Abstract
Myocardial infarction (MI) and subsequent adverse remodeling cause heart failure. Previously we demonstrated a role for Kit ligand (KL) in improving cardiac function post-MI. KL has two major isoforms; KL-1 is secreted whereas KL-2 is predominantly membrane bound. We demonstrate here first that KL-2-deficient mice have worse survival and an increased heart/bodyweight ratio post-MI compared to mice with reduced c-Kit receptor expression. Next we synthesized recombinant lentiviral vectors (LVs) that engineered functional expression of murine KL-1 and KL-2. For in vivo analyses, we directly injected these LVs into the left ventricle of membrane-bound KL-deficient Sl/Sld or wild-type (WT) mice undergoing MI. Control LV/enGFP injection led to measurable reporter gene expression in hearts. Injection of LV/KL-2 attenuated adverse left ventricular remodeling and dramatically improved survival post-MI in both Sl/Sld and WT mice (from 12 to 71% and 35 to 73%, respectively, versus controls). With regard toward beginning to understand the possible salutary mechanisms involved in this effect, differential staining patterns of Sca-1 and Ly49 on peripheral blood (PB) cells from therapeutically treated animals was found. Our data show that LV/KL-2 gene therapy is a promising treatment for MI.
Introduction
Recent advances in understanding of the molecular mechanisms of cardiovascular disease, the role of stem cells in cardiac regeneration, and in gene delivery approaches allow thematic convergence for the development of novel treatments for heart disease. Although gene therapy has mainly been thought of as a treatment for cancer or inherited single-gene disorders, recent studies have shown that this therapeutic approach has the capability to treat multifactorial diseases, including myocardial infarction (MI).1,2 Lentiviral vectors (LVs) are efficient gene delivery agents that have the capability to infect a variety of cell types including postmitotic cells. LVs have been approved for clinical utility and recent studies have demonstrated the use of these vectors in the treatment of cardiovascular disease.3
Adverse left ventricular remodeling post-MI triggers heart failure; it is important to prevent this outcome. Cytokine therapy post-MI is an attractive schema because such treatment might regenerate cardiac tissue and protect against adverse left ventricular remodeling.4,5,6,7,8 For example, Woldbaek et al. have shown that mRNA expression of Kit ligand (KL or SCF), the ligand for the steel receptor tyrosine kinase (c-Kit) receptor, is decreased in the heart post-MI.9 Furthermore, we have previously reported on detailed cardiac rescue and remodeling mechanisms post-MI involving the c-Kit receptor axis.10
KL has two isoforms, KL-1 and KL-2, which are formed by alternative splicing. KL-2 is missing a predominant extramembrane cleavage site11 and is largely membrane bound. These two isoforms of KL have differential effects on the survival and proliferation of hematopoietic cells;12,13 observations which are reinforced by the altered phenotype of Sl/Sld mice, which have only soluble KL. Importantly, membrane-associated KL has also demonstrated more potent and sustained signaling than its secreted counterpart.14
Recently, we reported α-galactosidase A correction in the hearts of animals in a Fabry disease model by direct intraventricular injection of a recombinant LV.15 That study on an inherited disorder provided a conceptual platform for the broadening of this therapeutic schema to impact acquired disorders as well. The aims of this present study were to develop novel recombinant LVs that engineer expression of KLs and to investigate the effects of direct left ventricular injection of vectors post-MI in mice. Effective vectors were generated and functional KL expression was documented in vitro. Direct injection of a LV that engineered expression of enGFP led to appreciable functional transductions of cardiac tissue. Next we observed that the overexpression of KL-2 by direct cardiac injection prevents adverse remodeling and dramatically improves survival post-MI both in KL-2-deficient mice and in wild-type (WT) animals. Increased survival was also correlated with differential expression of cell surface antigens Ly49 and Sca-1 on peripheral blood (PB) cells. These results open the door to the development of this therapeutic modality for the treatment of cardiovascular disease.
Results
Decreased survival and worsened cardiac function in Sl/Sld mice post-MI compared with W/Wv mice
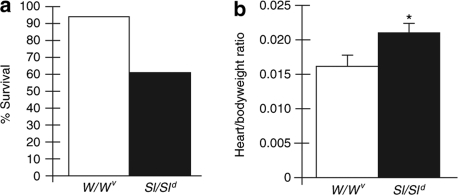
Our previous studies have shown that null c-Kit mutation w/w- viable (W/Wv) mice have diminished heart function and greater cardiac dilatation than WT mice 35 days after MI.10 We also demonstrated that these effects could be rescued by transplantation of WT bone marrow cells.10 To focus our present studies on dissecting the contributions of individual components of the KL/c-Kit receptor axis, we first performed MIs on W/Wv and Sl/Sld mice. Sl/Sld mice produce only soluble KL.11 Figure 1 shows the results of preliminary studies providing survival percentages and heart/bodyweight ratio calculations measured at 5 weeks after MI. Clear differences were seen. Sl/Sld mice have markedly decreased survival percentages and an increased heart/bodyweight ratio in surviving animals at killing than W/Wv mice—indicating worsened outcomes post-MI.
Figure 1.
Comparison of functional outcomes in W/Wv mice (n = 10) and Sl/Sld mice (n = 10) that have undergone myocardial infarction (MI). (a) Percent survival measured at 5 weeks after MI. (b) Heart/bodyweight ratio of surviving animals measured at 5 weeks after MI. *P < 0.05.
KL overexpression in transduced Sl/Sl4 and TF-1 cells
Next, we developed novel LVs that engineer expression of KL-1 or KL-2 (LV/KL-1 and LV/KL-2, respectively). LV/KL-2 has an 84-bp deletion that removes the major proteolytic cleavage site; a minor cleavage site closer to the transmembrane domain is still maintained. LV/enGFP16 was used as a control in vitro. VSV-g-pseudotyped LVs were generated and titered as before.16 LVs were used to infect a KL-deficient murine stromal cell line, (Sl/Sl4 cells; ref. 17), at an MOI of 10. Nontransduced Sl/Sl4 cells were negative for KL expression while ~95% of infected cells expressed KL-1 and KL-2, respectively, as measured by flow cytometry analyses (Figure 2a). KL expression and protein relative molecular weights were confirmed by western blots performed on transduced Sl/Sl4 cell lysates (Figure 2b). Subsequently, the supernatant from pools of infected Sl/Sl4 cells was analyzed by ELISA to determine the concentration of cleaved KL (Figure 2c). As expected, more secreted KL-1 was observed in similarly infected cells because of the inclusion of the major cleavage site. Finally, the bioactivity of KL generated by LV/KL-1 or LV/KL-2 infection was verified by its ability to support the growth of a KL-dependent cell line, TF-1 (ref. 18). Here transduced TF-1 cell pools were shown to proliferate faster than nontransduced cell pools, indicating production of functional KL (data not shown).
Figure 2.
LV/KL-1 and LV/KL-2 efficiently infect Sl/Sl4 cells, a KL-deficient cell line. Transgene expression in infected cells was confirmed by (a) flow cytometry (KL expression in NT, LV/KL-1, and LV/KL-2 infected cells; 0.98%, 94.9%, and 96.3% respectively) and (b) western blot. For the western blot, LV/enGFP and nontransduced Sl/Sl4 cell extracts were used as controls. β-actin levels were evaluated as a protein loading control. (c) Cleaved KL in the supernatant of infected Sl/Sl4 cell pools was measured by ELISA. NT; nontransduced.
LV-mediated enGFP expression in infarcted hearts
To evaluate marking transgene expression mediated by a single LV administration in compromised recipients, WT mice received an MI-generating ligation and then phosphate buffered saline (PBS) or LV/enGFP were directly injected into their hearts in a minimal volume. Mice were killed 7 days after the MI. As expected, the enGFP signal was detected in the LV/enGFP-treated hearts and not in the PBS-treated hearts (Figure 3a,b). To avoid misleading outcomes due to possible autofluorescence, immunostaining was also performed for enGFP with a monoclonal antibody and then these signals merged with the enGFP fluorescence signals (Figure 3c–e). Relative fluorescence intensity was also calculated from a number of mounts of whole tissue samples derived from animals injected with serial dilutions of LV/enGFP. Figure 3f demonstrates, as could be predicted, that increased amounts of LV/enGFP injected leads to increased fluorescence intensity in cardiac samples. Finally, we also determined the relative cellular transduction rate from a number of fields for each mouse tissue mount (Figure 3g). These data indicate that ~5% of cells were functionally transduced by this direct LV injection method. Combined with our previous data,15 this indicates that this direct injection LV system is efficient for gene transfer into the heart.
Figure 3.
enGFP expression in the hearts of WT mice directly injected with LV/enGFP and receiving an MI. (a) PBS control– injected hearts and (b) LV/enGFP-injected hearts were examined for enGFP signal (green) and also stained with enGFP antibody (Alexa546, red). (c) enGFP signal at higher magnification. (d) Immunostaining for enGFP expression. (e) Overlay of c and d showing co-localization of the enGFP signal and enGFP staining. (f) Relative fluorescence intensity of the enGFP signal in hearts receiving direct injections of various doses of LV/enGFP. For each group 14 fields were analyzed per mouse injected (n = 2 or three animals per group). (g) Estimation of the transduction rate in cardiac tissue samples from LV/enGFP-injected mice. For the PBS-injected control group six fields were analyzed per mouse (n = 2 mice). For the LV-enGFP group 8 or 9 fields were analyzed per mouse (n = 2 mice).
KL expression in the heart
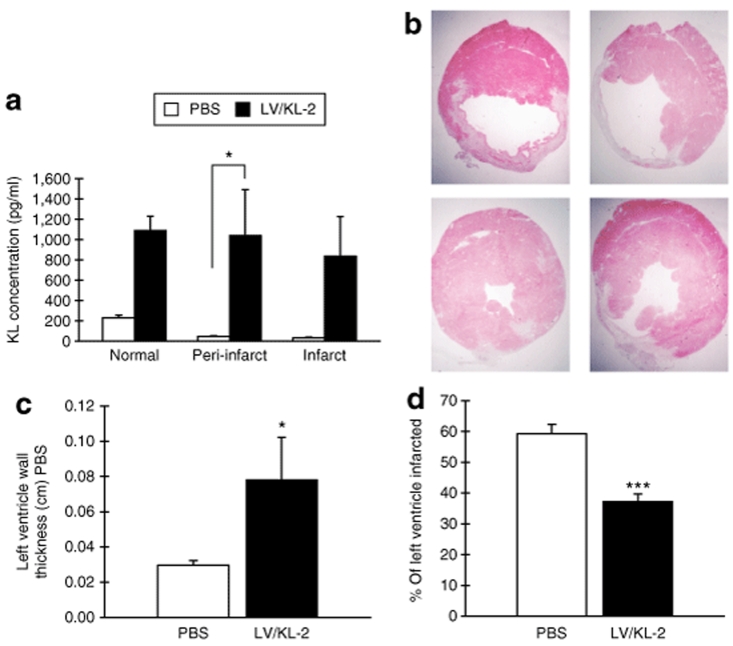
To determine the effect of LV-mediated KL overexpression on recovery post-MI, our gene therapy strategy was tested in both Sl/Sld and in WT mice. These mice were randomized into six groups, receiving either MI or a sham operation. Within minutes after MI or sham operation, injection of the KL LVs was performed. Therapeutic transgene expression, mediated by direct vector injection, was then quantified. KL-2 expression was determined by ELISA from heart tissue of LV/KL-2-injected WT mice at 3 days after MI. KL-2 was observed to be overexpressed in the left ventricle of the LV/KL-2-injected hearts, including both the infarct and peri-infarct regions, compared to controls (Figure 4a). These results demonstrate that LV/KL-2 efficiently infects cardiac tissue from a single direct vector administration and generates KL-2 protein expression in vivo.
Figure 4.
KL-2 expression in hearts and morphometric effects of LV/KL-2 gene therapy for MI. (a) Cardiac tissue KL expression as measured by ELISA at 3 days after MI in WT mice. Levels of KL are significantly greater in LV/KL-2-treated mice (n = 3) compared with PBS-treated mice (n = 3) in all areas of the heart. (b) Morphometrics (representative shown) demonstrate a smaller infarcted area in the left ventricle in LV/KL-2-treated WT mice compared with the PBS-treated group. (c) Left ventricular wall thickness and (d) the percent of left ventricle infarcted are significantly improved in LV/KL-2-treated WT mice (n = 6) at 35 days after MI compared with the PBS-treated group (n = 6). *P < 0.05, ***P < 0.001 compared with PBS-treated WT group.
LV/KL-2 administration substantially reduces infarct size and prevents adverse cardiac remodeling post-MI
Morphometric analysis was performed on injected WT mice at 7 and 35 days after MI. Representative results are shown in Figure 4b. In the LV/KL-2-treated group, left ventricular infarct sizes were significantly reduced (37.2 ± 2.5% versus 59.3 ± 3.0%, LV/KL-2 versus PBS respectively, P < 0.001) and left ventricular wall thickness was significantly improved at 35 days after MI (0.78 ± 0.24 mm versus 0.30 ± 0.03 mm, LV/KL-2 versus PBS respectively, P < 0.05) (Figure 4c,d).
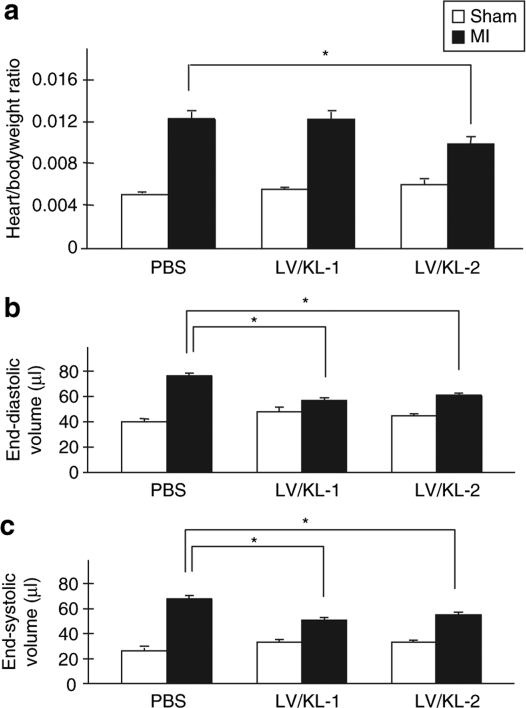
Following cardiac remodeling post-MI, left ventricles are commonly dilated. In our study, we observed that the heart/bodyweight ratio in the LV/KL-2-treated group was significantly decreased compared with the PBS-treated group in WT mice (0.0099 ± 7.2 × 10‐4 versus 0.0123 ± 6.8 × 10‐4, LV/KL-2 versus PBS respectively, P < 0.05) (Figure 5a). Furthermore, at 35 days after MI in WT mice, left ventricular end-diastolic volume and left ventricular end-systolic volume were reduced in LV/KL-1 and LV/KL-2-treated WT mice compared with PBS-treated WT mice (Figure 5b,c). These functional results show that LV/KL treatment prevented adverse cardiac remodeling in mice.
Figure 5.
Physiological profiles following LV/KL-2 treatment for MI. LV/KL-2-treated WT mice (n = 8) demonstrate marked improvement in (a) Heart/bodyweight ratio, (b) left ventricular end-diastolic volume (LVEDP), and (c) left ventricular end-systolic volume (LVESV) compared with the PBS-treated group (n = 3). *P < 0.05, ***P < 0.001.
Direct LV/KL-2 injection markedly improves survival in Sl/Sld and WT mice post-MI
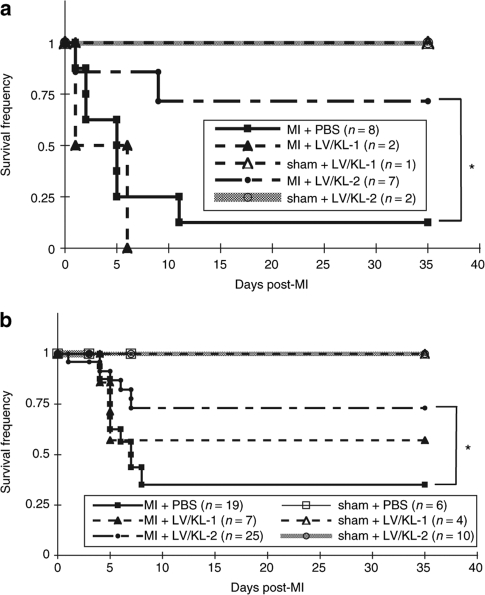
PBS-injected Sl/Sld mice demonstrated 12% survival at 35 days after MI (Figure 6a). In addition, animals receiving LV/KL-1 also had decreased survival frequencies (Figure 6a). In contrast, survival was greatly improved in Sl/Sld mice treated with LV/KL-2, with 71% survival observed at 35 days (P < 0.041 versus PBS group) (Figure 6a). This result itself implicates the c-Kit receptor/KL axis as an important component of recovery post-MI and demonstrates that constructive manipulation of facets of that pathway can dramatically improve survival. Finally, we examined this therapeutic strategy in a more relevant model. We hypothesized that such robust repair mechanisms mediated by direct LV delivery of KL-2 could also work even in the context of existing normal recovery operations. Similar functional analyses were then performed in WT mice. Here we observed that survival of WT mice following MI was also significantly improved from 35 to 73.1% (P < 0.05) following LV/KL-2 treatment (Figure 6b).
Figure 6.
Direct LV/KL-2 injection enhances survival post-MI. LV/KL- 2-treated (a) Sl/Sld and (b) WT mice demonstrate dramatically improved survival post-MI compared with PBS-treated mice. *P < 0.05, ***P < 0.02.
Flow cytometric analyses of PB and immunohistochemistry from LV-injected and control mice post-MI
Toward gaining insights into mechanism, PB was obtained from surviving mice at days 0, 3, 7, 14, and 35 post-MI. MI is associated with a deleterious inflammatory response. Indeed, our previous results demonstrated that NK cells mediated cardiac survival and repair post-MI,10 therefore mononuclear cells were analyzed for expression of a directed series of cell surface markers including Ly49 (NK and NK-T cells), CD11c (NK, monocytes, macrophages, subsets of T and B cells), CD94 (NK, NK-T, activated CD8+ T cells), CD117 (c-Kit), CD34 (endothelial cells and short-term reconstituting HSCs), and Sca-1 (HSCs, activated lymphocytes). Note that data from Sl/Sld mice receiving PBS post-MI could only be collected through 14 days because of high mortality. For some cell surface markers such as CD11c and CD34, values at day 35 were not different from starting values and were not dramatically impacted by the addition of LV/KL-2 (data not shown). This phenomenon also largely occurred with CD117 expression and CD94 expression (data not shown).
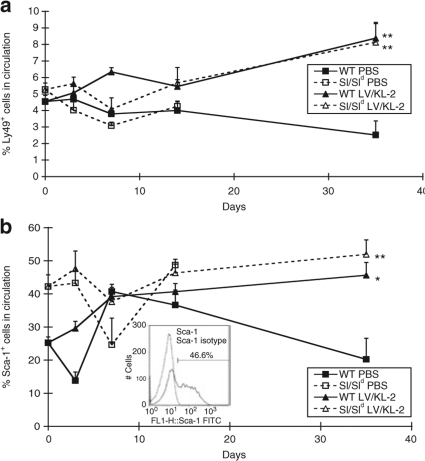
In contrast, for Ly49 expression starting values between the Sl/Sld and WT mice were quite similar (~5% of PB cells) as shown in Figure 7a. Yet at 14 days after MI these values started to diverge. Finally at 35 days both the Sl/Sld and WT mice receiving LV/KL-2 had significantly increased levels of Ly49+ cells in the PB (8.1 ± 2.4% and 6.8 ± 3.2%, respectively) in comparison with WT animals receiving PBS post-MI (2.5 ± 1.5%; P < 0.006) (Figure 7a).
Figure 7.
Results of flow cytrometric analyses for Ly49 and Sca-1 staining of peripheral blood from WT and Sl/Sld mice treated with PBS or LV/KL-2 following MI. (a) Ly49+ cell mobilization over 35 days. (b) Sca-1+ cell mobilization over 35 days. Inset: Sample flow cytometry analysis for Sca-1 staining with isotype control. *P < 0.05, **P < 0.01.
Another class of responses were observed with the Sca-1 analyses. Concerning Sca-1, differences were seen in positive-staining percentages at baseline between WT and Sl/Sld animals at day 0 (~25% versus ~42%, respectively) (Figure 7b). These differences were largely maintained over the period of analysis. That is, except for those WT animals treated with the LV/KL-2. There at day 35, values for the WT animals treated with LV/KL-2 were dramatically different than those from animals treated with PBS only following MI (39.2 ± 13.5% positive versus 20.2 ± 11.0% positive, respectively; P < 0.034). Further subset analysis of this group, i.e., co-staining for Sca-1 and CD34 jointly, did not reveal differences in the numbers of cells dually positive for those markers, however (data not shown).
Discussion
We have demonstrated that the LV gene delivery system is a promising approach for the treatment of MI; especially when the KL/c-Kit signaling axis (specifically KL-2) is accessed. LVs are able to transduce nondividing cells19 such as cardiomyocytes, integrate into the genome, and provide long-term transgene expression.20 Indeed, Fleury et al. have shown efficient LV transduction and transgene expression in vitro in adult rat cardiomyocytes.21 Lower levels of sustained expression of KL-2 as generated by LV-mediated delivery may provide more benefit over time as repair is an ongoing process, compared to the higher bolus amounts of cytokines that would be produced using other transient delivery systems such as recombinant adenoviruses, for example. Outcomes from experiments designed to test this hypothesis would be interesting and provide further insights into mechanisms and timing of cardiac repair.
Our in vitro results demonstrate generation of effective recombinant LVs that drive expression of KLs. Our in vivo results demonstrate that LV-mediated KL-2 overexpression mediated by a single gene delivery event targeted directly into the murine myocardium dramatically improves hemodynamics and morphometrics (Figures 4 and 5, respectively) as well as survival of both KL-2-deficient and even WT mice post-MI (Figure 6). Indeed the robustness of this response is striking in the WT group given that normal signaling and repair pathways are still present in these animals. These results imply that membrane-bound KL is an important molecule in recovery and in prevention of adverse cardiac remodeling post-MI. These findings thus extend into the therapeutic realm results from our previous studies demonstrating the importance of systemic KL and c-Kit signaling in remodeling and rescue of the infarcted heart.10 Note that we have also performed studies with imatinib mesylate (Gleevec), which is a pleiotropic agent that also blocks c-Kit function, and found a significant increase in animal mortality after MI over untreated animals (data not shown).
The mechanism whereby cytokine therapy prevents adverse cardiac remodeling has yet to be fully elucidated. It has been proposed that bone marrow cells are homing to the infarcted heart and transdifferentiate into cardiomyocytes post-MI.5,22,23 These cells may play an important role in cardiac remodeling post-MI. Recently, Dawn et al. have suggested that mobilization of cardiac stem cells themselves by cytokines could be an additional or alternative mechanism.8,24 Along these lines, it bears mentioning that we observed improved survival with LV/KL-2 compared to animals treated with LV/KL-1 (Figure 6a), although many fewer animals were tested in the second case. This may indicate a more pronounced requirement for the membrane-bound form of KL in cardiac rescue and remodeling post-MI, perhaps acting through an autocrine manner. These results may delineate a novel function for this isoform. On the other hand, we also show in Figure 2 that some detectable KL is secreted even from the form missing the major cleavage site. Thus perhaps both autocrine and paracrine mechanisms are at play here. In support of this hypothesis we see alterations in the nature of circulatory PB cells in treated animals (Figure 7). One difference between the two forms is in the duration and intensity of signaling, which is increased with KL-2 (ref. 14). KL-1 and KL-2 may also thus stimulate different homing or remodeling signals in this context.
For clinical application, further experiments need to be performed in order to address any pertinent safety concerns—such as any side effects with sustained overexpression of KL—and the optimal timing of vector administration following MI. Transcriptionally targeted vectors exploiting cardiac-specific promoters25,26 built into the next generation of recombinant LVs constructed for this purpose, for example, would decrease the likelihood of possible off-target expression effects. Likewise, the tropism of the vector may be made more restricted or the delivery system itself may be modified to further increase the potency of this approach. Concerning the timing of vector administration, these present proof-of-principle studies were undertaken wherein the MI and the vector delivery were performed immediately sequentially to address technical concerns and Animal Care Committee issues. Future studies, perhaps in rats (or other larger animal models) to relieve these concerns and optimize the timing of LV/KL-2 delivery to maximize beneficial outcomes following MI in the context of real world timing will be undertaken.
In summary, this is the first study to show improvement in survival using a novel direct LV gene therapy strategy and provides the platform for further development of this approach for potential treatment of MI.
Materials and Methods
LVs. HIV-1 based recombinant LVs were constructed by replacing the enhanced GFP (enGFP) in pHR′-cPPT-EF-GW-SIN plasmid with the KL-1 or KL-2 cDNAs (LV/KL-1 or LV/KL-2). This construction method was previously described.16 The KL-1 cDNA was subcloned out from C57Bl/6 mice. Briefly, total RNA was isolated from stroma cells using the TRIzol reagent (Invitrogen, Carlsbad, CA). First strand cDNA was synthesized from 1 µg total RNA using the SuperScript First Strand Synthesis System (Invitrogen). The specific KL-1 cDNA was amplified by PCR using primers based on the published mouse KL-1 mRNA sequence (NM_013598). Forward primer: 5′-CGCTGCCTTTCCTTATGAAG-3′ and Reverse primer: 5′-CGTCCACAATTACACCTCTTG-3′. KL-1 amplicons of ~850 bp were obtained after amplification for 35 cycles (denaturing at 94 °C, 45 seconds; annealing at 57 °C, 45 seconds; elongation at 72 °C, 60 seconds) using Platinum Taq DNA polymerase High Fidelity reagents (Invitrogen). The mouse KL-1 cDNA product was subcloned using the PCR-Script Amp cloning Kit (Stratagene, La Jolla, CA).
KL-2 lacks the codon for exon 6 of the KL-1 sequence. To remove exon 6 from the KL-1 cDNA, inverse PCR was performed on the pPCR-Script/KL-1 plasmid template. The forward primer targeted the 3′ end of exon 5 of the KL-1 cDNA and the reverse primer targeted the 5′ end of exon 7 of KL-1 cDNA. Forward primer; 5′-CTTTCTCGGGACCTAATGTTG-3′: Reverse primer; 5′-GGAAAGCCGCAAAGGCCC-3′. Inverse PCR was performed using Platinum Taq DNA polymerase High Fidelity reagents for 35 cycles. Each cycle consisted of the following steps: denaturation at 94 °C, 30 seconds; annealing at 54 °C, 5 minutes; extension at 68 °C, 5 minutes each. The inverse PCR product was then self-ligated to make plasmid, which contains the KL-2 cDNA. This product (pPCR-Script/KL-2) was transformed into XL10-Gold Ultracompetent cells (Stratagene) and verified by DNA sequencing.
Vesicular stomatitis virus glycoprotein-pseudotyped (VSV-g) LVs, including an enGFP control vector (LV/enGFP) used in vitro, were generated by transient transfection of 293T cells (obtained form Michele Calos, Stanford University) using the three-plasmid system (LV plasmid construct, packaging plasmid pCMVΔR8.91, and the VSV-g envelope-coding plasmid pMD.G) with FuGENE6 (Roche, Indianapolis, IN). Virus supernatant were harvested at 48 hours and concentrated at 50,000g for 2 hours. The concentrated viral supernatants were serially diluted and titered on 293T cells. p24 antigen levels also were determined by an HIV-1 p24 ELISA (PerkinElmer, Waltham, MA).
Functional expression of KL transgenes in the Sl/SL4 and TF-1 cell lines. The Sl/Sl4 cell line was purchased from ATCC (Manassas, VA). Sl/Sl4 cells were cultured in Dulbecco's Modified Eagle Medium with 10% fetal bovine serum, 100 IU of penicillin/ml and 100 µg of streptomycin/ml. A half million Sl/Sl4 cells were infected a single time with LV/KL-1, LV/KL-2, or LV/enGFP at an MOI of 10 in the presence of 8 µg/ml protamine sulfate. Flow cytometric analyses were performed 2 days later. Transduced Sl/Sl4 cells were also lysed in sample buffer and cell lysates were resolved by SDS-PAGE and transferred onto polyvinylidene difluoride filters (Millipore, Billerica, MA). Filters were blocked with 10% skim milk in PBS with 0.1% Tween20 for 1 hour at RT. KL was detected with biotinylated anti-mouse KL antibody (BAF455; R&D Systems, Minneapolis, MN), used at 0.2 µg/ml. Equal protein loading was confirmed with an anti-β-actin antibody (A5441; Sigma Aldrich, St Louis, MO) diluted 1:10,000. Blots were probed with secondary anti-goat (diluted 1:5,000, sc-2020; Santa Cruz Biotechnology, Santa Cruz, CA) or anti-mouse (diluted 1:1,000, NA931; GE Healthcare, Piscataway, NJ) horseradish peroxidase–conjugated antibodies. Protein bands were detected using an enhanced Chemiluminesence kit (Perkin Elmer, Waltham, MA).
Transduced or nontransduced Sl/Sl4 cells were seeded in a 10-cm dish in 10 ml of DMEM with 10% fetal bovine serum. Supernatant was harvested 2 days later and KL concentration was measured using the SCF ELISA kit (R&D Systems).
TF-1 cells, which are a human erythroblast cell line, were obtained from ATCC. TF-1 cells were infected with LV/KL-1 and LV/KL-2 at an MOI of 5. Three days after transduction, infected TF-1 cells were harvested and the cells were washed with PBS twice. TF-1 cells were incubated with biotinylated anti-mouse SCF antibody (R&D Systems) at 4 °C for 20 minutes and washed twice with PBS. These cells were incubated with streptavidin-phycoerythrin (BD Biosciences, San Jose, CA) at 4 °C for 20 minutes and washed with PBS twice. Expression of KL-1 and KL-2 on infected TF-1 cells was determined by flow cytometry and found to be 68.9 and 49.2 %, respectively (data not shown).
Experimental animals. WBB6F1/J-KitW/KitW‐v (W/Wv) mice, WCB6F1/J KitlSl kitlSl-d (Sl/Sld) mice, and their littermates (WT) were purchased from the Jackson Laboratory (Bar Harbor, ME). Animal experiments were performed under protocols approved by the University Health Network Animal Care Committee.
Induction of MI and cardiac injection of LVs. MI was induced by permanent ligation of the left anterior descending coronary artery as described previously.27 Forty microliters of LVs (corresponding to 2.8 µg/ml p24 antigen) or PBS were injected into the left ventricle as before.15
In vivo study plan. In experiment 1, Sl/Sld (n = 20) and WT (n = 37) mice were randomized into six groups. Mice received either an MI or sham operation and then PBS or LVs encoding KL-1 or KL-2 were injected directly into the left ventricle. Mice were monitored for morbidity and mortality. The percentages of death from the MI/injection procedure itself and controls, in Sl/Sld+MI, Sl/Sld+sham, WT+MI, and WT+sham groups, were 52.9, 50, 10.5, and 4.8%, respectively. At 35 days after MI, mice were killed after the evaluation of cardiac function. In experiment 2, WT mice (n = 34) were subjected to MI or sham operation and PBS or LV/KL-2 were injected. Some mice were randomly killed for analyses on 3, 7, and 35 days after MI.
In experiment 3, to check the efficiency of LV-mediated gene transfer into the heart, WT mice received an MI operation and were injected with PBS or LV/enGFP (n = 3 for each group) and were killed for immunohistochemistry 7 days after MI.
Flow cytometric analyses and antigen retrieval staining. PB was collected at 0, 3, 7, 14, and 35 days after MI. Cell surface expression of marker proteins on PB cells were analyzed by flow cytometry. Twenty microliters of PB cells were lysed with Red Blood Cell Lysing Buffer (Sigma) and incubated with antibody against Ly49, CD11c, CD94, CD117, Sca-1, and CD34 (all antibodies were purchased from BD Biosciences). PB cells were analyzed on a FACSCalibur flow cytometer (BD, Franklin Lakes, NJ).
To perform antigen retrieval staining for enGFP, heart sections were incubated in HistoVT One (Nacalai Tesque, Kyoto, Japan) for 20 minutes at 70 °C. The tissues were permeablized with PBS-T (0.1% Triton X-100 in PBS). The sections were incubated with anti-GFP monoclonal antibody (Nacalai Tesque, clone GF090R), and subsequently incubated with Alexa546-labeled goat anti-rat IgG antibody (Molecular Probes, Eugene, OR).
Cardiac function and morphometric evaluation. Cardiac function was analyzed using a Millar pressure volume conductance catheter. Morphometrics were analyzed as described before.27 Briefly, hearts were harvested, rinsed with PBS, and embedded in OCT. Tissue was sectioned at 5 µm and stored at ‐80 °C until analysis. Digitally captured pathological images were used to evaluate the left ventricular area, wall thickness, and percent-infarcted area.
Statistical analyses. All statistical analyses were performed using a two-sample Student's t-test assuming unequal variance. For Kaplan–Meier curves, a logrank test was used to evaluate significance.
Acknowledgments
This work was supported, in part, by the Heart and Stroke Foundation of Ontario and the Canadian Institutes of Health Research. We thank Makoto Yoshimitsu (Kagoshima University) for invaluable assistance.
REFERENCES
- Sasano T, McDonald AD, Kikuchi K., and , Donahue JK. Molecular ablation of ventricular tachycardia after myocardial infarction. Nat Med. 2006;12:1256–1258. doi: 10.1038/nm1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano KF, Pola R, Murayama T, Curry C, Kawamoto A, Iwakura A, et al. Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat Med. 2005;11:1197–1204. doi: 10.1038/nm1313. [DOI] [PubMed] [Google Scholar]
- Higuchi K., and , Medin JA. Lentiviral vectors for gene therapy of heart disease. J Cardiol. 2007;49:1–11. [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H, Fujita J, Kinjo K, Matsuzaki Y, Tsuma M, Miyatake H, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–3587. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, et al. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med. 2005;11:305–311. doi: 10.1038/nm1199. [DOI] [PubMed] [Google Scholar]
- Dawn B, Guo Y, Rezazadeh A, Huang Y, Stein AB, Hunt G, et al. Postinfarct cytokine therapy regenerates cardiac tissue and improves left ventricular function. Circ Res. 2006;98:1098–1105. doi: 10.1161/01.RES.0000218454.76784.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldbaek PR, Hoen IB, Christensen G., and , Tonnessen T. Gene expression of colony-stimulating factors and stem cell factor after myocardial infarction in the mouse. Acta Physiol Scand. 2002;175:173–181. doi: 10.1046/j.1365-201X.2002.00989.x. [DOI] [PubMed] [Google Scholar]
- Ayach BB, Yoshimitsu M, Dawood F, Sun M, Arab S, Chen M, et al. Stem cell factor receptor induces progenitor and natural killer cell-mediated cardiac survival and repair after myocardial infarction. Proc Natl Acad Sci USA. 2006;103:2304–2309. doi: 10.1073/pnas.0510997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman LK. The biology of stem cell factor and its receptor C-kit. Int J Biochem Cell Biol. 1999;31:1037–1051. doi: 10.1016/s1357-2725(99)00076-x. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Williams DA, Gotoh A, Nishimaki J, Broxmeyer HE., and , Toyama K. Membrane-bound Steel factor induces more persistent tyrosine kinase activation and longer life span of c-kit gene-encoded protein than its soluble form. Blood. 1995;85:641–649. [PubMed] [Google Scholar]
- Caruana G, Ashman LK, Fujita J., and , Gonda TJ. Responses of the murine myeloid cell line FDC-P1 to soluble and membrane-bound forms of steel factor (SLF) Exp Hematol. 1993;21:761–768. [PubMed] [Google Scholar]
- Caruana G, Cambareri AC., and , Ashman LK. Isoforms of c-KIT differ in activation of signaling pathways and transformation of NIH3T3 fibroblasts. Oncogene. 1999;18:5573–5581. doi: 10.1038/sj.onc.1202939. [DOI] [PubMed] [Google Scholar]
- Yoshimitsu M, Higuchi K, Dawood F, Rasaiah VI, Ayach B, Chen M, et al. Correction of cardiac abnormalities in fabry mice by direct intraventricular injection of a recombinant lentiviral vector that engineers expression of alpha-galactosidase A. Circ J. 2006;70:1503–1508. doi: 10.1253/circj.70.1503. [DOI] [PubMed] [Google Scholar]
- Yoshimitsu M, Sato T, Tao K, Walia JS, Rasaiah VI, Sleep GT, et al. Bioluminescent imaging of a marking transgene and correction of Fabry mice by neonatal injection of recombinant lentiviral vectors. Proc Natl Acad Sci USA. 2004;101:16909–16914. doi: 10.1073/pnas.0407572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toksoz D, Zsebo KM, Smith KA, Hu S, Brankow D, Suggs SV, et al. Support of human hematopoiesis in long-term bone marrow cultures by murine stromal cells selectively expressing the membrane-bound and secreted forms of the human homolog of the steel gene product, stem cell factor. Proc Natl Acad Sci USA. 1992;89:7350–7354. doi: 10.1073/pnas.89.16.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Tange T, Terasawa T, Chiba S, Kuwaki T, Miyagawa K, et al. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol. 1989;140:323–334. doi: 10.1002/jcp.1041400219. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gage FH, Trono D., and , Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleury S, Simeoni E, Zuppinger C, Deglon N, von Segesser LK., and , Kappenberger L, et al. Multiply attenuated, self-inactivating lentiviral vectors efficiently deliver and express genes for extended periods of time in adult rat cardiomyocytes in vivo. Circulation. 2003;107:2375–2382. doi: 10.1161/01.CIR.0000065598.46411.EF. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, et al. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci USA. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Sigmund CD., and , Lin JJ-C. Identification of cis elements in the cardiac troponin T gene conferring specific expression in cardiac muscle of transgenic mice. Circ Res. 2000;86:478–484. doi: 10.1161/01.res.86.4.478. [DOI] [PubMed] [Google Scholar]
- Su H, Joho S, Huang Y, Barcena A, Arakawa-Hoyt J, Grossman W, et al. Adeno-associated viral vector delivers cardiac-specific and hypoxia-inducible VEGF expression in ischemic mouse hearts. Proc Natl Acad Sci USA. 2004;101:16280–16285. doi: 10.1073/pnas.0407449101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Dawood F, Wen WH, Chen M, Dixon I, Kirshenbaum LA, et al. Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfuction. Circulation. 2004;110:3221–3228. doi: 10.1161/01.CIR.0000147233.10318.23. [DOI] [PubMed] [Google Scholar]