Abstract
Our objective was to study the expression and function of stromal interaction molecule 1 (STIM1), an endoplasmic reticulum protein recently identified as the calcium sensor that regulated Ca2+-released activated channels in T cells. STIM1 was found to be upregulated in serum-induced proliferating human coronary artery smooth muscle cells (hCASMCs) as well as in the neointima of injured rat carotid arteries. Growth factors–induced proliferation was significantly lower in hCASMC transfected with STIM1 siRNA than in those transfected with scrambled siRNA (increase relative to 0.1% S: 116 ± 12% and 184 ± 16%, respectively, P < 0.01). To assess the role of STIM1 in preventing vascular smooth muscle cells (VSMCs) proliferation in vivo, we infected balloon-injured rat carotid arteries with an adenoviral vector expressing a short hairpin (sh) RNA against rat STIM1 mRNA (Ad-shSTIM1). Intima/media ratios reflecting the degree of restenosis were significantly lower in Ad-shSTIM1- infected arteries than in Ad-shLuciferase-infected arteries (0.34 ± 0.02 vs. 0.92 ± 0.11, P < 0.006). Finally, we demonstrated that silencing STIM1 prevents activation of the transcription factor NFAT (nuclear factor of activated T cell). In conclusion, STIM1 appears as a major regulator of in vitro and in vivo VSMC proliferation, representing a novel and original pharmacological target for prominent vascular proliferative diseases.
Introduction
Vascular smooth muscle cells (VSMCs) proliferation is the major underlying biological process of pathological conditions such as in-stent restenosis. Intracoronary stenting can improve procedural success and reduce restenosis following percutaneous transluminal coronary angioplasty, but can also increase the rate of thrombotic complications including stent thrombosis. Such concern has been enhanced by the introduction of drug-eluting stents, which, however, resulted in a dramatic reduction in the incidence of restenosis.1 Drugs released from drug-eluting stents exert biological effects leading to inhibition of cell proliferation. Consequently, although aimed to prevent smooth muscle cell proliferation and migration, such drugs can also impair reendothelialization.2 There is thus a need to uncover new modalities for therapeutic treatments to prevent restenosis. Identification of signaling pathways that regulate VSMC phenotype and proliferative responses is then an active field of research.
Calcium, a ubiquitous intracellular signal that regulates many different cellular processes, plays an important role in VSMC proliferation.3,4 Compelling evidence has been presented that a sustained increase in cytosolic calcium is required to activate calcineurin, a calcium/calmodulin-dependent serine/threonine-specific protein phosphatase that regulates VSMC proliferation.4,5 Calcium entry triggered by loss of calcium from endoplasmic reticulum calcium stores, a process called store-operated calcium entry (SOCE), has been demonstrated to consistently increase calcium intracellular level. STIM1 (STromal Interaction Molecule 1) has been recently identified by RNA interference-based screening studies in drosophila as an essential component of SOCE.6,7 STIM1 is a transmembrane protein located in the ER where it exerts a calcium sensor function. Indeed, STIM1 has a Ca2+-binding EF hand motif located within the ER lumen. In case of Ca2+ store depletion, STIM1 moves to the plasma membrane where it activates calcium entry via store-operated calcium channels.7,8,9,10 Jousset et al.9 have shown that in cells containing normal amount of STIM1, the large flux of Ca2+ ions through SOC channels clusters exceeds the capacity of SERCA. However, in physiological conditions, the excess is taken up by mitochondria and then redirected to SERCA.9 Several data suggest that STIM1 may be also a key regulator of canonical transient receptor potential channels (TRPCs),11 which play an important role in SOCE. Finally, in a genome-wide drosophila RNAi screen, STIM1 silencing was associated with a strong repression of nuclear factor of activated T cell (NFAT) activity.12 The calcium-dependent serine/threonine protein phosphatase calcineurin has been identified as a central pro-proliferative factor in VSMC.3,13,14,15,16,17 Recently, STIM1 expression was reported in human airway smooth muscle and in vascular SMC.18,19,20
The aim of this study was to test whether STIM1 is present in arterial smooth muscle cells and whether silencing STIM1 could prevent restenosis in vivo and to determine the molecular mechanism involved in inhibition of proliferation.
Results
STIM1 was expressed in VSMCs
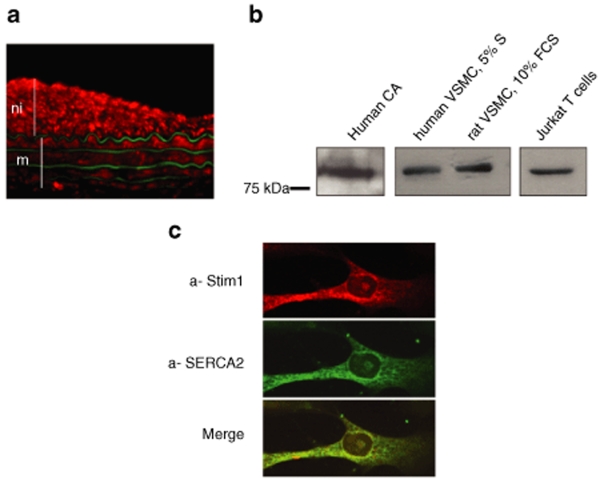
Immunofluorescence analysis of balloon-injured rat carotid arteries (a well-characterized model of SMC proliferation) revealed that STIM1 was expressed in the media as well as in highly proliferative SMC in the neointima (Figure 1a). The expected 90 kDa protein (the same molecular weight than the protein observed in human Jurkat T cell) was present in both VSMCs isolated from human coronary artery smooth muscle cell (hCASMC) and in rat aorta smooth muscle cell (Figure 1b). Confocal immunofluorescence analysis in isolated VSMCs revealed a predominant endoplasmic reticulum distribution of STIM1, which was similar to the one of SERCA2, an endoplasmic reticulum marker (Figure 1c). Negative controls are shown in supplementary data (Supplementary Figure S1).
Figure 1.
STIM1 is expressed in vascular smooth muscle cells (VSMCs). (a) Detection of STIM1 by immunofluorescence in a rat balloon-injured carotid artery. STIM1 is labeled in red, with a-STIM1; the green fluorescence represents autofluorescence of elastin and identifies the media. m: media, ni: neointima (b) Western blots of total extracts from human coronary artery (CA), human and rat VSMC and of Jurkat T cells hybridized with anti-GOK/Stim. (c) Confocal imaging of hCASMC labeled with anti-STIM1 and anti-SERCA2 (IID8).
STIM1 was upregulated in proliferative VSMC
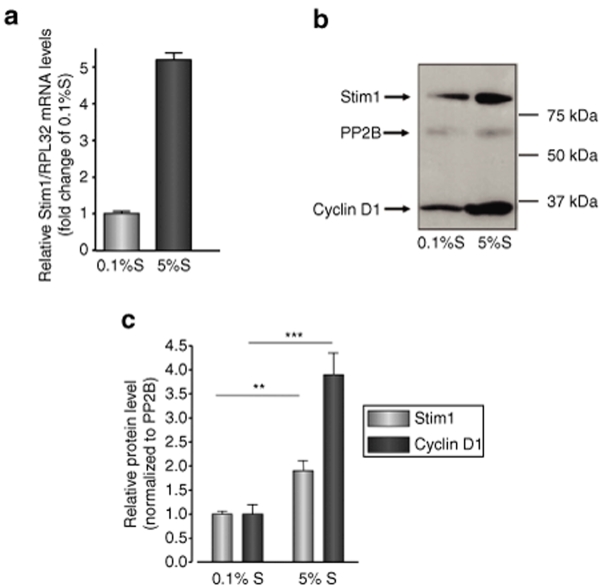
Relative expression level of STIM1 mRNA was obtained by quantitative real-time PCR in quiescent (0.1 % supplement mix, S, cultured hCASMC) and proliferative (5% S cultured hCASMC), showing a 5.2 ± 0.3-fold upregulation in proliferative condition (Figure 2a).
Figure 2.
STIM1 is upregulated in proliferative VSMC. (a) Relative STIM1 mRNA levels normalized to RPL32 mRNA in quiescent (0.1%S) and proliferative (5% S) hCASMC. (b) Western blot showing expression of STIM1, calcineurin (PP2B), and cyclin D1 in quiescent (0.1%S) and proliferative (5% S) hCASMC. (c) STIM1 (gray bars) and cyclin D1 (black bars) protein levels normalized to PP2B level in quiescent and proliferative hCASMC. **P < 0.01; ***P < 0.001.
Semiquantitative evaluation of STIM1 protein level 72 hours after exposition to supplement mix was obtained by integrated density analysis of immunoblotting, showing a 1.9 ± 0.3-fold overexpression in proliferative condition (P < 0.01), which correlated with the overexpression of the SMC proliferation marker cyclinD1 (Figure 2b,c). Of note, STIM1 protein level extracted 48 hours after exposition to supplement mix was not different from 0.1% protein level, which suggests that overexpression needs at least 72 hours to be seen on immunoblotting (Supplementary Figure S2).
RNA interference-induced STIM1 silencing inhibited hCASMC proliferation in vitro
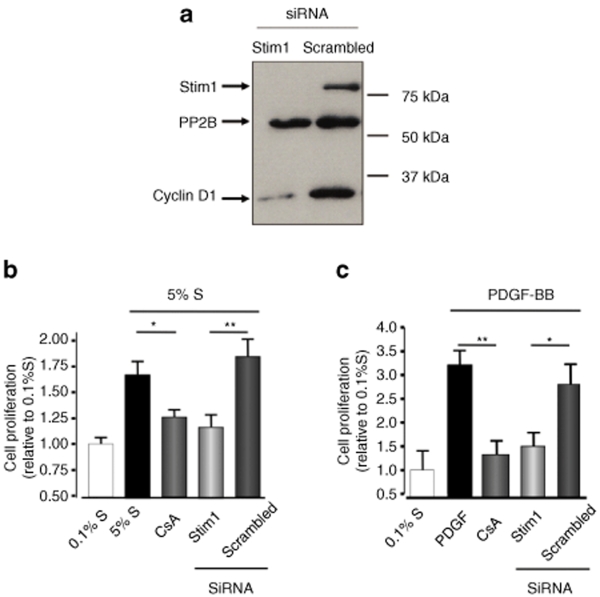
To further investigate the role of STIM1 in hCASMC proliferation, we used a RNAi-based strategy to specifically silence STIM1 expression. Two siRNA common to human and rat STIM1 mRNA were designed. STIM1 siRNA transfection (50 nmol/l) in cultured hCASMC induced a potent silencing of mRNA and protein: 72 hours after transfection, STIM1 mRNA was decreased by 91 ± 3% and the protein by 95 ± 4% (Figure 3a) compared to scrambled siRNA-transfected cells.
Figure 3.
STIM1 knockdown inhibited hCASMC proliferation in vitro. (a) Western blot showing the disappearance of STIM1 and the reduction of cyclin D1 expression 72 hours after transfection with STIM1 siRNA compared to the negative control (scrambled) siRNA. (b) Proliferation (measured by BrDU incorporation) of hCASMC in presence of 5% supplement mix or (c) 50 nmol/l PDGF-BB in control cells or cells transfected with STIM1 or scrambled siRNA for 72 hours, or treated with 5 µmol/l cyclosporin A (CsA) for 24 hours. *P < 0.05; **P < 0.01.
Supplement mix–induced proliferation was significantly lower in hCASMC transfected with STIM1 siRNA than in those transfected with scrambled siRNA (increase relative to 0.1% S: 116 ± 12% and 184 ± 16%, respectively, P < 0.01, Figure 3b). Such inhibition was similar to the one observed with cyclosporine A, a classical calcineurin inhibitor. An identical pattern was observed when hCASMC were stimulated with the platelet-derived growth factor (platelet-derived growth factor-BB), a more specific stimulator of NFAT-mediated signaling in VSMC (Figure 3c). Similar results were obtained with alternatively designed and validated STIM1 siRNA (data not shown). Finally, we observed that STIM1 silencing did not induce apoptosis of hCASMC (Supplementary Figure S3).
Adenoviral vector expressing specific STIM1 shRNA prevented in vivo neointima formation in rat injured carotid artery
To assess the role of STIM1 in preventing VSMC proliferation in vivo, we then infected balloon-injured rat carotid arteries with an adenoviral vector expressing a short hairpin RNA against rat STIM1 mRNA (Ad-shSTIM1, Figure 4a). The capacity of Ad-shSTIM1 to silence STIM1 expression was verified in vitro on rat arterial SMC. Seven days after infection, STIM1 protein level was reduced by 81 ± 10% in cells infected with Ad-shSTIM1 compared to cells infected with the same adenovirus expressing a luciferase shRNA (Ad-shLuc) (Supplementary Figure S4).
Figure 4.
Adenoviral vector expressing specific STIM1 shRNA prevented in vivo neointima formation in rat injured carotid artery. (a) Sequence of STIM1 shRNA. (b) Representative hematoxylin-eosin sections of noninjured (NI) and injured carotid arteries infected with Ad-sh Luciferase or Ad-sh STIM1, 14 days after surgery. (c) Average intima/media thickness ratios for the above three groups (***P < 0.001 compared with Ad-shLuc). M indicates media; ni, neointima; and ad, adventitia (n = 5 for noninjured carotid, n = 4 for Ad-shLuc and n = 6 for Ad-shSTIM1). (d) PCR analysis of DNA extracted from the vessels in the respective conditions. (e) Immunofluorescence analysis of expression of STIM1 in carotid arteries 14 days after injury.
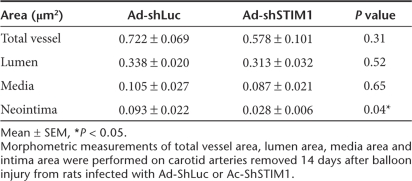
Two weeks after injury and infection with 1011 viral genomes of either Ad-shSTIM1 or Ad-shLuc, rats were sacrificed and morphometric analysis of injured carotids was performed on hematoxylin/eosin-stained cross-sections (Figure 4b). The degree of restenosis was determined by measuring intima and media areas and by calculating the intima/media (I/M) area ratio. I/M ratios were significantly lower in Ad-shSTIM1-infected arteries than in Ad-shLuc-infected arteries (0.34 ± 0.02 vs. 0.92 ± 0.11, P < 0.006, Figure 4c). The morphological analysis showed that intima formation was significantly reduced in Ad-ShSTIM1-infected arteries (Table 1). Similar data were obtained by measuring intima and media thickness (data not shown). To confirm adenoviral infection, carotid DNA was extracted from each sample and adenovirus DNA was detected by PCR with specific primers. A specific band was typically detected in carotids infected with either Ad-ShLuc or Ad-ShSTIM1 but not in noninfected carotids (Figure 4d). We also analyzed sections from carotids infected with another adenovirus encoding for shSTIM plus a fluorescent dye (DsRed) under the control of two different promoters. As shown in Supplementary Figure S5, DsRed was detected in the media of infected carotid arteries showing the efficient gene transfer in our model (Supplementary Figure S5). Finally, as an approach to certify STIM1 silencing in this in vivo model, immunofluorescence analysis showed that STIM1 was strongly expressed in the media and intima of carotids infected with Ad-shLuc (Figure 4e). We did not observe such expression of STIM1 in carotids infected with Ad-shSTIM1 as well as in noninjured carotids (Figure 4e). Such result suggests that STIM1 expression was efficiently decreased following in vivo administration of Ad-shSTIM1. These results show that inhibition of STIM1 activity in turn inhibits VSMC proliferation in vitro and balloon injury-induced neointima formation in vivo.
Table 1.
Morphological analysis of vessels
STIM1 silencing inhibited TRP single-channel activity
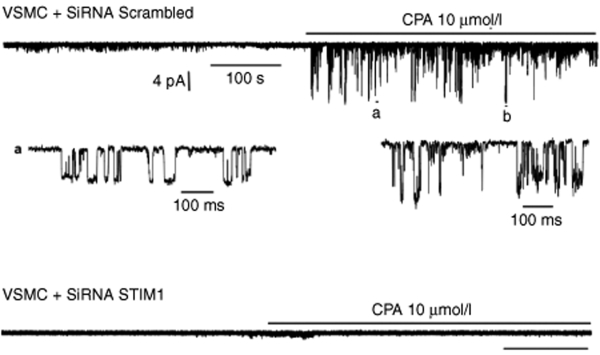
Channel activity was recorded over long periods of time in the cell membrane of hCASMCs cultured in the presence of serum and growth factors and transfected with either scrambled siRNA or STIM1 siRNA (Figure 5). We have use cyclopiazonic acid (CPA) to manipulate the intracellular Ca2+ level. By inhibiting SERCA, CPA induces SR/ER Ca2+ depletion. This will activate Ca2+ entry through store-operated channels or Ca2+ release–activated channels. The potential applied to the patch was maintained at −80 mV. When recorded in cells transfected with scrambled siRNA, application to the cell of CPA, (10 µmol/l) markedly enhanced activity of nonselective cation channels, as exemplified in Figure 5, upper trace. Short segments extracted from the above recording exhibited the gating pattern of these channels which shared similitude with the reported TRP channel activity in identical recording conditions.21 The larger conductance level was 103 ± 8 pS (n = 6). However, recordings from cells transfected with STIM1 siRNA, in same conditions, failed to exhibit channel activity upon application of CPA (Figure 5, lower trace), with a NPo of 0.0003 ± 0.00018 (n = 15) compared to 0.095 ± 0.06 (n = 8), for control; P < 0.001. Identical results were observed in cells infected with Ad-shSTIM1 compared to cells infected with Ad-shLuc (data not shown).
Figure 5.
STIM1 silencing inhibited TRP single-channel activity. Representative single-channel activity recordings obtained from cell-attached membranes on hCASMC transfected with either scrambled siRNA (upper trace) or either STIM1 siRNA (lower trace) at a patch membrane potential maintained to −80 mV. Extracts a and b are time-scale expansion of the channel activity from the above trace.
RNA interference–induced STIM1 silencing prevented NFAT nuclear translocation and activity
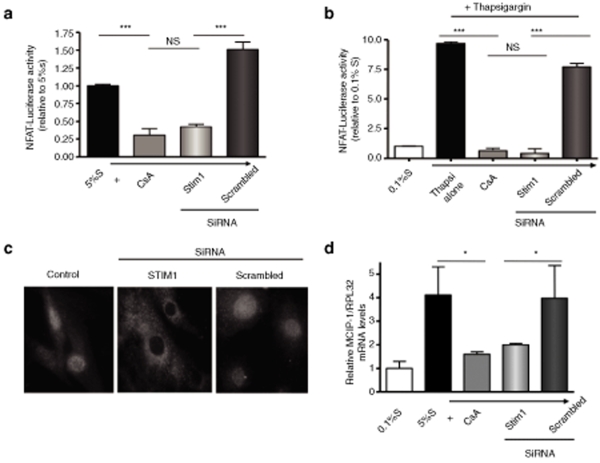
To determine the pathway relating STIM1 to proliferation, we tested the activity of the Ca2+-regulated transcription factor NFAT. NFAT activity was evaluated by measuring the activity of a NFAT-driven luciferase construct cotransfected with either STIM or scrambled siRNA. STIM1 siRNA-transfected cells had a much lower luciferase activity than scrambled siRNA-transfected cells (relative value of control hCASMC in 5% S 42 ± 4% vs. 151 ± 10%, P < 0.001), comparable to that of CsA-treated cells (30 ± 9%, P = NS) (Figure 6a). This effect was also observed in response to thapsigargin (TG): TG increased NFAT activity compared to control and the TG-dependent activation of NFAT was drastically decreased in STIM1 siRNA-transfected cells (Figure 6b).
Figure 6.
RNA interference-induced STIM1 silencing prevented NFAT nuclear translocation and activity. (a,b) Measurement of NFAT activity using a NFAT-driven luciferase construct in control cells as in a or cells treated with thapsigargin (1 µmol/l for 6 hours) as in b (P < 0.001). (c) Localization of NFAT protein by immunofluorescence. (d) Measurement of the relative MCIP mRNA level normalized to RPL32 mRNA. Cyclosporin A (CsA) is used as a negative control (*P < 0.05).
In control cells (5% S) as well as in scrambled siRNA- transfected cells, NFAT was mainly in the nucleus, whereas in STIM1 siRNA-transfected cells NFAT was in the cytosol (Figure 6c). Finally we measured the expression of MCIP1 (modulatory calcineurin interacting protein1) a gene driven by NFAT. MCIP1 mRNA was increased in the presence of growth supplement (5% S). Inhibition of calcineurin by CsA prevented MCIP1 expression, as expected. MCIP1 mRNA level was much lower in STIM1 siRNA-transfected cells than in scrambled siRNA-transfected cells (P < 0.05, Figure 6d). Together these data indicate that silencing STIM1 resulted in NFAT inactivation.
Discussion
We demonstrated that STIM1 is a major regulator of VSMC proliferation in vitro and in vivo, representing a novel and original pharmacological target for prominent vascular proliferative diseases. First, we observed that STIM1 expression is increased in proliferative VSMC as stated by its high expression in the neointima of injured rat carotid arteries and the overexpression in serum- and growth factors–induced proliferating VSMC. Second, STIM1 silencing suppressed serum-induced hCASMC proliferation confirming previous results by Takahashi et al.20 More important, we now established that STIM1 silencing by specific RNA interference inhibited restenosis in vivo in a model of carotid angioplasty in the rat, demonstrating its potential interest as a therapeutic target to prevent restenosis after vascular interventions.
By using cell-attached recordings on membranes from proliferating hCASMC, we demonstrated that STIM1-knockdown completely blocked cationic channels, which are activated following CPA application. Takahashi reported that STIM1 siRNA-inhibited TG-induced Ca2+ transient.20 This is in agreement with a role of STIM1 in activation of store-operated Ca2+ entry (SOCE) or Ca2+ release–activated channels (ICrac). Several proteins were shown to be component of SOCE: STIM1, STIM2, Orai1, and TRPCs.22 Gene inactivation23 and RNAi24 studies supported a role of TRPCs in SOCE. STIM1 carboxyl-terminus was shown to activate native SOC and TRPC1 channels11 but silencing STIM1 still inhibited SOCE in TRPC1-deficient cells.18 This indicates that TRPC1 is not the only channel involved in SOCE. Recently, Liao et al. proposed that Orai proteins, by interacting with TRPCs, act as regulatory subunits that confer STIM1-mediated store depletion sensitivity to TRP channels.21 In this study, Orai1 was shown to physically interact with TRPC3 and TRPC6.
SOCE is upregulated in pulmonary artery smooth muscle cells in culture.25 In agreement with an increasing role of SOCE in proliferating cells, TRPC1 is shown to be upregulated during proliferation of smooth muscle cells in culture26 and in neointimal hyperplasia in vivo,27 whereas antisense inhibition of TRPC1 attenuated proliferation.28 In addition, platelet-derived growth factor stimulates pulmonary SMC proliferation through activation of TRPC6.29 Because SOCE results from the activation of multiple channels, it will be difficult to reduce SOCE by targeting a single-channel type. The originality of this study is that we targeted a regulator of SOCE and not the channel itself.
STIM1 is also a partner of the sarco/endoplasmic reticulum Ca2+ ATPase, SERCA. Ablation of STIM1 inhibited TG-evoked Ca2+ entry without altering resting Ca2+ levels, Ca2+ release transients, or the membrane potential in normal cells.6,9 Furthermore, ablation of STIM1 neither inhibited SERCA activity nor prevented Ca2+ store refilling when cells were stimulated with physiological agonists.9 However, our results clearly showed that STIM1 is upregulated in proliferating hCASMC. STIM1 is also upregulated in proliferating rat mesenteric artery smooth muscle cells.30 Furthermore, it is well established that SERCA2a is repressed in these conditions.13,31 This suggests that an imbalance between the influx of Ca2+ through the membrane and the capacity of SERCA to take up Ca2+ in the ER appears in proliferating SMC.32 This should result in an increase in cytosolic [Ca2+], but basal cytosolic [Ca2+] was not increased in proliferating VSMC31 nor in cells treated with STIM siRNA,9,20 suggesting that the increase in Ca2+ is localized probably near the plasma membrane. Localized [Ca2+] increase may activate various signaling pathways through activation of kinases or phosphatases.33,34
We demonstrated that silencing STIM1 completely inhibited the NFAT activity. Such result is in agreement with its identification as a strong negative regulator of NFAT in a large RNAi screening in drosophila.12 Recent data have demonstrated that TRPC3 and TRPC6 are essential in the activation of the calcineurin-NFAT signaling pathway that drives pathologic cardiac remodeling.35,36,37 The sustained Ca2+ influx due to activation of SOC is necessary to activate calcineurin, a Ca2+/calmodulin-dependent phosphatase, which dephosphorylates many proteins; one such protein is the transcription factor NFAT. NFAT dephosphorylation results in its rapid import into the nucleus and thus to the activation of pro-proliferative or prohypertrophic gene expression program in numerous in vitro and in vivo models.3,4,14,15,16,17,32,38
In conclusion, silencing STIM1, by preventing store-operated channel activation of the calcineurin/NFAT transcription pathway, is an original way to inhibit VSMC proliferation and neointimal formation.
Material and Methods
Human VSMCs culture. Fragments of left anterior descending coronary artery were dissected from human explanted hearts. VSMCs were isolated from the medial layer by enzymatic digestion. Briefly, fragments of media were incubated in SMCBM2 (PromoCell, Heidelberg, Germany) with collagenase (CLS2, 50 U/ml; Worthington, Lakewood, NJ) and pancreatic elastase (0.25 mg/ml; MP Biomedicals, Illkirch, France) for 4–6 hours at 37 °C. After periods of 30 minutes, the suspension was centrifuged at 1,000 rpm for 3 minutes, and the cells were collected and placed in SMCBM2 supplemented with 20% supplement mix (S) (Promocell). The cells obtained in the first 30-minutes period were discarded. Those obtained in the other cycles were pooled and cultured in SMCBM2 containing S (5%) and antibiotics at 37 °C and 5% of CO2. Cells were used between passages 2 and 8.
Protein and RNA expression. Immunofluorescence was performed on paraformaldehyde-fixed tissue sections or on methanol-fixed isolated cells using a polyclonal antihuman STIM1 antibody (provided by J Roos or the commercially available a-STIM1: GOK), or an anti-SERCA2 (IID8, Ab2817; Abcam, Cambridge, MA). Immunofluorescence analysis on tissues was done after paraformaldehyde fixation using GOK 1/100. Total cell extracts proteins were prepared using RIPA and separated on a 10% acrylamide gel. The following antibodies were used: GOK/Stim1 (no. 610954; BD Transduction Laboratories), a-NFATc1 (K-18, Santa Cruz sc-1149; Santa Cruz Biotechnology, Heidelberg, Germany), PP2B (BD Transduction Laboratories, San Jose, CA; no. 610259), and cyclin D1 (BD Pharmingen, no. 556470).
siRNA and Ad-shRNA constructs. Silencing RNA were designed by using the academic Web-based siRNA design program SiSearch. The sense sequence is 5′-GGGAAGACCUCAAUUACCAdtdt-3′. A nonsilencing siRNA with no homology with mammalian genes (All Stars Negative Control, Qiagen, Germantown, MD) was used in parallel (scrambled siRNA).
The shRNA was designed using the SiSearch program to recognize rat and human STIM1 mRNA sequences. Oligonucleotides were annealed and ligated via BamHI/EcoRI into pSIREN vector using Knockout RNAi Systems technology (Clontech, Mountain View, CA). The recombinant pSIREN was then transformed in Escherichia coli, using Fusion-Blue Competent Cells (Clontech). The fragment was cut out with PI-Sce I/I-Ceu I and inserted into Adeno-X Viral DNA via PI-Sce I/I-Ceu I using Adeno-X Expression System 1 (Clontech). The resulting adenovirus was transfected into HEK293 cells and propagated, thereby generating the AdVs-designated Ad-shSTIM1 and Ad-shLuc. To better appreciate viral gene transfer into rat carotid arteries, another adenovirus containing the same sequence of shRNA, not only targeting STIM1 but also expressing the fluorescent dyer DsRed (under the control of a CMV promotor), was obtained using Knockout Adenoviral RNAi System 2 (Clontech), and the RNAI-ready pSIREN-DNR-DsRed-Express Donor Vector (Clontech). This vector was designated as Ad-ShSTIM1-DsRED.
To test the efficiency of the adenoviral constructs, VSMC were isolated from the media of the thoracic aorta of male Wistar rats and cultured as previously described.31
Cell proliferation. Human smooth muscle cells were cultured in 96-well tissue culture plates for 3 days in Smooth Muscle Cell Basal Medium 2 supplemented with 5% S. Medium containing 0.1% S was used for the growth-arrest control. Cells were incubated with siRNA for 72 hours in medium containing 5% S. BrdU was added for the last 16 hours. The plates were washed, and a colorimetric BrdU cell proliferation assay was performed according to the manufacturer's instructions (Roche, Basel, Switzerland).
Cell transfection. Cells were transfected with siRNA (50 nmol/l) in S-free medium for 6 hours, then the medium was replaced with S-containing medium for a further 66 hours. Transfection was performed using Lipofectamine 2000 (Invitrogen, Paisley, UK) or electroporation using Amaxa Nucleofector technology according to the manufacturer's instructions.
Single-channel recordings and data analysis. Unitary TRPC currents were recorded by the mean of the patch-clamp technique in the cell- attached configuration with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA). The patch pipettes were filled with (mmol/l) the following: 104 NaCl, 0.1 EGTA, and 10 HEPES at pH 7.4. The extracellular bath solution contained (mmol/l) the following: 145 KCl, 2 MgCl2, 0.1 CaCl2, and 10 HEPES at pH 7.4. Experiments were performed at 20–24 °C. Single-channel records were analyzed with Acquis1 software (G. Sadoc, CNRS, Gif/Yvette, France), to determine channel unitary current amplitude and activity (NPo).
Reporter gene assays. Cells were cotransfected with the siRNA (50 nmol/l) and the NFAT-Luciferase reporter gene plasmid (Stratagene, La Jolla, CA) by electroporation using Amaxa Nucleofector technology according to the manufacturer's instructions. After 6 hours the medium was replaced with medium containing 5% S for 66 hours. The results are the means of three independent experiments performed in triplicate. Luciferase activity was measured by using a kit from Promega (Madison, WI). The relative luciferase activity was the value obtained after normalization to total protein and expressed as a percent of the control value.
Rat carotid artery injury and histomorphometry. The animals were treated in accordance with our institutional guidelines. The left external carotid artery of adult male Wistar rats (CERJ, France) weighing 350–400 g was injured as previously described.3 Nontagged adenoviruses containing shSTIM1 or shLuciferase were incubated for 30 minutes after angioplasty. Two weeks after surgery, the carotids were collected. The luminal area (area circumscribed by the intimal border), intimal area (area between the lumen and the internal elastic lamina), medial area (area between the internal and the external elastic lamina), total vessel area, and intima to media ratio (I/M) (intimal area divided by medial area) were determined by computerized digital planimetry, using a videomicroscope with a dedicated image-analyzing software (Lucia Software; Nikon, Tokyo, Japan). DNA from injured rat carotids was extracted using standard procedures (Puregene DNA Purification Kit from Gentra (Minneapolis, MN)). Adenovirus expression was then controlled by PCR using a sense primer targeting the inserted expression cassette (5′-TCTTGTGGAAAGGACGAGGA-3′) and an antisense primer located in the adenoviral DNA (5′-ATCAAACGAGTTGGTGCTCA-3′). To better appreciate viral transfer, the same in vivo experiment was then realized with AdshSTIM1-DsRed. Viral gene transfer was controlled using direct immunofluorescence analysis of dsRed (Λ = 594) in rat carotid arteries cross sections.
Statistical analysis. All quantitative data are reported as means ± SEM. Statistical analysis was performed with the Prism software package (GraphPad v4). One-way analysis of variance or nonparametrical tests (Mann–Whitney test) when appropriate were used to compare continuous parameter. Post hoc t-test comparisons were performed to identify which group differences accounted for significant overall analysis of variance results. Differences were considered significant when P < 0.05.
Supplementary MaterialFigure S1. Immunofluorescence of a rat carotid artery (a) or an isolated cell (b).Figure S2. STIM1 is not overexpressed in 5% SM after 48 hours.Figure S3. STIM1 silencing is not associated with hCASMC apoptosis.Figure S4. Adenovirus-shSTIM1 decreased STIM1 mRNA and protein in rat SMC.Figure S5. Analysis of DsRed immunofluorescence in Ad-shSTIM1-DsRed infected carotid artery.
Supplementary Material
Immunofluorescence of a rat carotid artery (a) or an isolated cell (b).
STIM1 is not overexpressed in 5% SM after 48 hours.
STIM1 silencing is not associated with hCASMC apoptosis.
Adenovirus-shSTIM1 decreased STIM1 mRNA and protein in rat SMC.
Analysis of DsRed immunofluorescence in Ad-shSTIM1-DsRed infected carotid artery.
Acknowledgments
We thank Dr Jack Ross (La Jolla, CA) for providing the polyclonal anti human STIM1 antibody. This work is supported by Fondation Leducq through the CAERUS network (research agreement 05CVD03 to AML). YS is the recipient of a PhD fellowship from the French MENRS.
REFERENCES
- Garg P., and , Mauri L. The conundrum of late and very late stent thrombosis following drug-eluting stent implantation. Curr Opin Cardiol. 2007;22:565–571. doi: 10.1097/HCO.0b013e3282f02100. [DOI] [PubMed] [Google Scholar]
- Luscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115:1051–1058. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- Lipskaia L, del Monte F, Capiod T, Yacoubi S, Hadri L, Hours M, et al. Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ Res. 2005;97:488–495. doi: 10.1161/01.RES.0000180663.42594.aa. [DOI] [PubMed] [Google Scholar]
- Lipskaia L., and , Lompre AM. Alteration in temporal kinetics of Ca2+ signaling and control of growth and proliferation. Biol Cell. 2004;96:55–68. doi: 10.1016/j.biolcel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Cahalan MD, Zhang SL, Yeromin AV, Ohlsen K, Roos J., and , Stauderman KA. Molecular basis of the CRAC channel. Cell Calcium. 2007;42:133–144. doi: 10.1016/j.ceca.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba Y, Hayashi K, Fujii Y, Mizushima A, Watarai H, Wakamori M, et al. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousset H, Frieden M., and , Demaurex N. STIM1 knockdown reveals that store-operated Ca2+ channels located close to sarco/endoplasmic Ca2+ ATPases (SERCA) pumps silently refill the endoplasmic reticulum. J Biol Chem. 2007;282:11456–11464. doi: 10.1074/jbc.M609551200. [DOI] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa DL, Beck A, Nadler MJ, Koblan-Huberson M, et al. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, et al. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Sharma S, Nardone J, Tanasa B, Iuga A, Srikanth S, et al. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature. 2006;441:646–650. doi: 10.1038/nature04631. [DOI] [PubMed] [Google Scholar]
- Lipskaia L, Pourci ML, Delomenie C, Combettes L, Goudouneche D, Paul JL, et al. Phosphatidylinositol 3-kinase and calcium-activated transcription pathways are required for VLDL-induced smooth muscle cell proliferation. Circ Res. 2003;92:1115–1122. doi: 10.1161/01.RES.0000074880.25540.D0. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang C, Dronadula N, Li Q., and , Rao GN. Blockade of nuclear factor of activated T cells activation signaling suppresses balloon injury-induced neointima formation in a rat carotid artery model. J Biol Chem. 2005;280:14700–14708. doi: 10.1074/jbc.M500322200. [DOI] [PubMed] [Google Scholar]
- Nilsson LM, Sun ZW, Nilsson J, Nordstrom I, Chen YW, Molkentin JD, et al. Novel blocker of NFAT activation inhibits IL-6 production in human myometrial arteries and reduces vascular smooth muscle cell proliferation. Am J Physiol Cell Physiol. 2007;292:C1167–C1178. doi: 10.1152/ajpcell.00590.2005. [DOI] [PubMed] [Google Scholar]
- Yellaturu CR, Ghosh SK, Rao RK, Jennings LK, Hassid A., and , Rao GN. A potential role for nuclear factor of activated T-cells in receptor tyrosine kinase and G-protein-coupled receptor agonist-induced cell proliferation. Biochem J. 2002;368:183–190. doi: 10.1042/BJ20020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Sliedregt-Bol K, Overkleeft H, van der Marel GA, van Berkel TJ., and , Biessen EA. Therapeutic potential of a synthetic peptide inhibitor of nuclear factor of activated T cells as antirestenotic agent. Arterioscler Thromb Vasc Biol. 2006;26:1531–1537. doi: 10.1161/01.ATV.0000225286.30710.af. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Kalwa H, Storch U, Mederos YSM, Salanova B, Pinkenburg O, et al. Pressure-induced and store-operated cation influx in vascular smooth muscle cells is independent of TRPC1. Pflugers Arch. 2007;455:465–477. doi: 10.1007/s00424-007-0314-3. [DOI] [PubMed] [Google Scholar]
- Peel SE, Liu B., and , Hall IP. A key role for STIM1 in store operated calcium channel activation in airway smooth muscle. Respir Res. 2006;7:119. doi: 10.1186/1465-9921-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Watanabe H, Murakami M, Ono K, Munehisa Y, Koyama T, et al. Functional role of stromal interaction molecule 1 (STIM1) in vascular smooth muscle cells. Biochem Biophys Res Commun. 2007;361:934–940. doi: 10.1016/j.bbrc.2007.07.096. [DOI] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL., and , Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci USA. 2007;104:4682–4687. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, et al. Emerging perspectives in store-operated Ca2+ entry: roles of Orai, Stim and TRP. Biochim Biophys Acta. 2006;1763:1147–1160. doi: 10.1016/j.bbamcr.2006.08.050. [DOI] [PubMed] [Google Scholar]
- Freichel M, Vennekens R, Olausson J, Stolz S, Philipp SE, Weissgerber P, et al. Functional role of TRPC proteins in native systems: implications from knockout and knock-down studies. J Physiol. 2005;567:59–66. doi: 10.1113/jphysiol.2005.092999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba T, Watanabe H, Murakami M, Takahashi Y, Iino K, Kuromitsu S, et al. Upregulation of TRPC1 in the development of cardiac hypertrophy. J Mol Cell Cardiol. 2007;42:498–507. doi: 10.1016/j.yjmcc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Golovina VA. Cell proliferation is associated with enhanced capacitative Ca(2+) entry in human arterial myocytes. Am J Physiol. 1999;277:C343–C349. doi: 10.1152/ajpcell.1999.277.2.C343. [DOI] [PubMed] [Google Scholar]
- Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, et al. Upregulated TRP and enhanced capacitative Ca(2+) entry in human pulmonary artery myocytes during proliferation. Am J Physiol Heart Circ Physiol. 2001;280:H746–H755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- Kumar B, Dreja K, Shah SS, Cheong A, Xu SZ, Sukumar P, et al. Upregulated TRPC1 channel in vascular injury in vivo and its role in human neointimal hyperplasia. Circ Res. 2006;98:557–563. doi: 10.1161/01.RES.0000204724.29685.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney M, Yu Y, Platoshyn O, Zhang S, McDaniel SS., and , Yuan JX. Inhibition of endogenous TRP1 decreases capacitative Ca2+ entry and attenuates pulmonary artery smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L144–L155. doi: 10.1152/ajplung.00412.2001. [DOI] [PubMed] [Google Scholar]
- Yu Y, Sweeney M, Zhang S, Platoshyn O, Landsberg J, Rothman A, et al. PDGF stimulates pulmonary vascular smooth muscle cell proliferation by upregulating TRPC6 expression. Am J Physiol Cell Physiol. 2003;284:C316–C330. doi: 10.1152/ajpcell.00125.2002. [DOI] [PubMed] [Google Scholar]
- Berra-Romani R, Mazzocco-Spezzia A, Pulina MV., and , Golovina VA. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol. 2008;295:C779–C790. doi: 10.1152/ajpcell.00173.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallot O, Combettes L, Jourdon P, Inamo J, Marty I, Claret M, et al. Intracellular Ca(2+) handling in vascular smooth muscle cells is affected by proliferation. Arterioscler Thromb Vasc Biol. 2000;20:1225–1235. doi: 10.1161/01.atv.20.5.1225. [DOI] [PubMed] [Google Scholar]
- Lipskaia L, Hulot JS., and , Lompre AM.Role of sarco/endoplasmic reticulum calcium content and calcium ATPase activity in the control of cell growth and proliferation Pflugers Arch 2008. epub ahead of print [DOI] [PubMed]
- Dolmetsch RE, Lewis RS, Goodnow CC., and , Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Feske S, Srikanth S, Hogan PG., and , Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42:145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, et al. TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116:3114–3126. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H, Wilkin BJ, Bodi I., and , Molkentin JD. Calcineurin-dependent cardiomyopathy is activated by TRPC in the adult mouse heart. Faseb J. 2006;20:1660–1670. doi: 10.1096/fj.05-5560com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onohara N, Nishida M, Inoue R, Kobayashi H, Sumimoto H, Sato Y, et al. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006;25:5305–5316. doi: 10.1038/sj.emboj.7601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun. 2004;322:1178–1191. doi: 10.1016/j.bbrc.2004.07.121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunofluorescence of a rat carotid artery (a) or an isolated cell (b).
STIM1 is not overexpressed in 5% SM after 48 hours.
STIM1 silencing is not associated with hCASMC apoptosis.
Adenovirus-shSTIM1 decreased STIM1 mRNA and protein in rat SMC.
Analysis of DsRed immunofluorescence in Ad-shSTIM1-DsRed infected carotid artery.