S, Larocque D, et al. Immunization against the transgene but not the TetON switch reduces expression from gutless adenoviral vectors in the brain. Mol Ther. 2008;16:343–351. doi: 10.1038/sj.mt.6300375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G., and , Ptashne M. Negative effect of the transcriptional activator GAL4. Nature. 1988;334:721–724. doi: 10.1038/334721a0. [DOI] [PubMed] [Google Scholar]
- Yueh YG, Yaworsky PJ., and , Kappen C. Herpes simplex virus transcriptional activator VP16 is detrimental to preimplantation development in mice. Mol Reprod Dev. 2000;55:37–46. doi: 10.1002/(SICI)1098-2795(200001)55:1<37::AID-MRD6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Shockett PE., and , Schatz DG. Diverse strategies for tetracycline-regulated inducible gene expression. Proc Natl Acad Sci USA. 1996;93:5173–5176. doi: 10.1073/pnas.93.11.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha BA, Sibley CM., and , Ristuccia AM. Doxycycline. Ther Drug Monit. 1982;4:115–135. doi: 10.1097/00007691-198206000-00001. [DOI] [PubMed] [Google Scholar]
- Stieger K, Belbellaa B, Le Guiner C, Moullier P., and , Rolling F. In vivo gene regulation using tetracycline-regulatable systems. Adv Drug Deliv Rev. 2009;61:527–541. doi: 10.1016/j.addr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman B, Perez OA., and , Zell D. Update on rosacea and anti-inflammatory-dose doxycycline. Drugs Today. 2007;43:27–34. doi: 10.1358/dot.2007.43.1.1025697. [DOI] [PubMed] [Google Scholar]
- Tatarewicz SM, Wei X, Gupta S, Masterman D, Swanson SJ., and , Moxness MS. Development of a maturing T-cell-mediated immune response in patients with idiopathic Parkinson's disease receiving r-metHuGDNF via continuous intraputaminal infusion. J Clin Immunol. 2007;27:620–627. doi: 10.1007/s10875-007-9117-8. [DOI] [PubMed] [Google Scholar]
- Peden CS, Manfredsson FP, Reimsnider SK, Poirier AE, Burger C, Muzyczka N, et al. Striatal readministration of rAAV vectors reveals an immune response against AAV2 capsids that can be circumvented. Mol Ther. 2009;17:524–537. doi: 10.1038/mt.2008.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Reimsnider S, Manfredsson FP, Muzyczka N., and , Mandel RJ. Time course of transgene expression after intrastriatal pseudotyped rAAV2/1, rAAV2/2, rAAV2/5, and rAAV2/8 transduction in the rat. Mol Ther. 2007;15:1504–1511. doi: 10.1038/sj.mt.6300227. [DOI] [PubMed] [Google Scholar]
- Burger C, Nguyen FN, Deng J., and , Mandel RJ. Systemic mannitol-induced hyperosmolality amplifies rAAV2-mediated striatal transduction to a greater extent than local co-infusion. Mol Ther. 2005;11:327–331. doi: 10.1016/j.ymthe.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Manfredsson FP, Burger C, Sullivan LF, Muzyczka N, Lewin AS., and , Mandel RJ. rAAV-mediated nigral human parkin over-expression partially ameliorates motor deficits via enhanced dopamine neurotransmission in a rat model of Parkinson's disease. Exp Neurol. 2007;207:289–301. doi: 10.1016/j.expneurol.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Liu Y, Okada T, Shimazaki K, Sheykholeslami K, Nomoto T, Muramatsu S, et al. Protection against aminoglycoside-induced ototoxicity by regulated AAV vector-mediated GDNF gene transfer into the cochlea. Mol Ther. 2008;16:474–480. doi: 10.1038/sj.mt.6300379. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ, et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–167. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]
- Peden CS, Burger C, Muzyczka N., and , Mandel RJ. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J Virol. 2004;78:6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tümer N, Scarpace PJ, Dogan MD, Broxson CS, Matheny M, Yurek DM, et al. Hypothalamic rAAV-mediated GDNF gene delivery ameliorates age-related obesity. Neurobiol Aging. 2006;27:459–470. doi: 10.1016/j.neurobiolaging.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. Belmont, CA: Brooks/Cole; 1968. [Google Scholar]
- Ruz N, Zabala M, Kramer MG, Campanero MA, Dios-Viéitez MC., and , Blanco-Príeto MJ. Rapid and simple determination of doxycycline in serum by high-performance liquid chromatography. Application to particulate drug delivery systems. J Chromatogr A. 2004;1031:295–301. doi: 10.1016/j.chroma.2003.12.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Early vector designs. Prior to the construction of the single vector tet-off construct used in the main study several permutations of tet-on vectors were tested (a-b). The dual vector system (a) consisted of a vector containing the rtTA2 reverse transactivator and the tTS trans-silencer under the control of the constitutive CBA promoter separated by an internal ribosome entry site. The second vector consisted of the GDNF transgene behind the same tet responsive promoter used in the main study. (c). During the initial injection paradigm the vectors were injected at a 1:1 ratio. However, inducibility was remarkably low yielding only a roughly two-fold increase over background levels. Furthermore, injection of the tet-GDNF alone resulted in significantly higher levels of GDNF indicating a basal promoter activity, perhaps due to an endogenous promoter function of the ITRs which is actively repressed when tTS is present (*, p < 0.05 vs. white bar vs. black bar). Consequently, increasing the ratio of the rtTA/tTS vector to 2:1 resulted in background GDNF levels comparable to that of control (c, right portion). (d). A dose-response evaluation of the double vector injected at a 2:1 ratio indicated maximum inducibility at 400 mg/kg DOX (administered by time-release subcutaneous pellets; *, p < 0.05 vs. uninjected control, dagger, p <0.05 vs. all other DOX doses). (e). Basal expression (no DOX) of GDNF in striatum injected with tet-GDNF only. (f) Basal GDNF protein expression (no DOX) after addition of the rtTA/tTS vector in a 2:1 ratio that yielded background GDNF protein measurements. This demonstrates that even when ELISA measurements of striatal GDNF indicate background levels, there is still leaky expression from this regulated vector combination. Furthermore, the bi-directional construct shown in (b) displayed no inducibility and GDNF levels in the absence of DOX were similar to that seen with expression of GDNF shown in (e, data not shown). In addition, replacing the AAV2 ITRs with AAV5 ITRs only reduced background expression levels slightly (data not shown).