Abstract
Cholesterol metabolism is tightly controlled by members of the sterol regulatory element-binding protein (SREBP) family of transcription factors. Here we demonstrate that the ubiquitination and degradation of SREBPs depend on their transcriptional activity. Mutations in the transactivation or DNA-binding domains of SREBPs inhibit their transcriptional activity and stabilize the proteins. The transcriptional activity and degradation of these mutants are restored when fused to heterologous transactivation or DNA-binding domains. When SREBP1a was fused to the DBD of Gal4, the ubiquitination and degradation of the fusion protein depended on coexpression of a promoter–reporter gene containing Gal4-binding sites. In addition, disruption of the interaction between WT SREBP and endogenous p300/CBP resulted in inhibition of SREBP-dependent transcription and stabilization of SREBP. Chemical inhibitors of transcription reduced the degradation of transcriptionally active SREBP1a, whereas they had no effect on the stability of transcriptionally inactive mutants, demonstrating that transcriptional activation plays an important role in the degradation of SREBPs. Thus, transcription-dependent degradation of SREBP constitutes a feedback mechanism to regulate the expression of genes involved in cholesterol metabolism and may represent a general mechanism to regulate the duration of transcriptional responses.
Members of the sterol regulatory element-binding protein (SREBP) family of transcription factors control cholesterol and lipid metabolism and play critical roles during adipocyte differentiation (1–4). In addition, SREBP1c is an important regulator of insulin-dependent gene expression (5, 6). Two genes, srebp1 and srebp2, encode three different SREBP proteins, SREBP1a, -1c, and -2 (7). Newly synthesized SREBPs are inserted into the nuclear and endoplasmic reticulum membranes and are transcriptionally inactive. When cellular sterol concentrations are lowered, the N-terminal domain of SREBP is released from membranes by a two-step proteolytic cascade (8, 9). This transcriptionally active fragment of SREBP is translocated to the nucleus and binds to the promoters of SREBP target genes. SREBPs interact with various transcription factors and coactivators, such as p300/CBP and ARC/DRIP, and these interactions are required for their transcriptional activity (10–12).
Many transcription factors, particularly those involved in the control of cell growth, are unstable proteins targeted for degradation by the ubiquitin–proteasome system (13–15). It has been suggested that certain transactivation domains (TADs) confer instability to heterologous proteins and that components of the proteasome can be recruited to promoters through interactions with transcriptional regulators (16–19). However, the link between the transcriptional activity of native transcription factors and their degradation has been unclear. Here we demonstrate that members of the SREBP family of transcription factors are ubiquitinated and degraded through a transcription-dependent pathway involving the proteasome. These effects require both the transactivation and DNA-binding activity of SREBPs, demonstrating that transcriptional activation is critical for these processes. Thus, transcription-dependent degradation of transcription factors constitutes a feedback mechanism to regulate the stability of transcription factors and, thereby, the duration of transcriptional responses.
Materials and Methods
Cell Culture. All tissue culture media and antibiotics were obtained from Invitrogen and Sigma. HEK 293T, HepG2, HeLa, and Cos7 cells were obtained from the American Type Culture Collection. All cells were maintained at 37°C in DMEM supplemented with 10% FCS/50 units/ml penicillin/50 μg/ml streptomycin, in 5% CO2.
Reagents and Antibodies. Peroxidase-conjugated sheep anti-mouse and donkey anti-rabbit IgG and protein G Sepharose were obtained from Amersham Biosciences. Anti-Flag antibody (M5), actinomycin D, and α-amanitin were obtained from Sigma. Monoclonal anti-Myc (9E10), anti-SREBP1a (E4 and 2A4), anti-Gal4 DBD (RK5C1), antitubulin (TU-02), rabbit antihemagglutinin (anti-HA) (Y-11), and rabbit anti-SREBP1a (H-160) antibodies were obtained from Santa Cruz Biotechnology.
Plasmids and DNA Transfections. The expression vectors for Flag- and Myc-tagged SREBP1a (amino acid residues 2–490) and SREBP2 (amino acid residues 2–485) in the mammalian expression vector pcDNA3 (Invitrogen) have been described (20). Point mutants in SREBP1a and -2 were generated by site-directed mutagenesis (QuikChange, Stratagene). SREBP1a deletion mutants were generated by PCR. The DNA-binding domain (DBD) (amino acid residues 1–147) of Gal4 was amplified by PCR from the pM vector (Clontech) and cloned into pcDNA3 to generate pcDNA3-Gal4. This plasmid was subsequently used to generate the various SREBP1a-Gal4 constructs. To create the V8- and V8m-SREBP1a constructs, oligonucleotides corresponding to three copies of the minimal VP16 TAD [WT (V8), DFDLDMLG; mutant (V8m), DADADMLG] were annealed and cloned at the N terminus of the different SREBP1a constructs in pcDNA3 (21). All other expression vectors have been described (10, 20, 22). The HMG-CoA synthase promoter–reporter construct (SYNSRE-luc) has been described (20). The pG5tk-luc reporter gene contains five copies of the Gal4-binding site upstream of a minimal thymidine kinase promoter in pGL3basic (Promega). Transient transfections were performed by using the MBS transfection kit (Stratagene).
Immunoprecipitation and Immunoblotting. Cells were lysed in buffer A [50 mM Hepes (pH 7.2)/150 mM NaCl/1 mM EDTA/20 mM NaF/2 mM sodium orthovanadate/1% (wt/vol) Triton X-100/10% (wt/vol) glycerol/1 mM PMSF/10 mM sodium butyrate/1% aprotinin) and cleared by centrifugation. In experiments in which the ubiquitination of SREBP1a was analyzed, buffer A was supplemented with SDS (0.1%) and sodium deoxycholate (0.5%). Immunoprecipitations were carried out by adding the appropriate antibodies plus protein G-Sepharose beads, followed by incubation at 4°C. The immunoprecipitates were washed, resolved by SDS/PAGE, and transferred to nitrocellulose membranes (Millipore). The membranes were incubated with the appropriate antibodies, washed, and incubated with horseradish peroxidase-coupled anti-rabbit or anti-mouse IgG. After washing, the blots were visualized by Western blotting. To ensure that equal amounts of protein were loaded in each well, the levels of tubulin in the samples were estimated by Western blotting by using antitubulin antibodies.
Luciferase and β-Galactosidase Assays. Cos7 cells were transiently transfected with the SYNSRE-luc or G5tk-luc promoter–reporter genes in the absence or presence of expression vectors for SREBP1a or -2, either WT or the indicated mutants. After 36 h, luciferase activities were determined in duplicate samples as described by the manufacturer (Promega). The pCH110 vector encoding the β-galactosidase gene under the control of the SV40 promoter (Amersham Biosciences) was used as an internal control for transfection efficiency. Luciferase values (relative light units) were calculated by dividing the luciferase activity by the β-galactosidase activity. The data represent means ± SD of three independent experiments performed in duplicates.
Results
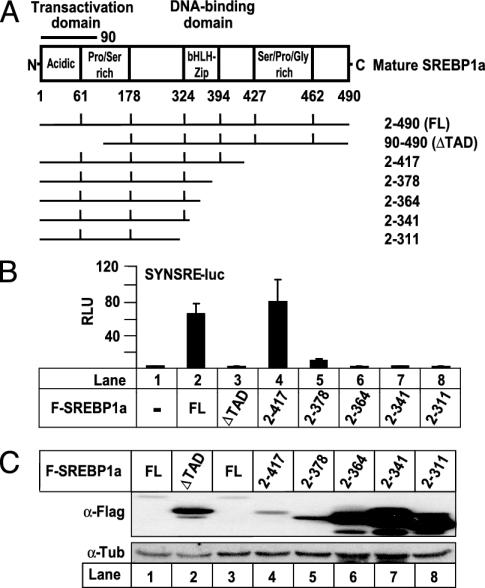
The levels of transcriptionally active SREBPs are extremely low in most tissues and cells. We were interested in the mechanisms and factors involved in controlling the levels of transcriptionally active SREBPs. In an initial screen using SREBP1a deletion mutants, we found a correlation between the transcriptional activity of SREBP1a and its degradation, i.e., all transcriptionally inactive mutants accumulated to high levels, whereas transcriptionally active proteins were rapidly degraded (Fig. 1). Mature SREBP1a (amino acid residues 2–490) activated the SREBP-responsive SYNSRE-luc promoter–reporter gene (Fig. 1B). Removal of the N-terminal TAD (ΔTAD in Fig. 1) inhibited the transcriptional activity of SREBP1a. Interestingly, the levels of WT SREBP1a were very low, whereas the transcriptionally inactive ΔTAD protein accumulated to high levels (Fig. 1C). Similarly, deletions that affected the basic helix–loop–helix leucine zipper domain (bHLH-Zip in Fig. 1 A) inhibited the transcriptional activity of SREBP1a and stabilized the protein (Fig. 1 B and C). We did not detect any ubiquitinated SREBP1a in these assays, suggesting that the protein is deubiquitinated in the nondenaturing lysis buffer.
Fig. 1.
Correlation between the transcriptional activity of SREBP1a and its degradation. (A) Schematic illustration of transcriptionally active SREBP1a (FL) and the deletions used to map degradation signals in SREBP1a. (B) Cos7 cells were transfected with SYNSRE-luc in the absence or presence (5.0 ng) of Flag-SREBP1a, either WT or the indicated deletion mutants. Thirty-six hours after transfection, the luciferase activity was measured. (C) Flag-SREBP1a (0.25 μg), either WT or the indicated deletion mutants, was expressed in 293T cells. The amount of SREBP1a in cell lysates was detected by Western blotting by using anti-Flag antibodies (α-Flag).
To analyze this observation further, we generated two separate mutations in SREBP1a and -2 that interfered with the transcriptional activities of these proteins (Fig. 2A). The MLQ sequence is found in the TAD of SREBPs and is critical for their interaction with the p300/CBP coactivators (10, 20). Mutation of this sequence blocked the interaction between SREBPs and p300 (see Fig. 7, which is published as supporting information on the PNAS web site) and interfered with SREBP-dependent transcription (Fig. 2B). Both SREBP1a and -2 accumulated to high levels when the MLQ sequence was mutated (Fig. 2C). The core DBD of SREBP1 and -2 contain two arginine residues and one tyrosine residue that are in close contact with DNA (23). When the tyrosine residue and one of the arginine residues were mutated in SREBP1a and -2 (SREBP1a-DBD–/– and SREBP2-DBD–/–, respectively), the proteins did not bind DNA (see Fig. 7) and failed to activate transcription from a SREBP-dependent promoter–reporter gene (Fig. 2B). Real-time PCR analysis demonstrated that the MLQ and DBD–/– mutants of SREBP1a were unable to activate endogenous target genes (data not shown). The DBD–/– mutants of both SREBP1a and -2 were stabilized and accumulated to high levels when expressed in 293T cells (Fig. 2C). The MLQ and DBD–/– mutants of SREBP1a were also stabilized in Cos7 cells (data not shown). The observation that mutations in either the TAD or DBD stabilize SREBPs demonstrates that both the transactivation and DNA-binding activities of SREBPs are required for their degradation. SREBPs form dimers, and the MLQ and DBD–/– mutants of SREBP1a inhibited SREBP-dependent transcription in a dominant-negative manner (Fig. 2D). To test whether this effect could influence the stability of SREBP1a, Myc-SREBP1a, either WT or the indicated mutants, were expressed in the absence or presence of an excess of Flag-SREBP1a, either WT or the indicated mutants (Fig. 2E). Overexpression of the MLQ and DBD–/– mutants of SREBP1a stabilized the WT protein (compare lanes 1, 3, and 4 in Fig. 2E). Surprisingly, overexpression of WT SREBP1a destabilized the MLQ and DBD–/– mutants (compare lane 5 with 6 and lane 9 with 10 in Fig. 2E), indicating that the WT protein confers some transcriptional activity to the mutants. This idea was supported by the observation that the WT/MLQ dimer could interact with p300 (presumably through the WT TAD) and that the WT/DBD–/– dimer was able to interact with DNA (Fig. 8, which is published as supporting information on the PNAS web site). Although weaker than those of the WT homodimer, these interactions should confer transcriptional activity to the mutant proteins and could explain why the mutants are destabilized in response to overexpression of WT SREBP1a. The experiments presented in Fig. 2 D and E were also performed with SREBP2 with similar results (data not shown). Thus, our results indicate that one of the proteins in the SREBP dimer can regulate the degradation of its partner and that the transcriptional activity of the complex will determine its stability.
Fig. 2.
Mutations that block the transcriptional activity of SREBPs stabilize the proteins. (A) Illustration of the SREBP constructs used in this study. (B) Cos7 cells were transfected with SYNSRE-luc and increasing amounts (2.5 and 5.0 ng) of Flag-SREBP1a or Flag-SREBP2, either WT or the indicated mutants. Thirty-six hours after transfection, luciferase activity was measured. (C) Flag-SREBP1a or Flag-SREBP2 (0.25 μg), either WT or the indicated mutants, were expressed in 293T cells. The levels of SREBPs in cell lysates were detected by anti-Flag antibodies. (D) Cos7 cells were transfected with SYNSRE-luc and Flag-SREBP1a (2.5 ng) in the absence or presence (1.0, 2.5, 5.0, and 10 ng) of the indicated Myc-tagged constructs. Thirty-six hours after transfection, luciferase activity was measured. (E) Myc-SREBP1a (0.25 μg), either WT or the indicated mutants, were expressed in 293T cells in the absence or presence (1.0 μg) of Flag-SREBP1a, either WT or the indicated mutants. The levels of SREBP1a were detected with anti-Flag and anti-Myc antibodies.
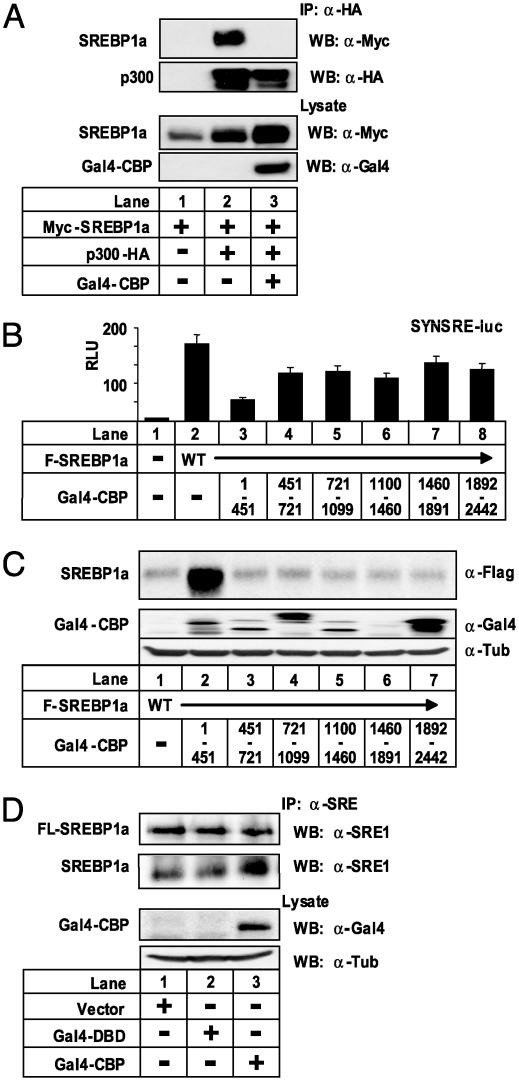
The transcriptional activities of SREBPs depend on interactions with the coactivators p300/CBP (10, 11). We wanted to use this fact to inhibit SREBP-dependent transcription without introducing mutations in the SREBP proteins. We hypothesized that overexpression of the SREBP-interaction domain of CBP would interfere with the interaction between SREBP and endogenous p300/CBP and thereby inhibit SREBP-dependent transcription. Initially, we confirmed that the interaction between p300 and SREBP1a was disrupted when a Gal4 fusion of the SREBP-interaction domain of CBP was overexpressed in 293T cells (Fig. 3A). To test whether SREBP-dependent transcription was affected when the interaction between SREBP and p300/CBP was disrupted, cells were transfected with a SREBP-responsive promoter–reporter gene and plasmids containing various fragments of CBP fused to the DBD of Gal4 (this domain confers a nuclear targeting signal to the CBP fragments). Overexpression of the SREBP-interaction domain of CBP (amino acid residues 1–451) interfered with the transcriptional activity of SREBP1a (Fig. 3B). In line with our hypothesis, expression of this domain of CBP in 293T cells stabilized cotransfected SREBP1a (Fig. 3C). Identical results were obtained for SREBP2 (see Fig. 9, which is published as supporting information on the PNAS web site). Likewise, the amount of endogenous mature SREBP1a in HepG2 cells was enhanced in cells expressing the N-terminal fragment of CBP (Fig. 3D). Taken together, these results indicate that interactions with coactivators confer both transcriptional activity and instability to members of the SREBP family of transcription factors.
Fig. 3.
SREBP1a is stabilized when its interaction with p300/CBP is disrupted. (A) Myc-SREBP1a (0.25 μg) was expressed in 293T cells in the absence or presence of p300-HA (1.0 μg) and a Gal4-tagged version of the SREBP-interaction domain of CBP (Gal4-CBP) (0.25 μg). Lysates were immunoprecipitated with anti-HA antibodies. Immunoprecipitated SREBP1a and p300 were detected with anti-Myc and anti-HA antibodies, respectively. The levels of Gal4-CBP and Myc-SREBP1a in cell lysates were determined by Western blotting. (B) Cos7 cells were transfected with SYNSRE-luc and Flag-SREBP1a (5.0 ng) in the absence or presence (100 ng) of the indicated Gal4-CBP fragments. Thirty-six hours after transfection, luciferase activity was measured. (C) Flag-SREBP1a (0.25 μg) was expressed in 293T cells in the absence or presence (0.25 μg) of the indicated Gal4-CBP fragments. The levels of SREBP1a and Gal4-CBP in cell lysates were detected with anti-Flag and anti-Gal4 antibodies, respectively. (D) HepG2 cells were transfected with the empty expression vector, the Gal4-DBD, or the SREBP-interaction domain of CBP (Gal4-CBP) (5.0 μg). Lysates from cells grown in lipoprotein-deficient media were immunoprecipitated with anti-SREBP1a antibodies. The levels of membrane-associated (FL-SREBP1a) and mature (SREBP1a) SREBP1a in the immunoprecipitates were determined with anti-SREBP1a antibodies. The amount of Gal4-CBP was detected with anti-Gal4 antibodies.
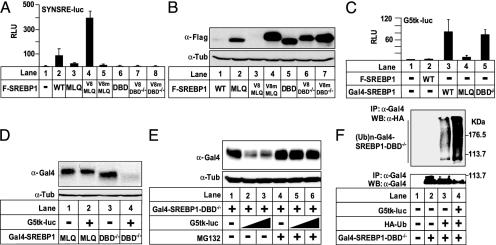
It has been demonstrated that the region in c-Myc that promotes ubiquitin-mediated proteolysis overlaps with its TAD (24). Subsequently, it was demonstrated that a number of TADs signal ubiquitin-mediated proteolysis and confer instability to heterologous proteins (21, 25, 26). Based on these studies and our earlier results, we speculated that SREBP proteins containing the MLQ mutation would be destabilized if their transcriptional activity was restored. To test this, three copies of the minimal TAD from the viral transcription factor VP16 (V8 in Fig. 4) was fused to the MLQ or DBD–/– mutants of SREBP1a. As controls, fusion proteins were also created that contained three copies of the VP16 TAD containing two point mutations that block the transcriptional activity of the TAD (V8m in Fig. 4). The V8 unit restored transcriptional activity to SREBP1a-MLQ (V8-MLQ in Fig. 4A), whereas it had no effect on the transcriptional activity of the DBD–/– mutant (V8-DBD–/– in Fig. 4A). Addition of V8m did not affect the transcriptional activity of either construct. The MLQ and DBD–/– versions of SREBP1a were stable and accumulated to high levels in cells (Fig. 4B). More importantly, and in line with our hypothesis, the transcriptionally active V8-MLQ construct was rapidly degraded, whereas the transcriptionally inactive V8-DBD–/– remained stable (Fig. 4B). Addition of the transcriptionally inactive TAD (V8m) did not affect the stability of any of the proteins. Thus, a functional TAD is not sufficient to confer instability to SREBP1a in the absence of DNA binding. Rather, the ability of the V8 TAD to confer instability to SREBP1a depends on the transcriptional potential of the fusion protein, which requires a functional DBD in SREBP1a.
Fig. 4.
Degradation of SREBP1a depends on its DBD and TAD. (A) Cos7 cells were transfected with SYNSRE-luc and Flag-SREBP1a (5.0 ng), either WT or the indicated mutants. Where indicated, SREBP1a was fused to three copies of the minimal VP16-TAD, either WT (V8) or transactivation-deficient (V8m). Thirty-six hours after transfection, luciferase activity was measured. (B) Flag-SREBP1a (0.25 μg), either WT or the mutants used in A, were expressed in 293T cells. The amount of SREBP1a in cell lysates was detected with anti-Flag antibodies. (C) Cos7 cells were transfected with G5tk-luc (0.25 μg) and Flag- or Gal4-tagged SREBP1a (5.0 ng), either WT or the indicated mutants. Thirty-six hours after transfection, luciferase activity was measured. (D) Gal4-SREBP1a (0.1 μg), either the MLQ or DBD–/– mutant, was expressed in 293T cells in the absence or presence (1.0 μg) of G5tk-luc. The levels of SREBPs in cell lysates were detected with anti-Gal4 antibodies. (E) Gal4-SREBP1a-DBD–/– (0.1 μg) was expressed in 293T cells in the absence or presence of G5tk-luc (1.0 μg). Where indicated, cells were treated with MG132 (50 μM) for 8 h before lysis. The amount of SREBP1a in cell lysates was detected with anti-Gal4 antibodies. (F) Gal4-SREBP1a-DBD–/– (0.25 μg) was expressed in 293T cells in the absence or presence of HA-ubiquitin (0.5 μg) and G5tk-luc (2.5 μg). SREBP1a was immunoprecipitated from cell lysates and resolved by SDS/PAGE. Ubiquitinated SREBP1a was detected with anti-HA antibodies. The levels of SREBP1a in the immunoprecipitates were determined with anti-Gal4 antibodies.
To further test our hypothesis, we wanted to restore DNA-binding and transcriptional activity to the SREBP1a-DBD–/– mutant. To do this, the DBD of Gal4 (Gal4-DBD) was fused to the N terminus of SREBP1a-DBD–/–. As controls, similar fusion proteins were also created with WT SREBP1a and the MLQ mutant. The transcriptional activities of these SREBP1a proteins were subsequently determined with a Gal4-responsive promoter–reporter gene. Addition of the Gal4-DBD conferred transcriptional activity to the SREBP1a construct containing the DBD–/– mutation, whereas it had no effect on the transcriptional activity of the MLQ mutant (Fig. 4C). The MLQ and DBD–/– versions of Gal4-SREBP1a were stable and accumulated to high levels in the absence of a Gal4-responsive promoter (Fig. 4D). In line with our hypothesis, the Gal4-SREBP1a-DBD–/– construct was rapidly degraded in response to cotransfection of a promoter–reporter gene containing Gal4-binding sites (G5tk-luc in Fig. 4D), reflecting the fact that this protein is able to activate the reporter gene. However, the transcriptionally inactive Gal4-SREBP1a-MLQ construct remained stable, even in the presence of the Gal4-responsive promoter. Thus, DNA binding alone is not sufficient to confer instability to SREBP1a. Rather, the ability of the DBD of Gal4 to confer instability to SREBP1a depends on the transcriptional potential of the fusion protein, which requires a functional TAD in SREBP1a.
Earlier work suggests that SREBP proteins are targeted by ubiquitination, indicating that they are degraded by the proteasome (20, 27). This was confirmed by our observation that WT SREBP1a was stabilized by proteasome inhibitors (see Fig. 10, which is published as supporting information on the PNAS web site). To test whether transcription-dependent degradation of SREBP depends on the ubiquitin–proteasome pathway, Gal4-SREBP1a-DBD–/– was expressed in 293T cells in the absence or presence of the G5tk-luc reporter gene (Fig. 4E). Gal4-SREBP1a-DBD–/– was degraded in a dose-dependent manner in response to cotransfection of the Gal4-responsive reporter gene, and this was inhibited by the proteasome inhibitor MG132, indicating that proteasome activity is required for transcription-dependent degradation of SREBP1a. This hypothesis received further support from our observation that the ubiquitination of Gal4-SREBP1a-DBD–/– was greatly enhanced in response to cotransfection of G5tk-luc (Fig. 4F).
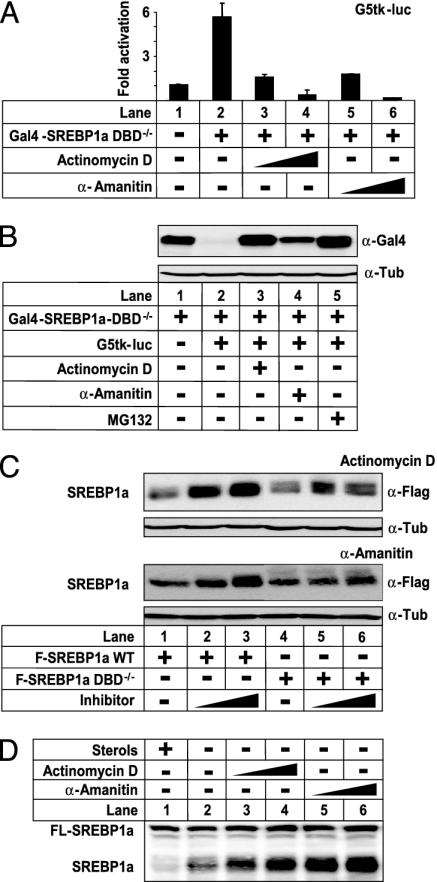
The results presented in Figs. 1, 2, 3, 4 indicate that the polyubiquitination and degradation of SREBPs is correlated to their transcriptional potential. However, whether transcriptional activation per se was required for these processes was unknown. To address this issue, we analyzed the effect of two inhibitors of transcription, actinomycin D and α-amanitin, on the stability of SREBP1a (Fig. 5). Both compounds inhibited the transcriptional activity of Gal4-SREBP1a-DBD–/– in a dose-dependent manner (Fig. 5A). In line with our hypothesis, both actinomycin D and α-amanitin reduced the degradation of Gal4-SREBP1a-DBD–/– in response to the G5tk-luc promoter–reporter gene (Fig. 5B), indicating that the transcriptional activity of SREBP1a is important for its degradation. This hypothesis received further support from our observation that both inhibitors stabilized WT SREBP1a when this protein was expressed in 293T cells, whereas they had no effect on the transcriptionally inactive DBD–/– mutant (Fig. 5C). Interestingly, the amount of endogenous mature SREBP1a was greatly enhanced in HeLa cells treated with either actinomycin D or α-amanitin, whereas neither compound affected the levels of the premature form of the protein (Fig. 5D). Taken together, our results demonstrate that the degradation of the SREBP family of transcription factors is regulated by a transcription-dependent mechanism involving the proteasome.
Fig. 5.
SREBP1a is stabilized when transcription is inhibited. (A) Cos7 cells were transfected with G5tk-luc (0.25 μg) and Gal4-SREBP1a-DBD–/– (5.0 ng). Where indicated, cells were treated with increasing amounts of actinomycin D (10 and 50 nM) or α-amanitin (0.2 and 1.0 μM) for 8 h before lysis. Thirty-six hours after transfection, luciferase activity was measured. (B) Gal4-SREBP1a-DBD–/– (0.125 μg) was expressed in 293T cells in the absence (lane 1) or presence (lanes 2–5) of G5tk-luc (62.5 ng). Where indicated, cells were treated with actinomycin D (10 nM), α-amanitin (1 μM), or MG 132 (50 μM) 8 h before lysis. The amount of SREBP1a in cell lysates was detected with anti-Gal4 antibodies. (C) Flag-SREBP1a, either WT (0.25 μg) or the DBD–/– mutant (0.1 μg), was expressed in 293T cells. Where indicated, cells were treated with increasing amounts of actinomycin D (Upper; 10 and 25 nM) or α-amanitin (Lower; 1.0 and 2.5 μM) 8 h before lysis. The amount of SREBP1a in cell lysates was detected with anti-Flag antibodies. (D) HeLa cells were grown in lipoprotein-deficient serum in the presence (lane 1) or absence (lanes 2–6) of cholesterol and 25-hydroxycholesterol (50 and 5.0 μg/ml, respectively). Where indicated, cells were treated with increasing amounts of actinomycin D (10 and 50 nM) or α-amanitin (0.2 and 1.0 μM) for 8 h before lysis. The amount of SREBP1a in cell lysates was detected with anti-SREBP1a antibodies. The migration of unprocessed (FL-SREBP1a) and mature (SREBP1a) SREBP1 is indicated.
Discussion
In summary, the data presented in this study demonstrate that the SREBP transcription factors are ubiquitinated and degraded through a transcription-dependent pathway involving the proteasome, thereby terminating the transcriptional signal. These effects require both a functional TAD and DBD in SREBP, demonstrating that transcriptional activation is of critical importance.
It has been demonstrated that the TAD in c-Myc is important for its ubiquitin-mediated proteolysis (24). However, and in contrast to SREBP, deletion of the DBD enhanced the ubiquitination of c-Myc and destabilized the protein, indicating that the transcriptional activity of c-Myc is not required for its degradation. Subsequently, it was demonstrated that a number of TADs signal ubiquitin-mediated proteolysis and confer instability to heterologous proteins (21, 25, 26). Mutations in the TAD (MLQ) or DBD (DBD–/–) blocked the transcriptional activity of SREBPs and stabilized the proteins. The transcriptional activity of the MLQ mutant of SREBP1a could be restored if the protein was fused to a minimal TAD from the viral transcription factor VP16. Interestingly, the VP16 TAD also conferred instability to SREBP1a, and this effect required a functional DBD in SREBP1a, indicating that degradation depends on the transcriptional activity of the fusion protein.
When the SREBP1a-DBD–/– mutant was fused to the DBD of Gal4, the fusion protein was able to transactivate a promoter–reporter gene containing Gal4-binding sites. The Gal4 DBD enhanced the ubiquitination and degradation of SREBP1a-DBD–/– only in the presence of a DNA template containing Gal4-binding sites. In addition, the Gal4 DBD destabilized SREBP1a only if the protein contained a functional TAD. It has been demonstrated that the VP16 TAD confers instability to the DBD of Gal4 (25). Interestingly, this effect depended on the DNA-binding activity of Gal4. Thus, a functional TAD or DBD is not sufficient to confer instability to SREBPs. Rather, the degradation of this family of transcription factors depends on their transcriptional activity, which requires both the TAD and the DBD. This was confirmed by our observation that inhibitors of transcription reduced the degradation of transcriptionally active SREBP1a, whereas they had no effect on the stability of transcriptionally inactive proteins.
Components of the proteasome are recruited to some promoters, and proteasome activity is required for the function of certain transcription factors (17–19). SREBPs are stabilized by proteasome inhibitors, and we have demonstrated that transcription-dependent degradation of SREBP1a involves the ubiquitin–proteasome system. Further studies are required to determine whether components of the proteasome are recruited to SREBP-regulated genes and whether the transcriptional activity of SREBPs targets other proteins in the transcriptional complex for degradation, which would constitute a very efficient feedback mechanism to terminate transcriptional responses. It will also be important to identify the ubiquitin ligases involved in these processes. It is likely that these factors are components of the basic transcriptional machinery or associated with p300/CBP (28, 29).
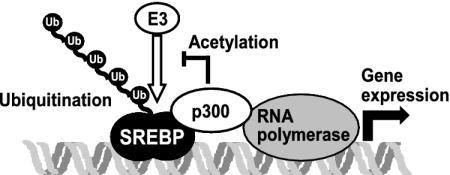
We have demonstrated that members of the SREBP family of transcription factors are ubiquitinated and degraded through a transcription-dependent pathway involving the proteasome (Fig. 6). The signals involved in these processes will require further study. However, we have previously demonstrated that p300/CBP-mediated acetylation of specific lysine residues in SREBP regulates its stability by interfering with the ubiquitination of these same residues (20). Thus, p300/CBP plays a dual role in the regulation of the stability of SREBPs. The expression of SREBP-regulated genes depends on SREBP-mediated recruitment of p300/CBP. However, the transcriptional activity of the SREBP-p300/CBP complex targets SREBP for degradation. In parallel, SREBP is acetylated by p300/CBP, which stabilizes the transcriptional complex and promotes SREBP-dependent gene expression. Consequently, the balance between transcription-dependent degradation and coactivator-mediated acetylation will determine the stability of SREBPs (Fig. 6). A number of proteins have recently been found to be stabilized by coactivator-mediated acetylation, including Smad7, E2F1, p53, and c-Myc, suggesting that the balance between these two pathways may be a general mechanism to regulate the stability and function of transcriptional regulators (22, 30–32). Further studies are required to analyze the timing and regulation of these two processes. It is possible that mono- and/or polyubiquitination of SREBPs are required for their transcriptional activity, and that they are degraded as a consequence of the transcriptional process, which would be in line with the hypothesis that the activity of transcription factors is licensed by ubiquitination (26). This hypothesis gained further support from the recent demonstration that the ubiquitin ligase Skp2 is required not only for the ubiquitination of Myc but also for its transcriptional activity (33, 34). Thus, the role of ubiquitination, ubiquitin ligases, and components of the proteasome in transcription may be an important area of future study. It is intriguing that both the activation and termination of SREBP-dependent transcription is regulated by proteolysis. Together, these pathways enable cells to rapidly respond to changes in cholesterol levels and control the duration of SREBP-dependent gene expression. Thus, transcription-dependent degradation of transcription factors constitutes a feedback mechanism to control gene expression.
Fig. 6.
Members of the SREBP family of transcription factors are ubiquitinated and degraded through a transcription-dependent pathway. Expression of SREBP-regulated genes depends on p300/CBP. However, the transcriptional activity of SREBP targets it for degradation by the ubiquitin–proteasome system. In parallel, SREBP is acetylated by p300/CBP on lysine residues that are otherwise ubiquitinated, stabilizing SREBP and prolonging the duration of the transcriptional signal.
Supplementary Material
Acknowledgments
J.E. dedicates this paper to Professor Gustav Dallner on the occasion of his 70th birthday. We thank G. Akusjärvi and C.-H. Heldin for reading the manuscript and members of the laboratory for stimulating discussions and suggestions. This work was in part supported by grants from the Swedish Research Council and the Novo Nordisk Foundation (to J.E.) and Magnus Bergvalls Stiftelse (to A.S.). J.E. is a Research Fellow of the Royal Swedish Academy of Sciences through a grant from the Knut and Alice Wallenberg Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DBD, DNA-binding domain; SREBP, sterol regulatory element-binding protein; TAD, transactivation domain; HA, hemagglutinin.
References
- 1.Edwards, P. A. & Ericsson, J. (1999) Annu. Rev. Biochem. 68, 157–185. [DOI] [PubMed] [Google Scholar]
- 2.Osborne, T. F. (2000) J. Biol. Chem. 275, 32379–32382. [DOI] [PubMed] [Google Scholar]
- 3.Rosen, E. D., Walkey, C. J., Puigserver, P. & Spiegelman, B. M. (2000) Genes Dev. 14, 1293–1307. [PubMed] [Google Scholar]
- 4.Brown, M. S. & Goldstein, J. L. (1999) Proc. Natl. Acad. Sci. USA 96, 11041–11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimomura, I., Bashmakov, Y., Ikemoto, S., Horton, J. D., Brown, M. S. & Goldstein, J. L. (1999) Proc. Natl. Acad. Sci. USA 96, 13656–13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foretz, M., Guichard, C., Ferre, P. & Foufelle, F. (1999) Proc. Natl. Acad. Sci. USA 96, 12737–12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards, P. A., Tabor, D., Kast, H. R. & Venkateswaran, A. (2000) Biochim. Biophys. Acta 1529, 103–113. [DOI] [PubMed] [Google Scholar]
- 8.Rawson, R. B., Zelenski, N. G., Nijhawan, D., Ye, J., Sakai, J., Hasan, M. T., Chang, T. Y., Brown, M. S. & Goldstein, J. L. (1997) Mol. Cell 1, 47–57. [DOI] [PubMed] [Google Scholar]
- 9.Sakai, J., Rawson, R. B., Espenshade, P. J., Cheng, D., Seegmiller, A. C., Goldstein, J. L. & Brown, M. S. (1998) Mol. Cell 2, 505–514. [DOI] [PubMed] [Google Scholar]
- 10.Ericsson, J. & Edwards, P. A. (1998) J. Biol. Chem. 273, 17865–17870. [DOI] [PubMed] [Google Scholar]
- 11.Naar, A. M., Beaurang, P. A., Robinson, K. M., Oliner, J. D., Avizonis, D., Scheek, S., Zwicker, J., Kadonaga, J. T. & Tjian, R. (1998) Genes Dev. 12, 3020–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naar, A. M., Beaurang, P. A., Zhou, S., Abraham, S., Solomon, W. & Tjian, R. (1999) Nature 398, 828–832. [DOI] [PubMed] [Google Scholar]
- 13.Glickman, M. H. & Ciechanover, A. (2002) Physiol. Rev. 82, 373–428. [DOI] [PubMed] [Google Scholar]
- 14.Desterro, J. M., Rodriguez, M. S. & Hay, R. T. (2000) Cell. Mol. Life Sci. 57, 1207–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conaway, R. C., Brower, C. S. & Conaway, J. W. (2002) Science 296, 1254–1258. [DOI] [PubMed] [Google Scholar]
- 16.Muratani, M. & Tansey, W. P. (2003) Nat. Rev. Mol. Cell Biol. 4, 192–201. [DOI] [PubMed] [Google Scholar]
- 17.Ferdous, A., Gonzalez, F., Sun, L., Kodadek, T. & Johnston, S. A. (2001) Mol. Cell 7, 981–991. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez, F., Delahodde, A., Kodadek, T. & Johnston, S. A. (2002) Science 296, 548–550. [DOI] [PubMed] [Google Scholar]
- 19.Reid, G., Hubner, M. R., Metivier, R., Brand, H., Denger, S., Manu, D., Beaudouin, J., Ellenberg, J. & Gannon, F. (2003) Mol. Cell 11, 695–707. [DOI] [PubMed] [Google Scholar]
- 20.Giandomenico, V., Simonsson, M., Gronroos, E. & Ericsson, J. (2003) Mol. Cell. Biol. 23, 2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salghetti, S. E., Muratani, M., Wijnen, H., Futcher, B. & Tansey, W. P. (2000) Proc. Natl. Acad. Sci. USA 97, 3118–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gronroos, E., Hellman, U., Heldin, C. H. & Ericsson, J. (2002) Mol. Cell 10, 483–493. [DOI] [PubMed] [Google Scholar]
- 23.Parraga, A., Bellsolell, L., Ferre-D'Amare, A. R. & Burley, S. K. (1998) Structure (London) 6, 661–672. [DOI] [PubMed] [Google Scholar]
- 24.Salghetti, S. E., Kim, S. Y. & Tansey, W. P. (1999) EMBO J. 18, 717–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molinari, E., Gilman, M. & Natesan, S. (1999) EMBO J. 18, 6439–6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salghetti, S. E., Caudy, A. A., Chenoweth, J. G. & Tansey, W. P. (2001) Science 293, 1651–1653. [DOI] [PubMed] [Google Scholar]
- 27.Hirano, Y., Yoshida, M., Shimizu, M. & Sato, R. (2001) J. Biol. Chem. 276, 36431–36437. [DOI] [PubMed] [Google Scholar]
- 28.Brower, C. S., Sato, S., Tomomori-Sato, C., Kamura, T., Pause, A., Stearman, R., Klausner, R. D., Malik, S., Lane, W. S., Sorokina, I., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 10353–10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grossman, S. R., Deato, M. E., Brignone, C., Chan, H. M., Kung, A. L., Tagami, H., Nakatani, Y. & Livingston, D. M. (2003) Science 300, 342–344. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Balbas, M. A., Bauer, U. M., Nielsen, S. J., Brehm, A. & Kouzarides, T. (2000) EMBO J. 19, 662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito, A., Kawaguchi, Y., Lai, C. H., Kovacs, J. J., Higashimoto, Y., Appella, E. & Yao, T. P. (2002) EMBO J. 21, 6236–6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vervoorts, J., Luscher-Firzlaff, J. M., Rottmann, S., Lilischkis, R., Walsemann, G., Dohmann, K., Austen, M. & Luscher, B. (2003) EMBO Rep. 4, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von der Lehr, N., Johansson, S., Wu, S., Bahram, F., Castell, A., Cetinkaya, C., Hydbring, P., Weidung, I., Nakayama, K., Nakayama, K. I., et al. (2003) Mol. Cell 11, 1189–1200. [DOI] [PubMed] [Google Scholar]
- 34.Kim, S. Y., Herbst, A., Tworkowski, K. A., Salghetti, S. E. & Tansey, W. P. (2003) Mol. Cell 11, 1177–1188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.