Abstract
Helper-dependent adenoviral vectors (HDAd) are devoid of all viral coding sequences and are thus an improvement over early generation Ad because they can provide long-term transgene expression in vivo without chronic toxicity. However, high vector doses are required to achieve efficient hepatic transduction by systemic intravenous injection, and this unfortunately results in dose-dependent acute toxicity. To overcome this important obstacle, we have developed a minimally invasive method to preferentially deliver HDAd into the liver of nonhuman primates. Briefly, a balloon occlusion catheter was percutaneously positioned in the inferior vena cava to occlude hepatic venous outflow. HDAd was injected directly into the occluded liver via a percutaneously placed hepatic artery catheter. Compared to systemic vector injection, this approach resulted in substantially higher hepatic transduction efficiency using clinically relevant low vector doses and was accompanied by mild-to-moderate acute but transient toxicities. Transgene expression was sustained for up to 964 days. These results suggest that our minimally invasive method of delivery can significantly improve the vector's therapeutic index and may be a first step toward clinical application of HDAd for liver-directed gene therapy.
Introduction
The liver is an important target for gene therapy because the fenestrated endothelium permits exposure to intravenously delivered vector, hepatocytes are well suited for secretion of therapeutic proteins into the circulation for systemic delivery and the liver is the affected organ in many genetic disorders. Helper-dependent adenoviral vectors (HDAd), which are deleted of all viral genes, show tremendous potential for liver-directed gene therapy because they can mediate long-term transgene expression without chronic toxicity leading to sustained phenotypic correction of several disease models.1 For example, in rodents, a single intravenous injection of HDAd can result in life-long phenotypic correction of genetic diseases.2,3 In large animals, hepatic transduction by a single injection of HDAd results in long-term transgene expression for the duration of observation of at least 1–2 years.4,5,6,7 Hepatic transduction by HDAd is not associated with chronic toxicity because of the absence of viral gene expression.2,3,4,5,6,7 Unfortunately, to achieve efficient hepatocyte transduction by peripheral intravenous, hepatic artery (HA) or portal vein injections, high vector doses are required. Studies have shown a nonlinear dose response, with low doses yielding low to undetectable levels of transgene expression, but with higher doses resulting in disproportionately high levels of transgene expression in both mice8,9 and nonhuman primates.10,11,12 For example, in nonhuman primates, doses of ≤5 × 1011 viral particles (vp)/kg resulted in no detectable hepatic transduction11 and a dose of 1 × 1012 vp/kg resulted in <1% hepatic transduction.11,12 In humans, a dose range of 2 × 109 to 6 × 1011 vp/kg was administered in ornithine transcarbamylase–deficient patients and hepatocyte transduction was detected in only 7 of 17 subjects.13 Moreover, the frequency of hepatocyte transduction in those seven positive samples was all <1% with no apparent dose response.13 In nonhuman primates, substantial hepatic transduction was not observed until high doses of ≥5 × 1012 vp/kg were administered.11,12,14,15 Unfortunately, these high doses resulted in dose-dependent activation of the innate immune response, characterized by increases in proinflammatory cytokines resulting in acute toxicity with potentially severe and lethal consequences in nonhuman primates and humans.11,12,14,16,17,18,19 In nonhuman primates, a dose of 1 × 1013 vp/kg is lethal, 11,12,14 while a dose of 6 × 1011 vp/kg was lethal in one of two humans.18 Although the mechanism of Ad-mediated innate immune activation remains to be fully elucidated, the severity is clearly dose-dependent.11,12,14,20 This is the major obstacle currently hindering clinical usefulness of HDAd for liver-directed gene therapy.
Considering the steep threshold for hepatocyte transduction and dose-limiting acute toxicity, strategies to achieve efficient hepatocyte transduction using clinically relevant low vector doses are needed to advance liver-directed HDAd mediated clinical gene therapy. Our first attempt to achieve this objective in baboons involved injecting HDAd directly into the surgically isolated liver following a laparotomy.5 Although that method resulted in high efficiency hepatocyte transduction, it was quite invasive. Subsequently, we developed a method to mimic hydrodynamic delivery of HDAd into baboons.7 That method also resulted in high efficiency hepatocytes transduction but was far less invasive. In this article, we describe an improved balloon occlusion catheter–based method which permits even greater hepatocyte transduction efficiency at clinically relevant low doses of 1 × 1011, 3 × 1010, and even 1 × 1010 vp/kg.
Results
Balloon occlusion catheter–based delivery of HDAd
In an attempt to achieve efficient hepatocyte transduction with low vector doses, we have developed a minimally invasive, balloon occlusion catheter–based method of delivering HDAd preferentially into the liver of baboons (Figure 1). In this method, a sausage-shaped balloon is percutaneously introduced into the inferior vena cava (IVC) and positioned so that when inflated it occludes hepatic venous outflow. Immediately upon balloon inflation, the HDAd, diluted to a volume of 5 ml saline, is injected (at a rate of 0.5 ml/15 seconds) directly into the liver via a percutaneously positioned HA catheter (Figure 1). The vector is permitted to dwell within the occluded liver for 7.5 or 15 minutes and then the balloon is deflated and the catheters are removed from the body. The 7.5 or 15 minutes IVC occlusion results in transient systemic hypotension caused by obstruction of the venous return to the heart from the lower extremities. The hypotension was successfully minimized with systemic infusion of 20 ml/kg of saline before balloon inflation and with phenylephrine titration as needed during the time of occlusion. Systemic systolic blood pressure decreased 20–50% for the majority of the baboons following balloon inflation. With the exception of baboon 16997, the procedure was uneventful, well tolerated and all animals returned to their healthy, preinjection state quickly after recovery from general anesthesia. In the case of baboon 16997, the take-off of the HA from the aorta was in an unusual position, almost at the level of the renal artery. Because of this anatomical variation and the limited equipment at the nonhuman primate facility, extensive manipulation was needed to securely cannulate the HA. As a consequence, there was considerable vasospasm, perivascular extravasation, and the occurrence of an intrahepatic perivascular hematoma. As described in Discussion, this complication would have been unlikely to occur in a fully equipped human catherization laboratory.
Figure 1.
A sausage-shaped balloon catheter is positioned in the inferior vena cava (IVC) under fluoroscopic guidance. Inflation of the balloon with contrast medium results in hepatic venous outflow occlusion from the hepatic veins (HVs). The HDAd is administered by injection through a percutaneously positioned hepatic artery (HA) catheter. Fluoroscopic image of balloon inflation with contrast medium from baboon 16855 is shown on the left. Fluoroscopic image of injection of contrast medium into the HA from baboon 11891 is shown on the right.
Level and duration of transgene expression
Using the balloon occlusion catheter–based delivery method described above, four baboons (15225, 15586, 16534, and 16432) were injected with 1 × 1011 vp/kg of HDΔ21.7E4PEPCK-bAFP-WL, a HDAd expressing the baboon α-fetoprotein (bAFP), a nontoxic, nonimmunogenic, secreted reporter protein under the control of a liver-restricted expression cassette.5 The vector was left to dwell within the occluded liver for 15 minutes for baboons 15225 and 15586 or 7.5 minutes for baboons 16534 and 16432. Table 1 summarizes the injection conditions for all baboons. Blood samples were obtained from the baboons at various times postinjection of vector to determine serum bAFP levels. All four animals in which the vector was delivered by the balloon occlusion method yielded high levels of serum bAFP which were detectable for at least 846 days for 15225 and 15586 (Figure 2a, black and white circles, respectively), at least 651 days for 16534 (Figure 2a, black squares) and at least 547 days for 16432 (Figure 2a, white squares). The baseline bAFP level, maximum bAFP level and the level of bAFP at the end of the observation period for each animal is shown in the Supplementary Table S1. An occlusion time of 15 minutes may have resulted in slighlty higher levels of transgene expression compared to 7.5 minutes, although this difference was small. To ascertain the potential improvement provided by the balloon occlusion, two controls were performed. For the first control, the same vector, at the same dose was administered to baboon 12139 by a simple peripheral intravenous injection (Figure 2a, white triangles). For another control, the same vector, at the same dose was administered to baboon 11891 by HA injection without balloon occlusion (Figure 2a, black triangles). As shown in Figure 2, the level of serum bAFP for these two animals were comparable, consistent with previous reports that no difference in hepatic transduction was observed between these two routes of vector administration.10,11 Transgene expression was detectable for at least 903 days in these two animals (Figure 2, black and white triangles). See Supplementary Table S1 for bAFP levels at baseline, peak, and at the end of the observation period. Importantly, the level of bAFP for these two animals (12139 and 11891) was approximately tenfold lower than for the four animals (15225, 15586, 16534, and 16432) that received the vector by the balloon method indicating that the balloon occlusion catheter–based method results in substantially higher efficiency hepatocyte transduction. Another two control baboons (16920 and 16728) underwent the balloon occlusion procedure with a 15 minutes dwell time as shown in Figure 1, but were injected with saline instead of vector. As expected, no increase in serum bAFP was observed for either 16920 (Figure 2a, black diamonds) or 16728 (Figure 2a, white diamonds) that underwent the balloon occlusion procedure but were injected with saline instead of vector.
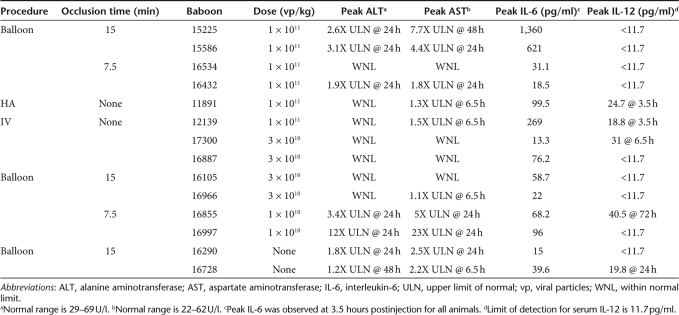
Table 1.
Summary of baboons, procedure, dose, and toxicity
Figure 2.
Transgene levels and toxicity measurements for baboons injected with 1 × 1011 viral particles (vp)/kg. (a) Serum baboon α-fetoprotein (bAFP) levels, (b) alanine aminotransferase (ALT), (c) aspartate aminotransferase (AST), and (d) interleukin-6 (IL-6) levels in baboons injected 1 × 1011 vp/kg of HDΔ21.7E4PEPCK-bAFP-WL or saline. Numbers in parenthesis represent the normal range, if known.
In considering clinical applications, administration of lower doses is critical for safety because Ad-mediated acute toxicity is dose-dependent. Therefore, we sought to evaluate the utility of the balloon catheter–based method for delivering low clinically relevant vector doses. For these experiments, 3 × 1010 vp/kg HDΔ21.7E4PEPCK-bAFP-WL was administered to two baboons (17300 and 16887) by simple systemic peripheral intravenous injections (Table 1). As expected, little to no transgene expression was detected in 17300 (Figure 3a, black circles) or 16887 (Figure 3a, white circles) because of the low dose given by this route of administration. In contrast, when the same low dose of the same vector was administered to two baboons (16105 and 16966) using the balloon occlusion catheter–based approach (Figure 3a, black and white squares, respectively), an increased level of transgene expression of up to 80-fold was observed compared to peripheral intravenous injection of 17300 and 16887. In addition, transgene expression persisted in 16105 and 16966 for the duration of the observation period of at least 964 and 963 days, respectively (Figure 3a, black and white squares). More importantly, when an even lower dose of 1 × 1010 vp/kg was administered to two baboons (16855, 16997) by the balloon occlusion catheter approach (Figure 3a, black and white triangles, respectively), the level of transgene expression was up to 30-fold higher than was observed for 17300 and 16887 given the higher 3 × 1010 vp/kg dose by peripheral intravenous injection (Figure 3a, black and white circles, respectively). As well, transgene expression in 16997 was detectable for the duration of the observation period of at least 337 days (Figure 3a, white triangles). See Supplementary Table S1 for bAFP levels at baseline, peak, and at the end of the observation period. Eleven weeks after vector injection, 16855 was found with his left arm degloved, with severe muscle trauma and infection, and exposed bone and nerve. This event was unrelated to our procedure and was a consequence of fighting with another baboon. Owing to poor condition the animal was euthanized. Nevertheless, up to this point, transgene expression was detectable for at least 57 days (Figure 3a, black triangles). Taken together, these results indicate that (i) clinically relevant low vector doses are ineffective, resulting in little to no transgene expression, when delivered by systemic peripheral intravenous injection and (ii) the balloon occlusion catheter–based method of delivering the same low dose results in substantially higher levels of persistent transgene expression.
Figure 3.
Transgene levels and toxicity measurements for baboons injected with 3 × 1010 and 1 × 1010 viral particles (vp)/kg. (a) Serum baboon α-fetoprotein (bAFP) levels, (b) alanine aminotransferase (ALT), (c) aspartate aminotransferase (AST), and (d) interleukin-6 (IL-6) levels in baboons injected 3 × 1010 or 1 × 1010 vp/kg of HDΔ21.7E4PEPCK-bAFP-WL. Numbers in parenthesis represent the normal range, if known.
Toxicity
Ad vectors cause hepatotoxicity and therefore we assessed aspartate aminotransferase (AST) and alanine aminotransferase (ALT) at various times after vector injection. Acute elevation in AST and ALT was observed in all animals (Figures 2b,c and 3b,c) and the peak levels are summarized in Table 1. With one exception (16997), all elevations were mild to moderate. In contrast, 16997 had severe elevations in AST (Figure 3b, white triangles) and ALT (Figure 3c, white triangle) which may have been related to the procedural complications mentioned above (addressed below). For all animals, including 16997, ALT and AST elevations were transient, returning toward the normal range by 48–72 hours after injection (Figures 2b,c and 3b,c). Based on the AST and ALT data presented in Figures 2 and 3 and Table 1, the following general observations were made: there did not appear to be an obvious correlation between vector dose and the magnitude of transaminase elevation, at least not at the relatively low doses used. In general, there did not appear to be an obvious correlation between 15 minutes versus 7.5 minutes of balloon occlusion and the magnitude of transaminase elevation. However, there appeared to be a correlation between balloon occlusion, regardless of whether it was 15 or 7.5 minutes, and the magnitude of transaminase elevation. Therefore, we conclude that balloon occlusion itself is responsible, at least in part, for the increase in AST and ALT. As described above, extensive manipulation was required to cannulate the HA of 16997. This was due to an unusual anatomical variation of the HA and limited equipment at the baboon facility. This combination led to extensive and unnecessary manipulation, vasospasm, extravasation, and the development of a transient intrahepatic perivascular hematoma. This complication is the most likely cause of the high AST and ALT levels in 16997 and as described in Discussion, would be unlikely to occur with the equipment normally available in a well equipped human catheterization laboratory.
Ad vectors are known to induce an acute innate inflammatory response characterized by an elevation of serum interleukin-6 (IL-6) and IL-12.14 Therefore, these proinflammatory cytokines were measured at various times after vector injection and presented in Figures 2d and 3d, and the peak levels are summarized in Table 1. Assessment of IL-6 revealed an acute elevation with peak levels observed at 3.5 hours after vector injection. In general, all animals that received vector had higher levels of IL-6 than 16920 and 16728 (Figure 2d, black and white diamond, respectively) that underwent the balloon procedure but received saline instead of vector. Therefore, elevations in IL-6 were mainly attributed to the vector. Nevertheless, the peak levels of IL-6 were mild to modest for all vector-injected animals, and more importantly, all were transient, returning toward baseline by 6.5 hours after injection. Serum IL-12 was acutely elevated in less than half of the vector-injected baboons and in one of two mock-injected baboons (Table 1). In all cases, IL-12 elevations were very mild, transient, and did not appear to correlate with vector dose or the balloon procedure.
Thrombocytopenia is a known adverse side effect of Ad.21 However, platelet counts remained within the normal range for all animals at all time points (Supplementary Figure S1). We attribute this to the low vector doses injected.
Histopathology
Liver samples were obtained at 7 or 8 days and at 28 days postprocedure by needle biopsy from the baboons that underwent the balloon procedure but were injected with saline instead of vector (16290 and 16728) for hematoxylin and eosin histology to determine whether the balloon procedure was associated with hepatic abnormalities. 16290 showed few (0–2) small foci of lymphocytic infiltrates per lobule at 8 days (Figure 4a) and only one small focus of portal triaditis at 28 days postprocedure (Figure 4b). 16728 showed normal histology at 7 days (Figure 4c) and rare foci (averaging <1 per lobule) of lymphocytic infiltration at 28 days (Figure 4d). The microscopic analysis of liver biopsy samples obtained 28 days postprocedure from animals that received vector (17300, 16887, 16855, and 16997) revealed no abnormalities (data not shown). 16105 showed an infiltrate of lymphocytes in one portal tract, minimal lobular inflammation, and only one small focus of steatosis at day 28 (Figure 4e). 16966 showed multiple small foci of portal lymphocytes, and rare lobular foci at day 28 (Figure 4f). It is difficult to determine the cause or significance of these minor histological abnormalities because there is no obvious correlation with the balloon procedure, the vector dose or the laboratory findings. Nevertheless, the histological abnormalities are rare and minor and we consider them clinically insignificant.
Figure 4.
Histopathology of baboon livers. Hematoxylin and eosin histology of liver from baboon 16290 at (a) day 8 and (b) day 28, baboon 16728 at (c) day 7 and (d) day 28, (e) baboon 16105 at day 28 and (f) baboon 16966 at day 28 showing rare and minor abnormalities.
Discussion
We have developed a balloon occlusion catheter approach to deliver HDAd into the liver of nonhuman primates. Using this approach we have demonstrated that robust levels of transgene expression can be achieved with clinically low vector doses. In contrast, the same vector at the same dose administered systemically by peripheral vein or HA yielded no detectable transgene expression. Significantly, the doses used here, 1 × 1011, 3 × 1010, and 1 × 1010 vp/kg are all well below the lethal dose of 6 × 1011 vp/kg used in the OTC gene therapy trial.13,18 We believe that these low doses are clinically relevant, given that 2 × 1011 vp/kg was relatively well tolerated in the ornithine transcarbamylase trial.13
The animals in this study showed acute and transient transaminase elevation, which for all animals, except 16997, were relatively mild to moderate. The severity of these abnormalities did not correlate with length of occlusion (7.5 or 15 minutes) or vector dose, but there was evidence that it was related to the balloon procedure itself instead of the vector. We believe that this variability is simply because of variable responses of the animals to the procedure and unavoidable variability in the procedure itself. Nevertheless, with the exception of 16997, the severity of AST and ALT elevations were mild to moderate and all were transient. The balloon procedure is minimally invasive, well tolerated and therefore potentially acceptable in certain risk-benefit settings. An acute elevation in the proinflammatory serum cytokine IL-6 was observed and this appeared to be related to the vector rather than the balloon procedure. However, IL-6 elevation was relatively mild and transient, returning quickly to undetectable levels. In contrast to the other animals, 16997 had more severe elevations in AST and ALT which we attribute to the intrahepatic perivascular hematoma caused by the extensive manipulation as a consequence of the unusual HA anatomy and the limited fluoroscopic and expendable equipment at the nonhuman primate facility. It is important to emphasize that this complication would be highly unlikely in clinical application because modern, human catheterization laboratories possess biplane imaging with digital recording of any angiography and with instantaneous biplane replay and “freeze frame” capabilities to produce a “road map” in three dimensions of the vessel being cannulated. In contrast, the 15-year-old fluoroscopy equipment available at the nonhuman primate facility is obsolete, with a fixed single plane position, with no freeze frame or replay capability and overall marginal image quality. Furthermore, there would be an extensive inventory of expendable supplies (catheters, wires, etc.) in a human catheterization laboratory to permit selection of the optimal size and/or shaped catheter or a different type wire to accommodate all unusual circumstances. In contrast, the nonhuman primate facility, which is not set up as a cardiac laboratory, understandably has a very limited selection of expendable catheterization supplies. Thus, the Baylor investigators brought most of the supplies needed to perform the procedure to the nonhuman primate facility. However, for practical reasons, the amount and selection of these supplies was necessarily limited. Consequently, we were obliged to use whatever was brought with us, which, in this particular case, was not ideal.
We have previously published a pseudo-hydrodynamic method of HDAd administration. In that method, two spherical balloons are inflated in the IVC, one above and one below the hepatic veins to occlude hepatic venous outflow. Following 30 minutes of occlusion, the balloons were deflated and the vector was administered by peripheral intravenous injection. An increase in transgene expression using pseudo-hydrodynamic injection was attributed to an increase in intrahepatic pressure generated during hepatic venous outflow occlusion.7 This same increase in intrahepatic pressure is observed in our current method and almost certainly contributes to the observed increase in transgene expression as compared to systemic vector injection. However, compared to the pseudo-hydrodynamic method, our current balloon occlusion method resulted in four- to sevenfold higher transgene expression using the same vector at the same dose. We attribute this increase to direct injection of the vector into the liver vasculature by HA injection and dwell time within the occluded liver, thus prolonging potential vector-hepatocyte contact.
One undesirable side effect of IVC occlusion is the transient hypotension caused by obstruction of the venous return to the heart which was observed in the majority of the baboons. This potential complication has also been reported in humans during IVC occlusion for regional chemotherapy22 although at a reduced incidence compared to baboons; only 3 of 10 patients experienced hypotension. The lack of hypotension in these seven patients may have been due to the presence of a collateral venous system returning blood from the abdomen to the chest (which would not affect the efficacy of our procedure because hepatic venous outflow would remain obstructed) and this may be more prevalent in humans than in baboons. We observed no obvious difference between 7.5 or 15 minutes of occlusion in terms of transgene expression, laboratory abnormalities or histopathology. However, for potential clinical application, 7.5 minutes would be preferred to reduce the period of hypotension and length of time under anesthesia. However, it is important to note that the duration of hypotension is short, caused no other acute abnormalities (arrhythmias, renal or clinically measurable central nervous system damage) is completely and immediately reversible upon balloon deflation, and the baboons appear to have suffered no ill long-term effects. Whether this potential transient hypotension will be clinically acceptable will be dictated by the risk:benefit ratio.
In summary, we have developed a clinically attractive method for efficient targeting of low dose HDAd into the liver of nonhuman primates. This method resulted in higher efficiency hepatic transduction as compared to systemic injections. Although acute elevations in liver enzymes were observed, they were all mild and transient, and they were also observed in the mock-injected animals. Given the minimal toxicity and long-term transgene expression, this approach is extremely encouraging for clinical gene therapy applications for liver diseases.
Materials and Methods
HDAds. The HDAd, HDΔ21.7E4PEPCK-bAFP-WL, contains a liver-restricted bAFP expression cassette and has been described previously.5 HDAd was produced in 116 cells23 with the helper virus AdNG16324 as described elsewhere.23 Helper virus contamination levels were determined as described elsewhere23 and were found to be <0.05%. DNA analyses of HDAd genomic structure was confirmed as described elsewhere.23 All vector preparations were tested using Multi-test Limulus Amebocyte Lysate (Pyrogent; BioWhittaker, Walkersville, MD) for the presence of endotoxin and were found to be below the limit of detection (endotoxin <0.5 EU/ml).
Nonhuman primates studies. Adult male baboons (Papio sp.) between 22 and 34 kg were used. Blood was collected from all animals as described previously7 before vector injection for blood cell counts, blood chemistries and serum cytokines to establish baseline levels. Serum levels of neutralizing anti-Ad5 antibodies were determined preinjection as described elsewhere25 and were undetectable for all animals. Blood was collected at 1, 3, 6, 24, and 72 hours postvector, then weekly for the first month and monthly thereafter for blood cell counts, blood chemistries, serum cytokines, and to determine serum bAFP levels. Serum IL-6 concentrations were determined by Specialty Laboratories (Santa Monica, CA). IL-12 was measured by enzyme-linked immunosorbent assay according to manufacturer's instructions (BioSource, Camarillo, CA). Serum bAFP was measured as previously reported.5 Needle biopsies of the liver were taken from some baboons at 7 or 8 days and/or 28 days postvector for hematoxylin and eosin histology.
Balloon catheter delivery. A 4 French sheath was placed in the right femoral vein, an 11 French sheath in the left femoral vein and a 4 French sheath in the left femoral artery by standard sterile percutaneous technique. A 20-gauge arterial catheter was placed in the femoral artery for continuous blood pressure monitoring during the procedure. The 8 × 3 cm2 balloon occlusion catheter was custom made by NuMED (Hopkinton, NY) and was introduced into the right femoral vein sheath and positioned in the IVC with its tip just within the IVC-right arterial junction. The balloon was then inflated with saline diluted contrast solution. Positioning of the balloon was confirmed by injection of a small amount of contrast solution into an intrahepatic vein with the balloon catheter inflated demonstrating complete occlusion of the hepatic venous outflow while monitored on fluoroscopy. Saline of 20 ml/kg was injected systemically before balloon inflation to minimize hypotension. The baboons were given phenylephrine by titration as needed during the occlusion time to further minimize the hypotension. Immediately after balloon inflation, HDAd, in 5 ml saline, was injected at a rate of 0.5 ml/15 seconds through a catheter previously placed percutaneously in the HA, and the balloon was deflated 15 or 7.5 minutes after inflation. The baboons were given 50 mg/kg of heparin just before balloon inflation and 2–4 mg of protamine sulfate was given to neutralize the effect of the heparin at the end of the procedure. Hemostasis was achieved by prolonged manual compression over the puncture sites (15–45 minutes) after the catheter/balloon/sheath removal.
Supplementary MaterialTable S1. Serum bAFP levels at baseline, peak and at the end of the observation period.Figure S1. Platelet counts from baboons injected with (A) 1 x 1011 vp/kg or (B) 3 x 1010 vp/kg or 1 x 1010 vp/kg of HDΔ21.7E4PEPCK-bAFP-WL. Numbers in parenthesis represent the normal range.
Supplementary Material
Serum bAFP levels at baseline, peak and at the end of the observation period.
Platelet counts from baboons injected with (A) 1 x 1011 vp/kg or (B) 3 x 1010 vp/kg or 1 x 1010 vp/kg of HDΔ21.7E4PEPCK-bAFP-WL. Numbers in parenthesis represent the normal range.
Acknowledgments
This work was supported by the National Institutes of Health Grants P50 HL59314 (A.L.B.), R01 DK067324 (P.N.), K99 DK077447 (N.B.-P.), the Texas Affiliate of the American Heart Association 0765032Y (N.B.-P.), and the Texas Digestive Disease Core Pilot/Project award P30DK056338 (N.B.-P.). We thank the Morphology Core Laboratory of the Gulf Coast Digestive Disease Center and Angela Major for the liver hematoxylin and eosin histology. We also thank Allen Tower and Doug Villnave of NuMED for expeditiously developing and supplying the balloon catheters.
REFERENCES
- Brunetti-Pierri N., and , Ng P. Progress towards the clinical application of helper-dependent adenoviral vectors for liver and lung gene therapy. Curr Opin Mol Ther. 2006;8:446–454. [PubMed] [Google Scholar]
- Kim IH, Jozkowicz A, Piedra PA, Oka K., and , Chan L. Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci USA. 2001;98:13282–13287. doi: 10.1073/pnas.241506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toietta G, Mane VP, Norona WS, Finegold MJ, Ng P, McDonagh AF, et al. Lifelong elimination of hyperbilirubinemia in the Gunn rat with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci USA. 2005;102:3930–3935. doi: 10.1073/pnas.0500930102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Nichols TC, McCorquodale S, Merricks E, Palmer DJ, Beaudet AL, et al. Sustained phenotypic correction of canine hemophilia B after systemic administration of helper-dependent adenoviral vector. Hum Gene Ther. 2005;16:811–820. doi: 10.1089/hum.2005.16.811. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Ng T, Iannitti DA, Palmer DJ, Beaudet AL, Finegold MJ, et al. Improved hepatic transduction, reduced systemic vector dissemination, and long-term transgene expression by delivering helper-dependent adenoviral vectors into the surgically isolated liver of nonhuman primates. Hum Gene Ther. 2006;17:391–404. doi: 10.1089/hum.2006.17.391. [DOI] [PubMed] [Google Scholar]
- Morral N, O'Neal W, Rice K, Leland M, Kaplan J, Piedra PA, et al. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci USA. 1999;96:12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Stapleton GE, Palmer DJ, Zuo Y, Mane VP, Finegold MJ, et al. Pseudo-hydrodynamic delivery of helper-dependent adenoviral vectors into non-human primates for liver-directed gene therapy. Mol Ther. 2007;15:732–740. doi: 10.1038/sj.mt.6300102. [DOI] [PubMed] [Google Scholar]
- Tao N, Gao GP, Parr M, Johnston J, Baradet T, Wilson JM, et al. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol Ther. 2001;3:28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- Schiedner G, Hertel S, Johnston M, Dries V, van Rooijen N, Kochanek S. Selective depletion or blockade of Kupffer cells leads to enhanced and prolonged hepatic transgene expression using high-capacity adenoviral vectors. Mol Ther. 2003;7:35–43. doi: 10.1016/s1525-0016(02)00017-5. [DOI] [PubMed] [Google Scholar]
- Sullivan DE, Dash S, Du H, Hiramatsu N, Aydin F, Kolls J, et al. Liver-directed gene transfer in non-human primates. Hum Gene Ther. 1997;8:1195–1206. doi: 10.1089/hum.1997.8.10-1195. [DOI] [PubMed] [Google Scholar]
- Nunes FA, Furth EE, Wilson JM., and , Raper SE. Gene transfer into the liver of nonhuman primates with E1-deleted recombinant adenoviral vectors: safety of readministration. Hum Gene Ther. 1999;10:2515–2526. doi: 10.1089/10430349950016852. [DOI] [PubMed] [Google Scholar]
- Morral N, O'Neal WK, Rice K, Leland MM, Piedra PA, Aguilar-Cordova E, et al. Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons. Hum Gene Ther. 2002;13:143–154. doi: 10.1089/10430340152712692. [DOI] [PubMed] [Google Scholar]
- Raper SE, Yudkoff M, Chirmule N, Gao GP, Nunes F, Haskal ZJ, et al. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum Gene Ther. 2002;13:163–175. doi: 10.1089/10430340152712719. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Palmer DJ, Beaudet AL, Carey KD, Finegold M., and , Ng P. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum Gene Ther. 2004;15:35–46. doi: 10.1089/10430340460732445. [DOI] [PubMed] [Google Scholar]
- Varnavski AN, Zhang Y, Schnell M, Tazelaar J, Louboutin JP, Yu QC, et al. Preexisting immunity to adenovirus in rhesus monkeys fails to prevent vector-induced toxicity. J Virol. 2002;76:5711–5719. doi: 10.1128/JVI.76.11.5711-5719.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruve DA, Cotter MJ, Zaiss AK, White LR, Liu Q, Chan T, et al. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J Virol. 2004;78:5966–5972. doi: 10.1128/JVI.78.11.5966-5972.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell MA, Zhang Y, Tazelaar J, Gao GP, Yu QC, Qian R, et al. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol Ther. 2001;3:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Muruve DA, Barnes MJ, Stillman IE., and , Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chirmule N, Gao GP, Qian R, Croyle M, Joshi B, et al. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]
- O'Neal WK, Zhou H, Morral N, Aguilar-Cordova E, Pestaner J, Langston C, et al. Toxicological comparison of E2a-deleted and first-generation adenoviral vectors expressing alpha1-antitrypsin after systemic delivery. Hum Gene Ther. 1998;9:1587–1598. doi: 10.1089/hum.1998.9.11-1587. [DOI] [PubMed] [Google Scholar]
- Berkenstadt H, Ben-Ari G., and , Perel A. Hemodynamic changes during a new procedure for regional chemotherapy involving occlusion of the thoracic aorta and inferior vena cava. J Clin Anesth. 1998;10:636–640. doi: 10.1016/s0952-8180(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Palmer D., and , Ng P. Improved system for helper-dependent adenoviral vector production. Mol Ther. 2003;8:846–852. doi: 10.1016/j.ymthe.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Palmer DJ., and , Ng P. Physical and infectious titers of helper-dependent adenoviral vectors: a method of direct comparison to the adenovirus reference material. Mol Ther. 2004;10:792–798. doi: 10.1016/j.ymthe.2004.06.1013. [DOI] [PubMed] [Google Scholar]
- Croyle MA, Chirmule N, Zhang Y., and , Wilson JM. “Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J Virol. 2001;75:4792–4801. doi: 10.1128/JVI.75.10.4792-4801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serum bAFP levels at baseline, peak and at the end of the observation period.
Platelet counts from baboons injected with (A) 1 x 1011 vp/kg or (B) 3 x 1010 vp/kg or 1 x 1010 vp/kg of HDΔ21.7E4PEPCK-bAFP-WL. Numbers in parenthesis represent the normal range.