Abstract
The tumor-tropic properties of neural stem cells (NSCs) led to the development of a novel strategy for delivering therapeutic genes to tumors in the brain. To apply this strategy to the treatment of brain metastases, we made a human NSC line expressing cytosine deaminase (F3.CD), which converts 5-fluorocytosine (5-FC) into 5-fluorouracil, an anticancer agent. In vitro, the F3.CD cells significantly inhibited the growth of tumor cell lines in the presence of the prodrug 5-FC. In vivo, MDA-MB-435 human breast cancer cells were implanted into the brain of immune-deficient mouse stereotactically, and F3.CD cells were injected into the contralateral hemisphere followed by systemic 5-FC administration. The F3.CD cells migrated selectively into the brain metastases located in the opposite hemisphere and resulted in significantly reduced volumes. The F3.CD and 5-FC treatment also decreased both tumor volume and number of tumor mass significantly, when immune-deficient mouse had MDA-MB-435 cells injected into the internal carotid artery and F3.CD cells were transplanted into the contralateral brain hemisphere stereotactically. Taken together, brain transplantation of human NSCs, encoding the suicide enzyme CD, combined with systemic administration of the prodrug 5-FC, is an effective treatment regimen for brain metastases of tumors.
Introduction
Brain metastasis is the most common intracranial tumor type in adults. In the resting state, the brain receives 15–20% of the body's blood flow, which increases the chance of circulating tumor cells reaching the brain.1,2 Consequently, brain metastases are common in cancer patients with an incidence rate of 12–35%.3,4,5 A solitary brain metastasis can be managed by local treatment modalities such as surgery or irradiation. However, many patients with brain metastasis harbor two or more metastases.1 In these cases, systemic chemotherapy is the sole treatment option and the intact blood–brain barrier (BBB) is largely impermeable to most chemotherapeutic drugs.
The discovery of the inherent tumor-tropic properties of neural stem cells (NSCs) potentially provides a novel approach to overcoming the primary challenge in developing chemotherapy regimens.6 A recent study showed that NSCs have the ability to target cytosine deaminase (CD) to brain metastases, where CD can convert the systemically administrated prodrug 5-fluorocytosine (5-FC) into the toxic anticancer agent 5-fluorouracil (5-FU).7 However, in this particular study,7 the NSCs were injected directly into the carotid artery. This delivery method cannot be used in the clinic nor can it be used to determine the migration potential of NSCs into brain metastases. It, therefore, remains to be determined whether NSCs migrate specifically to brain metastases and which injection time points result in the best therapeutic results. These questions should be addressed in order to realize the potential of NSC gene therapy.
We previously used an immortalized human NSC line (HB1.F3), expressing suicide genes, to target brain tumors. Using this approach, tumor burdens were significantly reduced in animal models with glioblastoma, medulloblastoma, and metastatic neuroblastoma, demonstrating the tumor tropic of the HB1.F3 cells.8,9,10,11 In this study, the NSC line was engineered to express the therapeutic CD gene (F3.CD) and shown to have the capacity to migrate selectively to brain metastases, where it significantly reduced the tumor burden after administration of the prodrug 5-FC.
Results
HB1.F3.CD—a human NSC line expressing CD
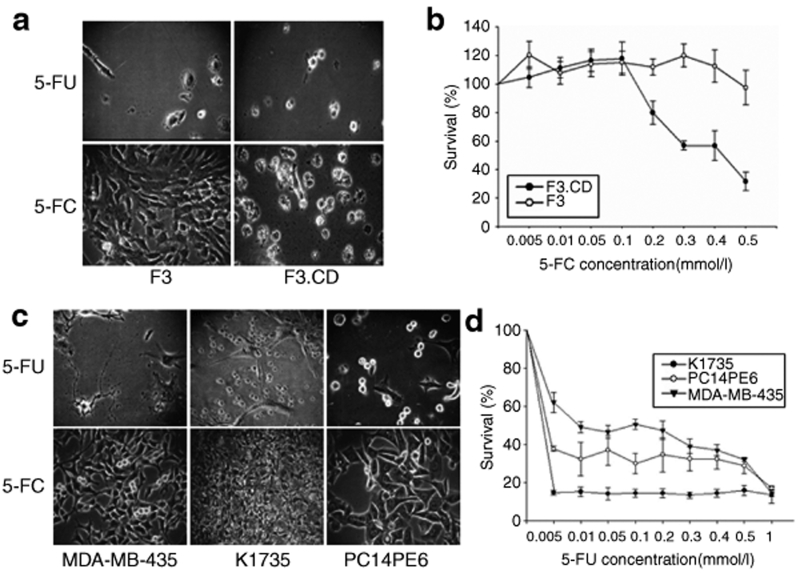
Expression of the CD transcript in F3.CD was confirmed by reverse transcription–PCR. As expected, the CD transcript was absent in the parental HB1.F3 (F3) cells (Supplementary Figure S1b). Next, sensitivity to 5-FC or 5-FU was determined for the F3.CD cells and compared with that of the parental F3 cells. The F3.CD cells were sensitive to 5-FC exposure at concentrations >0.2 mmol/l (P < 0.05, Figure 1a,b), which confirmed the enzymatic conversion of the prodrug 5-FC to the cytotoxic 5-FU. After 24 hours of 5-FC treatment, the F3.CD cells showed signs of mild toxicity, and after 48 hours, the toxic effect was very clear (Supplementary Figure S2).
Figure 1.
Cytosine deaminase (CD) from HB1.F3.CD (F3.CD) cells effectively converts the prodrug 5-fluorocytosine (5-FC) to 5- fluorouracil (5-FU). (a) F3 and F3.CD cells were treated with 0.5 mmol/l 5-FC or 5-FU for 4 days. (b) The cytotoxic effects of 5-FU and 5-FC were quantified by cell viability assays (0–1 mmol/l 5-FC or 5-FU for 48 hours). Each group, n = 4. (c,d) Influences of 5-FC and 5-FU on MDA-MB-435, K1735, and PC14PE6 cells were also checked using the same experimental methods.
MDA-MB-435 (a human breast cancer cell line), PC14PE6 (a human lung adenocarcinoma cell line), and K1735 cells (a mouse melanoma cell line) were not affected by 5-FC treatment even at 0.5 mmol/l, consistent with the lack of CD expression in these cells (data not shown). In contrast, all the cell lines were sensitive to 5-FU (Figure 1c,d). Among the tumor cell lines, the lowest sensitivity was observed with the MDA-MB-435 cells.
F3.CD coculture inhibits tumor cell growth
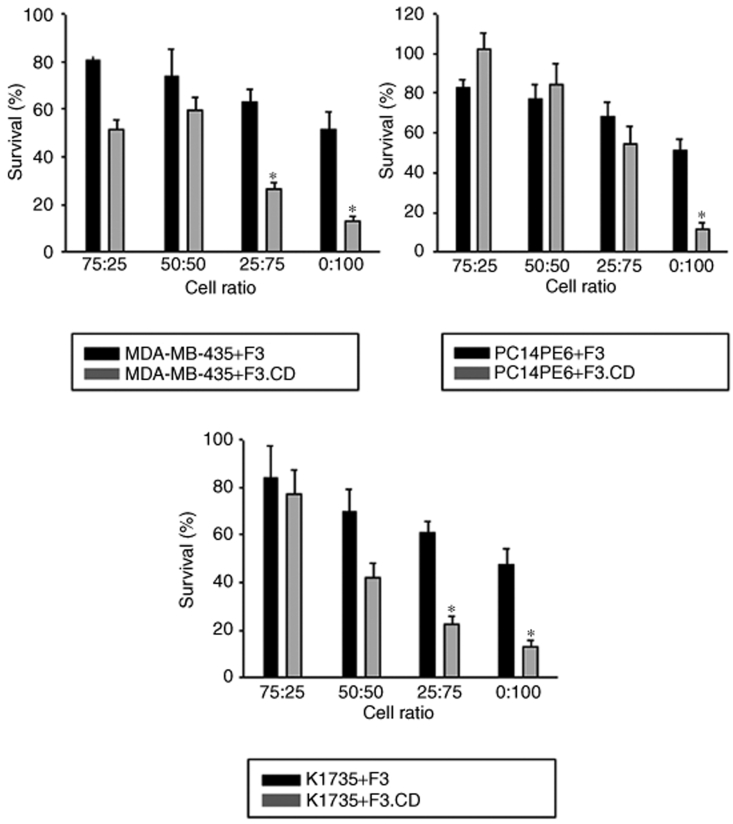
To confirm the “bystander effect” of the CD produced by the F3.CD cells, a coculture system was used to determine the effect on viability of cocultured tumor cells. In coculture with F3.CD cells, 0.5 mmol/l 5-FC significantly inhibited the growth of MDA-MB-435, PC14PE6, and K1735 cells (P < 0.05, Figure 2). This effect was not observed with the parental F3 cells. Similarly, without addition of 5-FC, the F3.CD coculture had no effect on the tumor cell growth (data not shown). These results show that F3.CD cells convert sufficient amounts of 5-FC to 5-FU to effectively kill MDA-MB-435, PC14PE6, and K1735 cells in vitro.
Figure 2.
5-Fluorouracil (5-FU) derived from 5-fluorocytosine (5-FC) was cytotoxic in vitro. The “bystander effect” of the cytosine deaminase (CD) produced by the F3.CD cells was confirmed using a coculture system. MDA-MB-435, PC14PE6, or K1735 cells and F3 or F3.CD cells were seeded in 96-well plates (total 5 × 103 cells per well, tumor cell:F3 or F3.CD cell = 100:0, 75:25, 50:50, 25:75, or 0:100). After 24 hours in coculture, cells were treated with 0.5 mmol/l 5-FC for 72 hours and cell viability determined (each group, n = 3). Survival (%) = percentage of the control viability (tumor cell only, = 100%). Columns = mean, bars = SD, *P < 0.05.
In vivo therapeutic efficacy of F3.CD cells
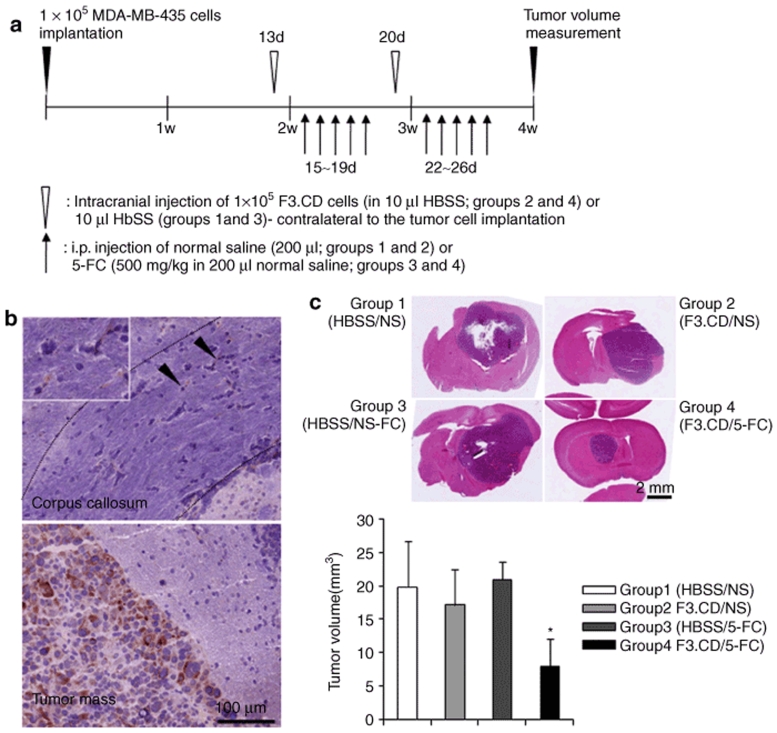
Next, the migrating potential and therapeutic efficacy of the F3.CD cells were determined. F3.CD cells were injected into the brain of MDA-MB-435 tumor-bearing animals, contralateral to the tumor cell implantation. The distribution of F3.CD cells and tumor volumes were determined in brain tissue 2 days after the last 5-FC treatment (Figure 3a). A large number of CD-immunoreactive F3.CD cells were identified in the tumor bed and at the tumor-normal parenchyma interface. Some migrating F3.CD cells were found in the corpus callosum (Figure 3b). Histological analysis showed significantly reduced tumor volumes in the brains of 5-FC-treated F3.CD animals: (Figure 3c; group 4, mean ± SD = 7.89 ± 4.18 mm3) compared the control groups [group 1, Hanks' balanced salt solution (HBSS) injection + saline, 19.81 ± 6.86 mm3; group 2, F3.CD injection + saline, 17.14 ± 5.24 mm3; group 3, HBSS injection + 5-FC, 20.94 ± 2.55 mm3]. The tumor sizes were reduced up to 60%. No abnormalities in the brain parenchyma surrounding the tumors were in treated animals.
Figure 3.
Treatment with F3.CD cells and 5-fluorocytosine (5-FC) has a significant in vivo therapeutic effect. (a) Timeline for the metastatic brain tumor animal model and subsequent treatment using F3.CD cells and 5-FC. (b) F3.CD cells were injected into the brain contralateral to the tumor-bearing hemisphere and localized using an anti-CD antiserum. Closed arrow heads indicated cytosine deaminase (CD)-immunoreactive F3.CD cells in the corpus callosum (upper panel) and the inset shows a magnified area. (c) Compared with the other groups, mice treated with F3.CD and 5-FC (group 4) showed significantly small tumor volumes (P < 0.05). Columns = mean, bars = SE. HBSS, Hanks' balanced salt solution; i.p., intraperitoneal.
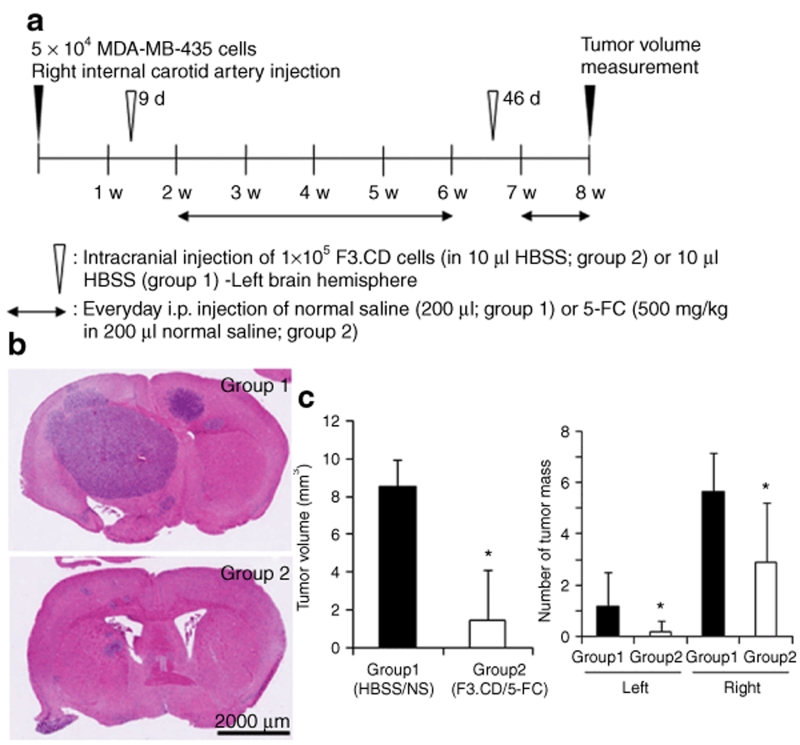
We confirmed the in vivo therapeutic efficacy of the F3.CD cells using another brain metastasis animal model, which was made by injection of MDA-MB-435 cells into the right internal carotid artery (ICA) of immune-deficient mouse. F3.CD cells were implanted into left brain hemisphere stereotactically and 5-FC was administrated systemically (Figure 4a). Both tumor volumes and numbers of tumor masses of treated mice were reduced significantly [Figure 4b,c; group 2, sum of tumor volumes = 1.46 ± 2.67 mm3 (mean ± SD), number of tumor masses in right and left brain hemispheres = 2.90 ± 2.33 and 0.20 ± 0.42, respectively], compared with those of control mice (group 1, HBSS injection + saline, sum of tumor volumes = 8.54 ± 1.42 mm3, number of tumor masses in right and left brain hemisphere = 5.67 ± 1.50 and 1.17 ± 1.33, respectively). Although F3.CD cells were implanted contralateral to the tumor cell injection, number of brain metastasis masses was decreased significantly in both hemispheres (Figure 4c).
Figure 4.
F3.CD cells have significant in vivo therapeutic effects on blood-born brain metastasis. (a) Timeline for the blood-born brain metastasis animal model and subsequent treatment using F3.CD cells and 5-fluorocytosine (5-FC). (b) Volume of MDA-MB-435 brain tumor mass and tumor mass number were determined 2 days after the last 5-FC treatment. (c) Compared with the control mice [group 1, Hanks' balanced salt solution (HBSS) injection + saline], mice treated with F3.CD and 5-FC (group 2) showed significantly smaller tumor volume (left panel) and less tumor mass number in both hemispheres (right panel). Columns = mean, bars = SD. Left, left hemisphere; right, right hemisphere. *P < 0.05. i.p., intraperitoneal.
In vivo effect of F3.CD injection on large brain metastases
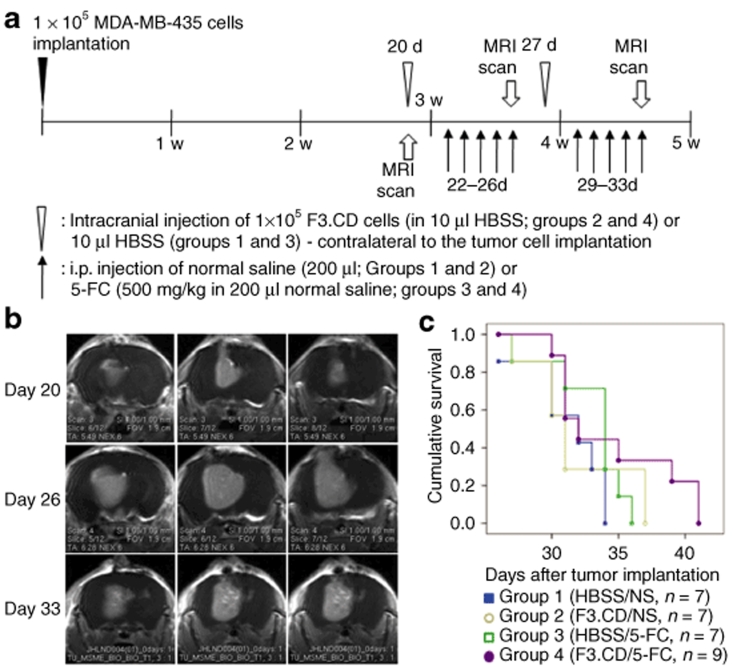
To test whether the F3.CD cells had therapeutic effects on brain metastasis when the F3.CD and 5-FC treatments started after brain metastasis grew large, the F3.CD and 5-FC treatments were started 1 week later than in the experiments described above. The therapeutic effects were determined by magnetic resonance imaging scans and survival analysis (Figure 5a). Some animals treated with F3.CD and 5-FC (group 4) showed tumor volume reduction in the magnetic resonance imaging scans (Figure 5b), but there was no difference in survival [Figure 5c; group 1 (HBSS injection + saline), median survival after tumor cell implantation, 32 days (26–34 days); group 2 (F3.CD injection + saline), 31 days (27–37 days); group 3 (HBSS injection + 5-FC), 34 days (27–36 days); group 4 (F3.CD injection + 5-FC, 32 days (30–41 days)—P = 0.221].
Figure 5.
F3.CD cells had little therapeutic effect on large brain metastasis. To test whether F3.CD had therapeutic effects on metastatic brain tumors when the F3.CD and 5-fluorocytosine (5-FC) treatment started after brain metastasis grew large, the treatment was started 1 week later than in the experiment above (Figure 3a). (a) Timeline for the experiment. The therapeutic effect was determined using (b) magnetic resonance imaging (MRI) scans and (c) survival analysis. (b) Some animals treated with F3.CD and 5-FC (group 4) showed mild tumor volume reduction. (c) Survival analysis was performed using the Kaplan–Meier and log rank tests. There was no difference in survival (P = 0.221). Cumulative survival = ratio of survive animal. HBSS, Hanks' balanced salt solution; i.p., intraperitoneal.
Direct intratumoral injection of F3.CD cells
When there is a large brain metastasis, F3.CD cells can be injected into the tumor mass directly. This route could reverse the limited effect of F3.CD cells on large brain metastasis because F3.CD cells could be delivered to tumor mass much more effectively. Accordingly, we injected F3.CD cells into MDA-MB-435 brain tumor mass directly (Supplementary Materials and Methods) and tested in vivo therapeutic effect (Supplementary Figure S3a). When F3.CD cells were injected 2 weeks after the tumor cell implantation (Supplementary Figure S3b), mice treated with F3.CD cells and 5-FC showed significantly small tumor volumes (group 2, mean ± SD = 1.95 ± 0.73 mm3), compared with the control group (group 1, HBSS injection + saline, 8.46 ± 2.17 mm3). However, F3.CD cells injected into tumor mass 3 weeks after the tumor cell implantation could not prolong survival time [Supplementary Figure S3c; group 1 (HBSS injection + saline), median survival after tumor cell implantation, 29 days (25–33 days); group 2 (F3.CD injection + 5-FC), 26 days (24–35 days)—P = 0.545].
Discussion
Brain metastases most commonly originate from melanoma, lung, breast, and colon cancer. Unfortunately, brain metastases are very difficult to treat because most drugs cannot penetrate the BBB and often multiple areas of the brain are affected. It is essential that patients with multiple brain metastases undergo systemic chemotherapy and/or whole brain radiation therapy. However, whole brain radiation therapy is hazardous and impairs cognitive function, and as mentioned, most effective chemotherapeutic agents cannot penetrate the BBB and reach the tumor.
The cytotoxic drug 5-FU is effective in the treatment of brain metastases, but it suffers from the inability to penetrate across BBB. However, the 5-FU prodrug 5-FC readily penetrates across BBB or into brain parenchyme.12,13 The 5-FC is converted to 5-FU by CD, an enzyme that is not encoded by the human genome. Therefore, if CD could be delivered to or expressed in brain metastases, the 5-FC prodrug would have great potential in the treatment of brain metastases. To test the feasibility of this approach, we used NSCs to target CD to brain metastases and determined the effect on CD on tumor volume.
Brain metastases are different from primary brain tumors such as gliomas and medulloblastomas because they originate from cells that do not reside in the brain. Therefore, even if NSCs selectively migrate into primary brain tumors,14,15,16,17,18,19,20,21 this does not mean NSCs will target brain metastases. Furthermore, it is possible that individual brain metastases differ in their ability to attract NSCs. Here, we show that NSCs injected into the ipsilateral hemisphere migrate into breast tumor metastases in the contralateral hemisphere. This suggests that breast tumor brain metastases attract NSCs as well as primary brain tumors. This is consistent with another study showing that NSCs injected into the ICA-targeted melanoma brain metastases.7 Recently, we demonstrated that intravenously administered HB1.F3 human NSCs, expressing either a carboxylesterase suicide enzyme or the immune moderator interferon-β gene, selectively targeted and migrated to metastatic neuroblastoma, completely relieving the tumor burden.8,9,10 This shows that NSCs have selective tumor-tropic migrating potential and can be used as therapeutic gene delivery vehicles in various brain metastases.
In this study, we tested the hypothesis that NSCs carrying a therapeutic gene have therapeutic effects on brain metastases. The NSCs showed significant therapeutic effects in two different breast cancer brain metastasis animal models; tumor cells were directly implanted into the brain or blood-born metastases were made by injection of tumor cells into the ICA. We expected that the NSCs could also make therapeutic effects when the F3.CD and 5-FC treatment started after brain metastasis grew large. However, the NSCs did not prolong the survival of the animals, and direct intratumoral injection of the NSCs, a more efficient delivery protocol of the NSCs, also failed to show significant therapeutic effects. NSCs carrying a therapeutic gene have significant therapeutic effects on brain metastasis; however, improved therapeutic methods such as more frequent administration, combination with conventional anticancer treatments or engineering of NSCs to carry multiple therapeutic genes with different modes of action,18,19,20 need to be developed to potentiate the therapeutic effects further.
Early detection methods in the clinic have made large brain metastasis rare and it should be removed urgently because it provokes critical mass effect. For these reasons, large brain metastasis has little chance to get nonsurgical treatments. Instead, small multiple brain metastases, the most popular form of brain metastasis,1 should be managed with systemic methods for they cannot be managed surgically. Small multiple brain metastases usually show radio- and/or chemo-resistance, accordingly, they recur in spite of variable conventional anticancer treatments. Our novel treatment strategy using NSCs as a therapeutic gene carrier showed significant therapeutic effects on multiple small brain metastases. Therefore, this protocol could be valuable in the clinical settings. In addition, it could be used as an adjuvant treatment after excision of large brain metastasis against undetectable remnant cancer cells.
The F3 human NSC line was immortalized with v-myc22,23,24,25 and is an ideal vehicle for carrying suicide genes or immune moderator genes to tumor targets, including primary brain tumors and brain metastases.8,9,10,21 So far, this study has shown no abnormalities originating from the F3.CD cells, but tumor formation must be considered a risk because of the v-myc oncogene in the F3 human NSCs. Nevertheless, many new techniques such as induction of pluripotent stem cells from adult human fibroblasts26,27 and generation of NSC-like cells from adult human bone marrow28,29 are providing potential sources of immune-compatible NSCs for use in adult humans. Therefore, NSCs show great promise as anticancer therapeutics using NSCs.
In summary, our results demonstrate that targeting of the CD gene to brain metastases by NSCs can be successfully combined with systemic administration of 5-FC to treat brain metastases.
Materials and Methods
Cell culture. HB1.F3 (F3) is an immortalized human NSC line. It was derived from human fetal brain (the ventricular zone) at 15 weeks of gestation and immortalized using an amphotropic, replication-incompetent retroviral vector containing v-myc.22,23,24,25 F3 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS), 2 mmol/l L-glutamine, 100 units/ml penicillin, 100 µg/ml streptomycin, and 0.25 µg/ml amphotericin B (Invitrogen, Grand Island, NY). The human breast cancer cell line MDA-MB-435, the human lung adenocarcinoma cell line PC14PE6, and the mouse melanoma cell line K1735, were maintained in Dulbecco's modified Eagle's medium containing 5% FBS, 10% FBS, and RPMI-1640 containing 10% FBS, respectively.
Engineering of the human NSC line HB1.F3.CD. The clonal HB1.F3.CD (F3.CD) line was derived from the parental F3 line. An expression plasmid encoding Escherichia coli CD was constructed using the retroviral pBabePuro backbone and the 1.5 kb CD cDNA. Vectors were packaged by cotransfection of pA317 cells with the CD puro plasmid (Supplementary Figure S1a) and the MV12 envelope-coding plasmid. CD puro retroviral supernatant was used for multiple infections of F3 cells. Transduced F3.CD cells were selected with 3 µg/ml puromycin (Invitrogen) over 4 weeks. Successful transduction of the F3.CD cells was confirmed by reverse transcription–PCR (Supplementary Figure S1b) using the primer pair: sense, 5′-GCGCGAGTCACCGCCAGCCACACCACGGC-3′ antisense, 5′-GTTTGTAATCGATGGCTTCTGGCTGC-3′).
To confirm the activity of CD in F3.CD, the cytotoxic effects of 5-FC and 5-FU were analyzed using a cell viability assay. The sensitivity to 5-FC and 5-FU was also assessed for F3, MDA-MB-435, PC14PE6, and K1735 cells. Next, 1 × 105 and 5 × 103 cells were plated in each well of 6- and 96-well plates, respectively. After 24 hours, cells in the 6-well plates were treated with 0.5 mmol/l 5-FC or 5-FU (Sigma, St. Louis, MO) at 37 °C for 4 days, and the status of the cells was observed using a microscope. Cells in 96-well plates were treated with 5-FC or 5-FU, using final concentrations ranging from 0.005 to 1 mmol/l, incubated at 37 °C for 1–4 days, and cell viability was determined with a colorimetric assay (Cell Counting Kit-8; Dojindo Molecular Technologies, Gaithersburg, MD).
In vitro “bystander effect” experiments. MDA-MB-435, PC14PE6, or K1735 cells and F3 or F3.CD cells were seeded in 96-well plates (total 5 × 103 cells per well) (tumor cells: F3 or F3.CD cells = 100:0, 75:25, 50:50, 25:75, or 0:100). MDA-MB-435 (human breast cancer) and F3 or F3.CD, PC14PE6 (human lung cancer) and F3 or F3.CD, and K1735 (mouse melanoma) and F3 or F3.CD were maintained in Dulbecco's modified Eagle's medium with 3% FBS. After 24 hours, 0.5 mmol/l 5-FC was added to the mixed cell cultures and 72 hours later, cell viability was determined as described above.
Brain metastasis animal models. Animal experiments in this study have been approved by the Review Board of Samsung Biomedical Research Institute. For direct tumor cell implantation into the brain, anesthetized BALB/c-nu mice (6 weeks old) were secured in a rodent stereotactic frame and a hollow guide screw implanted into a small drill hole made at 2 mm left and 1 mm anterior to the bregma, and 1 × 105 MDA-MB-435 human breast cancer cells in 10 µl HBSS were injected through this guide screw into the white matter at a depth of 2 mm [anterior/posterior (AP) +1.0 mm, medial/lateral (ML) +1.7 mm, dorsal/ventral (DV) −3.2 mm].
To produce blood-born brain metastasis animal model, the right ICA of anesthetized BALB/c-nu mice (6 weeks old) was prepared. A ligature of 4-0 silk suture was placed on the distal part of the common carotid artery, and a second ligature was placed and loosely tied on the proximal portion of ICA. The proximal ICA between the two ligatures was nicked with a pair of microscissors, and a <30-gauge polyethylene cannula was inserted into the lumen. Next, 5 × 104 MDA-MB-435 cells in 100 µl HBSS were injected slowly through the cannula, the second ligature was tightened, and the skin was closed.
In vivo therapeutic efficacy of F3.CD human NSCs. Furthermore, 13 and 20 days (direct tumor cell brain implantation animal model, Figure 3a) or 9 and 46 days (blood-born brain metastasis animal model, Figure 4a) after MDA-MB-435 tumor cell implantation, animals were subjected to contralateral injection (AP +1.0 mm, ML −1.7 mm, DV −3.2 mm) of 10 µl HBSS (direct tumor cell brain implantation animal model; groups 1 and 2, n = 10 for each group, blood-born brain metastasis animal model; group 1, n = 4) or 1 × 105 F3.CD cells in 10 µl HBSS (direct tumor cell brain implantation animal model; groups 3 and 4, n = 10 for each group, blood-born brain metastasis animal model; group 2, n = 5). Furthermore, 48 hours after the injection, the groups 1 and 3 and 2 and 4 received intraperitoneal injections of normal saline (200 µl) or 5-FC (500 mg/kg in 200 µl normal saline), respectively, everyday for 5 days (direct tumor cell brain implantation animal model, Figure 3a) or everyday (blood-born brain metastasis animal model, Figure 4a).
Two days after the last injection, brains were removed and cut into 4–6-mm thick slices. For tumor volume measurement (largest width2 × largest length × 0.5), the brain slices were fixed in 10% formalin/phosphate-buffered saline, embedded in paraffin, sectioned into 4-µm coronal sections using a microtome and stained with hematoxylin and eosin. For immunohistochemistry, brain slices were embedded in optimal cutting temperature compound (Miles, Elkhart, IN), frozen rapidly in liquid nitrogen, and cut into 8-µm coronal sections using a cryostat. Immunohistochemistry was performed using the free-floating method as previously described.30 A rabbit anti-CD polyclonal antibody (1:1,000; Dr K. Aboody, City of Hope Medical Center, Duarte, CA), followed by the avidin–biotin complex method (Vector Laboratories, Burlingame, CA), and visualized using 3,3′-diaminobenzidine (Sigma).
In vivo therapeutic efficacy of HB1.F3.CD cells on large brain metastatic tumors. After a period of 20 and 27 days after MDA-MB-435 human breast cancer cell implantation, animals were subjected to contralateral injection (AP +1.0 mm, ML −1.7 mm, DV −3.2 mm) of 10 µl HBSS (groups 1 and 2, n = 7 for each group) or 1 × 105 F3.CD human NSCs in 10 µl HBSS (groups 3 and 4, n = 7 for group 3, n = 9 for group 4) (Figure 5a). And 48 hours after the injection, animals of the groups 1 and 3 and 2 and 4 received intraperitoneal injection of normal saline (200 µl) or 5-FC (500 mg/kg in 200 µl normal saline), respectively, everyday for 5 days (Figure 5a). In vivo tumor size was determined by magnetic resonance imaging scans (T1-weighted image, Bruker Biospec 4.7 T, horizontal 4.7 T/30 cm magnet; Bruker BioSpin, Ettlingen, Germany) at 20, 26, and 33 days post-transplantation of the tumor cells. Survival times of the animals were also determined.
Statistics. Statistical comparisons of groups were performed using the Student's t-test. Survival analysis was performed using the Kaplan–Meier and log rank tests. P values <0.05 were considered statistically significant.
Supplementary MaterialFigure S1. Establishment of HB1.F3 human neural stem cells expressing the cytosine deaminase (CD) gene.Figure S2. CD activity in F3.CD human NSCs.Figure S3. Intratumoral injection did not increase in vivo therapeutic effects of F3.CD on large brain metastasis.Materials and Methods.
Supplementary Material
Establishment of HB1.F3 human neural stem cells expressing the cytosine deaminase (CD) gene. (a) A plasmid encoding the E. coli CD cDNA (1.5 kb fragment) was constructed using the retroviral pBabePuro backbone (pMSCVpuro/CD vector). LTR. Long term repeat; SV40, SV40 promoter; PUROR, Puromycin resistance gene. (b) Successful transduction of CD was confirmed by reverse transcription-PCR (559 bp). GAPDH controls confirmed equal RNA loading.
CD activity in F3.CD human NSCs. The cytotoxic effect of 5-FC on F3.CD was determined using a cell viability assay. 5 × 103 F3.CD cells were seeded in each well of a 96-well plate. After 24 hours, cells were treated with 5-FC (Final concentrations = 0.005 ∼ 0.5 mM), incubated at 37°C for 24 (a), 48 (b), or 72 (c) hours and cell viability determined.
Intratumoral injection did not increase in vivo therapeutic effects of F3.CD on large brain metastasis. (a) Timeline for the experiment. To test if intratumoral injection increased in vivo therapeutic effects of F3.CD cells on large metastatic brain tumor, F3.CD cells were injected into MDA-MB-435 brain tumor masses directly 2 (b) or 3 (c) weeks after the tumor cell implantation. (b) When F3.CD cells were injected 2 weeks after the tumor cell implantation, mice treated with F3.CD cells and 5-FC (group 2) showed significantly small tumor volumes, compared with the control group (HBSS injection + saline, group 1). Columns = mean, bars = SD. * P < 0.05. (c) When F3.CD cells were injected 3 weeks after the tumor cell implantation, there was no difference in survival (P = 0.545). Cumulative survival = ratio of survive animal.
Acknowledgments
This study was supported by the Canadian Myelin Research Initiative (S.U.K.), the Korean Ministry of Education, Science and Technology grants M103KV010026 (S.U.K.), a Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2007-313-E00212) (D.-H.N.) and the SRC program of KOSEF (Research Center for Women's Diseases) (D.-H.N.).
REFERENCES
- Delattre JY, Krol G, Thaler HT., and , Posner JB. Distribution of brain metastases. Arch Neurol. 1988;45:741–744. doi: 10.1001/archneur.1988.00520310047016. [DOI] [PubMed] [Google Scholar]
- Biswas G, Bhagwat R, Khurana R, Menon H, Prasad N., and , Parikh PM. Brain metastasis-evidence based management. J Cancer Res Ther. 2006;2:5–13. doi: 10.4103/0973-1482.19768. [DOI] [PubMed] [Google Scholar]
- Arbit E., and , Wronski M. Clinical decision making in brain metastases. Neurosurg Clin North Am. 1996;7:447–457. [PubMed] [Google Scholar]
- Posner JB., and , Chernik NL. Intracranial metastases from systemic cancer. Adv Neurol. 1978;19:579–592. [PubMed] [Google Scholar]
- Chang SD., and , Adler JR., Jr Current treatment of patients with multiple brain metastases. Neurosurg Focus. 2000;9:e5. [PubMed] [Google Scholar]
- Schmidt NO, Przylecki W, Yang W, Ziu M, Teng Y, Kim SU, et al. Brain tumor tropism of transplanted human neural stem cells is induced by vascular endothelial growth factor. Neoplasia. 2005;7:623–629. doi: 10.1593/neo.04781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboody K, Najbauer J, Schmidt NO, Yang W, Wu JK, Zhuge Y, et al. Targeting of melanoma brain metastases using engineered neural stem/progenitor cells. Neuro Oncol. 2006;8:119–126. doi: 10.1215/15228517-2005-012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimato S, Natsume A, Takeuchi H, Wakabayashi T, Fujii M, Ito M, et al. Human neural stem cells target and deliver therapeutic gene to experimental leptomeningeal medulloblastoma. Gene Ther. 2007;15:1132–1142. doi: 10.1038/sj.gt.3302932. [DOI] [PubMed] [Google Scholar]
- Aboody K, Bush R, Garcia E, Metz M, Najbour J, Justus K, et al. Development of a tumor-selective approach to treat metastatic cancer. PLoS One. 2006;1:e23. doi: 10.1371/journal.pone.0000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danks MK, Yoon KJ, Bush RA, Remack JS, Wierdl M, Tsurkan L, et al. Tumor-targeted enzyme/prodrug therapy mediates long-term disease-free survival of mice bearing disseminated neuroblastoma. Cancer Res. 2007;67:22–25. doi: 10.1158/0008-5472.CAN-06-3607. [DOI] [PubMed] [Google Scholar]
- Dickson PV, Hamner JB, Burger RA, Garcia E, Ouma AA, Kim SU, et al. Intravascular administration of tumor tropic neural progenitor cells permits targeted delivery of interferon-beta and restricts tumor growth in a murine model of disseminated neuroblastoma. J Pediatr Surg. 2007;42:48–53. doi: 10.1016/j.jpedsurg.2006.09.050. [DOI] [PubMed] [Google Scholar]
- Daneshmend TK., and , Warnock DW. Clinical pharmacokinetics of systemic antifungal drugs. Clin Pharmacokinet. 1983;8:17–42. doi: 10.2165/00003088-198308010-00002. [DOI] [PubMed] [Google Scholar]
- Vermes A, Guchelaar HJ., and , Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother. 2000;46:171–179. doi: 10.1093/jac/46.2.171. [DOI] [PubMed] [Google Scholar]
- Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97:12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barresi V, Belluardo N, Sipione S, Mudo G, Cattaneo E., and , Condorelli DF. Transplantation of prodrug-converting neural progenitor cells for brain tumor therapy. Cancer Gene Ther. 2003;10:396–402. doi: 10.1038/sj.cgt.7700580. [DOI] [PubMed] [Google Scholar]
- Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, et al. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. doi: 10.1038/74710. [DOI] [PubMed] [Google Scholar]
- Brown AB, Yang W, Schmidt NO, Carroll R, Leishear KK, Rainov NG, et al. Intravascular delivery of neural stem cell lines to target intracranial and extracranial tumors of neural and non-neural origin. Hum Gene Ther. 2003;14:1777–1785. doi: 10.1089/104303403322611782. [DOI] [PubMed] [Google Scholar]
- Ehtesham M, Kabos P, Kabosova A, Neuman T, Black KL., and , Yu JS. The use of interleukin 12-secreting neural stem cells for the treatment of intracranial glioma. Cancer Res. 2002;62:5657–5663. [PubMed] [Google Scholar]
- Ehtesham M, Kabos P, Gutierrez MA, Chung NH, Griffith TS, Black KL, et al. Induction of glioblastoma apoptosis using neural stem cell mediated delivery of tumor necrosis factor-related apoptosis-inducing ligand. Cancer Res. 2002;62:7170–7174. [PubMed] [Google Scholar]
- Herrlinger U, Woiciechowski C, Sena-Esteves M, Aboody KS, Jacobs AH, Rainov NG, et al. Neural precursor cells for delivery of replication-conditional HSV-1 vectors to intracerebral gliomas. Mol Ther. 2000;1:347–357. doi: 10.1006/mthe.2000.0046. [DOI] [PubMed] [Google Scholar]
- Kim SK, Cargioli TG, Machluf M, Yang W, Sun Y, Al-Hashem R, et al. PEX producing human neural stem cells inhibit tumor growth in a mouse glioma model. Clin Cancer Res. 2005;11:5965–5970. doi: 10.1158/1078-0432.CCR-05-0371. [DOI] [PubMed] [Google Scholar]
- Cho T, Bae JH, Choi HB, Kim SS, McLarnon JG, Suh-Kim H, et al. Human neural stem cells: electrophysiological properties of voltage-gated ion channels. Neuroreport. 2002;13:1447–1452. doi: 10.1097/00001756-200208070-00020. [DOI] [PubMed] [Google Scholar]
- Flax JD, Aurora S, Yang C, Simonin C, Wills AM, Billinghurst LL, et al. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol. 1998;16:1033–1039. doi: 10.1038/3473. [DOI] [PubMed] [Google Scholar]
- Kim SU. Human neural stem cells genetically modified for brain repair in neurological disorders. Neuropathology. 2004;24:159–171. doi: 10.1111/j.1440-1789.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- Ourednik V, Ourednik J, Flax JD, Zawada WM, Hutt C, Yang C, et al. Segregation of human neural stem cells in the developing primate forebrain. Science. 2001;293:1820–1824. doi: 10.1126/science.1060580. [DOI] [PubMed] [Google Scholar]
- Kim SK, Kim SU, Park IH, Bang JH, Aboody KS, Wang KC, et al. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res. 2006;12:5550–5556. doi: 10.1158/1078-0432.CCR-05-2508. [DOI] [PubMed] [Google Scholar]
- Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Hermann A, Gastl R, Liebau S, Popa MO, Fiedler J, Boehm BO, et al. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci. 2004;117:4411–4422. doi: 10.1242/jcs.01307. [DOI] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ., and , Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2006;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Establishment of HB1.F3 human neural stem cells expressing the cytosine deaminase (CD) gene. (a) A plasmid encoding the E. coli CD cDNA (1.5 kb fragment) was constructed using the retroviral pBabePuro backbone (pMSCVpuro/CD vector). LTR. Long term repeat; SV40, SV40 promoter; PUROR, Puromycin resistance gene. (b) Successful transduction of CD was confirmed by reverse transcription-PCR (559 bp). GAPDH controls confirmed equal RNA loading.
CD activity in F3.CD human NSCs. The cytotoxic effect of 5-FC on F3.CD was determined using a cell viability assay. 5 × 103 F3.CD cells were seeded in each well of a 96-well plate. After 24 hours, cells were treated with 5-FC (Final concentrations = 0.005 ∼ 0.5 mM), incubated at 37°C for 24 (a), 48 (b), or 72 (c) hours and cell viability determined.
Intratumoral injection did not increase in vivo therapeutic effects of F3.CD on large brain metastasis. (a) Timeline for the experiment. To test if intratumoral injection increased in vivo therapeutic effects of F3.CD cells on large metastatic brain tumor, F3.CD cells were injected into MDA-MB-435 brain tumor masses directly 2 (b) or 3 (c) weeks after the tumor cell implantation. (b) When F3.CD cells were injected 2 weeks after the tumor cell implantation, mice treated with F3.CD cells and 5-FC (group 2) showed significantly small tumor volumes, compared with the control group (HBSS injection + saline, group 1). Columns = mean, bars = SD. * P < 0.05. (c) When F3.CD cells were injected 3 weeks after the tumor cell implantation, there was no difference in survival (P = 0.545). Cumulative survival = ratio of survive animal.