Abstract
The reparative properties of bone marrow stromal cells (BMSCs) have been attributed in part to the paracrine action of secreted factors. We isolated typical human BMSCs by plastic adherence and compared them with BMSC sub-populations isolated by magnetic-activated cell sorting against CD133 (CD133-derived BMSCs, CD133BMSCs) or CD271 [p75 low-affinity nerve growth factor receptor (p75LNGFR), p75BMSCs]. Microarray assays of expressed genes, and enzyme-linked immunosorbent assays (ELISAs) of selected growth factors and cytokines secreted under normoxic and hypoxic conditions demonstrated that the three transit-amplifying progenitor cell populations were distinct from one another. CD133BMSC-conditioned medium (CdM) was superior to p75BMSC CdM in protecting neural progenitor cells against cell death during growth factor/nutrient withdrawal. Intracardiac (arterial) administration of concentrated CD133BMSC CdM provided neuroprotection and significantly reduced cortical infarct volumes in mice following cerebral ischemia. In support of the paracrine hypothesis for BMSC action, intra-arterial infusion of CD133BMSC CdM provided significantly greater protection against stroke compared with the effects of CD133BMSC (cell) administration. CdM from CD133BMSCs also provided superior protection against stroke compared with that conferred by CdM from p75BMSCs or typically isolated BMSCs. CD133 identifies a sub-population of nonhematopoietic stem/progenitor cells from adult human bone marrow, and CD133BMSC CdM may provide neuroprotection for patients with stroke.
Introduction
Adult mammalian bone marrow contains hematopoietic stem cells (HSCs) and progenitor cells that produce all of the major blood cell lineages.1,2,3 The field of HSC biology has benefited greatly from functional reconstitution assays in mice in which fractionated cell subsets can be transplanted into irradiated recipients to determine cell lineage relationships.3 In this manner, characterization of cell surface epitopes, and transplantation of HSCs and downstream progenitors identified the two functionally distinct branches of the hematopoietic system that derive from common myeloid progenitor cells4 and common lymphoid progenitor cells.5
Adult bone marrow also contains nonhematopoietic stem and progenitor cells that contribute to the hematopoietic microenvironment.6,7,8,9,10 An elegant recent study demonstrated that CD146+ cells isolated from human bone marrow contained nonhematopoietic stem-like cells that could be expanded and serially transplanted to transfer ectopic hematopoietic microenvironments.10 In its native environment, the nonhematopoietic bone marrow stem cell is likely to produce the progenitor cells commonly described as mesenchymal stem cells or multipotent stromal cells, which, in part, contribute structurally to the endosteal and sinusoidal compartments of the marrow that comprise HSC niches.10,11,12 Multipotent stromal cells are adherent in culture, are identified by their ability to differentiate into stromal cells, osteoblasts, adipocytes, and chondrocytes,7 and support the ex vivo maintenance of HSCs.13 Bone marrow stromal cells (BMSCs) play a critical role in regulating HSC proliferation, differentiation, and quiescence in vivo by signaling via the “stem cell niche synapse” through which growth factors, cytokines, and immunomodulatory factors are exchanged.12,14 In addition to regulating hematopoiesis, some nonhematopoietic progenitor cells may also enter the circulation and serve as a “continuous reservoir” of replacement cells and/or reparative cells for nonhematopoietic tissues.15 Despite several decades of research, in contrast to HSC biology where different progenitor cell lineages are known by distinct cell surface epitopes and functions, the progeny of the nonhematopoietic stem cell remain relatively poorly defined.
BMSCs have received increasing attention as expandable cells that can be used for cell and gene therapy.15 They have been demonstrated to provide functional benefits in a wide variety of animal models for tissue injury and disease such as myocardial infarction,16,17 hindlimb ischemia,18 and stroke.19 Beneficial results have also been reported in patients that received BMSCs.20,21,22 However, low levels of long-term BMSC engraftment reported in both animals and patients indicate that cell replacement is not the predominant mechanism responsible for the benefits conferred by BMSC administrations. Rather, it has been suggested that BMSCs are secreting “factories” that rescue cells, repair tissues and provide improved functional outcomes by virtue of their secretion of a multitude of growth factors, cytokines, and immunomodulatory molecules.23
BMSCs are commonly isolated from bone marrow aspirates by density gradient centrifugation to obtain mononuclear cells and then by simple adherence to tissue culture plastic and rapid growth in supportive mediums.6,7,24 These conditions, however, do not necessarily select for any particular progenitor cell population, and it is not clear that the BMSCs isolated by different laboratories actually represent the same cells. The lack of standardization may, in part, lead to differing results reported by some investigators that administer BMSCs to treat similar animal models of tissue injury and disease. It is generally assumed that the transit-amplifying progenitors that expand from adherent bone marrow cultures and that possess a defined set of cell surface epitopes25 are functionally equivalent.
In the interest of developing safe and effective cell-based therapies with predictable effects in vivo, we isolated nonhematopoietic progenitor cells directly from human bone marrow mononuclear cells by magnetic-activated cell sorting against two different cell surface epitopes that had previously been reported to isolate multipotent MSC-like cells from mobilized peripheral blood and cord blood (CD133, Prominin 1) (ref. 26), and bone marrow [CD271, p75 low-affinity nerve growth factor receptor (p75LNGFR)].27 Although similar to typically isolated human BMSCs in several ways, we found that the CD133-derived BMSCs (CD133BMSCs) and the p75LNGFR-derived BMSCs (p75BMSCs) differed from typical BMSCs and from each other in terms of their levels of secreted growth factors and cytokines. In addition, the BMSCs, CD133BMSCs, and p75BMSCs had different secretion responses when exposed to a hypoxic environment, suggesting that the nonhematopoietic bone marrow stem cell may produce different progenitors that reside in different marrow environments. Our results raise the possibility that nonhematopoietic stem/progenitor cell sub-populations may be reproducibly isolated and exploited in tissue- or injury-specific therapies based on their differential growth factor and cytokine secretion.
Results
Cell surface epitopes
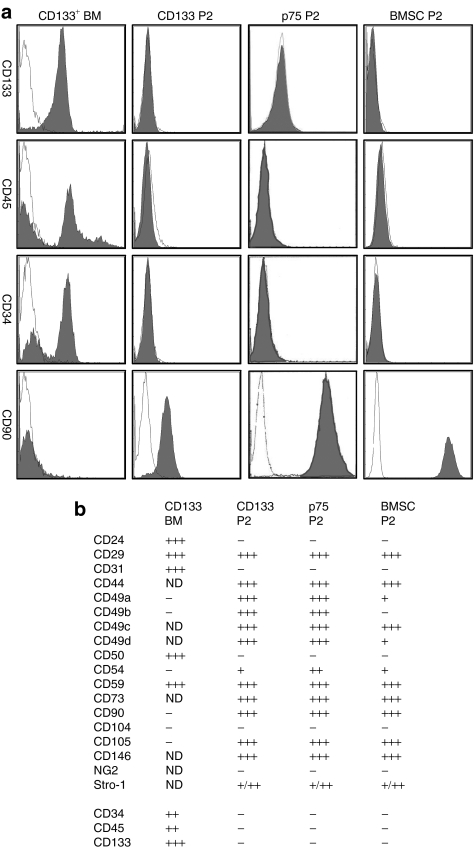
The frequency of CD133+ cells in total aspirated human bone marrow mononuclear cells was about 0.24% (n = 9 donors, Supplementary Table S1). Analysis of cell surface epitopes demonstrated that the freshly isolated CD133+ cells were >90% CD133+, 58% CD45+, 72% CD34+, 57% CD24+, 44% ABC G2+ [ATP-binding cassette, subfamily G (WHITE), member 2], and negative for CD49a, CD49b, CD90, and CD105 (Figure 1). The adherent CD133+ cells had the unusual morphology noted by Kuçi et al.28 in that they had blebs on the membrane surfaces in addition to three kinds of pseudopodia referred to as tenopodia (long, thin), magnupodia (short, thick), and lobopodia (spoon-like). In 5–7 days, the polyploid cells gave rise to colonies of cells with morphology similar to the spindle-shaped morphology of BMSCs and expanded rapidly (Supplementary Figure S1). Of note, the surface epitopes of the CD133+ cells and the p75LNGFR+ cells changed, as they adhered and expanded to generate CD133BMSCs and p75BMSCs (Figure 1). At passage 2 (P2), the CD133BMSCs were no longer positive for CD133, CD45, CD34, CD31, ABCG2, or CD24. Similarly, P2 p75BMSCs were no longer positive for the p75LNGFR epitope used to initially isolate the cells (data not shown). P2 cultures of CD133BMSCs, p75BMSCs, and typical BMSCs were all negative for CD133, CD45, and CD34 (Figure 1). The BMSC marker Stro-1 was variably expressed on P2 CD133BMSCs, p75BMSCs, and typical BMSCs (Figure 1 and Supplementary Figure S2).
Figure 1.
Cell surface epitopes. (a) Illustrating changes in cell surface epitope expression that occur in freshly isolated CD133+ cells from human bone marrow (CD133 BM) before and after they adhere to generate CD133BMSCs. Over time, the CD133 BM cells lose the expression of CD133 and acquire the expression of typical adherent BMSC markers such as CD90 (Thy 1) and CD105 (endoglin). Passage 2 CD133BMSCs (CD133 P2), p75BMSCs (p75 P2), and typical BMSCs (BMSC P2) are negative for CD34 and the pan-hematopoietic marker CD45. Antibody staining is shown in dark fill and isotype staining is shown in white fill. (b) Summary for all epitopes tested. Two donors were stained for each cell type. +, 1–25% cells positive; ++, 25–50% cells positive; +++, 50–100% cells positive; BMSC, bone marrow stromal cell; ND, not determined.
Expanding in serum-containing medium, all of the cells became positive for CD90 (Thy 1, Supplementary Figure S3) and CD105 (endoglin), epitopes that are characteristically expressed on BMSCs (Figure 1). The CD133BMSCs and p75BMSCs also expressed CD29, CD44, and CD59 as did P2 BMSCs. The expanded CD133BMSCs and p75BMSCs became positive for the integrin epitopes CD49a and CD49b that are initially expressed on typical P0 BMSCs but are lost as BMSCs are expanded. Among the epitopes that we examined, we found none that could clearly distinguish between the cultured CD133BMSCs and the p75BMSCs. P2 CD133BMSCs, P2 p75BMSCs, and P2 BMSCs, all expressed high levels of CD146 (melanoma cell adhesion molecule), the recently described marker for the human nonhematopoietic bone marrow stem cell (Figure 1).
Differentiation of the CD133BMSCs and p75BMSCs
The expanded CD133BMSCs, p75BMSCs, and BMSCs all had similar morphologies during culture and through several passages (Figure 2a–c). To assay the differentiation potential of the CD133BMSC and p75BMSC cultures, frozen vials of P1 and P2 cells were thawed, plated at 1,000 cells/cm2, expanded for 5 days, and then transferred to mediums to induce osteogenic, adipogenic, or chondrogenic differentiation. The CD133BMSCs readily differentiated into osteoblasts, adipocytes, and chondrocytes under the same culture conditions used to differentiate typical BMSCs (Figure 2d–f and Supplementary Figure S4). In contrast, the p75BMSCs differentiated into osteoblasts and adipocytes but were not chondrogenic (Figure 2g–i). They formed micromass pellets but did not possess typical chondrocyte morphology (Figure 2i).
Figure 2.
Multipotent differentiation of CD133BMSCs and p75BMSCs. (a–c) Phase contrast photomicrographs of cultured CD133BMSCs, p75BMSCs, and typical BMSCs. Bar = 50 µm. (d–f) Differentiation of CD133BMSCs into osteoblasts, adipocytes, and chondrocytes, respectively. Bar = 1 mm. (g–i) Differentiation of p75BMSCs into osteoblasts and adipocytes, but not chondrocytes. The p75BMSCs form a micromass pellet (i) but do not possess typical chondrocyte morphology. g, bar = 1 mm; h,i, bar = 62.5 µm. Calcification is stained by Alizarin Red S (d,g). Lipid is stained by Oil Red O (e,h). Sulfated proteoglycans are stained by toluidine blue sodium borate (f,i). BMSC, bone marrow stromal cell.
Growth rates of CD133BMSCs, p75BMSCs, and BMSCs under normoxic and hypoxic conditions
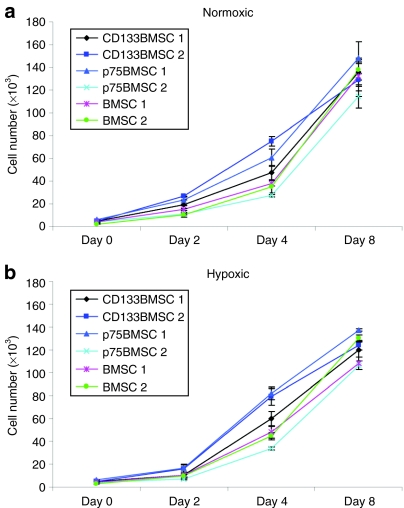
To determine the proliferative capacity of CD133BMSCs, p75BMSCs, and typical BMSCs under normoxic and hypoxic conditions (1% oxygen), we plated cells from two donors for each cell type (100 cells/cm2) and measured cell numbers on days 0, 2, 4, and 8. A two-way analysis of variance was performed to compare the growth rates of CD133BMSCs, p75BMSCs, and BMSCs. Growth rates from day 2 through day 8 for both conditions were similar for CD133BMSCs, p75BMSCs, and BMSCs, and no significant differences were found between normoxic and hypoxic cell counts at any day for any cell type (Figure 3a,b).
Figure 3.
Growth of CD133BMSCs, p75BMSCs, and BMSCs under normoxic and hypoxic conditions. BMSCs isolated by typical plastic adherence as well as those isolated by magnetic-activated cell sorting against CD133 (CD133BMSC) or p75LNGFR (p75BMSC) grow equally well under (a) normoxic and (b) hypoxic (1% oxygen) conditions. Cell growth data are shown for two donors for each cell type over 8 days. Cells from all of the donors were plated at 100 cells/cm2 and allowed to grow for 2 days in a normoxic incubator prior to moving half of the plates to a hypoxic incubator to begin the assay (day 0). There was no significant difference in cell growth rates for either condition (two-way analysis of variance). BMSC, bone marrow stromal cell; p75LNGFR, p75 low-affinity nerve growth factor receptor.
Microarray assays of expressed genes
Hierarchical clustering of microarray data sets demonstrated that the transcriptional profiles of freshly isolated CD271+ (p75LNGFR) cells and CD133+ cells from human bone marrow were more similar to each other than to P2 BMSCs, P2 CD133BMSCs, or P2 p75BMSCs (see cluster diagram and heat map patterns 1 and 2; Figure 4). This was not surprising, as the freshly sorted cells were likely to contain hematopoietic and endothelial stem/progenitor cells. Although sharing many expressed genes in common with P2 BMSCs (pattern 7, Figure 4), several sets of differentially expressed genes demonstrated that the P2 p75BMSCs and the P2 CD133BMSCs possessed unique gene expression profiles compared with typically isolated BMSCs (patterns 4, 5, 6, 8, and 9; Figure 4). Overall, the expressed genes from the p75BMSCs and the CD133BMSCs clustered together, apart from the BMSC profile, indicating that they were more similar to each other than to typical BMSCs (see cluster diagram; Figure 4). Of interest, three major gene expression patterns identified upregulated sets of transcripts that were differentially expressed by the p75BMSCs (pattern 6), the CD133BMSCs (pattern 9), and the BMSCs (pattern 4; Figure 4, Supplementary Table S2). In addition, the transcription of a cluster of expressed collagen genes was upregulated in the p75BMSCs that included Col3A1, Col4A1, Col5A1, Col7A1, Col11A1, and Col12A1; “collagen”, P ≤ 0.001. Notably, one of the significant Gene Ontology terms upregulated for CD133BMSCs (pattern 9) was “growth factor activity”, P ≤ 0.01.
Figure 4.
Microarray assays of expressed genes. Top: hierarchical clustering for gene expression for p75LNGFR+ and CD133+ cells freshly isolated from human bone marrow mononuclear cells and passage 2 (P2) BMSCs, CD133BMSCs, and p75BMSCs cultured in complete culture medium. The overall transcriptional profiles of the p75BMSC and CD133BMSC sub-populations are more similar to each other than to the profile for typical BMSCs. Middle: heat map depicting gene expression. Note, nine major patterns with patterns 6, 9, and 4 in particular demonstrating differentially upregulated genes for p75BMSCs, CD133BMSCs, and BMSCs, respectively (see also Supplementary Table S2). Bottom: the numbers of transcripts contained in each pattern are listed with the numbers of uniquely expressed genes for each pattern shown in parentheses. BMSC, bone marrow stromal cell; p75LNGFR, p75 low-affinity nerve growth factor receptor.
Growth factor and cytokine secretion responses
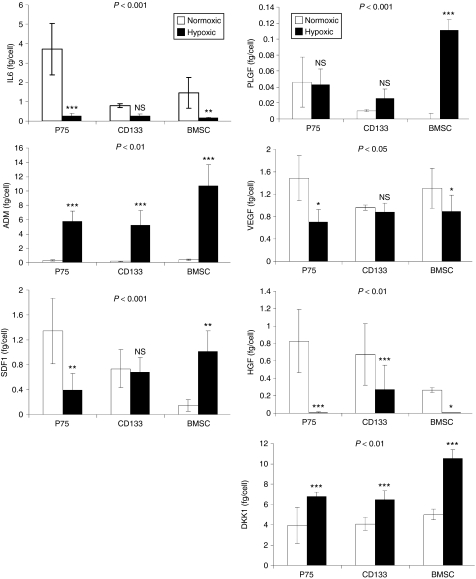
To examine the secretion responses of p75BMSCs, CD133BMSCs, and BMSCs for selected growth factors and cytokines, we ran enzyme-linked immunosorbent assays (ELISAs) on mediums conditioned by each cell type at 50 and 90% cell confluence, and under normoxic or hypoxic conditions (Figure 5, Supplementary Figures S5–S7). Significant differences in protein/peptide secretion between the three progenitor cell populations were determined by repeated measures analysis of variance. Analysis of estimated marginal means for protein/peptide secretion on a per cell basis demonstrated that the three progenitor cell populations responded to hypoxia exposure in a significantly different manner at both cell densities (Figure 5 and Supplementary Figure S5). Under the conditions that we used to generate conditioned medium (CdM), the secretion of epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), and platelet-derived growth factor-AB (PDGF-AB) was beyond the limits of detection (<2 pg/ml), and the secreted levels of β-nerve growth factor (β-NGF) and leukemia inhibitor factor (LIF) were low (<30 pg/ml) (data not shown). Bonferroni pairwise comparisons were used to determine differences between the cell populations in their levels of particular secreted factors at a given cell confluence and under normoxic or hypoxic conditions. As many of the cellular secretion responses were similar at both 50 and 90% confluence, only summarized ELISA data for cells at 90% confluence are reported in the results. Summarized ELISA data for cells at 50% confluence and the complete raw ELISA data sets both in fg/cell and pg/ml are provided in Supplementary Figures S5–S7.
Figure 5.
Growth factor/cytokine secretion under normoxic and hypoxic conditions. Levels of selected secreted proteins/peptides for cells grown to 90% confluence. N = 3 donors in each case. Enzyme-linked immunosorbent assay (ELISA) data were normalized for cell number. Statistical significance (P values) pictured at the tops of the graphs indicate the degree to which the cell populations responded differently from each other when exposed to hypoxia (repeated measures analysis of variance). The asterisks denote differences in protein/peptide secretion levels for a particular cell type in normoxic versus hypoxic conditions. ADM, adrenomedullin; BMSC, bone marrow stromal cell; DKK1, Dickkopf-1; HGF, hepatocyte growth factor; IL6, interleukin 6; NS, not significant; PLGF, placental growth factor; SDF1, stromal-derived factor 1; VEGF, vascular endothelial growth factor. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. All ELISA measurements were performed in triplicate.
Interleukin 6 (IL6). Secreted IL6 regulates HSC numbers, and is also expressed and released from tissues during inflammatory responses.29 Under normoxic conditions, the p75BMSCs secreted significantly greater amounts of IL6 than did either the CD133BMSCs or the BMSCs (90% confluence, versus CD133BMSC, P ≤ 0.001; versus BMSC, P = 0.006; Figure 5). IL6 secretion levels for the CD133BMSCs and BMSCs under normoxic conditions were not significantly different. At 90% confluence under hypoxic conditions, secretion of IL6 by CD133BMSCs was not different from IL6 secretion under normoxia, whereas IL6 secretion from p75BMSCs and BMSCs significantly decreased (p75BMSC, 14.9-fold decrease, P ≤ 0.001; BMSC, 8.7-fold decrease, P = 0.01; Figure 5).
Adrenomedullin (ADM). Adrenomedullin is a secreted vasodilating peptide that acts also to reduce cellular oxidative stress and apoptosis.30 ADM secretion was significantly increased by all of the progenitor cell populations under hypoxic conditions compared with their secretion levels under normoxic conditions regardless of cell density (90% confluence, p75BMSC, 22.0-fold increase, P ≤ 0.001; CD133BMSC, 33.2-fold increase, P ≤ 0.001; BMSC, 29.9-fold increase, P ≤ 0.001; Figure 5). Under hypoxic conditions, ADM secretion by p75BMSCs and CD133BMSCs was not significantly different, whereas the typical BMSCs secreted significantly higher levels of ADM than did the other cell types (90% confluence, versus p75BMSC, P = 0.017; versus CD133BMSC, P = 0.009; Figure 5).
Stromal-derived factor-1α (SDF1). Stromal-derived factor 1 controls, in part, HSC retention within and migration out of the bone marrow microenvironment.31 The secretion responses for SDF1 clearly differed between the typical BMSCs and the epitope-isolated sub-populations. Under hypoxia at 90% confluence, the p75BMSCs decreased their SDF1 secretion (3.4-fold decrease, P = 0.002; Figure 5). In contrast, CD133BMSCs did not alter their SDF1 secretion levels, and the BMSCs significantly increased their SDF1 secretion (6.95-fold increase, P = 0.005; Figure 5).
Placental growth factor (PLFG). Placental growth factor (PLGF) is a vascular endothelial growth factor (VEGF) family member that binds VEGFR1 and functions in pathological angiogenesis.32 The BMSCs demonstrated a dramatic increase in placental growth factor secretion in response to hypoxia compared with the other cell types that did not respond significantly (90% confluence, p75BMSC, not significant; CD133BMSC, not significant; BMSC, P ≤ 0.001; Figure 5). Under hypoxic conditions, typical BMSCs secreted greater levels of placental growth factor than did either other cell type (90% confluence, versus p75BMSC, P ≤ 0.001; versus CD133BMSC, P ≤ 0.001; Figure 5). The secreted levels of placental growth factor did not differ between p75BMSCs and CD133BMSCs under hypoxic conditions.
Vascular endothelial growth factor (VEGF). VEGF has numerous biological effects that include angiogenesis,32 cellular protection, and mobilization of bone marrow–derived cells. In our studies, VEGF secretion generally decreased for all three progenitor cell populations under hypoxic conditions as compared with secretion under normoxic conditions (90% confluence, p75BMSC, 2.1-fold decrease, P ≤ 0.001; CD133BMSC, not significant; BMSC, 1.5-fold decrease, P = 0.021; Figure 5). At 90% confluence, none of the populations differed significantly from each other in terms of VEGF secretion under normoxic or hypoxic conditions.
Hepatocyte growth factor (HGF). HGF has diverse roles in angiogenesis, cell survival, and cancer cell metastasis.33 At 90% confluence under normoxic conditions, the p75BMSCs did not differ significantly in HGF secretion compared with that of the CD133BMSCs but secreted significantly more HGF than did the typical BMSCs (P = 0.037, Figure 5). HGF secretion generally decreased in response to hypoxia for all three cell populations (90% confluence, p75BMSC, 165-fold decrease, P ≤ 0.001; CD133BMSC, 2.5-fold decrease, P ≤ 0.001; BMSC, 26.6-fold decrease, P = 0.024; Figure 5).
Dickkopf-1 (DKK1). DKK1 is a negative regulator of Wnt signaling and functions in a paracrine manner to regulate BMSC entry into the cell cycle.34 In general, DKK1 secretion for all three cell populations increased in response to hypoxia exposure (90% confluence, p75BMSC, 1.7-fold increase, P ≤ 0.001; CD133BMSC, 1.6-fold increase, P ≤ 0.001; BMSC, 2.1-fold increase, P ≤ 0.001; Figure 5). Under hypoxic conditions, the BMSCs secreted significantly greater levels of DKK1 than did either of the other cell types (90% confluence, versus p75BMSC, P ≤ 0.001; versus CD133BMSC, P ≤ 0.001; Figure 5).
Neural progenitor cell viability in CdMs from CD133BMSCs, p75BMSCs, and BMSCs
Neural stem cells were isolated as neurospheres from the brains of postnatal day 4 GFP mice (Figure 6a). They readily differentiated into immature βIII-tubulin-positive neurons and GFAP+ astrocytes after plating on laminin/poly-D-lysine-coated cell ware and incubation for 1 week in the appropriate differentiation mediums (Figure 6b,c). For neural progenitor cell protection assays, we dissociated neurosphere cultures into single cell suspensions and plated the cells onto laminin/poly-D-lysine-coated cell ware as neural progenitor cells (NPCs) in neural stem cell/NPC growth medium containing EGF, bFGF, heparin, and B27. After 2 days of adherent growth, the growth medium was switched to serum-free alpha minimum essential medium or serum-free 1× CdM from CD133BMSCs, p75BMSCs, or BMSCs for 48 hours. CD133BMSC CdM provided significant protection against growth factor/nutrient withdrawal–induced cell death compared with serum-free medium (P ≤ 0.01, Figure 6d). The level of NPC protection provided by CD133BMSC CdM did not differ from that conferred by BMSC CdM. However, CD133BMSC CdM protected significantly greater numbers of NPCs when compared with the protection provided by p75BMSC CdM (P ≤ 0.01, Figure 6d). These results indicated that CD133BMSC CdM contained different types or levels of secreted factors that benefited NPC survival compared with p75BMSC CdM. Notably, we found that CdM from p75BMSCs, CD133BMSCs, and BMSCs all lacked appreciable amounts of EGF and bFGF (<2 pg/ml), implying that other factors or combinations of factors secreted by CD133BMSCs were responsible for protecting the NPCs.
Figure 6.
Protection of neural progenitor cells (NPCs) from cell death induced by growth factor withdrawal. (a) Neural stem cells isolated as neurospheres from day 4 postnatal GFP mice. (b) Differentiation of NPCs into immature neurons (βIII-tubulin-positive; ALEXA 594, red). (c) Differentiation of NPCs into astrocytes (GFAP+ ALEXA 594, red). (d) Percent survival for NPCs after plating onto laminin/poly-D-lysine coated cell ware and growth factor withdrawal for 48 hours during incubation in serum-free alpha minimum essential medium (SFM), or conditioned medium (CdM) from p75BMSCs (p75, n = 3 donors), CD133BMSCs (CD133, n = 3 donors), or typical BMSCs (BMSC, n = 2 donors). MTS measurements were performed in triplicate. **P ≤ 0.01 compared with SFM, †P ≤ 0.01 compared with p75 CdM. BMSC, bone marrow stromal cell; GFAP, glial fibrillary acidic protein; GFP, green fluorescent protein; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium.
Effects of CdM administration following cerebral ischemia
To determine whether the factors secreted by an epitope-isolated sub-population would provide benefits in the context of tissue injury, we administered CD133BMSCs or concentrated CD133BMSC CdM to immunodeficient mice 1 day after permanent ligation of the middle cerebral artery. Distal ligation of the middle cerebral artery provided a reproducible model of cortical stroke (Figure 7a). In comparison, we administered concentrated CdM from p75BMSCs, BMSCs, and dermal fibroblasts. Each of the agents was infused slowly into the left ventricle of the heart in a 100 microliter volume (intra-arterial). The CdMs were generated from 90% confluent cells and were concentrated in a manner to normalize protein concentrations. By analysis of variance, the protein concentrations of the individual CdMs did not differ significantly (p75BMSC CdM, 1.97 ± 0.02 mg/ml; CD133BMSC CdM, 2.16 ± 0.19 mg/ml, BMSC CdM, 2.06 ± 0.06 mg/ml; fibroblast CdM, 1.75 ± 0.19 mg/ml). Of note, whereas the CD133BMSC CdM and the BMSC CdM were both concentrated to 40-fold to reach the protein determination values above, the p75BMSC CdM and the fibroblast CdM required 48.5-fold and 53.5-fold concentration, respectively, to reach similar values.
Figure 7.
Protection against cerebral ischemia. (a) Representative 2,3,5-triphenyltetrazolium chloride (TTC) stains to indicate viable cortical tissue in sham-operated animal, and 1 or 3 days after middle cerebral artery ligation (MCAL). In sham surgery, the needle is passed under the middle cerebral artery, but the suture is not tied. Bar = 1 mm. (b) Representative cresyl violet stains of brain sections from immunodeficient mice that underwent middle cerebral artery ligation surgery and treatment 24 hours later with PBS, 2 million human CD133BMSCs (cells), or concentrated CdM from p75BMSCs, CD133BMSCs, typical BMSCs (BMSC), or human dermal fibroblasts. PBS vehicle, CD133BMSCs, or CdM from the different cell types was injected into the left ventricle of the heart (intra-arterial, 100 µl). Animals were euthanized 48 hours following treatment for analysis. Bar = 1 mm. (c) Quantification of the cortical infarct volumes for PBS-treated (PBS, n = 5), p75BMSC CdM–treated (p75, n = 5), CD133BMSC-treated (cells, n = 6), CD133BMSC CdM–treated (CD133, n = 5), BMSC CdM–treated (BMSC, n = 5), and fibroblast CdM–treated mice (Fibro, n = 6). *P < 0.05 compared with PBS, **P < 0.01 compared with PBS. Statistics were determined by analysis of variance with Bonferroni post hoc testing. BMSC, bone marrow stromal cell; CdM, conditioned medium; PBS, phosphate-buffered saline.
Infusion of p75BMSC CdM, CD133BMSCs (cells), or fibroblast CdM did not significantly reduce cortical infarct volumes after stroke (Figure 7). In contrast, CdM from typically isolated BMSCs and CD133BMSC CdM both significantly reduced infarct volumes at 3 days after middle cerebral artery ligation, with CD133BMSC CdM providing the greatest level of protection against cerebral ischemia (phosphate-buffered saline, 2.1 ± 0.86 mm3, n = 5; BMSC CdM, 0.49 ± 0.40 mm3, n = 5, P < 0.05; CD133BMSC CdM, 0.25 ± 0.15 mm3, n = 6, P < 0.01; Figure 7).
Discussion
Our results demonstrate that human bone marrow contains different sub-populations of nonhematopoietic progenitor cells that can be enriched on the basis of CD133 and CD271 expression. Our transcriptional profiling and protein secretion data show that both sub-populations are significantly different from the total adherent transit-amplifying progenitor cells that are commonly denoted “MSCs.”
CD133 is expressed by adult stem/progenitor cells from many tissues including HSCs and hemangioblasts,35 endothelial progenitor cells,36 liver stem cells,37 neural stem cells,38 and stem-like cancer initiating cells.35 Mutations in CD133 (PROM1) lead to photoreceptor disk malformations and macular degeneration in patients,39 although the precise function of CD133 for stem/progenitor cells is unknown. We report here that CD133BMSCs from adult human bone marrow differentiate ex vivo into osteoblasts, adipocytes, and chondrocytes.
CD271 (p75LNGFR) functions in pan-neurotrophin signaling during development and is expressed by germline stem cells.40 In adults, the p75LNGFR is expressed by several types of stem/progenitor cells including keratinocyte stem cells and neural stem cells.41,42 Immunohistochemical assays using antibodies against p75LNGFR were initially reported to stain recticular cells in sections of human bone.43 Subsequent studies used magnetic sorting against the p75LNGFR to isolate adherent BMSC-like cells that expanded in culture and differentiated into osteoblasts and adipocytes.27 We found that expanded p75BMSCs differentiated into osteoblasts and adipocytes, but not into chondrocytes under the same culture conditions used to differentiate CD133BMSCs and BMSCs.
Quirici et al. reported variable expression of CD133 on 20–80% of human bone marrow cells that had been freshly isolated on the basis of cell surface p75LNGFR.27 Therefore, despite the significant differences that we observed between CD133BMSCs and p75BMSCs by microarrays, and ELISAs, there might be some degree of overlap between cell enrichments using either epitope. Both the CD133 and p75LNGFR epitopes were lost by P2 following adherent culture expansion, implying that cultured nonhematopoietic stem cells may generate transit-amplifying progenitor cells that lose the expression of primitive surface markers and then acquire the expression of more differentiated markers such as CD90 (Thy1) and CD105 (endoglin).
Several other groups have described additional epitopes that prospectively isolate BMSCs from bone marrow with varying degrees of efficiency including CD49b, CD90, CD105 (low efficiency),44 STRO-1 (high efficiency),45 CD73, CD130, CD146, CD200, integrin αV/β5 (high efficiency),44 and also CD140b, CD340, and CD349 (ref. 46). Sacchetti et al. reported recently that CD146 (melanoma cell adhesion molecule) expression identified cell populations from human bone marrow that contained a nonhematopoietic stem cell capable of self-renewal and of providing an ectopic hematopoietic microenvironment (HME) in mice.10 In addition, CD146+ cells re-isolated from the primary ectopic HME could be expanded and used to transfer a second ectopic HME to a different animal (an indication of stem cell activity). They found that bone marrow osteoblasts and dermal fibroblasts did not express the CD146 epitope. All of the CD133BMSCs, p75BMSCs, and typical BMSCs used in our studies expressed high levels of CD146. Based on their expression of CD146, we anticipate that each of these cell populations may contain some level of self-renewing nonhematopoietic stem-like cells, although their relative efficiency to reconstitute the HME in vivo remains to be explored. Our micromass pellet results for chondrogenesis indicate that the CD133BMSC and p75BMSC sub-populations may act differently in HME formation assays. In addition, as secreted growth factors and cytokines such as IL6 play critical roles in hematopoiesis,29 our ELISA results suggest that functional differences between the CD133BMSC and p75BMSC sub-populations, and the typically isolated BMSCs in the regulation of hematopoiesis may become apparent in future studies using the HME transfer model. Finally, clonal assays of multipotency and single cell HME transfer studies will both be necessary to establish whether the CD133BMSC and p75BMSC sub-populations contain truly multipotent and self-renewing nonhematopoietic bone marrow stem cells.
Gnecchi et al. found that CdM from AKT-modified rat BMSCs reduced cell death and improved cardiac function after intramuscular injection following myocardial infarction.47 Secreted HGF from adipose-derived murine MSCs was recently shown to be vasoprotective and to improve blood flow in a model of hindlimb ischemia.48 To determine whether the secreted factors from an epitope-isolated human nonhematopoietic progenitor sub-population would provide benefits in the context of ischemic tissue injury, we administered concentrated CD133BMSC CdM to immunodeficient mice with cerebral ischemia. In support of the paracrine hypothesis of MSC action, we found that intra-arterial infusion of CD133BMSC CdM provided protection against stroke. The neuroprotection conferred by the Cd133BMSC CdM was superior to that from p75BMSCs and typically isolated BMSCs (in fact, the p75BMSC CdM was not protective). Clearly, it will be of interest to identify the protective ligands contained in the CD133BMSC CdM and their mechanisms of action.
Our results demonstrate that CD133BMSC CdM can be delivered arterially in a single administration after cerebral ischemia without the need for the cells themselves. Because it does not require major histocompatibility complex matching, CdM may be therapeutically effective when used in a heterologous manner with appropriate virus screening. Our observations with human CD133BMSC CdM are also of clinical interest because cell aggregation may emerge as a problem in cell therapy. This is likely much less of a problem for administration of circulating cells such as hematopoietic cells as it could be for cultured adherent cells such as BMSCs that are relatively large and produce extracellular matrix that promotes cell aggregation. These issues may lead to emboli upon intravenous or intra-arterial administration of the cells.49 For regenerative medicine, it will be of particular interest to identify sub-populations of bone marrow–derived or tissue-specific nonhematopoietic stem/progenitor cells that possess unique repertoires of secreted factors and protect or repair tissue after injury.
Materials and Methods
Isolation and preparation of cells. Under institutional review board approval, BMSCs were isolated from bone marrow aspirates, expanded, and banked as frozen vials of cells (Tulane Center for the Preparation and Distribution of Adult Stem Cells). The distribution center is now located at Texas A&M University, Temple TX (http://www.medicine.tamhsc.edu/irm/msc-distribution.html). Briefly, 2–10 cc iliac crest aspirates were obtained from healthy human donors. Mononuclear cell fractions were obtained by discontinuous Ficoll density gradient centrifugation and extraction of the buffy coat (Ficoll-Paque PLUS; GE Healthcare, Piscataway, NJ). All cells were cultured in Nunclon Delta–coated 15 cm2 dishes (Nunc; Thermo Fisher Scientific, Rochester, NY). For the isolation of typical plastic adherent BMSCs, the mononuclear cell fraction was cultured directly in complete culture medium (CCM) containing alpha minimum essential medium (Invitrogen, Carlsbad, CA), 20% fetal bovine serum (lot selected for rapid growth of BMSCs; Atlanta Biologicals, Lawrenceville, GA), 100 units/ml penicillin, 100 µg/ml streptomycin, and 2 mmol/l L-glutamine (Mediatech, Herndon, VA). To isolate the CD133+ cells and p75+ cells from total bone marrow mononuclear cells, we performed magnetic-activated cell sorting using antibodies conjugated to dextran-coated iron beads according to the manufacturer's instructions [CD133 microbeads, CD271 microbeads (p75LNGFR); Miltenyi Biotec, Auburn, CA].
Phenotypic analysis by flow cytometry. Pellets of 105 to 0.5 × 106 cells were suspended in 0.5 ml phosphate-buffered saline and were incubated for 30 minutes at 4 °C with monoclonal mouse antihuman antibodies that were pretitered for flow cytometry. All antibodies except those against CD133 (Miltenyi Biotec), and CD105 and NG2 (Beckman Coulter, Miami, FL) were purchased from BD Biosciences (San Diego, CA). After labeling, the cells were washed twice with phosphate-buffered saline and analyzed by closed stream flow cytometry (Epics XL; Beckman Coulter; LSR II; Becton Dickinson, Franklin Lakes, NJ).
Microarray assays. For microarray assays of expressed genes, freshly isolated bone marrow mononuclear cells from different aspirate donors were sorted for CD133+ cells or p75LNGFR+ cells (magnetic-activated cell sorting) for RNA isolation (High Pure RNA isolation kit; Roche Applied Science, Indianapolis, IN). To obtain enough material from the fresh cells for microarray assays, ileac crest aspirates from each side (left/right) of a given donor were sorted, and the cells were lysed and combined. For cultured cells, P1 BMSCs, CD133BMSCs, and p75BMSCs (n = 2 donors per cell type) were seeded in 15 cm2 dishes in CCM at 100 cells/cm2, incubated until they reached 60 to 70% confluency (5–7 days), and lifted with trypsin/EDTA for RNA isolation (P2). Microarray methods including sample preparation, analysis by dChip, hierarchical clustering, and analyses for Gene Ontologies are provided in the Supplementary Materials and Methods.
Proliferation assays. Passage 3 BMSCs, CD133BMSCs, and p75BMSCs (n = 3 per cell type) were expanded in CCM, lifted and plated at 100 cells/cm2 in 6-well plates (Nunclon, Nunc; Thermo Fisher Scientific). Cells were grown in CCM under normoxic or hypoxic (1% oxygen) conditions for 2, 4, or 8 days prior to sampling (incubator model 3130; Thermo Electron Corporation, Houston, TX). At each time point, cells were lifted with trypsin/EDTA (Mediatech), pelleted, and frozen at −80 °C. For cells of each donor, 2 wells of the 6-well plate were combined and considered as a replicate (n = 3 per plate, per time point). Cell numbers were quantified by dye labeling of nucleic acids (CyQUANT; Invitrogen) in triplicate using a fluorescence plate reader (Biotek Synergy HT; BioTek Instruments, Winooski, VT).
Differentiation assays. Confluent cultures were prepared by plating P1 CD133BMSCs and p75BMSCs at 1,000 cells/cm2 and incubated for 5 days in CCM. The cultures were then transferred to either osteogenic medium or adipogenic medium. For chondrogenic differentiation, cells were harvested with trypsin/EDTA, and micromass pellet cultures were prepared by centrifugation of 200,000 cells at 1000 g for 8 minutes in 15 ml conical tubes. Pellets were cultured at 37 °C with 5% CO2 in 500 µl chondrogenic media.
Production of CdMs. Three donors each of typical BMSCs, CD133BMSCs, p75BMSCs, and fibroblasts were used to produce CdMs. Cells were seeded from frozen cryotubes containing ~1 million cells at passages 2 or 3, and then expanded, split, and utilized for CdM collection at passages 4 or 5. Cells were passaged two times with media changes every 3–4 days until 50 and 90% confluence was achieved for each donor. At these densities, the cells were washed twice with phosphate-buffered saline, and the medium was switched to serum-free alpha minimum essential medium. At the time of serum-free medium application, duplicate plates from each donor at both densities were placed in incubators set to 37 °C, 5% CO2, and normal atmospheric oxygen (21%) or 1% oxygen. After 48 hours of incubation, the CdM was collected, filtered (0.2 µm polyethersulfone membrane, NALGENE MF75; Thermo Fisher Scientific), and frozen at −80 °C. The cells at the time of CdM collection were lifted with trypsin/EDTA, pelleted, and frozen (−80 °C). Cell numbers were quantified by dye labeling of nucleic acids as above. For in vivo studies, CdMs were concentrated to 40-fold with a Labscale TFF diafiltration system using filters with a 5 kd cutoff (Millipore, Bedford, MA). Therefore, only medium components above 5 kd were concentrated (base medium components and salts remained at 1×).
ELISAs of secreted growth factors and cytokines. We performed sandwich ELISAs to quantify the levels of selected growth factors and cytokines secreted by typical BMSCs, CD133BMSCs, and p75BMSCs. Detailed ELISA procedures are provided in Supplementary Materials and Methods.
Additional methods. For more details including protocols for the neural progenitor cell viability assays and the middle cerebral artery ligation surgeries, please see Supplementary Materials and Methods.
SUPPLEMENTARY MATERIALFigure S1. Culture of adherent CD133-positive and CD133-derived cells (CD133BMSCs).Figure S2. Cell surface phenotyping for Stro-1 from 3 different CD133BMSC, p75BMSC, and BMSC donors.Figure S3. Cell surface phenotyping for CD90 from 3 different CD133BMSC, p75BMSC, and BMSC donors.Figure S4. Osteogenic, adipogenic, and chondrogenic differentiation of typical BMSCs.Figure S5. Summarized ELISA data for cells at 50% confluence.Figure S6. ELISA data for selected growth factors secreted by p75BMSCs, CD133BMSCs, and BMSCs under normoxic and hypoxic conditions (1% oxygen).Figure S7. ELISA data for cytokines secreted by p75BMSCs, CD133BMSCs, and BMSCs under normoxic and hypoxic conditions (1% oxygen).Table S1. Yields of CD133-positive cells from bone marrow aspirates.Table S2. Microarray GO terms.Materials and Methods.
Supplementary Material
Culture of adherent CD133-positive and CD133-derived cells (CD133BMSCs).
Cell surface phenotyping for Stro-1 from 3 different CD133BMSC, p75BMSC, and BMSC donors.
Cell surface phenotyping for CD90 from 3 different CD133BMSC, p75BMSC, and BMSC donors.
Osteogenic, adipogenic, and chondrogenic differentiation of typical BMSCs.
Summarized ELISA data for cells at 50% confluence.
ELISA data for selected growth factors secreted by p75BMSCs, CD133BMSCs, and BMSCs under normoxic and hypoxic conditions (1% oxygen).
ELISA data for cytokines secreted by p75BMSCs, CD133BMSCs, and BMSCs under normoxic and hypoxic conditions (1% oxygen).
Yields of CD133-positive cells from bone marrow aspirates.
Microarray GO terms.
Acknowledgments
We thank Alan Tucker, Tulane University Health Sciences Center, New Orleans, LA and Colette Charland, University of Vermont, Burlington, VT for technical assistance with cell phenotyping. We thank Nabuo Nagai, Kinki University School of Medicine, Osaka, Japan for assistance with the permanent middle cerebral artery ligation stroke model. We thank Darwin Prockop, Texas A&M University Health Science Center, Temple, TX for critical reading of the manuscript and for providing bone marrow stromal cells. This work was supported by R01 HL085210 National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (J.L.S.) and P20 RR016435 NIH/National Center for Research Resources (R Parsons, Center of Biomedical Research Excellence Principal Investigator (PI), J.L.S., PI project 3).
REFERENCES
- Till JE., and , McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- Till JE, McCulloch EA, Siminovitch L. A stochastic model of stem cell proliferation, based on the growth of spleen colony-forming cells. Proc Natl Acad Sci U S A. 1964;51:29–36. doi: 10.1073/pnas.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Uchida N., and , Weissman IL. The biology of hematopoietic stem cells. Annu Rev Cell Dev Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- Akashi K, Traver D, Miyamoto T., and , Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- Kondo M, Weissman IL., and , Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF., and , Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- Muguruma Y, Yahata T, Miyatake H, Sato T, Uno T, Itoh J, et al. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood. 2006;107:1878–1887. doi: 10.1182/blood-2005-06-2211. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C., and , Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kohara H, Noda M., and , Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Dexter TM, Simmons P, Purnell RA, Spooncer E., and , Schofield R. The regulation of hemopoietic cell development by the stromal cell environment and diffusible regulatory molecules. Prog Clin Biol Res. 1984;148:13–33. [PubMed] [Google Scholar]
- Wilson A., and , Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Gregory CA., and , Spees JL.2003One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues Proc Natl Acad Sci USA 100suppl. 1): 11917–11923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, et al. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007;354:700–706. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Koç ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- Phinney DG., and , Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG., and , Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Tondreau T, Meuleman N, Delforge A, Dejeneffe M, Leroy R, Massy M, et al. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23:1105–1112. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- Quirici N, Soligo D, Bossolasco P, Servida F, Lumini C., and , Deliliers GL. Isolation of bone marrow mesenchymal stem cells by anti-nerve growth factor receptor antibodies. Exp Hematol. 2002;30:783–791. doi: 10.1016/s0301-472x(02)00812-3. [DOI] [PubMed] [Google Scholar]
- Kuçi S, Wessels JT, Bühring HJ, Schilbach K, Schumm M, Seitz G, et al. Identification of a novel class of human adherent CD34- stem cells that give rise to SCID-repopulating cells. Blood. 2003;101:869–876. doi: 10.1182/blood-2002-03-0711. [DOI] [PubMed] [Google Scholar]
- Jenkins BJ, Roberts AW, Najdovska M, Grail D., and , Ernst M. The threshold of gp130-dependent STAT3 signaling is critical for normal regulation of hematopoiesis. Blood. 2005;105:3512–3520. doi: 10.1182/blood-2004-09-3751. [DOI] [PubMed] [Google Scholar]
- Kato J, Tsuruda T, Kita T, Kitamura K., and , Eto T. Adrenomedullin: a protective factor for blood vessels. Arterioscler Thromb Vasc Biol. 2005;25:2480–2487. doi: 10.1161/01.ATV.0000184759.91369.f8. [DOI] [PubMed] [Google Scholar]
- Broxmeyer HE. Chemokines in hematopoiesis. Curr Opin Hematol. 2008;15:49–58. doi: 10.1097/MOH.0b013e3282f29012. [DOI] [PubMed] [Google Scholar]
- Tjwa M, Luttun A, Autiero M., and , Carmeliet P. VEGF and PlGF: two pleiotropic growth factors with distinct roles in development and homeostasis. Cell Tissue Res. 2003;314:5–14. doi: 10.1007/s00441-003-0776-3. [DOI] [PubMed] [Google Scholar]
- Lesko E., and , Majka M. The biological role of HGF-MET axis in tumor growth and development of metastasis. Front Biosci. 2008;13:1271–1280. doi: 10.2741/2760. [DOI] [PubMed] [Google Scholar]
- Gregory CA, Singh H, Perry AS., and , Prockop DJ. The Wnt signaling inhibitor dickkopf-1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J Biol Chem. 2003;278:28067–28078. doi: 10.1074/jbc.M300373200. [DOI] [PubMed] [Google Scholar]
- Mizrak D, Brittan M., and , Alison MR. CD133: molecule of the moment. J Pathol. 2008;214:3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- Urbich C., and , Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao HL, et al. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007;204:1973–1987. doi: 10.1084/jem.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Chen Y, Lillo C, Chien J, Yu Z, Michaelides M, et al. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J Clin Invest. 2008;118:2908–2916. doi: 10.1172/JCI35891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LL, Townsend J., and , Anderson RA. The human fetal testis is a site of expression of neurotrophins and their receptors: regulation of the germ cell and peritubular cell population. J Clin Endocrinol Metab. 2003;88:3943–3951. doi: 10.1210/jc.2003-030196. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Endo K., and , Kinoshita S. Identification of human oral keratinocyte stem/progenitor cells by neurotrophin receptor p75 and the role of neurotrophin/p75 signaling. Stem Cells. 2007;25:628–638. doi: 10.1634/stemcells.2006-0494. [DOI] [PubMed] [Google Scholar]
- Young KM, Merson TD, Sotthibundhu A, Coulson EJ., and , Bartlett PF. p75 neurotrophin receptor expression defines a population of BDNF-responsive neurogenic precursor cells. J Neurosci. 2007;27:5146–5155. doi: 10.1523/JNEUROSCI.0654-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattoretti G, Schiró R, Orazi A, Soligo D., and , Colombo MP. Bone marrow stroma in humans: anti-nerve growth factor receptor antibodies selectively stain reticular cells in vivo and in vitro. Blood. 1993;81:1726–1738. [PubMed] [Google Scholar]
- Delorme B, Ringe J, Gallay N, Le Vern Y, Kerboeuf D, Jorgensen C, et al. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood. 2008;111:2631–2635. doi: 10.1182/blood-2007-07-099622. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Graves SE, Ohta S., and , Simmons PJ. The STRO-1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994;84:4164–4173. [PubMed] [Google Scholar]
- Bühring HJ, Battula VL, Treml S, Schewe B, Kanz L., and , Vogel W. Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci. 2007;1106:262–271. doi: 10.1196/annals.1392.000. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- Cai L, Johnstone BH, Cook TG, Liang Z, Traktuev D, Cornetta K, et al. Suppression of hepatocyte growth factor production impairs the ability of adipose-derived stem cells to promote ischemic tissue revascularization. Stem Cells. 2007;25:3234–3243. doi: 10.1634/stemcells.2007-0388. [DOI] [PubMed] [Google Scholar]
- Lee RH, Seo MJ, Pulin AA, Gregory CA, Ylostalo J., and , Prockop DJ. The CD34-like protein PODXL and alpha6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood. 2009;113:816–826. doi: 10.1182/blood-2007-12-128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Culture of adherent CD133-positive and CD133-derived cells (CD133BMSCs).
Cell surface phenotyping for Stro-1 from 3 different CD133BMSC, p75BMSC, and BMSC donors.
Cell surface phenotyping for CD90 from 3 different CD133BMSC, p75BMSC, and BMSC donors.
Osteogenic, adipogenic, and chondrogenic differentiation of typical BMSCs.
Summarized ELISA data for cells at 50% confluence.
ELISA data for selected growth factors secreted by p75BMSCs, CD133BMSCs, and BMSCs under normoxic and hypoxic conditions (1% oxygen).
ELISA data for cytokines secreted by p75BMSCs, CD133BMSCs, and BMSCs under normoxic and hypoxic conditions (1% oxygen).
Yields of CD133-positive cells from bone marrow aspirates.
Microarray GO terms.