Abstract
Arginine-rich cell-penetrating peptides (CPPs), including human immunodeficiency virus type 1 (HIV-1) Tat (48–60) and oligoarginines, have been applied as carriers for delivery of cargo molecules, because of their capacity to internalize into cells and penetrate biological membranes. Despite the fact that they have been extensively studied, the factors required for the efficient internalization of CPPs are still unclear. In this report, we evaluated the internalization efficiencies of seven CPPs derived from DNA/RNA-binding peptides, and discovered that a peptide derived from the flock house virus (FHV) coat protein was internalized most efficiently into Chinese hamster ovary (CHO-K1), HeLa, and Jurkat cells. Comparison of the factors facilitating the internalization with those of the Tat peptide revealed that the FHV peptide induces macropinocytosis much more efficiently than the Tat peptide, which leads to its high cellular uptake efficiency. Additionally, the strong adsorption of the FHV peptide on cell membranes via glycosaminoglycans (GAGs) was shown to be a key factor for induction of macropinocytosis, and these steps were successfully monitored by live imaging of the peptide internalization into cells in relation to the actin organization. The remarkable methods of FHV peptide internalization thus highlighted the critical factors for internalizations of the arginine-rich CPPs.
Introduction
Significant progress in understanding the molecular basis of disease processes by technological advances in genomics and proteomics research has opened up the possibility of using many different proteins and genes for defined therapeutic functions.1 However, the difficulty in gaining access for these molecules to the cell interior through the plasma membrane often limits their applicability as pharmaceutical agents. For overcoming such limitations, methodologies designed to enhance intracellular delivery using cell-penetrating peptides (CPPs) have been increasingly investigated (for review, see ref. 2). Arginine-rich CPPs, such as human immunodeficiency virus type 1 (HIV-1) Tat (48–60)3 and oligoarginine peptides,4,5 have been shown to efficiently internalize into various types of cells without any significant cytotoxicity and also to deliver membrane impermeable cargo such as bioactive proteins, peptides, nucleic acids, magnetic beads, and quantum dots.6
In recent studies investigating CPP internalization, macropinocytosis defined as actin organization and plasma membrane ruffling leading to the subsequent engulfment of large volumes of extracellular fluid7,8,9 has been shown to be important for entry into cells.10,11,12,13 Membrane-associated proteoglycans, including heparan sulfate (HS), have also been shown to be crucial for internalizations13,14,15,16 suggesting the attachment of arginine-rich CPPs with negatively charged glycosaminoglycans (GAGs) on plasma membranes via electrostatic interactions is an initial step for internalization of arginine-rich CPPs.13,15,17 In support of this, we and others have shown that the activation of the small guanosine triphosphatase Rac1 and organization of the actin (leading to lamellipodia formations)9,18 is induced by the arginine-rich CPPs and this triggers macropinocytosis or a macropinocytosis-like pathway.13,19 These results suggest the possibility that the interaction of the arginine-rich CPPs with these proteoglycans induces the intracellular signals that lead to actin organization and macropinocytosis. However, the factors required for the efficient internalization of the CPPs are still unclear.
We previously reported that many DNA/RNA-binding arginine-rich peptides efficiently internalize into cells similarly to the Tat peptide, and these included HIV-1 Rev (34–50) and flock house virus (FHV) coat (35–49) peptide.5 There, the internalized peptides were quantified using cell lysates obtained by the addition of a surfactant to the peptide-treated cells after phosphate-buffered saline (PBS) washing, and the result suggested a higher internalization efficiency of the Tat peptide compared with the FHV peptide. However, we and others later realized that these arginine-rich peptides often yield high extent of cell-surface adsorption and nonspecific binding to culture dishes and quartz cuvettes used for quantification (e.g., 14, 16), and quantification of internalized peptides can be affected by these factors.
In this report, we have re-evaluated the internalization efficiencies of these arginine-rich DNA/RNA-binding peptides. To avoid artifacts due to peptide binding, we carefully analyzed the cellular uptake of the peptides using fluorescence-activated cell sorting (FACS) so as to minimize these effects. We find that the FHV peptide internalizes with the highest efficiency among the examined peptides. Detailed analysis of the internalization methods of the FHV peptide revealed that this is mediated by relatively high cell-surface adsorption leading to enhanced macropinocytic uptake and cytosolic distribution of the peptide compared with that of the Tat peptide. In addition, a strong interaction of the peptide with membrane-associated proteoglycans is crucial for the eventual induction of macropinocytosis as well as cytosolic distribution of the peptide.
Results
Efficiency in cellular uptake of arginine-rich CPPs
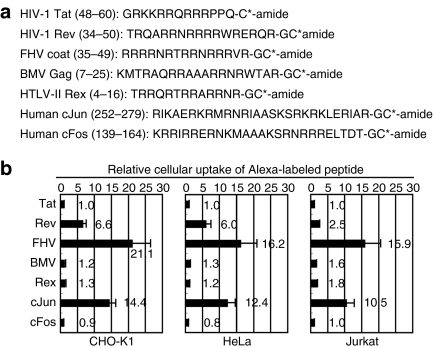
We previously reported that many of DNA/RNA-binding arginine-rich peptides5 efficiently internalize into cells, and that the number of arginine residues is important for their internalizations.5 In this study, FACS-based quantification of the cellular uptake of these arginine-rich peptides was conducted using Chinese hamster ovary (CHO-K1), human cervical cancer (HeLa), and human T-cell leukemia (Jurkat) cells (Figure 1). Figure 1a shows the amino acid sequences of the peptides examined. Each peptide has 6–11 arginine residues, and some of them also contain lysine residues. The cells were treated with these Alexa488-labeled arginine-rich peptides (5 µmol/l) for 30 minutes at 37 °C in serum-containing medium, followed by trypsinization and FACS analysis as stated in the Materials and Methods section. The data show that FHV peptide showed a 21 times higher uptake by the CHO-K1 cells than that of the HIV-1 Tat (48–60) peptide which has been widely used for intracellular delivery2 (Figure 1b). The superior cellular uptake of the FHV peptide was also confirmed (~16 times more than that of the Tat peptide) in HeLa and Jurkat cells.
Figure 1.
Examples of arginine-rich peptides and the cellular uptake efficiency. (a) Amino acid sequences of arginine-rich peptides derived from DNA/RNA-binding proteins. The side chain of the C-terminal cysteine (C*) was fluorescently labeled. (b) Relative cellular uptake of arginine-rich peptides by CHO-K1, HeLa, and Jurkat cells. These cells were treated with each peptide (5 µmol/l) for 30 minutes at 37 °C, and then FACS analyses were conducted as stated in the Materials and Methods section. The amount of the respective peptides taken up by the cells was normalized to the result of Tat peptide as a standard. Data represent the average (±SD) of three experiments. CHO, Chinese hamster ovary; FACS, fluorescence-activated cell sorting; FHV, flock house virus.
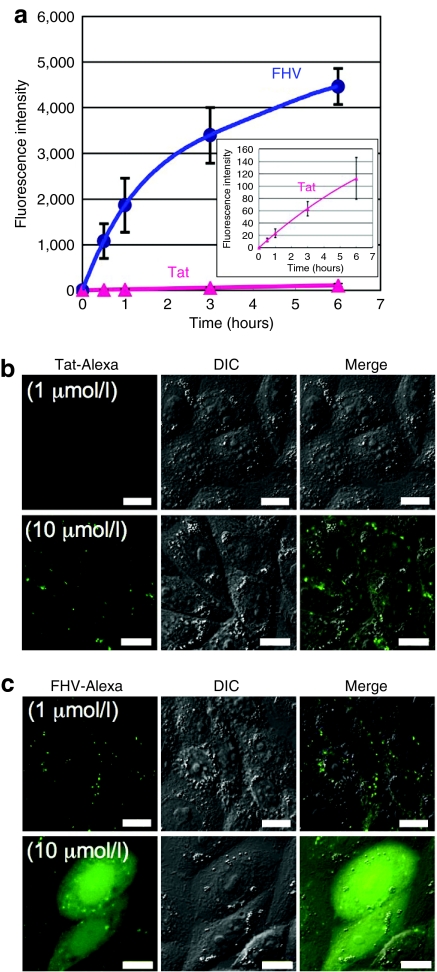
Detailed comparative analysis of cellular uptake was then conducted between the FHV peptide and the Tat peptide. Figure 2a shows the results of the time-course study on the cellular uptake of these peptides. CHO-K1 cells were incubated with Alexa488-labeled FHV (FHV-Alexa) or Tat (Tat-Alexa) peptides (1 µmol/l) for 0–6 hours at 37 °C, followed by FACS analysis. A rapid increase in the cellular uptake of the FHV peptide was observed in the first 1 hour, and the amount of the intracellular FHV peptide kept increasing for 6 hours without showing any saturation (Figure 2a). On the other hand, the cellular uptake of the Tat peptide was considerably lower than that of the FHV peptide (Figure 2a). The FHV peptide internalized ~80 times and 40 times more than that of the Tat peptide after 1 hour and 6 hours treatments of each peptide, respectively.
Figure 2.
Superior cellular uptake of the FHV peptide to the Tat peptide. (a) Time course of cellular uptake of Alexa488-labeled FHV (blue) or Tat (red) peptides (1 µmol/l) by CHO-K1 cells as analyzed by FACS. Data represent the average (±SD) of three experiments. Confocal microscopic observation of CHO-K1 cells treated with (b) Alexa488-labeled Tat or (c) FHV peptide (1 or 10 µmol/l) for 10 minutes at 37 °C. Bar = 10 µm. FACS, fluorescence-activated cell sorting; FHV, flock house virus.
Intracellular localization of the FHV-Alexa or Tat-Alexa peptide in the CHO-K1 cells at 10 minutes after the peptide treatment in the presence of 10% heat-inactivated fetal bovine serum was analyzed using a confocal laser scanning microscope (Olympus, Tokyo, Japan) (Figure 2b,c). For the FHV peptide, endosome-like punctate signals were observed in cells incubated with 1 µmol/l peptide. However, diffuse fluorescent signals in the cytosol and nucleus were obtained with the 10 µmol/l peptide (Figure 2c). On the other hand, only a punctate signal was observed even in the 10 µmol/l Tat-treated cells (Figure 2b). The above results suggest that the FHV peptide has a higher ability to translocate into cytosol. Co-incubation of cells with this peptide and propidium iodide, a membrane impermeable probe, yielded no significant labeling of cells with propidium iodide (data not shown), suggesting integrity of the plasma membrane during these experiments.
We previously reported that dodecaarginine (R12) showed a similar concentration-dependent cytosolic distribution. The peptide labels endocytic structures at relatively low concentrations, but upon raising the peptide concentration to exceed a certain threshold, the fraction labeling the cytosol increases dramatically.20 Enhanced cytosolic accumulation of R12 seemed to be accomplished via direct penetration of the peptide through plasma membranes, together with endosomal escape of the peptide. Time-course imaging of the cells revealed that the initial translocation of the R12 peptide did not occur throughout the cell but at defined locations, and this labeling then diffused through the cytosol and nucleus. Similar observation was reported by others using R9 peptide.21 We here observed a similar method of cytosolic labeling initiating from specific cell locations for the FHV peptide (Supplementary Figure S1). Therefore, at 1 µmol/l, endocytosis including macropinocytosis would be dominant pathways for the cellular uptake of the FHV peptide whereas a pathway involving direct translocation through plasma membrane to achieve effective cytosolic distribution of the peptide could be activated together with endocytic pathways at 10 µmol/l.
There was a significant suppression of cytosolic distribution of the R12 peptide when it was incubated with cells in the presence of serum.20 If the FHV peptide has less of an affinity for serum proteins compared with Tat, this could be a reason for the superiority of the former peptide in translocating into cytosol. To explore this possibility, comparative binding of FHV and Tat peptides to serum proteins was evaluated using ultrafiltration as previously reported.20 FHV- and Tat-Alexa (10 µmol/l each) were incubated in a serum (10%)-containing or a serum-free medium for 5 minutes at 37 °C and ultrafiltered (molecular weight cutoff: 30,000). The serum binding of the peptides were assessed by the amount of the peptides in the filtrates (unbound peptides) and unfiltered samples (serum protein-bound peptides). The FHV peptide bound to serum protein much more strongly (81% of total FHV peptide) as compared with the Tat peptide (51% of total Tat peptide). To explain this it could be reasoned that, despite the fact that the FHV peptide shows a higher affinity to serum proteins, if it has an even higher affinity for molecules on cell surfaces, then there would be a net accumulation of the peptide on the cell surface followed by internalization. As discussed in Figure 4, membrane-associated GAGs can be listed among cell-surface molecules that show high affinity for FHV peptide.
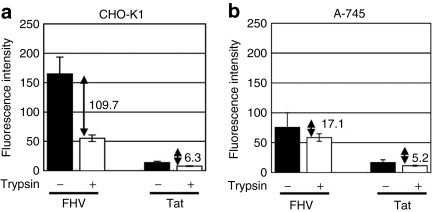
Figure 4.
High extent of accumulation of the FHV peptide on cell surface. Peptide binding assay of FHV-Alexa or Tat-Alexa (1 µmol/l) on (a) CHO-K1 or (b) A-745 cells conducted as stated in the Materials and Methods section. Data represent the average (±SD) of three experiments. Differences in fluorescence intensity between with or without trypsin treatment are shown. CHO, Chinese hamster ovary; FHV, flock house virus.
We assume that the stronger interaction of the FHV peptide to the cell surface lead to an enhanced ability to penetrate into the cytosol. In fact, diffuse signals of Tat-Alexa in the cytosol and nucleus was observed when the cells were treated with Tat-Alexa (20 µmol/l) in the absence of serum (Supplementary Figure S2) or by incubating with a higher concentration of peptide (50 µmol/l) even in the presence of serum (Supplementary Figure S2). The longer incubation (~ 6 hours) of CHO-K1 cells with the Tat-Alexa (10 µmol/l) in serum-containing medium only yielded endosome-like punctate signals. Two possibilities of cytosolic accumulation of the arginine-rich peptides, namely through endocytosis and endosomal escape, and via direct translocation through plasma membranes, can be considered.20,22 However, further studies are necessary to precisely discriminate the contribution of these factors to the extent of cytosolic labeling.
Efficient internalization of FHV peptide by macropinocytosis
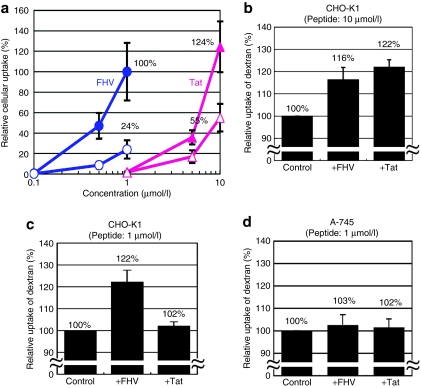
It has been reported that the induction of macropinocytosis is crucial for cellular uptake of the arginine-rich CPPs.10,11,12,13 For assessing the significance of this pathway during internalization of the FHV peptide, a macropinocytosis inhibitor, 5-(N-ethyl-N-isopropyl)amirolide (EIPA),7,23 was used for the evaluation of the CPP internalizations. CHO-K1 cells were incubated with different concentrations of the FHV-Alexa or Tat-Alexa peptide (0.1–10 µmol/l) for 30 minutes at 37 °C. Based on a FACS analysis, there was a one order of difference in both peptides for attaining the same uptake amount (Figure 3a). Almost the same amount of peptides was internalized by the treatment of the 1 µmol/l FHV peptide with the 10 µmol/l Tat peptide (Figure 3a). EIPA caused a significant suppression in the cellular uptake of 1 µmol/l FHV peptide as well as 10 µmol/l Tat.
Figure 3.
Potent macropinocytosis induction by the FHV peptide and the importance of membrane-associated proteoglycans. (a) Comparison of cellular uptake in different concentrations of FHV-Alexa or Tat-Alexa peptides with or without the pretreatment of the macropinocytosis inhibitor, EIPA. CHO-K1 cells were treated with 0.1–10 µmol/l peptides for 30 minutes, and then FACS analyses were conducted. Solid circle, FHV-Alexa; open circle, FHV-Alexa with pretreatment of EIPA; solid triangle, Tat-Alexa; open triangle, Tat-Alexa with pretreatment of EIPA. (b–d) Cellular uptake of FITC-labeled dextran (70 kd) in the presence of FHV or Tat peptides. CHO-K1 cells in the presence of each (b) 10 µmol/l or (c) 1 µmol/l peptide. (d) A-745 cells in the presence of each 1 µmol/l peptide. Cells treated with FITC-dextran in the absence of peptides were taken as controls. The amount of the respective peptides (a) or FITC-dextran (b–d) taken up by the cells was normalized to the results of FHV-Alexa (a) or controls (b–d). The relative cellular uptake of FITC-dextran in the absence of peptides in A-745 cells was 104% of the case of CHO-K1 cells by FACS analysis. Data represent the average (±SD) of three experiments. CHO, Chinese hamster ovary; EIPA, 5-(N-ethyl-N-isopropyl)amirolide; FACS, fluorescence-activated cell sorting; FHV, flock house virus; FITC, fluorescein-isothiocyanate.
To confirm the FHV peptide can induce macropinocytosis at 1 µmol/l, we examined the cellular uptake of dextran (70 kd), whose endocytosis is increased in cells undergoing macropinocytosis10,24 (Figure 3b–d). Figure 3b,c shows the cellular uptake (CHO-K1) of the fluorescein-isothiocyanate (FITC)-labeled dextran (FITC-dextran) in the presence of the FHV or Tat peptide (10 or 1 µmol/l). A significant increase (16 and 22%) was observed in the cellular uptake of FITC-dextran by treatment with the respective 10 µmol/l peptides (Figure 3b). However, in contrast to the fact that the FHV peptide evoked a 22% increase in the cellular uptake of the FITC-dextran at 1 µmol/l, no significant increase of the FITC-dextran uptake was observed by the Tat (1 µmol/l) treatment (Figure 3c). We also assessed the cellular uptake of large sized FITC-dextran (250 kd), because larger probes preferentially label macropinosome for distinction from pinocytosis using the small sized endosomes.25 In the case of the FITC-dextran (250 kd), coadministration of the FHV peptide (1 µmol/l) also enhanced the uptake of the FITC-dextran (10% increase), whereas the Tat peptide (1 µmol/l) did not enhance the uptake (data not shown). In GAG deficient A-745 cells, both peptides failed to enhance the cellular uptake of the FITC-dextran (Figure 3d). The importance of GAGs for internalization of the arginine-rich peptides has been reported.13,14,15,16 Cellular uptake of the FHV-Alexa peptide by all the GAG deficient A-745 cells was 63% lower than that by the wild-type CHO-K1 cells based on the FACS analysis, when the cells were treated with the FHV-Alexa (1 µmol/l) for 30 minutes at 37 °C (data not shown). These results show that the FHV peptide more efficiently induces macropinocytosis when compared to the Tat peptide, and that the GAGs are also important for the macropinocytosis induction by the FHV peptide.
High affinity of FHV peptide to membrane-associated proteoglycans that leads to high efficiency of internalization
The differences in the affinities of cell-surface adsorption might explain the differences in the potency of macropinocytosis induction by these peptides. We thus evaluated the difference in adsorption of the FHV or Tat peptide on the cell surface (Figure 4a,b). Wild-type CHO-K1 or GAG-deficient A-745 cells were detached by treatment with ethylenediaminetetraacetic acid, so as to retain the membrane-associated proteins. These cells were then treated with the FHV-Alexa or Tat-Alexa peptide (1 µmol/l) for 10 minutes at 4 °C, so that endocytosis, including macropinocytosis, is inhibited. The cells were then treated with trypsin to remove the cell-surface adsorbed peptides (see Materials and Methods section). The differences in the fluorescent intensity by the trypsinized and nontrypsinized cells correlate to the accumulation of each peptide on the cell surface. The difference in the fluorescence intensity in the trypsinized and nontrypsinized cells was 18 times greater in the case of the FHV-treated cells than the Tat-treated cells, suggesting the more abundant adsorption of the FHV peptide (Figure 4a). However, in the case of the GAG-deficient A-745 cells, the effects of trypsinization was much smaller (Figure 4b), showing that the amount of peptide adsorption on the cell surface was lower compared with wild-type CHO-K1 cells. These results suggest that the FHV peptide can more strongly attach to the cell membrane via GAGs than the Tat peptide, and this may be one of the reasons for the effective induction of macropinocytosis and internalization of the FHV peptide.
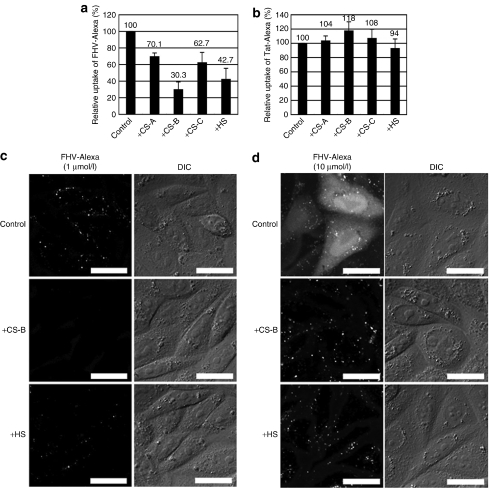
We also explored the internalization of the FHV or Tat peptide in the presence of sulfated polysaccharides (i.e., GAGs) for assessing the interaction with peptides and effects on the peptide internalization as previously reported14,26 (Figure 5). These arginine-rich peptides were mixed with 100 µg/ml GAGs [chondroitin sulfate (CS)-A, CS-B, CS-C, HS] prior to the treatment of the CHO-K1 cells. Figure 5a,b shows the cellular uptake of FHV-Alexa (1 µmol/l) and Tat-Alexa (1 µmol/l), respectively, in the presence of GAGs as analyzed by FACS. These results show that sulfated polysaccharides had a significant effect on the internalization of the FHV peptide (30–70% decrease) (Figure 5a). There was only a negligible effect by the Tat peptide under these experimental conditions (Figure 5b) and the slight differences in the amount of cell surface adsorbed Tat on the CHO-K1 and A-745 cells may be similarly explained. Differences in the sites of the sulfation define the class of the CSs and difference in the density of the sulfate on the sugar may produce the difference in affinities of the FHV peptides for the carbohydrates. Two hydroxyl moieties per one disaccharide unit are sulfated in CS-B whereas only one in CS-A and CS-C. Confocal microscopic observations showed a reduced cellular uptake of the FHV peptide (1 µmol/l) in the presence of the GAGs (Figure 5c). In addition, the strong inhibition of cellular uptake of the FHV peptide by addition of GAGs in the serum-containing medium may also imply a higher affinity of the peptide to GAGs compared with serum proteins. This would not contradict the notion that despite the presence of serum, the FHV peptide may more efficiently accumulate on the cell surface by interaction with GAGs than the Tat peptide and thus attain a higher efficiency of internalization.
Figure 5.
Reduced cellular uptake of the FHV peptide in the presence of glycosaminoglycans. Internalization of (a) FHV-Alexa or (b) Tat-Alexa (each 1 µmol/l) in the presence of glycosaminoglycans (100 µg/ml) (CS-A, CS-B, CS-C, HS) into CHO-K1 cells, analyzed by FACS. Cells treated in the absence of the peptides were taken as controls. The amount of the respective peptides taken up by the cells was normalized to the results of controls as standards. (c,d) Confocal microscopic observation of CHO-K1 cells treated with FHV-Alexa peptide [(c) 1 µmol/l or (d) 10 µmol/l] in the presence of CS-B or HS (100 µg/ml). (a–d) The cells were treated with each Alexa-labeled peptide for 10 minutes at 37 °C before the FACS or confocal microscopic analysis. (a,b) Data represent the average (±SD) of three experiments. Bar = 10 µm. CHO, Chinese hamster ovary; CS, chondroitin sulfate; FHV, flock house virus; HS, heparan sulfate.
More significant differences in the cellular localization of the FHV peptide were observed when the peptide concentration was increased to 10 µmol/l. As shown in Supplementary Figure S1, a pathway to induce direct internalization of the FHV peptide through plasma membranes into cytosol seemed to be activated at this peptide concentration. The addition of GAGs would diminish the concentration of the peptide on the cell surface thus reducing the amount that penetrated the bilayer to label the cytosol (Figure 5d). This result was well in accordance with our recent finding that cytosolic diffusion of the arginine-rich peptide is highly dependent on their effective concentration on the cell surface.20 Significant decrease in the cytosolic signals of the FHV peptide was also observed by the pretreatment of the cells with macropinocytosis inhibitors (Supplementary Figure S3). Cytochalasin D (depolymerization of actin cytoskeleton),10,27 EIPA, and nocodazole (depolymerization of microtubules)28,29 were employed as macropinocytosis inhibitors. Pretreatment with these agents inhibited the internalization of FHV-Alexa by ~25–35% (Supplementary Figure S3a). Confocal microscopic observations also showed significant reduction in diffuse cytosolic labeling of the FHV-Alexa peptide in cells treated with these drugs (Supplementary Figure S3b). The above results suggest that certain fraction of the FHV peptide may reach cytosol via endosomal escape after uptake by macropinocytosis when treated with cells at 10 µmol/l, suggesting that accumulation of the sufficient concentration of the arginine-rich peptides even on endosomal membranes may accelerate the translocation through the endosomal membranes. Considering that cytosolic diffusion was analyzed 10 minutes after the peptide treatment in Supplementary Figure S3b, release from macropinosomes may be quite rapid.
Visualization of processes for FHV peptide adsorption on cell surface leading to actin organization process
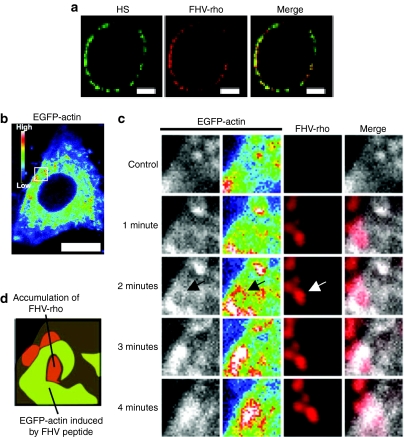
Interaction of the FHV peptide with membrane-associated proteoglycans leads to actin reorganization and macropinocytosis, and massive accumulation of the rhodamine-labeled FHV peptide signal with that from the HS-specific antibody on cell membranes of the CHO-K1 cells was observed using confocal microscopy (Figure 6a). To analyze the methods of actin organization by the peptide, a live-cell confocal microscopic analysis was conducted using the CHO-K1 cells, expressing a fused protein of actin with the enhanced green fluorescent protein (EGFP) in the presence of the rhodamine-labeled FHV peptide (FHV-rho) (1 µmol/l) (Figure 6b–d). After incubation for 1 minute, accumulations of rhodamine signals on the cell surface appeared. However, no significant actin organization was observed in this period. At 2 minutes, high intensity signals of the EGFP-actin were observed around the accumulating points of the rhodamine signals. At 3–4 minutes, intense signals of the EGFP-actin overlapped on the rhodamine signals, leading influx of the rhodamine signals into the cell. Thus, by the live-cell imaging during the process of peptide adsorption on the cell membrane, it was strongly suggested that the accumulation of the FHV peptide via the GAGs on the cell membrane led to the induction of the actin rearrangement to induce macropinocytosis.
Figure 6.
Massive accumulation of the FHV peptide on cell surface leads actin rearrangement. (a) Confocal microscopic observation of CHO-K1 cells treated with anti-HS antibody and rhodamine-labeled FHV peptide (FHV-rho) (1 µmol/l) at 4 °C (green, HS; red, FHV peptide). Bar = 5 µm. (b,c) Confocal microscopic observations of EGFP-actin expressed CHO-K1 cells treated with FHV-rho (1 µmol/l) at 37 °C under 5% CO2. Nontreatment (control) (b,c), and ~4 minutes treatment (c) with the peptide. Intensity of EGFP-actin is represented by dark blue (low) and white (high). Bar = 10 µm. Arrow shows the high intensity of EGFP-actin signals around the massive adsorption of FHV-rho. (d) Schematic illustration of 2 minutes treatment in c. Green, high levels of EGFP-actin; red, accumulation of FHV-rho. EGFP, enhanced green fluorescent protein; FHV, flock house virus; HS, heparan sulfate.
Also, the actin rearrangement by the treatment of the FHV peptide was examined in the GAG-deficient A-745 cells (Supplementary Figure S4). CHO-K1 and A-745 cells were treated with the FHV or Tat peptide (10 µmol/l) for 2.5 minutes at 37 °C, then fixed with 4% paraformaldehyde and stained with phalloidin-TRITC for visualizing the actin cytoskeleton (Supplementary Figure S4). In CHO-K1 cells, the FHV peptide induced rearrangement of actin, but no significant effect on the actin structure was observed by similarly treating A-745 cells with the FHV and Tat peptide. These results strongly suggest that the accumulation of the FHV peptide via membrane-associated proteoglycans is essential for the induction of actin rearrangement.
Discussion
In this study, we show very efficient internalization, in a number of cell types, of the FHV peptide compared to other DNA/RNA-binding arginine-rich peptides. Kameyama et al. and Sugita et al. have already reported that the transduction efficacy was Rev > Tat peptide in various types of cells (HeLa, HaCaT, A431, Jurkat, MOLT-4, HL60) and these peptides internalized into the cells by a macropinocytosis pathway.30,31 We now confirm by FACS analysis that the Rev (34–50) peptide internalized into CHO-K1, HeLa, and Jurkat cells about 2.5–6.6 times more than the Tat (48–60) peptide, and furthermore, the FHV peptide internalized 2.7–6.4 times more than the Rev peptide. The FHV peptide more efficiently induces macropinocytosis compared with the Tat peptide (Figure 3), and this efficient macropinocytosis induction leads to its high internalization into cells.
The FHV peptide adsorbed on the CHO-K1 cell membrane about 17 times higher than that of the Tat peptide (Figure 4a), and HS proteoglycans play a crucial role in the accumulation of the peptide on the plasma membrane (Figures 4 and 6a). In addition to this, internalization of the FHV peptide was suppressed in the presence of GAGs in cell culture medium, because the side chains of the arginine residues in the FHV peptide amino acid sequence interacted with the GAGs in the cell culture medium before the adsorption of the peptides on the cell surface, which leads to its poor internalization into cells as previously reported,14,26 whereas there was almost no effect in the case of the Tat peptide under this experimental condition (Figure 5). Most notably, internalization of the FHV peptide was suppressed in the presence of CS-B, which has two sulfate groups in a unit [IdoUA(2S)α1-3GalNAc(4S)] and is considered to strongly interact with the FHV peptide via hydrogen bonds between the arginines and sulfates. This result suggests that the FHV peptide more strongly interacts with the GAGs than that of the Tat peptide, and the higher accumulation of the FHV peptide on the cell membrane may efficiently induce signaling pathways for induction of macropinocytosis.
We have reported that the number of arginine residues in cationic peptides play crucial roles in mediating the internalization method and efficacy.5,20 Undoubtedly this would be one predominant factor in explaining the superior internalization efficiency of FHV peptide (containing 11 arginines) over the Tat peptide (seven arginines). Actually, their modes of internalization may be reminiscent to those of the R12 and R8 peptides, respectively. However, the Rev (34–50) peptide has 10 residues (close to 11) but is less efficient in internalization compared with FHV peptide (Figure 1). The cJun and cFos peptides have similar numbers of arginines but show considerably different internalization efficiency. Therefore, further studies are needed to clarify the effect of factors other than the numbers of arginines on the internalization efficiency of the arginine-rich peptides.
Invasion of host cells by some viruses is also through macropinocytosis.32,33 Macropinocytosis stimulation of the adenovirus requires contact of the virus with cell-surface receptors, including the αv integrin subunits,23,34 and HS proteoglycans play a crucial role in the accumulation of viruses on the cell surface.35 Additionally, cytoplasmic domains of proteoglycans have been shown to interact with the actin cytoskeleton,36,37,38 and the possibility has been suggested that intracellular domains of proteoglycans play a crucial role in the bacterial internalization (phagocytosis) by mediating signal transduction or anchorage to the cytoskeleton.39,40,41,42 The importance of membrane-associated proteoglycans has also been noted for internalization of the HIV-1 Tat protein and arginine-rich peptides,13,14,15,16,26 and interaction of the arginine-rich CPPs with proteoglycans is a requirement for induction of macropinocytosis.13 However, it is still unclear whether the signal transduction of macropinocytosis by arginine-rich CPPs starts directly from accumulation of peptides via membrane-associated proteoglycans or other receptors that are linked to signaling pathways and aid in the uptake of growth factors and viruses.
In Figure 6, accumulation of the FHV peptide via HS proteoglycans resulting in induction of actin organization was confirmed by live-cell imaging. Ziegler and Seelig reported that arginine-rich CPPs formed particles with GAGs having radius of >70 nm, suggesting that these clusters consisted of several GAG chains aggregating with the peptides rather than a single GAG chain saturated with the maximum number of ligands.43 Additionally, clustering of the proteoglycan (syndecan-4) has been shown to initiate signaling cascades that lead to PKCα and Rac1 activation that result in actin organizations.44,45,46,47 Thus it is likely that strong adsorption and extensive accumulation of the FHV peptide to membrane-associated proteoglycans leads to their clustering that then induces the necessary signaling for actin reorganization and macropinocytosis.
When the CHO-K1 cells were treated with the FHV-Alexa (10 µmol/l) in serum-containing medium, rapid diffusion of the fluorescence from specific parts of the cells into the cytosol and nucleus was observed in a short time period (10 minutes) (Figure 2c and Supplementary Figure S1) and this suggested that the pathways of direct internalization of the FHV peptide through plasma membranes into cytosol are activated at this concentration. This cytosolic diffusion of the FHV-Alexa peptide was suppressed to a certain extent by treatment with macropinocytosis inhibitors (Supplementary Figure S3b). These results suggest the possibility that a certain fraction of cytosolic labeling may be via endosomal escape at relatively early stage of the macropinocytosis. However, at these high concentrations of 10 µmol/l it is still difficult to clearly dissect the contribution endosomal escape and direct translocation through plasma membranes to cytosolic labeling. At lower FHV concentration, or with peptides having lower affinity to cell surfaces, detailed methods of endosomal escape after their macropinocytic uptake still remain ambiguous.
In summary, the efficient cellular uptake of the FHV peptide are thus accomplished by (i) its strong adsorption on cell membranes via interaction with proteoglycans that lead (ii) local induction of actin organization and macropinocytosis. At the elevated peptide concentration, (iii) efficient translocation pathway through the plasma membranes becomes available. Thus, for the efficient induction of cellular uptake, a strategy of an inducible strong interaction of CPPs with cell surface molecules including GAGs should be a desired strategy. The methods of FHV peptide internalization thus highlighted the critical factors for internalizations of the arginine-rich CPPs including adsorption, macropinocytosis induction, and translocation through cell membranes.
Materials and Methods
Peptide synthesis and fluorescent label. All the peptides were chemically synthesized by 9-fluorenylmethyloxycarbonyl solid-phase peptide synthesis on a Rink amide resin as already described.48 The amino acid derivatives and Rink amide resin were purchased from the Peptide Institute (Osaka, Japan) and Shimadzu Biotech (Kyoto, Japan). Deprotection of the peptide and cleavage from the resin were conducted by treatment with a trifluoroacetic acid/ethanedithiol mixture (95:5) at room temperature for 3 hours. For preparation of the fluorescently labeled peptides, a cysteine residue or glycyl cysteine sequence was introduced at the C-terminus of each peptide and fluorescent labeling was conducted by treatment with 1.5 equivalent of Alexa 488 C5 maleimide sodium salt (Invitrogen, Eugene, OR) or tetramethylrhodamine-5-maleimide (Invitrogen) in a dimethyl formamide/methanol mixture (1:1) for 1.5 hours at room temperature, followed by reverse-phase high-performance liquid chromatography purification. The structures of the synthesized peptides were confirmed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry.
Cell culture. CHO cells [CHO-K1 cell lines, wild type; pgsA-745 (A-745) cell lines, all GAG deficient] were purchased from the American Type Culture Collection (Manassas, VA), and cultured in F-12 nutrient mixture (Ham's F-12) containing 10% heat-inactivated fetal bovine serum (Biological Industries, Kibbutz Beit Haemek, Israel). Human cervical cancer-derived HeLa cells and T-lymphocyte Jurkat cells were purchased from the Riken BRC Cell Bank (Ibaraki, Japan) and cultured in α-minimal essential medium containing 10% heat-inactivated bovine serum (Invitrogen) and in RPMI medium containing 10% heat-inactivated fetal bovine serum, respectively. Cells were grown on 100-mm dishes and incubated at 37 °C under 5% CO2.
Confocal microscopic observation. CHO-K1 cells (4.0 × 105 cells/well) were plated on 35-mm glass-bottomed dishes (Iwaki, Tokyo, Japan) and cultured in Ham's F-12 medium containing 10% heat-inactivated fetal bovine serum for 48 hours. After complete adhesion, the cell culture medium was removed, and then the cells were incubated at 37 °C in fresh medium (200 µl) containing the fluorescently labeled peptides. The cells were then washed with PBS (3×), and fresh medium not containing any peptides (1 ml/well) was added. Distribution of the fluorescently labeled peptides in the cells was analyzed using an FV300 or FV1000 confocal scanning laser microscope (Olympus) equipped with a ×60 objective without fixing the cells to avoid artifactual localization of the internalized peptides.11,49
For staining by the HS-specific antibody, the cells were incubated for 15 minutes at 4 °C in serum-free Ham's F-12 medium. The cells were then treated with the monoclonal HS (104 epitope) antibody (5 µg/ml) for 30 minutes at 4 °C. After the treatment, the cells were washed with serum-free Ham's F-12 medium, then treated with Alexa Fluor 488 goat anti-mouse immunoglobin M (4 µg/ml) for 30 minutes at 4 °C. The cells were washed again with serum-free Ham's F-12 medium, and then incubated with the rhodamine-labeled FHV peptide (1 µmol/l) for 10 minutes at 4 °C. Distributions of fluorescent signals were analyzed as already described.
For observation of the actin fused with the EGFP (EGFP-actin), the DNA fragment coding EGFP, which was flanked with AgeI and XhoI, was constructed using the standard PCR technique with pEGFP-N1 as the template, and cloned into the pDsRed-monomer-actin vector (Clonetech, Mountain View, CA) to obtain the plasmid (pEGFP-actin). CHO-K1 cells (4.0 × 105 cells/well) were then transfected with pEGFP-actin (800 ng) using Lipofectamine 2000 (Invitrogen) according to manufacturer instructions, followed by incubation for 24 hours prior to analysis.
Phalloidin-TRITC staining was conducted as previously described.11
Flow cytometry. CHO-K1, A-745 cells (both 2.0 × 105 cells/well), and HeLa cells (1.0 × 105 cells/well) were plated into a 24-well microplate (Iwaki) and cultured for 24 hours in Ham's F-12 containing 10% heat-inactivated fetal bovine serum (CHO-K1, A-745) or α-minimal essential medium containing 10% heat-inactivated bovine serum (HeLa). After complete adhesion, the cells were incubated at 37 °C with synthesized peptides dissolved in fresh serum-containing medium (200 µl) prior to washing with PBS. The cells were then treated with 0.01% trypsin at 37 °C for 10 minutes prior to the addition of PBS (200 µl), and centrifuged at 3,000 r.p.m. (800 g) for 3 minutes at 4 °C. After the supernatant was removed, the cells were washed with PBS (400 µl) and centrifuged at 3,000 r.p.m. for 3 minutes at 4 °C. After this washing cycle was repeated, the cells were suspended in PBS (400 µl) and subjected to fluorescence analysis on a FACScalibur (BD Biosciences, Franklin Lakes, NJ) flow cytometer (FACS) using 488-nm laser excitation and a 515–545-nm emission filter.
Cellular uptake of FITC-dextran. The CHO-K1 cells (2.0 × 105 cells/well) were cultured in a 24-well microplate at 37 °C for 24 hours in Ham's F-12 containing 10% heat-inactivated fetal bovine serum, the culture medium was replaced by serum-free medium and the cells were then cultured for 24 hours. After this period, the cells were treated with serum-free medium containing FITC-dextran (2 mg/ml) (70 kd, Invitrogen; 250 kd, Sigma, St Louis, MO) in the presence or absence of FHV or the Tat peptide (1 or 10 µmol/l) at 37 °C for 30 minutes. The intensity of the fluorescent signals in cells was then analyzed using FACS as stated earlier.
Analysis of cell-surface peptide adsorption. The CHO-K1 or A-745 cells (2.0 × 105 cells/well) were seeded into a 24-well plate and cultured for 24 hours in Ham's F-12 containing 10% heat-inactivated fetal bovine serum. After this period, the cells were washed twice with PBS (200 µl), and treated with ethylenediaminetetraacetic acid (2 mmol/l) for 10 minutes at 37 °C. The detached cells were centrifuged at 3,000 r.p.m. for 3 minutes at 4 °C. The supernatant was removed and Ham's F-12 medium containing the fluorescently labeled FHV or Tat peptide (1 µmol/l) and 10% serum were added. The cells were incubated for 10 minutes at 4 °C, centrifuged at 3,000 r.p.m. for 3 minutes at 4 °C. The supernatant was then removed, the cells were treated with 0.01% trypsin or PBS without trypsin for 10 minutes at 37 °C. The cells were centrifuged at 3,000 r.p.m. for 3 minutes at 4 °C, and the supernatant was exchanged with fresh PBS (400 µl) and subjected to analysis by a FACScalibur (BD Biosciences) flow cytometer using 488-nm laser excitation and a 515–545-nm emission filter.
Treatment with macropinocytosis inhibitors. For the confocal microscopic observations and FACS analysis, the cells were pretreated with macropinocytosis inhibitors (cytochalasin D, 5 µmol/l, 15 minutes; EIPA, 100 µmol/l, 30 minutes; nocodazole, 20 µmol/l, 20 minutes), then treated with each peptide prior to confocal microscopic observation and FACS analysis as previously described.
Interaction of arginine-rich peptides with serum proteins. To evaluate the amount of the peptides adsorbed to serum proteins, an ultrafiltration method was conducted.20 Briefly, Alexa488-labeled FHV or Tat peptide was diluted to 10 µmol/l in Ham's F-12 medium with or without 10% heat-inactivated fetal bovine serum, and incubated for 5 minutes at 25 °C. Each peptide solution was then subjected to ultrafiltration using Microcon YM-30 centrifugal filter (Millipore, Billerica, MA) (molecular weight cutoff 30,000) at 14,000 g for 12 minutes to separate the free peptide (unbound with serum) from the fraction adsorbed to serum proteins. Peptide concentration in each fraction was determined as previously reported.20
SUPPLEMENTARY MATERIALFigure S1. Time-course study of FHV-Alexa internalization into CHO-K1 cells.Figure S2. Effects of serum and peptide concentration on internalization of the Tat peptide into cells.Figure S3. Effects of macropinocytosis inhibitors on penetration of the FHV peptide into cells.Figure S4. Actin organization in CHO-K1 cells and its absence in A-745 cells following incubation with the FHV and Tat peptides.
Supplementary Material
Time-course study of FHV-Alexa internalization into CHO-K1 cells.
Effects of serum and peptide concentration on internalization of the Tat peptide into cells.
Effects of macropinocytosis inhibitors on penetration of the FHV peptide into cells.
Actin organization in CHO-K1 cells and its absence in A-745 cells following incubation with the FHV and Tat peptides.
REFERENCES
- Platis D., and , Labrou NE. Chemical and genetic engineering strategies to improve the potency of pharmaceutical proteins and enzymes. Curr Med Chem. 2008;15:1940–1955. doi: 10.2174/092986708785132924. [DOI] [PubMed] [Google Scholar]
- Futaki S. Membrane permeable peptide vectors: chemistry and functional design for the therapeutic applications. Adv Drug Deliv Rev. 2008;60:447. doi: 10.1016/j.addr.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Vivès E, Brodin P., and , Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- Rothbard JB, Garlington S, Lin Q, Kirschberg T, Kreider E, McGrane PL, et al. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat Med. 2000;6:1253–1257. doi: 10.1038/81359. [DOI] [PubMed] [Google Scholar]
- Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, Ueda K, et al. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem. 2001;276:5836–5840. doi: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- Futaki S. Oligoarginine vectors for intracellular delivery: design and cellular-uptake mechanisms. Biopolymers. 2006;84:241–249. doi: 10.1002/bip.20421. [DOI] [PubMed] [Google Scholar]
- Swanson JA., and , Watts C. Macropinocytosis. Trends Cell Biol. 1995;5:424–428. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]
- Jones AT. Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides. J Cell Mol Med. 2007;11:670–684. doi: 10.1111/j.1582-4934.2007.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JA. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol. 2008;9:639–649. doi: 10.1038/nrm2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadia JS, Stan RV., and , Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- Nakase I, Niwa M, Takeuchi T, Sonomura K, Kawabata N, Koike Y, et al. Cellular uptake of arginine-rich peptides: roles for macropinocytosis and actin rearrangement. Mol Ther. 2004;10:1011–1022. doi: 10.1016/j.ymthe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Kaplan IM, Wadia JS., and , Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J Control Release. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Nakase I, Tadokoro A, Kawabata N, Takeuchi T, Katoh H, Hiramoto K, et al. Interaction of arginine-rich peptides with membrane-associated proteoglycans is crucial for induction of actin organization and macropinocytosis. Biochemistry. 2007;46:492–501. doi: 10.1021/bi0612824. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Futaki S, Niwa M, Tanaka S, Ueda K., and , Sugiura Y. Possible existence of common internalization mechanisms among arginine-rich peptides. J Biol Chem. 2002;277:2437–2443. doi: 10.1074/jbc.M110017200. [DOI] [PubMed] [Google Scholar]
- Fuchs SM., and , Raines RT. Pathway for polyarginine entry into mammalian cells. Biochemistry. 2004;43:2438–2444. doi: 10.1021/bi035933x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JP, Melikov K, Brooks H, Prevot P, Lebleu B., and , Chernomordik LV. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J Biol Chem. 2005;280:15300–15306. doi: 10.1074/jbc.M401604200. [DOI] [PubMed] [Google Scholar]
- Ziegler A., and , Seelig J.2004Interaction of the protein transduction domain of HIV-1 TAT with heparan sulfate: binding mechanism and thermodynamic parameters Biophys J 861 Pt 1): 254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarius M, Lee CH., and , Anderson RA. Supervised membrane swimming: small G-protein lifeguards regulate PIPK signalling and monitor intracellular PtdIns(4,5)P2 pools. Biochem J. 2006;398:1–13. doi: 10.1042/BJ20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbal-Chaloin S, Gondeau C, Aldrian-Herrada G, Heitz F, Gauthier-Rouvière C., and , Divita G. First step of the cell-penetrating peptide mechanism involves Rac1 GTPase-dependent actin-network remodelling. Biol Cell. 2007;99:223–238. doi: 10.1042/BC20060123. [DOI] [PubMed] [Google Scholar]
- Kosuge M, Takeuchi T, Nakase I, Jones AT., and , Futaki S. Cellular internalization and distribution of arginine-rich peptides as a function of extracellular peptide concentration, serum, and plasma membrane-associated proteoglycans. Bioconjug Chem. 2008;19:656–664. doi: 10.1021/bc700289w. [DOI] [PubMed] [Google Scholar]
- Duchardt F, Fotin-Mleczek M, Schwarz H, Fischer R., and , Brock R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic. 2007;8:848–866. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Fretz MM, Penning NA, Al-Taei S, Futaki S, Takeuchi T, Nakase I, et al. Temperature-, concentration- and cholesterol-dependent translocation of L- and D-octa-arginine across the plasma and nuclear membrane of CD34+ leukaemia cells. Biochem J. 2007;403:335–342. doi: 10.1042/BJ20061808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier O, Boucke K, Hammer SV, Keller S, Stidwill RP, Hemmi S, et al. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J Cell Biol. 2002;158:1119–1131. doi: 10.1083/jcb.200112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcone S, Cocucci E, Podini P, Kirchhausen T, Clementi E., and , Meldolesi J.2006Macropinocytosis: regulated coordination of endocytic and exocytic membrane traffic events J Cell Sci 119Pt 22): 4758–4769. [DOI] [PubMed] [Google Scholar]
- Araki N, Johnson MT., and , Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi M, Rusnati M, Presta M., and , Giacca M. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J Biol Chem. 2001;276:3254–3261. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- Sampath P., and , Pollard TD. Effects of cytochalasin, phalloidin, and pH on the elongation of actin filaments. Biochemistry. 1991;30:1973–1980. doi: 10.1021/bi00221a034. [DOI] [PubMed] [Google Scholar]
- Racoosin EL., and , Swanson JA. M-CSF-induced macropinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J Cell Sci. 1992;102 Pt 4:867–880. doi: 10.1242/jcs.102.4.867. [DOI] [PubMed] [Google Scholar]
- Kee SH, Cho EJ, Song JW, Park KS, Baek LJ., and , Song KJ. Effects of endocytosis inhibitory drugs on rubella virus entry into VeroE6 cells. Microbiol Immunol. 2004;48:823–829. doi: 10.1111/j.1348-0421.2004.tb03614.x. [DOI] [PubMed] [Google Scholar]
- Kameyama S, Horie M, Kikuchi T, Omura T, Tadokoro A, Takeuchi T, et al. Acid wash in determining cellular uptake of Fab/cell-permeating peptide conjugates. Biopolymers. 2007;88:98–107. doi: 10.1002/bip.20689. [DOI] [PubMed] [Google Scholar]
- Sugita T, Yoshikawa T, Mukai Y, Yamanada N, Imai S, Nagano K, et al. Improved cytosolic translocation and tumor-killing activity of Tat-shepherdin conjugates mediated by co-treatment with Tat-fused endosome-disruptive HA2 peptide. Biochem Biophys Res Commun. 2007;363:1027–1032. doi: 10.1016/j.bbrc.2007.09.077. [DOI] [PubMed] [Google Scholar]
- Maréchal V, Prevost MC, Petit C, Perret E, Heard JM., and , Schwartz O. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J Virol. 2001;75:11166–11177. doi: 10.1128/JVI.75.22.11166-11177.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J., and , Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Mathias P, Cheresh DA., and , Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Xie J, Chiang L, Contreras J, Wu K, Garner JA, Medina-Kauwe L, et al. Novel fiber-dependent entry mechanism for adenovirus serotype 5 in lacrimal acini. J Virol. 2006;80:11833–11851. doi: 10.1128/JVI.00857-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootjans JJ, Zimmermann P, Reekmans G, Smets A, Degeest G, Dürr J, et al. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc Natl Acad Sci USA. 1997;94:13683–13688. doi: 10.1073/pnas.94.25.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AR, Woods DF, Marfatia SM, Walther Z, Chishti AH, Anderson JM, et al. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J Cell Biol. 1998;142:129–138. doi: 10.1083/jcb.142.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda A., and , Couchman JR. Regulation of cytoskeletal organization by syndecan transmembrane proteoglycans. Matrix Biol. 2003;22:25–33. doi: 10.1016/s0945-053x(03)00010-6. [DOI] [PubMed] [Google Scholar]
- Grassmé HU, Ireland RM., and , van Putten JP. Gonococcal opacity protein promotes bacterial entry-associated rearrangements of the epithelial cell actin cytoskeleton. Infect Immun. 1996;64:1621–1630. doi: 10.1128/iai.64.5.1621-1630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehio C, Freissler E, Lanz C, Gómez-Duarte OG, David G., and , Meyer TF. Ligation of cell surface heparan sulfate proteoglycans by antibody-coated beads stimulates phagocytic uptake into epithelial cells: a model for cellular invasion by Neisseria gonorrhoeae. Exp Cell Res. 1998;242:528–539. doi: 10.1006/excr.1998.4116. [DOI] [PubMed] [Google Scholar]
- Hsueh YP, Yang FC, Kharazia V, Naisbitt S, Cohen AR, Weinberg RJ, et al. Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses. J Cell Biol. 1998;142:139–151. doi: 10.1083/jcb.142.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freissler E, Meyer auf der Heyde A, David G, Meyer TF., and , Dehio C. Syndecan-1 and syndecan-4 can mediate the invasion of OpaHSPG-expressing Neisseria gonorrhoeae into epithelial cells. Cell Microbiol. 2000;2:69–82. doi: 10.1046/j.1462-5822.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- Ziegler A., and , Seelig J. Binding and clustering of glycosaminoglycans: a common property of mono- and multivalent cell-penetrating compounds. Biophys J. 2008;94:2142–2149. doi: 10.1529/biophysj.107.113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A., and , Couchman JR. Syndecan-4 and focal adhesion function. Curr Opin Cell Biol. 2001;13:578–583. doi: 10.1016/s0955-0674(00)00254-4. [DOI] [PubMed] [Google Scholar]
- Dovas A, Yoneda A., and , Couchman JR.2006PKCbeta-dependent activation of RhoA by syndecan-4 during focal adhesion formation J Cell Sci 119Pt 13): 2837–2846. [DOI] [PubMed] [Google Scholar]
- Tkachenko E, Elfenbein A, Tirziu D., and , Simons M. Syndecan-4 clustering induces cell migration in a PDZ-dependent manner. Circ Res. 2006;98:1398–1404. doi: 10.1161/01.RES.0000225283.71490.5a. [DOI] [PubMed] [Google Scholar]
- Bass MD, Roach KA, Morgan MR, Mostafavi-Pour Z, Schoen T, Muramatsu T, et al. Syndecan-4-dependent Rac1 regulation determines directional migration in response to the extracellular matrix. J Cell Biol. 2007;177:527–538. doi: 10.1083/jcb.200610076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futaki S, Niwa M, Nakase I, Tadokoro A, Zhang Y, Nagaoka M, et al. Arginine carrier peptide bearing Ni(II) chelator to promote cellular uptake of histidine-tagged proteins. Bioconjug Chem. 2004;15:475–481. doi: 10.1021/bc034181g. [DOI] [PubMed] [Google Scholar]
- Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, et al. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time-course study of FHV-Alexa internalization into CHO-K1 cells.
Effects of serum and peptide concentration on internalization of the Tat peptide into cells.
Effects of macropinocytosis inhibitors on penetration of the FHV peptide into cells.
Actin organization in CHO-K1 cells and its absence in A-745 cells following incubation with the FHV and Tat peptides.