Abstract
Osteoporosis is a systemic skeletal disorder characterized by reduced bone mineral density (BMD) and increased risk of fracture. We studied the effects of transplantation of mesenchymal stem cells (MSCs) overexpressing receptor activator of nuclear factor-κB (RANK)-Fc and CXC chemokine receptor-4 (CXCR4) using retrovirus on ovariectomy (OVX)-induced bone loss in mice. Ten-week-old adult female C57BL/6 mice were divided into six groups as follows: Sham-operated mice treated with phosphate-buffered saline (PBS) (Sham-op + PBS); OVX mice intravenously transplanted with syngeneic MSCs overexpressing RANK-Fc-DsRED and CXCR4-GFP (RANK-Fc + CXCR4); RANK-Fc-DsRED and GFP (RANK-Fc + GFP); CXCR4-GFP and DsRED (CXCR4 + RED); DsRED and GFP (RED + GFP); or treated with PBS only (OVX + PBS). Measurement of BMD showed that introduction of RANK-Fc resulted in significant protection against OVX-induced bone loss compared to treatment with PBS (−0.1% versus −6.2%, P < 0.05) at 8 weeks after cell infusion. CXCR4 + RED group also significantly prevented bone loss compared to OVX + PBS group (2.7% versus −6.2%, P < 0.05). Notably, the effect of RANK-Fc + CXCR4 was greater than that of RANK-Fc + GFP (4.4% versus −0.1%, P < 0.05) while it was not significantly different from that in CXCR4 + RFP group (4.4% versus 2.7%, P = 0.055) at 8 weeks. Transplantation of MSCs with control virus (RED + GFP group) also resulted in amelioration of bone loss compared to OVX + PBS group (−1.7% versus −6.2%, P < 0.05). Fluorescence-activated cell sorting (FACS) and real-time quantitative PCR (qPCR) analysis for GFP from bone tissue revealed enhanced cell trafficking to bone by co-overexpression of CXCR4. In conclusion, we have demonstrated that intravenous transplantation of syngeneic MSCs overexpressing CXCR4 could promote increased in vivo cell trafficking to bone in OVX mice, which could in itself protect against bone loss but also enhance the therapeutic effects of RANK-Fc.
Introduction
Osteoporosis is a common skeletal disorder, which is composed of increased bone turnover, progressive bone loss, microarchitectural deterioration, and finally increased risk of fracture.1 As life expectancy increases, the prevalence of osteoporosis and related fractures increase rapidly around the world. Most of the current treatment strategies for osteoporosis is focused on antiresorptive agents, which inhibit bone resorbing activity of osteoclast. Although these conventional therapies have been shown to effectively increase bone mineral density (BMD) and reduce risk of fractures,2 there have been ongoing concerns regarding its long-term safety. Indeed, estrogen therapy has been shown to be associated with increased risk of breast cancer or thromboembolism3 and long-term bisphosphonate treatment may lead to severe suppression of bone turnover resulting in spontaneous fracture.4 Therefore, the need for development of new therapeutic modalities based on biological mechanisms that could physiologically modulate bone metabolism in its microenvironment is necessary.
Mesenchymal stem cell (MSC)–mediated gene therapy has been investigated as an attractive option for treatment of a number of diseases including articular cartilage damage,5,6 hemophilia,7 or myocardial infarction.8 MSCs can be obtained by bone marrow aspiration and can be expanded by ex vivo culture, thereby making them good vehicles for in vivo gene delivery. It has been well established that MSCs showed good in vitro transduction efficacy and long-term in vivo survival after retroviral transduction.9 Although osteoporosis has been listed one of the top 10 potential targets of stem cell therapy,10 systemic delivery of MSCs for osteoporosis treatment has not been studied.
Receptor activator of nuclear factor-κB ligand (RANKL), receptor activator of nuclear factor-κB (RANK), and osteoprotegerin (OPG) play a critical role in the regulation of bone remodeling by affecting osteoclastogenesis.11 RANKL, a membrane-bound protein of osteoblast and other bone marrow stromal cells, binds to RANK on osteoclast precursor cells and stimulates osteoclast differentiation. The RANK–RANKL interaction activates NF-κB signaling, which activates differentiation of osteoclast precursor to osteoclasts. OPG is a soluble decoy receptor secreted by osteoblasts and stromal cells, which binds to RANKL, sequestrates it from RANK, and inhibits osteoclast formation.12 In animal models, null mutations of OPG resulted in severe osteoporosis12 while overexpression of OPG13 or deletion of RANKL or RANK showed high bone mass.14,15,16 Based on these evidences, RANKL/RANK/OPG system has been a target for developing new drugs for osteoporosis.17
RANK-Fc is a recombinant protein of the extracellular domain of RANK fused to the Fc region of human IgG and functions as a soluble antagonist against RANKL and has a longer half-life than soluble RANK.18 With its ability to specifically bind to RANKL, treatment with RANK-Fc has been shown to block osteoclast differentiation and activation both in vitro and in vivo19 and improve osteolytic lesion in multiple myeloma.20 Previously, we have shown that intraperitoneal transplantation of MSCs overexpressing RANK-Fc prevented ovariectomy (OVX)-induced bone loss in mice model, although the effect was marginal.21
CXC chemokine receptor-4 (CXCR4) is a dynamic seven–transmembrane receptor of stromal cell–derived factor-1 (SDF-1), regulated by environmental factors such as cytokines, chemokines, adhesion molecules, and proteolytic enzymes.22 SDF-1 is highly expressed in bone marrow, especially by bone marrow endothelial cells23,24,25 and regarded as a chemotactic agent for lymphoid, myeloid, and immature CD34+ cells.26,27,28 SDF-1/CXCR4 interaction is a well-known regulator of cell trafficking and homing29 and upregulation of CXCR4 expression could improve the engraftment and repopulation of human stem cells in nonobese diabetic/severe combined immunodeficiency mice.30
In this study, we investigated the effects of intravenous infusion of syngeneic MSCs overexpressing RANK-Fc and/or CXCR4 on the prevention of OVX-induced bone loss in mice model. We found that MSCs overexpressing RANK-Fc effectively prevented OVX-induced bone loss and co-overexpression of CXCR4 resulted in greater protection.
Results
Induction of serum level of SDF-1 following OVX
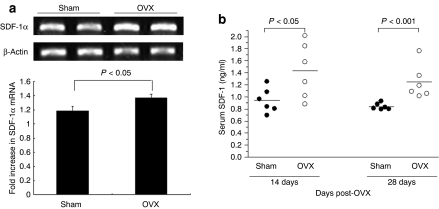
As previous study showed that estrogen positively regulates SDF-1 in human breast and ovarian cancer cells in vitro,31 we wanted to identify whether bone marrow expression or serum level of SDF-1 changes after OVX. Ten-week-old female mice underwent OVX or Sham operation (n = 6, respectively). Mice were killed and cells were harvested from bone marrow culture. Reverse transcription-PCR analysis showed that SDF-1α mRNA level increased by 16% following OVX (Figure 1a), contrary to the previous results from cancer cells in vitro.31 Moreover consistent with reverse transcription-PCR results, serum SDF-1α levels were significantly increased by ~1.5-fold at both 14 and 28 days after OVX, although there was a substantial overlap in the individual values between two groups (Figure 1b). The overlap in the SDF-1 levels between two groups tended to disappear from day 14 to day 28. These results prompted us to exploit the SDF-1α/CXCR4 system for enhancing MSC delivery to bone marrow in OVX mice.
Figure 1.
Expression of SDF-1 in bone and serum in ovariectomized (OVX) mice. (a) RT-PCR analysis of SDF-1 from bone. Both Sham-operated group (n = 6) and OVX group (n = 6) were used for evaluating gene expression levels of SDF-1. mRNA coding for SDF-1 was detected by PCR amplification of cDNA fragment from reverse-transcribed total RNA prepared from whole mouse femur 28 days after Sham-op or OVX. (b) Blood samples were collected on days 14 and 28 after operation, serum was separated by centrifuge, and total SDF-1 levels were determined by enzyme-linked immunosorbent assay. cDNA, complementary DNA; RT-PCR, reverse transcription-PCR; SDF-1, stromal cell–derived factor-1.
Establishment of cells stably co-overexpressing RANK-Fc and CXCR4
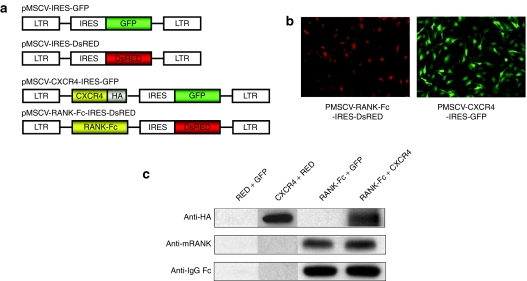
Having confirmed the upregulation of SDF-1 by OVX, we next generated MSCs overexpressing RANK-Fc, CXCR4, or both RANK-Fc and CXCR4. Toward this end, murine stem cell virus (MSCV)–based retrovirus encoding each gene or empty virus were sequentially transduced to MSCs obtained from syngeneic mice femurs (Figure 2a). Successful transduction of retrovirus was assessed by visualization of GFP or DsRED, which is coexpressed through internal ribosome entry site with the CXCR4 or RANK-Fc, respectively, using fluorescent microscope (Figure 2b). Fluorescence-activated cell sorting (FACS) analysis also showed that transduction efficiencies were within 88.5–94.2% (data not shown). Only GFP and DsRED positive cells were selected using FACS and used for further study. In addition, the cellular expression of CXCR4 or RANK-Fc was confirmed by western blot analysis using anti-HA or anti-RANK/anti-IgG-Fc antibodies, respectively, with protein isolated from whole cell lysates of transduced MSCs (Figure 2c).
Figure 2.
Preparation of mesenchymal stem cells (MSCs) to express RANK-Fc with or without CXCR4 by retroviral infection. (a) MSCV-LTR is used to drive the coexpression of RANK-Fc or CXCR4 and GFP or DsRED through bicistronic cassettes containing IRES. (b) Fluorescent microscopic examination of the cells overexpressing CXCR4 and RANK-Fc that coexpress GFP from pMSCV-CXCR4-GFP or DsRED from pMSCV-RANK-Fc-DsRED, respectively. (c) Western blot analysis of cellular expression of CXCR4 and RANK-Fc in vitro. Cell lysates from confluent cultures of MSCs transduced with indicated retrovirus were analyzed by sodium dodecyl sulfate–polyacrylamaide gel electrophoresis (SDS-PAGE) and western blotting using HA, mRANK, or IgG-Fc antibody. CXCR4, CXC chemokine receptor-4; DsRED, Discosoma sp. red fluorescent protein; GFP, green fluorescent protein gene; IRES, internal ribosomal entry site of the encephalomyocarditis virus; MSCV-LTR, Moloney murine leukemia virus long terminal repeat; RANK-Fc, receptor activator of nuclear factor-κB-Fc.
Transplantation of MSCs overexpressing RANK-Fc and CXCR4 increased BMD in OVX mice
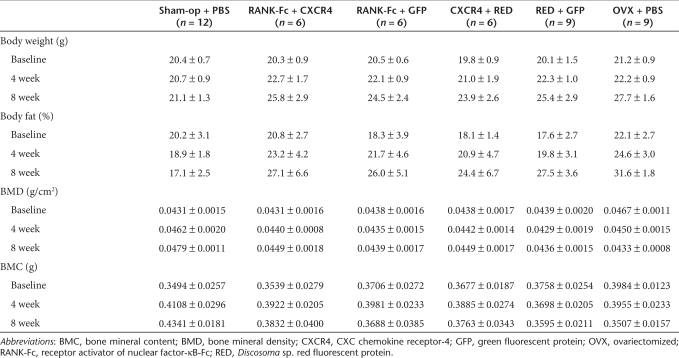
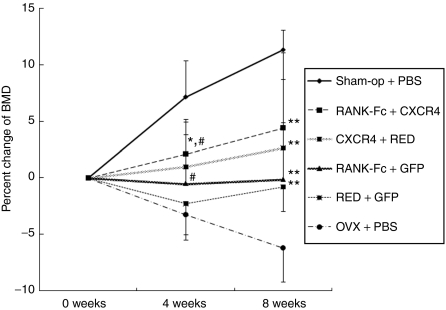
Ten-week-old female C57BL/6 immunocompetent mice were used for this experiment. To ensure successful induction of osteoclast-mediated bone loss, sublethal or lethal irradiation was not performed in these animals. After OVX or Sham operation (Sham-op), mice underwent either syngeneic MSC transplantation, which was genetically modified as above, or phosphate-buffered saline (PBS) injection intravenously. We divided the animals into six groups as follows: (i) Sham-op mice treated with PBS (Sham-op + PBS; n = 12), four groups (2, 3, 4, and 5) of OVX mice injected with MSCs transduced with dual retrovirus, (ii) pMSCV-RANK-Fc-IRES-DsRED and pMSCV-CXCR4-IRES-GFP (RANK-Fc + CXCR4; n = 6), (iii) pMSCV-RANK-Fc-IRES-DsRED and pMSCV-IRES-GFP (RANK-Fc + GFP; n = 6), (iv) pMSCV-IRES-DsRED and pMSCV-CXCR4-IRES-GFP (CXCR4 + RED; n = 6), (v) pMSCV-IRES-DsRED and pMSCV-IRES-GFP and (RED + GFP; n = 9), and (vi) OVX mice treated with PBS (OVX + PBS; n = 9). Among the six study groups, all OVX mice (other than Sham-op group) showed marked increase in fat percent and body weight, which indicated successful induction of estrogen deficiency (Table 1). Measurement of BMD by dual energy X-ray absorptiometry revealed that OVX + PBS groups showed decrease in BMD 8 weeks after OVX as expected (Table 1, Figure 3). Introduction of MSCs overexpressing RANK-Fc only (RANK-Fc + GFP) could significantly attenuate OVX-induced bone loss at 8 weeks (compare RANK-Fc +GFP with OVX + PBS group, −0.1% versus −6.2%, P < 0.05; Figure 3). Co-overexpression of CXCR4 along with RANK-Fc resulted in obvious increase in BMD during study period (2.1% at 4 weeks and 4.4% at 8 weeks) so that the difference between RANK-Fc + CXCR4 and OVX + PBS group reaches 10.6% at 8 weeks. Within the RANK-Fc groups, RANK-Fc + CXCR4 group showed significantly higher BMD compared to RANK-Fc + GFP group at 4 weeks (P < 0.05) and the difference persists until 8 weeks although it did not reach significance at 8 weeks (P = 0.21). Remarkably, CXCR4 + RED group also prevented bone loss compared to OVX + PBS group (1.0% versus −3.2% at 4 weeks, P < 0.05, 2.7% versus −6.2% at 8 weeks, P < 0.05) to the extent that the percent BMD changes in CXCR4 + RED and RANK-Fc + CXCR4 group were only marginally different at 4 weeks (1.0% versus 2.1%, P = 0.055) and 8 weeks (2.7% versus 4.4%, P = 0.055). These results not only were in agreement with our hypothesis that overexpression of CXCR4 could improve the effect of gene therapy with RANK-Fc but also suggest that CXCR4 overexpression itself can be utilized for enhancing MSC effects. Intriguingly, RED + GFP group also prevented bone loss compared to OVX + PBS group (−1.7% versus −6.2% at 8 weeks, P < 0.05) indicating that MSCs per se may also play some role in OVX-induced osteoporosis in mice model.
Table 1.
Body weight, body fat percentage, BMD, and BMC changes of the animals
Figure 3.
Effects of MSC injection on bone mineral densities in the animals. Percent changes of bone mineral density. Ten-week-old female C57BL/6 mice were Sham-operated or ovariectomized (OVX) and randomized into six groups; Sham-op + PBS mice (n = 12), RANK-Fc + CXCR4 mice (n = 6), RANK-Fc + GFP mice (n = 6), CXCR4 + RED mice (n = 6), RED + GFP mice (n = 9), or OVX + PBS mice (n = 9). All mice were injected with MSCs transduced with indicated retrovirus or PBS at day 0 and 7 and BMD (g/cm2) were measured with PIXImus densitometer (GE Lunar) on weeks 0, 4, and 8. Data are expressed means ± SE. *P < 0.05, versus RANK-Fc + GFP, at 4 weeks, #P < 0.05 versus OVX + PBS, at 4 weeks, **P < 0.05, versus OVX + PBS, at 8 weeks, Sham-op + PBS; Sham-operated and intravenously injected with PBS, RANK-Fc + CXCR4; OVX and intravenously injected with MSCs overexpressing RANK-Fc and CXCR4, RANK-Fc + GFP; OVX and intravenously injected with MSCs overexpressing RANK-Fc and GFP, CXCR4 + RED; OVX and intravenously injected with MSCs overexpressing CXCR4 and RED, RED + GFP; OVX and intravenously injected with MSCs overexpressing RED and GFP, OVX + PBS; OVX and intravenously injected with PBS. BMD, bone mineral density; CXCR4, CXC chemokine receptor-4; GFP, green fluorescent protein gene; MSC, mesenchymal stem cells; PBS, phosphate-buffered saline; RANK-Fc, receptor activator of nuclear factor-κB-Fc.
Expression of RANK-Fc in mouse serum
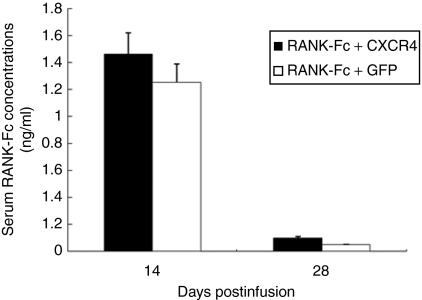
Next, we investigated whether MSCs transduced with retrovirus encoding RANK-Fc could secrete RANK-Fc in mouse serum after systemic injection. As shown in Figure 4, the production of RANK-Fc in serum was evident at 14 days after the first injection in both RANK-Fc + CXCR4 (1.46 ± 0.16 ng/ml) and RANK-Fc + GFP groups (1.25 ± 0.14 ng/ml), and then the levels decreased below to 0.10 ng/ml at 28 days. There was no significant difference in the RANK-Fc levels between two groups. No serum RANK-Fc was detected in control animals (data not shown).
Figure 4.
Production of RANK-Fc from transplanted MSCs in mouse serum. Mice sera from RANK-Fc + CXCR4 or RANK-Fc + GFP group were obtained on days 14 and 28 after first injection of MSCs and serum RANK-Fc level was measured by enzyme-linked immunosorbent assay using mRANK and human IgG-Fc antibody. Data are expressed as mean ± SE, n = 6 per group. CXCR4, CXC chemokine receptor-4; GFP, green fluorescent protein gene; MSC, mesenchymal stem cells; RANK-Fc, receptor activator of nuclear factor-κB-Fc.
MSC trafficking with CXCR4 overexpression
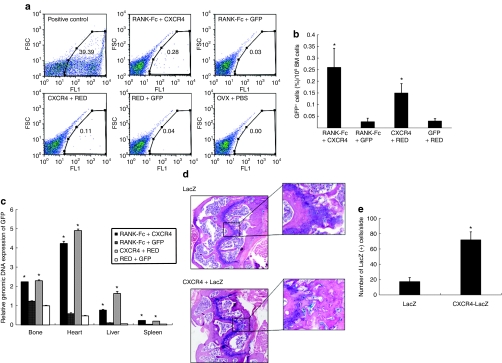
To identify the direct evidence of MSC trafficking, three mice from each group that underwent MSC injection were killed 48 hours after injection as a parallel experiment. FACS analysis showed that the fraction of GFP-positive cells in bone marrow of mice injected with CXCR4-overexpressing MSCs 9.8-fold (RANK-Fc + CXCR4 versus RANK-Fc + GFP; P < 0.05) or 5-fold (CXCR4 + RED versus RED + GFP; P < 0.05) higher than that in mice given CXCR4 negative cells, indicating that CXCR4-overexpressing cells have better capability to navigate to bone marrow compared to CXCR4 negative cells (Figure 5a,b).
Figure 5.
MSCs cell trafficking after transplantation. (a,b) FACS analysis of GFP staining in the bone marrow of OVX mice that were injected with MSCs overexpressing RANK-Fc + CXCR4, RANK-Fc + GFP, CXCR4 + RED, or RED + GFP recovered at 48 hours after injection. Samples from OVX mice injected with PBS were used as a negative control. For positive control, cells were collected immediately after direct intrafemoral injection of RED + GFP cells. The experiment was repeated three times, showing similar results. (a) Representative flow cytometry analysis is shown. (b) Comparison of the percentage of GFP-positive cells (%) per 105 bone marrow cells. *P < 0.05, versus RANK-Fc + GFP or RED + GFP. (c) The relative copy numbers of GFP DNA measured by real-time quantitative PCR analysis. Tissues from the OVX mice that underwent intravenous injection of MSCs transduced with retrovirus encoding RANK-Fc + CXCR4, RANK-Fc + GFP, CXCR4 + RED, or RED + GFP were harvested 48 hours after injection and genomic DNA were extracted for the analysis of GFP by real-time qPCR. The values are calculated based on the standard curve equation. *P < 0.05, versus RED + GFP or RANK-Fc + GFP. (d,e) X-Gal stain of MSCs in vivo. Femur from mice that underwent OVX and intravenous injection of MSCs transduced with retrovirus encoding LacZ or CXCR4 + LacZ were isolated and decalcified. (d) Distal femur sections stained with X-Gal and counterstained with hematoxylin and eosin were examined under microscope (original magnification ×100). (e) The total numbers of X-Gal positive cells in the sections were counted. Five fields from three different mice for each cell type. *P < 0.05, versus LacZ. BM, bone marrow; CXCR4, CXC chemokine receptor-4; FSC, forward scatter; GFP, green fluorescent protein; PBS, phosphate-buffered saline; RANK-Fc, receptor activator of nuclear factor-κB-Fc.
Next, we performed real-time quantitative PCR (qPCR) analysis for GFP using genomic DNA isolated from femur, heart, liver, or spleen in these four groups of mice at 48 hours after cell injection. Real-time qPCR showed a significantly higher signal intensity of GFP from RANK-Fc + CXCR4 and CXCR4 + RED group compared to RANK-Fc + GFP or RED + GFP group in all tissues (Figure 5c). Among the tissues we tested, the signal intensity from heart was the most prominent.
Finally, we tried to identify the transplanted cells in vivo and to verify the effects of CXCR4 overexpression using a histological method. However, as bone tissue itself produces a significant amount of autofluorescence, demonstrating fluorescence from the infused GFP-positive cells has many inherent difficulties. Therefore, for this particular experiment, we transduced MSCs with retroviruses that express LacZ instead of GFP. Systemic injection of MSCs transduced with pMSCV-CXCR4-IRES-LacZ (CXCR4 + LacZ) or pMSCV-IRES-LacZ (LacZ) retrovirus was performed and femurs were harvested 48 hours after cell injection. Consistent with the PCR data, ex vivo X-Gal stain revealed that positively stained cells were definitely increased in CXCR4 + LacZ group principally in areas of metaphysis compared to the mice injected with MSCs overexpressing LacZ alone (Figure 5d,e). Collectively, these data suggest that CXCR4 overexpression could help the MSCs to navigate to bone, which could partly explain the greater protection of bone loss by transplantation of MSCs overexpressing both RANK-Fc and CXCR4.
Discussion
In this study, we have demonstrated that transplantation of MSC retrovirally co-transduced with RANK-Fc and CXCR4 resulted in protection of OVX-induced bone loss in mouse model. The extent of protection by RANK-Fc and CXCR4 was greater compared to transplantation of MSCs that produce RANK-Fc alone. Moreover, transplantation of MSC overexpressing CXCR4 alone also demonstrated significant protection against OVX-induced bone loss, suggesting that co-overexpression of CXCR4 contributed to the enhanced efficacy of MSC therapy.
In a previous study, we have shown that RANK blockade by RANK-Fc could prevent OVX-induced bone loss by intraperitoneal injection of MSCs transduced with retrovirus encoding RANK-Fc 21. In that study, although RANK-Fc producing MSCs have clearly prevented bone loss, the effect was marginal, increasing just ~4% of BMD compared to the control group. We reasoned that the lack of robust effects was attributable to the relatively poor trafficking of transplanted cells. To overcome these limitations, we have modified our experimental models. First, we changed the route of cell injection from intraperitoneal to intravenous so that the injected MSCs can be directly delivered to bone marrow space following systemic circulation thereby affecting skeletal microenvironment with secreted RANK-Fc by paracrine manners. Second, we have overexpressed CXCR4 along with the RANK-Fc to facilitate trafficking to bone. Indeed, with these two modified manners, we could achieve more potent bone-protecting effects of RANK-Fc gene therapy for OVX-induced bone loss in mice. Compared with the 4.6% gain in BMD by intraperitoneal transplantation of MSC secreting RANK-Fc alone,21 intravenous injection of MSCs that simultaneously overexpress RANK-Fc and CXCR4 resulted in >10% increase at the same duration. Although 10% increase in BMD is far from complete recovery to the level of Sham-op group, this is substantial in view of the fact that treatment with bisphosphonate, a standard therapy for osteoporosis, in human leads to 5–6% increase in BMD in 24–36 months associated with prevention of major fractures by >50% (refs. 32,33).
Although we have demonstrated significant protection against OVX-induced bone loss by MSC transplantation, the animals in our study seem to be in growing phase in view of the steady increase in BMD in Sham-op group. As bone length, mass, and mechanical properties reach mature levels by 12 weeks in mice,34 10- to 12-week-old animals are commonly used to demonstrate OVX-induced bone loss. However, adult peak bone density is typically achieved by 4 months and femurs continue to lengthen for 4 or 8 months thereafter.35 Therefore, performance of OVX at 10-week-old in our study has in fact induced premature menopause but resulted in significant bone loss nonetheless, as was demonstrated in OVX + PBS group.
SDF-1/CXCR4 interaction has been recently exploited in diverse aspects of stem cell therapies. Overexpression of CXCR4 in human hematopoietic stem cell line36 or CD34+ progenitors37 improved chemotaxis, migration, and homing while blocking CXCR4/SDF-1 interaction led to inhibition of homing and repopulation.30,38 Besides hematopoietic stem cell transplantation, increasing CXCR4 expression in MSCs by stimulating cytokines, including FMS-like tyrosine kinase-3 ligand, stem cell factor, interleukin-6, hepatocyte growth factor, and interleukin-3, improved the engraftment of MSC to bone marrow, assisting the recovery of hematopoiesis after sublethal irradiation of nonobese diabetic/severe combined immunodeficiency mice.39 Moreover, in mouse model of myocardial infarction, increased CXCR4 expression40,41 resulted in enhanced migration of cells to the infarcted sites and improved cardiac performance. Therefore, the enhanced BMD response by co-overexpressing RANK-Fc and CXCR4 demonstrated in our study is in good agreement with these previous observations, underscoring the critical role of CXCR4 in modulating MSC trafficking. Furthermore, we found that OVX has induced SDF-1α gene expression from cultured bone marrow mononuclear cells. Although there was a substantial degree of overlap, mean serum SDF-1 measurement also exhibited corresponding changes, which could further augment the effects of MSCs overexpressing CXCR4. Significant overlap in the SDF-1 levels may indicate that estrogen deficiency induced by OVX is not the only factor that controls the SDF-1 levels. Alternatively, given the clear difference in SDF-1 mRNA levels from bone marrow cells, it is possible that serum levels of SDF-1 may not accurately reflect the levels in bone marrow environment.
Notably, we have demonstrated that MSCs overexpressing CXCR4 alone (CXCR4 + RED group) were also able to protect against OVX-induced bone loss, which was comparable to the effects of MSCs overexpressing RANK-Fc alone. This result suggests that augmentation of RANK-Fc effect by co-overexpression of CXCR4 may be attributable to the CXCR4 itself rather than indirectly affecting the function of RANK-Fc by CXCR4.
Our study has limitation in that we were not able to demonstrate long-term engraftment of infused GFP-positive cells in vivo. In addition, the serum level of RANK-Fc peaked at 2 weeks but decreased thereafter. However, both FACS analysis of the cells from bone marrow and PCR amplification of genomic DNA clearly showed that the infused cells have successfully navigate to bone in short term. Although FACS analysis revealed that only 0.26% of the bone marrow cells have GFP positive signals in mice receiving RANK-Fc + CXCR4, it may result from the dilution of the cells with all different kinds of cell populations and is still 9.8-fold higher compared to those harvested from RANK-Fc only group. Moreover, short-term histological analysis after injecting LacZ-positive MSCs also demonstrated localization of cells in the metaphysis, which could partly explain the mechanism of the BMD gain. Disappearance of serum RANK-Fc after 2 weeks might suggest that the infused cells are not actually engrafting but lodging and then dying or moving on. The steady effects on BMD up to 8 weeks in this context are difficult to interpret. It might be possible that the initial secretion of RANK-Fc in the microenvironment by the transplanted cells induced sustained blockade of RANK–RANKL interaction with resultant inhibition of bone resorption even after the cells disappeared.
The trafficking efficiency of MSCs overexpressing CXCR4 in our study was much lower than that from previous study using CXCR4-overexpressing hematopoietic stem cell, in which sublethal irradiation was performed before transplantation. As irradiation of bone marrow has been shown to induce SDF-1 expression,23 lack of this procedure may also have contributed to the low trafficking efficiency. Another interesting finding is that substantial amount of GFP signal was observed in heart from the mice transplanted with MSCs overexpressing CXCR4 (both RANK-Fc + CXCR4 and CXCR4 + RED). Although differential expression of SDF-1 has not been well studied, Gleichmann et al. have reported that heart tissue exhibited the strongest expression of SDF-1γ, an isoform of SDF-1, which shares almost identical amino acid sequence with SDF-1α.42 We speculate that this higher endogenous expression of SDF-1γ in heart could lead to avid retention of MSCs overexpressing CXCR4. Therefore, notwithstanding the potential benefit of CXCR4 overexpression, the inherent nonspecificity and scarcity of stem cell trafficking is something to overcome to establish an efficient therapeutic modality by using the stem cell transplantation.
Interestingly, transplantation of MSCs transduced with control virus (RED + GFP) has also resulted in significant protection against OVX-induced bone loss. Therapeutic effects of MSC transplantation per se without genetic modification have also been observed in fracture43,44 or local ischemic heart disease.45,46 Moreover, in rabbit osteoporosis model, local application of MCS could strengthen the osteoporotic bone in vivo.47 Although these previous studies demonstrated the effects of locally applied MSCs, the beneficial effect of MSCs in our study was observed when we gave the cells systemically. In the absence of RANK-Fc secretion, the small number of MSCs with control virus may have differentiated into the osteoblasts, which could account for the protection against bone loss. Another potential explanation is that engrafted MSCs could favorably condition the bone marrow microenvironment to alleviate the OVX-induced bone loss. The latter hypothesis is supported by a recent study that showed supportive role of MSCs in hematopoietic stem cell engraftments.39 The favorable effects of MSCs per se, i.e., without genetic modification, can provide a promising future for cell therapy given the potential risk of retroviral transduction of MSCs, including uncontrolled proliferation or oncogenesis that can occur depending on the integration of the virus.
In summary, we demonstrated that RANK-Fc protects against bone loss in mice model of osteoporosis and co-overexpression of CXCR4 resulted in greater protection with improved MSC trafficking to bone. Moreover, the bone protective effects of MSCs per se were validated in OVX-induced bone loss model. These results support the use of CXCR4 transduced MSCs as an efficient cellular vehicle for gene therapy. Furthermore, we could speculate that MSCs per se with or without RANK-Fc expression, might be one of the potential therapeutic tools for osteoporosis.
Materials and Methods
Animals. Female C57BL/6 mice aged 10 weeks were purchased from Japan SLC Lab (Hamamatsu, Japan). All animal experiments were performed under approval from the Institutional Animal Care and Use Committee of Seoul National University and complied with the National Research Council's “Guidelines for the Care and Use of Laboratory Animals” (revised 1996).
Experimental design to study the effects of OVX on SDF-1α. Mice were randomly divided into two groups and underwent either Sham operation (Sham; n = 6) or bilateral OVX (OVX; n = 6). Sera were collected on the day 14 and 28 after operation. Twenty-eight days after operation, all mice were killed and femurs from each group were dissected free of surrounding tissue and stored at −70 °C with liquid nitrogen.
Retroviral vector construction and establishment of MSCs. The mouse CXCR4 cDNA in eukaryotic expression vector pCMV-CXCR4 was obtained from Takashi Nagasawa (Kyoto University, Japan), and the pMT-RANK-Fc vector containing a DNA sequence encoding the extracellular domain of mouse RANK (Met1–Pro213) fused via a linker to the Fc region of human immunoglobulin G1 (IgG1) was provided by Jaerang Rho (Chungnam National University, Daejon, Korea).
The MSCV-based retroviral vector, pMSCV-IRES-GFP was a kind gift from Neil A. Clipstone (Northwestern University, Chicago, IL) and has been described previously.48 To generate retroviral vectors that coexpress DsRED or LacZ downstream of IRES, DsRED or LacZ cDNA was PCR amplified using pCMV-DsRED or pCMV-LacZ as parental plasmids and ligated into the pMSCV-IRES-GFP vector in place of GFP, giving pMSCV-IRES-DsRED or pMSCV-IRES-LacZ, respectively. The mouse CXCR4 cDNA with HA epitope tag at the C-terminus was prepared using PCR from pCMV-CXCR4 as a template and ligated into the BglII/EcoRI site of plasmid pMSCV-IRES-GFP or BglII/XhoI site of pMSCV-IRES-LacZ, giving pMSCV-CXCR4-IRES-GFP or pMSCV-CXCR4-IRES-LacZ, respectively (Figure 2a). The retroviral vector encoding RANK-Fc was generated by introducing the RANK-Fc cDNA into the EcoRI/NotI site of pMSCV-IRES-DsRED, giving pMSCV-RANK-Fc-IRES-DsRED. The nucleotide sequence of the plasmids were confirmed by direct sequencing. The retroviral vectors express RANK-Fc or CXCR4 and marker genes, i.e., GFP, DsRED, or LacZ, from a single bicistronic mRNA under the transcriptional control of MSCV-LTR allowing identification of gene expression using the fluorescence (GFP or DsRED) or LacZ.
Mouse MSCs were established by explant culture of bone marrow cells from C57BL/6 mouse femur as previously described21 and P2 to P3 MSCs were use for retroviral transduction. All MSCs used in this experiment were co-transduced with two types of retrovirus harboring GFP or RED, with two rounds of transduction for each construct. Viral transduction has been performed as described previously.21
Experimental design to study the effects of MSC transplantation on OVX-induced bone loss. Ten-week-old female C57BL/6 mice were divided into six groups as described the Results section. On study day −1, under general anesthesia with ketamine (50 mg/kg) and xylazine (15 mg/kg), bilateral OVX or Sham-op was performed by standard method49 for “OVX” or “Sham-op” groups, respectively. On study day 0, total 6–7 × 105 MSCs in 100 µl of PBS were injected into the lateral tail veins of mice within 5–6 seconds. On day 7, second dose of MSCs were injected. Forty-eight hours after infusion of second dose of cells, three mice from cell injection groups were used for cell trafficking assay. BMD (g/cm2) and body composition using a Lunar PIXImus densitometer (software version 2.0; GE Lunar, Madison, WI) were measured as previously described21 on weeks 0, 4, and 8 with remaining mice. Blood samples were obtained from retro-orbital plexus on week 4 and 8 under anesthesia with diethyl ether and sera were separated and stored at −70 °C until analysis.
Enzyme-linked immunosorbent assay of SDF-1 and RANK-Fc. SDF-1 levels were analyzed by double-antibody sandwich enzyme-linked immunosorbent assay method according to the directions of the manufacturer (sensitivity, 31.25 pg/ml; range, 62.5–5,000 pg/ml; R&D systems, Minneapolis, MN). RANK-Fc enzyme-linked immunosorbent assay was performed as described previously.21
Analysis of MSC trafficking
FACS for GFP: Forty-eight hours after MSC infusion, cells were recovered from bone marrow and were analyzed for the presence of GFP-positive cells by FACS (FACS Vantage SE; Becton Dickinson, Franklin Lakes, NJ) using 106 cells per sample. Cells from nontransplanted mice were used to exclude false positive cells. For positive control, direct intrafemoral injection of RED + GFP cells were perform and b cells were collected immediately after injection.
DNA PCR for GFP: Tissues from heart, liver, and spleen were removed and frozen with liquid nitrogen. Femurs were fixed with 4% paraformaldehyde at 4 ºC for 24 hours immediately after extraction. To detect GFP by real-time qPCR, the soft tissues including heart, liver, and spleen were placed in a lysis buffer containing 100 mmol/l NaCl, 100 mmol/l Tris–Cl (pH 8.0), 25 mmol/l EDTA (pH 8), 0.5% sodium dodecyl sulfate, and 0.1 mg/ml proteinase K (Gentra Systems, Minneapolis, MN). The bones were homogenized, and then lysis buffer was added. Genomic DNA was extracted using standard phenol–chloroform extraction method as described previously.21 The β-actin gene was selected as a housekeeping gene to normalize variations in DNA content. SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) was used as reaction medium in a total volume of 30 µl. Real-time qPCR reactions were performed in an ABI Prism 7000 Sequence Detection System (Applied Biosystems). The precycling steps were 2 minutes at 50 °C and 10 minutes at 95 °C, followed by 15 seconds at 95 °C and 1 minute at 60 °C for 40 cycles. An automatic threshold over background was selected, and the cycle corresponding to its surpass (Ct) was defined. For each reaction, Cts for different GFP were normalized with the corresponding β-actin Ct by substraction (DCt). The sequences of sense and antisense primers are as follows; 5′-CACATGAAGCAGCACGACTT-3′ and 5′-AGTTCACCTTGATGCCGTTC-3′ for GFP, 5′-TGGGTATGGAATCCT GTGGC-3′ and 5′-CCAGACAGCACTGTGTTGGC-3′ for β-actin.
Histological examination of MSCs overexpressing LacZ: To demonstrate the MSCs in situ, 10-week-old C57BL/6 mice underwent intravenous injection with MSCs transduced with MSCV-IRES-LacZ or MSCV-CXCR4-IRES-LacZ and were killed 48 hours after MSC infusion. After 24 hours fixation, femurs were rinsed in PBS for 24 hours and then decalcified in 0.25 mol/l EDTA at 4 °C for 72 hours. Each femur was immersed in 5 ml 1 mol/l X-Gal in PBS containing 20 mmol/l potassium ferricyanide, 20 mmol/l potassium ferrocyanide, and 1 mmol/l magnesium chloride at 37 °C for 8 hours.50 After staining, femur was removed from X-Gal, rinsed with PBS for 24 hours and embedded in paraffin. Sagittal sections were taken at 5 µm thickness, mounted on slides and stained with hematoxylin and eosin.
Statistical methods. Data were assessed using Statistical Package for the Social Sciences (SPSS, Chicago, IL) software (version 11.0), and summarized with descriptive statistics (mean, standard error). The differences between each group at each time-point were analyzed with the nonparametric Kruskal–Wallis test. All tests were two-sided, and a significance level of 5% was assigned.
Acknowledgments
This work was supported by a grant from Seoul National University Hospital (grant # 03-2005-023-0).
REFERENCES
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. [Google Scholar]
- Miller PD. Anti-resorptives in the management of osteoporosis. Best Pract Res Clin Endocrinol Metab. 2008;22:849–868. doi: 10.1016/j.beem.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA., and , Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90:1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- Gelse K, von der Mark K, Aigner T, Park J., and , Schneider H. Articular cartilage repair by gene therapy using growth factor-producing mesenchymal cells. Arthritis Rheum. 2003;48:430–441. doi: 10.1002/art.10759. [DOI] [PubMed] [Google Scholar]
- Park J, Ries J, Gelse K, Kloss F, von der Mark K, Wiltfang J, et al. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: a comparison of adenoviral vectors and liposomes. Gene Ther. 2003;10:1089–1098. doi: 10.1038/sj.gt.3301960. [DOI] [PubMed] [Google Scholar]
- Van Damme A, Chuah MK, Dell'accio F, De Bari C, Luyten F, Collen D, et al. Bone marrow mesenchymal cells for haemophilia A gene therapy using retroviral vectors with modified long-terminal repeats. Haemophilia. 2003;9:94–103. doi: 10.1046/j.1365-2516.2003.00709.x. [DOI] [PubMed] [Google Scholar]
- Tang J, Xie Q, Pan G, Wang J., and , Wang M. Mesenchymal stem cells participate in angiogenesis and improve heart function in rat model of myocardial ischemia with reperfusion. Eur J Cardiothorac Surg. 2006;30:353–361. doi: 10.1016/j.ejcts.2006.02.070. [DOI] [PubMed] [Google Scholar]
- Porada CD, Zanjani ED., and , Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther. 2006;1:365–369. doi: 10.2174/157488806778226821. [DOI] [PubMed] [Google Scholar]
- Perry D. Patients' voices: the powerful sound in the stem cell debate. Science. 2000;287:1423. doi: 10.1126/science.287.5457.1423. [DOI] [PubMed] [Google Scholar]
- Wada T, Nakashima T, Hiroshi N., and , Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, et al. osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess TL, Qian Y, Kaufman S, Ring BD, Van G, Capparelli C, et al. The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol. 1999;145:527–538. doi: 10.1083/jcb.145.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci USA. 2000;97:1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- Hamdy NA. Targeting the RANK/RANKL/OPG signaling pathway: a novel approach in the management of osteoporosis. Curr Opin Investig Drugs. 2007;8:299–303. [PubMed] [Google Scholar]
- Zhang J, Dai J, Yao Z, Lu Y, Dougall W., and , Keller ET. Soluble receptor activator of nuclear factor κB Fc diminishes prostate cancer progression in bone. Cancer Res. 2003;63:7883–7890. [PubMed] [Google Scholar]
- Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA. 1999;96:3540–3545. doi: 10.1073/pnas.96.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse RN, Sordillo EM, Yaccoby S, Wong BR, Liau DF, Colman N, et al. Multiple myeloma disrupts the TRANCE/ osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci USA. 2001;98:11581–11586. doi: 10.1073/pnas.201394498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Cho SW, Her SJ, Yang JY, Kim SW, Kim SY, et al. Retrovirus-mediated gene transfer of receptor activator of nuclear factor-κB-Fc prevents bone loss in ovariectomized mice. Stem Cells. 2006;24:1798–1805. doi: 10.1634/stemcells.2005-0480. [DOI] [PubMed] [Google Scholar]
- Lapidot T., and , Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002;16:1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, Sandbank J, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331–1339. doi: 10.1172/JCI10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Kikutani H., and , Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci USA. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K, Kobayashi M, Wang J, Ohiro Y, Hamada J, Cho Y, et al. Selective transendothelial migration of hematopoietic progenitor cells: a role in homing of progenitor cells. Blood. 1999;93:149–156. [PubMed] [Google Scholar]
- Bleul CC, Schultze JL., and , Springer TA. B lymphocyte chemotaxis regulated in association with microanatomic localization, differentiation state, and B cell receptor engagement. J Exp Med. 1998;187:753–762. doi: 10.1084/jem.187.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiuti A, Webb IJ, Bleul C, Springer T., and , Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier AJ, van der Laan LJ, Hildbrand P, Siani MA, Thompson DA, Dawson PE, et al. Presentation of chemokine SDF-1 α by fibronectin mediates directed migration of T cells. Blood. 2000;96:2682–2690. [PubMed] [Google Scholar]
- Kucia M, Reca R, Miekus K, Wanzeck J, Wojakowski W, Janowska-Wieczorek A, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- Hall JM., and , Korach KS. Stromal cell-derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Mol Endocrinol. 2003;17:792–803. doi: 10.1210/me.2002-0438. [DOI] [PubMed] [Google Scholar]
- Cranney A, Tugwell P, Adachi J, Weaver B, Zytaruk N, Papaioannou A, et al. Meta-analyses of therapies for postmenopausal osteoporosis. III. Meta-analysis of risedronate for the treatment of postmenopausal osteoporosis. Endocr Rev. 2002;23:517–523. doi: 10.1210/er.2001-3002. [DOI] [PubMed] [Google Scholar]
- Cranney A, Wells G, Willan A, Griffith L, Zytaruk N, Robinson V, et al. Meta-analyses of therapies for postmenopausal osteoporosis. II. Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr Rev. 2002;23:508–516. doi: 10.1210/er.2001-2002. [DOI] [PubMed] [Google Scholar]
- Ferguson VL, Ayers RA, Bateman TA., and , Simske SJ. Bone development and age-related bone loss in male C57BL/6J mice. Bone. 2003;33:387–398. doi: 10.1016/s8756-3282(03)00199-6. [DOI] [PubMed] [Google Scholar]
- Beamer WG, Donahue LR, Rosen CJ., and , Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18:397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- Porecha NK, English K, Hangoc G, Broxmeyer HE., and , Christopherson KW. Enhanced functional response to CXCL12/SDF-1 through retroviral overexpression of CXCR4 on M07e cells: implications for hematopoietic stem cell transplantation. Stem Cells Dev. 2006;15:325–333. doi: 10.1089/scd.2006.15.325. [DOI] [PubMed] [Google Scholar]
- Kahn J, Byk T, Jansson-Sjostrand L, Petit I, Shivtiel S, Nagler A, et al. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103:2942–2949. doi: 10.1182/blood-2003-07-2607. [DOI] [PubMed] [Google Scholar]
- Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Li J, Liao L, Chen B, Li B, Chen L, et al. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92:897–904. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Ou L, Zhou X, Li F, Jia X, Zhang Y, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571–579. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T., and , Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77:134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- Gleichmann M, Gillen C, Czardybon M, Bosse F, Greiner-Petter R, Auer J, et al. Cloning and characterization of SDF-1γ, a novel SDF-1 chemokine transcript with developmentally regulated expression in the nervous system. Eur J Neurosci. 2000;12:1857–1866. doi: 10.1046/j.1460-9568.2000.00048.x. [DOI] [PubMed] [Google Scholar]
- Taguchi K, Ogawa R, Migita M, Hanawa H, Ito H., and , Orimo H. The role of bone marrow-derived cells in bone fracture repair in a green fluorescent protein chimeric mouse model. Biochem Biophys Res Commun. 2005;331:31–36. doi: 10.1016/j.bbrc.2005.03.119. [DOI] [PubMed] [Google Scholar]
- Devine MJ, Mierisch CM, Jang E, Anderson PC., and , Balian G. Transplanted bone marrow cells localize to fracture callus in a mouse model. J Orthop Res. 2002;20:1232–1239. doi: 10.1016/S0736-0266(02)00051-7. [DOI] [PubMed] [Google Scholar]
- Hahn JY, Cho HJ, Kang HJ, Kim TS, Kim MH, Chung JH, et al. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;51:933–943. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- Grauss RW, Winter EM, van Tuyn J, Pijnappels DA, Steijn RV, Hogers B, et al. Mesenchymal stem cells from ischemic heart disease patients improve left ventricular function after acute myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293:H2438–H2447. doi: 10.1152/ajpheart.00365.2007. [DOI] [PubMed] [Google Scholar]
- Wang Z, Goh J, Das De S, Ge Z, Ouyang H, Chong JS, et al. Efficacy of bone marrow-derived stem cells in strengthening osteoporotic bone in a rabbit model. Tissue Eng. 2006;12:1753–1761. doi: 10.1089/ten.2006.12.1753. [DOI] [PubMed] [Google Scholar]
- Neal JW., and , Clipstone NA. Calcineurin mediates the calcium-dependent inhibition of adipocyte differentiation in 3T3-L1 cells. J Biol Chem. 2002;277:49776–49781. doi: 10.1074/jbc.M207913200. [DOI] [PubMed] [Google Scholar]
- Bellino FL. Nonprimate animal models of menopause: workshop report. Menopause. 2000;7:14–24. doi: 10.1097/00042192-200007010-00004. [DOI] [PubMed] [Google Scholar]
- Price J. Retroviruses and the study of cell lineage. Development. 1987;101:409–419. doi: 10.1242/dev.101.3.409. [DOI] [PubMed] [Google Scholar]