Abstract
The thymus is the primary site of T-cell development and plays a key role in the induction of self-tolerance. We previously showed that the intrathymic (IT) injection of a transgene-expressing lentiviral vector (LV) in mice can result in the correction of a T cell–specific genetic defect. Nevertheless, the efficiency of thymocyte transduction did not exceed 0.1–0.3% and we were unable to detect any thymus transduction in macaques. As such, we initiated studies to assess the capacity of recombinant adeno-associated virus (rAAV) vectors to transduce murine and primate thymic cells. In vivo administration of AAV serotype 2–derived single-stranded AAV (ssAAV) and self-complementary AAV (scAAV) vectors pseudotyped with capsid proteins of serotypes 1, 2, 4, 5, and 8 demonstrated that murine thymus transduction was significantly enhanced by scAAV2/8. Transgene expression was detected in 5% of thymocytes and, notably, transduced cells represented 1% of peripheral T lymphocytes. Moreover, IT administration of scAAV2/8 particles in macaques, by endoscopic-mediated guidance, resulted in significant gene transfer. Thus, in healthy animals, where thymic gene transfer does not provide a selective advantage, scAAV2/8 is a unique tool promoting the in situ transduction of thymocytes with the subsequent export of gene-modified lymphocytes to the periphery.
Introduction
The thymus is the primary site of T-cell development. T-cell differentiation in this organ is initiated by the entry of hematopoietic progenitors, migrating from the bone marrow. The maturation of progenitors to T cells, requiring ~28 days,1 occurs via interactions with thymic stromal cells and cytokine stimulation. T-cell differentiation can be divided into three major stages defined by the expression of CD4 and CD8 markers. The most immature or double-negative (DN) stage is characterized by the lack of expression of CD4 and CD8 markers and represents 2–5% of thymocytes. DN cells progress to a stage wherein there is a dual expression of these markers (double-positive, DP, 80–90%) before lineage commitment to either CD4 or CD8 cells (single-positive, SP, 5–15%) that emigrate from the thymus to the periphery. Schematically, the transition from DN to DP and DP to SP stages is regulated by the rearrangement and selection of a functional T-cell receptor, respectively. The selection step plays a key role in the induction of a broad repertoire of self-tolerant T cells. This is achieved by the deletion of autoreactive T cells and the generation of regulatory T cells (reviewed in ref. 2). Because of these properties, the thymus is a critical site wherein T-cell responsiveness and tolerance can be manipulated.
Targeting the thymus with an antigen of interest can result in the induction of tolerance. Indeed, this has been achieved by the direct injection of soluble antigen,3 of viral vectors harboring an antigen of interest,4,5 or even of entire cells.6 Moreover, we previously demonstrated the feasibility of an in situ correction of a genetic immunodeficiency by direct intrathymic (IT) injection of a lentiviral vector (LV) expressing the deficient gene.7 Nevertheless, the efficiency of thymocyte transduction in our studies was extremely low, not exceeding 0.1–0.3%. Moreover, attempts to transpose this LV-based thymocyte transduction strategy to macaques were unfortunately not successful; despite endoscopic-guided injection of high-titer vesicular stomatitis virus G-protein–pseudotyped LV virions into the macaque thymus, we were unable to detect any transduction (data not shown). Because of the potential value of an IT gene–transfer strategy for immune modulation, it is important to develop tools for efficient thymus transduction in mice and primates.
Recombinant adeno-associated virus (rAAV) vectors hold great promise for gene-transfer therapies. Particles of high titer and purity can be produced, and in the vast majority of cases, administration is not associated with pathogenicity or toxicity (reviewed in ref. 8). In contrast with retroviral-based vectors, rAAV vectors are capable of infecting dividing as well as nondividing cells and insertional mutagenesis has only been observed in the liver.9 Moreover, as compared to adenovirus-based vectors, rAAV does not induce a significant immune response and is not associated with any human disease.
The rAAV vectors were initially developed as single-stranded (ss) viral DNA vectors. The transduction efficiency of these “conventional” rAAV vectors, based on the AAV2 serotype, is known to be tissue dependent with significant gene transfer in various tissues10,11,12,13,14,15 and only low-level infection of hematopoietic cells.16,17,18 Notably though, several other AAV serotypes, differing from the AAV2 serotype in their expression of distinct capsid proteins, have been isolated. These divergent AAV serotypes display different tissue and cell tropisms, and as such, modulating the serotype may significantly enhance the ability to transduce a specific organ/cell type with an AAV-derived vector.19,20,21,22,23 Efficient transduction by conventional ssAAV vector serotypes has also been limited by the need to convert the ss genome into a transcriptionally active double-stranded form.24 The ability to package the rAAV genome as a self-complementary (sc) duplex strand structure has significantly increased transduction efficiencies in numerous tissues.25,26,27,28,29 More recently, it has been shown that these sc rAAV vectors, which bypass the requirement for host cell–mediated viral second-strand DNA synthesis, markedly increase the transduction efficiency in murine hematopoietic stem cells.23,30 However, studies assessing rAAV-mediated thymocyte and thymus transduction have yet to be reported.
In an attempt to achieve efficient gene transfer in the thymus, we evaluated in situ IT gene transfer using ss and sc rAAV-2 vectors cross-packaged into the capsids of other AAV serotypes. These experiments, performed both in mice and macaques, demonstrated that the scAAV2 vector pseudotyped with the AAV8 capsid (scAAV2/8) is significantly more efficient than other serotypes in thymus transduction. Notably, the level of gene transfer was markedly higher than that which could be obtained with LV vectors, approaching 5% of all thymocytes. Moreover, and in marked contrast with LV vectors, transduction of the thymocyte subsets reflected their relative proportion in the thymus. Importantly, thymic gene transfer resulted in the emigration of transduced T cells to the periphery, with up to 1% of splenic/lymph node (LN) T cells expressing the harbored transgene. The high efficiency of in situ rAAV IT gene transfer in mice as well as macaques indicates the important potential of this approach in modulating T-cell differentiation and immunity.
Results
scAAV2/8 vectors efficiently transduce murine thymocytes in vivo
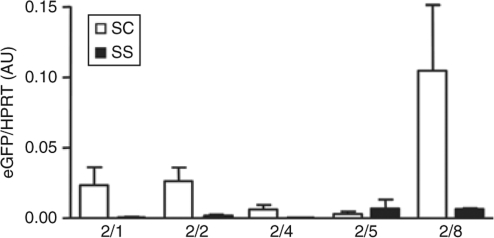
To determine whether AAV vectors are capable of in situ transduction of murine thymocytes, we produced scAAV and ssAAV vectors pseudotyped with capsid proteins of different serotypes (AAV2/1, AAV2/2, AAV2/4, AAV2/5, and AAV2/8). All these AAV vectors harbored the enhanced green fluorescent protein (eGFP) cDNA under the control of a constitutive promoter (phosphoglycerate kinase). The 10 AAV vectors were injected either directly into the thymus (IT) of 3- to 4-week-old mice or administered intravenously (IV). The eGFP expression in the thymus was quantified by real-time reverse transcription–PCR (RT-PCR) at short and longer time points (3 days and 1 month). At both time points, IT administration of scAAV vectors resulted in a significantly higher expression of the eGFP transgene than ssAAV transduction (Figure 1 and data not shown). Among the scAAV vectors, there was at least a fivefold higher level of eGFP expression following administration of the 2/8 serotype as compared to all other serotypes tested (Figure 1 and data not shown). Importantly, the route of administration had a major impact on thymus transduction as transgene expression in the thymus was significantly lower following IV rAAV administration, independently of the serotype tested (0.04 ± 1.2 versus 1.05 ± 0.26 for AAV2/8, for example; data not shown). As such, in all subsequent experiments, we chose to administer the scAAV2/8 vector via the IT route.
Figure 1.
Influence of encapsidated genome configuration and capsid serotype on in vivo thymus transduction efficiency. Single-stranded adeno-associated virus (ssAAV) and self-complementary AAV (scAAV) vectors based on the AAV2 backbone and expressing the enhanced green fluorescent protein (eGFP) transgene were pseudotyped with capsid proteins of serotypes 1, 2, 4, 5, and 8. 1 × 1010 vector genomes (vg)–containing particles of the indicated ss and scAAV vectors were injected directly into the thymi of 3- to 4-week-old mice. Thymocytes were harvested from killed animals 30 days following AAV administration. In vivo expression of eGFP was assessed by quantitative reverse transcription–PCR in triplicate samples and all data were normalized to hypoxanthine-guanine phosphoribosyl transferase (HPRT). Normalized means ± SD of arbitrary units (AU) are shown.
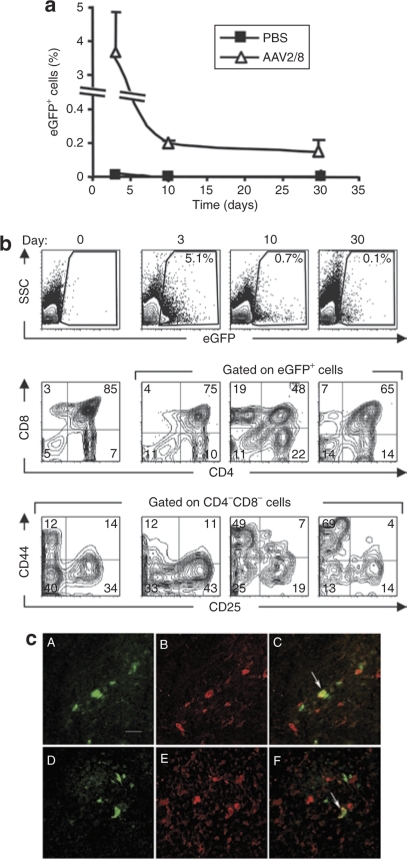
In order to characterize the kinetics of in vivo scAAV2/8 transduction as well as the phenotypes of the transduced cells, mice were killed at 3, 10, and 30 days following IT administration. The percentage of transduced cells, monitored as a function of eGFP expression, was maximal 3 days after scAAV2/8 administration (3.65 ± 1.2%), decreasing by day 10 and remaining stable until at least day 30 (0.2 ± 0.01 and 0.15 ± 0.07, respectively) (Figure 2a). At 3 days post IT administration of a scAAV2/8 vector, all thymocyte populations (Figure 2b) as well as epithelial cells and dendritic cells were transduced (Figure 2c). The percentages of scAAV-transduced thymocytes within the DN (CD4−CD8−), DP (CD4+CD8+), and SP mature (CD4+CD8− and CD4−CD8+) subsets ranged from 4 to 8% and reflected their overall presence in the thymus (DN: 12.8 ± 2.18, DP: 64.9 ± 8.32, SP4: 15.8 ± 2.91, SP8: 6.6 ± 3.59) (Figure 2b and data not shown). Moreover, the distribution of eGFP+ cells within the DN subpopulations, characterized as DN1 to DN4, was similar to that observed for the total DN subset (DN1: 9.5 ± 0.95, DN2: 5.6 ± 1.32, DN3: 51 ± 1.07, DN4: 33.9 ± 1.06) (Figure 2b). These data are in marked contrast with those obtained following in situ IT transduction using high-titer LV preparations (5 × 108 transducing unit) wherein only 0.3% of DN and 0.1% of DP thymocytes were transduced (ref. 7 and data not shown).
Figure 2.
Kinetics of transgene expression and phenotype of transduced cells following in vivo intrathymic administration of scAAV2/8. (a) Mice were injected intrathymically with 4 × 1011 vector genomes–containing scAAV2/8 particles. Thymi were harvested from killed animals at days 3, 10, and 30 postinjection and the percentages of eGFP+ thymocytes were assessed by flow cytometry. The means ± SD of three animals are shown for each time point (b) Flow cytometry data showing the percentages of eGFP+ cells as a function of side scatter (SSC) are presented at days 0, 3, 10, and 30 (top row). The phenotype of the eGFP+ cells was assessed as a function of CD4 and CD8 expression, allowing double-negative (CD4−/CD8−), double-positive (CD4+/CD8+) and mature single-positive cells (CD4+, CD8+) to be distinguished (middle row). Double-negative (DN) cells were further phenotyped on the basis of CD44 and CD25 expression distinguishing DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44−CD25+), and DN4 (CD44−CD25−) subsets (bottom row). All dot plots are compared to the total thymocyte profiles (left panels). (c) Immunohistochemical analyses of thymic stromal cells from mice injected with scAAV2/8 vectors 10 days previously. Enhanced green fluorescent protein (eGFP) expression was evaluated in the medulla (A and D). Epithelial and dendritic cells were visualized by staining with a wide-spectrum anti-CTK pAb (B), and an anti-S100 pAb (E), respectively. Overlays of eGFP with CTK and S100 immunostaining are shown in C and F, respectively. Arrows indicate colocalization. Bar = 50 µm. PBS, phosphate-buffered saline; scAAV, self-complementary adeno-associated virus.
Between 3 and 30 days post scAAV2/8 transduction, there was an evolution in the distribution of the genetically modified thymocyte populations (Figure 2a,b). There was an initial increase in the relative transduction of mature SP thymocytes (reaching 40%, SP4: 23.9 ± 1.65, SP8: 19.47 ± 3.6 at day 10 for example), likely due to the differentiation of immature transduced progenitors. Surprisingly though, significant percentages of transduced DN cells were still detected at days 10 and 30 after in vivo transduction (11.5 ± 3.74 and 13.5 ± 3, respectively). Given the kinetics of thymocyte differentiation wherein cells can proceed from DN to mature SP cells in ~4 weeks,1 our data suggest that immature progenitors are transduced by AAV, and these transduced progenitors remain viable for at least 4 weeks following transduction, pursuing their differentiation pathway.
scAAV-transduced thymocytes differentiate and migrate to the periphery
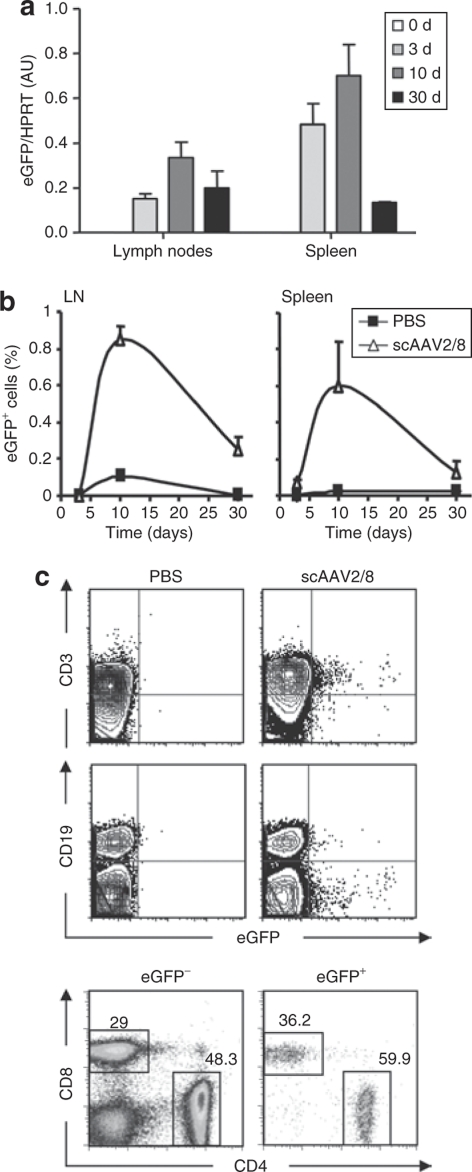
To determine whether thymocytes transduced in situ by scAAV2/8 differentiate and migrate into the peripheral circulation, LNs, and spleens of these mice were analyzed 3, 10, and 30 days after vector administration. Transduction of peripheral lymphocytes, assessed as a measure of eGFP expression, was assayed by both RT-PCR as well as flow cytometry. In both the LN and spleen, the emergence of eGFP-expressing cells followed a bell-shaped curve (Figure 3a,b). As expected, at day 3 post IT injection, the percentage of eGFP+ lymphocytes in the periphery was extremely low (<0.07%, data not shown). Importantly though, by 10 days postinjection, the percentage of eGFP+ cells in the spleen or LN reached ~1% of all cells. The significance of these data is notable as these mice have a full lymphocyte compartment and the transduced cells do not have a selective advantage as compared to their nontransduced counterpart. This latter point is corroborated by our finding that by day 30, there is a prominent decline in the percentage of eGFP+ cells, decreasing to 0.1–0.3%.
Figure 3.
Thymocytes transduced by in situ injection of a scAAV2/8 vector differentiate and migrate to the periphery. (a) Expression of the enhanced green fluorescent protein (eGFP) transgene was monitored in the lymph nodes (LNs) and spleens of mice intrathymically injected with the scAAV2/8 vector. Expression was assessed by quantitative reverse transcription–PCR at days 3, 10, and 30, and was normalized to hypoxanthine-guanine phosphoribosyl transferase. Normalized means ± SD of arbitrary units (AU) are shown for duplicate samples. (b) The percentages of LN and spleen cells expressing the eGFP transgene were also monitored by flow cytometry. The means ± SD of three animals are shown for each time point. (c) Following intrathymic scAAV2/8 administration, the phenotypes of transduced lymphocytes was monitored in LNs by assessing eGFP expression in CD3 and CD19 populations as compared to mock-transduced mice (phosphate-buffered saline) (upper panels). The expression of the eGFP transgene within the CD3 subset was further evaluated by CD4 and CD8 staining. The relative ratio of CD4 and CD8 cells within the nontransduced (eGFP−) and transduced (eGFP+) fractions are shown in representative dot plots (bottom panels). scAAV, self-complementary adeno-associated virus.
As expected, at all time points assessed, the transduced peripheral LN and splenic cells had a CD3+ phenotype with nearly undetectable numbers of transduced CD19+ B cells (0.02 ± 0.02% at day 10) (Figure 3c and data not shown). Moreover, there was no bias at the level of the CD4/CD8 ratio, which remained ~3:2 in both the eGFP– and eGFP+ subsets (Figure 3c, lower panel). These data indicate an equivalent selection, migration, and short-term persistence of AAV-transduced CD4 as well as CD8 lineage cells. The finding that these T lymphocytes had a naive phenotype (CD44low/CD62Lhigh, data not shown) provide further support of their recent migration from the thymus. Moreover, these results indicate that AAV transduction does not activate T lymphocytes per se resulting in their acquisition of an effector or memory phenotype.
Thymocyte transduction in macaques following IT injection of an scAAV2/8 vector
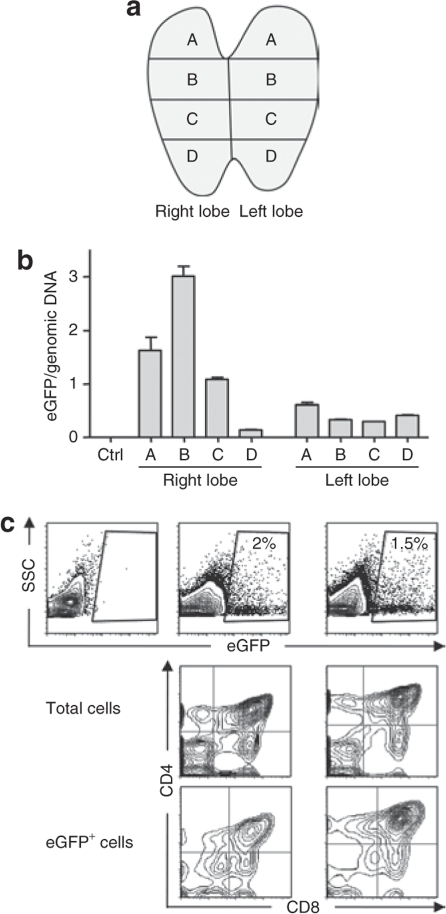
To begin to evaluate the clinical potential of an IT AAV-mediated gene therapy approach, we tested the efficiency of scAAV2/8 administration in macaques. We first assessed whether this technique was feasible by thoracoscopy. Indeed, we determined that this technique could be easily and safely applied to anesthetized macaques, with accurate targeting of the thymus requiring a total procedure time of <15 minutes. Following this validation step, 1 ml of scAAV2/8, containing 2 × 1013 vector genomes (vg), was administered over two to three injection sites in the right thymic lobe of two macaques. Serum samples were repeatedly collected as soon as 30 minutes postinjection and subsequently (6 hours, 1, 3, 7, 10, 21, and 30 days postinjection). Infectious rAAV particles were detected in serum samples obtained between 0.5 and 24 hours postinjection, but not at later time points, as assessed by a replication center assay (Table 1, sensitivity >5 × 102 particles/ml). Macaques were thymectomized 10 days later. Each thymic lobe was separated into four sections as shown in Figure 4a, and scAAV2/8 transduction in each section was quantified by PCR in order to measure the total quantity of scAAV2/8 genome in transduced cells rather than promoter activity per se (Figure 4b). Transduction varied between sections, most likely correlating with the injection zone. Notably, in the most efficiently transduced thymocyte section, the number of scAAV2/8 genomes reached 3 copies/cell (Figure 4b). In the noninjected left lobe, eGFP DNA was also detected, albeit at significantly lower levels. A similar distribution was found in the second macaque, but with lower vector DNA levels (data not shown). Analysis of the transduced cells by flow cytometry revealed a transduction of all thymocyte subsets (Figure 4c). It is notable that the level of macaque thymocyte transduction (~2%) was similar to that observed in the mouse.
Table 1.
mRCA to detect infectious particles in the serum following intrathymic scAAV2/8 administration in macaques
Figure 4.
In vivo administration of scAAV2/8 in a macaque thymus results in thymocyte transduction. (a) Three-year-old cynomolgus macaques were injected intrathymically in the right lobe with 2 × 1013 vector genomes–containing scAAV2/8 particles in two or three points. Ten days following injection, thymi were removed under general anesthesia and four sections were made from each lobe, shown here in the schematic diagram and labeled as A to D. (b) Transduction efficiencies in the various sections were assessed by quantitative PCR of the enhanced green fluorescent protein (eGFP) transgene. All values were normalized to Epo and the mean of eGFP copies per genomic DNA ± SD are shown for samples from the two macaques. (c) Flow cytometry data showing the percentages of eGFP+ cells as a function of side scatter (SSC) in the section B and A from right (right panels) and left (left panels) lobes, respectively, from a representative macaque injected 10 days previously with the scAAV2/8 vector (top row). The phenotypes of eGFP+ thymocytes in comparison to the total thymocyte population are shown as a function of CD4 and CD8 expression. scAAV, self-complementary adeno-associated virus.
Transduced T lymphocytes are detected in macaques following IT injection of an scAAV2/8 vector
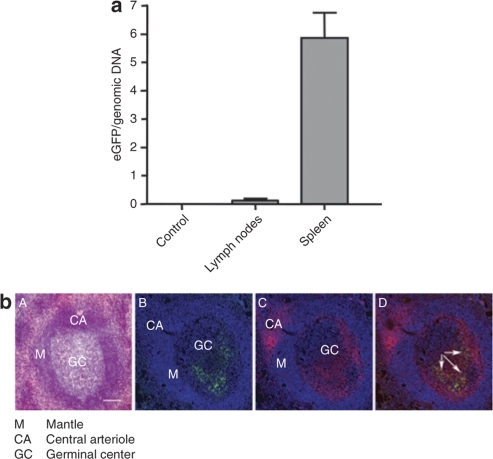
As scAAV2/8 vector transduction was detected in macaque thymocytes following IT injection, we were interested in determining whether this would translate to transgene expression in LNs and spleen, as observed in the mouse model. One month following IT injection of scAAV2/8, the two macaques underwent splenectomy and mesenteric LN biopsies. As shown in Figure 5a, eGFP DNA was detected in both the LN and spleen of the two macaques. CD4+ T cells expressing eGFP were found in the germinal center of splenic corpuscles (Figure 5b), and eGFP expression was detected in both CD4 and CD8 splenic T cells by flow cytometry (data not shown). Altogether these data demonstrate that IT scAAV2/8 administration results in the differentiation of transduced thymocytes in both mice and macaques followed by the rapid detection of eGFP-expressing mature T cells in peripheral lymphoid organs.
Figure 5.
In situ thymus injection of a scAAV2/8 vector in macaques results in the differentiation and migration of transduced thymocytes to peripheral lymphoid organs. (a) Expression of the enhanced green fluorescent protein (eGFP) transgene was monitored following in vivo intrathymic administration of a scAAV2/8 vector in macaques (right lobe). Expression was assessed by quantitative PCR in lymph nodes and spleen 30 days following scAAV2/8 injection. Expression was normalized to Epo and means of eGFP copies per genomic DNA ± SD are shown for samples from the two macaques. (b) Localization of eGFP+ cells in splenic corpuscles. A, HES staining with: GC, germinal center; M, mantle; CA, central arteriole. B, eGFP+ cells are located in the GC. C, CD4 labeling. D, merge of eGFP signal with CD4 labeling (colocalizations are indicated by arrows). Bar = 100 µm. scAAV, self-complementary adeno-associated virus.
Discussion
Expression of heterologous proteins in the thymus can be used as a therapeutic tool to modulate T-cell differentiation, activation, and selection. However, efficient gene transfer in the thymus is challenging because this organ is composed of a large number of cells (1 × 1010 to 5 × 1010 thymocytes in humans31) and >95% of all thymocytes are eliminated during positive and negative selection (reviewed in ref. 2). As such, gene or cell modification in the thymus is often attempted under conditions where the majority of thymocytes are eliminated (i.e., chemotherapy or irradition) or under conditions wherein the transgene provides a selective advantage to the transduced thymocyte. Indeed, using a lentiviral-based IT gene–transfer strategy, we were able to successfully introduce the ZAP-70 transgene in a murine model of ZAP-70-deficiency, in large part because the expression of this protein tyrosine kinase provided gene-corrected thymocytes with a selective advantage.7 Nevertheless, as indicated above, the level of gene transfer into thymocytes was extremely low, not exceeding 0.3%, and we were unable to translate this approach to macaques.
Here, we show that gene transfer mediated by rAAV vectors can result in high in vivo transduction efficiency in the thymus. Transduction with rAAV has been shown to be limited by the need to convert a ss form into an active double-stranded form.25,26,27,28,29 Indeed, for all rAAV serotypes, with the exception of 2/5, packaging the DNA cassette as complementary dimers resulted in significantly higher IT gene transfer. Furthermore, for the scAAV serotype 8 vector, thymic transduction was more than tenfold higher than that obtained with the comparable ssAAV vector. AAV8 vectors have previously been reported to transduce skeletal and cardiac muscle as well as liver with high efficiencies,32,33 and our data now demonstrate the efficacy of the scAAV8 vector in the thymus. Although rAAV vectors have only been sparsely used in the hematopoietic system, Srivastava and colleagues have recently shown that scAAV1 and scAAV7 vectors can efficiently transduce murine hematopoietic stem cells.30,34 Taken together, our data indicate that the transduction of hematopoietic stem cells and thymocytes are facilitated by different rAAV serotypes.
While significant in vivo expression from rAAV vectors generally requires at least 1–2 weeks, we detected transgene expression in 3–5% of thymocytes at early time points, 3 days following scAAV2/8 administration. This “rapid-onset” activity of scAAV2/8 has recently been reported in the liver and heart,35 and this characteristic is clearly extremely important for applications in the thymus, where the vast majority of thymocytes have a half-life of <7 days.36,37 Moreover, the efficiency of thymocyte transduction obtained following scAAV2/8 administration was ~1 log higher than that reported using vesicular stomatitis virus G-protein–pseudotyped LV vectors.7,38 In comparison with adenovirus vectors where transduction appears to be restricted to epithelial cells,39 scAAV2/8 transduced epithelial and dendritic cells. Intriguingly though, while we detected eGFP+ stromal cells as well as thymocytes by immunohistochemistry at 3 and 10 days post scAAV2/8 administration, this was not the case at 30 days. This is likely due to a higher eGFP expression at early time points and a lower sensitivity of our immunohistochemical analyses because at day 30 eGFP+ thymocytes could be detected by other methods including RT-PCR and flow cytometry.
As discussed above, scAAV2/8-transduced murine thymocytes were detected for at least 30 days following IT administration, albeit at lower levels (0.1%) than that observed at day 3. Notably, at day 30, ~15% of these cells were immature DN thymocytes. The dynamics of thymocyte differentiation is complex but a complete differentiation of hematopoietic progenitors to thymic emigrants can occur in 28 days.1 As such, our detection of transduced DN thymocytes at day 30 suggests that AAV2/8 virions can be trapped in the thymus in an infectious form for later infection of transiting thymocytes. The transduction of murine thymocytes was also associated with the presence of the rAAV genome in secondary lymphoid organs (spleen and LN). This is either due to the migration of transduced thymocytes and/or to a leak of the vector during the injection process. The first hypothesis is supported by the following data: (i) peripheral T-cell transduction was not detected following IV injection of scAAV2/8 (data not shown); and (ii) following IT injection, gene-modified T lymphocytes were observed, but the transgene was not detected in non–T hematopoietic subsets. Moreover, the kinetics of transgene expression are consistent with the emigration of transduced thymocytes. The highest level of transgene expression in the murine thymus was detected 3 days following administration, whereas the presence of gene-transduced peripheral lymphocytes was delayed, with the highest levels detected at day 10. In macaques, technical and ethical considerations made it difficult to evaluate a potential direct transduction of peripheral T lymphocytes following IT scAAV2/8 administration; the thymus and peripheral lymphoid organs could not be evaluated at multiple time points. However, it is important to note that scAAV2/8 injection resulted in a similarly high level of thymocyte transduction in macaques and mice (~2%).
Given the high level of death in the thymus together with the presence of a “full” preexisting nontransduced peripheral lymphocyte compartment, we were surprised to observe such a high level of transduced peripheral T cells (reaching 1% of the total pool in mice at day 10). This is likely explained by the observation that recent thymic emigrants have an advantage over resident peripheral lymphocytes for the first 3 weeks following their export; the substitution of peripheral T cells by recent thymic emigrant implies that 5% of the total pool is renewed each day.40,41 Thus, our data strongly suggest that the majority of differentiated scAAV2/8-modulated thymocytes emigrate to the periphery, resulting in a similar level of transduction between the thymus and the periphery.
Gene therapy strategies in animal models have resulted in several cures. However, treatment complications related to immune responses against the vector and/or the transgene have emerged as serious obstacles for the successful translation of gene therapy to humans. Indeed, following intramuscular administration of a rAAV vector in macaques, we detected a cytotoxic T lymphocyte response against the transgene.42 Among several potential strategies aimed at harnessing the immune response,43 inducing tolerance by introduction of exogenous genes in the thymus may be beneficial. IT inoculation of heterologous protein has been shown to induce T-lymphocyte tolerance, by deletion or inactivation of “autoreactive” T-cell clones.2 However, in the case of IT scAAV2/8 administration in macaques, our ability to detect vector in the serum during the first 24 hours postinjection indicates that the transgene was also delivered to the periphery. In accord with this observation, we were able to detect transduced cells in the liver 1 month post IT rAAV administration (data not shown). In this regard, it is interesting to note the elegant work from Iacomini and colleagues showing that the recirculation of homeostatically proliferating peripheral T cells into the thymus promotes central tolerance.44,45 As such, it will be important to test whether our approach can induce central tolerance in lymphodepleted animals wherein there will be a homeostatic proliferation of transduced T cells. Complementary studies aimed at achieving tolerance by a scAAV8-mediated strategy will focus on optimizing the IT injection protocol in order to best restrict transgene expression to the thymus.
In conclusion, we show that IT administration of scAAV2/8 results in the efficient transduction of the murine thymus. Notably, this therapeutic approach was easily translated from mice to nonhuman primates. Moreover, despite the presence of a preexisting T-cell pool, composed of >2.5 × 108 lymphocytes, comparable gene-transfer efficiencies were detected in the thymus and peripheral secondary lymphoid organs. Thus, scAAV2/8-mediated gene transfer is a promising tool for the further development of strategies aimed at modulating T-cell differentiation and the induction of tolerance.
Materials and Methods
rAAV constructs and production. The recombinant scAAV-GFP construct was derived from the pHPa-trs-SK plasmid-encoding eGFP (kindly provided by DM McCarty, University of North Carolina at Chapel Hill, Chapel Hill, NC) under the control of the cytomegalovirus promoter. This promoter was excised and replaced by the murine phosphoglycerate kinase promoter. The second pA signal (BGH pA signal) was excised. To construct the ssAAV-GFP plasmid, the entire PGK-GFP-SV40 fragment was excised from scAAV-GFP and inserted into pSub201.
The vector stocks, rAAV-1, rAAV-2, rAAV-4, rAAV-5, and rAAV-8, were produced by cotransfection of 293T cells with vector plasmid and the pDG plasmid,46 an adenoviral plasmid providing helper functions needed for rAAV assembly, as previously described.47 All vectors were purified by cesium chloride and dialyzed against Ca2+/Mg2+-containing phosphate-buffered saline (PBS). Vectors titers were determined by dot-blot to provide the number of particles/ml based on the quantification of viral DNA. The number of infectious particles/ml in the rAAV stocks was determined using a stable rep-cap HeLa cell line in a modified replication center assay, as described previously.48 Each scAAV vector was calculated as containing 2 copies of ss viral genomes.
IT and IV injections of recombinant ssAAV and scAAV vectors in mice and macaques. C57Bl/6 mice were maintained under specific pathogen-free conditions in the Institut de Génétique Moléculaire de Montpellier animal facility (Montpellier, France). Injections were performed in 3- to 4-week-old nonconditioned mice. IV injections (100 µl) were performed in the tail vein. For IT injections, rAAV vectors (1 × 1010 or 4 × 1011 vg) or PBS alone, were administered in a total volume of 10 µl, directly through the skin into the thoracic cavity immediately above the sternum, using a 0.3 ml 28-gauge 8-mm insulin syringe as previously described.7 Mice were anesthetized using an oxygen/isoflurane evaporator (Isotec3; TEM, Lormont, France). Mice were killed either 3, 10, or 30 days postinjection as indicated.
Two 3-year-old cynomolgus macaques (Macaca fascicularis) were purchased from BioPrim (Baziège, France). Neutralization assays were performed to assure that these animals did not harbor neutralizing antibodies against the rAAV-8 serotype. Animals were anesthetized with ketamine and medetomidine and maintained using an oxygen/isoflurane mix. Morphine and atropine were administered subcutaneously. The rAAV vector injections were performed under endoscopic guidance, resulting in the delivery of 2 × 1013 vg per animal at two to three sites in the right thymic lobe in a total volume of 1 ml. Animals were thymectomized 10 days following injection and spleen and LNs biopsies were obtained 30 days postinjection. Thymus, LN, and spleen biopsies from noninjected macaques were used as controls. All experiments were approved by the local animal facility Institutional Review Board in accordance with national guidelines.
Flow cytometry. Cells isolated from thymus, spleen, and LNs were stained with the appropriate fluorochrome-conjugated antibodies: anti-CD4, anti-CD8, anti-CD3, anti-CD44, anti-CD25, and anti-CD19 antibodies for murine cells and anti-CD4 and anti-CD8 for macaques cells (all from Becton Dickinson, France) as previously described.7 Briefly, nonspecific staining was blocked by incubating cells with an anti-FcγRII antibody (24G2; Becton Dickinson) in PBS containing 2% fetal calf serum. Cells were then washed and incubated with the indicated antibodies diluted in PBS containing 2% fetal calf serum at 4 °C. All acquisitions were performed on a FACSCalibur or FACSCanto (BD Biosciences, San Jose, CA) and analyses were performed using FlowJo software (TreeStar, San Carlos, CA).
Quantitative real-time PCR and RT-PCR. DNA was extracted using a genomic DNA purification kit (Promega, Madison, WY) while total RNA was isolated using TRIZol (Invitrogen, Cergy Pontoise, France). RNA was treated with Turbo DNAseI (Ambion, Austin, TX) and reverse transcribed into first strand cDNA using poly-dT oligonucleotide and Moloney murine leukaemia virus reverse transcriptase (Invitrogen). Quantitative PCRs were performed using the GenAmp 7700 sequence detection system (Applied Biosystems, Foster City, CA) with SYBR Green core reagents (Applied Biosystems). Primer sequences designed to amplify eGFP DNA and the eGFP cDNA are as follows: 5′-CACCATCTTCTTCAAGGACGA-3′ and 5′-CCATGATATAGACGTTGTGGCTG-3′, and 5′-CACCATCTTCTT CAAGGACGA-3′ and 5′-CCATGATATAGACGTTGTGGCTG-3′, respectively. Sequences for genomic Epo (5′-GCTCCACTCCGAACCATCAC-3′ and 5′-TCATCTGTCCCCTCTCCTGC-3′) and hypoxanthine-guanine phosphoribosyl transferase cDNA (5′-CGTGATTAGCGATGATGAA CC-3′ and 5′-ATCCAGCAGGTCAGCAAAGA-3′) are indicated. Thermal cycling conditions comprised a 2-minute incubation at 50 °C, a 10-minute denaturation at 95 °C, followed by 40 cycles of denaturation (95 °C for 15 seconds), annealing (60 °C for 1 minute), extension (Tm for 15 seconds), and 10 minutes at 72 °C. Tm was 79 °C for hypoxanthine-guanine phosphoribosyl transferase and the genomic and cDNA sequences of eGFP and 77 °C for Epo. For DNA samples, Ct values were compared to those obtained using dilutions of plasmids harboring eGFP and Epo sequences, respectively. Relative expression of eGFP, after normalization to hypoxanthine-guanine phosphoribosyl transferase values, was calculated as 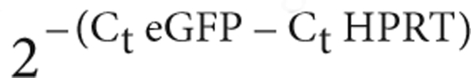 .
.
Immunohistochemistry. Thymus and spleen samples were embedded in inclusion compound (Sakura Finetek, Torrance, CA), frozen in isopentane cooled with liquid nitrogen and stored at −80 °C. Transverse thymus and spleen cryosections (10-µm) were cut using a Leica CM 3050S cryostat. Frozen sections were mounted on slides with mounting medium (Mowiol medium; Calbiochem, Strasbourg, France) and examined for eGFP expression using a laser-scanning confocal microscope (C1-Nikon TE-2000; Nikon, Belmont, CA) equipped with a blue argon laser emitting at 488 nm. Immunohistochemistry was performed on 10-µm sections. Nonspecific antigen binding was blocked in PBS containing 20% goat serum (blocking buffer). Sections were then stained by overnight incubation at 4 °C in blocking buffer containing the following primary antibodies at the indicated dilutions: anti-CTK pAb, 1:40 (Signet, Cambridge, MA), anti-S100 pAb, 1:100 (Dakocytomation, Trappes, France), and an anti-CD4 mAb, 1:25 (Becton Dickinson, San Diego, CA). After washing with PBS, sections were incubated with biotinylated goat antimouse or antirabbit antibodies (1:300; Dakocytomation), followed by an Alexa555 streptavidin complex (1:2,000; Molecular Probes, Eugene, OR). Sections were counterstained with topro-3 (1:1,000; Invitrogen, Cergy Pontoise, France) to stain nuclei, mounted and serially scanned using a confocal microscope (C1-Nikon TE-2000).
Acknowledgments
We are grateful to all the members of our laboratories for their careful and enthusiastic input throughout the course of this study. We thank Didier Trono for generously providing high-titer purified lentiviral enhanced green fluorescent protein virion stocks. Flow cytometry and animal experiments were made possible by the MRI-RIO imaging platform (GIS-IBISA, Languedoc-Roussillon) and T&TA core facilities, respectively. A.M. has been supported by a grant from the Association Française contre les Myopathies (AFM) and the Pays de Loire Collectivités Territoriales, R.V. by a fellowship from the Portuguese Foundation for Science and Technology (SFRH/BD/23553/2005), and O.A. by successive support from ATTACK and the AFM. N.T. and P.M. are supported by the Institut National de la Santé et de la Recherche Médicale and V.S.Z. by the Centre National de la Recherche Scientifique. This work was supported by grant R01AI059349 from the National Institute of Allergy and Infectious Diseases, the AFM (to V.S.Z., N.T., and P.M.), and the European Community (contract LSHC-CT-2005-018914 “ATTACK,” to N.T.). We also thank in Nantes, the Vector Core (www.vectors.nantes.inserm.fr), and the large animal facility (Centre de Boisbonne).
REFERENCES
- Porritt HE, Gordon K., and , Petrie HT. Kinetics of steady-state differentiation and mapping of intrathymic-signaling environments by stem cell transplantation in nonirradiated mice. J Exp Med. 2003;198:957–962. doi: 10.1084/jem.20030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald HR. Thymus organogenesis. Annu Rev Immunol. 2008;26:355–388. doi: 10.1146/annurev.immunol.26.021607.090408. [DOI] [PubMed] [Google Scholar]
- Khoury SJ, Sayegh MH, Hancock WW, Gallon L, Carpenter CB., and , Weiner HL. Acquired tolerance to experimental autoimmune encephalomyelitis by intrathymic injection of myelin basic protein or its major encephalitogenic peptide. J Exp Med. 1993;178:559–566. doi: 10.1084/jem.178.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMatteo RP, Chu G, Ahn M, Chang E, Barker CF., and , Markmann JF. Long-lasting adenovirus transgene expression in mice through neonatal intrathymic tolerance induction without the use of immunosuppression. J Virol. 1997;71:5330–5335. doi: 10.1128/jvi.71.7.5330-5335.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marodon G, Fisson S, Levacher B, Fabre M, Salomon BL., and , Klatzmann D. Induction of antigen-specific tolerance by intrathymic injection of lentiviral vectors. Blood. 2006;108:2972–2978. doi: 10.1182/blood-2006-03-010900. [DOI] [PubMed] [Google Scholar]
- Posselt AM, Barker CF, Friedman AL., and , Naji A. Prevention of autoimmune diabetes in the BB rat by intrathymic islet transplantation at birth. Science. 1992;256:1321–1324. doi: 10.1126/science.1598576. [DOI] [PubMed] [Google Scholar]
- Adjali O, Marodon G, Steinberg M, Mongellaz C, Thomas-Vaslin V, Jacquet C, et al. In vivo correction of ZAP-70 immunodeficiency by intrathymic gene transfer. J Clin Invest. 2005;115:2287–2295. doi: 10.1172/JCI23966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bec C., and , Douar AM. Gene therapy progress and prospects—vectorology: design and production of expression cassettes in AAV vectors. Gene Ther. 2006;13:805–813. doi: 10.1038/sj.gt.3302724. [DOI] [PubMed] [Google Scholar]
- Russell DW. AAV vectors, insertional mutagenesis, and cancer. Mol Ther. 2007;15:1740–1743. doi: 10.1038/sj.mt.6300299. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O'Malley KL, et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Xiao X, Samulski RJ, Li J, Ojaama K, Klein IL.Long-term gene transfer in porcine myocardium after coronary infusion of an adeno-associated virus vector Ann Thorac Surg 1996621669–1676.et al [DOI] [PubMed] [Google Scholar]
- McCown TJ, Xiao X, Li J, Breese GR., and , Samulski RJ. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 1996;713:99–107. doi: 10.1016/0006-8993(95)01488-8. [DOI] [PubMed] [Google Scholar]
- Griffey MA, Wozniak D, Wong M, Bible E, Johnson K, Rothman SM, et al. CNS-directed AAV2-mediated gene therapy ameliorates functional deficits in a murine model of infantile neuronal ceroid lipofuscinosis. Mol Ther. 2006;13:538–547. doi: 10.1016/j.ymthe.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Sondhi D, Peterson DA, Giannaris EL, Sanders CT, Mendez BS, De B, et al. AAV2-mediated CLN2 gene transfer to rodent and non-human primate brain results in long-term TPP-I expression compatible with therapy for LINCL. Gene Ther. 2005;12:1618–1632. doi: 10.1038/sj.gt.3302549. [DOI] [PubMed] [Google Scholar]
- Xiao X, Li J, McCown TJ., and , Samulski RJ. Gene transfer by adeno-associated virus vectors into the central nervous system. Exp Neurol. 1997;144:113–124. doi: 10.1006/exnr.1996.6396. [DOI] [PubMed] [Google Scholar]
- Hargrove PW, Vanin EF, Kurtzman GJ., and , Nienhuis AW. High-level globin gene expression mediated by a recombinant adeno-associated virus genome that contains the 3′ gamma globin gene regulatory element and integrates as tandem copies in erythroid cells. Blood. 1997;89:2167–2175. [PubMed] [Google Scholar]
- Malik P, McQuiston SA, Yu XJ, Pepper KA, Krall WJ, Podsakoff GM, et al. Recombinant adeno-associated virus mediates a high level of gene transfer but less efficient integration in the K562 human hematopoietic cell line. J Virol. 1997;71:1776–1783. doi: 10.1128/jvi.71.3.1776-1783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Hanawa H, Vandergriff J, Kelly P, Vanin EF., and , Nienhuis AW. Efficient gene transfer into human cord blood CD34+ cells and the CD34+CD38- subset using highly purified recombinant adeno-associated viral vector preparations that are free of helper virus and wild-type AAV. Gene Ther. 2000;7:183–195. doi: 10.1038/sj.gt.3301068. [DOI] [PubMed] [Google Scholar]
- Choi VW, McCarty DM., and , Samulski RJ. AAV hybrid serotypes: improved vectors for gene delivery. Curr Gene Ther. 2005;5:299–310. doi: 10.2174/1566523054064968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH., and , Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D., and , Kay MA. From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr Gene Ther. 2003;3:281–304. doi: 10.2174/1566523034578285. [DOI] [PubMed] [Google Scholar]
- Zhong L, Li W, Li Y, Zhao W, Wu J, Li B, et al. Evaluation of primitive murine hematopoietic stem and progenitor cell transduction in vitro and in vivo by recombinant adeno-associated virus vector serotypes 1 through 5. Hum Gene Ther. 2006;17:321–333. doi: 10.1089/hum.2006.17.321. [DOI] [PubMed] [Google Scholar]
- Fisher KJ, Kelley WM, Burda JF., and , Wilson JM. A novel adenovirus- adeno-associated virus hybrid vector that displays efficient rescue and delivery of the AAV genome. Hum Gene Ther. 1996;7:2079–2087. doi: 10.1089/hum.1996.7.17-2079. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN, et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P., and , Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ma HI, Li J, Sun L, Zhang J., and , Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- Hirata RK., and , Russell DW. Design and packaging of adeno-associated virus gene targeting vectors. J Virol. 2000;74:4612–4620. doi: 10.1128/jvi.74.10.4612-4620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GS, Schmidt M, Yan Z, Lindbloom JD, Harding TC, Donahue BA, et al. Virus-mediated transduction of murine retina with adeno-associated virus: effects of viral capsid and genome size. J Virol. 2002;76:7651–7660. doi: 10.1128/JVI.76.15.7651-7660.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina N, Han Z, Li X, Hu Z, Zhong L, Bischof D, et al. Recombinant self-complementary adeno-associated virus serotype vector-mediated hematopoietic stem cell transduction and lineage-restricted, long-term transgene expression in a murine serial bone marrow transplantation model. Hum Gene Ther. 2008;19:376–383. doi: 10.1089/hum.2007.143. [DOI] [PubMed] [Google Scholar]
- Weerkamp F, de Haas EF, Naber BA, Comans-Bitter WM, Bogers AJ, van Dongen JJ.Age-related changes in the cellular composition of the thymus in children J Allergy Clin Immunol 2005115834–840.et al [DOI] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J., and , Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- Han Z, Zhong L, Maina N, Hu Z, Li X, Chouthai NS, et al. Stable integration of recombinant adeno-associated virus vector genomes after transduction of murine hematopoietic stem cells. Hum Gene Ther. 2008;19:267–278. doi: 10.1089/hum.2007.161. [DOI] [PubMed] [Google Scholar]
- Zincarelli C, Soltys S, Rengo G., and , Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- Egerton M, Scollay R., and , Shortman K. Kinetics of mature T-cell development in the thymus. Proc Natl Acad Sci USA. 1990;87:2579–2582. doi: 10.1073/pnas.87.7.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-Vaslin V, Altes HK, de Boer RJ., and , Klatzmann D. Comprehensive assessment and mathematical modeling of T cell population dynamics and homeostasis. J Immunol. 2008;180:2240–2250. doi: 10.4049/jimmunol.180.4.2240. [DOI] [PubMed] [Google Scholar]
- Marodon G., and , Klatzmann D. In situ transduction of stromal cells and thymocytes upon intrathymic injection of lentiviral vectors. BMC Immunol. 2004;5:18. doi: 10.1186/1471-2172-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMatteo RP, Raper SE, Ahn M, Fisher KJ, Burke C, Radu A.Gene transfer to the thymus. A means of abrogating the immune response to recombinant adenovirus Ann Surg 1995222229–239–239–242.et aldiscussion [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzins SP, Godfrey DI, Miller JF., and , Boyd RL. A central role for thymic emigrants in peripheral T cell homeostasis. Proc Natl Acad Sci USA. 1999;96:9787–9791. doi: 10.1073/pnas.96.17.9787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida AR, Rocha B, Freitas AA., and , Tanchot C. Homeostasis of T cell numbers: from thymus production to peripheral compartmentalization and the indexation of regulatory T cells. Semin Immunol. 2005;17:239–249. doi: 10.1016/j.smim.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Favre D, Blouin V, Provost N, Spisek R, Porrot F, Bohl D, et al. Lack of an immune response against the tetracycline-dependent transactivator correlates with long-term doxycycline-regulated transgene expression in nonhuman primates after intramuscular injection of recombinant adeno-associated virus. J Virol. 2002;76:11605–11611. doi: 10.1128/JVI.76.22.11605-11611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss AK., and , Muruve DA. Immunity to adeno-associated virus vectors in animals and humans: a continued challenge. Gene Ther. 2008;15:808–816. doi: 10.1038/gt.2008.54. [DOI] [PubMed] [Google Scholar]
- Tian C, Bagley J., and , Iacomini J. Homeostatic expansion permits T cells to re-enter the thymus and deliver antigen in a tolerogenic fashion. Am J Transplant. 2007;7:1934–1941. doi: 10.1111/j.1600-6143.2007.01891.x. [DOI] [PubMed] [Google Scholar]
- Tian C, Bagley J, Forman D., and , Iacomini J. Induction of central tolerance by mature T cells. J Immunol. 2004;173:7217–7222. doi: 10.4049/jimmunol.173.12.7217. [DOI] [PubMed] [Google Scholar]
- Grimm D, Kay MA., and , Kleinschmidt JA. Helper virus-free, optically controllable, and two-plasmid-based production of adeno-associated virus vectors of serotypes 1 to 6. Mol Ther. 2003;7:839–850. doi: 10.1016/s1525-0016(03)00095-9. [DOI] [PubMed] [Google Scholar]
- Chenuaud P, Larcher T, Rabinovitz JE, Provost N, Joussemet B, Bujard H, et al. Optimal design of a single recombinant adeno-associated virus derived from serotypes 1 and 2 to achieve more tightly regulated transgene expression from nonhuman primate muscle. Mol Ther. 2004;9:410–418. doi: 10.1016/j.ymthe.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Salvetti A, Oreve S, Chadeuf G, Favre D, Cherel Y, Champion-Arnaud P, et al. Factors influencing recombinant adeno-associated virus production. Hum Gene Ther. 1998;9:695–706. doi: 10.1089/hum.1998.9.5-695. [DOI] [PubMed] [Google Scholar]