Abstract
We studied antiangiogenic and antilymphangiogenic effects of sVEGFR-1 (sFlt-1), sVEGFR-2 (sFlk-1/KDR), and sVEGFR-3 (sFlt-4) gene transfers and their combinations in intraperitoneal ovarian cancer xenograft mice (Balb/c-Anu, n = 55). Gene therapy was initiated when the presence of sizable tumors was confirmed in magnetic resonance imaging (MRI). Adenovirus-mediated gene transfer was performed intravenously via tail vein as follows: AdLacZ as a control (group I), AdsFlt-1 (group II), AdsKDR (group III), AdsFlt-4 (group IV) and two combination groups of AdsFlt-1 and AdsFlt-4 (group V) and AdsFlt-1, AdsKDR, and AdsFlt-4 (group VI). Antitumor effectiveness was assessed by sequential MRI, immunohistochemistry, microvessel density, overall tumor growth, and survival time. In combination group VI, intraperitoneal tumors were significantly smaller than in the control group at the end of the follow-up (P < 0.001). Furthermore, in group VI the microvessel density (microvessels/mm2) in tumor tissue and the total area of tumors covered by microvessels were significantly smaller than in the controls. One mouse in group V was cured. The combined antiangiogenic gene therapy with soluble VEGFRs reduced tumor growth, tumor vascularity, and ascites formation in ovarian cancer xenografts. The results suggest that the combined antiangiogenic gene therapy is a potential approach for the treatment of ovarian cancer patients.
Introduction
Ovarian cancer is the most lethal of the gynecological malignancies.1 Two-thirds of the patients with ovarian carcinoma have widely disseminated disease with intraperitoneal carcinosis and ascites at the time of diagnosis. Despite optimal surgery and chemotherapy, the prognosis of patients remains poor and new treatments are urgently needed. To this end, phase I gene therapy studies employing different strategies, such as suicide genes, targeting oncogenes, or restoring tumor suppressor genes, have shown the feasibility of such treatments, but the effectiveness to suppress tumor growth has been very limited. The only phase III trial carried out so far was closed after interim analysis because it showed no clinical benefit in patients receiving the combination of carboplatin and paclitaxel with intraperitoneal p53 gene therapy as compared to standard therapy.2
Angiogenesis, defined as new vessel formation, is crucial for tumor growth and metastasis.3 VEGF (vascular endothelial growth factor) is a potent angiogenic factor, which has been found to be overexpressed in various tumors,4 including ovarian tumors,5 and associated with poor prognosis. In addition to VEGF, also VEGF-B, -C, and -D have been suggested to play significant roles in ovarian tumorigenesis.6,7,8 VEGF family members meditate their effects through VEGF receptors 1, 2, and 3, also known as Flt-1, KDR/Flk-1, and Flt-4, respectively.9,10 VEGFR-1, which binds VEGF, VEGF-B, and placenta growth factor, is found in both vascular endothelial cells and macrophages. VEGFR-1 is a positive regulator of monocyte/macrophage migration and inflammation, and it stimulates angiogenesis, tumor growth and metastasis.11 Binding of VEGFR-2 by VEGF and processed forms of VEGF-C and -D is considered to be the main mediator of angiogenesis, vasculogenesis, and vascular permeability, whereas VEGFR-3 with high affinity to VEGF-C and -D, is required for lymphatic vessel development and functioning,9,10 and is essential for tumor spread via lymphatic vessels.12 Because VEGFs play key roles in tumor biology, VEGFs and their receptors are potential targets for cancer therapy.
Soluble VEGF receptors used in this study lack transmembrane domain and intracellular tyrosine kinase-containing parts and therefore they do not initiate signal transduction. All constructs contain an immunoglobulin Fc domain to ensure efficient dimerization of the soluble receptors. Both sVEGFR-1 and sVEGFR-2 sequester VEGF ligands and also form inactive heterodimers with transmembrane receptors.13,14,15 sVEGFR-3 has shown to bind VEGF-C and VEGF-D with the same efficiency as the full-length receptor and therefore competes with the binding of the ligands to their native receptors.16
The aim of this study was to combine antiangiogenic gene therapy in an ovarian cancer xenograft model that closely reflects the complexity of tumor progression, mimicks the diversity of human ovarian cancer, and shows a very aggressive behavior.17 Furthermore, we have applied magnetic resonance imaging (MRI) for the timing of gene therapy to treat sizable tumors, not a micrometastatic disease, and to follow tumor progression noninvasively in vivo.
Results
Transgene expression
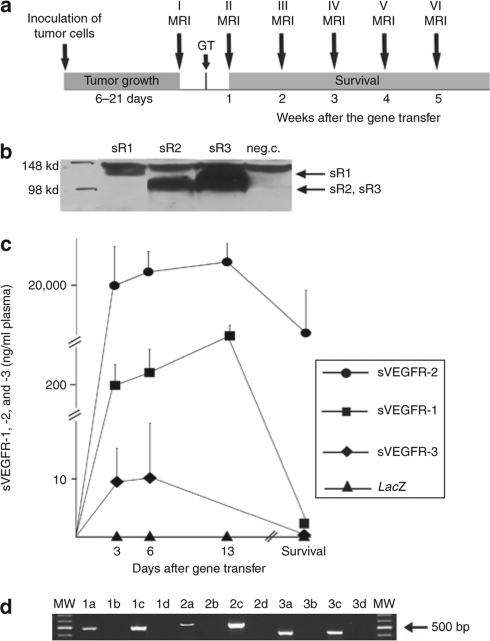
Outline of the study is shown in Figure 1a. Western blotting in vitro showed similar expression of sVEGFR-1, -2, and -3 in the medium of SKOV-3m cells after adenovirus transductions (Figure 1b). Plasma sVEGFR-1 and sVEGFR-2 levels were highest at day 13 after the gene therapy and the levels were higher than 1 ng/ml (ref. 18) throughout the follow-up. Plasma level of sVEGFR-2 was higher than 8,400 ng/ml at each time point. In the control group I, no signals were detected for the soluble receptors at any time point by enzyme-linked immunosorbent assay (Figure 1c). Reverse transcription–PCR with 35 cycles showed mRNA expression of all transgenes in liver samples 6 days after the gene transfer (Figure 1d).
Figure 1.
Protocol of the study and expressions of soluble VEGFRs. (a) Outline of the study. Tumors developed within 3 weeks after the inoculation of the tumor cells. The presence of all tumors was verified by MRI before starting gene therapy. Tumors were observed weekly until the death of the mice. GT, gene therapy; MRI, magnetic resonance imaging. (b) Expression levels of sVEGFR-1, sVEGFR-2, and sVEGFR-3 after adenoviral transduction in SKOV-3m cells were similar as measured by western blotting. (c) sVEGFR-1, sVEGFR-2, and sVEGFR-3 levels in plasma as determined by enzyme-linked immunosorbent assays in combination group VI (sVEGFR-1, sVEGFR-2, and sVEGFR-3). The transgene expression profiles were identical in the other groups. sVEGFR-1, sVEGFR-2, and sVEGFR-3 were not detected in AdLacZ control mice at any time point. Error bars = SEM. (d) Reverse transcription (RT)-PCR was used to confirm the expression of sVEGFR-1, -2, and -3 in mouse liver samples. Lanes 1a–d: sVEGFR-1 RT-PCR. 1a: liver sample 6 days after AdsFlt-1 gene transfer, 1b: liver sample 6 days after AdLacZ gene transfer, 1c: positive control, and 1d: no RT-control. Lanes 2a–d: sVEGFR-3 RT-PCR. 2a: Liver sample 6 days after AdsFlt-4 gene transfer, 2b: liver sample 6 days after AdLacZ gene transfer, 2c: positive control and 2d: no RT-control. Lanes 3a–d: sVEGFR-2 RT-PCR. 3a: liver sample 6 days after AdsKDR gene transfer, 3b: liver sample 6 days after AdLacZ gene transfer, 3c: positive control, and 3d: no RT-control.
Intraperitoneal tumor growth
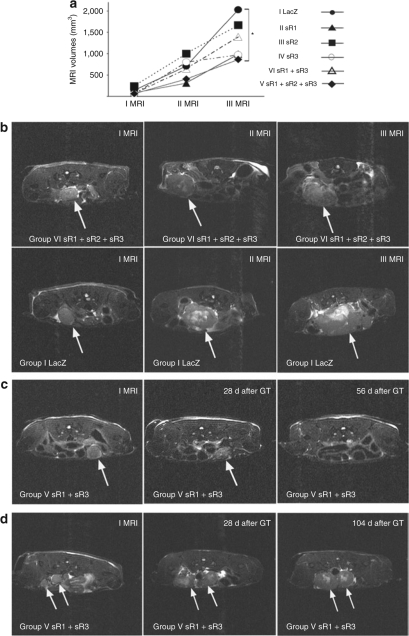
All mice developed intraperitoneal tumors within 3 weeks (6–21 days) after SKOV-3m cell inoculation. MRI was repeated weekly after the gene transfer. In the first and second MRI, there were no significant differences in tumor volumes between the control AdLacZ group and the gene therapy groups. At the time of the third MRI (2 weeks after the gene therapy), the mean tumor volume in group VI was significantly smaller (P = 0.035) than that of the control mice (866 ± 270 mm3 versus 2,026 ± 369 mm3) (Figure 2a,b). The final mean tumor weights at the end of the follow-up were also significantly smaller (P < 0.001) in the gene therapy group VI (2.3 ± 0.38 g), as compared to the control mice (4.5 ± 0.42 g) (Figure 3a). There was statistically significant correlation between tumor volumes at the time of third MRI and the final tumor weights (r = 0.8, P < 0.005).
Figure 2.
MRI measurements of tumor growth. (a) Measured by MRI, the mean tumor volumes (mm3) were significantly smaller in group VI (sVEGFR-1, -2, and -3) versus controls 2 weeks after the gene therapy (III MRI) *P < 0.05. (b) MRI pictures of the development of ovarian tumors in group VI compared to group I (AdLacZ). At the time of the first and the second MRI, there was no difference in tumor volumes, but in the third MRI tumors were significantly smaller in group VI. Tumors are marked with arrows. (c) MRI pictures of the cured mouse in group V (sVEGFR-1 and sVEGFR-3). An intraperitoneal ovarian tumor (arrow) was visible in MRI 18 days after the tumor cell injection. 28 days after the gene therapy, the tumor was shrunken (arrow) and 56 days after the gene therapy the tumor was not visible in MRI. (d) MRI pictures of the mouse in group V (sVEGFR-1 and sVEGFR-3) which had dormancy in tumor growth and a notably prolonged survival. Tumors are marked with arrows. MRI, magnetic resonance imaging.
Figure 3.
Tumor and ascites measurements. (a) At the end of the follow-up, the weights of the tumors were significantly smaller in both combination groups and in the mice treated with sVEGFR-3. (b) sVEGFR-2-treated mice did not form any ascites in the peritoneal cavity. (c) Microvessel density (MVD: microvessels/mm2) was significantly reduced in mice treated with sVEGFR-2 and with the combination of sVEGFR-1, -2, and -3. (d) combination gene therapy with sVEGFR-1, -2, and -3 significantly reduced the total area of tumors covered by microvessels (TVA: tumor vascular area). *P < 0.05; **P < 0.01; ***P < 0.001 versus LacZ, Error bars = SEM.
Formation of ascites
Formation of ascites was completely blocked in mice receiving sVEGFR-2 gene therapy and the difference was significant as compared to the AdLacZ control group (1.9 ± 0.42 ml), P = 0.005 (Figure 3b). In the other gene therapy groups, there were no significant differences in the amount of ascites as compared to the controls. However, in the combination group VI a distinct tendency toward lesser formation of ascites (0.85 ± 0.44 ml) was noted (Figure 3b).
Microvessel measurements
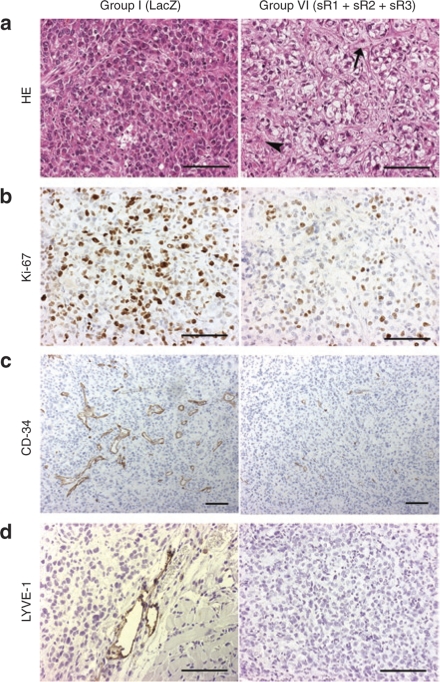
To detect the effect of gene therapy on intratumoral microvessels, microvessel density (MVD) and tumor vascular area (TVA) were measured. MVD (42.3 ± 6.4) and TVA (0.83 ± 0.14%) of tumors in group VI were significantly smaller than those of the controls (86.1 ± 6.5, P = 0.005 and 2.6 ± 0.24%, P = 0.005) (Figure 3c,d). An example of the smaller TVA in the combination gene therapy group VI (Figure 4c, right panel) as compared to the controls is shown in Figure 4c, left panel. The majority of intratumoral lymphatic vessels in the control group, stained with LYVE-1, were found at the edges of the tumors and only 15% in the central part (Figure 4d, left panel). In group VI, LYVE-1 positive lymphatic vessels were not detected (Figure 4d, right panel), and in sVEGFR-3-treated mice, only 2–3 lymphatic vessels per section were found (data not shown).
Figure 4.
Histology of intraperitoneal ovarian tumors. (a) Hematoxylin– eosin staining of serous adenocarcinoma in group I (AdLacZ, left panel). Focal necrosis (arrowhead) and connective tissue (arrow) were present in the tumor tissue in group VI (sVEGFR-1, -2, and -3, right panel). (b) more proliferating tumor cells were seen in control group I (left panel) than in group VI (right panel), 70–80% and 5–40%, respectively. Ki-67 staining. (c) CD-34 positive microvessels in tumor tissue. In group I (left panel), total vascular area was higher than in group VI (right panel). (d) LYVE-1 positive lymphatic vessels were seen in the periphery of tumors in group I (left panel) contrary to the treatment group VI (right panel). Magnification, ×200 (a, b, and d); ×100 (c). Bar = 100 µm.
Histology
The tumors were poorly differentiated (grade 3) serous cystadenocarcinomas with variable size of nucleus and limited stroma (Figure 4a, left panel). In the combination treatment group VI tumor tissue was partly replaced by connective tissue and morphology of the tumors was disturbed at the end of the follow-up (Figure 4a, right panel). In the combination gene therapy group cell proliferation index measured by Ki-67 staining was 5–40% compared to 70–80% in the control group, P = 0.003 (Figure 4b).
Survival and safety
The mean survival (days) in the study groups was as follows: 32 ± 2 in group I, 35 ± 2 in group II, 36 ± 2 in group III, 35 ± 2 in group IV, 55 ± 16 days in group V and 38 ± 2 in group VI. A trend for a prolonged survival in the combination gene therapy groups was observed, but the difference was not statistically significant. Indeed, in group V (sVEGFR-1 and sVEGFR-3) the mean survival was notably longer than in the control group (55 ± 16 versus 32 ± 2 days) with one animal being completely cured having no detectable tumor in MRI 56 days after the gene therapy or at autopsy (Figure 2c) and we noted dormancy in tumor growth with another mouse belonging to the same group (Figure 2d).
Safety was judged by the assessment of histological samples of liver, spleen, kidneys and lungs as well as by the analysis of plasma alanine aminotransferase and creatinine levels. Histologically, liver samples of both treated and control mice were normal 6 days after the gene transfer. At the end of the follow-up, there was evidence of regenerative changes in group VI liver samples and 25% of mice showed macroscopic alterations in the liver, which histologically consisted of local necrosis. Plasma alanine aminotransferase levels were elevated at the late stages of the disease in both treated and control groups (Table 1). There were no histological alterations in other organs and, accordingly, creatinine values were within normal range (Table 1).
Table 1.
Clinical chemistry after the gene transfer (mean ± SEM)
Discussion
We present here the first antiangiogenic cancer gene therapy study with a combination of three soluble VEGF receptors VEGFR-1, 2, and -3 which competitively inhibit the binding of all members of the VEGF family to their receptors. The aim of the study was to collect preclinical evidence of the efficacy and safety in advanced disease, not at a micrometastatic state, the latter being an unlikely clinical scenario for potential phase I studies in ovarian cancer. Overall, this combined adenoviral gene therapy appeared to inhibit, but not completely reverse, the growth of human intraperitoneal ovarian tumors in nude mice with a well-established disease, large solid tumors and ascites. Indeed, antiangiogenic therapy reduced tumor growth, tumor vascularity and ascites formation, as assessed by sequential MRI, histology, and immunohistochemistry.
The most potent effect of gene therapy on tumor growth was achieved in group VI, in which the mice received a combination of all three sVEGFRs. It seems that combination therapy has a more powerful antitumor effect than single gene therapy. This is also supported by the fact that one mouse in the combination group V was cured and another mouse also had a notably longer survival than any of the control mice. MVD, TVA, and the weights of tumors were also significantly reduced in the combination group VI suggesting that this is compatible with VEGF activity. However, in mice treated with sVEGFR-2, the MVD was significantly smaller than in the controls although the tumor weights at the end of the follow-up were not significantly smaller. This may imply that also other mechanisms than the antiangiogenic effect may be involved in mice receiving triple therapy. As an example, reduced lymphangiogenesis with an antitumor effect was detected in mice treated with sVEGFR-3.
VEGF is also called as a vascular permeability factor and it is thought to be essential for the development of malignant ascites.19 When sVEGFR-1 has been used in intraperitoneal ovarian cancer gene therapy studies, it has been reported to suppress the formation of ascites18,20 whereas similar results with regard to sVEGFR-2 are sparse. Wu et al. have recently used adenoviral sVEGFR-2 (ref. 21), but in their study ovarian cancer cells were injected subcutaneously, and therefore, the amount of ascites could not be evaluated. In the current model, sVEGFR-2 completely blocked the formation of ascites fluid and a similar trend showing reduced ascites was also found in mice treated with sVEGFR-1, which supports the view that VEGF plays a major role in the ascites formation. It has been shown that inhibition of lymphangiogenesis in transgenic embryos expressing sVEGFR-3 induces lymphedema16 and increased ascites formation is also present in genetically modified mice having malfunctional VEGFR-3 (ref. 22). In this study, sVEGFR-3, which binds VEGF-C and VEGF-D, increased the formation of ascites, which is cleared from the peritoneum via lymphatic channels. It is possible that the triple combination therapy may have caused more leakiness from the capillaries and the drainage capacity of the lymphatic vessels may have been reduced as compared to the single sVEGFR-2 therapy. However, in the combination treatment groups V and VI the amount of ascites was reduced compared to the controls.
Survival was only slightly prolonged in individual gene therapy groups, probably due to the very aggressive nature of the model with the first tumors arising within 6 days after the engraftment. Previous antiangiogenic studies have shown that combination of chemotherapy with gene therapy prolongs survival and such regimens warrant further study. Liver toxicity has been reported previously when adenoviral sVEGFR-1 was injected intravenously.23 At the time of the highest sVEGFR-1 protein levels in plasma 6 days after the gene transfer, the histology of liver samples was normal. However, regenerative changes were observed in the liver samples of the group VI at the end of the follow-up but these mice also had severe metastasized intraperitoneal carcinoma which may contribute to these findings. In this study, we have used the maximum levels of adenoviral sVEGFRs although lower levels of soluble receptor expression might reduce liver toxicity without compromising the treatment effect tissue. Takei et al.18 have shown that peritoneal dissemination of ovarian cancer in mice is inhibited at the level of 1 ng/ml of sVEGFR-1 in circulation, which was maintained in our study throughout the follow-up period with all three soluble receptors. Also, when sR2 was given alone, the very high levels in plasma only inhibited ascites (Figure 3b) but not the tumor growth. As ovarian cancer is an intraperitoneal disease usually with a number of cancer nodules of varying size, intravenous delivery of antiangiogenic genes and their products is expected to be the best strategy to target malignant cells or their vascular supply although the risk of systemic side effects also becomes relevant. The transgene expression was in line with previous studies on adenoviruses but to achieve longer expression times it would be interesting to combine adenoviral treatment with long-term expression vectors. The combination of sVEGFR-1, sVEGFR-2, and sVEGFR-3 was more effective in suppressing tumor growth than any of the agents alone. In that group, the interindividual variability was less than in the other gene therapy groups, where the changes in microvascular measurements were not detected in all tumors. This result is in line with previous preclinical studies utilizing an antiangiogenic approach using gene-based therapy against VEGF.20 The present model showed that even the combination of antiangiogenic and antilymphangiogenic therapy was insufficient to induce tumor reggression, even though tumor vasculature was reduced throughout the tumor after the gene delivery. It is plausible that the ability of the tumors to escape the treatment is because of the upregulation of other angiogenic factors such as PDGF-B (platelet-derived growth factor),24 Ang-1 or Ang-2 (ref. 25). Although antiangiogenic agents alone are unlikely to eradicate tumors completely, similar strategies have been applied in ovarian cancer clinical trials using antihuman VEGF monoclonal antibody bevacizumab,26 soluble hybrid decoy receptor VEGF-Trap,27 tyrosine kinase inhibitor AZD 2171 (ref. 28), recombinant human IL-12 (ref. 29) and thalidomide.30
To conclude, we achieved a significant in vivo antitumor response by the triple combination of antiangiogenic sVEGF receptors 1, 2, and 3 in a novel mouse model having an advanced disease state at the time of the treatment. These results warrant further development of the combined antiangiogenic gene therapy to define the best dose and schedule for such a treatment, and suggest that this approach could be utilized in clinics along with other anticancer therapies.
Materials and Methods
Cell line. Detailed characteristics of the SKOV-3m cell line have been described previously.17 SKOV-3m cells were cultured in McCoy's 5A medium (Sigma, Steinheim, Germany). Before in vivo inoculation the cells were trypsinized and counted.
Viral vectors. Adenoviral vectors encoding human sVEGF receptor-1-Ig fusion protein (AdsFlt-1),31,32,33 human sVEGF receptor-2-IgG fusion protein (AdsKDR),34 mouse sVEGF receptor-3-IgG fusion protein (AdsFlt-4),16,35 and LacZ (AdLacZ) as a control vector were used for the study. Replication-deficient E1-E3 deleted clinical GMP-grade adenoviruses were produced in 293 cells. Adenoviruses were analyzed to be free from helper viruses, lipopolysaccharides, and bacteriological contaminants.36,37 For western blotting, SKOV-3m cells were plated on 12-well plates at a density of 100,000 cells per well. After 24 hours of cultivation, adenoviral vectors encoding soluble VEGFR-Fc fusion proteins were added to the plates (multiplicity of infection 200). Culture media were changed after 16 hours and samples were taken 72 hours after transduction. Protein expression was analyzed using immunoblotting with antihuman IgG (Fc-specific) antibody I2136 (Sigma-Aldrich, St Louis, MO), donkey anti-goat IgG-HRP secondary antibody sc-2020 (Santa Cruz Biotechnology, Santa Cruz, CA), SuperSignal West Dura (Thermo Fisher Scientific, Rockford, IL) substrate, and CL-XPosure Films (Thermo Fisher Scientific, Rockford, IL).
Animal model. Eight to 10-weeks old (n = 55) Balb/cA-nu female nude mice were used for the studies. The mice were kept in a pathogen-free isolated unit at the National Experimental Animal Center of the University of Kuopio. The mice received chow and water ad libitum. Food, water, and sawdust bedding were autoclaved.
Ovarian carcinoma was produced by inoculating 1 × 107 SKOV-3m cells into the peritoneal cavity of the nude mice with a 22 G needle. Development of the ovarian carcinoma tumors was followed by sequential MRI (Figure 1a). When the first solid, measurable tumor was detected in MRI, gene transfer was done the following day. The mice were randomly divided into six groups: seven animals received AdsFlt-1 (1 × 109 pfu), six animals received AdsKDR (1 × 109 pfu), eight animals received AdsFlt-4 (1 × 109 pfu), 12 animals received AdsFlt-1 and AdsFlt-4 (1 × 109 pfu both vectors), nine animals received AdsFlt-1, AdsFlt-4, and AdsKDR (0.7 × 109 pfu each vector) and 13 control animals received AdLacZ (2 × 109 pfu) (Table 2). Gene transfer was performed intravenously via tail vein in the final volume of 200 µl in 0.9% saline. MRI was done weekly after gene transfer and tumor volumes were assessed. The overall follow-up time lasted until the appearance of significant symptoms necessitating killing or to the death (Figure 1a). At the time of death, all tumor tissue, liver, spleen, kidneys, and lungs were harvested and tumor masses were weighed. Ascites fluid was collected with a syringe. All animal studies were accepted by the Experimental Animal Committee of the University of Kuopio.
Table 2.
Characterization of the study groups
Histology, immunohistochemistry and microvessel measurements. Tissue samples were immersed in 4% paraformaldehyde for 4–6 h, followed by overnight immersion in 15% sucrose.38 The specimens were embedded in paraffin and 5 µm thick sections were processed for hematoxylin– eosin, Ki-67 (DakoCytomation, Glostrup, Denmark), CD-34 (HyCult biotechnology b.v., AA Uden, The Netherlands) and LYVE-1 (ReliaTech, Braunschweig, Germany) stainings.
Photographs of histological sections were taken and processed using an Olympus AX70 microscope (Olympus Optical, Tokyo, Japan), and analySIS (Soft Imaging System, Münster, Germany) and PhotoShop (Adobe) software. Mean microvessel area (µm2), MVD, and total microvascular area (%) of the tumors (TVA) were measured from CD34-immunostained sections using analySIS software at ×100 magnification in a blinded manner. Ten different fields which represented maximum microvessel areas were selected from each tumor. Necrotic areas were avoided. The total number of LYVE-1 positive lymphatic vessels per section was counted. Means ± SEM of the measurements are reported.
MRI. To follow the development of ovarian carcinoma and to measure tumor volumes, 9.4 T vertical magnet (Oxford Instruments, Oxford, UK) equipped with actively shielded field gradients (Magnex Scientific, Abdington, UK) interfaced to an s.m.i.s. console (Surrey Medical Imaging Systems, Guolford, UK) was used. The details of MRI imaging have been described previously. MRI was performed weekly after the first tumors were detectable.
Reverse transcription–PCR. Reverse transcription–PCR was used to confirm the transgene expression in mouse liver samples. The liver tissue was snap-frozen at the sixth day after the gene transfer in liquid nitrogen and stored at −70 °C for reverse transcription–PCR analysis. Total RNA was extracted using Trizol Reagent (Gibco BRL, Grand Island, NE) according to manufacturer's instructions. Total RNA was treated with DNaseI (Promega, Madison, WI) to remove any contaminating DNA and cDNA synthesis was performed with 2 µg of RNA with random hexamers. Primers for the amplification of sVEGFR-1 cDNA were forward: 5′-AGG CCA GAC ACT GCA TCT CC-3′ and reverse: 5′-GCT TCA CAG GTC AGA AGC CC-3′, for sVEGFR-3 cDNA forward: 5′-TGA AGG CAC AGA AGC TAG GCC-3′ and reverse: 5′-ACC TGA GTC GAA CTC AGC CC-3′ and for sVEGFR-2 forward: 5′-ACA GAG GGA CTT GGA CTG GC-3′ and reverse: 5′-TTC ACA GAA GAC CAT GCC AGC-3′, with amplicon sizes of 500 bp, 530 bp, and 480 bp, respectively. PCR mixtures consisted of 20 pmol of each primer, 0.2 mmol/l dNTPs (Promega,), 1.5 mmol/l MgCl2, Dynazyme EXT PCR buffer, 1.5 U of Dynazyme EXT DNA polymerase (Finnzymes, Helsinki, Finland) and 500 ng of cDNA sample. Amplifications were carried out with the following conditions: the first denaturation step at 95 °C for 3 minutes, followed by 35 cycles with 45 seconds at 95 °C, 45 seconds at 60 °C for sFlt-1 and sKDR or at 62 °C for sFlt-4, 45 seconds at 72 °C with the final step at 72 °C for 15 minutes.
Clinical chemistry and enzyme-linked immunosorbent assay analyses from plasma samples. Plasma samples were collected at day 3, 6, and 13 after the gene transfer and when the mice were killed. Alanine aminotransferase and creatinine were monitored using routine clinical chemistry assays at Kuopio University Hospital Central Laboratory. Enzyme-linked immunosorbent assays (Quantikine; R&D Systems, Minneapolis, MN) were used to detect the presence of human soluble VEGFRs in plasma samples.
Statistical analyses. Statistical significance was evaluated using Kruskall–Wallis test, followed by Mann–Whitney U-test with appropriate correction for multiple comparisons. Results are expressed as mean ± SEM. A value of P < 0.05 was considered as statistically significant. Bivariate correlations were analyzed by Spearman's test.
Acknowledgments
We thank Seija Sahrio, Sari Järveläinen, Tiina Koponen, Anne Martikainen, Anneli Miettinen, and Helena Kemiläinen for skillful technical assistance. This study was supported by Finnish Academy, Ludwig Institute for Cancer Research, EU Lymphangiogenomics network (LSHG-CT-2004-503573), Kuopio University Hospital (EVO grant 5185), The Finnish Medical Foundation, The Foundation of Finnish Cancer Institute, The Finnish Cultural Foundation of Northern Savo and Research Foundation of Orion Corporation.
REFERENCES
- Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- Zeimet AG., and , Marth C. Why did p53 gene therapy fail in ovarian cancer. Lancet Oncol. 2003;4:415–422. doi: 10.1016/s1470-2045(03)01139-2. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Konishi I, Mandai M, Kuroda H, Komatsu T, Nanbu K, et al. Expression of vascular endothelial growth factor (VEGF) in epithelial ovarian neoplasms: correlation with clinicopathology and patient survival, and analysis of serum VEGF levels. Br J Cancer. 1997;76:1221–1227. doi: 10.1038/bjc.1997.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowter HM, Corps AN, Evans AL, Clark DE, Charnock-Jones DS., and , Smith SK. Expression and localization of the vascular endothelial growth factor family in ovarian epithelial tumors. Lab Invest. 1997;77:607–614. [PubMed] [Google Scholar]
- Yokoyama Y, Charnock-Jones DS, Licence D, Yanaihara A, Hastings JM, Holland CM, et al. Vascular endothelial growth factor-D is an independent prognostic factor in epithelial ovarian carcinoma. Br J Cancer. 2003;88:237–244. doi: 10.1038/sj.bjc.6600701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida N, Yano H, Komai K, Nishida T, Kamura T., and , Kojiro M. Vascular endothelial growth factor C and vascular endothelial growth factor receptor 2 are related closely to the prognosis of patients with ovarian carcinoma. Cancer. 2004;101:1364–1374. doi: 10.1002/cncr.20449. [DOI] [PubMed] [Google Scholar]
- Olsson AK, Dimberg A, Kreuger J., and , Claesson-Welsh L. VEGF receptor signalling – in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- Takahashi H., and , Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 2005;109:227–241. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- He Z, Evelhoch JL, Mohammad RM, Adsay NV, Pettit GR, Vaitkevicius VK, et al. Magnetic resonance imaging to measure therapeutic response using an orthotopic model of human pancreatic cancer. Pancreas. 2000;21:69–76. doi: 10.1097/00006676-200007000-00054. [DOI] [PubMed] [Google Scholar]
- Kendall RL, Wang G., and , Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun. 1996;226:324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- Lin P, Sankar S, Shan S, Dewhirst MW, Polverini PJ, Quinn TQ, et al. Inhibition of tumor growth by targeting tumor endothelium using a soluble vascular endothelial growth factor receptor. Cell Growth Differ. 1998;9:49–58. [PubMed] [Google Scholar]
- Millauer B, Longhi MP, Plate KH, Shawver LK, Risau W, Ullrich A, et al. Dominant-negative inhibition of Flk-1 suppresses the growth of many tumor types in vivo. Cancer Res. 1996;56:1615–1620. [PubMed] [Google Scholar]
- Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- Sallinen H, Anttila M, Narvainen J, Orden MR, Ropponen K, Kosma VM, et al. A highly reproducible xenograft model for human ovarian carcinoma and application of MRI and ultrasound in longitudinal follow-up. Gynecol Oncol. 2006;103:315–320. doi: 10.1016/j.ygyno.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Takei Y, Mizukami H, Saga Y, Yoshimura I, Hasumi Y, Takayama T, et al. Suppression of ovarian cancer by muscle-mediated expression of soluble VEGFR-1/Flt-1 using adeno-associated virus serotype 1-derived vector. Int J Cancer. 2007;120:278–84. doi: 10.1002/ijc.22307. [DOI] [PubMed] [Google Scholar]
- Nagy JA, Masse EM, Herzberg KT, Meyers MS, Yeo KT, Yeo TK, et al. Pathogenesis of ascites tumor growth: vascular permeability factor, vascular hyperpermeability, and ascites fluid accumulation. Cancer Res. 1995;55:360–368. [PubMed] [Google Scholar]
- Hasumi Y, Mizukami H, Urabe M, Kohno T, Takeuchi K, Kume A, et al. Soluble FLT-1 expression suppresses carcinomatous ascites in nude mice bearing ovarian cancer. Cancer Res. 2002;62:2019–2023. [PubMed] [Google Scholar]
- Wu Y, Li ZY, Zhao X, Kan B., and , Wei YQ. Inhibition of ovarian tumor growth by gene therapy with recombinant soluble vascular endothelial growth factor receptor 2. Hum Gene Ther. 2006;17:941–948. doi: 10.1089/hum.2006.17.941. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, et al. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci USA. 2001;98:12677–12682. doi: 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahasreshti PJ, Kataram M, Wang MH, Stockard CR, Grizzle WE, Carey D, et al. Intravenous delivery of adenovirus-mediated soluble FLT-1 results in liver toxicity. Clin Cancer Res. 2003;9:2701–2710. [PubMed] [Google Scholar]
- Versnel MA, Haarbrink M, Langerak AW, de Laat PA, Hagemeijer A, van der Kwast TH, et al. Human ovarian tumors of epithelial origin express PDGF in vitro and in vivo. Cancer Genet Cytogenet. 1994;73:60–4. doi: 10.1016/0165-4608(94)90183-x. [DOI] [PubMed] [Google Scholar]
- Gilad AA, Israely T, Dafni H, Meir G, Cohen B, Neeman M. Functional and molecular mapping of uncoupling between vascular permeability and loss of vascular maturation in ovarian carcinoma xenografts: the role of stroma cells in tumor angiogenesis. Int J Cancer. 2005;117:202–211. doi: 10.1002/ijc.21179. [DOI] [PubMed] [Google Scholar]
- Aghajanian C. The role of bevacizumab in ovarian cancer--an evolving story. Gynecol Oncol. 2006;102:131–133. doi: 10.1016/j.ygyno.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Byrne AT, Ross L, Holash J, Nakanishi M, Hu L, Hofmann JI, et al. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin Cancer Res. 2003;9:5721–5728. [PubMed] [Google Scholar]
- Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, et al. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005;65:4389–400. doi: 10.1158/0008-5472.CAN-04-4409. [DOI] [PubMed] [Google Scholar]
- Hurteau JA, Blessing JA, DeCesare SL, Creasman WT. Evaluation of recombinant human interleukin-12 in patients with recurrent or refractory ovarian cancer: a gynecologic oncology group study. Gynecol Oncol. 2001;82:7–10. doi: 10.1006/gyno.2001.6255. [DOI] [PubMed] [Google Scholar]
- Eisen T, Boshoff C, Mak I, Sapunar F, Vaughan MM, Pyle L, et al. Continuous low dose Thalidomide: a phase II study in advanced melanoma, renal cell, ovarian and breast cancer. Br J Cancer. 2000;82:812–817. doi: 10.1054/bjoc.1999.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj S, Roy H, Karpanen T, He Y, Jauhiainen S, Hedman M, et al. Periadventitial angiopoietin-1 gene transfer induces angiogenesis in rabbit carotid arteries. Gene Ther. 2005;12:388–394. doi: 10.1038/sj.gt.3302426. [DOI] [PubMed] [Google Scholar]
- Pajusola K, Aprelikova O, Armstrong E, Morris S., and , Alitalo K. Two human FLT4 receptor tyrosine kinase isoforms with distinct carboxy terminal tails are produced by alternative processing of primary transcripts. Oncogene. 1993;8:2931–2937. [PubMed] [Google Scholar]
- Takayama K, Ueno H, Nakanishi Y, Sakamoto T, Inoue K, Shimizu K, et al. Suppression of tumor angiogenesis and growth by gene transfer of a soluble form of vascular endothelial growth factor receptor into a remote organ. Cancer Res. 2000;60:2169–2177. [PubMed] [Google Scholar]
- Roy H, Bhardwaj S, Babu M, Jauhiainen S, Herzig KH, Bellu AR, et al. Adenovirus-mediated gene transfer of placental growth factor to perivascular tissue induces angiogenesis via upregulation of the expression of endogenous vascular endothelial growth factor-A. Hum Gene Ther. 2005;16:1422–1428. doi: 10.1089/hum.2005.16.1422. [DOI] [PubMed] [Google Scholar]
- Veikkola T, Jussila L, Makinen T, Karpanen T, Jeltsch M, Petrova TV, et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;20:1223–1231. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laitinen M, Makinen K, Manninen H, Matsi P, Kossila M, Agrawal RS, et al. Adenovirus-mediated gene transfer to lower limb artery of patients with chronic critical leg ischemia. Hum Gene Ther. 1998;9:1481–1486. doi: 10.1089/hum.1998.9.10-1481. [DOI] [PubMed] [Google Scholar]
- Puumalainen AM, Vapalahti M, Agrawal RS, Kossila M, Laukkanen J, Lehtolainen P, et al. β-Galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum Gene Ther. 1998;9:1769–1774. doi: 10.1089/hum.1998.9.12-1769. [DOI] [PubMed] [Google Scholar]
- Yla-Herttuala S, Rosenfeld ME, Parthasarathy S, Glass CK, Sigal E, Witztum JL, et al. Colocalization of 15-lipoxygenase mRNA and protein with epitopes of oxidized low density lipoprotein in macrophage-rich areas of atherosclerotic lesions. Proc Natl Acad Sci USA. 1990;87:6959–6963. doi: 10.1073/pnas.87.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]