Mesenchymal stem cells (MSCs) show promise for gene delivery to treat various diseases such as anemia and stroke, as well as other oncological and neural disorders.1,2 In this issue of Molecular Therapy, Campeau et al.3 report on an experimental model that compares the responses of allogeneic and syngeneic hosts to the transfer of erythropoietin (EPO)-expressing MSCs. The studies were based on the premise that EPO could be delivered using genetically modified MSCs to treat anemia or myocardial infarction. The treatment of anemia with expanded autologous MSCs seems plausible, in that the chronic nature of anemia is compatible with the time needed to expand bone marrow−derived MSCs to sufficient numbers. However, in the case of acute disorders such as myocardial infarction and stroke,4,5 gene delivery interventions would have to be immediate, thereby eliminating autologous gene-modified MSCs as an option. Because MSCs have been reported to suppress allogeneic responses, in particular graft-versus-host disease,6,7,8 “off-the-shelf” sources of such cells have been proposed to treat various clinical disorders that require intervention at early time points.
Allogeneic MSCs are already being evaluated in the clinic to treat graft-versus-host responses and other autoimmune disorders. These treatments are based on the immunosuppressive properties of MSCs. MSCs, as third-party cells in the allogeneic hematopoietic stem cell transplantation setting, can function as immunouppressive cells. Similar immunosuppression would not be relevant in autologous transplantation where rejection would not be a problem. Despite this promise of MSCs as third-party cells, this type of application is different from the delivery of MSCs to an allogeneic host to deliver a therapeutic gene, such as EPO. Because the delivery of allogeneic MSCs has been reported only relatively recently, it is difficult to verify their safety in allogeneic hosts, such as their use as cellular vectors for gene delivery. Thus, the safety of the use of MSCs for therapeutic gene transfer remains to be established.
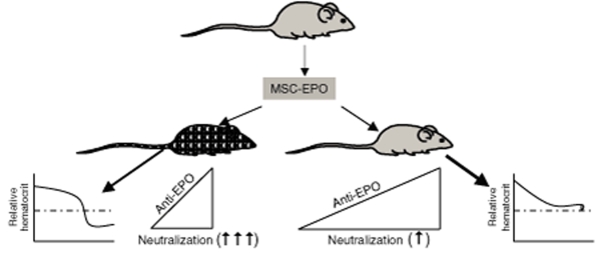
Campeau et al. now report unexpected phenotypic and immunological effects following the delivery of allogeneic MSCs engineered to express EPO into mice of different haplotypes: BALB/c and C57BL/6 (Figure 1). MSCs from C57BL/6 mice were engineered to express EPO using a retroviral system. The engineered cells were then injected subcutaneously into healthy syngeneic C57BL/6 mice and allogeneic BALB/C mice. In both cases there were transient increases in hematocrit. Although the baseline level of EPO was maintained following cell transfer in the syngeneic transplants, hematocrit levels soon decreased below baseline levels in the allogeneic transplants. The allogeneic mice showed rapid increases in antibodies against EPO (anti-EPO), whose levels were sustained for at least 7 weeks. In contrast, there was a gradual increase in anti-EPO levels in the syngeneic animals. At week 12 the significant differences in hematocrit levels between the two strains of mice could not be explained by differences in anti-EPO levels. The authors further explored this paradox by determining whether there were differences between the neutralizing abilities of the anti-EPOs in the two strains of mice. Using an EPO-responsive cell line, the authors compared the neutralization properties of anti-EPO in the sera of both strains of treated mice. Although anti-EPO from the allogeneic sera was able to neutralize EPO, the sera from the syngeneic sera showed only partial neutralization, suggesting differences in the avidity of the antibodies.
Figure 1.
Differential immune responses to EPO delivered by syngeneic and allogeneic mesenchymal stem cells (MSCs). MSCs from C57BL/6 mice (gray) were engineered to express erythroprotein (EPO) and then transplanted to syngeneic (gray) and allogeneic BALB/c mice (hatched). Whereas the 12-week levels of antibodies to EPO (anti-EPO) were similar in both hosts, the increase was acute in the allogeneic host (left triangle) as compared with a gradual increase in the syngeneic host (right triangle). Different neutralization properties were also observed for the two hosts. The allogeneic host produced anti-EPO that showed stronger avidity than the syngeneic host: ↑↑↑vs. ↑. The differences in the neutralizing properties of the sera from the two strains of mice correlate with the relative hematocrit levels (graphs at far left and far right).
To further understand the mechanism by which EPO expression induced anti-EPO in the allogeneic mice, the authors analyzed the MSCs for cytokine secretion. The major upregulated cytokine in the EPO-engineered MSCs, C-C motif chemokine 2, did not show evidence of involvement in the anti-EPO response, suggesting other mechanisms and/or involvement of other cytokines.
The findings by Campeau et al.3 are significant in that EPO is routinely delivered to individuals affected by disorders in which MSCs are reported to have roles in the pathogenesis, such as tumors and myeloproliferative disorders.9,10,11 For example, tumor progression has been reported in individuals with cancer who received EPO as a pro-erythropoietic agent for the treatment of cancer-related anemia.9,10 Because MSCs have also been implicated as a cellular support for tumor metastasis,2 these findings indicate the need to revisit the evaluation of EPO therapy in experimental models so as to improve treatment of anemia in individuals with cancer. Although Campeau et al. did not show antigen presentation by the EPO-expressing MSCs, it is possible that increased EPO levels in mice with a normal hematocrit could result in pathological responses. Specifically, the excess EPO might be processed as a foreign antigen and induce autoimmunity and the production of anti-EPO.12,13 If this premise were valid, it would be of interest to implant the engineered MSCs into animal models of anemia and then to compare the results to those achieved when implanting such cells into healthy mice with a normal hematocrit. Such studies might provide insight into whether the results of the present study may have originated with the supraphysiological levels of EPO. If anemic mice do not show increases in anti-EPO in response to transfer of the engineered MSCs, then “off-the-shelf” MSCs may still prove valuable for EPO delivery in individuals with EPO deficiencies.
It is interesting that the avidity of anti-EPO differed between the syngeneic and allogeneic transplants. Because the anti-EPO level gradually increased in the syngeneic animals, perhaps there is a selection for clones that produce antibody with weak avidity. If it could be determined that there is a mechanism to delete B-cell clones that produce anti-EPO with high avidity in syngeneic recipients, then adjuvant intervention may be possible to induce the deletion of such clones in allogeneic recipients, thereby eliminating anti-EPO with high avidity. Because the goal is to translate the studies to patients, a similar argument could be true for the delivery of EPO-engineered MSCs across an allogeneic barrier in humans.
Although the authors did not observe the induction of the expression of pro-inflammatory cytokines in the engineered MSCs, the studies nonetheless pointed to an immune-mediated mechanism that is likely to include interactions between MSCs and immune cells. The involvement of immune responses could have implications for EPO treatment for disorders in which there is already underlying immune activation, such as aplastic anemia, and for those in which there is already some dysfunction of endogenous MSCs.14,15
This report suggests caution with respect to the transplanting of genetically engineered MSCs across an allogeneic barrier. Interestingly, another recent report offers evidence for the reversion of the immunosuppressive properties of MSCs when the cells are transferred in vivo.16 The immune-stimulatory properties of MSCs, such as their antigen presentation and pro-inflammatory effects, may be equal in importance to their suppressive properties, such as recruitment of cells and inhibition of the graft-versus-host response. Whereas the literature is vast on the latter, information about the immune-enhancing properties of MSCs such as the expansion of T-suppressor MSCs is still scanty. Campeau et al. do not suggest that we must eliminate the use of “off-the-shelf” MSCs for gene delivery. However, they provide insightful evidence of the potential for untoward effects when MSCs are delivered into allogeneic recipients. The studies establish strong evidence for further preclinical research to attain safe delivery of off-the-shelf transplantation of MSCs, not only for gene delivery but also for other therapies, such as tissue repair.
REFERENCES
- Wang H., and , Chen X. Imaging mesenchymal stem cell migration and the implications for stem cell–based cancer therapies. Future Oncol. 2008;4:623–628. doi: 10.2217/14796694.4.5.623. [DOI] [PubMed] [Google Scholar]
- Gottfried ON., and , Dailey AT. Mesenchymal stem cell and gene therapies for spinal fusion. Neurosurgery. 2008;63:380–391. doi: 10.1227/01.NEU.0000324990.04818.13. [DOI] [PubMed] [Google Scholar]
- Campeau PM, Rafei M, François M, Birman E, Forner K-A., and , Galipeau J. Mesenchymal stromal cells engineered to express erythropoietin induce anti-erythropoietin antibodies and anemia in allorecipients. Mol Ther. 2009;16:369–372. doi: 10.1038/mt.2008.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., and , Chopp M. Neurorestorative treatment of stroke: cell and pharmacological approaches. NeuroRX. 2006;3:466–473. doi: 10.1016/j.nurx.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopp M, Li Y., and , Zhang J. Plasticity and remodeling of brain. J Neurol Sci. 2008;265:97–101. doi: 10.1016/j.jns.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Casiraghi F, Azzollini N, Cassis P, Imberti B, Morigi M, Cugini D, et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol. 2008;181:3933–3946. doi: 10.4049/jimmunol.181.6.3933. [DOI] [PubMed] [Google Scholar]
- Potian JA, Aviv H, Ponzio NM, Harrison JS., and , Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–3434. doi: 10.4049/jimmunol.171.7.3426. [DOI] [PubMed] [Google Scholar]
- Glennie S, Soeiro I, Dyson PJ, Lam EWF., and , Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- Gascon P. Safety update on erythropoiesis-stimulating agents: trials within and outside the accepted indications. Oncologist. 2008;13:4–10. doi: 10.1634/theoncologist.13-S3-4. [DOI] [PubMed] [Google Scholar]
- Newland AM., and , Black CD.Tumor progression associated with erythropoiesis-stimulating agents Ann Pharmacother 2008. in the press [DOI] [PubMed]
- Flores-Figueroa E, Montesinos JJ, Flores-Guzmán P, Gutierrez-Espindola G, Arana-Trejo RM, Castillo-Medina S, et al. Functional analysis of myelodysplastic syndromes–derived mesenchymal stem cells. Leukemia Res. 2008;32:1407–1416. doi: 10.1016/j.leukres.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Chan JL, Tang KC, Patel AP, Bonilla LM, Pierobon N, Ponzio NM, et al. Antigen-presenting property of mesenchymal stem cells occurs during a narrow window at low levels of interferon-γ. Blood. 2006;107:4817–4824. doi: 10.1182/blood-2006-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg J, Pommey S, Eliopoulos N., and , Galipeau J. Interferon-γ-stimulated marrow stromal cells: a new type of nonhematopoietic antigen-presenting cell. Blood. 2006;107:2570–2577. doi: 10.1182/blood-2005-07-2793. [DOI] [PubMed] [Google Scholar]
- Young NS, Scheinberg P., and , Calado RT. Aplastic anemia. Curr Opin Hematol. 2008;15:162–168. doi: 10.1097/MOH.0b013e3282fa7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacigalupo A, Valle M, Podestà M, Pitto A, Zocchi E, De Flora A, et al. T-cell suppression mediated by mesenchymal stem cells is deficient in patients with severe aplastic anemia. Exp Hematol. 2005;33:819–827. doi: 10.1016/j.exphem.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Prigozhina TB, Khitrin S, Elkin G, Eizik O, Morecki S., and , Slavin S. Mesenchymal stromal cells lose their immunosuppressive potential after allotransplantation. Exp Hematol. 2008;36:1370–1376. doi: 10.1016/j.exphem.2008.04.022. [DOI] [PubMed] [Google Scholar]