Abstract
Radiation resistance in a subset of prostate tumors remains a challenge to prostate cancer radiotherapy. The current study on the effects of radiation on prostate cancer cells reveals that radiation programs an unpredicted resistance mechanism by upregulating acid ceramidase (AC). Irradiated cells demonstrated limited changes of ceramide levels while elevating levels of sphingosine and sphingosine-1-phosphate. By genetically downregulating AC with small interfering RNA (siRNA), we observed radiosensitization of cells using clonogenic and cytotoxicity assays. Conversely, AC overexpression further decreased sensitivity to radiation. We also observed that radiation-induced AC upregulation was sufficient to create cross-resistance to chemotherapy as demonstrated by decreased sensitivity to Taxol and C6 ceramide compared to controls. Lower levels of caspase 3/7 activity were detected in cells pretreated with radiation, also indicating increased resistance. Finally, utilization of the small molecule AC inhibitor, LCL385, sensitized PPC-1 cells to radiation and significantly decreased tumor xenograft growth. These data suggest a new mechanism of cancer cell resistance to radiation, through upregulation of AC that is, in part, mediated by application of the therapy itself. An improved understanding of radiotherapy and the application of combination therapy achieved in this study offer new opportunities for the modulation of radiation effects in the treatment of cancer.
Introduction
Radiation therapy (RT) is an established modality for treatment of localized prostate cancer.1,2 Nevertheless, prostate cancer still has a significant local recurrence rate.3 Cancer cell death induced by ionizing radiation is understood to occur through DNA strand breakage, apoptosis induction, and generation of reactive oxygen species.4,5 Bioactive sphingolipids, namely, ceramide, sphingosine, and sphingosine-1-phosphate (S1P), have been recognized as important signaling initiators that regulate survival, proliferation, and cell death.6 A large body of evidence has demonstrated a role for ceramide generation as a mediator of radiation-induced apoptosis.7,8,9,10,11 Ceramide signaling following irradiation is dependent on the stress-activated protein kinase and Bcl-2 family–induced mitochondrial depolarization pathways.12,13 Defects in ceramide generation or rapid ceramide metabolism leads to increased formation of S1P and results in increased resistance to radiation-induced apoptosis.14,15,16 Restoration of ceramide accumulation in radioresistant cancer cells restores radiation sensitivity, confirming that ceramide is both a necessary and sufficient mediator of radiation-induced cell death.17,18
Most studies investigating radiation-induced ceramide generation have implicated hydrolysis of sphingomyelin as the source of ceramide.15,16,19,20,21 Ceramide generation from this pathway is independent of DNA damage and occurs within minutes.19 However, other studies have shown that radiation-induced DNA damage can activate de novo ceramide synthesis, which also leads to apoptosis.22 The addition of Fumonisin B1, a specific ceramide synthase inhibitor, abrogates DNA damage–induced death.22
Acid ceramidase (AC) is a catabolic lysosomal enzyme that deacylates ceramide and yields sphingosine, the substrate for sphingosine kinase-1 (SK1). Phosphorylation of sphingosine forms the potent mitogen S1P. The level of intracellular AC is an important determinant of the balance between cellular levels of ceramide, sphingosine, and S1P, and is integral in determining cell survival, growth, or death.19,20,23 Interest in AC protein levels and its role in cancer increased after studies from our lab revealed AC protein levels were elevated in primary prostate cancer tissues.24 Seelan et al.25 reported that AC mRNA overexpression was more frequently observed in advanced Gleason-grade prostate cancers consistent with our own unpublished findings. Altogether these data suggest that AC overexpression may be characteristic of more advanced and aggressive prostate cancers.24,26,27,28,29 The importance of AC overexpression is emphasized by our recent publication, which revealed that prostate cancer cells overexpressing AC exhibited increased proliferation, invasiveness, and resistance to chemotherapy.26
Although RT is commonly used for treatment of prostate cancer, it is complicated by the persistence of radioresistant cancer cells and dose-limiting toxicities in surrounding normal pelvic tissues. This frequently prevents complete tumor eradication, ultimately leading to failure of the therapy and systemic dissemination of the disease. Therefore, there is an imperative need to understand the mechanism of resistance and improve RT success rate. The current study demonstrates a new mechanism of radioresistance that is due to upregulation of AC in response to RT, which results in prevention of ceramide accumulation and blunted apoptotic response. This leads to reduced effectiveness of the therapy and also provides an explanation for the observed insensitivity of irradiated cells to chemotherapy such as Taxol. RNA interference and LCL385-mediated AC inhibition demonstrated that radioresistance is reversible, suggesting AC is a novel target for radio- and chemo-sensitization. The strategy described in the current study that inhibition of AC can be translated into improved therapeutic outcomes will assist the radiation oncologist to improve radiation control in radiation-responsive tumors, enhance control in radiation-resistant tumors, and have the added benefit of decreasing adverse events.
Results
Sphingosine and S1P, but not ceramide, are upregulated by ionizing radiation in PPC-1 cells
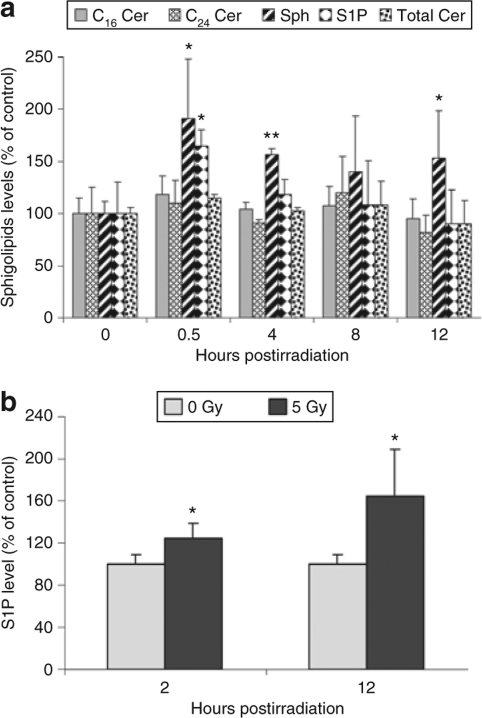
In order to determine whether ionizing radiation modulates sphingolipid metabolism in prostate cancer cells, sphingolipid levels were determined in PPC-1 cells at early time points following exposure to γ radiation (5 Gy). As shown in Figure 1a, there was no significant quantitative change in total ceramide levels after irradiation, although there was a slight but transient elevation of C16 ceramide at 0.5 and 4 hours. However, sphingosine levels increased to 191% of baseline within 30 minutes postirradiation, and this upregulation persisted for at least 12 hours. S1P was acutely increased within 30 minutes of irradiation and returned to basal levels by 8 hours. Because S1P is a powerful signaling ligand that tends to counteract the function of long-chain ceramides, it was of interest to determine whether S1P generation in irradiated prostate cancer cells also resulted in secretion of S1P into the culture media. The acute increase and gradual return to basal levels of intracellular sphingosine and S1P was coincident with increased secretion of S1P into the media (Figure 1b). Starting from 2 hours postirradiation, there was significant difference in the amount of S1P in the culture media of irradiated cells, and by 12 hours there was a 65% increase in S1P in media compared to nonirradiated controls, indicating that S1P was available to exert both autocrine and paracrine signaling effects, both of which are known to counteract the effect of intracellular ceramides.30
Figure 1.
Sphingosine and sphingosine-1-phosphate (S1P), but not ceramide (Cer), are upregulated by ionizing radiation. PPC-1 prostate cancer cell cultures were irradiated (mock or 5 Gy) and collected at the indicated time points after irradiation. Cell pellets and culture media were prepared and lipids were extracted for mass spectrometry as mentioned in Materials and Methods. (a) Intracellular C16 Cer (shaded bars), C24 Cer (hatched bars), sphingosine (Sph, striped bars), S1P (diamond bars), and total Cer (dotted bars) expression levels are represented as percent of nonirradiated cells. (b) S1P levels in PPC-1 culture media from irradiated or control cells. Results shown are mean ± SD of three replicates from one of two independent, representative experiments. *P < 0.05, **P < 0.01 compared with nonirradiated cells.
Ionizing radiation induces activation and upregulation of AC, but not SK1
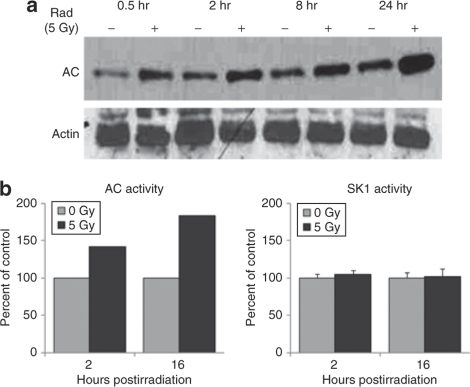
Ceramide catabolism is the major source of intracellular sphingosine, and the ceramidases, predominantly AC, are the rate-limiting enzymes in this process. Western blotting of PPC-1 cell lysates demonstrated that ionizing radiation (single dose of 5 Gy) rapidly upregulated AC protein expression, which persisted through 24 hours (Figure 2a). Increased AC activity levels by enzymatic assay were also detected (Figure 2b). However, there was no change in SK1 enzyme activity between irradiated and nonirradiated cells at the indicated time points (Figure 2b). These results suggest that radiation-induced upregulation of AC, but not SK1, protein expression, and enzyme activity may account for the upregulation of sphingosine and S1P observed in Figure 1.
Figure 2.
Ionizing radiation induces upregulation of acid ceramidase (AC), but not sphingosine kinase-1 (SK1). PPC-1 prostate cancer cells were irradiated (5 Gy) and collected during the first 24 hours of irradiation. (a) Protein lysates were subjected to western blot analysis for AC protein expression. (b) Protein lysates were isolated at 2 and 16 hours following irradiation, and in vitro AC and SK1 enzymatic activities were evaluated as described in Materials and Methods.
AC silencing reverses the insensitivity of PPC-1 cells to ionizing radiation
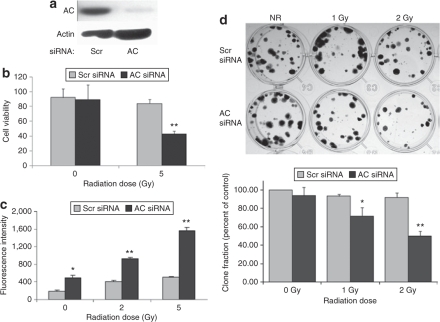
We have now demonstrated the elevation of AC enzyme activity and protein levels in irradiated PPC-1 cancer cells, which has the potential to prevent ceramide signaling and induction of cell death.30 To genetically confirm involvement of AC in radiation resistance, we used small interfering RNA (siRNA) to downregulate AC protein expression (Figure 3a). Sphingolipid analysis indicated a reduction of sphingosine and concomitant elevation of ceramide, including all ceramide species, as a result of AC inhibition by siRNA (data not shown). Cells were exposed to a single 5 Gy dose of radiation, and the combination of AC silencing and ionizing radiation resulted in a 58% decrease in cell viability at 48 hours as assessed by MTS assay (Figure 3b). A caspase 3/7 activity assay confirmed these data, indicating that cells with downregulation of AC were more sensitive to radiation-induced apoptosis as judged by elevation of executioner caspase activity (Figure 3c). Moreover, by clonogenic assay, the effect of AC silencing in combination with ionizing radiation was observed to significantly reduce clonogenicity at 1 and 2 Gy compared to scrambled (Scr) siRNA-treated cells (Figure 3d). This can be replicated by the addition of exogenous C6 ceramide which causes a significant dose-dependent reduction in clonogenic survival, indicating intracellular ceramide elevation affects both short- and long-term cell deaths (data not shown).
Figure 3.
Acid ceramidase (AC) silencing reverses the insensitivity of PPC-1 cells to ionizing radiation. PPC-1 cells were plated in 35-mm2 dishes at a concentration of 1 × 105 cells/dish. Following overnight incubation, cells were transfected with small interfering RNA (siRNA) targeting either AC or a scrambled (Scr)-sequence control. (a) AC protein expression was evaluated by western blot 30 hours following siRNA transfection. (b) Twenty-four hours after siRNA transfection, cells were irradiated at 0 or 5 Gy, and cell death was monitored at 48 hours postirradiation by MTS assay. Cell viability data are represented as percent of untreated cells. (c) Caspase 3/7 activity was determined 24 hours after irradiation to measure the apoptotic status in irradiated or control cells. (d) Irradiated and control cells were seeded at 200 cells/well and cultured for 14 days. Cultures were then fixed and stained with crystal violet. Representative examples are presented. The bar graph below represents quantification by colony scoring. Results are representative of the average ± SD of three independent experiments, each performed as four replicates. *P < 0.05, **P < 0.01 compared with Scr-sequence-transfected cells.
AC overexpression reduces PPC-1 cell sensitivity to ionizing radiation
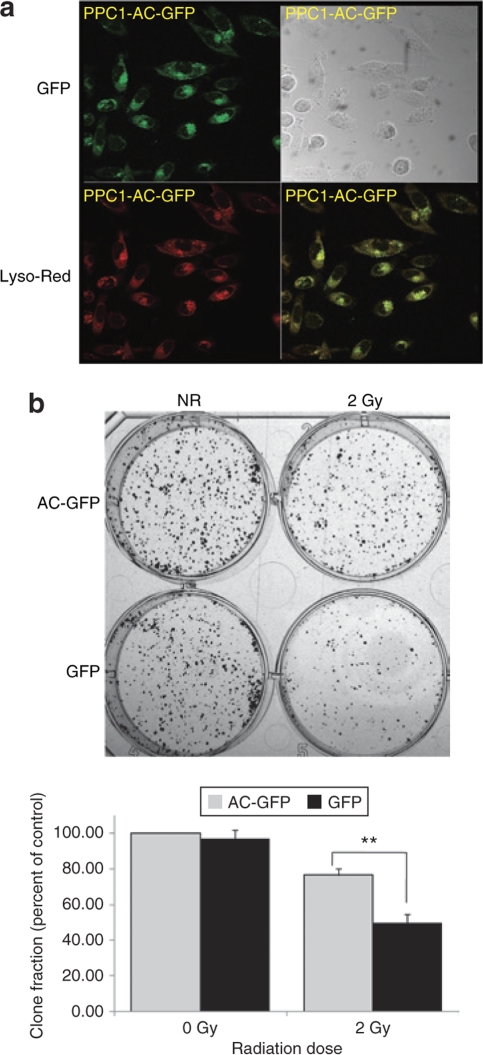
Upon evincing reversal of prostate cancer cell insensitivity to radiation through AC silencing, we were interested to determine whether cells that overexpress AC demonstrate increased radioresistance. PPC-1 cells were transfected with plasmids expressing either green fluorescent protein (GFP) or an AC–GFP fusion protein. Confocal microscopy revealed the intracellular localization of AC–GFP (shown as green) tracked to the lysosome (indicated by LysoTracker Red) as expected (yellow overlay; Figure 4a). When cells received 2 Gy of radiation, increased clonogenic survival was observed in AC overexpressing cells compared to GFP control-transfected cells (Figure 4b).
Figure 4.
Acid ceramidase (AC) over-expression reduces PPC-1 cell sensitivity to ionizing radiation. PPC-1 cells were transfected with plasmid constructs expressing either an AC-GFP fusion protein or green fluorescent protein (GFP) (control). Stably transfected clones were selected and validated for AC overexpression by western blot. (a) Confocal microscopic images of AC-GFP (green), LysoTracker Red–labeled lysosomes (red), or overlay of the two colors is shown and indicates the proper AC colocalization (yellow) within the lysosome. (b) AC overexpressing PPC-1 cells and control cells were irradiated and a clonogenic assay was performed as described in Materials and Methods. After 2 weeks in culture, cells were fixed and stained with crystal violet. Representative cell cultures are depicted. The bar graph below represents quantification by colony scoring. Results are presented as percent of nonirradiated control cells. Results are representative of the average ± SD of two independent experiments, each performed in triplicate. **P < 0.01 compared to GFP-transfected cells.
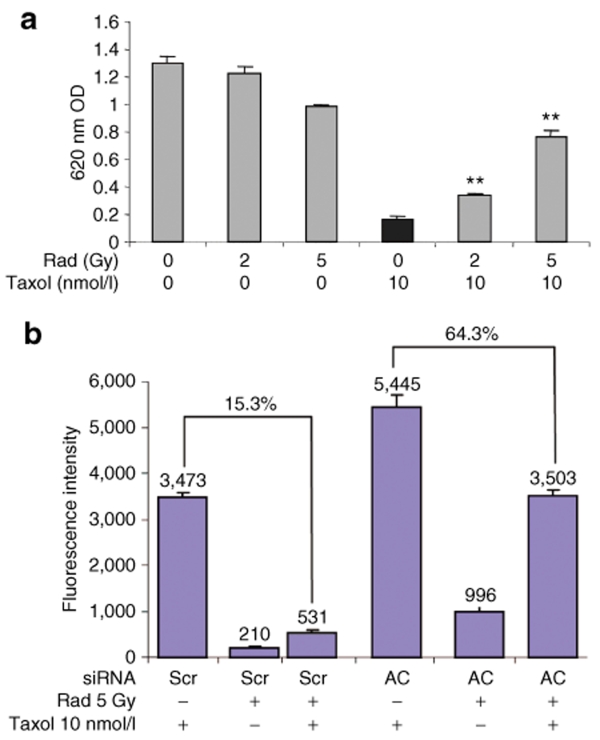
Radiation desensitizes PPC-1 cells to Taxol treatment by upregulation of AC
Interest in the efficacy of taxanes for treating prostate cancer has been sparked by the TAX 32731 and SWOG 99-1632 studies that demonstrated docetaxel increased survival of patients with advanced prostatic disease. As a result, taxane chemotherapy has been examined in combination with standard treatment modalities including ionizing radiation.33,34 However, recent reports demonstrate an antagonism between radiation- and taxane-induced cell death.35,36,37,38,39 The current study reveals a similar antagonism when PPC-1 cells are treated with Taxol following exposure to radiation. After application of Taxol for 48 hours, it was observed that cells pretreated with radiation at doses between 0 and 5 Gy exhibited a dose-dependent survival advantage, proportional to the radiation dose (Figure 5a). Altering the sequence of administering radiation and Taxol did not change these results. Interest in this phenomenon was extended to other cytotoxic agents including C6 ceramide and doxorubicin. Doxorubicin produced modest to marked improvement in cell killing, whereas C6 ceramide-induced cell killing was reduced by radiation as previously observed for Taxol (data not shown).
Figure 5.
Radiation desensitizes PPC-1 cells to Taxol treatment by upregulation of acid ceramidase (AC). (a) PPC-1 cells were irradiated at 0, 2, or 5 Gy followed 2 hours later by treatment with 10 nmol/l Taxol. After 48 hours of chemotherapy, attached cells were fixed and evaluated by crystal violet staining intensity as measured by optical density (OD) at 620 nm. (b) PPC-1 cells were pretransfected with control or AC small interfering RNA (siRNA) 24 hours prior to radiation. Two hours after radiation, 10 nmol/l Taxol was applied. The caspase 3/7 activity assay was performed 24 hours after chemotherapy and results depicted as fluorescence intensity. Results are representative of the average ± SD of four independent experiments. Each data point is the average of three replicates. **P < 0.01 compared with Taxol treatment alone. Scr, scrambled.
To explore whether decreased Taxol sensitivity in irradiated cancer cells was due to AC upregulation, we evaluated the effect of AC siRNA on cells treated with Taxol and ionizing radiation (Figure 5b). Using a caspase 3/7 activity assay, we observed that the application of ionizing radiation followed by Taxol resulted in decreased caspase 3/7 activation as shown in Figure 5b, where only 15.3% activation was observed in cells compared with Taxol alone. However, the addition of AC siRNA, significantly reversed antagonism, achieving 64.3% of the level of caspase 3/7 activation observed in the absence of ionizing radiation (Figure 5b). Similar experiments treating irradiated PPC-1 cells with C6 ceramide or doxorubicin indicated decreased caspase activity only in cells treated with C6 ceramide. Genetic knockdown of AC resensitized irradiated cells to C6 ceramide as observed for Taxol in Figure 5. The same trend was not observed in doxorubicin-treated cells where the chemotherapy drug functions by other mechanisms that are only indirectly related to ceramide metabolism.
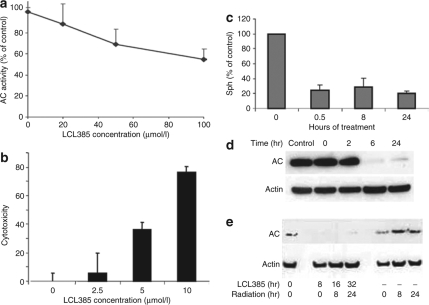
AC inhibitor LCL385 blocks radiation-induced AC upregulation
Cancer cells appear to respond to irradiation by increasing AC. This suggests cells use this mechanism to recover from stress, which makes AC a novel drug target when used in combination with radiation therapies. Using a rationally designed AC inhibitor, LCL385, it was observed that a concentration-dependent inhibition of AC activity occurred using an in vitro cell lysate assay (Figure 6a). The drug alone has therapeutic potential and induces cell death in a dose-dependent manner (Figure 6b). As shown in Figure 6c, a nontoxic dose of LCL385 (2.5 µmol/l) depleted sphingosine by 78% within 30 minutes of treatment, and this effect persisted for 24 hours. As a lysosomotropic agent, LCL385 caused lysosomal destabilization accompanied by proteolytic degradation of AC within 6 hours of treatment (Figure 6d), which confirms our previous observations with this class of drug.29 In order to determine whether AC inhibition/degradation sensitized cells to radiation, cells were pretreated with 2.5 µmol/l LCL385 for 8 hours followed by 5 Gy radiation. LCL385 pretreatment completely blocked radiation-induced AC upregulation and caused AC degradation (Figure 6e) similar to our previous observations using LCL204.29
Figure 6.
Acid ceramidase (AC) inhibitor LCL385 blocks radiation-induced AC upregulation. (a) Dose-dependent inhibitory effect of LCL385 on AC in vitro was performed through a cell-free assay using PPC-1 cell homogenates as the AC source and [3H-N] labeled C6 ceramide as the substrate, performed at pH 4.5. Data are plotted as percentage of AC inhibition compared to control cell lysate. (b) Cytotoxicity of LCL385 on PPC-1 cells was evaluated by MTS assay 24 hours following treatment at the indicated doses. Results represent percentage of cell death compared to cells with no treatment. (c) PPC-1 cells were treated with LCL385 (2.5 µmol/l). At the indicated time points sphingosine levels were measured in triplicate using mass spectrometry and plotted as the percent change compared to cells with no LCL385 treatment. (d) PPC-1 cells were treated with LCL385 (2.5 µmol/l) and protein lysates were isolated at the indicated time points and processed by western blotting for AC protein expression. (e) PPC-1 cells were treated with LCL385 (2.5 µmol/l) 8 hours prior to radiation as indicated. Protein lysates were collected at 8 or 24 hours after radiation administration and processed by western blotting to determine AC protein levels.
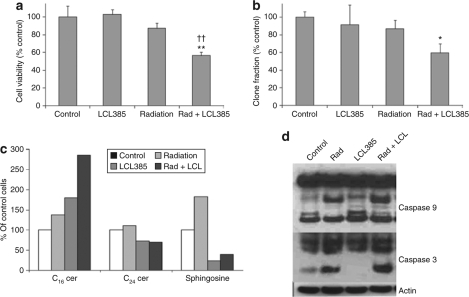
AC inhibition sensitizes PPC-1 cells to ionizing radiation
Although treatment with either LCL385 or radiation alone had only a mild effect on cell viability, PPC-1 cells pretreated with LCL385 at 2.5 µmol/l became 30% more sensitive to radiation-induced cell death within 48 hours of treatment (Figure 7a). Moreover, combination treatment of PPC-1 cells with LCL385 and ionizing radiation demonstrated similar reduction of clonogenic survival rates at 14 days (Figure 7b). Evaluation of radiation-induced changes in sphingolipid levels revealed that LCL385 abolished the upregulation of sphingosine within 30 minutes postirradiation, which persisted for at least 24 hours. Our results also demonstrated that pretreatment of PPC-1 cells with LCL385 followed by radiation significantly increased C16 ceramide levels compared to treatment with either radiation or LCL385 alone (Figure 7c). Combination treatment using radiation and LCL385 resulted in increased activity of both caspases 9 and 3 as detected by western blotting indicating combination treatment enhances cell death compared to either treatment alone (Figure 7d).
Figure 7.
Acid ceramidase (AC) inhibition sensitizes PPC-1 cells to ionizing radiation. PPC-1 cells were pretreated with LCL385 at 2.5 µmol/l followed 8 hours later by 5 Gy radiation. (a) Cell viability at 48 hours following irradiation was evaluated by crystal violet staining. Results are represented as percent of untreated control cells quantified by colorimetric absorbance at 620 nm. (b) Clonogenic survival between 10 and 14 days was evaluated as described in Materials and Methods. Results are presented as percent of nonirradiated crystal violet staining in control cells. (c) Sphingolipid species in treated cells at 30 minutes following radiation were analyzed by mass spectrometry. (d) Protein lysates were collected at 48 hours following irradiation and/or LCL385 treatment, and processed by western blot for assessment of caspases 3 and 9 activation. *P < 0.05; ** and ††P < 0.01 compared with radiation alone and LCL385 treatment alone, respectively.
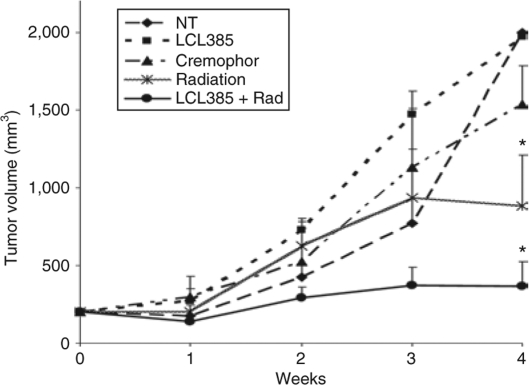
AC inhibitor LCL385 sensitizes PPC-1 tumor xenografts to ionizing radiation
In order to determine whether AC inhibition improves tumor sensitivity to ionizing radiation in vivo, PPC-1 tumor xenografts were generated in nude mice. Treatment was initiated when the xenografts reached 200 mm3. Animals received intraperitoneal injections of either LCL385 (30 mg/kg emulsified in Cremophor, as described in the figure legends) or suitable controls. Eight hours after drug administration, tumors were subjected to focal ionizing radiation (5 Gy) administered in the radiation oncology department. This treatment was performed twice weekly for 3 weeks (6 doses; 30 Gy total radiation). Tumor growth rates are presented in Figure 8. Tumors samples that received no treatment, Cremophor vehicle control, or LCL385 in Cremophor, were observed to grow toward the maximum allowed volume of 2,000 mm3. Tumors undergoing RT grew slower, reaching ~881 mm3 by 4 weeks, whereas animals receiving combination therapy with LCL385 pretreatment and ionizing radiation demonstrated a significantly slower growth rate reaching only 366 mm3 at 4 weeks.
Figure 8.
Acid ceramidase (AC) inhibitor LCL385 sensitizes PPC-1 tumor xenografts to ionizing radiation. Eight million PPC-1 tumor cells were injected subcutaneously into the flank of nu/nu mice. When tumors reached a mean size of 200 mm3, animals received 5 Gy of radiation (Varian 2100c linear accelerator) twice weekly for a total of six doses (30 Gy total) alone or in combination with LCL385 administered by intraperitoneal injection at 30 mg/kg in 400 µl of Cremophor/ethanol/phosphate-buffered saline 8 hours prior to radiation as described in Materials and Methods. LCL385, no treatment, irradiation, or Cremophor alone were controls used for comparison. Tumor volume was measured twice weekly and expressed as mentioned in Materials and Methods. Data points represent the mean change in tumor volumes relative to day 0 after tumors reached ~200 mm3 for each group of animals (n = 6 or 7). *P < 0.05 compared to the control group.
Discussion
The application of ionizing radiation for treating cancer is a well-established therapeutic modality for >750,000 patients per year. However, ~30% of radiation-treated cancers as well as most prostate cancer cell lines are relatively resistant to radiation-induced apoptosis as reported by others.40,41,42,43,44 Ceramide has been identified as a critical component of ionizing radiation-induced apoptosis.7,9,19,20 This was determined in patients with Niemann–Pick disease and acid sphingomyelinase-knockout mice, both of which exhibit high levels of resistance to ionizing radiation.15 In addition, it has also been reported that de novo synthesis of ceramide, catalyzed by the enzymes serine palmitoyl CoA synthase and ceramide synthase, is responsible for a secondary wave of ceramide synthesis associated with DNA damage repair.20 Thus, the generation of ceramide seems to be an important cellular response following radiation exposure. This article presents data relative to the role of AC in this process and indicates that increased ceramide degradation in irradiated cells results in radio insensitivity. Thus, these studies extend our previously published study on AC's role that demonstrated prostate cancer cells exhibit a highly aggressive tumor phenotype if AC is overexpressed.26
Sphingolipid analysis of irradiated cells revealed that both sphingosine and S1P are persistently upregulated in response to ionizing radiation, whereas total ceramide remains essentially unchanged (Figure 1). It is known that AC and SK1 are the two rate-limiting enzymes controlling the intracellular balance of ceramide and S1P. The Spiegel group10 reported that radiation led to a rapid 50% decrease in the activity of SK1 in TSU-Pr1 radiosensitive bladder cancer cells but not in radioresistant LNCaP cells. This suggests that SK1 plays an important role in the cellular radiation response. However, this study utilizing PPC-1 cells did not observe a change in SK1 enzyme activity. Instead, AC upregulation appeared to increase levels of sphingosine and S1P. Similar results were observed in PC-3 and DU145 prostate cells (data not shown).
Ceramidases are the primary enzymatic mechanism for formation of sphingosine. This suggests that elevation of AC activity by radiation functions to diminish the elevation of ceramide while increasing production of sphingosine, the substrate for SK1 that forms S1P. More important, cells with increased S1P create a tumor site that favors cancer survival by virtue of S1P's known mechanism of action.6
To confirm the involvement of AC in this mechanism of resistance, we developed both genetic and small-molecule drugs that inhibit AC function. When these drugs were applied, we observed by MTS that AC was responsible for radioresistance in these cells because downregulation of AC resensitized PPC-1 cells to radiation (Figure 3b). Long-term clonogenic survival assays indicated that inhibition of AC decreases irradiated cell survival and this was due to alteration of intracellular ceramide levels by AC inhibition. These studies were carried out in PPC-1 cells, which are p53 null, suggesting the mechanism of resistance is p53 independent. To further confirm our hypothesis that AC is responsible for radioresistance we demonstrated that PPC-1 cells overexpressing AC become more resistant to radiation when compared to control cells (Figure 4b). Our unpublished data from extended studies on treatment of PC-3 and PPC-1, two related and well-established prostate cancer cell lines exhibiting different levels of AC expression, indicate a potential role for high-endogenous AC expression as promoting a prosurvival phenotype having decreased sensitivity to radiation.
Studies published earlier from the laboratory demonstrated that AC overexpression also leads to resistance to chemotherapeutic drugs.26 We have now determined in this article that induction of AC by prior exposure to radiation reduces sensitivity to Taxol (Figure 5b) and supports the role of AC activity as the mechanism for insensitivity. This conclusion was reached by demonstrating that radiation elevated AC, which led to decreased sensitivity to Taxol compared with nonirradiated cells. This could be corrected using AC inhibition or AC-siRNA. Previous work from another laboratory demonstrated that γ radiation was able to inhibit paclitaxel-induced IkB-α degradation and Bcl-2 phosphorylation by arresting target cells in G2 phase that appeared to prevent cell death due to the taxane.35 The current report points out another possible pathway that involves AC activation as a means for developing resistance, and it is known that the ceramide pathway is involved in Taxol-induced cell cytotoxicity.45,46 Sphingolipid analysis demonstrated that AC siRNA permitted induction of the executioner long-chain ceramides and diminished production of very-long-chain ceramides, sphingosine, and S1P. Our data indicate that AC is activated by radiation in prostate cancer and contributes to radiation resistance via its ability to alter ceramide metabolism and through the same mechanism leads to insensitivity to chemotherapy as well. This also implies care must be taken in the sequencing of chemotherapy with RT.
Because treatment of patients with siRNA has a delivery issue, the laboratory has sought to develop small-molecule AC inhibitors47 that achieve similar results as AC-siRNA, i.e., inhibition of AC enzyme activity. At doses that are not intrinsically toxic to PPC-1 cells, we have now demonstrated that LCL385 prevents AC upregulation (Figure 6) and enhances cell killing in response to radiation treatment (Figure 7). These results are believed to be due to signaling by long-chain ceramides, such as C16, which are elevated following radiation, and distinguished from sphingosine and S1P, which are lower when AC activity is inhibited by LCL385 (Figure 7b). Finally, we were able to confirm these data in vivo and demonstrated that modulation of AC by LCL385 appears to be a reasonable approach to sensitizing prostate cancer cells to ionizing radiation (Figure 8).
In conclusion, prostate cancer is typically an indolent disease usually treated by surgery or radiation. In more aggressive, advanced, and recurrent disease, the combination of radiation and chemotherapy has been applied. Recently, Sanfilippo reported a phase I/II study of biweekly paclitaxel and radiation in androgen-ablated, locally advanced prostate cancer, with modest but encouraging results.34 However, this clinical trial used concomitant radiation/chemotherapy. The data in this article suggest a way to further improve clinical outcomes by application of AC inhibitors concomitant with standard therapy. In fact, our study provides new insight into why radiation and radiation/chemotherapy treatment of prostate cancer may have limited success because they demonstrate that activation of AC leads to both resistance to RT itself and to Taxol. Consequently, the data in this article provide an understanding about why combination treatment fails and suggest the clinical oncologist/radiation therapist reconsider their clinical strategies for combination therapy. Because AC is a druggable target, this article concludes that AC inhibition could be the basis for an innovative clinical approach for treating prostate and other cancers where AC has been shown to be overexpressed.
Materials And Methods
Cell line and reagents. The human prostate cell line PPC-1 was obtained from American Type Culture Collection (Manassas, VA) and maintained in RPMI 1640 media (Mediatech, Herndon, VA) supplemented with 10% heat-inactivated fetal calf serum (Hyclone, Logan, UT) and 1% penicillin/streptomycin (Mediatech). Cells were grown in 5% CO2 at 37 °C. LCL385 was synthesized in the Medical University of South Carolina Lipidomics Core Facility (Charleston, SC) as described48 and dissolved in 100% ethanol at a final concentration of 20 mmol/l.
Cell irradiation. Cells were cultured overnight in 100-mm2 plates (Greiner Bio One, Longwood, FL) at a density of 2 × 106/dish. γ Irradiation of cultured cells at variable doses was carried out at 25°C in a γ-cell 40 chamber containing two sources of 137Cs (model 143; J.L. Shepherd & Associates, San Fernando, CA) at a dose rate of 2.27 Gy/min.
Sphingolipid measurement. Cells were cultured overnight in 100-mm2 plates (Greiner Bio-One) at a density of 2 × 106/dish and treated as described in the figure legends. For sphingolipid analysis, lipids were extracted from frozen cell pellets and examined by mass spectrometry as previously described.49
Western blot. Cells were seeded in 60-mm2 plates (Greiner Bio One) in RPMI 1640/10% fetal calf serum at 5 × 105/dish cell density and treated accordingly. Protein isolation and immunoblot were performed as previously described.27
AC and SK1 activity assays. Detection of AC and SK1 intracellular enzymatic activities was performed according to our previously published protocols.50
In vitro siRNA transfection. In vitro siRNA transfection was performed using specifically designed siRNA reagents: 5′ AATCA ACCTATCCTCCTTCAG 3′, human AC siRNA; and 5′ AATTC TCCGAACGTGTCACGT 3′, Scr-sequence control siRNA. Both siRNA reagents were purchased from Qiagen (Valencia, CA). PPC-1 prostate cancer cells were seeded in 6-well plates (1.0 × 105 cells/well), and 66.7 nmol/l of either AC or Scr siRNA were mixed with Oligofectamine (Invitrogen, Carlsbad, CA) and transfected according to the manufacturer's instructions. Experiments were performed 24 hours after transfection. Knockdown efficiency was determined by western blotting.
Caspase 3/7 activity and MTS viability assays. Cells were seeded in 96-well plates at a density of 5 × 103 cells/well. Following treatment, caspase 3/7 activity was determined as per the manufacturer using the Apo-ONE assay (Promega, Madison, WI) according to the manufacturer's recommendations. Cell viability was determined as per the manufacturer using the tetrazolium-based (MTS) assay referred to as CellTiter 96 (Promega).
Clonogenic assay. Treated cells were washed with phosphate-buffered saline and trypsinized. All the groups were plated in triplicate at a density of 200 cells/well in 6-well plates and incubated for 2 weeks to allow colonies to develop. After 2 weeks, the medium was removed, and colonies were washed once with phosphate-buffered saline and fixed with 3.7% formaldehyde. Colonies were stained with crystal violet in 2% ethanol for 20 minutes and subsequently washed with deionized water to remove excess dye. Colonies of >50 cells or >1 mm in size were quantitated.
Animal studies. Animal protocols strictly followed both Institutional and the National Institutes of Health animal care rules and regulations. Nu/Nu athymic nude mice were kept in the animal facility at least 1 week prior to experimentation. Next, 8 × 106 PPC-1 cells suspended in 100 µl of phosphate-buffered saline were injected subcutaneously into the flank of the mouse. Upon first appearance of a 200-mm3 tumor, treatment was initiated. Chemical pretreatment of mice consisted of a single intraperitoneal injection of LCL385 (30 mg/kg) or vehicle control (Cremophor EL; BASF, Florham Park, NJ) alone. Eight hours later, focal ionizing radiation (5 Gy) was administered specifically to the tumors (Varian 2100c linear accelerator; Varian Medical Systems, North Charleston, SC) while mice were restrained in custom-designed holders. This treatment was performed twice weekly and mice were monitored daily. Tumor volumes were calculated as previously described.27
Statistics. Experiments were performed in triplicate unless otherwise stated. Results are expressed as the mean ± SD unless otherwise stated. Statistical significance of the mean was determined using Student's t-test.
Acknowledgments
This work was supported by the National Institutes of Health (NIH)/National Cancer Institute PO1 CA97132; Division of Laboratory Animal Research, NIH, C06 RR015455; and the Medical University of South Carolina (MUSC) Lipidomics Core, NIH, C06 RR018823. The technical assistance of Margaret Kelly of the MUSC Hollings Cancer Center Microscopy Core Facility is greatly appreciated. We are also thankful to Janie Nelson for excellent secretarial assistance, and S. Tucker Marrison, Xiaoyi Zhang, and Ben Gilbertson for their help in preparing this manuscript.
REFERENCES
- Mangar SA, Huddart RA, Parker CC, Dearnaley DP, Khoo VS., and , Horwich A. Technological advances in radiotherapy for the treatment of localised prostate cancer. Eur J Cancer. 2005;41:908–921. doi: 10.1016/j.ejca.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Peschel RE., and , Colberg JW. Surgery, brachytherapy, and external-beam radiotherapy for early prostate cancer. Lancet Oncol. 2003;4:233–241. doi: 10.1016/s1470-2045(03)01035-0. [DOI] [PubMed] [Google Scholar]
- Hanks GE, Hanlon AL, Epstein B., and , Horwitz EM. Dose response in prostate cancer with 8–12 years' follow-up. Int J Radiat Oncol Biol Phys. 2002;54:427–435. doi: 10.1016/s0360-3016(02)02954-1. [DOI] [PubMed] [Google Scholar]
- Ward JF. DNA damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol. 1988;35:95–125. doi: 10.1016/s0079-6603(08)60611-x. [DOI] [PubMed] [Google Scholar]
- Allan DJ. Radiation-induced apoptosis: its role in a MADCaT (mitosis-apoptosis-differentiation-calcium toxicity) scheme of cytotoxicity mechanisms. Int J Radiat Biol. 1992;62:145–152. doi: 10.1080/09553009214551951. [DOI] [PubMed] [Google Scholar]
- Ogretmen B., and , Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- Kolesnick R., and , Fuks Z. Radiation and ceramide-induced apoptosis. Oncogene. 2003;22:5897–5906. doi: 10.1038/sj.onc.1206702. [DOI] [PubMed] [Google Scholar]
- Vit JP., and , Rosselli F. Role of the ceramide-signaling pathways in ionizing radiation-induced apoptosis. Oncogene. 2003;22:8645–8652. doi: 10.1038/sj.onc.1207087. [DOI] [PubMed] [Google Scholar]
- Kimura K, Markowski M, Edsall LC, Spiegel S., and , Gelmann EP. Role of ceramide in mediating apoptosis of irradiated LNCaP prostate cancer cells. Cell Death Differ. 2003;10:240–248. doi: 10.1038/sj.cdd.4401145. [DOI] [PubMed] [Google Scholar]
- Nava VE, Cuvillier O, Edsall LC, Kimura K, Milstien S, Gelmann EP, et al. Sphingosine enhances apoptosis of radiation-resistant prostate cancer cells. Cancer Res. 2000;60:4468–4474. [PubMed] [Google Scholar]
- Hara S, Nakashima S, Kiyono T, Sawada M, Yoshimura S, Iwama T, et al. p53-Independent ceramide formation in human glioma cells during γ-radiation-induced apoptosis. Cell Death Differ. 2004;11:853–861. doi: 10.1038/sj.cdd.4401428. [DOI] [PubMed] [Google Scholar]
- Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, et al. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- Mu Z, Hachem P., and , Pollack A. Antisense Bcl-2 sensitizes prostate cancer cells to radiation. Prostate. 2005;65:331–340. doi: 10.1002/pros.20303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmura SJ, Nodzenski E, Kharbanda S, Pandey P, Quintans J, Kufe DW, et al. Down-regulation of ceramide production abrogates ionizing radiation-induced cytochrome c release and apoptosis. Mol Pharmacol. 2000;57:792–796. doi: 10.1124/mol.57.4.792. [DOI] [PubMed] [Google Scholar]
- Santana P, Pena LA, Haimovitz-Friedman A, Martin S, Green D, McLoughlin M, et al. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- Bruno AP, Laurent G, Averbeck D, Demur C, Bonnet J, Bettaieb A, et al. Lack of ceramide generation in TF-1 human myeloid leukemic cells resistant to ionizing radiation. Cell Death Differ. 1998;5:172–182. doi: 10.1038/sj.cdd.4400330. [DOI] [PubMed] [Google Scholar]
- Alphonse G, Bionda C, Aloy MT, Ardail D, Rousson R., and , Rodriguez-Lafrasse C. Overcoming resistance to gamma-rays in squamous carcinoma cells by poly-drug elevation of ceramide levels. Oncogene. 2004;23:2703–2715. doi: 10.1038/sj.onc.1207357. [DOI] [PubMed] [Google Scholar]
- Samsel L, Zaidel G, Drumgoole HM, Jelovac D, Drachenberg C, Rhee JG, et al. The ceramide analog, B13, induces apoptosis in prostate cancer cell lines and inhibits tumor growth in prostate cancer xenografts. Prostate. 2004;58:382–393. doi: 10.1002/pros.10350. [DOI] [PubMed] [Google Scholar]
- Haimovitz-Friedman A, Kan CC, Ehleiter D, Persaud RS, McLoughlin M, Fuks Z, et al. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994;180:525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmura SJ, Nodzenski E, Beckett MA, Kufe DW, Quintans J., and , Weichselbaum RR. Loss of ceramide production confers resistance to radiation-induced apoptosis. Cancer Res. 1997;57:1270–1275. [PubMed] [Google Scholar]
- Chmura SJ, Mauceri HJ, Advani S, Heimann R, Beckett MA, Nodzenski E, et al. Decreasing the apoptotic threshold of tumor cells through protein kinase C inhibition and sphingomyelinase activation increases tumor killing by ionizing radiation. Cancer Res. 1997;57:4340–4347. [PubMed] [Google Scholar]
- Liao WC, Haimovitz-Friedman A, Persaud RS, McLoughlin M, Ehleiter D, Zhang N, et al. Ataxia telangiectasia-mutated gene product inhibits DNA damage-induced apoptosis via ceramide synthase. J Biol Chem. 1999;274:17908–17917. doi: 10.1074/jbc.274.25.17908. [DOI] [PubMed] [Google Scholar]
- Kolesnick R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Invest. 2002;110:3–8. doi: 10.1172/JCI16127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris JS, Bielawska A, Day T, El-Zawahri A, ElOjeimy S, Hannun Y, et al. Combined therapeutic use of AdGFPFasL and small molecule inhibitors of ceramide metabolism in prostate and head and neck cancers: a status report. Cancer Gene Ther. 2006;13:1045–1051. doi: 10.1038/sj.cgt.7700965. [DOI] [PubMed] [Google Scholar]
- Seelan RS, Qian C, Yokomizo A, Bostwick DG, Smith DI., and , Liu W. Human acid ceramidase is overexpressed but not mutated in prostate cancer. Genes Chromosomes Cancer. 2000;29:137–146. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1018>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Saad AF, Meacham WD, Bai A, Anelli V, Elojeimy S, Mahdy AE, et al. The functional effects of acid ceramidase overexpression in prostate cancer progression and resistance to chemotherapy. Cancer Biol Ther. 2007;6:1455–1460. doi: 10.4161/cbt.6.9.4623. [DOI] [PubMed] [Google Scholar]
- Liu X, Elojeimy S, El-Zawahry AM, Holman DH, Bielawska A, Bielawski J, et al. Modulation of ceramide metabolism enhances viral protein apoptin's cytotoxicity in prostate cancer. Mol Ther. 2006;14:637–646. doi: 10.1016/j.ymthe.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Liu X, Zeidan YH, Elojeimy S, Holman DH, El-Zawahry AM, Guo GW, et al. Involvement of sphingolipids in apoptin-induced cell killing. Mol Ther. 2006;14:627–636. doi: 10.1016/j.ymthe.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Holman DH, Turner LS, El-Zawahry A, Elojeimy S, Liu X, Bielawski J, et al. Lysosomotropic acid ceramidase inhibitor induces apoptosis in prostate cancer cells. Cancer Chemother Pharmacol. 2008;61:231–242. doi: 10.1007/s00280-007-0465-0. [DOI] [PubMed] [Google Scholar]
- Hannun YA., and , Linardic CM. Sphingolipid breakdown products: anti-proliferative and tumor-suppressor lipids. Biochim Biophys Acta. 1993;1154:223–236. doi: 10.1016/0304-4157(93)90001-5. [DOI] [PubMed] [Google Scholar]
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- Kumar P, Perrotti M, Weiss R, Todd M, Goodin S, Cummings K, et al. Phase I trial of weekly docetaxel with concurrent three-dimensional conformal radiation therapy in the treatment of unfavorable localized adenocarcinoma of the prostate. J Clin Oncol. 2004;22:1909–1915. doi: 10.1200/JCO.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Sanfilippo NJ, Taneja SS, Chachoua A, Lepor H., and , Formenti SC. Phase I/II study of biweekly paclitaxel and radiation in androgen-ablated locally advanced prostate cancer. J Clin Oncol. 2008;26:2973–2978. doi: 10.1200/JCO.2007.14.4105. [DOI] [PubMed] [Google Scholar]
- Sui M, Dziadyk JM, Zhu X., and , Fan W. Cell cycle-dependent antagonistic interactions between paclitaxel and gamma-radiation in combination therapy. Clin Cancer Res. 2004;10:4848–4857. doi: 10.1158/1078-0432.CCR-03-0707. [DOI] [PubMed] [Google Scholar]
- Liebmann J, Cook JA, Fisher J, Teague D., and , Mitchell JB. Changes in radiation survival curve parameters in human tumor and rodent cells exposed to paclitaxel (Taxol) Int J Radiat Oncol Biol Phys. 1994;29:559–564. doi: 10.1016/0360-3016(94)90456-1. [DOI] [PubMed] [Google Scholar]
- Ingram ML., and , Redpath JL. Subadditive interaction of radiation and Taxol in vitro. Int J Radiat Oncol Biol Phys. 1997;37:1139–1144. doi: 10.1016/s0360-3016(96)00629-3. [DOI] [PubMed] [Google Scholar]
- Stromberg JS, Lee YJ, Armour EP, Martinez AA., and , Corry PM. Lack of radiosensitization after paclitaxel treatment of three human carcinoma cell lines. Cancer. 1995;75:2262–2268. doi: 10.1002/1097-0142(19950501)75:9<2262::aid-cncr2820750912>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Geard CR., and , Jones JM. Radiation and taxol effects on synchronized human cervical carcinoma cells. Int J Radiat Oncol Biol Phys. 1994;29:565–569. doi: 10.1016/0360-3016(94)90457-x. [DOI] [PubMed] [Google Scholar]
- Bowen C, Spiegel S., and , Gelmann E. Radiation-induced apoptosis mediated by retinoblastoma protein. Cancer Res. 1998;58:3275–3281. [PubMed] [Google Scholar]
- Algan O, Stobbe CC, Helt AM, Hanks GE., and , Chapman JD. Radiation inactivation of human prostate cancer cells: the role of apoptosis. Radiat Res. 1996;146:267–275. [PubMed] [Google Scholar]
- Li WX., and , Franklin WA. Radiation- and heat-induced apoptosis in PC-3 prostate cancer cells. Radiat Res. 1998;150:190–194. [PubMed] [Google Scholar]
- Kolesnick R, Haimovitz-Friedman A., and , Fuks Z. The sphingomyelin signal transduction pathway mediates apoptosis for tumor necrosis factor, Fas, and ionizing radiation. Biochem Cell Biol. 1994;72:471–474. doi: 10.1139/o94-063. [DOI] [PubMed] [Google Scholar]
- Kyprianou N, King ED, Bradbury D., and , Rhee JG. bcl-2 over-expression delays radiation-induced apoptosis without affecting the clonogenic survival of human prostate cancer cells. Int J Cancer. 1997;70:341–348. doi: 10.1002/(sici)1097-0215(19970127)70:3<341::aid-ijc16>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Chachisvilis M, Zhang YL., and , Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci USA. 2006;103:15463–15468. doi: 10.1073/pnas.0607224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles AG, Han TY, Liu YY, Hansen N, Giuliano AE., and , Cabot MC. Taxol-induced ceramide generation and apoptosis in human breast cancer cells. Cancer Chemother Pharmacol. 2001;47:444–450. doi: 10.1007/s002800000265. [DOI] [PubMed] [Google Scholar]
- Bielawska A, Bielawski J, Szulc ZM, Mayroo N, Liu X, Bai A, et al. Novel analogs of D-e-MAPP and B13. Part 2: signature effects on bioactive sphingolipids. Bioorg Med Chem. 2008;16:1032–1045. doi: 10.1016/j.bmc.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagan A, Wang C, Fibach E., and , Gatt S. Synthetic, non-natural sphingolipid analogs inhibit the biosynthesis of cellular sphingolipids, elevate ceramide and induce apoptotic cell death. Biochim Biophys Acta. 2003;1633:161–169. doi: 10.1016/s1388-1981(03)00122-7. [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Baes M, Busman M, Hannun YA., and , Van Veldhoven PP. Mass spectrometric analysis of ceramide perturbations in brain and fibroblasts of mice and human patients with peroxisomal disorders. Rapid Commun Mass Spectrom. 2004;18:1569–1574. doi: 10.1002/rcm.1520. [DOI] [PubMed] [Google Scholar]
- Zeidan YH, Pettus BJ, Elojeimy S, Taha T, Obeid LM, Kawamori T, et al. Acid ceramidase but not acid sphingomyelinase is required for tumor necrosis factor-[alpha]-induced PGE2 production. J Biol Chem. 2006;281:24695–24703. doi: 10.1074/jbc.M604713200. [DOI] [PubMed] [Google Scholar]