Abstract
Cancer stem cells (CSCs) are defined by their ability to (i) fully recapitulate the tumor of origin when transplanted into immunodeficient mouse hosts, and (ii) self-renew, demonstrated by their ability to be serially transplanted. These properties suggest that CSCs are required for tumor maintenance and metastasis; thus, it has been predicted that CSC elimination is required for cure. This prediction has profoundly altered paradigms for cancer research, compelling investigators to prospectively isolate CSCs to characterize the molecular pathways regulating their behavior. Many potential strategies for CSC-directed therapy have been proposed, but few studies have rigorously demonstrated their efficacy using in vivo models. Herein, we highlight recent studies that demonstrate the utility of CSC-directed therapies and discuss the implications of the CSC hypothesis to experimental design and therapeutic strategies.
Introduction
Despite the many advances in our understanding of human cancer development, our ability to develop clinically effective therapies based on this knowledge has been met with limited success.1,2,3 Although conventional therapies frequently initially control tumor growth, most patients ultimately relapse. When the first molecularly targeted cancer therapy—imatinib—was developed to treat chronic myeloid leukemia (CML), there was great hope that such rationally based strategies would lead to cure, but the initial excitement has been tempered by the realization that imatinib does not effect cure.4,5 This result is not surprising since imatinib does not target the quiescent hematopoietic stem cells (HSCs) that harbor the characteristic BCR-ABL translocation of CML.6,7,8 These findings underscore the need to isolate self-renewing and therapy-resistant cancer cells as well as to determine the molecular pathways that regulate their biological behavior. Identification of such molecular pathways offers the best hope for developing curative cancer therapies.
Over the past decade there have been extensive efforts to isolate cancer stem cells (CSCs) in solid and hematopoietic cancers (Table 1). Since the isolation and characterization of CSCs is the focus of numerous excellent review, we will focus instead on the data that address three predictions of the CSC hypothesis: (i) that CSCs are therapy-resistant cells; (ii) that CSC-directed therapies can effectively treat cancers; and (iii) that CSCs are relevant to both the biological behavior and clinical outcomes of cancers. Because experimental design is an important consideration when evaluating these data, we also discuss strategies for evaluating CSCs in each of these contexts.
Table 1.
Selected CSCs identified in primary tumor isolates
The CSC Hypothesis
It has been appreciated for more than a century that tumors are composed of morphologically heterogeneous cells, and by the mid-twentieth century researchers understood that cancer cells also exhibit functional heterogeneity both in vitro9,10,11,12 and in vivo.13,14,15 Despite this evidence for tumor cell functional heterogeneity, subsequent research emphasized the monoclonal nature of cancers with investigators reconciling tumor monoclonality with tumor heterogeneity by hypothesizing that the tumor microenvironment or the presence of clinically inapparent genetic subclones could explain the variable behavior of tumor cells.16,17 In addition, the practical difficulties in obtaining primary tumor tissue for research as well as the relative ease of manipulating tumor cell lines promoted research that largely disregarded functional differences in tumor cell subsets.
Although researchers had long-suspected that cancers may arise from stem or stem-like cells,18 it was not until the mid-1990s that a stem cell–like population was prospectively isolated from a human cancer. John Dick and colleagues showed that human acute myeloid leukemia (AML) contains a small percentage of cells (typically 0.1–1%) capable of transferring human AML into immunodeficient mouse hosts.19,20 The resulting leukemia recapitulates the morphologic and immunophenotypic heterogeneity of the original disease, and engrafted blasts can transfer disease into secondary recipients, formally establishing the presence of a self-renewing population. The leukemia-initiating cells, also known as leukemia stem cells (LSCs), could be FACS-purified by virtue of their cell surface phenotype (CD34+CD38−). Moreover, CD34+CD38+ cells did not engraft mice, consistent with a hierarchical organization in AML, with LSCs giving rise to non-LSCs, but not vice versa. Based on comparison of the immunophenotypes of LSCs to normal human HSCs and progenitors, it was predicted that AML LSCs arise from the earliest progenitors of the hematopoietic system.19,20 We had previously shown that human HSCs could be prospectively isolated from CD34+CD38lo cells based on the expression of CD90/Thy-1 (refs. 21,22) and that these cells were distinct from AML LSCs, which lack CD90 expression.23 These data suggest that AML LSCs are not likely to be derived from HSCs, but possibly from the recently described human multipotent progenitor.24,25
These seminal studies of AML LSCs laid the foundation for the CSC hypothesis which holds that, like normal tissue, cancers are maintained by a population of stem-like cells that exhibit the ability to self-renew as well as differentiate into downstream, non-self-renewing progenitors and mature cells.26 Based on the studies identifying AML LSCs and other CSCs, a consensus definition for CSCs has been established, with a recent AACR workshop declaring that “cancer stem cells can only be defined experimentally by their ability to recapitulate the generation of a continuously growing tumor.”27 In practical terms, this means that a candidate CSC population should exhibit the following properties: (i) the unique ability to engraft; (ii) the ability to recapitulate the tumor of origin both morphologically and immunophenotypically in xenografts; and (iii) the ability to be serially transplanted. Experimental demonstration of CSC properties requires the use of xenotransplantation systems to demonstrate both tumor-initiating potential and the ability to be serially transplanted, the gold-standard for demonstrating self-renewal capacity.27 In our laboratory, CSCs are defined by transplantation of cancer cell subsets into immunodeficient mice, typically at orthotopic sites (e.g., breast to breast, brain to brain, marrow to marrow). In our experience, secondary transplants usually grow faster than primary grafts, and tertiary transplants grow even faster, likely representing enrichment for more aggressive subsets of CSCs. This reminds us that even CSCs are susceptible to selection pressures and that more aggressive tumors (and probably all metastases), likely represent the end-result of a competition among CSCs in a given tumor.
As illustrated by the discovery of LSCs in AML, the characterization of stem and progenitor cells in normal tissue has expedited CSC isolation by providing candidate markers for purification of candidate CSC populations. Such prospective isolations of CSC allow their direct comparison to normal stem/progenitors, revealing important information about CSC regulation, CSC origins, and disease pathogenesis.26,28 For example, although LSCs in AML exhibit the human multipotent progenitor phenotype,24 malignant cells in blast crisis of CML are Lin-CD34+CD38+CD45RA+ CD123+, corresponding to a normal human granulocyte-macrophage progenitor. Moreover, in blast crisis of CML, phenotypic granulocyte-macrophage progenitors show aberrant activation of the Wnt/β-catenin signaling pathway, a self-renewal associated pathway not active in normal granulocyte-macrophage progenitors.29 These data indicate that LSCs may arise from normal progenitor populations, and that aberrant activation of self-renewal pathways may be a part of this process.
It is important to emphasize that the term “cancer stem cell” does not refer to the cell of origin. Instead, the term CSC refers to the properties shared with normal stem cells—self-renewal and the ability to initiate a hierarchy of more differentiated cells that cannot self-renew. Based on these properties, the CSC hypothesis makes two important predictions: (i) CSCs are required for tumor growth and metastasis; and, (ii) elimination of the CSCs is required for cure.26 Based on observations in the clinic as well as in normal stem cells, some investigators have introduced an important corollary to these predictions—that CSCs are also relatively resistant to conventional therapy. Needless to say, these predictions have challenged investigators to isolate CSCs in all tumor types and identify the genes that regulate their function and responses to conventional therapies.
Expanding the CSC Roster
Since the description of LSCs in AML, CSCs have been identified in numerous solid and hematopoietic cancers (Table 1). Although CSC populations have been described for numerous mouse cancer models and cell lines, we will largely limit our discussion to CSCs from primary human tumors.
The first solid tumor CSC was isolated from breast cancer by Michael Clarke's group.30 Although the normal human mammary stem cell still had not been isolated at the time of these studies, it had been appreciated that early multipotent epithelial progenitor cells express markers such as epithelial specific antigen (ESA) and CD44 (refs. 31,32,33,34). Using these markers, candidate CSC populations were isolated from dissociated primary breast tumors, FACS-purified, and transplanted into the mammary fat pads of NOD/SCID mice. Importantly, when separating tumor cells, a cocktail of lineage antibodies was used to exclude hematopoietic, mesenchymal, and endothelial components of the tumor. These studies showed that CD44+, CD24−/low cells were uniquely capable of transplanting disease to NOD/SCID mice. This population, representing 11–35% of cells in primary breast tumors, gave rise to tumors that recapitulated the morphologic and immunophenotypic features of the original tumor. In addition, these same cells could be sorted from the primary grafts and serially transplanted, demonstrating their self-renewal capacity.
Since the description of the breast CSCs, numerous other solid tumor CSCs have been identified. Although the degree of rigor to which these populations were isolated differed both with respect to purity as well as their ability to serially transplant disease, they all fulfilled the CSC criteria established by the AACR. CSCs in colon,35 pancreatic,36 prostate,37 and head-and-neck squamous cell38 carcinomas were enriched by virtue of their expression of CD44, a marker of progenitor cells in the basal layer of normal epithelium. Hepatocellular carcinoma CSCs were isolated based on the presence of CD90, a marker previously described on HSCs. This population could be further separated into highly tumorigenic and less-tumorigenic populations based on CD44 expression.39 CD133, a marker previously shown to enrich for immature progenitors in normal hematopoietic, neural, endothelial and epithelial tissues,40,41,42 was found to enrich tumor-initiating cells in glioblastoma and medulloblastoma,43,44,45,46 colon cancer,35,47,48,49 pancreatic cancer,50 and lung cancer.51 It is important to note that CSCs for some tumors have been described using different markers, e.g., CD44 and CD133 for both colon and pancreatic cancer, suggesting either that these CSC markers are co-expressed or may represent overlapping, yet distinct tumor cell populations. In colon cancer, CD133 does not identify CSCs in all patient samples, indicating that all tumors arising from a given tissue may not express the same surface markers.35 In addition, CD133 does not uniquely enrich for tumorigenic potential at metastatic sites, suggesting that expression of surface markers by CSCs may vary over time and/or by location.52 Clearly, more research will be required to determine the variability in CSC phenotypes for each type of tumor.
In some cases, markers not previously identified on normal stem/progenitors have been used to isolate tumorigenic populations. In the case of melanoma, tumorigenic cells were isolated using a candidate gene approach after identifying genes associated with melanocytic tumor progression (i.e., benign melanocytic nevi, primary cutaneous melanoma, metastases to lymph nodes, and metastases to viscera). One of these genes, ABCB5, a known chemoresistance mediator in melanoma, was expressed in only a minority of tumor cells (1.6–20.4%), and only ABCB5+ cells were capable of establishing primary grafts and serially transplanting disease in NOD/SCID mice. In addition, ABCB5+ cells gave rise to both ABCB5+ and ABCB5− cells, confirming a hierarchical organization among the tumor cells.53
Because CSC cell surface phenotypes may not apply to all cancers arising from the same tissue type, some groups have attempted to isolate CSCs based on functional activity. For example, high ALDH activity has been described in murine and human hematopoietic and neural stem and progenitor cells.54,55,56 Investigators have FACS-purified and functionally characterized human cancers based on ALDH expression, demonstrating that ALDH+ cells are enriched for CSCs in AML57,58 as well as in primary breast59 and colon cancer.60
CSCs have been identified in a number of hematopoietic diseases as well. Examples include acute B-cell lymphoblastic leukemia,46,61 T-cell lymphoblastic leukemia,62 the chronic phase of chronic myelogenous leukemia,63 and multiple myeloma.64,65 Similar to AML, CSCs in each of these lesions exhibit immunophenotypes that correspond to normal progenitor populations that are more primitive than the normal immunophenotypic counterparts of the nontumorigenic cells. For example, in multiple myeloma, the tumorigenic population exhibits a memory B-cell phenotype (CD19+CD27+CD138−), even though the predominant malignant population is composed of plasma cells that are CD19−CD138+.65 Similarly, in B-cell lymphoblastic leukemia and T-cell lymphoblastic leukemia, the LSC population expresses CD34 but lacks CD38 whereas the nontumorigenic blasts are CD38 positive.
CSC Controversies
Although tumorigenic populations have been isolated from numerous human cancers, the CSC hypothesis remains the target of numerous criticisms. The most common critique of the CSC hypothesis is that xenotransplantation systems only measure the ability of a human tumor cell to grow in a permissive mouse niche and do not reflect intrinsic properties of tumor cells in humans.66,67,68 To address the potential confounding variables introduced by xenotransplantation, some advocate using transgenic mouse cancer models and syngeneic transplantation systems to evaluate CSCs. Although such models remove potential cross-species barriers to engraftment and may more faithfully recapitulate CSC interactions with the microenvironment, the relevance of such models to human disease is unclear, particularly with respect to measuring tumor responses to CSC-directed therapies. Although there is no direct evidence that xenotransplantation systems have incorrectly identified human CSCs, studies have shown that CSC xenografts occupy the same niches as those of their normal stem cell counterparts, suggesting that at least some aspects of the tumor microenvironment are recapitulated in the xenograft setting.69,70 Although criticisms regarding the imperfect xenograft tumor environment may never be completely laid to rest, one must remember that at present there is no superior assay system to measure the tumorigenic potential of primary human tumor isolates. Hopefully, this issue will be resolved through the use of more “humanized” models in which mice are either engrafted with human tissue to serve as niches for CSC transplantation or engineered to produce human growth and/or survival factors that may be required for tumor engraftment.
The potential to be misled by xenotransplantation models was raised by a recent study of human AML LSCs. Dominique Bonnet's group observed that pretreatment of primary human cord blood and bulk AML blasts with anti-CD38 antibody before transplantation inhibited engraftment of both cell types in NOD/SCID mice, but not in NOD/SCID/IL2-Rγ deficient mice.71 They also showed that both CD34+CD38− and CD34+CD38+ blasts could engraft NOD/SCID/IL2-Rγ mice and that treatment of NOD/SCID recipients with intravenous immunoglobulin before transplantation partially abrogated the effect of anti-CD38 treatment. Together, these results suggested that anti-CD38 inhibition of engraftment may be mediated by natural killer cells. Unfortunately, these studies evaluated a limited number of AML samples and did not include limiting dilution experiments to assess LSC frequencies in both the CD34+CD38− and CD34+CD38+ populations. In addition, it was unclear why prior in vitro and in vivo studies using the same CD38 antibodies to sort normal human HSC/progenitor populations supported a similar hierarchy of human stem/progenitors.25 Although these findings need to be further evaluated, they illustrate the potential confounding variables introduced when using xenotransplantation systems to identify CSC populations.
A second concern regarding the CSC hypothesis is that cells purified from tumors on the basis of CSC markers may include malignant cells as well as stromal elements that are important for engraftment.66 A number of studies have addressed this argument by separating stromal cell populations from sorted CSC populations before xenotransplantation.30,35,36,38 In addition, a recent study showed that outgrowths derived from single CSC are sufficient to engraft tumors, strongly supporting the contention that that only CSC-derived elements are required for engraftment.72
Another criticism is raised by cancer researchers who believe that all tumor cells have tumorigenic potential and they point out that large numbers of “nontumorigenic” cells can initiate tumors.67,73 Although it will be difficult to definitively exclude this possibility because even the most rigorous separation techniques cannot routinely yield 100% pure cell populations, this criticism does not negate the fact that tumorigenic populations can be highly enriched. Thus, the importance of prospectively isolating CSCs and evaluating the molecular pathways that regulate their function has not been brought into question.
A fourth criticism raised against the CSC hypothesis is that some mouse and human cancers contain high frequencies of tumorigenic cells, which means that, unlike normal tissues, stem cells in cancer are not rare.67 We agree with this statement and note that it is likely that cancers exhibit heterogeneity with respect to CSC frequency, with more poorly differentiated cancers contain larger percentages of CSCs. However, in our opinion, instead of undermining the CSC hypothesis, the variable frequency of CSCs in different types of cancer underscores the need to identify CSCs for each tumor without altering the fundamental promise of the CSC hypothesis—that prospectively isolating and studying tumorigenic populations will expedite discovery of future CSC therapies.68
A final concern regarding the CSC hypothesis is that it is too simplistic because it ignores the complex interactions between tumor cells and their microenvironment. Certainly, this is an important criticism because solid tumor and epithelial tumors have been shown to interact with components of the microenvironment,74,75 In our view, supporting the CSC hypothesis and acknowledging that the CSC hypothesis may not provide a complete view of cancer biology should not be mutually exclusive acts. Instead, taking such a position leaves room for development of more comprehensive cancer models that account for the interplay between malignant cells and the tumor microenvironment based on investigations that explore both intrinsic and extrinsic properties of CSCs.
Ultimately, resolution of many of the issues raised by critics of the CSC hypothesis will require additional investigations, and such investigations will likely involve improving existing xenograft models or developing new experimental strategies (Figure 1). In the meantime we propose that the most important information to resolve lingering controversies regarding the CSC hypothesis would be the demonstration that CSCs are responsible for determining clinical responses to therapy as well as long-term patient outcomes, including disease-free and overall survival. Although such data are limited because of the relative youth of the CSC field, several studies already suggest that CSCs fulfill this prediction and are discussed later in this review.
Figure 1.
Strategies to evaluate CSC-directed therapy in preclinical models. Top: Treat bulk tumor or CSCs in vitro, then measure the therapeutic effect in vitro. Parameters to be measured include (i) decreased growth/increased cell death, (ii) CSC selectivity, and (iii) differentiation. Top: Pretreat bulk tumor or purified CSCs in vitro followed by transplantation. Parameters to be measured include level of engraftment (tumor burden), frequency of CSCs, and durability of effect. Ideally, transplanted mice should be monitored serially. Bottom: Treatment of previously engrafted tumors in vivo. Parameters to be measured include tumor growth/reduction, differentiation status, durability of effect, and ability of any residual disease to serial transplant. Ideally, serial transplants should be performed with equivalent numbers of tumor cells, either bulk or purified CSCs, to determine whether or not the effect of a drug is at the level of single cells or entire tumor cell populations.
CSC Responses to Conventional Therapy
It has been hypothesized that CSCs possess several characteristics that make them resistant to conventional chemo- and radiotherapy including high expression of drug transporters, relative cell cycle quiescence, high levels of DNA repair machinery, and resistance to apoptosis.76,77 Although these features have been demonstrated in numerous normal tissue stem cells, direct evidence for the presence of similar pathways in CSCs is still limited. Because this is an important consideration in designing potential CSC-directed therapeutics, several groups have attempted to experimentally address the question of whether CSCs constitute the therapy- resistant fraction in tumors.
Although tumorigenic populations have been variably enriched in some tumor cell lines by virtue of their ability to actively efflux Hoechst dyes (frequently referred to as “side-population” cells),78,79,80 reports of enrichment for CSC activity in side-population cells from primary tumors is far more limited. In AML there is evidence that LSCs are preferentially drug resistant compared to non-LSCs, similar to normal HSCs compared to committed progenitors. Costello et al. found that CD34+CD38− cells in both AML patients and normal patients exhibited decreased daunorubicin sensitivity compared to CD34+CD38+ cells, and that this difference correlated with higher levels of mRNA expression of the drug resistance-related genes LRP and MRP.81 The decrease in daunorubicin influx in CD34+CD38− cells was associated with increased proliferation and survival (reduced apoptosis) following treatment. Unfortunately, like many evaluations of drug susceptibility of CSCs, these studies lacked an in vivo correlate—testing whether or not daunorubicin-treated CD34+CD38− cells have the capacity to initiate leukemia.
Quiescence is typically thought to confer resistance to therapies that target highly proliferating cells. The importance of quiescence to CSC clinical behavior has not been investigated extensively, but human AML LSCs have been shown to reside almost exclusively within the G0 phase of the cell cycle.82,83 Although CSCs may be nonproliferative compared to non-CSCs, quiescence may not be sufficient to mediate drug resistance, as some studies have shown that CML-derived cell lines induced to cell cycle arrest were no less sensitive to imatinib-induced apoptosis than proliferating cells.6,84
In vitro studies of solid tumor CSCs indicate that they are relatively resistant to chemotherapeutic agents. For example, freshly isolated or cultured CD133+ cells from glioblastoma exhibited less cell death than their CD133− counterparts when treated with multiple chemotherapeutic agents. Intriguingly, the relative chemoresistance of primary CD133+ cells was not due to increased drug efflux activity, suggesting that defects in cell death pathways may play an important role in their chemoresistance.85 Microarray studies supported this idea, showing that CD133+ cells overexpress the DNA repair protein MGMT as well as genes that inhibit apoptosis including FLIP, Bcl-2, Bcl-XL, and numerous IAP family members.86 Similarly, treating CD133+ lung tumor spheres from non-small cell lung carcinomas with cisplatin, etoposide, paclitaxel, or gemcitabine in doses comparable to those achieved in patients resulted in minimal reduction of tumor cell viability even after 5 days of treatment.51 Primary CD133+ pancreatic CSCs also showed dramatically reduced sensitivity to gemcitabine compared to autologous CD133− cells.50 Unfortunately, none of these studies assessed whether or not the surviving cells maintained tumor-initiating potential.
Although development of CSC-directed therapies will require a better understanding of CSC responses to conventional therapies in the xenograft setting, to date, only a few studies have done so, One study directly assessed the response of AML LSCs to cytosine arabinoside (Ara-C), a standard chemotherapeutic agent for the treatment of AML. Ishikawa et al. demonstrated that Ara-C treatment of stably xenografted AML resulted in significant blast death; however, blasts in endosteal areas, thought by some to represent the HSC niche, were relatively spared.69 Bone marrow examination revealed that endosteal blasts preferentially exhibited a CD34+CD38− phenotype and were more quiescent than residual CD34+CD38+ blasts. Moreover, residual CD34+CD38− blasts could stably engraft secondary recipients. These data demonstrate that LSCs represent the Ara-C resistant fraction in AML and that the LSC phenotype is not altered with treatment, indicating that monitoring of CSC frequency using flow cytometric techniques during therapy may be a viable means of assessing drug efficacy.
Recent data demonstrate that some solid tumor CSCs are relatively resistant to conventional chemotherapy. Evaluation of disassociated tumor cells from breast cancer core biopsies before and after neoadjuvant chemotherapy revealed that the percentage of immunophenotypic CSCs (CD44+/CD24−/low) was significantly increased following therapy; however, treatment with lapatinib, an epidermal growth factor receptor (EGFR)/HER2 pathway inhibitor, led to a nonstatistically significant decrease in the percentage of immunophenotypic CSCs.87 In both treatment groups the frequency of CSC post-therapy directly correlated with the capacity to form mammospheres in vitro and initiate tumors in immunodeficient mice, consistent with CSC activity. Unfortunately, CSC reductions were not correlated with long-term clinical outcome. Nevertheless, these studies suggest that lapatinib, by virtue of its potential ability to target CSCs as well as non-CSCs, may be a useful therapy for the treatment of breast cancer in combination with standard neoadjuvant chemotherapy. In colorectal cancer, enrichment of CSC following chemotherapy was demonstrated using xenografted tumors. After allowing xenogeneic tumors to engraft, mice were treated with cyclophosphamide or irinotecan. Residual tumors following treatment were enriched for immunophenotypic CSCs (ESA+CD44+) and also showed increased frequencies of tumorigenic cells as demonstrated by in xenotransplantation assays.60 In addition, colorectal CSC expressed elevated ALDH1 expression and activity, and ALDH1 was an important mediator of resistance to cyclophosphamide, identifying a potential target for CSC-directed therapy.
The best studies of radioresistance in CSCs have been performed in glioblastoma. Rich and colleagues demonstrated that treatment of xenografted glioblastomas with radiation resulted in tumors enriched for CD133+ cells, the population previously shown to be enriched for glioblastoma CSCs.88 Following irradiation, both CD133+ and CD133− cells induced DNA damage responses, but CD133+ cells showed functional differences compared to CD133− cells including: (i) basal activation of the DNA damage repair pathway; (ii) greater efficiency in repairing the DNA damage; and (iii) less apoptosis following radiation by robustly inducing checkpoint kinases. Intriguingly, the cellular pathways implicated in mediating the radioresistance significantly overlap with those that regulate normal stem cell function (e.g., Wnt/β-catenin).
Attempts to assess the radiosensitivity of breast CSCs have utilized breast cancer cell lines. Using conditions for culturing mammospheres from primary breast cancer specimens, adherent cell lines were adapted to grow as spheroids containing increased numbers of CD44+/CD24− cells, previously shown to enrich for CSC activity in breast cancer. Following irradiation in vitro, the spheroids exhibited a higher fraction of surviving cells compared to the adherent cells, and the percentage of nonadherent CD44+/CD24− cells in monolayer cultures increased, suggesting that the expansion of spheroid cells was due to their relative radioresistance.89 Consistent with this observation, induction of reactive oxygen species and phosphorylation of H2AX, both indicators of a radiation response, were decreased in spheroids compared to adherent cells.
Although the number of studies directly testing the responses of CSCs to conventional therapies is limited, these early studies suggest that CSCs are relatively resistant to conventional therapies; however, their mechanisms of resistance may be varied. This underscores the importance of isolating CSCs from each tumor type and determining the molecular pathways that mediate their resistance. It is also important to consider that not all cells in currently defined CSC populations will make-up the therapy-resistant fraction in tumors. In such cases, additional markers may be required to separate true CSCs from contaminating populations.
General Considerations for CSC Therapeutics
Although the goal for any CSC-directed therapy is the eradication of all CSCs, the efficacy of single-agents may be limited by several factors. First, currently defined CSCs may not be homogeneously sensitive to any given therapy. Second, as discussed previously, CSC immunophenotypes may not be homogeneous, which may limit the efficacy of monoclonal antibody therapies directed at cell surface markers. Third, pathways shared by CSCs and normal stem cells may limit dosing due to toxicity to normal stem cells. Finally, and perhaps most important, there is no reason to expect that CSCs will be free from selection pressures and therefore therapy-resistant CSC clones may emerge.90 The idea that genetically resistant cells exist before initiation of treatment is not new;91,92,93 however, the implication for designing cancer treatment is significant since therapy with curative intent will likely require targeting of 2 or 3 independent CSC pathways to sufficiently reduce the probability of an escape mutant.
The development of combinatorial therapeutic strategies will likely rely on adding new CSC-directed therapies to established therapies. Thus, CSC-directed therapy would be given to patients who have already undergone standard induction therapy, at the nadir of the absolute CSC burden. Such combinatorial strategies may be designed to block multiple intrinsic and extrinsic (microenvironment) pathways that regulate CSC function. This raises the important question of whether or not CSCs and normal stem/progenitor cells use conserved self-renewal pathways. In that case, treatment strategies followed by transplantation may be required to rescue normal tissue function. Overall, we envision several potential strategies for administration of CSC-directed therapies: (i) stand-alone therapy in which the therapy targets CSCs but spares normal stem cells; (ii) stand-alone therapy in which the therapy targets CSCs but also targets normal stem cells; (iii) combination therapy—options no. 1 or no. 2 in combination with other drugs. Other combination strategies include pretreatment of a tumor with an agent that sensitizes CSCs or all tumor cells to subsequent therapy, or debulking agents given before treatment with CSC therapy (Figure 2).
Figure 2.
Potential CSC therapeutic strategies. CSC-directed therapeutic strategies may include stand-alone and combination therapies. (a) The CSC hypothesis predicts that conventional therapies fail to target CSCs, resulting in disease relapse. CSC therapies may exert a direct cytotoxic effect on CSCs or by inducing differentiation. (b) Combination therapies involve using conventional therapies or novel sensitizing agents in combination with CSC-directed therapies. Please refer to the text for descriptions of the cited examples.
In our view, preclinical validation of an effective CSC therapy requires demonstration that the intervention affects the two defining properties of CSCs—the ability to initiate tumors and serially transplant disease. Demonstration of therapeutic efficacy can be accomplished in a number of ways, each representing differing levels of rigor, and each more accurately reflecting clinical scenarios. Although treatment of CSCs in vitro and monitoring of subsequent engraftment is a necessary first step in evaluating therapies, this strategy may not accurately reflect in vivo responses to treatment since cells adapted to culture may not mimic primary CSC isolates. A more clinically relevant strategy involves therapeutic interventions that target primary CSCs in engraftment models. This can be accomplished in a number of ways: (i) demonstrate the ability of the intervention, either given before or at the same time as transplant, to block CSC engraftment; (ii) demonstrate the ability of the intervention to eliminate or block the growth of previously engrafted tumors; (iii) perform no. 2 as well as assess the ability of the treatment to abrogate the ability of the tumor to serially transplant (Figure 1). Approaches 2 and 3 would provide strong evidence that the intervention is effective, both in terms of its tumoricidal activity and its bioavailability. Elimination of the CSC population can be demonstrated by the inability of the treated tumor to reinitiate tumors in secondary recipients.
The CSC hypothesis also has important implications for the methods used to evaluate the clinical efficacy of CSC-directed therapies. Initial positive clinical responses to conventional therapies, usually measured over weeks to months following initiation of therapy, do not necessarily portend improved survival.94 Therefore, the traditional assumption in cancer therapeutics—that objective clinical responses (e.g., reduction in tumor size or burden) will translate into improved survival—may not necessarily hold for CSC therapies, which may not necessarily act by reducing tumor burden in the short-term. Thus, investigators must avoid study designs that solely rely on such traditional approaches since this may lead to premature abandonment of therapy. One such case may be the use of rituximab in the treatment of myeloma. Although rituximab is effective in treating B-cell lymphomas,95 its activity in multiple myeloma has been less impressive, despite evidence that the myeloma stem cell has the phenotype of a CD20 positive memory B cell.64,65 It is possible that longer treatment periods may be required to exhaust CSCs and observe clinical responses.
How, then, should one confront the difficulties in evaluating CSC-directed therapies? Certainly, in vivo xenotransplantation and in vitro preclinical models will allow investigators to anticipate the pattern of clinical responses and design clinical trials based on those results. For example, if a particular therapy selectively depletes CSCs in a xenograft model without significantly reducing tumor size during the initial response, investigators can design clinical trials with longer observation periods to measure outcomes. Mathematical models may also aid in planning clinical studies. For example, Michor et al. developed a mathematical model based on normal hematopoietic development to characterize the kinetics of the reduction of BCR-ABL positive cells in response to imatinib, the mainstay of CML therapy. The number of BCR-ABL positive transcripts during and following cessation of therapy was consistent with presence of LSCs that had not been eradicated with treatment.96,97 Using such models, investigators may be able to predict the minimum observation period required to monitor for recurrent disease following therapy cessation, or they may be able to predict when the maximum therapeutic effect of a particular debulking therapy is achieved so that CSC-directed therapies may be initiated at that time. Alternatively, investigators may establish simple tests that act as surrogates for measuring CSC activity during and after therapy. Since a major implication of the CSC hypothesis is that CSCs are responsible for metastasis, monitoring of circulating tumor cells or disseminated tumor cells, may represent an effective means of monitoring tumor responses to therapy. Although the relationship of circulating tumor cells and disseminated tumor cells with CSCs is not currently clear, circulating tumor cells and disseminated tumor cells appear to be similar to CSCs with respect to CSC surface marker expression, relative quiescence, and relative resistance to chemotherapy.98,99,100 Finally, clinical trials may be designed to include primary and secondary endpoints: the primary endpoint would be progression-free survival and the secondary endpoint might involve direct, serial measurement of CSC burden or ex vivo functional assays using isolated CSCs. Demonstrating a positive correlation between survival and CSC functional assays would validate such methods or future clinical use (Figure 2). Already, retrospective analyses have been performed for AML and gliobastoma, and they have demonstrated a correlation between CSC burden at the time of diagnosis and clinical endpoints, including achievement of remission as well as overall survival (see below).
Targeting Molecular Pathways in CSCs
Because both normal stem cells and CSCs self-renew and differentiate, perhaps it is not surprising that several pathways that regulate normal stem cell function may play important roles in CSC biology. These molecular pathways include Wnt/β-catenin, Hedgehog, Notch, Hox family members, Bmi-1, PTEN, telomerase, efflux transporters, and others.26,101,102,103 At this time, experimental evidence for CSC dependence on these pathways is limited, but because normal and malignant stem cells may share important functional pathways, it will be important to develop CSC-selective therapies that avoid potential significant side effects caused by inhibition of normal stem cell function. Recent data suggest that such selectivity is possible.
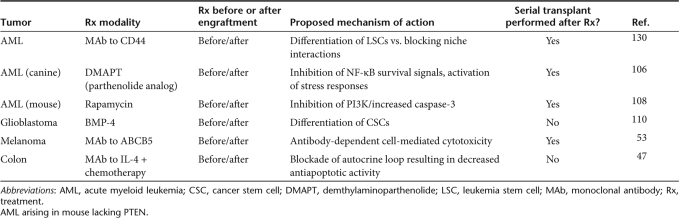
Studies in AML have shown that LSCs may exhibit differential sensitivity to small molecule inhibitors compared to their normal stem/progenitor counterparts. Craig Jordan's group has demonstrated the selective effect of several small molecule inhibitors on primary CD34+CD38− AML blasts. First, they showed that LSCs, but not normal HSCs, were susceptible to the apoptotic effects of combination therapy with the proteasome inhibitor MG-132 and the anthracycline idarubicin, presumably through inhibition of NF-κB activity.104 They have also shown that treatment with TDZD-8, a compound previously shown to be cytoprotective, kills leukemic blasts in vitro through induction of oxidative stress, but spares normal HSCs/progenitors.105 In other studies, they showed that naturally occurring (parthenolide) and synthesized (demthylaminoparthenolide—DMAPT) inhibitors of NF-κB induce apoptosis of CD34+CD38− blasts but spare normal HSCs.83,106 These studies were notable because the in vitro observations were verified in vivo by treating mice previously engrafted with canine AML with DMAPT. DMAPT treatment resulted reduced the number of blasts and a significant reduction in the ability of primary grafts to engraft secondary recipients. Blasts from DMAPT treated mice also showed substantial inhibition of NF-κB and activation of stress responses. When leukemic dogs were treated, DMAPT induced a rapid reduction in CD34+ blasts without a significantly altering in leukocyte counts. Blood cell morphology suggested that induced differentiation of blasts was likely responsible for the blast reduction. Unfortunately, these studies did not test the durability of remissions, which would have provided important information about DMAPT as a primary induction therapy. Overall, these small molecule inhibitor studies provide intriguing data that LSCs and HSCs can respond differentially to blockade of cell survival pathways.
The PTEN pathway was recently implicated in LSC survival as well. PTEN (phophatase and tensin homologue) is deleted in a number of human cancers, including hematopoietic malignancies.107 Recently, two groups demonstrated that deletion of PTEN in the mouse hematopoietic system results in AML following a progressive decline in the number of bone marrow HSCs.108,109 The dependence of leukemia development on this pathway was illustrated through the use of rapamycin, which acts as an inhibitor of the PI3K/PTEN signaling pathway. Treating leukemic blasts before, or following, stable engraftment in secondary hosts led to a dramatic decrease in leukemic burden.108 Moreover, this effect appeared to be specific for the leukemia since normal HSCs were unaffected as judged by stable blood counts.
Piccirillo et al. have shown that treating CSCs with differentiation factors can effectively deplete CSCs in human glioblastoma.110 In addition to showing that BMP-4 promotes differentiation of tumor cells in vitro, they also showed that CD133+ glioblastoma cells pretreated with BMP-4 before xenotransplant did not form invasive tumors. BMP-4 inhibited tumor growth, when administered either at the time of tumor implantation or 10 days later; however, a subset of mice still developed tumors 3 months after treatment, indicating that BMP treatment did not force all CSCs to differentiate. Unfortunately, the authors did not examine the efficacy of this treatment on established xenografts or on the ability of the residual tumor cells to initiate new tumors. Nevertheless, this report suggests that differentiation therapy is a promising noncytotoxic strategy to deplete CSC activity.
Others have studied the potential of depleting LSCs by inhibiting antiapoptotic pathways implicated in leukemogenesis. Using a combination of AML cell lines as well as primary AML cells, one group showed that the small molecule BH-3 mimetic ABT-737, which blocks the cytoprotective activity of BCL-2 family members, exhibits a dose-dependent inhibition on cell growth and proapoptotic activity in vitro.111 ABT-737 treatment in vitro rapidly induced apoptosis in CD34+ AML blasts and purified LSCs, and this effect was more pronounced when used in combination with other chemotherapeutic agents such as Ara-C and Dox, as well as the MEK1 inhibitor PD98059. Importantly, treatment of primary AML bulk blasts significantly reduced in vitro colony formation, whereas colony formation of normal bone marrow cells was unaffected, indicating that ABT-737 spared normal hematopoietic stem/progenitor cells. Although these studies did not test the effect of ABT-737 on primary human AML xenografts, the researchers showed that administration of ABT-737 to mice transplanted with a Raf-transformed leukemia cell line reduced tumor burden in mice by ~50%. Unfortunately, the durability of this response and the effect on leukemia transplantability was not tested.
The potential utility of administering sensitizing agents before chemotherapy has recently been demonstrated for colon cancer. Having previously established autocrine production of IL-4 as a survival factor in thyroid cancer by modulating death receptor and chemotherapy-induced apoptosis, Todaro et al. showed that treatment of CD133+ colon cancer cells or long-term spheroid cultures with a neutralizing antibody to IL-4 before treatment with oxiplatin, 5-FU, or TRAIL resulted in increased cell death.49 This result was confirmed in vivo by directly injecting tumors with IL-4 neutralizing antibodies, which was not sufficient to inhibit tumor growth alone, but effectively reduced tumor burden when followed by oxiplatin.
Although the most clinically relevant experimental validation for CSC-directed therapies would involve initiation of treatment after establishment of tumor xenografts, this method can be very laborious and time-consuming. A number of laboratories have used in vitro assays to assess the effect of therapy on CSCs using surrogate read-outs, including changes in tumor cell composition (as assessed by immunophenotype), proliferation, differentiation, or self-renewal capacity using clonogenic assays with serial replating. Using these types of assays, investigators have implicated the sonic hedgehog pathway in the maintenance of myeloma stem cells112 and Notch signaling in embyronal brain tumors such as medulloblastoma.113 It should be noted that such studies must be interpreted with caution since in vitro read-outs may not necessarily reflect in vivo function.
Using Monoclonal Antibodies to Target CSCs
Antibody therapies directed against tumor cell surface antigens have resulted in several clinical therapeutic successes. Monoclonal antibodies can exert antitumor activity by triggering immune effectors that cause target cell death (e.g., anti-CD20, Rituxan), by blocking or inhibiting signaling pathways initiated through their targets (anti-VEGF, Avastin), or by being conjugated to radiation emitters or cytotoxins that exert a direct cytotoxic effect (e.g., CD33/Mylotarg). Although antibodies can significantly ameliorate disease and lead to improved prognosis or survival, antibody therapies have not yet resulted in cures. It has been proposed that tumors escape the cytotoxic effect of antibodies by down-regulating target expression or by directly developing resistance.114,115,116 Given the variable success of antibody strategies, one must be reminded that cancers frequently show immunophenotypic heterogeneity, and thus antibodies failing to target all CSCs would be expected to lead to therapeutic failure. Indeed, this may be the case for gemtuzumab oxogamicin (Mylotarg), an anti-CD33 monoclonal antibody approved for patients with relapsed AML. Although most AML blasts express CD33, in some cases LSCs may not express CD33 or may not be contained exclusively among CD33+ cells.117,118 Longer-term follow-up will be required to determine whether or not Mylotarg can be used to effectively treat all AML patients.
At present, there are few examples of antibodies developed specifically against CSC antigens, but in at least one case, an antibody targeting a CSC antigen may induce direct tumor cell death. Schatton et al. demonstrated that treatment of previously xenografted melanomas with an anti-ABCB5 antibody resulted in significant reduction of tumor size, and that antibody treatment–induced tumor cell death through antibody-dependent cell-mediated cytotoxicity.53 In another example of a monoclonal antibody therapy designed to target CSCs, data suggest that such antibodies may be effective even if they recognize CSC epitopes shared with normal tissue stem cells. Work from John Dick's group indicates that CD44, a surface antigen highly expressed on all AML blasts and expressed on normal BM HSCs/progenitors at lower levels, can selectively block engraftment of AML LSCs but not normal HSCs when the cells are pretreated with the activating anti-CD44 antibody (H90) before transplant. In addition, H90 treatment of mice previously engrafted with human AML led to a significant reduction (83–100%) in disease burden. Interestingly leukemic engraftment was lower after in vivo treatment of leukemia as opposed to in vitro CD44 ligation, even when controlling for transplanted cell numbers, suggesting that H90 directly altered LSCs fate by either inducing their differentiation or by inhibiting their repopulation capacity by disrupting LSC interactions with the stromal microenvironment. Although it is unclear from these studies whether the H90-induced decrease in blasts represents a durable remission or cure, they do provide proof-of-principle that antibody therapies, even those recognizing epitopes shared by CSCs and normal tissue stem cells, may represent viable therapeutic strategies.
Although these studies suggest that a single antibody may be effective in eliminating CSCs, it is important to remember that single targets may not be sufficient to eliminate CSCs. Therefore, antibodies might be given in conjunction with standard therapies (e.g., R-CHOP for non-Hodgkin lymphoma) or as combinations of antibodies that target either the same or multiple surface molecules expressed by CSCs. Studies in non-Hodgkin lymphoma have shown that rituximab, given in combination with either anti-CD19 or anti-CD22 antibodies, acts synergistically to reduce tumor burden.116 The identification of additional CSC antigens raises the possibility of such combinatorial strategies.
Are CSCs Clinically Relevant?
Although CSCs have been postulated to be responsible for the clinical behavior of cancers, limited data directly support this contention. This is not surprising, given the relative youth of the CSC field; however, a number of recent studies have begun to evaluate the role of CSCs in determining patient outcomes.
In human AML, investigators have asked whether the frequency of LSCs correlates with clinical outcome. Although older studies failed to demonstrate a correlation between the percentage of CD34+ blasts with treatment outcome or overall survival,119,120,121 more recent studies have suggested a link between LSC burden and clinical behavior by showing that undifferentiated AML (FAB subtype M0) has a higher frequency of LSCs and a poorer prognosis than other FAB subtypes.122,123 A more recent study addressed the relationship between LSC frequency and clinical outcome more directly, demonstrating that the frequency of LSCs at diagnosis correlates with several clinical endpoints, including overall survival, disease-free survival, and relapse-free survival. The predictive value of LSC frequency was independent of white blood cell count at diagnosis, age, and FLT-3 status, while total CD34+ blast frequencies did not correlate with any of the survival parameters.124 Interestingly, the percentage of LSCs also correlated with the ability of AML to engraft NOD/SCID mice, suggesting that xenograft models may reflect important aspects of clinical behavior. This result was confirmed by another study which determined that the ability of AML to engraft was related to the cytogenetic risk group and independent of homing ability, increasing cell dose, intensive conditioning, more permissive recipients, or alternative tissue sources.125
Attempts have been made to correlate CSCs with clinical outcome for breast cancer as well. While quantification of CD44+/CD24−/low tumor cells in breast cancer by immunohistochemistry failed to demonstrate an association between CSC frequency and tumor progression or overall survival, a higher percentage of phenotypic CSCs was found in primary tumors with distant metastasis.126 More promising results were obtained when assessing ALDH1 expression by immunostains. ALDH1+ tumors were associated with high histology grade, ERBB2 overexpression, absence of estrogen and progesterone receptor expression, and expression of the basal-like cytokeratins CK5/6 (ref. 59). Although no correlation was found between ALDH positivity and age, tumor size, or lymph node metastasis, ALHD1+ tumors were associated with poor clinical outcomes and ALDH positivity was implicated as an independent prognostic factor when evaluated in a Cox multivariate analysis including ALDH1, tumor size, age, lymph node metastasis, histologic grade, ER, PR, Ki-67, and ERBB2 expression.
Because of the potential immunophenotypic heterogeneity of CSCs, others have taken a different approach to evaluate the role of CSCs in tumor behavior. Instead of directly quantifying the number of CSCs in tumors, mRNA signatures of unfractionated breast tumors were compared to mRNA signatures obtained from sorted breast CSCs. Patients with breast tumors whose gene signatures were more closely related to that of purified breast CSCs exhibited shorter disease-free intervals, increased relapse rates, and shorter life expectancies.34 Although CSCs were not quantified in these studies, these results suggest that the clinical behavior of breast tumors may be due to a higher fraction of functional CSCs that can be identified on the basis of gene expression. Similarly, recent studies have compared the gene signatures of poorly differentiated tumors to embryonic stem cell signatures. These studies reveal that poorly differentiated breast, bladder and brain tumors share a more embryonic stem cell–like signature, suggesting that their poor clinical behavior may be related to the expression of embryonic stem cell genes.127 Although the relevance of these findings to the CSC hypothesis is not clear, they suggest that CSCs may also express more embryonic stem cell–like genes that regulate CSC function.
Data for a number of solid tumors support the notion that measurable CSC-related parameters determine prognosis. Investigators used an immunohistochemical approach to demonstrate that the frequency and presence of clusters of CD133+ cells in gliomas were significant prognostic factors, with patients showing shorter progression-free survival and shorter overall survival periods independent of WHO grade, age, and extent of resection; the frequency of CD133+ cells was also a risk factor for tumor re-growth as well as time to malignant progression for WHO grade 2 and 3 tumors.128 Frequencies of CSCs in head-and-neck squamous cell carcinoma and melanoma have also been reported to correlate with clinical parameters. Head-and-neck squamous cell carcinomas showing >15% CD44+ tumor cells experience significantly higher rates of tumor recurrence and lower survival rates (L. Ailles, personal communication), while higher frequencies of ABCB5+ tumor cells are present in more advanced melanocytic lesions, suggesting a correlation between CSC burden and prognosis in melanoma.53
Final Words
Although CSCs have been identified in numerous human cancers, whether or not CSC-directed therapies will ultimately lead to cures remains an open question. Although this issue will, no doubt, be the focus of investigations for years to come, the studies described herein indicate that CSCs are likely to fulfill several of the predictions of the CSC hypothesis: (i) that CSCs are relative resistant to conventional therapies and (ii) that clinical outcomes will correlate with measurable CSC parameters including frequency, localization, and gene signatures. Based on these observations, we think CSC-directed therapies show great promise for improving clinical outcomes, but it will be important for researchers to verify these properties for CSCs in each tumor type since they likely will not be uniform with respect to their biologic properties, consistent with their heterogeneous molecular origins.
Since CSC therapies may not induce responses like conventional therapies, we think it is critical for all investigators to be aware of the numerous potential experimental approaches available to them when designing and evaluating their preclinical and clinical trials. Our hope is that others will follow the lead of those investigators who have performed the most rigorous studies by adopting preclinical experimental models that most closely resemble the clinical setting (Table 2). Although it is simpler (but necessary) to test the effect of therapies on CSCs in vitro, existing xenotransplantation models provide researchers with a powerful system to test the efficacy of CSC therapies and aid in the rational design of future clinical trials, whether such therapies are administered alone or in combination with existing therapies. Finally, we hope that investigators will strongly consider combinatorial approaches to CSC therapeutic development to directly confront the near inevitable resistance that will develop in response to single agent therapy. In the end, such well-designed studies offer the best chance for bringing new and effective therapies to cancer patients.
Table 2.
Potential CSC therapies validated in xenograft assays using primary tumor cells. Please see text for descriptions
Acknowledgments
We apologize to those investigators whose works have not been cited because of space limitations. We thank Drs Dalerba, Majeti, Luppen, and Pang for critically reviewing this work.
REFERENCES
- Vogelstein B., and , Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- Hanahan D., and , Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Fearon ER., and , Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Jones RJ, Matsui WH., and , Smith BD. Cancer stem cells: are we missing the target. J Natl Cancer Inst. 2004;96:583–585. doi: 10.1093/jnci/djh095. [DOI] [PubMed] [Google Scholar]
- Huntly BJ., and , Gilliland DG. Cancer biology: summing up cancer stem cells. Nature. 2005;435:1169–1170. doi: 10.1038/4351169a. [DOI] [PubMed] [Google Scholar]
- Graham SM, Jørgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- Hammond SM. MicroRNAs as oncogenes. Curr Opin Genet Dev. 2006;16:4–9. doi: 10.1016/j.gde.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Holtz MS, Slovak ML, Zhang F, Sawyers CL, Forman SJ., and , Bhatia R. Imatinib mesylate (STI571) inhibits growth of primitive malignant progenitors in chronic myelogenous leukemia through reversal of abnormally increased proliferation. Blood. 2002;99:3792–3800. doi: 10.1182/blood.v99.10.3792. [DOI] [PubMed] [Google Scholar]
- Price JE., and , Tarin D. Low incidence of tumourigenicity in agarose colonies from spontaneous murine mammary tumours. Differentiation. 1989;41:202–207. doi: 10.1111/j.1432-0436.1989.tb00748.x. [DOI] [PubMed] [Google Scholar]
- Gioanni J, Farges MF, Duplay H, Hery M, Zanghellini E, Schneider M, et al. In vitro clonogenicity in relation to kinetic and clinicopathological features of breast cancer. Bull Cancer. 1988;75:285–290. [PubMed] [Google Scholar]
- Sabbath KD, Ball ED, Larcom P, Davis RB., and , Griffin JD. Heterogeneity of clonogenic cells in acute myeloblastic leukemia. J Clin Invest. 1985;75:746–753. doi: 10.1172/JCI111756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JD., and , Lowenberg B. Clonogenic cells in acute myeloblastic leukemia. Blood. 1986;68:1185–1195. [PubMed] [Google Scholar]
- Bruce WR., and , Van Der Gaag H. A quantitative assay for the number of murine lymphoma cells capable of proliferation in vivo. Nature. 1963;199:79–80. doi: 10.1038/199079a0. [DOI] [PubMed] [Google Scholar]
- Park CH, Bergsagel DE., and , McCulloch EA. Mouse myeloma tumor stem cells: a primary cell culture assay. J Natl Cancer Inst. 1971;46:411–422. [PubMed] [Google Scholar]
- Southam C., and , Brunschwig A. Quantitative studies of autotransplantation of human cancer. Cancer. 1961;14:481–463. [Google Scholar]
- Lagos-Quintana M, Rauhut R, Lendeckel W., and , Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- Dalerba P, Cho RW., and , Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- Wicha MS, Liu S., and , Dontu G.Cancer stem cells: an old idea—a paradigm shift Cancer Res 2006661883, 1895–1890.discussion [DOI] [PubMed] [Google Scholar]
- Bonnet D., and , Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- Baum CM, Weissman IL, Tsukamoto AS, Buckle AM., and , Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci USA. 1992;89:2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrin RS, Atkinson K, Leemhuis T, Hanania E, Juttner C, Tierney K, et al. Transplantation of highly purified CD34+Thy-1+ hematopoietic stem cells in patients with metastatic breast cancer. Biol Blood Marrow Transplant. 2000;6:262–271. doi: 10.1016/s1083-8791(00)70008-5. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Weissman IL., and , Akashi K. AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc Natl Acad Sci USA. 2000;97:7521–7526. doi: 10.1073/pnas.97.13.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeti R, Park CY., and , Weissman IL. Identification of a Hierarchy of Multipotent Hematopoietic Progenitors in Human Cord Blood. Cell Stem Cell. 2007;1:635–645. doi: 10.1016/j.stem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF., and , Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Weissman I. Stem cell research: paths to cancer therapies and regenerative medicine. JAMA. 2005;294:1359–1366. doi: 10.1001/jama.294.11.1359. [DOI] [PubMed] [Google Scholar]
- Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ., and , Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J, Eaves CJ, Kuusk U., and , Emerman JT. Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast. Differentiation. 1998;63:201–213. doi: 10.1111/j.1432-0436.1998.00201.x. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson T, Villadsen R, Nielsen HL, Ronnov-Jessen L, Bissell MJ., and , Petersen OW. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 2002;16:693–706. doi: 10.1101/gad.952602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Wang X, Chen GY, Dalerba P, Gurney A, Hoey T, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356:217–226. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- Collins AT, Berry PA, Hyde C, Stower MJ., and , Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153–166. doi: 10.1016/j.ccr.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ., and , Collins AT. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- Yu S, Zhang JZ, Zhao CL, Zhang HY., and , Xu Q. Isolation and characterization of the CD133+ precursors from the ventricular zone of human fetal brain by magnetic affinity cell sorting. Biotechnol Lett. 2004;26:1131–1136. doi: 10.1023/B:BILE.0000035484.64499.ac. [DOI] [PubMed] [Google Scholar]
- Mizrak D, Brittan M., and , Alison MR. CD133: molecule of the moment. J Pathol. 2008;214:3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, et al. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castor A, Nilsson L, Astrand-Grundström I, Buitenhuis M, Ramirez C, Anderson K, et al. Distinct patterns of hematopoietic stem cell involvement in acute lymphoblastic leukemia. Nat Med. 2005;11:630–637. doi: 10.1038/nm1253. [DOI] [PubMed] [Google Scholar]
- O'Brien CA, Pollett A, Gallinger S., and , Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, et al. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DA, Meyerrose TE, Wirthlin L, Craft TP, Herrbrich PE, Creer MH, et al. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104:1648–1655. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- Hess DA, Wirthlin L, Craft TP, Herrbrich PE, Hohm SA, Lahey R, et al. Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107:2162–2169. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong L, Stojkovic M, Dimmick I, Ahmad S, Stojkovic P, Hole N, et al. Phenotypic characterization of murine primitive hematopoietic progenitor cells isolated on basis of aldehyde dehydrogenase activity. Stem Cells. 2004;22:1142–1151. doi: 10.1634/stemcells.2004-0170. [DOI] [PubMed] [Google Scholar]
- Cheung AM, Wan TS, Leung JC, Chan LY, Huang H, Kwong YL, et al. Aldehyde dehydrogenase activity in leukemic blasts defines a subgroup of acute myeloid leukemia with adverse prognosis and superior NOD/SCID engrafting potential. Leukemia. 2007;21:1423–1430. doi: 10.1038/sj.leu.2404721. [DOI] [PubMed] [Google Scholar]
- Pearce DJ, Taussig D, Simpson C, Allen K, Rohatiner AZ, Lister TA, Bonnet D, et al. Characterization of cells with a high aldehyde dehydrogenase activity from cord blood and acute myeloid leukemia samples. Stem Cells. 2005;23:752–760. doi: 10.1634/stemcells.2004-0292. [DOI] [PubMed] [Google Scholar]
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dylla SJ, Beviglia L, Park IK, Chartier C, Raval J, Ngan L, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS ONE. 2008;3:e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CV, Evely RS, Oakhill A, Pamphilon DH, Goulden NJ., and , Blair A. Characterization of acute lymphoblastic leukemia progenitor cells. Blood. 2004;104:2919–2925. doi: 10.1182/blood-2004-03-0901. [DOI] [PubMed] [Google Scholar]
- Cox CV, Martin HM, Kearns PR, Virgo P, Evely RS., and , Blair A. Characterization of a progenitor cell population in childhood T-cell acute lymphoblastic leukemia. Blood. 2007;109:674–682. doi: 10.1182/blood-2006-06-030445. [DOI] [PubMed] [Google Scholar]
- Eisterer W, Jiang X, Christ O, Glimm H, Lee KH, Pang E, et al. Different subsets of primary chronic myeloid leukemia stem cells engraft immunodeficient mice and produce a model of the human disease. Leukemia. 2005;19:435–441. doi: 10.1038/sj.leu.2403649. [DOI] [PubMed] [Google Scholar]
- Matsui W, Huff CA, Wang Q, Malehorn MT, Barber J, Tanhehco Y, et al. Characterization of clonogenic multiple myeloma cells. Blood. 2004;103:2332–2336. doi: 10.1182/blood-2003-09-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui W, Wang Q, Barber JP, Brennan S, Smith BD, Borrello I, et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008;68:190–197. doi: 10.1158/0008-5472.CAN-07-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RP.Identifying cancer stem cells in solid tumors: case not proven Cancer Res 2006661891, 1890–1895.discussion [DOI] [PubMed] [Google Scholar]
- Kelly PN, Dakic A, Adams JM, Nutt SL., and , Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- Kennedy JA, Barabe F, Poeppl AG, Wang JC., and , Dick JE.Comment on “Tumor growth need not be driven by rare cancer stem cells” Science 20073181722, 1722author reply [DOI] [PubMed] [Google Scholar]
- Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–1321. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Taussig DC, Miraki-Moud F, Anjos-Afonso F, Pearce DJ, Allen K, Ridler C, et al. Anti-CD38 antibody-mediated clearance of human repopulating cells masks the heterogeneity of leukemia-initiating cells. Blood. 2008;112:568–575. doi: 10.1182/blood-2007-10-118331. [DOI] [PubMed] [Google Scholar]
- Vermeulen L, Todaro M, de Sousa Mello F, Sprick MR, Kemper K, Perez Alea M, et al. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci USA. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MF., and , Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Liotta LA., and , Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- Ailles LE., and , Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460–466. doi: 10.1016/j.copbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Dean M, Fojo T., and , Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- Jordan CT., and , Guzman ML. Mechanisms controlling pathogenesis and survival of leukemic stem cells. Oncogene. 2004;23:7178–7187. doi: 10.1038/sj.onc.1207935. [DOI] [PubMed] [Google Scholar]
- Mimeault M, Hauke R, Mehta PP., and , Batra SK. Recent advances in cancer stem/progenitor cell research: therapeutic implications for overcoming resistance to the most aggressive cancers. J Cell Mol Med. 2007;11:981–1011. doi: 10.1111/j.1582-4934.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Ang BT., and , Pervaiz S. Cancer stem cell: target for anti-cancer therapy. Faseb J. 2007;21:3777–3785. doi: 10.1096/fj.07-8560rev. [DOI] [PubMed] [Google Scholar]
- Ward RJ., and , Dirks PB. Cancer Stem Cells: At the Headwaters of Tumor Development. Annu Rev Pathol. 2007;2:175–189. doi: 10.1146/annurev.pathol.2.010506.091847. [DOI] [PubMed] [Google Scholar]
- Costello RT, Mallet F, Gaugler B, Sainty D, Arnoulet C, Gastaut JA, et al. Human acute myeloid leukemia CD34+/CD38− progenitor cells have decreased sensitivity to chemotherapy and Fas-induced apoptosis, reduced immunogenicity, and impaired dendritic cell transformation capacities. Cancer Res. 2000;60:4403–4411. [PubMed] [Google Scholar]
- Guan Y, Gerhard B., and , Hogge DE. Detection, isolation, and stimulation of quiescent primitive leukemic progenitor cells from patients with acute myeloid leukemia (AML) Blood. 2003;101:3142–3149. doi: 10.1182/blood-2002-10-3062. [DOI] [PubMed] [Google Scholar]
- Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, et al. Nuclear factor-κB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- Holyoake T, Jiang X, Eaves C., and , Eaves A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94:2056–2064. [PubMed] [Google Scholar]
- Eramo A, Ricci-Vitiani L, Zeuner A, Pallini R, Lotti F, Sette G, et al. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ. 2006;13:1238–1241. doi: 10.1038/sj.cdd.4401872. [DOI] [PubMed] [Google Scholar]
- Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Phillips TM, McBride WH., and , Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- Lagasse E. Cancer stem cells with genetic instability: the best vehicle with the best engine for cancer. Gene Ther. 2008;15:136–142. doi: 10.1038/sj.gt.3303068. [DOI] [PubMed] [Google Scholar]
- Lederberg J., and , Lederberg EM. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952;63:399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria SE., and , Delbruck M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei E 3rd, Karon M, Levin RH, Freireich EJ, Taylor RJ, Hananian J, et al. The effectiveness of combinations of antileukemic agents in inducing and maintaining remission in children with acute leukemia. Blood. 1965;26:642–656. [PubMed] [Google Scholar]
- Huff CA, Matsui WH, Douglas Smith B., and , Jones RJ. Strategies to eliminate cancer stem cells: clinical implications. Eur J Cancer. 2006;42:1293–1297. doi: 10.1016/j.ejca.2006.01.045. [DOI] [PubMed] [Google Scholar]
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- Michor F. Chronic myeloid leukemia blast crisis arises from progenitors. Stem Cells. 2007;25:1114–1118. doi: 10.1634/stemcells.2006-0638. [DOI] [PubMed] [Google Scholar]
- Michor F, Hughes TP, Iwasa Y, Branford S, Shah NP, Sawyers CL, et al. Dynamics of chronic myeloid leukaemia. Nature. 2005;435:1267–1270. doi: 10.1038/nature03669. [DOI] [PubMed] [Google Scholar]
- Sleijfer S, Gratama JW, Sieuwerts AM, Kraan J, Martens JW., and , Foekens JA. Circulating tumour cell detection on its way to routine diagnostic implementation. Eur J Cancer. 2007;43:2645–2650. doi: 10.1016/j.ejca.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Riethdorf S, Wikman H., and , Pantel K. Review: Biological relevance of disseminated tumor cells in cancer patients. Int J Cancer. 2008;123:1991–2006. doi: 10.1002/ijc.23825. [DOI] [PubMed] [Google Scholar]
- Pantel K, Brakenhoff RH., and , Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:329–340. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- Lobo NA, Shimono Y, Qian D., and , Clarke MF. The Biology of Cancer Stem Cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- Huntly BJ., and , Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer. 2005;5:311–321. doi: 10.1038/nrc1592. [DOI] [PubMed] [Google Scholar]
- Krause DS., and , Van Etten RA. Right on target: eradicating leukemic stem cells. Trends Mol Med. 2007;13:470–481. doi: 10.1016/j.molmed.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman ML, Swiderski CF, Howard DS, Grimes BA, Rossi RM, Szilvassy SJ, et al. Preferential induction of apoptosis for primary human leukemic stem cells. Proc Natl Acad Sci USA. 2002;99:16220–16225. doi: 10.1073/pnas.252462599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman ML, Li X, Corbett CA, Rossi RM, Bushnell T, Liesveld JL, et al. Rapid and selective death of leukemia stem and progenitor cells induced by the compound 4-benzyl, 2-methyl, 1,2,4-thiadiazolidine, 3,5 dione (TDZD-8) Blood. 2007;110:4436–4444. doi: 10.1182/blood-2007-05-088815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman ML, Rossi RM, Neelakantan S, Li X, Corbett CA, Hassane DC, et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007;110:4427–4435. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahia PL, Aguiar RC, Alberta J, Kum JB, Caron S, Sill H, et al. PTEN is inversely correlated with the cell survival factor Akt/PKB and is inactivated via multiple mechanism haematological malignancies. Hum Mol Genet. 1999;8:185–193. doi: 10.1093/hmg/8.2.185. [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Peacock CD, Wang Q, Gesell GS, Corcoran-Schwartz IM, Jones E, Kim J, et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci USA. 2007;104:4048–4053. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66:7445–7452. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- Golay J, Lazzari M, Facchinetti V, Bernasconi S, Borleri G, Barbui T, et al. CD20 levels determine the in vitro susceptibility to rituximab and complement of B-cell chronic lymphocytic leukemia: further regulation by CD55 and CD59. Blood. 2001;98:3383–3389. doi: 10.1182/blood.v98.12.3383. [DOI] [PubMed] [Google Scholar]
- Lopez-Guillermo A., and , Mercadal S. The clinical use of antibodies in haematological malignancies. Ann Oncol. 2007;18 Suppl 9:ix51–ix57. doi: 10.1093/annonc/mdm294. [DOI] [PubMed] [Google Scholar]
- Buelow R., and , van Schooten W. The future of antibody therapy. Ernst Schering Found Symp Proc. 2006;4:83–106. doi: 10.1007/2789_2007_040. [DOI] [PubMed] [Google Scholar]
- Taussig DC, Pearce DJ, Simpson C, Rohatiner AZ, Lister TA, Kelly G, et al. Hematopoietic stem cells express multiple myeloid markers: implications for the origin and targeted therapy of acute myeloid leukemia. Blood. 2005;106:4086–4092. doi: 10.1182/blood-2005-03-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth AW, Florian S, Printz D, Sotlar K, Krauth MT, Fritsch G, et al. Expression of the target receptor CD33 in CD34+/CD38−/CD123+ AML stem cells. Eur J Clin Invest. 2007;37:73–82. doi: 10.1111/j.1365-2362.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- Kyoda K, Nakamura S, Hattori N, Takeshima M, Nakamura K, Kaya H, et al. Lack of prognostic significance of CD34 expression in adult AML when FAB M0 and M3 are excluded. Am J Hematol. 1998;57:265–266. doi: 10.1002/(sici)1096-8652(199803)57:3<265::aid-ajh24>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Selleri C, Notaro R, Catalano L, Fontana R, Del Vecchio L., and , Rotoli B. Prognostic irrelevance of CD34 in acute myeloid leukaemia. Br J Haematol. 1992;82:479–481. doi: 10.1111/j.1365-2141.1992.tb06452.x. [DOI] [PubMed] [Google Scholar]
- Bradstock K, Matthews J, Benson E, Page F., and , Bishop J. Prognostic value of immunophenotyping in acute myeloid leukemia. Australian Leukaemia Study Group. Blood. 1994;84:1220–1225. [PubMed] [Google Scholar]
- Costello R, Mallet F, Chambost H, Sainty D, Arnoulet C, Gastaut JA, et al. The immunophenotype of minimally differentiated acute myeloid leukemia (AML-M0): reduced immunogenicity and high frequency of CD34+/CD38− leukemic progenitors. Leukemia. 1999;13:1513–1518. doi: 10.1038/sj.leu.2401519. [DOI] [PubMed] [Google Scholar]
- Haferlach T, Schoch C, Löffler H, Gassmann W, Kern W, Schnittger S, et al. Morphologic dysplasia in de novo acute myeloid leukemia (AML) is related to unfavorable cytogenetics but has no independent prognostic relevance under the conditions of intensive induction therapy: results of a multiparameter analysis from the German AML Cooperative Group studies. J Clin Oncol. 2003;21:256–265. doi: 10.1200/JCO.2003.08.005. [DOI] [PubMed] [Google Scholar]
- van Rhenen A, Feller N, Kelder A, Westra AH, Rombouts E, Zweegman S, et al. High stem cell frequency in acute myeloid leukemia at diagnosis predicts high minimal residual disease and poor survival. Clin Cancer Res. 2005;11:6520–6527. doi: 10.1158/1078-0432.CCR-05-0468. [DOI] [PubMed] [Google Scholar]
- Pearce DJ, Taussig D, Zibara K, Smith LL, Ridler CM, Preudhomme C, et al. AML engraftment in the NOD/SCID assay reflects the outcome of AML: implications for our understanding of the heterogeneity of AML. Blood. 2006;107:1166–1173. doi: 10.1182/blood-2005-06-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M., and , Brauch H. Prevalence of CD44+/CD24−/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11:1154–1159. [PubMed] [Google Scholar]
- Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, et al. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 2008;14:123–129. doi: 10.1158/1078-0432.CCR-07-0932. [DOI] [PubMed] [Google Scholar]
- Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- Jin L, Hope KJ, Zhai Q, Smadja-Joffe F., and , Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]