Abstract
Gene transfer to the retina using recombinant adeno-associated viral (rAAV) vectors has proven to be an effective option for the treatment of retinal degenerative diseases in several animal models and has recently advanced into clinical trials in humans. To date, intracellular trafficking of AAV vectors and subsequent capsid degradation has been studied only in vitro, but the fate of AAV particles in transduced cells following subretinal injection has yet to be elucidated. Using electron microscopy and western blot, we analyzed retinas of one primate and four dogs that had been subretinally injected with AAV2/4, -2/5, or -2/2 serotypes and that displayed efficient gene transfer over several years. We show that intact AAV particles are still present in retinal cells, for up to 6 years after successful gene transfer in these large animals. The persistence of intact vector particles in the target organ, several years postadministration, is totally unexpected and, therefore, represents a new and unanticipated safety issue to consider at a time when gene therapy clinical trials raise new immunological concerns.
Introduction
Recombinant adeno-associated viral (rAAV) vectors, which are among the most efficient vehicles for prolonged gene transfer, have been used to deliver therapeutic genes to correct defects in animal models of various human disorders. Proof of principle for gene replacement therapy using rAAV in several animal models of retinal degeneration, including the RPE65–/– dogs,1,2,3 has been shown, and the first clinical trials for this type of therapy has recently started in the United Kingdom and in the United States.4,5,6
The manner by which AAVs enter and persist in host cells has been extensively studied in vitro, and can be divided into several steps, including cellular uptake, endosomal trafficking, endosomal escape, capsid degradation, and nuclear transfer of viral DNA.
AAV particles interact with the cell surface in a receptor-mediated fashion. These receptors are serotype specific and can be omnipresent or specific for a certain cell type. The main receptor for AAV2 is heparan sulfate proteoglycan, which is present on a variety of different cell types and explains in part the broad host range of this serotype.7 Three coreceptors have been identified for AAV2 so far, αVβ5 integrin,8 human fibroblast growth factor receptor,9 and hepatocyte growth factor receptor.10 In addition, several receptors have been found for other serotypes, including 2,3 O-linked sialic acid as coreceptor for AAV4 (ref. 11), and 2,3 N-linked sialic acid and platelet-derived growth factor receptor-α as coreceptors for AAV5 (refs. 11,12,13). The 2,3 and 2,6 N-linked sialic acids are the coreceptors for AAV serotypes 1 and 6 (ref. 14) and the 37/67 kDa laminin receptor was shown to be the main receptor for AAV8 and plays a role in the cellular uptake of serotypes 2, 3, and 9 (ref. 15).
AAV2 and AAV5 particles enter cultured cells by clathrin-mediated endocytosis,16 and are found in HeLa cells in early endosomes immediately after entry. These cellular compartments traffic through the cytoplasm and rapidly approach a perinuclear location, where they mature into late endosomes. In contrast to AAV2, serotype 5 capsids, in addition to being found in the endosomes, can also be found in the trans-Golgi apparatus, indicating differences in endosomal trafficking between serotypes.17 Moreover, it has been shown that intracellular trafficking of AAV2 particles is different in a LN229 glioma cell line compared to HeLa cells, demonstrating that trafficking of AAV particles is dependent on the cell type studied.18 Experiments on lung epithelial cells showed that endosomal processing of AAV2 particles is impaired when cellular uptake happens on the apical surface,19 a mechanism that was not observed when using AAV5 particles.20
In cell lines such as HeLa, 293 HEK, or HepG2, the endosomal compartments are acidified during intracellular translocation,21,22 a maturation step that exposes VP1/VP2 N-terminal sequences containing nuclear localization signals and a pospholipase A2 domain.23,24,25 These changes seem to be necessary for the endosomal escape of AAV particles.
Studies on HeLa and 293 HEK cell lines and on airway epithelia revealed that having reached perinuclear localization, capsids from serotypes 2, 5, 7, and 8 are ubiquitinylated and directed to the proteasome, the cellular protein degradation machinery.26,27,28 Indeed, blocking proteasomal degradation increases transduction efficiency of AAV2 and 5 vectors,21,29 which supports the hypothesis that capsid degradation happens before or during nuclear entry. Recently, epidermal growth factor receptor protein tyrosine kinase has been identified as a potential inhibitor of ubiquitinylation of AAV2 capsids, which in turn facilitates nuclear transport by limiting proteasome-mediated degradation of AAV vectors.30
However, little is known about this crucial step during infection, the transfer of viral genome across the nuclear envelope. Some groups claim that the whole capsid enters the nucleus,31,32 whereas other groups state that capsid uncoating happens in the cytoplasm and only the viral DNA enters the nucleus.33,34 Interestingly, intranuclearly injected antibodies specifically targeting the viral capsid abolished transduction of HeLa and 293 HEK by AAV2, supporting the hypothesis that entire particles enter the nucleus prior to uncoating.24 Because the mechanisms of nuclear entry and capsid degradation are still unclear, the question of whether all AAV particles are degraded or not following transduction of a cell has yet to be answered in vivo.
The purpose of this study was to evaluate, several years after a single vector administration, the status of AAV particles in the retina of dogs and primates that successfully underwent gene transfer experiments. Using transmission electron microscopy (EM), including direct observation and immunogold labeling, and western blot analysis, we performed screenings of retinas from one primate and four dogs, following subretinal injection of AAV serotypes 4, 5, and 2.
Results
Detection of AAV-like particles by EM in the retina of dogs and nonhuman primates several years post-subretinal administration
The macaque designated P1 was subretinally injected in the right eye with the AAV2/4.hRPE65.TetOn.epo vector (1 × 1010 particles, Table 1), which displayed regulated EPO expression upon doxycycline induction over the duration of the experiment,35 and was killed 2.5 years postvector administration. Semithin sections of both eyes were stained with toluidin blue (Figure 1a) and ultrathin sections were screened for the presence of viral particles by transmission EM (Figure 1b–f).
Table 1.
Detection of AAV particles by electron microscopy and western blot
Figure 1.
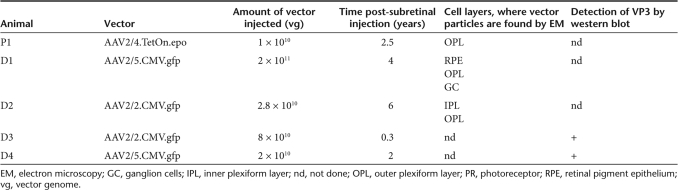
Detection of adeno-associated viral (AAV) particles in the treated retina of P1. (a) Semithin section of the right, injected retina. Bar = 200 µm. (b–e) Ultrathin sections of the right, injected retina at different magnifications. (b) Portion of the retina, where viral particles were found (indicated by an arrow). Bar = 20 µm. (c) Magnification of the area indicated in b. The arrow marks a synaptic ribbon structure. The squares indicate the location of the images in d and e. Bar = 1 µm. (d) Vector particles are found inside of synaptic invaginations. Bar = 200 nm. (e) Vector particles are found outside of synaptic invaginations, within the photoreceptor. Asterisks mark synaptic vesicles with a medium diameter of 50 nm. Bar = 200 nm. The insert represents a magnification to identify the hexagonal structure and the size of the particles of 21 nm. Bar = 100 nm. (f) Ultrathin section of the left, noninjected retina. The arrow marks a synaptic ribbon structure. Asterisks mark synaptic vesicles with a medium diameter of 50 nm. Bar = 200 nm. (g) Schematic representation of the anatomic structures within rod spherule and cone pedicule. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer (photoreceptor nuclei); IS, inner segments (of photoreceptors); OS, outer segments (of photoreceptors); RPE, retinal pigment epithelium; SR, synaptic ribbon; SI, synaptic invagination.
Electron-dense particles were observed in the injected area of the right eye within the outer plexiform layer (OPL) of the retina (Figure 1b–e). The size (21 nm) and a typical hexagonal shape similar to viral particles supported their identification as AAV particles (Figure 1e, inset). The majority of AAV particles was found in closed compartments (Figure 1c,d). The presence of synaptic ribbon structures (arrow in Figure 1c) confirms that these compartments are synaptic invaginations of horizontal and bipolar cells into either the rod photoreceptor spherule or the cone photoreceptor pedicule (Figure 1g). Viral particles can be found within and outside of these invaginations, indicating that they localize both pre- and postsynapticly within photoreceptors and in horizontal and bipolar cells. AAV particles were not detected in any other retinal layer including the retinal pigment epithelium (RPE).
Screenings of ultrathin retinal slices from the contralateral noninjected eye did not reveal the presence of AAV particles in the OPL or in any other layer of the retina (Figure 1f).
To further elucidate the identity of the observed AAV particles, we performed immunogold labeling on ultrathin sections from the same epon blocks of P1. For this purpose, we used the monoclonal mouse anti-AAV4 capsid protein antibody (ADK4) that specifically reacts with intact AAV4 particles. ADK4 recognizes a conformational epitope of assembled capsids, not present in denatured capsid proteins and native but unassembled capsid proteins.36 To verify the capacity of ADK4 to detect AAV4 particles using immunogold labeling, it was first tested on the injected vector suspension (Figure 2a,b).
Figure 2.
Identification of adeno-associated viral (AAV)4 particles in the treated retina of P1 by immunogold labeling. (a) AAV4 particles of the injected vector suspension observed by direct electron microscopy. (b) AAV4 particles of the vector suspension detected by immunogold labeling using mouse monoclonal anti-AAV4 antibody ADK4 and goat antimouse antibodies conjugated to 6 nm gold particles. Bar = 50 nm. (c) Epon section of P1 showing the OPL area. The square indicates the area highlighted in d. Bar = 500 nm. (d) Magnification of the area indicated in c. Vector particles are marked with the conjugated gold particles similar to b. Bar = 100 nm.
On epon sections of P1, AAV4 immunogold-labeled particles were detected in the OPL, confirming the direct observation of the EM screening (Figure 2c,d). The relatively low number of marked particles in the immunogold labeling experiment compared to the EM screening is due to the processing technique of the retinal tissue, as glutaraldehyde fixation and epon embedding provides very good ultrastructural preservation and vector particle visualization but causes also a significantly reduced antigenicity of any given epitope.37 No AAV4 particles were detected in the contralateral noninjected eye of P1 (data not shown).
Following these intriguing results, we wanted to know whether similar observations could be made for other AAV serotypes, especially the clinically relevant serotypes 5 and 2.
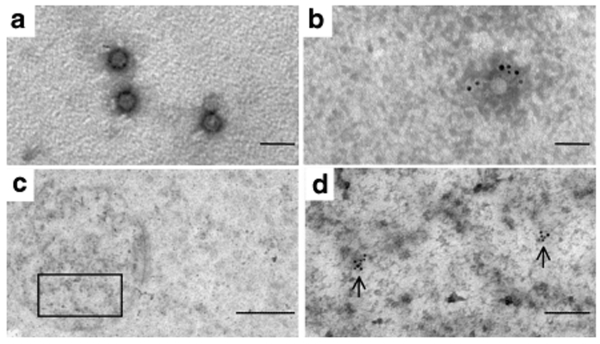
Two dogs, D1 and D2, which had been subretinally injected in the right eye with rAAV2/5.CMV.gfp and rAAV2/2.CMV.gfp, and which displayed stable GFP expression over time,38 were killed 4 and 6 years postvector administration, respectively. The total amount of vector particles injected was 2 × 1011 and 2.8 × 1010 particles for AAV2/5 and AAV2/2, respectively (Table 1). Both eyes were processed and analyzed by direct EM observation similarly to the primate eyes. Ultrathin sections of the retinas revealed electron-dense particles in several layers of the treated retina of both animals (Figures 3 and 4). In D1, these particles, of the same size and shape as those observed in the macaque retina, were again found in the synaptic invaginations of horizontal and bipolar cells into rod spherules or cone pedicules (Figure 3a–c). In addition, AAV5 particles were found in the RPE (Figure 3d–f) and in ganglion cells (Figure 3g–i).
Figure 3.
Detection of adeno-associated viral (AAV) particles in the treated retina of D1. (a–c) Ultrathin sections of the portion of the retina representing the outer nuclear layer at different magnifications. (a) Portion of the retina representing the outer plexiform layer. The arrow indicates a synaptic ribbon structure. The square marks the area of higher magnification in b. Bar = 1 µm. (b) Vector particles are found inside of synaptic invaginations. Bar = 200 nm. (c) Magnification of b to identify the hexagonal structure and the size of the particles of 21 nm. Bar = 100 nm. (d–f) Ultrathin sections of the portion of the retina representing the RPE at different magnifications. (d) Portion of the retina where viral particles are found (indicated by a square). Bar = 20 µm. (e) Magnification of the area indicated in d. Vector particles are found inside closed compartments (indicated by an arrow). Bar = 1 µm. (f) Magnification of e. Bar = 200 nm. (g–i) Ultrathin sections of the portion of the retina representing the ganglion cells. (g) Portion of the retina where viral particles are found (indicated by a square). Bar = 20 µm. (h) Magnification of the area indicated in g. Vector particles are found inside closed compartments. Bar = 2 µm. (i) Magnification of h. Bar = 200 nm. RPE, retinal pigment epithelium; OS, outer segments (of photoreceptors); IS, inner segments (of photoreceptors); IPL, inner plexiform layer; GCL, ganglion cell layer.
Figure 4.
Detection of adeno-associated viral (AAV) particles in the treated retina of D2. (a–c) Ultrathin sections of the portion of the retina representing the INL. (a) Portion of the retina where viral particles are found (indicated by a square). Bar = 20 µm. (b) Magnification of the area indicated in a. Vector particles are found inside of cellular structures (arrow). Bar = 1 µm. (c) Magnification of b to identify the hexagonal structure and the size of the particles of 21 nm. Bar = 100 nm. (d–f) Ultrathin sections of the portion of the retina representing the OPL. (d) Portion of the retina where viral particles are found (indicated by a square). Bar = 20 µm. (e) Magnification of the area indicated in d. Vector particles are found inside closed compartments (square). Bar = 1 µm. (f) Magnification of e. Bar = 200 nm. INL, inner nuclear layer; IPL, inner plexiform layer; OPL, outer plexiform layer.
In D2, the majority of the particles were detected in the inner plexiform layer (Figure 4a–c) and a small amount of AAV2 particles was detected in the OPL (Figure 4d–f). No particles were found in the contralateral noninjected eyes of D1 and D2. For all animals, AAV particles were mainly found within the site of administration (bleb), but a few were also found outside of the injected area (data not shown).
Detection of the AAV VP3 capsid protein, by western blot analysis, in the retina of dogs and nonhuman primates several years post-subretinal administration
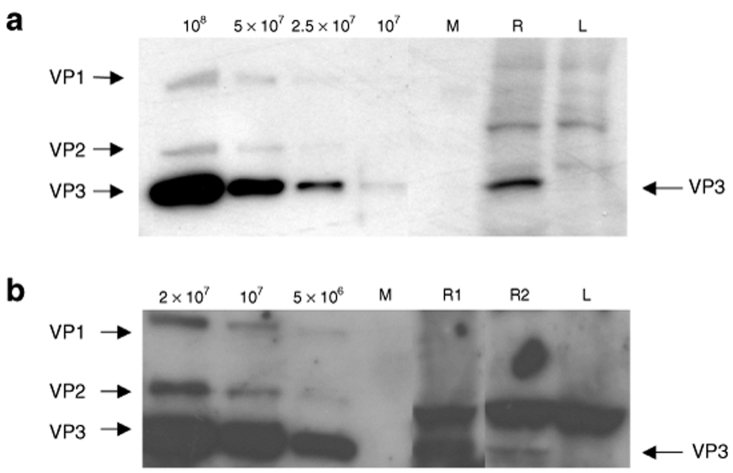
To verify the findings of the EM screening, additional retinas were analyzed by western blot using antibodies specific to the capsid proteins of AAV (VP1, VP2, and VP3). Two dogs, D3 and D4, which had been subretinally injected into the right eye with rAAV2/2.CMV.gfp and rAAV2/5.CMV.gfp, and which displayed stable GFP expression over time, were killed at either 3 months or 2 years, respectively, postvector administration. The total amount of vector injected was 8 × 1010 and 2 × 1010 particles for AAV2/2 and AAV2/5, respectively (Table 1).
Total protein was extracted from the left and the right retina, and the monoclonal antibody B1 was used to detect the AAV2 VP capsid proteins.39 The detection limit for AAV2 capsids using B1 antibody was in the range of 1 × 107 to 5 × 107 (Figure 5a). The polyclonal antibody anti-AAV VP1 + VP2 + VP3 commercialized by Progen was used to detect AAV5 capsid proteins (Progen, Heidelberg, Germany). AAV5 capsids were detected ranging from ~1 × 106 to 5 × 106 (Figure 5b). Due to the fact that the stochiometric ratio of the capsid proteins to form one icosahedral AAV capsid is 1:1:10 for VP1:VP2:VP3, the band for the VP3 protein is always the strongest on a western blot (Figure 5, left lanes). As a result, the VP3 protein could be detected by western blot in the right injected retina of both animals, D3 and D4 (Figure 5a, lane R; and Figure 5b, lanes R1, R2). In addition, in dog D4, AAV5 capsid proteins were detected inside the injected area (Figure 5b, lane R1) and, to a lesser extent, outside of the injected area (Figure 5b, lane R2). For both dogs, no VP3 protein was detected in the contralateral noninjected retina (Figure 5a,b, lane L).
Figure 5.
Detection of adeno-associated viral (AAV) VP3 capsid protein in the treated retina of D3 and D4. (a) Western blot analysis of 60 µg of total protein extracted from the right, treated retina (R) and the left, untreated retina (L) of Dog D3, which had been injected with an AAV2/2 vector. The marker lane (M) separates the sample lanes from a sensitivity assay for the antibody B1 (left). For this assay, AAV2/2 particles at different concentrations were mixed with 60 µg of total protein extracted from the retina of an untreated control animal. (b) Western blot analysis of 60 µg of total protein extracted from the right, treated retina inside the injected area (R1), and outside of the injected area (R2), and form the left, nontreated retina (L) of Dog D4, which had been injected with an AAV2/5 vector. The marker lane (M) separates the sample lanes from a sensitivity assay for the antibody Progen (left). For this assay, AAV2/5 particles at different concentration were mixed with 60 µg of total protein extracted from the retina of an untreated control animal.
These results confirm the presence of AAV particles in the subretinally injected eye several years postadministration of the vector. In accordance with what has been observed by EM, the western blot analysis suggest that most of the AAV particles detected are located within the injected area (the bleb) with fewer particles being detected outside the targeted area.
Discussion
In this study, a total of five large animals has been screened for the presence of AAV particles in the retina following several years of successful AAV-mediated retinal gene transfer. Using EM in one macaque and two dogs, AAV particles of three different serotypes (AAV4, -5, and -2, respectively) were found in retinal cells of the treated retina, mainly in neuronal synapses, up to 6 years post-subretinal injection. This has been further validated by immunogold labeling of the vector particles in the treated retina of P1 using a monoclonal antibody that specifically binds to intact AAV4 particles. These EM observations have also been confirmed by the detection of the AAV VP3 capsid protein in the rAAV2- or rAAV5-treated retinas of two dogs by using western blot analysis. These results demonstrate the persistence of intact AAV particles in the targeted retina several years after subretinal injection of the vector.
To our knowledge, this is the first report wherein intact AAV particles are shown to persist in cells in vivo several years postvector administration. This is in striking contrast to the current hypothesis, which postulates that all capsid proteins are degraded by the proteasomal machinery. In vitro studies showed that having reached perinuclear localization, capsids from serotypes 2, 5, 7, and 8 are ubiquitinylated and directed to the proteasome, the cellular protein degradation machinery.26,27,28
The presence of AAV particles in the treated retina is not likely due to unwanted AAV replication, as AAV vectors cannot replicate in animals in the absence of helper virus and virus rep/cap proteins that can propagate AAV2 ITRs. By using a replication center assay, we confirmed that the batches of rAAV used in this study were not contaminated with adenovirus or rep-positive AAV.40
Another hypothesis is that the particles detected could be wild-type AAV particles following naturally acquired infection of the animals. However, the fact that these particles were systematically observed in the injected retina and not in the noninjected control retina is an important argument against a naturally acquired infection with wt AAV.
A very interesting finding was the presence of AAV vector particles inside and, to a lesser extent, outside of the injected area. In all studies published to date on the transfer of genes into the retina using known AAV serotypes, expression of the transgene was always limited to the injected area. One exception was the recent finding by our group that AAV serotype 8 allows the expression of transgenes outside of the injected area in the retina of rats and dogs.41
The results of the present study suggest that even though transgene expression following subretinal injection of AAV serotypes 2, 4, and 5 is limited to the injected area, some of the vector particles are transported outside of the injection zone.
For all treated retinas, vector particles were found in cells that do not display expression of the transgene. For rAAV2/4 that only transduces the RPE,42 vector particles were detected in the OPL (P1) (Figure 6a). For AAV2/5 (D1) and rAAV2/2 (D2) that display expression of the transgene in both the RPE and the photoreceptors,43 viral particles were found in the RPE, in the OPL and ganglion cell layer for D1 (Figure 6b) and in the inner plexiform layer and OPL for D2 (Figure 6c). These results suggest that expression of the transgene in a specific cell does not depend only on the ability of the vector to enter into this cell but may also be conditioned by the capacity of the vector to be processed by the endocytic pathway and to deliver the viral genome into the nucleus of the cell. The presence of AAV5 particles in ganglion cells and AAV2 particles in the inner plexiform layer, which are more distant from the injection site, is somewhat difficult to explain. One hypothesis would be a transsynaptic transport from photoreceptors to these cells.
Figure 6.
Schematic drawing of the retina and depiction of the localization of the adeno-associated viral (AAV) particles found by electron microscopy. (a) Localization of AAV particles in the retina of P1, after subretinal injection of a rAAV2/4 vector. (b) Localization of AAV particles in the retina of D1 after subretinal injection of an AAV2/5 vector. (c) Localization of AAV particles in the retina of D2 after subretinal injection of an AAV2/2 vector. The red spotted circles indicate the location, where particles are found. The green circles indicate the cells in which transgene expression can be observed. IPL, inner plexiform layer; OPL, outer plexiform layer; RPE, retinal pigment epithelium.
It was previously observed that following intravitreal or subretinal injection of AAV2/2 vectors in dogs or primates, vector DNA was detected along the visual pathway in the brain.44,45 More recently, we demonstrated that following subretinal injection of an AAV2/8 vector, we observed the expression of GFP in all the cells of the neuroretina and in distal neurons of the lateral geniculate nucleus contralateral to the injected eye.41 All together, these previous observations suggested that AAV particles can travel by axonal and transsynaptic transport from the retina to the brain via the visual pathway.
In this study, the hypothesis of transsynaptic transport of vector particles in the retina is strongly supported by the presence of AAV particles presynapticly within the rod spherule and/or cone pedicule and postsynapticly in the connecting invaginations of horizontal and bipolar cells (OPL).
The persistence of virions several years post-subretinal injection may have important consequences not only for ongoing clinical trials targeting the retina, but also for other organs, as it has been generally accepted, until now, that unprocessed viral particles would be rapidly degraded. Very recently, it has been suggested that persistence of intact vector particles and expression of transgenes may occur simultaneously in the same cell as vector DNA can uncoat independently of capsid degradation.46 Regarding gene transfer to the retina, following subretinal injection, the vector is administered into a confined compartment of the eye, into the subretinal space. This unique characteristic of retinal gene transfer may have facilitated the detection of vector particles within the targeted organ. For evident anatomical reason, detection of AAV particles following gene transfer in the muscle, in the brain, or in the liver might be more challenging as the vector is rapidly diluted and disseminated within the entire organ and/or body.
In a recent clinical trial of hepatic AAV factor IX gene transfer into hemophilia B patients, transgene expression started to decline 4–6 weeks after vector administration.47 Apparently, this was caused by rejection of transduced hepatocytes by AAV capsid-specific memory CD8+ T cells reactivated by AAV vectors.48 The finding that AAV virions can persist intact in vivo raises the possibility that it could happen in immune competent cells, which in turn could potentially favor the induction and the maintenance of an immune response against the AAV capsid.
Regarding preclinical studies in the canine or primate retina, no detectable immune responses against AAV2/2 or AAV2/4 capsids leading to loss of transgene expression have been observed in large animal models,35,45,49 which could be in part explained by the immunoprivileged status of the eye. However, considering that the cytotoxic immune response against AAV capsids in the liver of humans was not observed in the preclinical studies in dogs and nonhuman primates,48,50 one cannot exclude such immune response following retinal gene transfer in humans. The three clinical trials that have started recently in the United Kingdom and the United States for gene replacement therapy as treatment of retinal dystrophy caused by defects in the RPE65 gene4,5,6 should evaluate this important issue. To date, at 12 months postvector administration, no immune response against viral capsid protein was observed in any treated patient.
In this study, we demonstrate for the first time that intact AAV particles can persist within transduced and nontransduced cells of the retina several years post-subretinal administration of the vector in primates and dogs that underwent efficient gene transfer. As the medical field deals with clearance of recombinant vectors, this discovery opens the concept of in vivo intracellular clearance as a potentially critical factor to consider for the design of gene therapy clinical trials.
Materials and Methods
Production of AAV vector. Generation of AAV2/4.TetOn.epo, AAV2/2.CMV.gfp, and AAV2/5.CMV.gfp has been described earlier.35,38 All vectors were produced as previously described51 in the Vector Core at the University Hospital of Nantes (http://www.vectors.nantes.inserm.fr), and the titer was determined by dot blot and expressed as vector genomes/ml.40 All vector batches used were characterized using a modified replication center assay.40 No infectious adenovirus or rep-positive AAV were detected.
Subretinal delivery. The beagle dogs (D1–D4) used in this study were purchased from the Centre d'Elevage du Domaine des Souches, Mezilles, France. Primate P1 was purchased from BioPrim, Baziège, France. All animals were cared for in accordance to the ARVO statement for the use of animals in ophthalmic and vision research. Injection protocols were approved by the Institutional Animal Care and Use Committee of the University of Nantes.
Surgical procedure was conducted by a transvitreal approach in the right eye as described previously by Weber et al.42
Electron microscopy. Animals were euthanized by an intravenous overdose of anesthesia, eyes were removed, and immediately fixed in 0.1 M cacodylate-buffered Karnovsky's solution (2.5% glutaraldehyde and 1% paraformaldehyde). Forty-eight hours later, the anterior chamber, lens, and vitreous were removed and the remaining retina again fixed in Karnovsky's solution for 48 hours. The retina was then postfixed in 1% osmium tertoxide (2 hours) at pH 7.3, dehydrated in graded ethanols, and embedded in the EMbed-812 epoxy resin (all reagents from Science Services, Munich/Germany, automated LYNX-Tissue processor Leica, Bensheim/Germany). After 48 hours heat polymerization at 60 °C, semithin (0.8 µm) tissue sections were cut, stained with toluidine blue and basic fuchsin and analyzed under a light microscope.
After selection of the appropriate area on the semithin section, the epon block was trimmed for ultrathin sectioning. Ultrathin (80 nm) sections were cut with a diamond knife on a Reichert Ultracut-S ultramicrotome and stained with aqueous 2% uranyl acetate and lead citrate solutions for 10 minutes each. The sections were examined in a LEO912AB electron microscope (Zeiss, Oberkochen, Germany) operating at 80 k in a zero-loss mode.
Immunogold labeling. The immunogold labeling was carried out using the automated LYNX-Tissue processor (Leica, Bensheim, Germany) in a modified negative staining approach on AAV2/4 vector stock solution or on epon sections.
A formvar-coated 400 mesh nickel grid was placed on a suspension drop containing the AAV2/4 vector for 10 minutes at room temperature. Subsequently, the grid was incubated for 1 hour with the monoclonal mouse ADK4 antibody (anti-AAV4 particles antibody, kindly provided by Jürgen Kleinschmidt, German Cancer Research Center, Heidelberg, Germany) diluted 1:2 in 1% phosphate buffered saline (PBS)/bovine serum albumin (BSA) with continuous agitation in the IGL processor. After washing, the grid was incubated for 30 minutes in 1% PBS/BSA buffer containing a 1:20 dilution of goat antimouse IgG gold conjugates (6 nm gold particles; Aurion, Wageningen, the Netherlands). An additional fixation step in 2% glutaraldehyde in PBS followed, and after washing in ddH2O the negative staining with 1% phosphotungstic acid in water for 1 minute at room temperature was performed.
The postembedding immunogold labeling was done on sections of the epon-embedded material as described above with preceding epon section etching and antigen retrieval. The pretreatment of the ultrathin sections on formvar-coated nickel grids consisted of etching with 2% sodium metaperiodate for 4 minutes at room temperature, washing, and the antigen retrieval with 10 mmol/l sodium citrate buffer at pH 6.0 for 1 hour at room temperature. After extensive washing, the grids were quenched with 1% PBS/BSA and incubated with the monoclonal mouse ADK4 antibody diluted 1:2 or 1:10 in 1% PBS/BSA. The secondary goat antimouse IgG gold conjugate (6 and 10 nm gold particles; Aurion) was applied in a 1:20 dilution for 1 hour at room temperature. Nonspecific binding was blocked by washing in PBS, followed by an additional postfixation in 2% glutaraldehyde, and the sections were finally stained with 1% aqueous uranyle acetate for 1 minute.
All immunogold-labeled sections were examined in a LEO912AB electron microscope (Zeiss, Oberkochen, Germany) operating at 80 k in a zero-loss mode.
Western blot analysis. Two dogs, D3 and D4, were euthanized by an intravenous overdose of anesthesia and the eyes were removed. The eyes were cut through the pars plana, and the anterior segment and the lens were removed. The eye cup was cut peripherally in four sections and flattened. In dog D3, the injected part of the retina was separated from the rest of the retina. Tissues were stored at −80 °C until further processing.
Retinas were homogenized by using a Polytron Homogenizer (Kinematica AG, Littau/Lucerne, Switzerland) twice for 15 seconds at 30,000 rpm. After incubation on ice for 1 hour with repeated aspirations through a syringe every 15 minutes, the sample was centrifuged at 14,000 rpm for 15 minutes at 4 °C. The supernatant was removed into a new vial, and the protein concentration was measured using a BC assay kit (Uptima, Interchim Montlucon, France).
Migration of total protein extraction was performed on 6% Tris-Glycine PAGE gels (Invitrogen, Carlsbad, CA) and proteins were transferred to a Hybond nitrocellulose membrane (Amersham, Paris, France). The membrane was stained with Ponceau Red (Simga-Aldrich, Saint-Quentin Falavier, France) to verify the correct transfer of the proteins.
Nonspecific antigen binding was blocked by overnight incubation of the membrane at 4 °C in blocking buffer (0.2% Tween/PBS, 5% milk powder) followed by a second overnight incubation at 4 °C in blocking buffer containing the following primary antibodies at the indicated dilution: B1 (monoclonal mouse anti-AAV VP proteins) at 1:20 (kindly provided by Jürgen Kleinschmidt, German Cancer Research Center, Heidelberg, Germany); Progen (polyclonal rabbit anti-AAV VP1+VP2+VP3) at 1/500 (Progen, Heidelberg, Germany). After being washed three times in 0.2%Tween/PBS for 5 minutes at room temperature, the membrane was incubated for 1 hour at room temperature with Peroxidase-conjugated goat antimouse immunoglobulin G antibody at 1:2000 (Dakocytomation #P0447, Trappes Cedex, France) or Peroxidase-conjugated donkey antirabbit immunoglobulin G antibody (H+L) at 1:2000 (Jackson 711-035-152, Charles River Laboratories, l'Arbresle Cedex, France). The membrane was again washed three times in 0.2%Tween/PBS, followed by enhanced chemiluminescence detection using the ECL western blotting substrate (Thermoscientific Rockford, IL).
Acknowledgments
We thank Matthew Ellinwood (Department of Animal Science, Iowa State University, Ames, Iowa, USA) for critical reading and editing. We thank Jürgen Kleinschmidt (German Cancer Research Center, Heidelberg, Germany) for providing the B1 and the ADK4 antibodies. We thank Beate Voll and Heiko Sigmund for outstanding technical assistance regarding electron microscopy. We also thank the Vector Core (http://www.vectors.nantes.inserm.fr) at the University of Nantes, the Association Française contre les Myopathies, the INSERM, and the Fondation pour la Thérapie Génique en Pays de la Loire.
REFERENCES
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- Narfstrom K, Katz ML, Ford M, Redmond TM, Rakoczy E., and , Bragadottir R. In vivo gene therapy in young and adult RPE65–/– dogs produces long-term visual improvement. J Hered. 2003;94:31–37. doi: 10.1093/jhered/esg015. [DOI] [PubMed] [Google Scholar]
- Le Meur G, Stieger K, Smith AJ, Weber M, Deschamps JY, Nivard D, et al. Restoration of vision in RPE65-deficient Briard dogs using an AAV serotype 4 vector that specifically targets the retinal pigmented epithelium. Gene Ther. 2007;14:292–303. doi: 10.1038/sj.gt.3302861. [DOI] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth W, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, et al. Phase I trial of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results. Hum Gene Ther. 2008;7:7. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerford C., and , Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerford C, Bartlett JS., and , Samulski RJ. αVβ5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- Qing K, Mah C, Hansen J, Zhou S, Dwarki V., and , Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- Kashiwakura Y, Tamayose K, Iwabuchi K, Hirai Y, Shimada T, Matsumoto K, et al. Hepatocyte growth factor receptor is a coreceptor for adeno-associated virus type 2 infection. J Virol. 2005;79:609–614. doi: 10.1128/JVI.79.1.609-614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaludov N, Brown KE, Walters RW, Zabner J., and , Chiorini JA. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J Virol. 2001;75:6884–6893. doi: 10.1128/JVI.75.15.6884-6893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RW, Yi SM, Keshavjee S, Brown KE, Welsh MJ, Chiorini JA, et al. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J Biol Chem. 2001;276:20610–20616. doi: 10.1074/jbc.M101559200. [DOI] [PubMed] [Google Scholar]
- Di Pasquale G, Davidson BL, Stein CS, Martins I, Scudiero D, Monks A, et al. Identification of PDGFR as a receptor for AAV-5 transduction. Nat Med. 2003;9:1306–1312. doi: 10.1038/nm929. [DOI] [PubMed] [Google Scholar]
- Wu Z, Miller E, Agbandje-McKenna M., and , Samulski RJ. α2,3 and α2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J Virol. 2006;80:9093–9103. doi: 10.1128/JVI.00895-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akache B, Grimm D, Pandey K, Yant SR, Xu H., and , Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol. 2006;80:9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JS, Wilcher R., and , Samulski RJ. Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors. J Virol. 2000;74:2777–2785. doi: 10.1128/jvi.74.6.2777-2785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantel-Schaal U, Hub B., and , Kartenbeck J. Endocytosis of adeno-associated virus type 5 leads to accumulation of virus particles in the Golgi compartment. J Virol. 2002;76:2340–2349. doi: 10.1128/jvi.76.5.2340-2349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajusola K, Gruchala M, Joch H, Luscher TF, Yla-Herttuala S., and , Bueler H. Cell-type-specific characteristics modulate the transduction efficiency of adeno-associated virus type 2 and restrain infection of endothelial cells. J Virol. 2002;76:11530–11540. doi: 10.1128/JVI.76.22.11530-11540.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Yue Y, Yan Z, McCray PB., Jr, and , Engelhardt JF. Polarity influences the efficiency of recombinant adeno-associated virus infection in differentiated airway epithelia. Hum Gene Ther. 1998;9:2761–2776. doi: 10.1089/hum.1998.9.18-2761. [DOI] [PubMed] [Google Scholar]
- Zabner J, Seiler M, Walters R, Kotin RM, Fulgeras W, Davidson BL, et al. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J Virol. 2000;74:3852–3858. doi: 10.1128/jvi.74.8.3852-3858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douar AM, Poulard K, Stockholm D., and , Danos O. Intracellular trafficking of adeno-associated virus vectors: routing to the late endosomal compartment and proteasome degradation. J Virol. 2001;75:1824–1833. doi: 10.1128/JVI.75.4.1824-1833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleker S, Sonntag F., and , Kleinschmidt JA. Mutational analysis of narrow pores at the fivefold symmetry axes of adeno-associated virus type 2 capsids reveals a dual role in genome packaging and activation of phospholipase A2 activity. J Virol. 2005;79:2528–2540. doi: 10.1128/JVI.79.4.2528-2540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girod A, Wobus CE, Zadori Z, Ried M, Leike K, Tijssen P, et al. The VP1 capsid protein of adeno-associated virus type 2 is carrying a phospholipase A2 domain required for virus infectivity. J Gen Virol. 2002;83:973–978. doi: 10.1099/0022-1317-83-5-973. [DOI] [PubMed] [Google Scholar]
- Sonntag F, Bleker S, Leuchs B, Fischer R., and , Kleinschmidt JA. Adeno-associated virus type 2 capsids with externalized VP1/VP2 trafficking domains are generated prior to passage through the cytoplasm and are maintained until uncoating occurs in the nucleus. J Virol. 2006;80:11040–11054. doi: 10.1128/JVI.01056-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger JC, Snowdy S., and , Samulski RJ. Separate basic region motifs within the adeno-associated virus capsid proteins are essential for infectivity and assembly. J Virol. 2006;80:5199–5210. doi: 10.1128/JVI.02723-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Zak R, Luxton GW, Ritchie TC, Bantel-Schaal U., and , Engelhardt JF. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J Virol. 2002;76:2043–2053. doi: 10.1128/jvi.76.5.2043-2053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Zhang L, Yan Z., and , Engelhardt JF. Intracellular trafficking of adeno-associated viral vectors. Gene Ther. 2005;12:873–880. doi: 10.1038/sj.gt.3302527. [DOI] [PubMed] [Google Scholar]
- Denby L, Nicklin SA., and , Baker AH. Adeno-associated virus (AAV)-7 and -8 poorly transduce vascular endothelial cells and are sensitive to proteasomal degradation. Gene Ther. 2005;12:1534–1538. doi: 10.1038/sj.gt.3302564. [DOI] [PubMed] [Google Scholar]
- Yan Z, Zak R, Zhang Y, Ding W, Godwin S, Munson K, et al. Distinct classes of proteasome-modulating agents cooperatively augment recombinant adeno-associated virus type 2 and type 5-mediated transduction from the apical surfaces of human airway epithelia. J Virol. 2004;78:2863–2874. doi: 10.1128/JVI.78.6.2863-2874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Zhao W, Wu J, Li B, Zolotukhin S, Govindasamy L, et al. A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol Ther. 2007;15:1323–1330. doi: 10.1038/sj.mt.6300170. [DOI] [PubMed] [Google Scholar]
- Sanlioglu S, Benson PK, Yang J, Atkinson EM, Reynolds T., and , Engelhardt JF. Endocytosis and nuclear trafficking of adeno-associated virus type 2 are controlled by rac1 and phosphatidylinositol-3 kinase activation. J Virol. 2000;74:9184–9196. doi: 10.1128/jvi.74.19.9184-9196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seisenberger G, Ried MU, Endress T, Buning H, Hallek M., and , Brauchle C. Real-time single-molecule imaging of the infection pathway of an adeno-associated virus. Science. 2001;294:1929–1932. doi: 10.1126/science.1064103. [DOI] [PubMed] [Google Scholar]
- Xiao W, Warrington KH, Jr, Hearing P, Hughes J., and , Muzyczka N. Adenovirus-facilitated nuclear translocation of adeno-associated virus type 2. J Virol. 2002;76:11505–11517. doi: 10.1128/JVI.76.22.11505-11517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux K, Goerlitz N, Schlemminger S, Perabo L, Goldnau D, Endell J, et al. Green fluorescent protein-tagged adeno-associated virus particles allow the study of cytosolic and nuclear trafficking. J Virol. 2005;79:11776–11787. doi: 10.1128/JVI.79.18.11776-11787.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger K, Le Meur G, Lasne F, Weber M, Deschamps JY, Nivard D, et al. Long-term doxycycline-regulated transgene expression in the retina of nonhuman primates following subretinal injection of recombinant AAV vectors. Mol Ther. 2006;13:967–975. doi: 10.1016/j.ymthe.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Kuck D, Kern A., and , Kleinschmidt JA. Development of AAV serotype-specific ELISAs using novel monoclonal antibodies. J Virol Methods. 2007;140:17–24. doi: 10.1016/j.jviromet.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Brorson SH. pH-dependent effect of heat-induced antigen retrieval of epoxy section for electron microscopy. Micron. 2002;33:481–482. doi: 10.1016/s0968-4328(01)00040-3. [DOI] [PubMed] [Google Scholar]
- Le Meur G, Weber M, Pereon Y, Mendes-Madeira A, Nivard D, Deschamps JY, et al. Postsurgical assessment and long-term safety of recombinant adeno-associated virus-mediated gene transfer into the retinas of dogs and primates. Arch Ophthalmol. 2005;123:500–506. doi: 10.1001/archopht.123.4.500. [DOI] [PubMed] [Google Scholar]
- Wistuba A, Weger S, Kern A., and , Kleinschmidt JA. Intermediates of adeno-associated virus type 2 assembly: identification of soluble complexes containing Rep and Cap proteins. J Virol. 1995;69:5311–5319. doi: 10.1128/jvi.69.9.5311-5319.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvetti A, Oreve S, Chadeuf G, Favre D, Cherel Y, Champion-Arnaud P, et al. Factors influencing recombinant adeno-associated virus production. Hum Gene Ther. 1998;9:695–706. doi: 10.1089/hum.1998.9.5-695. [DOI] [PubMed] [Google Scholar]
- Stieger K, Colle MA, Dubreil L, Mendes-Madeira A, Weber M, Le Meur G, et al. Subretinal delivery of recombinant AAV serotype 8 vector in dogs results in gene transfer to neurons in the brain. Mol Ther. 2008;16:916–923. doi: 10.1038/mt.2008.41. [DOI] [PubMed] [Google Scholar]
- Weber M, Rabinowitz J, Provost N, Conrath H, Folliot S, Briot D, et al. Recombinant adeno-associated virus serotype 4 mediates unique and exclusive long-term transduction of retinal pigmented epithelium in rat, dog, and nonhuman primate after subretinal delivery. Mol Ther. 2003;7:774–781. doi: 10.1016/s1525-0016(03)00098-4. [DOI] [PubMed] [Google Scholar]
- Auricchio A, Kobinger G, Anand V, Hildinger M, O'Connor E, Maguire AM, et al. Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum Mol Genet. 2001;10:3075–3081. doi: 10.1093/hmg/10.26.3075. [DOI] [PubMed] [Google Scholar]
- Provost N, Le Meur G, Weber M, Mendes-Madeira A, Podevin G, Cherel Y, et al. Biodistribution of rAAV vectors following intraocular administration: evidence for the presence and persistence of vector DNA in the optic nerve and in the brain. Mol Ther. 2005;11:275–283. doi: 10.1016/j.ymthe.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Acland GM, Aguirre GD, Aleman TS, Schwartz SB, Cideciyan AV, et al. Safety of recombinant adeno-associated virus type 2-RPE65 vector delivered by ocular subretinal injection. Mol Ther. 2006;13:1074–1084. doi: 10.1016/j.ymthe.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Murphy SL, Bhagwat A, Edmonson S, Zhou S., and , High KA. High-throughput Screening and Biophysical Interrogation of Hepatotropic AAV. Mol Ther. 2008;30:30. doi: 10.1038/mt.2008.210. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- Lebherz C, Auricchio A, Maguire AM, Rivera VM, Tang W, Grant RL, et al. Long-term inducible gene expression in the eye via adeno-associated virus gene transfer in nonhuman primates. Hum Gene Ther. 2005;16:178–186. doi: 10.1089/hum.2005.16.178. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Hasbrouck NC, Basner-Tschkarajan E, Edmonson SA, Hui DJ, Sabatino DE, et al. Modulation of tolerance to the transgene product in a non-human primate model of AAV-mediated gene transfer to liver. Blood. 2007;3:3. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]