Abstract
We report the development of a gene replacement strategy for very long–chain acyl-CoA dehydrogenase (VLCAD) deficiency. VLCAD is a mitochondrial enzyme involved in fatty acid β-oxidation, a key step in energy production during times of fasting or stress. Deficiency of VLCAD classically presents as hepatic dysfunction, hypoglycemia, cardiomyopathy, rhabdomyolysis, and/or sudden death. While dietary therapy for VLCAD deficiency has proven beneficial in preventing some symptoms, a risk of metabolic catastrophic decompensation remains throughout life during times of increased energy demand. We designed a recombinant adeno-associated virus (AAV) expressing the human VLCAD gene (AAV8-hVLCAD). To demonstrate its in vivo activity, AAV8-hVLCAD was administered via the tail vein to VLCAD-knockout mice. A reduction in accumulated serum long-chain acylcarnitines and increased fasting tolerance judged on blood glucose concentrations were observed as of 11 days postinjections through >100 days. Western analysis of liver, skeletal muscle, and heart extracts using PEP1 anti-hVLCAD antibody revealed short-term hVLCAD expression in the liver and muscle and longer-term expression in heart. This demonstrates the ability of human VLCAD to correct the biochemical phenotype of VLCAD-deficient mice.
Introduction
Acyl-CoA dehydrogenases (ACDs) are an important group of mitochondrial enzymes involved in energy homeostasis by sequential cleavage of two-carbon acetyl-CoAs from long-chain fatty acids in the fatty acid β-oxidation pathway. The various ACD deficiencies have a wide range of clinical presentations from mild hypotonia in adults to sudden unexpected death.1 With early detection and institution of therapy the initial outcomes are improved for most patients, yet long-term morbidity of affected individuals can be triggered by acute illness or noncompliance with dietary recommendations resulting in rapid, life-threatening metabolic decompensation. This has led to the development of gene therapy strategies for long-, medium-, and short-chain acyl-CoA dehydrogenase deficient mouse models.2,3,4 Very long–chain acyl-CoA dehydrogenase (VLCAD) is one of the ACDs involved in β-oxidation of fatty acids. It is a 70-kDa protein that forms a homodimer and is associated with the inner-mitochondrial membrane.5,6 Deficiency of the VLCAD enzyme has been estimated to affect between 1:100,000 and 1:120,000 individuals.7,8,9 While historically considered a rare disorder, the introduction of acylcarnitine profiling into expanded newborn screening programs has revealed the actual incidence may be as high as 1:42,500.10 VLCAD is highly expressed in liver, heart, and skeletal muscle, and when completely or partially deficient causes variable phenotypes from neonatal hypoglycemia, severe cardiomyopathy, and precipitous death, to a milder form presenting as myopathy and/or rhabdomyolysis in adolescence or adulthood.11,12,13,14,15,16,17 Unfortunately, the diagnosis is all too often delayed until the first critical illness or on postmortem biochemical evaluation18 and currently available therapeutic options, including avoidance of fasting with frequent meals, a high-carbohydrate and low-fat diet, and supplementation with l-carnitine, are unable to address significant potential long-term risks of this disorder.1,19,20,21 While the pathophysiology of VLCAD deficiency is not yet entirely understood, many of the symptoms are attributable to lack of energy production. Furthermore, the accumulation of long-chain acylcarnitine species has been suggested to be arrhythmogenic and associated with cardiac dysfunction.15 The highly complex, variable, and uncertain clinical course of VLCAD deficiency makes it a candidate for gene therapy. Treatment of VLCAD deficiency beyond dietary manipulation would serve as a potentially preventable cause of cardiomyopathy and provide long-term stabilization of clinical symptoms with reduced risk of decompensation and chronic morbidity and mortality.
Serotype 2 adeno-associated virus (AAV) has been described as an efficient in vivo delivery vector for transgenes and their hepatic expression in the treatment of metabolic diseases.22,23,24,25 Following the description of serotype 8, recombinant pseudotypes were constructed and have been shown to be highly effective in transducing skeletal and cardiac muscle.26,27 Here we report the development of a pseudotype AAV2/8 recombinant vector containing a plasmid construct we previously described carrying human VLCAD driven by a CMV promoter that targets the mitochondria, is expressed in the murine liver, and corrects the acylcarnitine profile in vitro.28 This recombinant vector (AAV8-hVLCAD) is able to correct the biochemical phenotype after transfection of VLCAD-deficient mice and represents a further step toward the development of in vivo gene replacement therapy for VLCAD deficiency.
Results
In vivo expression of hVLCAD
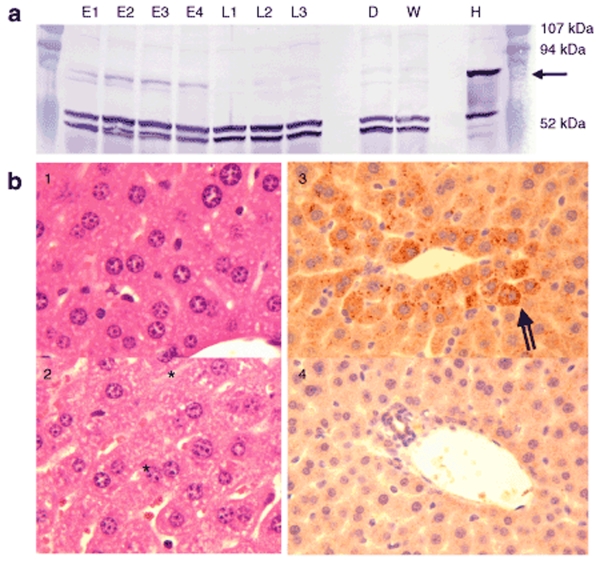
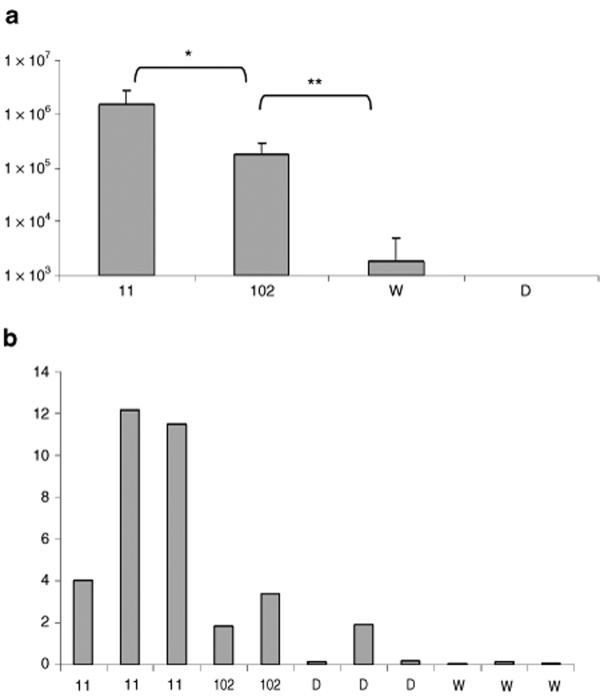
We investigated the expression of hVLCAD in liver, skeletal muscle, and cardiac muscle. Vector purification (see also Supplementary Materials and Methods) resulted in a 90% purification of the final preparation with concentrations ranging from 2 to 5 × 1013 vector genomes/ml (Supplementary Figure S1a). Electron microscopy demonstrated formation of appropriately sized intact viral particles (Supplementary Figure S1b). To investigate hepatic expression we injected seven mice with AAV8-hVLCAD and then killed four mice in the early treatment group at 11 days while the three remaining mice in the late treatment group were followed until 102 days. We used the PEP1 antibody which has high reactivity to the N-terminal region of human VLCAD and does not detect native murine VLCAD in wild-type mice.28 Western blot of livers from mice injected with AAV8-hVLCAD demonstrated the presence of hVLCAD in the early treatment group although this band was absent in the late group (Figure 1a). After prolonged fasting the untreated VLCAD-deficient mice developed subtle changes on liver histology consistent with steatosis (Figure 1b–2) that did not appear to be present in the early treatment group (Figure 1b–1). Immunohistochemical staining of liver using PEP1 antibody demonstrated overexpression of hVLCAD in this early group (Figure 1b–3) as compared to untreated mice (Figure 1b–4). Quantitative PCR revealed a modest decrease in the total number of vector sequences in treated murine livers from the early group as compared to late group mice (P < 0.05). However, even in the late group the total number of vector sequences detected was higher than in wild-type and untreated control mice (P < 0.0001; Figure 2a). Analysis through reverse transcriptase quantitative PCR of hepatic cDNA expression using GADPH as a control revealed a relative higher percentage of hepatocytes expressing AAV8-hVLCAD in the early groups than those levels seen in the late and control group animals (Figure 2b). The elevation of a single knockout mouse in Figure 2b represented a poor amplification of the control GADPH primers.
Figure 1.
In vivo hepatic expression of hVLCAD. (a) Western blot prepared from four early (E1–E4) and three late (L1–L3) group mice. Controls from knockout VLCAD-deficient (D) and wild-type (W) mouse liver with normal human liver (H) run in parallel. Each lane represents 20 µg protein after Lubrol extraction and hybridization with PEP1 anti-hVLCAD antibody. The arrow on the right indicates the position of the 70-kDa hVLCAD. Prestained molecular weight standards are shown in the column on the right. The intense bands that are seen around 50 kDa represent nonspecific hybridization not related to VLCAD and serve as a loading control. (b) Histological examination of treated (1,3) and untreated (2,4) murine liver after H&E staining (1,2) and immune staining with PEP1 (3,4; 1:500 dilution) in the early treatment group. Asterisk indicates mild steatosis. Striped arrow indicates PEP1 antibody binding to hVLCAD.
Figure 2.
Quantitative PCR. (a) Quantization of AAV8-hVLCAD genomes in total genomic DNA in liver tissue from treated mice at 11 (N = 3) and 102 (N = 2) days as compared to control wild-type (W, N = 3) and knockout (D, N = 3) mice. The y-axis represents average AAV genomes detected per microgram total genomic DNA. Error bars represent one standard deviation. Single asterisk indicates P value <0.05; double asterisk indicates P < 0.0001. (b) RT-qPCR of individual mice (×100, y-axis) at 11 and 102 days in treated mice and controls. The y-axis represents the relative percentage of AAV8 genomes detected per GADPH genome (×100). Additional control samples from this study omitting reverse transcriptase were negative and not shown.
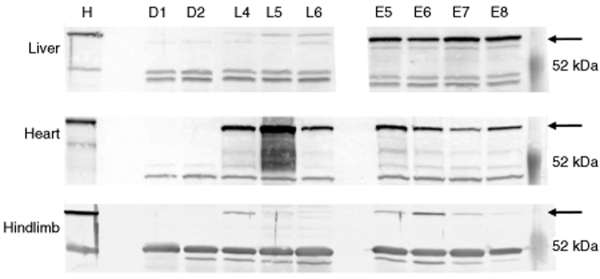
Figure 3.
Liver, heart, and hindlimb expression of hVLCAD. Western blots from four mice killed at 11 days postinjection (E5–E8) and three mice were killed at 102 days postinjection (L4–L6). Control lanes were run in parallel representing control extracts from liver, heart, and hindlimb muscle from knockout VLCAD-deficient mice (D1, D2) or normal controls from either human liver, heart, or skeletal muscle (H). Each lane represents 20 µg protein after Lubrol extraction and hybridization with PEP1 anti-hVLCAD antibody. The arrow on the right indicates the position of the 70-kDa hVLCAD. Prestained molecular weight standards are shown in the column on the right.
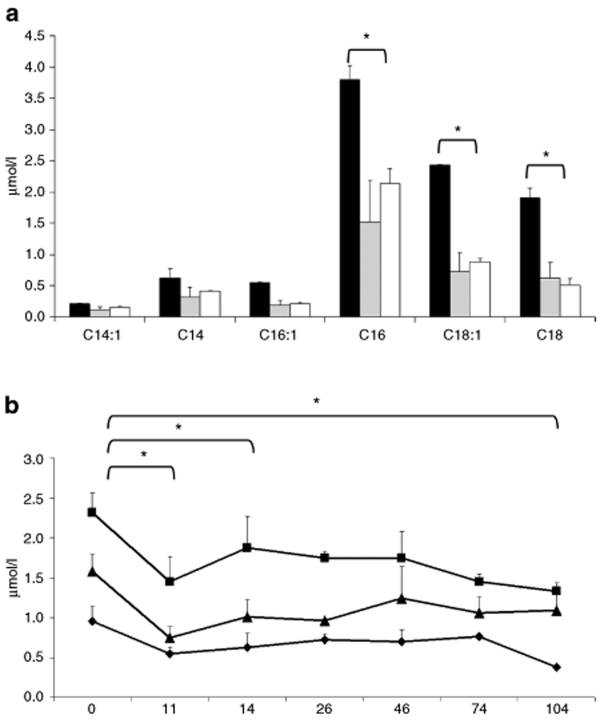
Figure 4.
Acylcarnitine analysis. (a) Short-term study following VLCAD-deficient mice injected with AAV8-hVLCAD (N = 2, gray column) with blood collected at 14 days postinjection following a 24-hour fast compared with control wild-type (N = 3, white) and deficient (N = 2, black) mice. Asterisks indicate P value <0.05 between untreated mice and injected mice. There was no significant difference between injected and wild-type mice. (b) Long-term study of AAV8-hVLCAD-injected VLCAD-deficient mice were with blood collected from early and late treatment groups (N = 7) at days 0, 11, and 14. Days 26 through 104 represent only the late treatment group (N = 3). C16- (square), C18:1- (triangle), and C18- (diamond) acylcarnitine levels. Measurements indicate average of all injected mice at that time point. Error bars represent one standard deviation. Asterisks indicate P value <0.05 for comparisons between 0 and 11, 14, or 104 days for C16-acylcarnitine.
A third experimental group was followed to examine expression of hVLCAD in cardiac and skeletal muscle at early and late time points by western blot (Figure 3). The early group demonstrated strong hVLCAD expression levels in heart and liver but only low levels were seen in skeletal muscle (Figure 3, E5–E8). Further comparison of the late group revealed continued or increasing expression in the heart, but there was only minimal expression in liver and skeletal muscle (Figure 3, L4–L6).
Biochemical correction of VLCAD deficiency
To assess the ability of hVLCAD to correct the defect in fatty acid β-oxidation in the knockout mouse model we analyzed acylcarnitines in dried blood spots at specified time points. VLCAD-knockout mice are known to demonstrate primary elevations in C16 and C18 saturated and unsaturated acylcarnitine species.29 A short-term comparison study showed following administration of AAV8-hVLCAD to knockout mice there was a significant decrease in accumulated long-chain acylcarnitines to lower levels equivalent to those seen in wild-type mice at 14 days (P < 0.05, Figure 4a). A long-term study was performed by injecting 3 × 1012 vector genomes into knockout mice. Following injection the treated mice revealed a rapid and statistically significant drop in C16, C18, and C18:1 acylcarnitine species by 11 days postinjection (P ≤ 0.0002, Figure 4b). A small reaccumulation of acylcarnitines was observed at 14 days, but levels still remained lower than pretreatment values (P < 0.05). A relative stabilization at these lower levels (P ≤ 0.01) was noted at the subsequent time points.
Correction of fasting-induced hypoglycemia
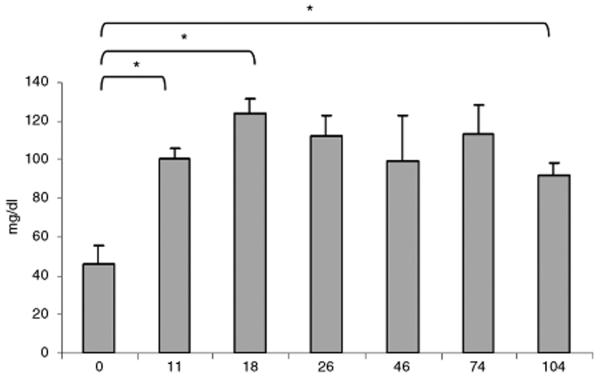
Hypoglycemia is a typical finding and major source of morbidity in VLCAD deficiency in humans. It is triggered by increased energy demands due to fasting or metabolic stress. VLCAD-deficient mice demonstrate a milder phenotype and only develop hypoglycemia after prolonged fasting or cold-induced stress.29 We found considerable difficultly in demonstrating a profound hypoglycemia without causing significant mortality from cold-induced stress; however, a prolonged 24-hour fast at normal room temperature allowed us to safely stress knockout mice, which exhibited moderate hypoglycemia, and longitudinal study. By 11 days postinjection both early and late group mice did not develop hypoglycemia following fasting, and there was a significant rise in glucose levels (P < 0.0001, Figure 5). Until the end of the experiment the late group continued to demonstrate fasting serum glucose levels at physiological levels and at significantly higher concentrations than before treatment (P < 0.0001).
Figure 5.
Correction of fasting-induced hypoglycemia. VLCAD-deficient mice injected with AAV8-hVLCAD. Blood was collected at days 0 and 11 for both early and late groups (N = 7). Days 18 to 104 represent the late group only (N = 3). Levels indicate average serum glucose measurement levels of all mice at that time point. Error bars represent 1 SD. Asterisks indicate P value <0.0001 for comparisons between 0 and 11, 18, or 104 days. Nonfasting, untreated VLCAD-knockout mice and wild-type mice had serum glucose measurements in the 70–140 mg/dl range (data not shown).
Discussion
This study demonstrates for the first time in vivo correction of VLCAD deficiency in an animal model by gene therapy using a recombinant AAV2/8 vector containing a well-characterized human VLCAD cDNA expression cassette and a CMV promoter.28 The recombinant vector was produced in sufficient quantities through triple co-transfection of 293 cells and cesium chloride purification. Following tail vein injection of the vector, hVLCAD is strongly expressed in hepatocytes correlating with rapid correction of the enzymatic block in long-chain fatty acid β-oxidation and prevention of stress-induced hypoglycemia. This is consistent with our prior studies showing hVLCAD is properly imported into the mitochondria of knockout mice as a functional homodimer.30 The small increase of acylcarnitines shortly after treatment may be related to decreased expression of hVLCAD in hepatocytes. While the CMV promoter is downregulated in the murine liver,31 the decreasing numbers of vector DNA seen on quantitative analysis in the liver and decreased hVLCAD expression on western blot at 102 days is consistent with loss of vector DNA as reported by Wang et al.27 The reason for the poor hVLCAD expression in skeletal muscle is uncertain as the AAV2/8 vector is known to efficiently cross the blood-muscle barrier. Our injection dose may have been insufficient or our specific cDNA construct may not have been appropriately translated in muscle.
Cardiomyopathy is a common complication of VLCAD deficiency and major contributor to morbidity and mortality.13,16 The pathophysiology of VLCAD-associated cardiomyopathy is unclear, but has been thought to be related to toxicity from long-chain acylcarnitine accumulation rather than a singular block in energy metabolism. In contrast to findings in liver and skeletal muscle, western blot analysis revealed increased expression of hVLCAD in heart muscle at 102 days as compared to 11 days. This expression cannot be solely explained by the use of the CMV promoter as this effect has been seen with the AAV2/8 serotype using other promoters.27 The continued strong expression in the myocardium is likely due to a more persistent circular monomer form of the vector DNA.27 Similar vectors have demonstrated a comparable phenomenon with expression levels continually rising at 6 months.32 While it may be tempting to hypothesize that this increased hVLCAD expression may have been sufficient to correct the acylcarnitine profile, further investigation of myocardial expression is needed. This specific tropism of the AAV2/8 serotype provides a unique tool to further explore the tissue-specific pathophysiology of long-chain acylcarnitine accumulation in VLCAD. Overall though, extrahepatic expression may have provided sufficient enzymatic activity to provide prolonged correction of the biochemical phenotype.
The murine VLCAD-knockout model has a milder phenotype compared to that seen in humans with complete VLCAD deficiency, although with sufficient stress systemic pathology is observed in mice as well. This may be explained by differences in substrate specificity and/or differential tissue expression between humans and mice.29 While we were able to obtain evidence of biochemical correction in mice, their milder phenotype will present difficulty when translating these studies to humans. Preliminary experiments have also shown the presence of vector antibody formation in treated mice. While AAV-mediated gene transfer has not been associated with significant inflammatory responses,33 further study is needed to determine the effect of these antibodies upon VLCAD expression and the correction of the biochemical phenotype. However, liver-directed AAV-mediated gene transfer has shown induced tolerance of therapeutic genes,33 and this may play a role in facilitating murine expression of human VLCAD. The use of alternative promoters such as liver-specific or native promoters may also allow prolonged expression in the liver and other tissues and evasion of the immune response.34
Overall, these studies demonstrate that human VLCAD protein is able to function in murine liver and provide evidence of successful gene therapy for VLCAD deficiency. We show strong initial hepatic and later onset myocardial hVLCAD expression leading to lower long-chain acylcarnitine levels. This robust cardiac hVLCAD expression provides an opportunity to further examine the tissue-specific pathology of cardiac dysfunction in VLCAD deficiency.35 These findings provide the basis for further development of a human gene replacement strategy and for the study of long-term transgenic hVLCAD expression and its effects on the complex pathophysiology of VLCAD deficiency.
Methods
The materials and methods for vector production, purification, and characterization and quantitative analysis are described in the Supplementary Materials and Methods.
VLCAD-deficient mice. VLCAD-knockout mice29 were handled and housed at Mayo Clinic's animal facilities with approval by the Institutional Animal Care and Use Committee. For the initial comparison group, knockout mice were injected with 200 µl of 8.55 × 1011 vector genomes/ml via the tail veins and followed in parallel with untreated knockout and wild-type mice. Mice were monitored until 14 days postinjection, fasted for 24 hours, and then blood was collected prior to the mice being euthanized. For longer-term study groups, at 6–8 weeks of age, blood was collected from knockout mice prior to injection with 200 µl of 2.42 × 1013 vector genomes/ml on day zero and at 10, 14, 26, 46, 74, and 104 days postinjection. Mice were randomly placed into two groups and euthanized at either 14 days for the early or 104 days for the late analysis at which point the heart, hindlimb skeletal muscle, and liver were quickly removed for snap-freezing in liquid nitrogen or embedding in paraffin. Additional experimental mouse groups were injected with AAV8-hVLCAD using similar protocols for follow-up studies.
Biochemical analysis. Blood was collected via retroorbital bleed and then spotted onto S&S 903 Specimen collection paper (Schleicher and Schuell, Keene, NH). Acylcarnitine species were quantified from a single 3 mm punch by tandem mass spectrometry as previously described.29 Glucose levels were measured by applying a drop of blood to the test strip of a ReliOn handheld glucometer (Solartek, Bedford, MA).
Western blot and immunohistochemistry. Western blots were performed as previously described using PEP1 antihuman VLCAD antibody.28 Liver sections were placed on glass slides and stained with hematoxylin and eosin for routine histology. Paraffin-embedded liver was deparaffinized, quenched, steamed in citrate, incubated with PEP1 primary antibody then biotinylated secondary antibody, and finally detected using a modified protocol from the VECTASTAIN Universal Quick kit (Vector Laboratories, Burlingame, CA) by the Tissue and Cell Molecular Analysis Core Facility of the Mayo Clinic Cancer Center. Images were acquired at ×40 magnification using a Leica DFC320 microscope (Leica Microsystems, Wetzlar, Germany).
Statistical analysis. All analyses to compare the significance of measured levels were completed using the unpaired t-test.
Supplementary MaterialMaterials and Methods.Figure S1. Characterization of AAV8-hVLCAD. (a) Silver stain image of purified viral stock (left lane) demonstrating three purified primary AAV proteins at 62, 73, and 87 kDa (arrows) as compared to unpurified stock (right lane). (b) Transmission electron microscopy demonstrating purified intact viral particles.
Supplementary Material
Characterization of AAV8-hVLCAD. (a) Silver stain image of purified viral stock (left lane) demonstrating three purified primary AAV proteins at 62, 73, and 87 kDa (arrows) as compared to unpurified stock (right lane). (b) Transmission electron microscopy demonstrating purified intact viral particles.
Acknowledgments
This article is dedicated to the memory of David B. Schowalter (1960–2007). The authors have no financial conflicts of interest to declare. We thank Steven J. Russell, Jill M. Thompson, and the Molecular Medicine Program at the Mayo Clinic College of Medicine (Rochester, MN). We are also indebted to James M. Wilson (University of Pennsylvania, Philadelphia, PA) for providing the recombinant AAV2/8 replication plasmid and Philip A. Wood (University of Alabama, Birmingham, AL) for providing VLCAD-knockout mice. This study was supported by funding from the American Heart Association (0234938N) and Mayo Clinic College of Medicine clinical research funds.
REFERENCES
- Rinaldo P, Matern D., and , Bennett MJ. Fatty acid oxidation disorders. Annu Rev Physiol. 2002;64:477–502. doi: 10.1146/annurev.physiol.64.082201.154705. [DOI] [PubMed] [Google Scholar]
- Schowalter DB, Matern D., and , Vockley J. In vitro correction of medium chain acyl CoA dehydrogenase deficiency with a recombinant adenoviral vector. Mol Genet Metab. 2005;85:88–95. doi: 10.1016/j.ymgme.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Conlon TJ, Walter G, Owen R, Cossette T, Erger K, Gutierrez G, et al. Systemic correction of a fatty acid oxidation defect by intramuscular injection of a recombinant adeno-associated virus vector. Hum Gene Ther. 2006;17:71–80. doi: 10.1089/hum.2006.17.71. [DOI] [PubMed] [Google Scholar]
- Beattie SG, Goetzman E, Tang Q, Conlon T, Campbell-Thompson M, Matern D, et al. Recombinant adeno-associated virus-mediated gene delivery of long chain acyl coenzyme A dehydrogenase (LCAD) into LCAD-deficient mice. J Gene Med. 2008;10:1113–1123. doi: 10.1002/jgm.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T, Souri M, Ushikubo S, Kamijo T, Yamaguchi S, Kelley RI, et al. Purification of human very-long-chain acyl-coenzyme A dehydrogenase and characterization of its deficiency in seven patients. J Clin Invest. 1995;95:2465–2473. doi: 10.1172/JCI117947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss AW, Powell CK, Hale DE, Anderson MM, Ahuja A, Brackett JC, et al. Molecular basis of human mitochondrial very-long-chain acyl-CoA dehydrogenase deficiency causing cardiomyopathy and sudden death in childhood. Proc Natl Acad Sci USA. 1995;92:10496–10500. doi: 10.1073/pnas.92.23.10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zytkovicz TH, Fitzgerald EF, Marsden D, Larson CA, Shih VE, Johnson DM, et al. Tandem mass spectrometric analysis for amino, organic, and fatty acid disorders in newborn dried blood spots: a two-year summary from the New England Newborn Screening Program. Clin Chem. 2001;47:1945–1955. [PubMed] [Google Scholar]
- Wilcken B, Wiley V, Hammond J., and , Carpenter K. Screening newborns for inborn errors of metabolism by tandem mass spectrometry. N Engl J Med. 2003;348:2304–2312. doi: 10.1056/NEJMoa025225. [DOI] [PubMed] [Google Scholar]
- Chace DH, Kalas TA., and , Naylor EW. The application of tandem mass spectrometry to neonatal screening for inherited disorders of intermediary metabolism. Annu Rev Genomics Hum Genet. 2002;3:17–45. doi: 10.1146/annurev.genom.3.022502.103213. [DOI] [PubMed] [Google Scholar]
- Spiekerkoetter U, Sun B, Zytkovicz T, Wanders R, Strauss AW., and , Wendel U. MS/MS-based newborn and family screening detects asymptomatic patients with very-long-chain acyl-CoA dehydrogenase deficiency. J Pediatr. 2003;143:335–342. doi: 10.1067/S0022-3476(03)00292-0. [DOI] [PubMed] [Google Scholar]
- Vianey-Saban C, Divry P, Brivet M, Nada M, Zabot MT, Mathieu M, et al. Mitochondrial very-long-chain acyl-coenzyme A dehydrogenase deficiency: clinical characteristics and diagnostic considerations in 30 patients. Clin Chim Acta. 1998;269:43–62. doi: 10.1016/s0009-8981(97)00185-x. [DOI] [PubMed] [Google Scholar]
- Hoffman JD, Steiner RD, Paradise L, Harding CO, Ding L, Strauss AW, et al. Rhabdomyolysis in the military: recognizing late-onset very long-chain acyl Co-A dehydrogenase deficiency. Mil Med. 2006;171:657–658. doi: 10.7205/milmed.171.7.657. [DOI] [PubMed] [Google Scholar]
- Bertrand C, Largilliere C, Zabot MT, Mathieu M., and , Vianey-Saban C. Very long chain acyl-CoA dehydrogenase deficiency: identification of a new inborn error of mitochondrial fatty acid oxidation in fibroblasts. Biochim Biophys Acta. 1993;1180:327–329. doi: 10.1016/0925-4439(93)90058-9. [DOI] [PubMed] [Google Scholar]
- Bonnet D, de Lonlay P, Gautier I, Rustin P, Rotig A, Kachaner J, et al. Efficiency of metabolic screening in childhood cardiomyopathies. Eur Heart J. 1998;19:790–793. doi: 10.1053/euhj.1997.0818. [DOI] [PubMed] [Google Scholar]
- Bonnet D, Martin D, Pascale De L, Villain E, Jouvet P, Rabier D, et al. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation. 1999;100:2248–2253. doi: 10.1161/01.cir.100.22.2248. [DOI] [PubMed] [Google Scholar]
- de Lonlay-Debeney P, Fournet JC., and , Bonnet D. Fatty acid β-oxidation deficiency masquerading as fulminant myocarditis. Int J Cardiol. 1998;65:287–289. doi: 10.1016/s0167-5273(98)00122-3. [DOI] [PubMed] [Google Scholar]
- Kluge S, Kuhnelt P, Block A, Merkel M, Gocht A, Lukacs Z, et al. A young woman with persistent hypoglycemia, rhabdomyolysis, and coma: recognizing fatty acid oxidation defects in adults. Crit Care Med. 2003;31:1273–1276. doi: 10.1097/01.CCM.0000045201.10682.F6. [DOI] [PubMed] [Google Scholar]
- Chace DH, DiPerna JC, Mitchell BL, Sgroi B, Hofman LF., and , Naylor EW. Electrospray tandem mass spectrometry for analysis of acylcarnitines in dried postmortem blood specimens collected at autopsy from infants with unexplained cause of death. Clin Chem. 2001;47:1166–1182. [PubMed] [Google Scholar]
- Vockley J., and , Whiteman DA. Defects of mitochondrial β-oxidation: a growing group of disorders. Neuromuscul Disord. 2002;12:235–246. doi: 10.1016/s0960-8966(01)00308-x. [DOI] [PubMed] [Google Scholar]
- Solis JO., and , Singh RH. Management of fatty acid oxidation disorders: a survey of current treatment strategies. J Am Diet Assoc. 2002;102:1800–1803. doi: 10.1016/s0002-8223(02)90386-x. [DOI] [PubMed] [Google Scholar]
- Straussberg R, Harel L, Varsano I, Elpeleg ON, Shamir R., and , Amir J. Recurrent myoglobinuria as a presenting manifestation of very long chain acyl coenzyme A dehydrogenase deficiency. Pediatrics. 1997;99:894–896. doi: 10.1542/peds.99.6.894. [DOI] [PubMed] [Google Scholar]
- Xiao W, Berta SC, Lu MM, Moscioni AD, Tazelaar J., and , Wilson JM. Adeno-associated virus as a vector for liver-directed gene therapy. J Virol. 1998;72:10222–10226. doi: 10.1128/jvi.72.12.10222-10226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Zhou S, Nakai H, Thomas CE, Storm TA, Fuess S, et al. Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood. 2003;102:2412–2419. doi: 10.1182/blood-2003-02-0495. [DOI] [PubMed] [Google Scholar]
- Koeberl DD, Sun BD, Damodaran TV, Brown T, Millington DS, Benjamin DK, Jr, et al. Early, sustained efficacy of adeno-associated virus vector-mediated gene therapy in glycogen storage disease type Ia. Gene Ther. 2006;13:1281–1289. doi: 10.1038/sj.gt.3302774. [DOI] [PubMed] [Google Scholar]
- Moscioni D, Morizono H, McCarter RJ, Stern A, Cabrera-Luque J, Hoang A, et al. Long-term correction of ammonia metabolism and prolonged survival in ornithine transcarbamylase-deficient mice following liver-directed treatment with adeno-associated viral vectors. Mol Ther. 2006;14:25–33. doi: 10.1016/j.ymthe.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH., and , Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- Merritt JL 2nd, Matern D, Vockley J, Daniels J, Nguyen TV., and , Schowalter DB. In vitro characterization and in vivo expression of human very-long chain acyl-CoA dehydrogenase. Mol Genet Metab. 2006;88:351–358. doi: 10.1016/j.ymgme.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Cox KB, Hamm DA, Millington DS, Matern D, Vockley J, Rinaldo P, et al. Gestational, pathologic and biochemical differences between very long-chain acyl-CoA dehydrogenase deficiency and long-chain acyl-CoA dehydrogenase deficiency in the mouse. Hum Mol Genet. 2001;10:2069–2077. doi: 10.1093/hmg/10.19.2069. [DOI] [PubMed] [Google Scholar]
- Souri M, Aoyama T, Hoganson G., and , Hashimoto T. Very-long-chain acyl-CoA dehydrogenase subunit assembles to the dimer form on mitochondrial inner membrane. FEBS Lett. 1998;426:187–190. doi: 10.1016/s0014-5793(98)00343-3. [DOI] [PubMed] [Google Scholar]
- Kay MA, Baley P, Rothenberg S, Leland F, Fleming L, Ponder KP, et al. Expression of human alpha 1-antitrypsin in dogs after autologous transplantation of retroviral transduced hepatocytes. Proc Natl Acad Sci USA. 1992;89:89–93. doi: 10.1073/pnas.89.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomeque J, Chemaly ER, Colosi P, Wellman JA, Zhou S, Del Monte F, et al. Efficiency of eight different AAV serotypes in transducing rat myocardium in vivo. Gene Ther. 2007;14:989–997. doi: 10.1038/sj.gt.3302895. [DOI] [PubMed] [Google Scholar]
- Zaiss AK., and , Muruve DA. Immunity to adeno-associated virus vectors in animals and humans: a continued challenge. Gene Ther. 2008;15:808–816. doi: 10.1038/gt.2008.54. [DOI] [PubMed] [Google Scholar]
- Franco LM, Sun B, Yang X, Bird A, Zhang H, Schneider A, et al. Evasion of immune responses to introduced human acid α-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol Ther. 2005;12:876–884. doi: 10.1016/j.ymthe.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Werdich AA, Baudenbacher F, Dzhura I, Jeyakumar LH, Kannankeril PJ, Fleischer S, et al. Polymorphic ventricular tachycardia and abnormal Ca2+ handling in very-long-chain acyl-CoA dehydrogenase null mice. Am J Physiol Heart Circ Physiol. 2007;292:H2202–H2211. doi: 10.1152/ajpheart.00382.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterization of AAV8-hVLCAD. (a) Silver stain image of purified viral stock (left lane) demonstrating three purified primary AAV proteins at 62, 73, and 87 kDa (arrows) as compared to unpurified stock (right lane). (b) Transmission electron microscopy demonstrating purified intact viral particles.