Abstract
Stem cells are a promising resource for gene therapy. Adipose tissue–derived stem cells (ADSCs) offer advantages because of their abundance and ease of isolation. However, it is difficult to transduce genes into ADSCs by common transfection methods, especially nonviral methods. We report here the use of a new electroporation method, termed “microporation,” to transduce plasmids into human ADSCs (hADSCs). We determined optimal conditions that led to efficient transfection of >76.1% of the microporated hADSCs with only minimal cell damage or cytotoxicity. We demonstrated the expression of both enhanced green fluorescent protein (EGFP) and luciferase from different promoters in microporated hADSCs. More important, the microporated hADSCs retained their multipotency and reporter gene expression was maintained for >2 weeks in vitro and in vivo. We further showed that a Tet-ON-inducible gene expression system could be microporated into hADSCs and that this system was functional following transplantation of the microporated cells into nude mice. Taken together, our data demonstrate that microporation allows a highly efficient transfection of hADSCs, without impairing their stem cell properties.
Introduction
Stem cells are clonogenic and self-renewing, and can give rise to specialized cell types.1 Two kinds of stem cell may be defined according to the tissue source: embryonic stem cells and adult stem cells. Embryonic stem cells are pluripotent cells that can give rise to the various cell types of the body. Generally, adult stem cells are limited by the number and type of differentiated cell types that they can become. For cell-based tissue regeneration, a potential advantage of using stem cells from an adult is that the patient's own cells could be expanded in culture and then reintroduced into the patient without the problem of tissue rejection by the immune system.2,3 Mesenchymal stem cells are isolated from mesodermal organs, such as bone marrow, umbilical cord blood, and fat tissue.4,5,6 They have the ability to differentiate into mesodermal cell lineages, such as muscle, bone, cartilage, and fat under the appropriate culture conditions.7,8 Therefore, mesenchymal stem cells are a good cell source for tissue regeneration and gene therapy.
The biology of adult mesenchymal stem cells and their potential use in gene therapy have provided opportunities for therapeutic use in tissue regeneration. Recently, stem cells were successfully transduced with therapeutic genes via viral vectors. However, the possibility of inducing toxicity or immune and inflammatory responses by the viral vector is a major concern.2,3 In addition, virus-based methods are time consuming owing to technical difficulties and specific safety requirements, especially when working with human cells.9 Gene delivery by nonviral methods, including native DNA, liposomes, cationic polymers, and electroporation, is less efficient than virus-mediated DNA delivery. Typically, transfection efficiency by nonviral methods is limited to 20–25%.10 Furthermore, adult mesenchymal stem cells tend to resist transgene delivery by classic nonviral methods, as do primary cultured cells.11,12 Still, nonviral methods have several advantages, such as lower manufacturing costs, no (or weak) immunogenic responses with repeated administration, and they are generally safe.13 Therefore, improving the transfection efficiency of nonviral methods for adult mesenchymal stem cells should prove beneficial to cell therapy.
We present here a new transfection method, termed microporation, which we show can efficiently transduce plasmid DNA into human adipose tissue–derived stem cells (hADSCs). We established optimal transfection conditions associated with minimal cell damage. These conditions are associated with a transfection efficiency of up to 76.1% 24 hours after microporation. More important, the transfected hADSCs retain their multipotency. Furthermore, hADSCs microporated with a Tet-ON regulated enhanced green fluorescent protein (EGFP) expression plasmid showed regulated transgene expression by exposure to doxycycline (Dox) following transplantation of the transduced cells into nude mice.
Results
Determination of optimal transfection conditions by microporation
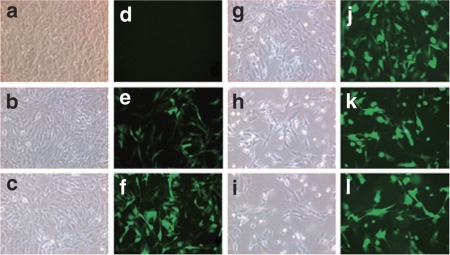
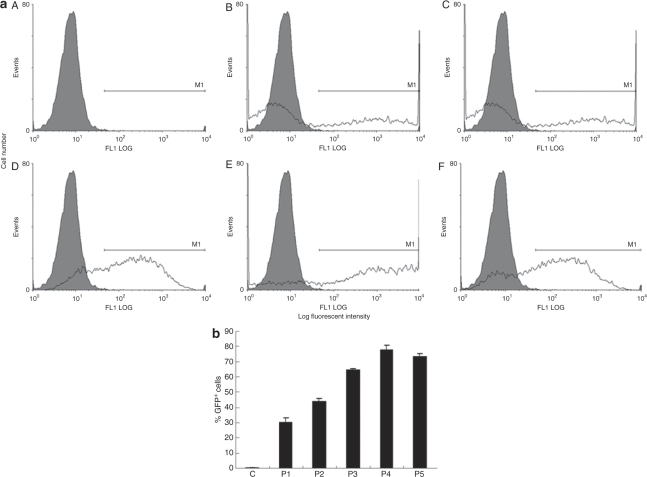
We investigated a nonviral transfection method for hADSCs using microporator technology. First, we followed the protocol provided by the manufacturer. To further determine the best microporation conditions, equivalent quantities of both hADSCs and the pEGFP-N1 plasmid were used to test five different pulsing programs: program 1 (P1), 900 V, 20 ms, one pulse; P2, 900 V, 20 ms, two pulses; P3, 1,500 V, 20 ms, one pulse; P4, 1,500 V, 20 ms, two pulses; and P5, 1,800 V, 20 ms, one pulse. EGFP expression was visualized 24 hours after microporation by fluorescence microscopy (Figure 1). All of the microporation conditions tested with the exception of P1 led to high transfection efficiency of the hADSCs. P2 generated a greater transfection efficiency than P1, with the only difference between these programs being the number of pulses (Figure 1e and f). However, we observed a similar transfection efficiency when using either P3 or P4, which also only differed by the number of pulses (Figure 1j and k). We further observed that cell morphology was round and that the cells were unable to spread following microporation at the higher voltages and with increasing pulse number (Figure 1h and i). Next, the EGFP-positive microporated cells (EGFP+ cells) were analyzed by flow cytometry (Figure 2a). The EGFP+ cells represented at least 30% of the transfected hADSCs under each microporation condition. The fraction of EGFP+ cells using P1, P2, P3, P4, and P5 conditions corresponded to 30.5 ± 2.7%, 44.4 ± 1.2%, 64.8 ± 0.8%, 77.8 ± 2.9%, and 73.9 ± 1.6%, respectively (Figure 2b). As such, the results of the fluorescence microscopic analysis were consistent with the results of the flow cytometric analysis. At the same voltage, the percentage of EGFP+ cells increased by ~13–14% when employing a second microporation pulse (e.g., compare P1 versus P2 and P3 versus P4).
Figure 1.
Human adipose tissue–derived stem cells (hADSCs) were transfected with 1.5 µg of pEGFP-N1 using a microporator. After 24 hours, the expression of enhanced green fluorescent protein (EGFP) was analyzed using fluorescence microscopy, and hADSCs were microporated using different voltage and pulse conditions: (a and d), control; (b and e), program 1; (c and f), program 2; (g and j), program 3; (h and k), program 4; and (i and l), program 5; (a–c) and (g–i), phase contrast images; (d–f) and (j–l), EGFP fluorescence.
Figure 2.
Flow cytometric analysis of transfection efficacy. (a) Human adipose tissue–derived stem cells were transfected with plasmid pEGFP-N1 and enhanced green fluorescent protein (EGFP)-expressing cells were detected 24 hours later by flow cytometry. Cells were microporated using different voltage and pulse conditions: A, control; B, program 1; C, program 2; D, program 3; E, program 4; and F, program 5. Microporated (white) and control groups (gray) are illustrated in each panel. (b), Comparison of the fraction of GFP+ cells estimated following fluorescence-activated cell-sorting analysis.
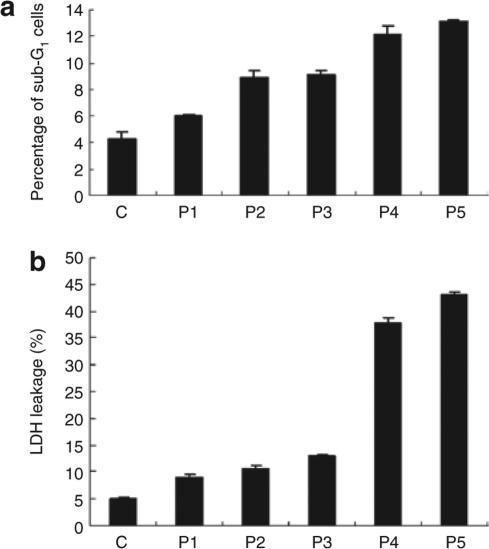
hADSCs morphology was altered following microporation at the higher voltages and with increasing pulse number (Figure 1a). To determine whether this change in cell morphology was caused by cytotoxic effects of microporation, we monitored the sub-G1 cell population in cells following microporation. After microporation, the cells were allowed to adhere overnight. Subsequently, dead cells, debris, and viable cells were collected for flow cytometric analysis. The percentage of sub-G1 cells increased after microporation (Figure 3). In addition, cell death was estimated using the lactate dehydrogenase assay. Lactate dehydrogenase leakage of hADSCs following microporation under conditions P1–P5 was significantly elevated by 8.9 ± 0.6% (P1), 10.6 ± 0.6% (P2), 12.9 ± 0.4% (P3), 37.8 ± 0.9% (P4), and 43.1 ± 0.5% (P5), respectively, compared to the control cells (Figure 3). The data indicated that microporation could have cytotoxic effects on hADSCs and that these effects correlated with increased voltage and pulse number. Overall, the P3 appeared to be the optimal transfection condition for hADSCs, owing to a greater transfection efficiency and a relatively lower cytotoxic effect. As a result, we used the P3 conditions for all subsequent studies.
Figure 3.
Analysis of cytotoxic effects of microporation of human adipose tissue–derived stem cells. (a) Flow cytometric analysis. Cells were microporated using different voltage and pulse conditions. After 24 hours, cells were fixed and stained with propidium iodide, followed by fluorescence-activated cell-sorting analysis of DNA content. Sub-G1 populations were defined as the portion of cells with DNA content <2N. The experiment was repeated three times independently. The results are expressed as the mean ± SD. (b) Cells were microporated using different voltage and pulse conditions. After 24 hours, lactate dehydrogenase (LDH) activities were detected as described in Materials and Methods. Results are derived from at least three separate experiments and expressed as the mean ± SD.
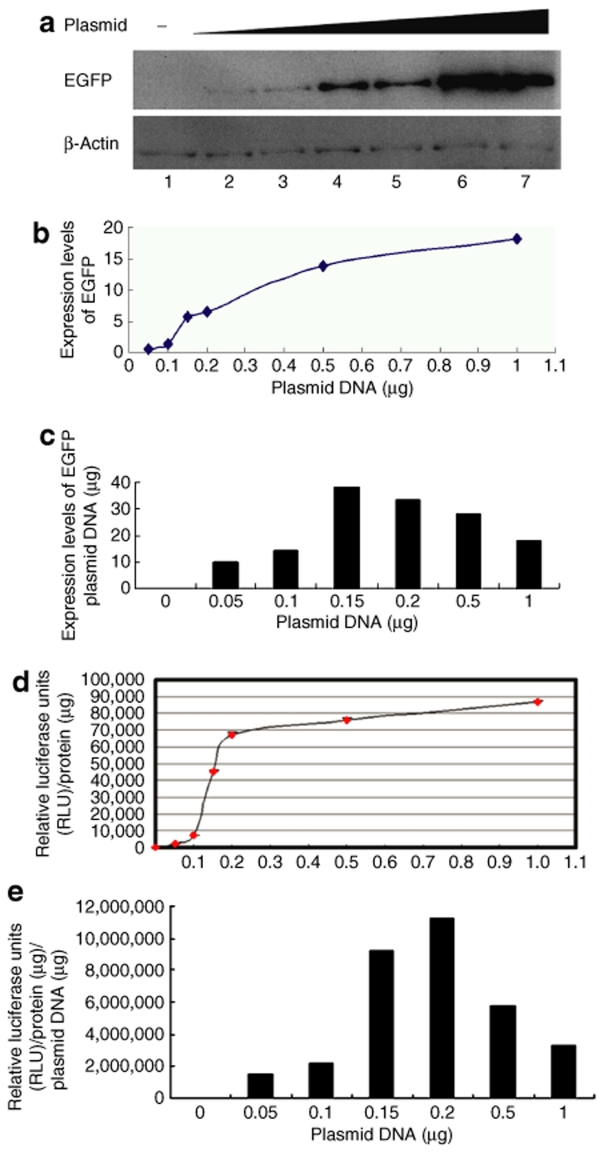
Next, we determined the most efficient dose of plasmid for microporation-mediated gene expression by measuring reporter gene expression following microporation of different concentrations of reporter plasmid DNA into hADSCs. Two days after microporation of the EGFP reporter plasmid, EGFP expression was analyzed by western blot analysis (Figure 4a). As determined by image quantification, the expression levels of EGFP increased with increasing concentrations of plasmid DNA (Figure 4b). The data showed that 1.5 to 2 µg of DNA was the most effective dose for EGFP expression (Figure 4c). Next, hADSCs were microporated with different concentrations of the luciferase reporter plasmid. Luciferase activity also increased with increasing concentrations of reporter plasmid DNA (Figure 4d). Consistent with the EGFP expression analysis, the highest luciferase activities were achieved with the use of 1.5–2 µg of plasmid DNA/106 cells/reaction (Figure 4e). Therefore, microporation is a very reliable method for plasmid DNA transfection into hADSCs.
Figure 4.
Determination of transfection efficiency by microporation of different concentrations of reporter DNA into human adipose tissue–derived stem cells (hADSCs). (a) hADSCs were transfected without or with pCMV-EGFP-N1 at different doses (0.05, 0.1, 0.15, 0.2, 0.5, or 1 µg) as described in Materials and Methods, and the expression level of enhanced green fluorescent protein (EGFP) was determined by western blot analysis with anti-EGFP antibody. (b) The graph shows densitometric analysis of EGFP expression, normalized to β-actin expression. (c) Calculation of relative EGFP expression per dose of plasmid DNA in hADSCs. (d) hADSCs were transfected with pRL-TK at different doses (0.05, 0.1, 0.15, 0.2, 0.5, or 1 µg), and luminescence was measured with TopCount NXTTM. Luciferase activities were normalized to protein content and expressed in relative luciferase units (RLUs) as the mean ± SD of three independent samples. (e) Calculation of the relative luciferase activity per dose of reporter plasmid DNA following microporation into hADSCs.
Microporation allows gene expression in hADSCs without impairing their ability to differentiate
The preceding experiments demonstrated that microporation is an efficient method to induce a high level of transgene expression in hADSCs and that it may be superior to other transfection systems. Because hADSCs can give rise to both osteogenic and adipogenic lineages,14,15 it is important to evaluate whether the phenotype and/or functional properties are altered in microporated hADSCs. Thus, we next evaluated the differentiation potential of microporated hADSCs under culture conditions that induced osteogenic or adipogenic differentiation in vitro.
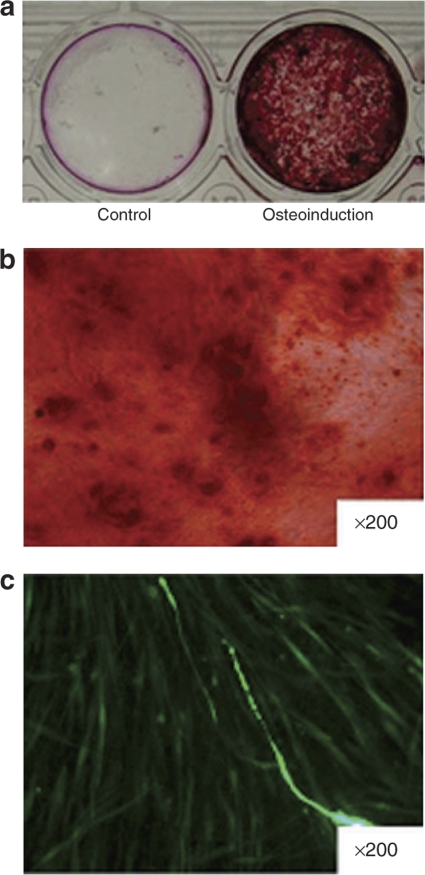
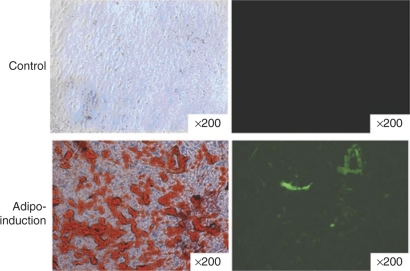
After osteogenic induction for 14 days, microporated hADSCs exhibited a significant increase in mineralization by Alizarin red staining when compared with the noninduced control group (Figure 5a and b). Meanwhile, the expression of EGFP was maintained in hADSCs during the osteoinductive conditions (Figure 5c). We next evaluated the adipogenic differentiation of transfected hADSCs. Microporated hADSCs were cultured in adipogenic medium for 12 days, resulting in their adipocytic differentiation as determined by Oil-red O staining (Figure 6). The expression of EGFP was maintained in the microporated hADSCs during adipoinductive conditions. Collectively, the data suggest that microporated hADSCs maintain their multipotent properties. In addition, the expression of EGFP was maintained following osteoinductive and adipoinductive conditions for longer periods of time (14 days).
Figure 5.
Microporation of human adipose tissue–derived stem cells (hADSCs) does not affect osteoblastic differentiation. Furthermore, hADSCs were transfected with the reporter plasmid, pCMV-EGFP-N1. After 24 hours, cells were cultured in control medium or osteogenic medium for 14 days. (a) Following fixation, the cells were stained by Alizarin red as described in Materials and Methods. (b) Representative high magnification fields of Alizarin red staining confirmed mineralized nodule formation in the osteo-induction group. (c) Evaluation of enhanced green fluorescent protein (EGFP) expression in microporated hADSCs. EGFP expression was detected by fluorescence microscopy. Images were taken using ×200 objectives in b and c.
Figure 6.
Microporation of human adipose tissue–derived stem cells (hADSCs) does not affect their potential for adipogenic differentiation, and hADSCs were transfected with reporter plasmid, pCMV-EGFP-N1. After 24 hours, the cells were cultured in control medium (upper row) or adipogenic medium (lower row) for 12 days. Following fixation, the cells were stained with Oil-red as described in Materials and Methods. High magnification fields of Oil-red staining confirmed oil body formation in the adipo-induction group. Enhanced green fluorescent protein (EGFP) expression was detected by fluorescence microscopy. Images were taken using ×200 objectives.
In vivo expression of regulated EGFP
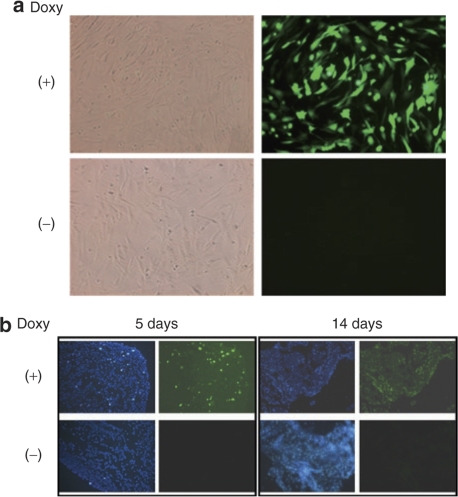
To test the potential for application of microporated hADSCs for cell therapy in vivo, we transplanted hADSCs microporated with a Tet-ON inducible expression system into nude mice and monitored reporter transgene expression. The Tet-ON system was used to be able to regulate the expression of EGFP in hADSCs in response to Dox treatment of animals receiving the cell transplants. We first demonstrated that the induction of EGFP expression could be observed in vitro following Dox treatment of hADSCs transiently co-transfected with pCMV-rtTA and pBI-EGFP by microporation (Figure 7a, upper row). Conversely, EGFP expression could not be detected in microporated cells that were not exposed to Dox (Figure 7a, lower row). One day after co-transfection by microporation, the hADSCs were transplanted subcutaneously on the back of nude mice. Five days later, we observed the expression of EGFP in animals that had access to drinking water containing Dox, with the expression of EGFP maintained for 2 weeks (Figure 7b). In contrast, the expression of EGFP was not observed in animals that did not have access to Dox in their drinking water (Figure 7b). Therefore, we suggest that microporation of hADSCs with an inducible expression system may have application in the development of cell therapies that make use of hADSCs.
Figure 7.
Microporation of plasmids encoding the Tet-ON system into human adipose tissue–derived stem cells (hADSCs) and testing of enhanced green fluorescent protein (EGFP) expression in vitro and in vivo in response to Dox treatment. First, hADSCs were microporated with pCMV-rtTA and pBI-EGFP plasmids. (a) Following microporation, cells were cultured in the presence or absence of Dox for 24 hours. A strong fluorescent signal was observed in the microporated hADSCs cultured in the presence of Dox (right panel, upper row). In contrast, EGFP expression could not be detected in the Dox (−) group (right panel, lower row). The corresponding phase contrast images are shown in the left panels. Images were taken using ×200 objectives. (b) One day after co-transfection of the pCMV-rtTA and pBI-EGFP plasmids, hADSCs were transplanted subcutaneously on the back of nude mice. The mice were then exposed to drinking water that either contained Dox (200 µg/ml) or not. Cells were harvested at the indicated times. After preparation of frozen sections, EGFP expression was detected by fluorescence microscopy. Images were taken using ×40 objectives.
Discussion
Ex vivo gene therapy is a promising strategy to treat disease. Adipose tissue–derived stem cells are an excellent resource for the development of this type of therapy, as well as for tissue regeneration, due to the ease of their isolation and their inherent and efficient capacity for self-renewal. The multipotency of ADSCs has been demonstrated.2,16 However, the possibility of delivery of therapeutic transgenes by transplantation of genetically engineered hADSCs appears limited by the resistance of ADSCs to current transfection methods, including lipofection.17,18 Broadening the application of hADSCs in cell therapy requires methods that increase the transfection efficiency of hADSCs, especially through nonviral means. In this study, we have demonstrated that microporation is an excellent method for gene transfection into hADSCs.
The microporator is a newly designed electroporator. It makes use of a new electroporation technology using a pipette tip as an electroporation space and a gold-coated electrode surface. This device generates a uniform electric field with minimal heat production, metal ion dissolution, or oxide formation, which are harmful to cells during electroporation. In this study, we established optimal conditions for transfection of hADSCs by microporation. These conditions generated minimal cell damage in hADSCs and led to a high transfection efficiency of up to 76.1% 24 hours after microporation. More important, microporated hADSCs retained their multipotency and reporter gene expression for >2 weeks. In vivo, hADSCs microporated with a Tet-ON–regulated EGFP expression system exhibited inducible transgene expression. The use of the Tet-ON–regulated system of transgene expression should allow sufficient control of transgene expression so as to enhance the safety of the use of engineered hADSCs in cell therapy.
Safety and efficiency are major concerns for stem cell therapy involving the delivery of therapeutic transgenes. Although viral vectors can lead to more stable transgene expression and higher transfection efficiencies compared to nonviral vectors,13,19 viral vectors are associated with the risk of inducing recipient toxicity, as well as immune and inflammatory responses. In addition, virus-based methods are difficult to set up because of the rigid requirements for specific safety conditions, especially when using human cells.9 In contrast, nonviral methods give rise to a typical transfection efficiency of 20–25%, which is less efficient than viral vector–mediated DNA delivery.10 However, there are several advantages to nonviral methods, including lower manufacturing costs, the lack of or presence of a weak immunogenic response following repeated administration, and the lack of concern of generating replication-competent virus by recombination.13 Therefore, developing safe and efficient methods for gene delivery is the main challenge of gene therapy. Here, we showed that microporation is suitable for transducing plasmid DNA into hADSCs with high transfection efficiency (Figures 1 and 2). Furthermore, unlike traditional electroporation methods, microporation induces minimal cell death in hADSCs. Therefore, the method should allow for the generation of a sufficient quantity of transfected hADSCs for cell therapy without the need for extensive cell culturing.
Chung et al. have reported that different promoter systems may have different abilities to express reporter genes in embryonic stem cells.20 We show here that both the cytomegalovirus (CMV) and thymidine kinase (TK) promoters can express reporter genes efficiently (Figure 4). After co-transfection, expression of the Tet-ON-inducible EGFP was also detected in hADSCs both in vivo and in vitro (Figure 4). Thus, the different promoter systems that we employed were able to express reporter genes in hADSCs. Also, microporation of EGFP and luciferase constructs as reporters resulted in similar transfection efficiencies under optimal transfection conditions. These findings suggest that existing promoter and reporter gene systems are fully compatible with the microporation method described in this report.
We further demonstrated that microporation did not give rise to a toxic effect on the transfected cells. Recently, nucleofection, another modified electroporation method, achieved nearly 10-fold greater transient and stable transfection efficiency in mouse ES cells than electroporation.21 It has been further demonstrated that nucleofection can be used to transfect several primary cell types, including mouse T cells,22 neurons,23 keratinocytes,24 and stem cells of human and mouse origin.25,26 However, cell viability of the nucleofected MSCs was generally very low (from 15 to 40%).25,27 Although Aluigi et al.25 have shown that nucleofection achieved ~70% transfection efficiency in hMSCs, the reported transfection efficiency was overestimated due to the assumption that, in their study, debris and dead cells were eliminated from the culture medium of transfected cells before being attached to culture dishes after overnight incubation.
To increase the application performance of microporation, the transfection efficiency in different stem cells from different tissues should be investigated. We have analyzed the transfection efficacies of microporated human bone marrow mesenchymal stem cells. Similar transfection efficiencies were obtained in human bone marrow mesenchymal stem cells, suggesting that microporation is a good method for gene transfer into stem cells and that it deserves more investigation to evaluate its application in gene therapy (Supplementary Figure S1).
We have demonstrated that microporation is an excellent method for gene transfection into hADSCs. The stem cell plasticity of hADSCs was maintained after microporation. Microporated ADSCs could be induced to differentiate into adipocyte and osteoblast lineages and were shown to maintain transgene expression following differentiation. Our data also showed that Dox-regulated EGFP expression could be observed both in vitro and in vivo on microporation. Therefore, gene delivery by microporation is a safe and efficient means for prolonged transgene expression and may enhance the feasibility of transgenic stem cell therapy in the future.
Materials and Methods
Materials. A Tet-ON system comprising pCMV-rtTA and pBI-EGFP was purchased from Clontech (BD Biosciences, San Jose, CA). An EGFP expression vector, pCMV-EGFP-N1, was also purchased from Clontech (BD Biosciences). A reporter pRL-TK vector contained a herpes simplex virus–TK promoter region derived from the pRL-TK reporter vector containing the Renilla luciferase gene (Promega, Madison, WI). The monoclonal anti-EGFP antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β-actin monoclonal antibody was obtained from Upstate Biotechnology. Horseradish peroxidase–conjugated secondary antibody was obtained from Amersham Biosciences, Buckinghamshire, UK. The enhanced chemiluminescence kit was purchased from Millipore (Billerica, MA). Collagenase, 4′,6-diamidino-2-phenylindole, Alizarin red, and Oil-red O were purchased from Sigma. The microporator instrument was purchased from Digital Bio Technology (Digitalbiotechnology, Seoul, South Korea).
Isolation of human adipose tissue–derived mesenchymal stem cells (hADSCs). Subcutaneous adipose tissue was obtained from patients during THA or internal fixation for fractures. Furthermore, hADSCs were isolated as previously reported.28 Briefly, adipose tissues were washed with sterile phosphate-buffered saline (PBS) at least three times to remove the majority of erythrocytes. Then, the tissue was treated with collagenase (1 mg/ml) in Dulbecco's modified Eagle's medium at 37 °C on a shaker. After incubation overnight, the collagenase solution was removed and the pellet was washed with PBS. Subsequently, cells were cultured in keratinocyte medium (Invitrogen, Carlsbad, CA) with 5% fetal bovine serum, 2 mmol/l N-acetyl-L-cysteine, and 200 µmol/l L-ascorbic acid-2-phosphate in a 5% CO2 incubator. The medium was changed every other day, and the cells were allowed to grow until near confluence, which was defined as passage “0.” The passage number of hADSCs used in the experiments ranged from 3 to 10.
Osteogenic and adipogenic differentiation. To induce osteogenic differentiation, cells were treated with osteogenic medium for 2 weeks. Osteogenic medium consists of Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 10 mmol/l β-glycerol phosphate, 50 µmol/l ascorbic acid, and 100 µg/ml penicillin/streptomycin. Calcium phosphate mineral deposition was determined by Alizarin red S staining. Briefly, the cells were washed twice with PBS and fixed with 10% formaldehyde for 30 minutes. The fixed cells were incubated with 2% Alizarin red S for 10 minutes and then washed three times with deionized water to remove nonspecific staining. The nontransfected cells were stained for the control experiment.
For induction of adipogenic differentiation, the cells were cultured in adipogenic medium (Dulbecco's modified Eagle's medium with 10% fetal bovine serum, 100 µg/ml penicillin/streptomycin, 10−6 mol/l dexamethasone, 0.5 mmol/l 3-isobutyl-1-methylxanthine, and 5 µg/ml insulin). Medium was then changed every 2 days.29 To evaluate adipogenic differentiation, cells were stained with Oil-red O on the 12 th day. The cells were fixed with 10% formaldehyde for 30 minutes and washed with water and then with 60% isopropyl alcohol. After being washed, cells were incubated in Oil-red O–staining solution: 1.8 mg/ml in 60% isopropyl alcohol for 30 minutes. The excess staining solution was removed by washing with 60% isopropyl alcohol and water.
Flow cytometric analysis. Cells were detached and flushed with PBS to prevent any aggregation, 24 h after microporation. For DNA content analysis, cells were fixed with ice-cold 70% alcohol and incubated at 4 °C for a minimum of 30 minutes. Cell membranes were permeated with 0.1% TritonX-100, and RNA was digested with 20 µg/ml DNase-free RNAase at 37 °C for 1 hour. Cells were then stained with 50 µg/ml propidium iodide in the dark. For EGFP expression analysis, cells were washed with PBS and immediately fixed with 2% paraformaldehyde. Cells were filtered with a filter with a pore size of 41 µm just before the analysis. DNA content and EGFP-positive cells were measured by using a laser flow cytometer (EPICS Elite; Coulter, Hialeah, FL). The cell cycle distribution was analyzed by Wincycle software (EPICS Elite, Coulter). Cells with DNA content less than that of G1 phase cells (sub-G1) were assumed to be apoptotic.30
Lactate dehydrogenase assay. Lactate dehydrogenase leakage from cells was measured to quantify cytotoxicity by using the Cytotoxicity Detection kit (Roche, Penzberg, Germany). After microporation, the supernatants and cell layers of the cultures were collected for the assay according to the manufacturer's instructions. Absorbance was measured with an enzyme-linked immunosorbent assay reader with a 490 nm filter.31
Luciferase activity. Various doses of the reporter plasmid, pRL-TK, were transfected into hADSCs. The cells were harvested 24 hours later. Luciferase activity was assayed on total cell extracts using a commercial kit (Promega) and luminescence was measured with TopCount NXT. Protein was determined by the Bradford colorimetric method (Bio-Rad). Luciferase activities were normalized to total protein content and expressed in relative luciferase units as the mean ± SD of three independent samples.
Microporation transfection. Subconfluent hADSCs were harvested and washed with PBS. Cells were resuspended in Resuspension buffer R at a density of ~1 × 107 cells/ml and incubated with various concentrations of plasmids. Then, microporation was performed at room temperature using different programs: P1, 900 voltage, 20 ms, one pulse; P2, 900 voltage, 20 ms, two pulses; P3, 1,500 voltage, 20 ms, one pulse; P4, 1,500 voltage, 20 ms, two pulse; and P5, 1,800 voltage, 20 ms, one pulse. After electroporation, cells were distributed into 35-mm cell culture dishes and placed at 37 °C in a 5% CO2-humidified atmosphere. Cells were harvested at 24 hours and washed twice with PBS. Cell pellets were stored at −70 °C until use for western blot and luciferase activity determination.
Green fluorescence of EGFP was visualized using a Nikon TE300 microscope. Images were created by Image-Pro Plus 5.0 (Media Cybernetics, Silver Spring, MD) and digitized directly from the microscope images using a CCD (Evolution VF Cooled Monochrome; Media Cybernetics).
Western blots. Western blotting was performed as described previously.32 Briefly, equal amounts of cell extracts were separated through a 10% sodium dodecyl sulfate polyacrylamide gel and transferred onto Hybond-C membranes. The membranes were blocked with 5% nonfat milk in PBST buffer (PBS and 0.1% Tween 20) and probed with anti-EGFP and anti-β-actin antibodies. The blots were then incubated with horseradish peroxidase–conjugated goat antimouse immunoglobulin G and visualized using the enhanced chemiluminescence system. Images were captured and quantitative densitometric analyses were performed using Lab Works Image Acquisition and analysis software (UVP, Upland, CA).
In vivo gene expression analysis. The method of cell transplantation was described previously33 with minor modifications. Furthermore, hADSCs were co-transfected with pCMV-rtTA and pBI-EGFP plasmids. After 24 hours, cells were trypsinized and then washed with PBS. After centrifugation, 105cells were resuspended in cold serum-free Dulbecco's modified Eagle's medium and mixed with an equal volume of cold Matrigel (10 µg/ml). A total volume ~0.3 ml was subcutaneously injected into both back flanks of nude mice. Each mouse was implanted in six locations. There were two experimental groups: Group 1 (control) did not receive Dox in the drinking water, whereas group 2 received Dox in the drinking water (200 µg/ml). The drinking water contained 2.5% sucrose, and the water bottles were wrapped with aluminum foil. The bottles of water were changed every 3 days. The mice were killed 5 or 14 days after the injection. The Matrigel plugs were embedded in OCT compound and quickly frozen in liquid nitrogen. The frozen materials were cut into 6- to 7-µm-thick sections using a cryostat (Leica CM1900, Wetzlar, Germany). The sections were rehydrated in cold PBS. The nuclei were counterstained with 4′,6-diamidino-2-phenylindole (2 ng/ml) for 5 minutes and mounted. Images were acquired by microscopy.
Supplementary MaterialFigure S1. hBMSCs were transfected with 1.5 µg of pEGFP-N1 using a microporator.
Twenty-four hours post microporation, the expression of EGFP was analyzed under fluorescence microscopy. A, hBMSCs were microporated using different voltage and pulse conditions: a and d, control; b and e, program 1, c and f, program 2; g and j, program 3; h and k, program 4; and i and l, program 5. a-c and g-i, phase contrast images; d-f and j-l, EGFP fluorescence.
Supplementary Material
hBMSCs were transfected with 1.5 µg of pEGFP-N1 using a microporator.
Twenty-four hours post microporation, the expression of EGFP was analyzed under fluorescence microscopy. A, hBMSCs were microporated using different voltage and pulse conditions: a and d, control; b and e, program 1, c and f, program 2; g and j, program 3; h and k, program 4; and i and l, program 5. a-c and g-i, phase contrast images; d-f and j-l, EGFP fluorescence.
Acknowledgments
This study was supported by the Ministry of Economic Affairs under grant (96-EC-17-A-17-S1-041) and the National Health Research Institutes under grant (NHRI-EX96-9615EP), in Taiwan. We thank Chung-Hwan Chen and Bertrand Chin-Ming Tan for the helpful discussion and for critically reading the manuscript.
REFERENCES
- Moraleda JM, Blanquer M, Bleda P, Iniesta P, Ruiz F, Bonilla S, et al. Adult stem cell therapy: dream or reality. Transpl Immunol. 2006;17:74–77. doi: 10.1016/j.trim.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Schaffler A., and , Buchler C. Concise review: adipose tissue-derived stromal cells—basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- Hattori H, Ishihara M, Fukuda T, Suda T., and , Katagiri T. Establishment of a novel method for enriching osteoblast progenitors from adipose tissues using a difference in cell adhesive properties. Biochem Biophys Res Commun. 2006;343:1118–1123. doi: 10.1016/j.bbrc.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Kern S, Eichler H, Stoeve J, Kluter H., and , Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- Seo MJ, Suh SY, Bae YC., and , Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258–264. doi: 10.1016/j.bbrc.2004.12.158. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Shahdadfar A, Fronsdal K, Haug T, Reinholt FP., and , Brinchmann JE. In vitro expansion of human mesenchymal stem cells: choice of serum is a determinant of cell proliferation, differentiation, gene expression, and transcriptome stability. Stem Cells. 2005;23:1357–1366. doi: 10.1634/stemcells.2005-0094. [DOI] [PubMed] [Google Scholar]
- Tomanin R., and , Scarpa M. Why do we need new gene therapy viral vectors? Characteristics, limitations and future perspectives of viral vector transduction. Curr Gene Ther. 2004;4:357–372. doi: 10.2174/1566523043346011. [DOI] [PubMed] [Google Scholar]
- Vetere A, Marsich E, Di Piazza M, Koncan R, Micali F., and , Paoletti S. Neurogenin3 triggers beta-cell differentiation of retinoic acid-derived endoderm cells. Biochem J. 2003;371:831–841. doi: 10.1042/BJ20021524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dass CR. Cytotoxicity issues pertinent to lipoplex-mediated gene therapy in-vivo. J Pharm Pharmacol. 2002;54:593–601. doi: 10.1211/0022357021778817. [DOI] [PubMed] [Google Scholar]
- Jo J., and , Tabata Y. Non-viral gene transfection technologies for genetic engineering of stem cells. Eur J Pharm Biopharm. 2008;68:90–104. doi: 10.1016/j.ejpb.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Oligino TJ, Yao Q, Ghivizzani SC., and , Robbins P. Vector systems for gene transfer to joints. Clin Orthop Relat Res. 2000. pp. S17–S30. [DOI] [PubMed]
- Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Chen X, Yan Z, Liu L, Tang W, Zheng X, et al. Multilineage differentiation of adipose-derived stromal cells from GFP transgenic mice. Mol Cell Biochem. 2006;285:69–78. doi: 10.1007/s11010-005-9056-8. [DOI] [PubMed] [Google Scholar]
- Zaragosi LE, Billon N, Ailhaud G., and , Dani C. Nucleofection is a valuable transfection method for transient and stable transgene expression in adipose tissue-derived stem cells. Stem Cells. 2007;25:790–797. doi: 10.1634/stemcells.2006-0235. [DOI] [PubMed] [Google Scholar]
- Young AT, Lakey JR, Murray AG., and , Moore RB. Gene therapy: a lipofection approach for gene transfer into primary endothelial cells. Cell Transplant. 2002;11:573–582. [PubMed] [Google Scholar]
- Phillips JE, Gersbach CA., and , Garcia AJ. Virus-based gene therapy strategies for bone regeneration. Biomaterials. 2007;28:211–229. doi: 10.1016/j.biomaterials.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Chung S, Andersson T, Sonntag KC, Bjorklund L, Isacson O., and , Kim KS. Analysis of different promoter systems for efficient transgene expression in mouse embryonic stem cell lines. Stem Cells. 2002;20:139–145. doi: 10.1634/stemcells.20-2-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmipathy U, Pelacho B, Sudo K, Linehan JL, Coucouvanis E, Kaufman DS, et al. Efficient transfection of embryonic and adult stem cells. Stem Cells. 2004;22:531–543. doi: 10.1634/stemcells.22-4-531. [DOI] [PubMed] [Google Scholar]
- Lai W, Chang CH., and , Farber DL. Gene transfection and expression in resting and activated murine CD4 T cell subsets. J Immunol Methods. 2003;282:93–102. doi: 10.1016/j.jim.2003.07.015. [DOI] [PubMed] [Google Scholar]
- Cesnulevicius K, Timmer M, Wesemann M, Thomas T, Barkhausen T., and , Grothe C. Nucleofection is the most efficient nonviral transfection method for neuronal stem cells derived from ventral mesencephali with no changes in cell composition or dopaminergic fate. Stem Cells. 2006;24:2776–2791. doi: 10.1634/stemcells.2006-0176. [DOI] [PubMed] [Google Scholar]
- Distler JH, Jüngel A, Kurowska-Stolarska M, Michel BA, Gay RE, Gay S, et al. Nucleofection: a new, highly efficient transfection method for primary human keratinocytes*. Exp Dermatol. 2005;14:315–320. doi: 10.1111/j.0906-6705.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- Aluigi M, Fogli M, Curti A, Isidori A, Gruppioni E, Chiodoni C, et al. Nucleofection is an efficient nonviral transfection technique for human bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24:454–461. doi: 10.1634/stemcells.2005-0198. [DOI] [PubMed] [Google Scholar]
- Quenneville SP, Chapdelaine P, Rousseau J, Beaulieu J, Caron NJ, Skuk D, et al. Nucleofection of muscle-derived stem cells and myoblasts with phiC31 integrase: stable expression of a full-length-dystrophin fusion gene by human myoblasts. Mol Ther. 2004;10:679–687. doi: 10.1016/j.ymthe.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Hamm A, Krott N, Breibach I, Blindt R., and , Bosserhoff AK. Efficient transfection method for primary cells. Tissue Eng. 2002;8:235–245. doi: 10.1089/107632702753725003. [DOI] [PubMed] [Google Scholar]
- Lin TM, Tsai JL, Lin SD, Lai CS., and , Chang CC. Accelerated growth and prolonged lifespan of adipose tissue-derived human mesenchymal stem cells in a medium using reduced calcium and antioxidants. Stem Cells Dev. 2005;14:92–102. doi: 10.1089/scd.2005.14.92. [DOI] [PubMed] [Google Scholar]
- Nuttall ME, Patton AJ, Olivera DL, Nadeau DP., and , Gowen M. Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: implications for osteopenic disorders. J Bone Miner Res. 1998;13:371–382. doi: 10.1359/jbmr.1998.13.3.371. [DOI] [PubMed] [Google Scholar]
- Shrivastava A, Tiwari M, Sinha RA, Kumar A, Balapure AK, Bajpai VK, et al. Molecular iodine induces caspase-independent apoptosis in human breast carcinoma cells involving the mitochondria-mediated pathway. J Biol Chem. 2006;281:19762–19771. doi: 10.1074/jbc.M600746200. [DOI] [PubMed] [Google Scholar]
- Chang JK, Wu SC, Wang GJ, Cho MH., and , Ho ML. Effects of non-steroidal anti-inflammatory drugs on cell proliferation and death in cultured epiphyseal-articular chondrocytes of fetal rats. Toxicology. 2006;228:111–123. doi: 10.1016/j.tox.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Wang YH, Tsay YG, Tan BC, Lo WY., and , Lee SC. Identification and characterization of a novel p300-mediated p53 acetylation site, lysine 305. J Biol Chem. 2003;278:25568–25576. doi: 10.1074/jbc.M212574200. [DOI] [PubMed] [Google Scholar]
- Glondu M, Coopman P, Laurent-Matha V, Garcia M, Rochefort H., and , Liaudet-Coopman E. A mutated cathepsin-D devoid of its catalytic activity stimulates the growth of cancer cells. Oncogene. 2001;20:6920–6929. doi: 10.1038/sj.onc.1204843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
hBMSCs were transfected with 1.5 µg of pEGFP-N1 using a microporator.
Twenty-four hours post microporation, the expression of EGFP was analyzed under fluorescence microscopy. A, hBMSCs were microporated using different voltage and pulse conditions: a and d, control; b and e, program 1, c and f, program 2; g and j, program 3; h and k, program 4; and i and l, program 5. a-c and g-i, phase contrast images; d-f and j-l, EGFP fluorescence.