Abstract
Genetically engineered herpes simplex viruses (HSVs) can selectively infect and replicate in cancer cells, and are candidates for use as oncolytic therapy. This long-term report of a phase I trial examines vascular administration of HSV as therapy for cancer. Twelve subjects with metastatic colorectal cancer within the liver failing first-line chemotherapy were treated in four cohorts with a single dose (3 × 106 to 1 × 108 particles) of NV1020, a multimutated, replication-competent HSV. After hepatic arterial administration, subjects were observed for 4 weeks before starting intra-arterial chemotherapy. All patients exhibited progression of disease before HSV injection. During observation, levels of the tumor marker carcinoembryonic antigen (CEA) decreased (median % drop = 24%; range 13–74%; P < 0.02). One of three individuals at the 108 level showed a 39% radiologic decrease in tumor size by cross-section and 75% by volume. HSV infection was documented from liver tumor biopsies. After beginning regional chemotherapy, all patients demonstrated a further decrease in CEA (median 96%; range 50–98%; P < 0.008) and a radiologic partial response. Median survival for this group was 25 months. During follow-up, no signs of virus reactivation were found. Multimutated HSV can be delivered safely into the human bloodstream to produce selective infection of tumor tissues and biologic effects.
Introduction
There has been great interest in exploiting the natural life cycle of herpes simplex virus type 1 (HSV-1) for killing cancer. After this virus infects host cells, it uses the host DNA and protein synthetic machinery to replicate, then kills the host cell by lysis. The resultant release of progeny virus leads to further infection and killing of tumor, amplifying the virus' cancer-killing actions. This common virus is an attractive therapeutic candidate for a number of reasons. Because of wide human exposure, biology of HSV-1 is much studied and understood. The wild-type virus is well tolerated; most exposed patients do not demonstrate any apparent clinical syndrome. The minority of cases of clinical infection usually manifest as the common “cold sore.” The large genome of this DNA virus contains a limited number of genes that are essential for replication, leaving much room for genetic engineering to produce mutant viruses that may be even more attenuated in effects on normal tissues and may also more specifically infect and kill cancer. Furthermore, the presence of the herpes thymidine kinase (TK) gene renders this family of viruses sensitive to antivirals such as acyclovir. Thus, this class of vectors represents the only viruses under clinical investigation for which effective clinical antiviral therapy exists.
Virologists have attempted to alter wild-type HSV for use as a vaccine for decades.1,2 The first HSV specifically engineered for potential cancer therapy was reported in 1995.3 Our study represents the first vascular infusion of an engineered HSV for treatment of human disease. We had previously reported the short-term safety data related to such viral administration, demonstrating the safety of this approach.4 The current data confirm through long-term follow-up that no additional deleterious effects were noted. In addition, these data demonstrate selective infection of tumor and biologic antitumor effects.
Results
Baseline characteristics
Median age was 57 years (mean 55, range 32–68 years), with eight Caucasian and four African-American patients. There were nine men and three women. Median time from initial diagnosis of colorectal carcinoma was 12 months (mean 12, range 7–31 months). At baseline, all had a Karnofsky Performance Score of 100%. Two patients were seropositive for hepatitis A; three were positive for antibodies directed at the hepatitis B virus, but without hepatitis B antigens or any signs of active hepatitis or cirrhosis. All were HSV-1 seropositive; five tested seropositive for HSV-2. All patients had undergone primary tumor resection and received chemotherapy following the initial cancer diagnosis. Two had also received radiotherapy not involving the liver.
Chemotherapy history
All 12 subjects had previously failed 5-fluorouracil and leucovorin treatment: 11, 2, and 1 also failed irinotecan, capecitabine (Xeloda; Roche Pharmaceuticals, Nutley, NJ), and oxaliplatin therapy, respectively.
One month after viral infusion, all subjects were started on intra-arterial floxuridine plus dexamethasone via hepatic artery. Of these, seven were treated simultaneously with systemic irinotecan, two with systemic oxaliplatin, and three with both irinotecan and oxaliplatin.
Tumor viral appearance
In one patient [3 × 106 plaque-forming units (pfu)], NV1020 was detected by PCR and by immunohistochemistry in tumor tissue from the perfused lobe (Figure 1). NV1020 was also detected in biopsy samples by PCR from two patients in the highest dose cohort (1 × 108 pfu). In one of those two patients, NV1020 was detected in tumor tissue samples from both the perfused and nonperfused lobes. In the second of those patients, NV1020 was detected in normal liver samples from both lobes.
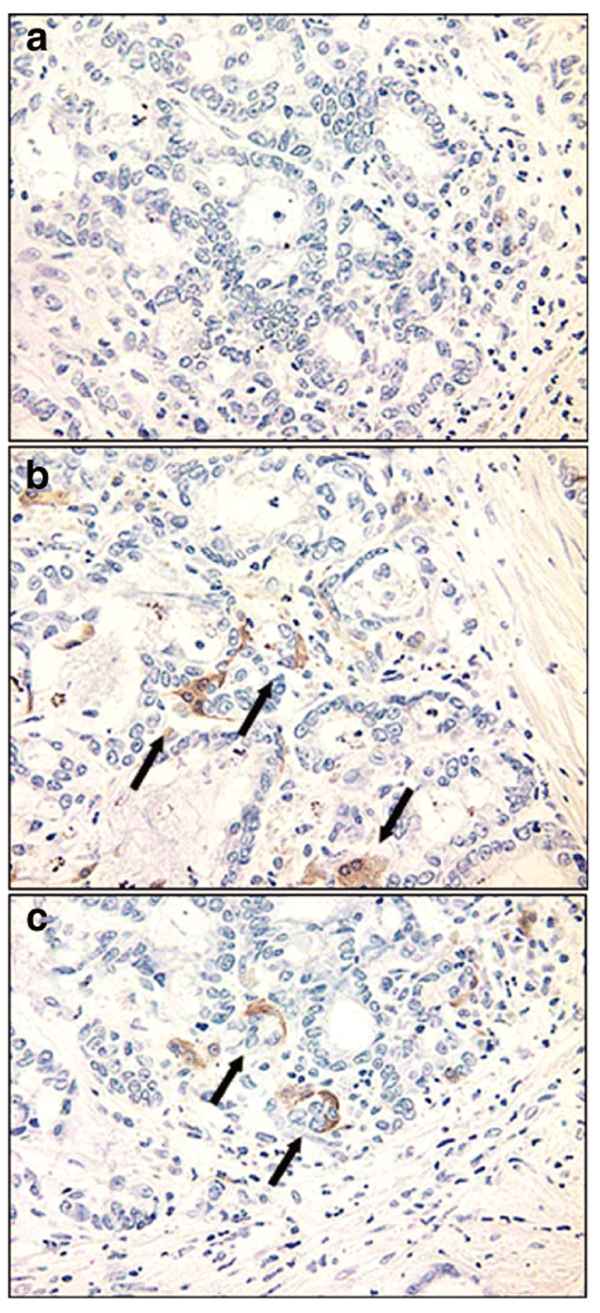
Figure 1.
Immunostaining for herpes simplex virus (HSV) antigens in tumor and liver from subjects dosed with NV1020. (a) Control tumor from a patient not dosed with virus. (b,c) Two separate tumors from two separate patients dosed with virus. Both patients were dosed with 3 × 106 plaque-forming units. The tumor stained positive for HSV (arrows) whereas all normal liver area stained negative to date.
Antitumor effects
Radiologic assessment. Antitumor activity was seen as early as 28 days after viral administration. There was a 39 (Figure 2) and 20% reduction in tumor size in 2 of 12 patients (1 × 108 pfu cohort) after a single infusion of NV1020 at day 28, before intrahepatic arterial infusion chemotherapy administration. Most patients were stable (n = 7) or had progression of disease (n = 3).
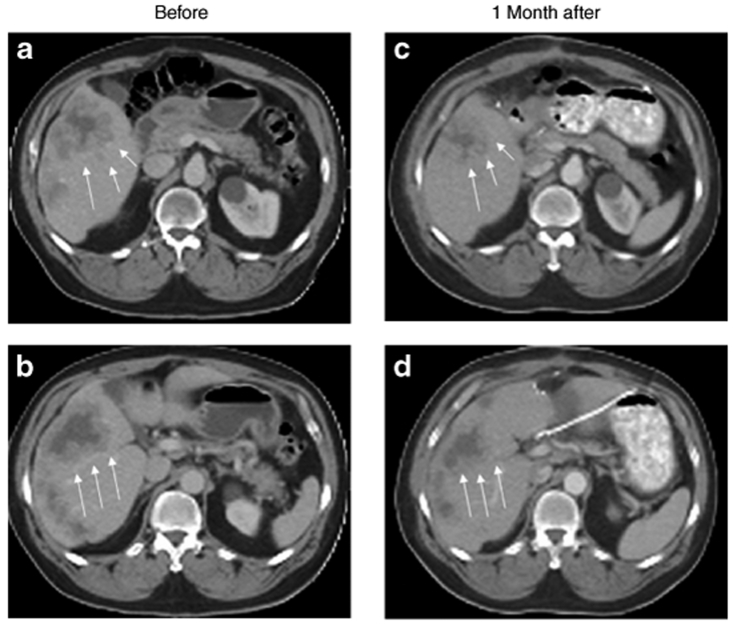
Figure 2.
Radiographic change in a single patient at a 1 × 108 plaque-forming unit dose. Two representative slices from a computed tomography scan done before (a,b) and comparative slices done 1 month after viral treatment (c,d) are shown for a patient at a 1 × 108 dose. The tumor (outlined by white arrows) has clearly decreased significantly in size. No other treatment besides viral injection was undertaken between the two sets of scans.
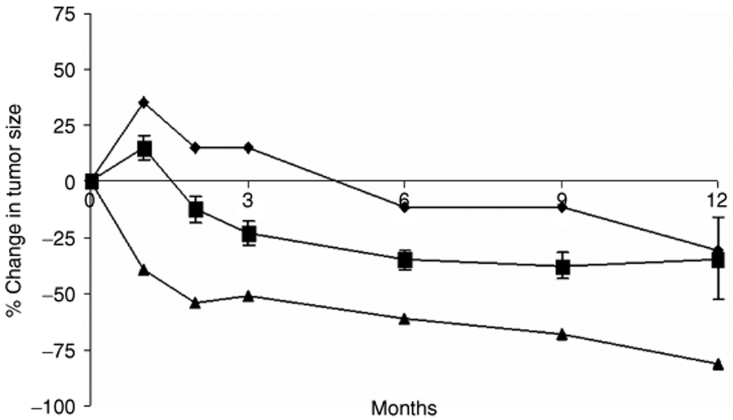
The subsequent change in tumor size is illustrated in Figure 3. By month 2 (1 month after start of chemotherapy) there was a 12% average decrease in tumor dimensions. This response continued, with a 22% decrease in tumor dimensions by month 3, and a sustained 35–37% decrease in months 6–12. All 12 patients had a partial response to subsequent chemotherapy. The maximum response varied from 39 to 81%. There did not seem to be a relationship between viral dose and subsequent response to chemotherapy.
Figure 3.
Changes in tumor size after treatment with virus and chemotherapy. Dimensions are given according to the Response Evaluation Criteria in Solid Tumor criteria. The minimum response (diamond), maximum response (triangle) and average ± SE (square) are shown.
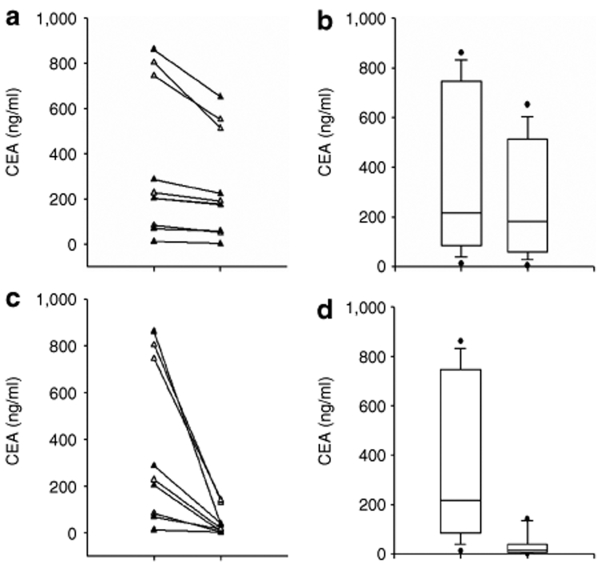
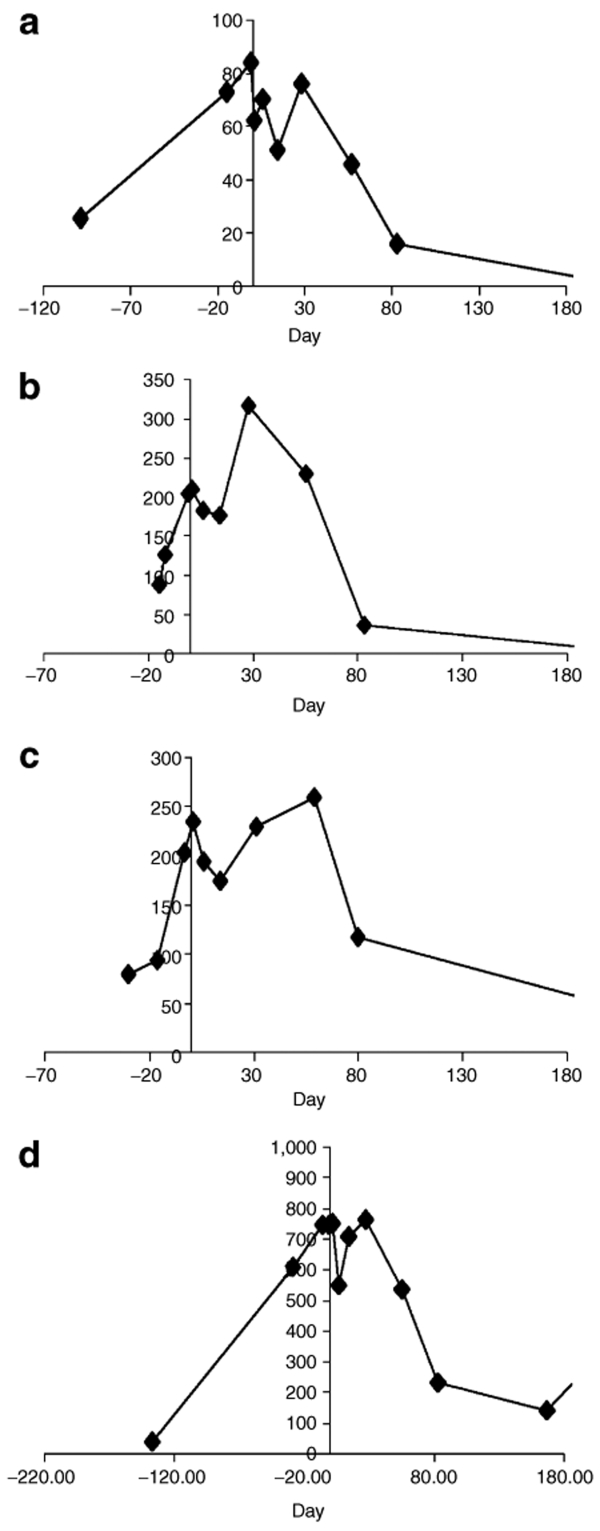
Carcinoembryonic antigen assessment. At baseline, 10 of 12 patients had markedly elevated carcinoembryonic antigen (CEA) levels (>50 ng/ml) with a mean CEA level of 298 ng/ml for all (Figure 4). By day 6, the majority (11 patients) displayed a decline in CEA concentration, and by day 14, this decline was still evident in 8 subjects. Figure 4a depicts the maximal drop in CEA in the first 28 days after NV1020 administration for each patient. Figure 4b presents box plots of these data. Drops varied between 13 and 74% (median 24%; P = 0.02, paired t-test). In most cases, CEA rebounded by the end of the first month. What is remarkable about these changes in CEA is that all subjects were failing chemotherapy and exhibited rising CEA levels at the time of viral treatment. Individual data from four representative patients are shown in Figure 5. Thus, not only was there an arrest of this increase in CEA, there was a decline.
Figure 4.
Changes in carcinoembryonic antigen (CEA) levels. (a) Maximum drop in CEA before chemotherapy in the first 28 days after NV1020 administration for each individual subject. Drops varied between 13 and 74% (median 24%). (b) Box plots of these data. P = 0.02 by paired t-test. (c) Maximum drop in CEA after chemotherapy. Drops varied between 50 and 98% (median 96%). (d) Box plots of these data. P = 0.008 by paired t-test.
Figure 5.
Carcinoembryonic antigen (CEA) changes in four representative patients. CEA levels, represented along the y-axis, are shown in nanograms per milliliter; the x-axis represents time. Zero time represents the time of virus injection.
Once chemotherapy started, CEA levels continued to decline. By month 3, following two chemotherapy cycles, most patients demonstrated a decrease in CEA levels of ≥50%. Median concentration for the group dropped from 204 (baseline) to 27.5 ng/ml at month 3 (P < 0.05, paired t-test). The maximal change in CEA in the first year after viral treatment is shown in Figure 4c,d. Figure 4c demonstrates the maximal drop in CEA after NV1020 administration and chemotherapy for each individual. Figure 4d presents box plots of these data. Drops varied between 50 and 98% (median 96%; P = 0.008, paired t-test). On the basis of CEA criteria, all patients responded after combination viral and chemotherapeutic treatment.
A relation between tumor regression and declines in CEA levels was observed. Patients (one each from the 3 × 107 and 1 × 108 pfu cohorts) showing a ≥50% decline in CEA levels with NV1020 alone also experienced tumor regression during the same period, as determined by computed tomography scan. By month 2, both patients had decreases in CEA levels of 38–50% and achieved a partial response. By month 3, all seven patients with radiologic evidence of tumor response also had a >50% reduction in CEA levels. An additional three patients with ≥50% decreases in CEA levels did not experience tumor regression. There was no evidence of a dose–response in the CEA level changes.
Long-term follow-up and survival
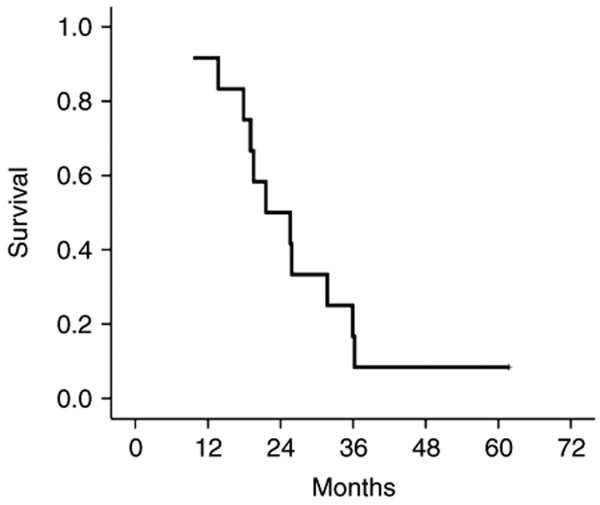
All subjects were followed for continued assessment of safety, clinical status, and HSV monitoring. One month after administration of NV1020, no additional adverse effects attributable to the test agent were noted. At the time of publication, 1 of the initial 12 subjects is still alive 62 months after dosing. Of note, this is the patient who had the highest response to NV1020. All others have died of disease progression. The survival curve is shown in Figure 6. The median survival of this entire group was 25 months (mean = 25 ± 3 months).
Figure 6.
Kaplan–Meier survival curve for the 12 study subjects.
During this follow-up period, no patient demonstrated signs of virus reactivation. No blood, urine, vaginal swabs, or rectal swabs cultured positive for HSV.
Discussion
One attractive attribute of HSV is its large genome, which can be altered to decrease its virulence against normal tissues. Using such genetic engineering, a number of investigators have attempted to further attenuate this common, naturally well-tolerated virus.3,5 Four such designer viruses specifically made for cancer therapy are currently undergoing clinical investigation.6,7 The origin of the NV1020 virus used for the current trial differs from these agents in that it was originally designed as a vaccine against herpes infections.8 We reasoned that since one goal of a viral vaccine program is production of viruses that are highly attenuated in terms of infecting normal tissue, such vaccines might be ideal cancer therapeutic agents if they are also cytotoxic for cancer cells. Indeed, NV1020 has proved to be such a candidate. As a vaccine, it was tested in 36 human volunteers in 1985 by single dose intramuscular injection without significant toxicity.9 It has been tested in intravascular injection in rodents and primates,10,11 and by direct liver injections, with acceptable toxicity profile. We previously reported the short-term effects of intra-arterial delivery.4 Doses ≤108 are well tolerated.12 The current follow-up study found no additional HSV toxicity, including no signs of viral reactivation or disseminated herpes infection even at the terminal phases of cancer. The current data also demonstrate significant biologic anticancer effects that may be attributable to the viral therapies.
A number of experimental models have demonstrated high efficacy of viral treatment by regional delivery. Administration of a single dose of oncolytic HSV by peritoneal perfusion in models of carcinomatosis often cured experimental animals with gastric cancer,13 colorectal cancer,14 and mesothelioma.15 Delivery of virus to the chest cavity by pleural perfusion was very effective in models of esophageal cancer,16 lung cancer,17 and mesothelioma.15 Carotid perfusion produced cures for experimental oral cancer.18 Indeed, vascular perfusion of liver as performed in one phase I study was highly effective in eradicating experimental liver cancer.19 It must be pointed out, however, that models of systemic delivery of HSV were also effective at treating and curing animal models of cancer.20 Our choice of regional delivery therefore was not based on a lack of preclinical data supporting the easier and more widely applicable systemic delivery.
Using a regional, intra-arterial route allows for delivery of the agent proximal to the target. Therefore, this route of administration was chosen to minimize systemic toxicities and exposure to neutralizing antibodies, while providing optimal conditions for biologic effects on the target tumors. This study also presented the opportunity to conduct the first study of intravascular delivery of HSV as treatment for any disease. The low doses of virus at which we encountered evidence of biologic activity are encouraging for possible efficacy of systemic delivery.
CEA is the standard tumor marker used to assess the presence of colorectal cancer.21 Although absolute levels vary greatly from patient to patient, the relative change in level in a single patient has been shown to correlate with tumor recurrence22,23,24 and tumor burden.25 Changes in CEA have also been shown to be indicative of tumor response to therapy that can be seen before radiologic changes. This is because killed tumor still requires time for resorption. In a setting of rising CEA before administration, it is very encouraging that all patients demonstrated a decrease in CEA in the first month after NV1020 administration. The greatest drop in CEA (75%) occurred in the individual who demonstrated the greatest shrinkage of tumor on scanning (39%). These data are highly suggestive of a biologic effect of the virus on cancer.
The normal liver has ~2 × 1011 cells. In this study, a minimal viral dose of 3 × 106 pfu was administered, and a random 1-g tumor sample taken at surgery. To detect virus in the tumor at any level is remarkable, given the low virus-to-cell ratio. To find virus by immunohistochemical assay (Figure 1) is highly suggestive of viral replication.
Immunogenicity is a concern with any viral therapeutic modality. Nearly 90% of human adults have active antibodies directed against HSV-1. Immunoreactivity may theoretically produce acute toxic effects as well as reduce agent efficacy. Potential immunoreaction has been seen clinically for viruses of greater immunogenicity such as adenovirus. We had previously reported that administration of virus in the current cohort did not produce a major immune reaction that could be detected either clinically or by acute cytokine profile.4 Preclinical data had already demonstrated that in animals that have preformed cellular and humoral immunity against HSV, gene transfer using herpes vectors20,26 and effective killing of tumor by oncolytic HSV are still accomplished, although a higher dose is necessary.20,26 In the current study, only seropositive patients were included. The current data demonstrate biologic activity in these patients, and it is likely that these effects will be seen in even lower doses in seronegative individuals.
Other oncolytic viruses are being considered for therapy. For adenoviruses, doses of 2 × 1013 pfu have been required to produce biologic effect.27 In the current study, we have documented biologic effect against a common cancer target at a dose of 3 × 106 pfu. These data demonstrate oncolytic HSV to be a potentially safe therapy at a dose that produces biologic effects. Preclinical data had suggested multiple dosing to be much more efficacious.20,28 Our current data encourage clinical studies of such multiple dosing in humans.
Materials and Methods
Study population and design. This phase I, open-label, dose-escalation study investigated the safety, tolerability and antitumor activity of a single hepatic arterial infusion of NV1020. Forty-eight patients with colorectal adenocarcinoma metastatic to the liver and refractory to first-line chemotherapy were screened, of which 12 eligible subjects were consented under an Institutional Review Board–approved protocol and enrolled for this trial. Declaration of Helsinki protocols was followed and patients provided written, informed consent. All were >18 years of age, and had unresectable liver metastases from histologically confirmed primary colorectal carcinoma with three or more metastatic lesions involving both lobes. All had failed first-line chemotherapy. All were awaiting intrahepatic arterial infusion pump insertion for subsequent regional chemotherapy. All subjects had a Karnofsky Performance Score >60%. All were seropositive for circulating HSV-1 antibodies. None had a history or signs of hepatic fibrosis or cirrhosis. In addition, no subject had any evidence of active herpes infection or a history of other malignancies. None was undergoing systemic antiviral or steroid therapy.
Viral agent. NV1020 is an oncolytic HSV-1 genetically altered to be replication competent but highly attenuated and less neurovirulent than its wild-type counterpart. The construction of NV1020 has been described in detail.19,29,30,31 This nonselected clonal derivative of R7020 was originally produced by Roizman and colleagues in the 1980s.11,32 It has a 15-kb deletion over the joint region of the HSV-1 genome, which encompasses the region coding for ICP0, ICP4, latency-associated transcripts and one copy of the neurovirulence gene (γ134.5). These deletions greatly attenuate virulence. As NV1020 was originally designed as a vaccine against HSV-1 and HSV-2 infection,8 a fragment of HSV-2 DNA from the HindIIIL region encoding for several glycoprotein genes was inserted into the deleted joint region. NV1020 also has a 700-bp deletion of the endogenous TK locus that prevents expression of overlapping transcripts belonging to the UL24 gene. An exogenous copy of the HSV-1 TK gene was inserted under control of the α4 promoter, providing sensitivity to antiviral drugs such as acyclovir. Clinical grade virus stocks were produced by BioReliance (Rockville, MD).
Treatment. NV1020 was diluted in normal saline to achieve a 10 ml total infusate volume. The 12 patients were grouped in four cohorts of three patients each. A single dose of NV1020 was infused into the right hepatic artery of each patient via a percutaneous catheter over 10 minutes. The cohorts received escalating doses of 3 × 106, 1 × 107, 3 × 107, and 1 × 108 pfu. No control or comparison group was used.
Three days after infusion, a laparotomy was performed, during which an intrahepatic arterial infusion pump was placed to deliver subsequent, regional chemotherapy. During surgery, normal liver and tumor were biopsied to assess levels of viral infection. Approximately 1 g each of liver and tumor was harvested from each side of the liver.
Subjects were observed for toxicity, and given no other tumor-directed treatment for a minimum of 28 days after viral infusion. Chemotherapy, typically intrahepatic arterial infusion floxuridine and intravenous irinotecan (CPT-11), was then administered. The acute toxic effects of such a viral infusion have been previously reported.4 Here, we report biologic changes noted in this trial and long-term follow-up.
Assessments.
Viral infection in tumor and liver tissue: Assessment was performed at an independent laboratory blinded to treatments. Freshly harvested tissues were immediately frozen at −70 °C, then shipped for sectioning and immunohistochemical analysis by staining for HSV antigens using polyclonal antibodies specific for HSV as previously described.19 Tissues were counterstained with hematoxylin and eosin.
To further ascertain persistence of infection and extent of dissemination, we performed quantitative PCR analysis on genomic DNA extracted from organs and tumor using an ABI Prism 7700 Sequence Detector (PE Biosystems, Foster City, CA), as previously described,33,34 using primers specific for a unique 111-bp fragment of the HSV ICP0 (immediate early gene).19
Tissue viral cultures were performed at a separate independent laboratory (University of Alabama, Birmingham, AL) also blinded to treatment doses and conditions. Freshly frozen (−70 °C) tissues were transported under sterile conditions. Tissues were homogenized and serial dilutions cultured on Vero cells through standard plaque assay.35
Long-term assessment of viral shedding: Long-term assessment of viral shedding was done monthly for the first 3 months and then every 3 months until the 1-year time point after injection with virus. Patients were assessed for circulating virus and mucosal shedding of virus. Swabs of oral mucous membranes, rectal and vaginal swabs, and blood sampling were done both for viral culture and PCR for virus.
CEA levels: CEA levels were measured in serum samples before and at serial time points after viral administration by monoclonal antibody–based immunoassay, a solid phase enzyme-linked immunosorbent assay based on the sandwich principle (Ortho Clinical Diagnostics, Rochester, NY). A CEA serum level ≥5 ng/ml was considered positive.
Radiologic assessment: Hepatic computed tomography scans were obtained at baseline, day 28, and months 2, 3, 6, 9, and 12 after infusion. Antitumor activity was assessed with the Response Evaluation Criteria in Solid Tumor using sums of largest diameters on serial radiologic assessments. Interpretation of radiologic images was performed by an independent group blinded to the treatments (Bio-Imaging Technologies, Newtown, PA). A 30% decrease was considered a partial response. A 20% increase in size was considered progression of disease. Stable disease was any disease not considered response or progression.
Clinical follow-up: Subjects were followed at least every 3 months until death. No subject was lost to follow-up.
Statistical methods. Comparison of CEA levels was performed by paired t-test. P < 0.05 was considered significant. Survival curves were depicted using the Kaplan–Meier method.36
Acknowledgments
This study was performed in New York, NY, USA.
REFERENCES
- Meignier B., and , Roizman B. Genetic engineering and properties of novel herpes simplex viruses for use as potential vaccines and as vectors of foreign genes. Adv Exp Med Biol. 1989;257:187–192. doi: 10.1007/978-1-4684-5712-4_17. [DOI] [PubMed] [Google Scholar]
- Roizman B, Warren J, Thuning CA, Fanshaw MS, Norrild B., and , Meignier B. Application of molecular genetics to the design of live herpes simplex virus vaccines. Dev Biol Stand. 1982;52:287–304. [PubMed] [Google Scholar]
- Mineta T, Rabkin SD, Yazaki T, Hunter WD., and , Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- Kemeny N, Brown K, Covey A, Kim T, Bhargava A, Brody LA, et al. A Phase I, open-label, dose-escalating study of a genetically engineered herpes simplex virus, NV1020, in subjects with metastatic colorectal carcinoma to the liver. Hum Gene Ther. 2006;17:1214–1224. doi: 10.1089/hum.2006.17.1214. [DOI] [PubMed] [Google Scholar]
- Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- Kwong YL, Chen SH, Kosai K, Finegold M., and , Woo SL. Combination therapy with suicide and cytokine genes for hepatic metastases of lung cancer. Chest. 1997;112:1332–1337. doi: 10.1378/chest.112.5.1332. [DOI] [PubMed] [Google Scholar]
- Harrow S, Papanastassiou V, Harland J, Mabbs R, Petty R, Fraser M, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11:1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- Meignier B., and , Roizman B. Herpes simplex virus vaccines. Antiviral Res. 1985. pp. 259–65. [DOI] [PubMed]
- Cadoz M, Micoud M, Seigneurin JM, Mallaret MR, Baccard C., and , Morand P. Program and Abstracts of the 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, DC, 11 October 1992; 1992. Phase I trial of R7020: a live attenuated recombinant herpes simplex virus (HSV) candidate vaccine. [Google Scholar]
- Meignier B, Martin B, Whitley RJ., and , Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020. II. Studies in immunocompetent and immunosuppressed owl monkeys (Aotus trivirgatus) J Infect Dis. 1990;162:313–321. doi: 10.1093/infdis/162.2.313. [DOI] [PubMed] [Google Scholar]
- Meignier B, Longnecker R., and , Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020: construction and evaluation in rodents. J Infect Dis. 1988;158:602–614. doi: 10.1093/infdis/158.3.602. [DOI] [PubMed] [Google Scholar]
- Fong Y, Jarnagin WR, Guilfoyle B, Tufaro F, Haag N, Glasschroeder B, et al. Results of a phase 1, dose-escalating study of the safety, tolerability, and anti-tumor activity of a single injection of a genetically engineered herpes simplex virus, NV1020, in subjects with hepatic colorectal metastases Am Soc Clin Oncol 2005. Abstract 242
- Bennett JJ, Delman KA, Burt BM, Mariotti A, Malhotra S, Zager J, et al. Comparison of safety, delivery, and efficacy of two oncolytic herpes viruses (G207 and NV1020) for peritoneal cancer. Cancer Gene Ther. 2002;9:935–945. doi: 10.1038/sj.cgt.7700510. [DOI] [PubMed] [Google Scholar]
- Stanziale SF, Stiles BM, Bhargava A, Kerns SA, Kalakonda N., and , Fong Y. Oncolytic herpes simplex virus-1 mutant expressing green fluorescent protein can detect and treat peritoneal cancer. Hum Gene Ther. 2004;15:609–618. doi: 10.1089/104303404323142051. [DOI] [PubMed] [Google Scholar]
- Kucharczuk JC, Randazzo B, Chang MY, Amin KM, Elshami AA, Sterman DH, et al. Use of a “replication-restricted” herpes virus to treat experimental human malignant mesothelioma. Cancer Res. 1997;57:466–471. [PubMed] [Google Scholar]
- Stiles B, Bhargava A, Adusumili P, Stanziale SF, Kim TH, Rusch VW, et al. The replication-competent oncolytic herpes simplex virus mutant virus NV1066 is effective in the treatment of esophageal cancer. Surgery. 2003;134:357–364. doi: 10.1067/msy.2003.244. [DOI] [PubMed] [Google Scholar]
- Stiles B, Adusumilli P, Bhargava A, Stanziale S, Kim T, Chan M, et al. Minimally-invasive localization of oncolytic herpes simplex viral therapy of micrometastatic pleural cancer. Cancer Gene Ther. 2006;13:53–64. doi: 10.1038/sj.cgt.7700860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carew JF, Kooby DA, Halterman MW, Federoff HJ., and , Fong Y. Selective infection and cytolysis of human head and neck squamous cell carcinoma with sparing of normal mucosa by a cytolytic herpes simplex virus type 1 (G207) Hum Gene Ther. 1999;10:1599–1606. doi: 10.1089/10430349950017608. [DOI] [PubMed] [Google Scholar]
- Kooby DA, Carew JF, Halterman MW, Mack JE, Bertino JR, Blumgart LH, et al. Oncolytic viral therapy for human colorectal cancer and liver metastases using a multimutated herpes simplex virus type-1 (G207) FASEB J. 1999;6:499–504. doi: 10.1096/fasebj.13.11.1325. [DOI] [PubMed] [Google Scholar]
- Delman KA, Bennett JJ, Zager JS, Burt BM, McAuliffe PF, Petrowsky H, et al. Effects of preexisting immunity on the response to herpes simplex-based oncolytic viral therapy. Hum Gene Ther. 2000;11:2465–2472. doi: 10.1089/10430340050207957. [DOI] [PubMed] [Google Scholar]
- Rocklin MS, Senagore A., and , Talbott T. Role of carcinoembryonic antigen and liver function tests in the detection of recurrent colorectal carcinoma. Dis Colon Rectum. 1991;34:794–797. doi: 10.1007/BF02051073. [DOI] [PubMed] [Google Scholar]
- Wong IH, Yeo W, Chan AT., and , Johnson PJ. Quantitative relationship of the circulating tumor burden assessed by reverse transcription-polymerase chain reaction for cytokeratin 19 mRNA in peripheral blood of colorectal cancer patients with Dukes' stage, serum carcinoembryonic antigen level and tumor progression. Cancer Lett. 2001;162:65–73. doi: 10.1016/s0304-3835(00)00630-3. [DOI] [PubMed] [Google Scholar]
- Choi MY, Lee KM, Chung JK, Lee DS, Jeong JM, Park JG, et al. Correlation between serum CEA level and metabolic volume as determined by FDG PET in postoperative patients with recurrent colorectal cancer. Ann Nucl Med. 2005;19:123–129. doi: 10.1007/BF03027391. [DOI] [PubMed] [Google Scholar]
- Webb A, Scott-Mackie P, Cunningham D, Norman A, Andreyev J, O'Brien M, et al. The prognostic value of CEA, beta HCG, AFP, CA125, CA19-9 and C-erb B-2, beta HCG immunohistochemistry in advanced colorectal cancer. Ann Oncol. 1995;6:581–587. doi: 10.1093/oxfordjournals.annonc.a059248. [DOI] [PubMed] [Google Scholar]
- Novis BH, Gluck E, Thomas P, Steele GD, Zurawski VR, Jr, Stewart R, et al. Serial levels of Ca 19-9 and CEA in colonic cancer. J Clin Oncol. 1986;4:987. doi: 10.1200/JCO.1986.4.6.987. [DOI] [PubMed] [Google Scholar]
- Herrlinger U, Kramm CM, Aboody-Guterman KS, Silver JS, Ikeda K, Johnston KM, et al. Pre-existing herpes simplex virus 1 (HSV-1) immunity decreases, but does not abolish, gene transfer to experimental brain tumors by a HSV-1 vector. Gene Ther. 1998;5:809–819. doi: 10.1038/sj.gt.3300643. [DOI] [PubMed] [Google Scholar]
- Nemunaitis J, Cunningham C, Buchanan A, Blackburn A, Edelman G, Maples P, et al. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther. 2001;8:746–759. doi: 10.1038/sj.gt.3301424. [DOI] [PubMed] [Google Scholar]
- Wong RJ, Kim SH, Joe JK, Shah JP, Johnson PA., and , Fong Y. Effective treatment of head and neck squamous cell carcinoma by an oncolytic herpes simplex virus. J Am Coll Surg. 2001;193:12–21. doi: 10.1016/s1072-7515(01)00866-3. [DOI] [PubMed] [Google Scholar]
- Petrowsky H, Roberts GD, Kooby DA, Burt BM, Bennett JJ, Delman KA, et al. Functional interaction between fluorodeoxyuridine-induced cellular alterations and replication of a ribonucleotide reductase-negative herpes simplex virus. J Virol. 2001;75:7050–7058. doi: 10.1128/JVI.75.15.7050-7058.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zager JS, Delman KA, Malhotra S, Ebright MI, Bennett JJ, Kates T, et al. Combination vascular delivery of herpes simplex oncolytic viruses and amplicon mediated cytokine gene transfer is effective therapy for experimental liver cancer. Mol Med. 2001;7:561–568. [PMC free article] [PubMed] [Google Scholar]
- Ebright MI, Zager JS, Malhotra S, Delman KA, Weigel TL, Rusch VW, et al. Replication-competent herpes virus NV1020 as direct treatment of pleural cancer in a rat model. J Thorac Cardiovasc Surg. 2002;124:123–129. doi: 10.1067/mtc.2002.122297. [DOI] [PubMed] [Google Scholar]
- Braun DK, Roizman B., and , Pereira L. Characterization of post-translational products of herpes simplex virus gene 35 proteins binding to the surfaces of full capsids but not empty capsids. J Virol. 1984;49:142–153. doi: 10.1128/jvi.49.1.142-153.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heid CA, Stevens J, Livak KJ., and , Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- Luoh SM, Di Marco F, Levin N, Armanini M, Xie MH, Nelson C, et al. Cloning and characterization of a human leptin receptor using a biologically active leptin immunoadhesin. J Mol Endocrinol. 1997;18:77–85. doi: 10.1677/jme.0.0180077. [DOI] [PubMed] [Google Scholar]
- Kemeny N., and , Fong Y. Holland JF, Frei E, Bast RC, Kufe DW, Morton DL., and , Weichselbaum RR. Cancer Medicine. 4 edn. Williams & Wilkins: Baltimore, MD; 1997. Treatment of liver metastases; pp. 1939–1954. [Google Scholar]
- Kaplan EL., and , Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]