Abstract
Baculovirus is a promising gene delivery vector but its widespread application is impeded as it only mediates transient transgene expression in mammalian cells. To prolong the expression, we developed a dual baculovirus system whereby one baculovirus expressed FLP recombinase while the other harbored an Frt-flanking cassette encompassing the transgene and oriP/EBNA1 derived from Epstein–Barr virus. After cotransduction of cells, the expressed FLP cleaved the Frt-flanking cassette off the baculovirus genome and catalyzed circular episome formation, then oriP/EBNA1 within the cassette enabled the self-replication of episomes. The excision/recombination efficiency was remarkably enhanced by sodium butyrate, reaching 75% in human embryonic kidney-293 (HEK293) cells, 85% in baby-hamster kidney (BHK) cells, 77% in primary chondrocytes, and 48% in mesenchymal stem cells (MSCs). The hybrid baculovirus substantially prolonged the transgene expression to ≈48 days without selection and >63 days with selection, thanks to the maintenance of replicons and transgene transcription. In contrast to the replicating episomes, the baculovirus genome was rapidly degraded. Furthermore, an osteoinductive growth factor gene was efficiently delivered into MSCs using this system, which not only prolonged the growth factor expression but also potentiated the osteogenesis of MSCs. These data collectively implicate the potential of this hybrid baculovirus system in gene therapy applications necessitating sustained transgene expression.
Introduction
Baculovirus has emerged as a promising gene delivery vector in recent years. Despite being a DNA virus that infects insects as its natural host, baculovirus efficiently transduces numerous mammalian cells with minimal cytotoxicity and possesses a number of distinct advantages (for review see ref. 1,2,3). Therefore, baculovirus has captured growing attention as a novel vector for in vitro and in vivo gene delivery,4,5,6 development of cell-based assays,7 surface display of eucaryotic proteins,8 delivery of vaccine immunogens,9 cancer therapy,10 and production of virus vectors.11,12 Aside from these applications, a recombinant baculovirus expressing bone morphogenetic protein-2 (BMP-2), Bac-CB, can restore the differentiation status of de-differentiated chondrocytes and stimulate in vitro formation of engineered cartilages.13 After implantation, these engineered cartilages are able to repair osteochondral defects in rabbit knees.14 Furthermore, Bac-CB transduction accelerates in vitro osteogenesis of mesenchymal stem cells (MSCs).15 These findings altogether implicate the potential of baculovirus in tissue engineering. Despite the wide spectrum of applications, one drawback associated with baculovirus is that due to its inability to replicate in mammalian cells the viral genome undergoes dilution upon cell proliferation and is subjected to degradation over time.16 As a result, most transgene expression typically extinguishes in ≤2 weeks. The short-term baculovirus-mediated transgene expression restricts its applications in conditions necessitating sustained expression.
FLP/Frt is a system derived form Saccharomyces cerevisiae in which the FLP recombinase recognizes the FLP recognition target (Frt) sites and catalyzes highly efficient site-specific recombination, resulting in the re-circularization of an extrachromosomal episome.17 To stably maintain the episome within the cells, the episome requires a proper origin of replication. Among the well-known replication origins, oriP derived from Epstein–Barr virus contains Epstein–Barr virus nuclear antigen-1 (EBNA1) binding sites and the binding of EBNA1 to oriP orchestrates the replication and segregation of the episomes to daughter cells.18 Plasmids containing oriP/EBNA1 have been exploited for long-term maintenance of transgenes and confer persistent expression in vitro19 and in vivo.20
Prompted by the need to extend baculovirus-mediated expression, we designed a hybrid baculovirus system that exploited the FLP/Frt-mediated recombination for circular episome formation and oriP/EBNA1 for the retention of episomes. The first baculovirus expressed FLP while the second baculovirus harbored an Frt-flanking transgene cassette that encompassed oriP/EBNA1. We hypothesized that after cotransduction the expressed FLP would cleave the transgene cassette from the baculovirus genome and catalyze intracellular episome formation, while oriP/EBNA1 would enable episomal self-replication. We first confirmed, and then optimized the FLP/Frt-mediated recombination and intracellular episome formation. The hybrid baculovirus system functioned in various mammalian cells and the resultant replicon enabled sustained transgene expression. Finally, the feasibility to employ this system in tissue engineering was explored by delivering the BMP-2 gene into MSCs and confirmed by the osteogenesis potentiated by the prolonged BMP-2 expression.
Results
Design of the hybrid baculovirus vector and confirmation of episome formation
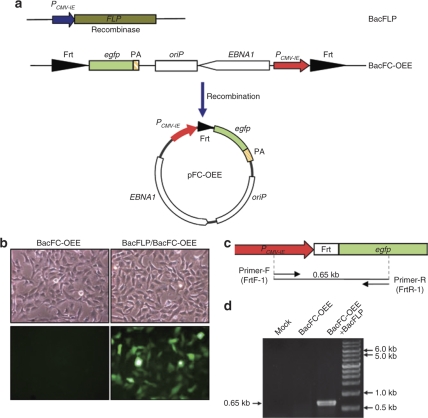
To explore the feasibility of FLP/Frt-mediated recombination in the context of baculovirus genome (≈134 kb), we constructed BacFLP expressing FLP under the control of cytomegalovirus immediate-early promoter (PCMV-IE), and BacFC-OEE harboring genes encoding enhanced green fluorescent protein (egfp), oriP, EBNA1, and PCMV-IE (Figure 1a). The transgene cassette was flanked by two Frt sites for FLP recognition and formation of the episomal pFC-OEE. Note that egfp was not driven by any promoter in the baculovirus genome, thus only after recombination and pFC-OEE formation could egfp be placed downstream of PCMV-IE. As such, the EGFP expression served as an indicator of FLP/Frt-mediated recombination.
Figure 1.
Baculovirus construction and confirmation of FLP/Frt-mediated recombination. (a) Schematic illustration of the constructs and formation of pFC-OEE. (b) Confirmation of recombination by EGFP expression. (c) Primers targeting PCMV-IE and egfp that flanked the recombined Frt sites. (d) Confirmation of recombination by PCR. HEK293 cells were transduced with BacFC-OEE (MOI 100) alone or cotransduced with BacFLP/BacFC-OEE (MOI 100/400), and analyzed at 2 dpt. Magnification, 200×. EGFP, enhanced green fluorescent protein; HEK293, human embryonic kidney-293; MOI, multiplicity of infection; PA, polyadenylation signal.
Human embryonic kidney-293 (HEK293) cells were first transduced with BacFC-OEE alone or cotransduced with BacFLP/BacFC-OEE at multiplicity of infection (MOI) 100/400. Figure 1b shows that at 2 days post-transduction (dpt) EGFP was expressed only in the cotransduced cells but not in the singly transduced cells, suggesting the occurrence of recombination only after cotransduction. To verify the formation of pFC-OEE, episomal DNA was extracted and subjected to PCR using primers targeting the egfp gene and PCMV-IE that flanked the newly joined Frt sites (FrtR-1 and FrtF-1, Figure 1c), which would amplify a 0.65 kb fragment. Figure 1d reveals no PCR products from the mock-transduced and BacFC-OEE-transduced cells, indicating the absence of re-circularized pFC-OEE. In contrast, a 0.65-kb fragment was amplified from the cotransduced cells, which was further sequenced to verify the identity (data not shown). These data collectively confirmed that BacFLP/BacFC-OEE cotransduction gave rise to FLP/Frt-mediated recombination and episome formation.
Improvement of FLP/Frt-mediated recombination and episome formation
To improve the recombination efficiency, HEK293 cells were cotransduced with BacFLP/BacFC-OEE (MOI 100/400) and cultured in the presence of sodium butyrate (a histone deacetylase inhibitor that induces chromatin remodeling21). The butyrate-containing medium was replaced with fresh medium at 15-hours post-transduction and the cells continued to be cultured until flow cytometry analysis (Figure 2a) at 2 dpt. Compared with the control (0 mmol/l), 5 mmol/l sodium butyrate tremendously increased the percentage of GFP+ (%GFP+) cells and the mean fluorescence intensity (MFI). Because only the circular pFC-OEE conferred EGFP expression, the data indicated that recombination occurred in 71% cells in the presence of 5 mmol/l sodium butyrate. Raising the butyrate concentration to 10 or 15 mmol/l failed to elevate the %GFP+ cells, despite the continued increase in MFI. Thus 5 mmol/l sodium butyrate was supplemented in all subsequent experiments as described above and the resultant %GFP+ cells indicated the recombination efficiency.
Figure 2.
Improvement of FLP/Frt-mediated recombination and episome formation. (a) Recombination efficiency and EGFP expression at various butyrate concentrations. (b) Recombination efficiency and EGFP expression at various virus dosages. In all experiments, the butyrate-containing DMEM medium was replaced with fresh medium 15 hours after transduction and continued to be cultured. The cells were harvested at 2 dpt and analyzed for the %GFP+ cells and MFI by flow cytometry. All data represent the averages of three independent culture experiments. DMEM, Dulbecco's modified Eagle's medium; EGFP, enhanced green fluorescent protein; MFI, mean fluorescence intensity.
We next assessed the optimal dosages for BacFLP and BacFC-OEE by cotransduction at various MOI combinations. The flow cytometry analyses performed at 2 dpt (Figure 2b) overtly depict the dependence of the %GFP+ cells and MFI on the MOI. Overall, the FLP/Frt-mediated recombination and episome-mediated EGFP expression culminated at MOI 50–200 for BacFLP and MOI 200–400 for BacFC-OEE, as evidence by the high %GFP+ cells (65–75%) and MFI (a.u. ≈3,175–5,471).
Episome formation in different mammalian cells
Whether this system functioned in other cells was explored by cotransducing various cell types with BacFLP/BacFC-OEE (MOI 100/400). Figure 3a depicts that the %GFP+ cells, and hence the recombination efficiency, exceeded 36% even for the difficult-to-transfect cell lines HepG2 and HuH-7. The recombination efficiency was the highest for baby-hamster kidney (BHK) cells (83%) and remained high for primary rabbit articular chondrocytes (77%) and human MSCs (48%). Note that baculovirus can transduce these cells at efficiencies >80%,22,23,24 thus the differences in the %GFP+ cells reflected the disparities in the recombination efficiencies within these cell types.
Figure 3.
Episome formation in different cells. (a) Recombination efficiency in various cell types. (b) Comparison of episome delivery efficiencies. The cells cultured in 6-well plates (5 × 105 cells/well) were cotransduced with BacFLP/BacFC-OEE (MOI 100/400) or transfected with 4.0 µg/well pFC-OEE-aq plasmid, and were harvested 2 days later for analyses. The absolute episome copy numbers were quantified by Q-PCR using primers targeting the egfp gene and PCMV-IE that flanked the newly joined Frt sites. The data represent the averages of three independent culture experiments. MOI, multiplicity of infection; Q-PCR, quantitative real-time PCR.
Because our system enabled efficient episome formation, we next compared the episome delivery efficiencies via baculovirus transduction and lipid transfection. HuH-7, MSCs, and chondrocytes cultured in six-well plates (≈5 × 105 cells/well) were cotransduced with BacFLP/BacFC-OEE at MOI 100/400, which corresponded to the use of ≈2.0 × 108 plaque-forming units for BacFC-OEE. For comparison, the cells were transfected with a control plasmid (≈4 µg/well) which mimicked the recombined episome pFC-OEE in size (6.4 kb) and transgene (PCMV-IE-Frt-egfp). Assuming that 1 plaque-forming unit is equivalent to 10–100 baculovirus particles,25,26 ≈0.2–2.0 × 1010 copies of BacFC-OEE and ≈6.5 × 1011 copies of the control plasmid were used per well. Therefore, ≈33–330 times more egfp genes were used for transfection than for transduction. Consequently, the absolute episome copy numbers as measured by quantitative real-time PCR (Q-PCR) were tremendously higher in the transfected HuH-7, MSCs, and chondrocytes than in the baculovirus-transduced counterparts (Figure 3b, upper panel). However, the resultant %GFP+ cells (Figure 3b, lower panel) were significantly higher for the transduced cells (42, 48, and 76% for HuH-7, MSCs, and chondrocytes, respectively) than for the transfected cells, demonstrating more effective transgene expression mediated by the baculovirus-delivered episomes.
Persistence of hybrid baculovirus-mediated transgene expression
Whether this system prolonged the transgene expression was evaluated by another hybrid baculovirus BacCON-CE (Figure 4a) which contained Frt-flanking oriP/EBNA1 for the retention of the re-circularized replicon pCON-CE. However, in BacCON-CE egfp was directly under the control of PCMV-IE for direct assessment of transduction efficiency and EGFP expression. Also BacCON-CE contained a neomycin-resistance gene (Neor) driven by SV40 promoter. HEK293 cells were cotransduced with BacFLP/BacCON-CE (MOI 100/400) and cultured with or without G418. As controls, the cells were transduced with BacCON-CE (MOI 400) alone or with a conventional baculovirus vector (Bac-CE) that transiently expressed EGFP under PCMV-IE (MOI 400).16 The cells were subcultured every 3–4 days upon confluency and subjected to flow cytometry at different times (Figure 4b).
Figure 4.
Persistence of transgene expression. (a) Schematic illustration of BacCON-CE and the formation of episomal pCON-CE. (b) Time-course profiles of %GFP+ cells and MFI. HEK293 cells in 12-well plates (2 × 105 cells/well) were cotransduced with BacFLP/BacCON-CE (MOI 100/400) and cultured with or without G418 (50 µg/ml). As controls, the cells were transduced with BacCON-CE (MOI 400) alone or with Bac-CE (MOI 400). All transduced cells were subcultured every 3–4 days by splitting the cells at a ratio of 1:5. The transgene expression data were measured by flow cytometry and represent the averages of 3 independent culture experiments. HEK293, human embryonic kidney-293; MFI, mean fluorescence intensity; MOI, multiplicity of infection.
Under these transduction conditions, the %GFP+ cells approached 99–100% initially for all groups. However, Bac-CE-mediated EGFP expression underwent a rapid decay, with ≈16 and ≈2% cells emitting fluorescence at 14 and 21 dpt, respectively. By defining the time point at which the %GFP+ cells dropped <5% as the expression duration16 and data extrapolation, Bac-CE led to an expression duration of ≈20 days. BacCON-CE transduction alone slightly prolonged the expression duration to ≈27 days. Without G418, BacFLP/BacCON-CE cotransduction considerably elevated the %GFP+ cells at 14 and 21 dpt to ≈66 and ≈50% with concomitant enhancement of MFI. More importantly, the cotransduction extended the expression duration to ≈48 days. Selection with G418 resulted in an even more remarkable improvement in gene expression duration and level, with the %GFP+ cells maintaining at 78 and 36% at 21 and 63 dpt, respectively. Therefore, the transgene expression was prolonged beyond 63 days.
To attest whether the persistent fluorescence truly stemmed from the long-term maintenance of pCON-CE, the experiments in Figure 4b were repeated and baculovirus genomic DNA and episomal pCON-CE were quantified by Q-PCR using the primers targeting endogenous baculovirus gp64 gene and the recombined Frt sites, respectively. egfp mRNA was quantified by quantitative real-time reverse transcriptase PCR (qRT-PCR) using primers targeting its mature domain. All DNA and mRNA levels were normalized against those obtained at 2 dpt to yield the relative copy numbers. The time point at which the relative copy numbers dropped <0.1% was defined as the cutoff.
Without G418, baculoviral genome copy numbers (Figure 5a) decreased rapidly and dropped below the cutoff at 21 dpt upon Bac-CE transduction or BacFLP/BacCON-CE cotransduction. In contrast, the pCON-CE DNA decayed at a slower rate and persisted for ≈45 days. With G418 selection the cotransduction still led to a steady decrease in the baculoviral genome copy number, but nevertheless gave rise to the stable maintenance (>3%) of pCON-CE for at least 63 days.
Figure 5.
Time-course profiles of DNA and egfp mRNA. The copy numbers of (a) viral and episomal DNA and (b) egfp mRNA were measured by Q-PCR and qRT-PCR, respectively. All data were normalized against those obtained at 2 dpt to yield the relative copy numbers. The data represent the averages of three independent culture experiments. The dotted line indicates the cutoff. Q-PCR, quantitative real-time PCR; qRT-PCR, quantitative real-time reverse transcription PCR.
Concurrent with the DNA decay, the egfp mRNA level (Figure 5b) in the Bac-CE-transduced cells decreased precipitously at as early as 7 dpt and vanished after ≈27 dpt. In the cotransduced cells, the relative egfp mRNA level declined at a remarkably slower rate even without G418, remaining >3.5 and 0.5% at 21 and 49 dpt, respectively. G418 supplementation substantially augmented the maintenance of egfp transcription, whose level ranged between 5 and 10% from 35 to 63 dpt.
Prolonged baculovirus-mediated BMP-2 expression potentiated in vitro osteogenesis
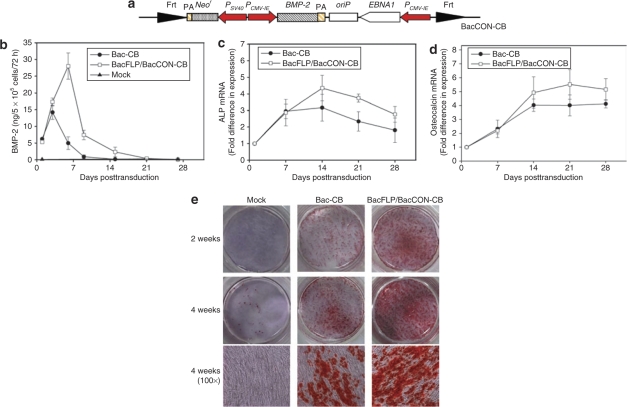
BMP-2 is an osteoinductive factor that triggers the differentiation of MSCs into osteoblasts and then osteocytes.15 To exploit this system for MSCs engineering and osteogenesis, we constructed BacCON-CB that resembled BacCON-CE except that egfp was replaced by BMP-2 gene (Figure 6a). Human MSCs were cotransduced with BacFLP/BacCON-CB (MOI 50/150), or transduced with a conventional baculovirus vector (Bac-CB, MOI 150) that transiently expressed BMP-2 under PCMV-IE.22 After transduction, the cells were cultured without passaging and subjected to medium exchange (no G418) every 3 days. At different times the medium was withdrawn for enzyme-linked immunosorbent assay.
Figure 6.
The hybrid baculovirus system prolonged BMP-2 expression and potentiated osteogenesis. (a) Schematic illustration of BacCON-CB. (b) Time-course profile of BMP-2 expression. (c) Relative ALP mRNA levels. (d) Relative osteocalcin mRNA levels. (e) Calcium deposition. Human MSCs were mock-transduced, singly transduced with Bac-CB (MOI 150) or cotransduced with BacFLP/BacCON-CB (MOI 50/150). Extracellular BMP-2 concentrations were measured by enzyme-linked immunosorbent assay at 1, 3, 6, 9, 15, 21, and 27 dpt to calculate the expression levels. The mRNA levels were measured by qRT-PCR and normalized against those from the mock-transduced MSCs at 1 dpt. The calcium deposition was stained by Alizarin red at weeks 2 and 4. The photographs indicating the wells stained at weeks 2 and 4, and the micrographs indicating the cells stained at week 4 (100×) are shown. All data are representative of three independent culture experiments. ALP, alkaline phosphatase; BMP-2, bone morphogenetic protein-2; MSC, mesenchymal stem cells; MOI, multiplicity of infection; qRT-PCR, quantitative real-time reverse transcriptase PCR.
Figure 6b reveals that BMP-2 expression by the mock-transduced MSCs was undetectable at all times. Bac-CB transduction led to the maximum BMP-2 expression at 3 dpt (14.0 ng/5 × 105 cells/72 hours), which then precipitously dropped below 1 ng/5 × 105 cells/72 hours at 9 dpt. BacFLP/BacCON-CB cotransduction not only augmented the maximum BMP-2 expression (≈27.8 ng/5 × 105 cells/72 hours) at 6 dpt, but also sustained the expression level higher than 1.0 ng/5 × 105 cells/72 hours for 15 days. qRT-PCR analyses (Figure 6c,d) attested that BacFLP/BacCON-CB cotransduction enhanced the transcription levels of the early (alkaline phosphatase) and late (osteocalcin) osteogenic differentiation markers when compared with Bac-CB transduction. The MSCs differentiation into osteocytes was also compared by calcium deposition as stained by Alizarin red at weeks 2 and 4. Figure 6e illustrates that the mock-transduced MSCs deposited virtually no calcium spots, indicating minimal spontaneous MSCs differentiation without BMP-2 stimulation. Bac-CB transduction triggered progressive calcium deposition with time, but calcium spots were still absent in a large portion of cells at week 4. In marked contrast, BacFLP/BacCON-CB cotransduction increased the density of calcium spots at weeks 2 and 4. Figure 6 confirmed that the hybrid baculovirus system prolonged BMP-2 expression in MSCs and potentiated the osteogenic differentiation into osteocytes.
Discussion
One major hurdle to the widespread application of baculovirus as a gene therapy vector is its nonreplicative nature in mammalian cells, which leads to transient expression. To address this issue, a baculovirus-adeno-associated virus (AAV) hybrid vector containing a gene cassette flanked by the AAV inverted terminal repeats was developed.27 Transduction of 293 cells with this hybrid vector expressing additional rep gene results in specific integration of the inverted terminal repeat-flanking cassette into AAVS1 site. Similar baculovirus-AAV hybrid vectors incorporating inverted terminal repeat-flanking cassette also extend transgene expression in the rat brain28 and human embryonic stem cells.29 However, this system relies on gene integration thus safety concerns may arise. Besides, this hybrid system fails to extend in vitro transgene expression in BHK cells (Y.-C. Hu unpublished results), as such it is probably cell-type specific.
To prolong the transgene expression and circumvent the safety concerns regarding gene integration, we developed a hybrid baculovirus vector that exploited FLP/Frt-mediated recombination and Epstein–Barr virus oriP/EBNA1 for sustained maintenance of the episomal replicon. Upon cotransduction with BacFLP, our hybrid baculovirus efficiently delivered the transgene cassette into cells and allowed for ensuing transgene excision from the baculovirus genome and episome formation (Figure 1). The excision/recombination was remarkably ameliorated by the histone deacetylase inhibitor sodium butyrate (Figure 2a), which concurred with the finding that sodium butyrate augments the excision of AAV inverted terminal repeat-flanking transgene cassette from baculovirus.12 For HEK293 cells, MOI 50–200 for BacFLP and MOI 200–400 for BacFC-OEE gave rise to fairly high recombination efficiencies (65–75%) and MFI (3,175–5,471 a.u.). This system was also applicable to mammalian cell lines and primary cells, attaining recombination efficiencies up to 85% in BHK cells, and 77% in difficult-to-transfect primary chondrocytes (Figure 3a). Consequently, our hybrid vector resulted in significantly more efficient episome delivery and episome-mediated gene expression when compared with transfection (Figure 3b).
More importantly, the split cassette and oriP/EBNA1 substantially prolonged the transgene expression duration to at least 63 days in the presence of selection (Figure 4b), potentially rendering this system attractive for continuous production of biologically active proteins while obviating the need to generate stable cell lines. Without selection the transgene expression persisted for ≈48 days, which was considerably prolonged as compared with ≈20 days when using the conventional vector Bac-CE (Figure 4b). Such sustained expression was attributed to the prolonged maintenance of the replicon (Figure 5a) and hence the transgene transcription (Figure 5b). Because it has been shown that the EBNA1 expression level is essential for the maintenance of oriP-containing genome,30 future optimization of the EBNA1 expression may further prolong the expression period. In sharp contrast to the persistent episomes, after unloading the payload the baculovirus genome was rapidly degraded (Figure 5a), thereby easing the concerns about the residual viral DNA residing in the transduced cells. These attributes may benefit the development of baculovirus as an appealing system alternative to transfection for replicon delivery into difficult-to-transfect primary cells.
Of note, BacCON-CE transduction alone led to rapid extinction of EGFP expression (Figure 4b), suggesting that the baculovirus carrying the oriP/EBNA1 cassette itself failed to extend the transgene expression. This observation contradicted the data reported previously whereby transduction of cells with a single baculovirus harboring the oriP/EBNA1 cassette was sufficient to support presistent expression.30 The exact reason accounting for the discrepancy remains to be investigated, but we believe that the whole baculovirus genome (≈134 kb), as compared with the split episome, is more susceptible to nuclease attack, which contributes to the more rapid degradation as observed in this study.
Although the transgene expression was gradually lost in the absence of selection, it is observed in various gene delivery systems exploiting oriP/EBNA119,31,32 and can be ascribed to: (i) the cells that did not harbor the recombined replicons predominated in the cell population after serial passaging; (ii) the oriP/EBNA1 system was insufficient to support stable retention of the replicon. The first problem may be tackled by improving the recombination efficiency so that the majority of cells, if not all, harbor the replicons. Because FLP exerts optimal activity at 30 °C,33 engineering the FLP to shift its optimal activity to 37 °C may help augment the recombination efficiency. The second problem might be alleviated by using a new replication origin. Because vectors incorporating the scaffold matrix attachment region (S/MAR) can be retained episomally for >100 generations in the absence of selection34 and are effective for persistent retention of transgenes in vivo,35 future extension of the expression may be achieved by replacing oriP/EBNA1 with S/MAR.
Compared with HEK293 cells, in human MSCs the recombination efficiency (≈48%, Figure 3a) was lower and the expression period was shorter (Figure 6b), concurring with the notion that the prolonged expression is cell-type dependent.30 Nevertheless, BacFLP/BacCON-CB cotransduction augmented the BMP-2 expression (Figure 6b) and extended the expression to at least 15 days in the absence of selection, which outlasted the duration (≈8 days) resulting from the conventional baculovirus vector (Bac-CB) and potentiated the osteogenesis (Figure 6b–e). The elevated expression can be attributed to the interaction of oriP and EBNA1 which enhances the expression of neighboring genes in the context of baculovirus genome.36 Although short-term BMP-2 expression is sufficient to promote ectopic bone formation in the nude mice model,15 a longer-term expression might be beneficial and required for the repair of massive bone defects (e.g., massive segmental bone fracture). Furthermore, it is proposed that MSCs should be differentiated into a specific lineage prior to implantation into patients owing to concerns that clinical use of undifferentiated stem cells may result in uncontrolled proliferation and hence tumor formation.37 As such, it may be preferable to culture the genetically engineered MSCs and initiate lineage-specific differentiation in vitro before implantation. In this regard, prolonged growth factor expression with this vector can not only potentiate in vitro differentiation, but also ensure sustained secretion of the growth factor after implantation, which exerts autocrine and paracrine effects (e.g., recruitment of other progenitor cells) in vivo to promote the tissue regeneration.
In summary, we developed a novel baculovirus vector that confers sustained expression in various mammalian cells including MSCs, and demonstrated its potential in tissue engineering. This system may also be used for continuous recombinant protein production in mammalian cells or for the treatment of other indications (e.g., cancer) requiring sustained expression. Although long-term expression can be achieved by other viral vectors such as retrovirus, lentivirus, and AAV, these vectors are fairly labor-intensive to use and have limited cloning capacity. Additionally, retrovirus and lentivirus are prone to integration into coding or regulatory regions of transcriptionally active genes,38,39 raising concerns about gene silencing and insertional mutagenesis. AAV-mediated integration is also associated with hepatocellular carcinoma in neonatal mice.40 Alternatively, stable expression can be mediated by transposons such as Sleeping Beauty41 and PiggyBac42, or by phage integrase such as ΦC31.43 However, they still may impose position effects and more experiments are required to scrutinize the possibility of insertional mutagenesis. In contrast to these integrating vectors, our hybrid baculovirus is based on extrachromosomal maintenance of the transgene cassette, which is less likely to cause gene silencing and insertional mutagenesis.18 Although such oriP/EBNA1-based replicon can be delivered via HSV-1 (herpes simplex virus type 1)44 and adenoviral vector,45 HSV-1 is immunogenic and cytotoxic to non-neuronal cells while adenovirus also mounts strong immune responses. Despite the advent of gutless adenoviral vectors to minimize the immune responses, the production and purification of gutless vectors are cumbersome. In contrast to these vectors, baculovirus is not pathogenic to humans and has a cloning capacity as large as 38 kb.46 Furthermore, recombinant baculovirus can be easily constructed and produced to high titers simply by infecting insect cells in Biosafety Level 1 laboratories. All these features support the use of this hybrid baculovirus vector for gene therapy.
Materials and Methods
Cell culture. Human cell lines HEK293, HepG2, HuH-7, HeLa, and BHK cell line were cultured using Dulbecco's modified Eagle's medium (Sigma, St Louis, MO) containing 10% fetal bovine serum (FBS; Gibco, Gaithersburg, MD) and 1% penicillin/streptomycin solution (Gibco). Primary articular chondrocytes were isolated from New Zealand White rabbits and cultured using Dulbecco's modified Eagle's medium.22 Human bone marrow-derived MSCs were obtained and cultured as described,23 using α-modified Eagle's medium (Hyclone, Ogden, UT) containing 20% FBS, 4 ng/ml basic fibroblast growth factor (R&D Systems, Minneapolis, MN), 100 U/ml penicillin, and 100 mg/ml streptomycin.
Donor plasmids. All baculovirus donor plasmids were constructed using pFastBac-DUAL plasmid (Invitrogen, Carlsbad, CA) as the backbone. The gene cassette encoding FLP under the control of cytomegalovirus immediate early (PCMV-IE) promoter was digested by SalI/PvuII from pOG44 (Invitrogen) and subcloned into pFastBac-DUAL to yield pBacFLP.
To generate the hybrid donor plasmids, a transfer plasmid was first constructed. The egfp gene (0.7 kb) was cleaved from pEGFP-N1 (Clontech, Mountain View, CA) and subcloned into pRep4 plasmid containing oriP/EBNA1 (Invitrogen) by SalI/XbaI treatment. The cassette comprised of egfp at the upstream of oriP/EBNA1 (6.0 kb) was then subcloned into pET-30a(+) (Novagen, San Diego, CA) by EcoRI/SalI digestion to yield pET-EGFP-oriP/EBNA1.
pFC-OEE was constructed in three stages. First, a DNA fragment composed of a multiple cloning site flanked by two Frt sites in a parallel orientation was PCR-amplified from pLOI222647 using two primers (5′-TGCCGGTACCATCGATGAATTGATCCGAA-3′ and 5′-TAGCC TGCAGACCAATTCGAAGTTCCTA-3′). The amplicon (0.25 kb) was subcloned into pFastBac-DUAL by KpnI/PstI digestion to yield pBac-Frt. Second, PCMV-IE (0.6 kb) was PCR-amplified from pcDNA3.1(+) (Invitrogen) using two primers (5′-TCAGGGATCCGCGTTGACATTGATTATTG-3′ and 5′-TGGACCCGGGAGTTAGCCAGAGAGCTCTG-3′) and subcloned into pBac-Frt by BamHI/SmaI treatment. The resultant pBac-Frt-RCMV thus contained PCMV-IE which was in an orientation opposite to the Frt direction. Third, the egfp-oriP/EBNA1 cassette in pET-EGFP-oriP/EBNA1 was digested with SalI/BglII and subcloned into pBac-Frt-RCMV with SalI/BamHI treatment to yield pFC-OEE (see Figure 1a).
pBacCON-CE was constructed in four stages. First, PCMV-IE promoter was amplified by PCR from pcDNA3.1(+) using two primers (5′-TCAG CCCGGGGCGTTGACATTGATTATTG-3′ and 5′-TGGAGGATCCAG TTAGCCAGAGAGCTCTG-3′) and the amplicon was subcloned into pBac-Frt by SmaI/BamHI digestion to yield pBac-Frt-LCMV. Second, the neomycin-resistance gene under the simian virus 40 (SV40) promoter was PCR-amplified from pcDNA3.1(+) and subcloned into pBac-Frt-LCMV to form pBac-Frt-Neor-LCMV. Third, the egfp gene under PCMV-IE was PCR-amplified from pBac-CE48 using two primers (5′-TCC GGTCGACTCATACCGTCCCACCATC-3′ and 5′-TGGATCTAGATTT CACTTATCTGGTTC-3′) and the amplicon was subcloned into pET-EGFP-oriP/EBNA1 by SalI/XbaI digestion to replace the promoterless egfp. Fourth, the whole cassette was cleaved with SalI/BglII and subcloned into pBacFrt-Neor-LCMV by SalI/BamHI treatment to yield pBacCON-CE (Figure 4a).
pBacCON-CB was constructed in a way similar to pBacCON-CE construction except that BMP-2 gene was cloned in lieu of egfp. The BMP-2 gene under the control of PCMV-IE was PCR-amplified from pBac-CB22 using two primers (5′-TCAGGTCGACGCGTTGACATTGATT ATTG-3′ and 5′-GGACTCTAGATATAGTTCTAGTGGT TGGC-3′).
Baculovirus preparation and transduction. The recombinant baculoviruses (BacFLP, BacFC-OEE, BacCON-CE, BacCON-CB) were constructed using the corresponding donor plasmids (pBacFLP, pFC-OEE, pBacCON-CE, pBacCON-CB) following the instructions of Bac-To-Bac system (Invitrogen). The recombinant baculovirus that transiently expressed EGFP (Bac-CE) or BMP-2 (Bac-CB) was constructed previously.22,48 All viruses were propagated and titered as described.22,48
The cells were transduced according to the protocol developed previously16,49 with minor modifications. Depending on the MOI, a certain volume of virus supernatant was pre-mixed with NaHCO3-deficient Dulbecco's modified Eagle's medium containing 10% FBS to adjust the final volume to 500 µl (per well). Transduction was initiated by adding the virus mixture to the cells in 6-well plates (5 × 105cells/well) and continued by gently shaking the plates for 6 hours (4 hours for MSCs) at room temperature. After the incubation period, the virus solution was withdrawn and the cells continued to be cultured. To enhance the FLP-mediated recombination efficiency (see Results), the cells were cultured with butyrate-containing medium for 15 hours, after which the medium was withdrawn and cells were cultured with normal medium.
For long-term expression experiments, HEK293 cells in 12-well plates (2 × 105 cells/well) were cotransduced with BacFLP/BacCON-CE and cultured in the presence or absence of 50 µg/ml G418 after 2 dpt. The cells were passaged at a 1:5 split ratio upon confluence. For MSCs, the cells in 6-well plates (5 × 105 cells/well) were cotransduced with BacFLP/BacCON-CB as described above. The cells were cultured with medium exchange every 3 days, but were not subcultured.
Detection of recombination and episome formation. The recombination and episome formation were confirmed by fluorescence microscopy and PCR at 2 dpt. The cells were observed by a fluorescence microscope and the DNA was extracted with Blood & Tissue Extraction Mini Kit (Viogen, Taipei, Taiwan) as the PCR template. The PCR primers FrtF-1 (5′-CATAGTAACGCCAATAGGGAC-3′) and FrtR-1 (5′-CAGATGAACTTCAGGGTCAGC-3′) were designed to probe the PCMV-IE promoter and egfp which flanked the newly re-joined Frt sites (Figure 1c). The PCR products were subjected to 1% agarose gel electrophoresis.
Analysis of transgene expression. The percentage of GFP+ cells (%GFP+ cells) and MFI were measured by flow cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ).16,23 The MFI is expressed in arbitrary unit (a.u.). The BMP-2 concentration in the culture medium was measured using an enzyme-linked immunosorbent assay kit (R&D Systems).
Plasmid transfection. The cells in 6-well plates (5 × 105 cells/well) were transfected with ≈4.0 µg/well control plasmid pFC-OEE-aq which mimicked the recombined episome pFC-OEE in size and transgene by lipofectamine 2000 (Invitrogen).
Q-PCR and qRT-PCR. The absolute episome copy number was quantified by Q-PCR using primers (forward: 5′-GGATCCATGATAAATTTAATTATTGATG-3′, reverse: 5′-GGTACCTTATTTAGTATATTTTAAGTG-3′) targeting the egfp gene and PCMV-IE that flanked the newly re-joined Frt sites in the episome. Total DNA was extracted using Blood & Tissue Extraction Mini Kit. Real-time PCR reactions were performed using ABI PRISM 7300 (Applied Biosystems, Foster City, CA) under the conditions as described previously.23 For each PCR, a no-template reaction was included as negative control. Copy number quantification was based on the external standard curve created using known amounts of pFC-OEE-aq.
The relative copy numbers of the baculoviral genome and the replicon were also quantified by Q-PCR. The baculoviral genome was probed using primers that targeted gp64 (forward: 5′-CGCCTTCAGCCATGGAAGT-3′, reverse: 5′-CCACCATGGAGAACACCAAGTT-3′). The replicon DNA was probed using primers that targeted the newly recombined Frt site (forward: 5′-ATCAATGTCAACGCGTATATCTG-3′, reverse: 5′-CATGT CTGTATACCCTCGACCA-3′). The housekeeping gene gapdh (internal control) was probed using another set of primers (forward: 5′-CTGG TCATCAATGGGAAAC-3′, reverse: 5′-CAAAGTTGTCATGGATGA-3′). Real-time PCR reactions were performed as described above.
The relative egfp transcription levels were quantified by qRT-PCR. Total mRNA was extracted using RNeasy Mini Kit (Qiagen, Valencia, CA) and reverse transcribed to cDNA using Omniscript RT kit (Qiagen). The cDNA was subjected to Q-PCR using primers targeting egfp (forward: 5′-TATATCATGGCCGACAACA-3′, reverse: 5′-TGTTCTGCTGGTAG TGGTCG-3′) and gapdh (forward: 5′-CCACCCATGGCAAATTCC-3′, reverse: 5′-TGGGATTTCCATTGATGACAA-3′). For relative quantification, the threshold cycle values were normalized against that of gapdh. All data were normalized against those obtained at 2 dpt.
The relative transcription levels of alkaline phosphatase and osteocalcin were quantified by qRT-PCR following the aforementioned procedures, except that the primers were specific for alkaline phosphatase (forward: 5′-TGCGGAAGAACCCCAAAG-3′, reverse: 5′-AT GGTGCCCGTGGTCAAT-3′) and osteocalcin (forward: 5′-AGGAGG GCAGCGAGGTAG-3′; reverse: 5′-GAAAGCCGATGTGGTCAGC-3′).
Alizarin red staining. The mineralization was confirmed by calcium phosphate deposition as stained by Alizarin red.15
Acknowledgments
We acknowledge the support from the National Tsing Hua University Booster Program (97N2511E1), National Science Council (NSC 97-2627-B-007-014, NSC 97-2622-E-007-009-CC3), VTY Joint Research Program, Tsou's Foundation (VGHUST97-P5-15), CGMH-NTHU Joint Research Program (96N2425E1), and National Health Research Institutes (NHRI-EX97-9412EI), Taiwan.
REFERENCES
- Hu Y-C. Baculoviral vectors for gene delivery: A review. Curr Gene Ther. 2008;8:54–65. doi: 10.2174/156652308783688509. [DOI] [PubMed] [Google Scholar]
- Hu Y-C. Baculovirus vectors for gene therapy. Adv Virus Res. 2006;68:287–320. doi: 10.1016/S0065-3527(06)68008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost TA, Condreay JP., and , Jarvis DL. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat Biotechnol. 2005;23:567–575. doi: 10.1038/nbt1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airenne KJ, Hiltunen MO, Turunen MP, Turunen AM, Laitinen OH, Kulomaa MS, et al. Baculovirus-mediated periadventitial gene transfer to rabbit carotid artery. Gene Ther. 2000;7:1499–1504. doi: 10.1038/sj.gt.3301269. [DOI] [PubMed] [Google Scholar]
- Abe T, Takahashi H, Hamazaki H, Miyano-Kurosaki N, Matsuura Y., and , Takaku H. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J Immunol. 2003;171:1133–1139. doi: 10.4049/jimmunol.171.3.1133. [DOI] [PubMed] [Google Scholar]
- Wang X, Wang C, Zeng J, Xu X, Hwang PYK, Yee W-C, et al. Gene transfer to dorsal root ganglia by intrathecal injection: effects on regeneration of peripheral nerves. Mol Ther. 2005;12:314–320. doi: 10.1016/j.ymthe.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Kost TA, Condreay JP, Ames RS, Rees S., and , Romanos MA. Implementation of BacMam virus gene delivery technology in a drug discovery setting. Drug Discov Today. 2007;12:396–403. doi: 10.1016/j.drudis.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Grabherr R, Ernst W, Oker-Blom C., and , Jones I. Developments in the use of baculoviruses for the surface display of complex eukaryotic proteins. Trends Biotechnol. 2001;19:231–236. doi: 10.1016/s0167-7799(01)01610-9. [DOI] [PubMed] [Google Scholar]
- Strauss R, Huser A, Ni S, Tuve S, Kiviat N, Sow PS, et al. Baculovirus-based vaccination vectors allow for efficient induction of immune responses against Plasmodium falciparum circumsporozoite protein. Mol Ther. 2007;15:193–202. doi: 10.1038/sj.mt.6300008. [DOI] [PubMed] [Google Scholar]
- Wang C-Y, Li F, Yang Y, Guo H-Y, Wu C-X., and , Wang S. Recombinant baculovirus containing the Diphtheria toxin A gene for malignant glioma therapy. Cancer Res. 2006;66:5798–5806. doi: 10.1158/0008-5472.CAN-05-4514. [DOI] [PubMed] [Google Scholar]
- Lesch HP, Turpeinen S, Niskanen EA, Mähönen AJ, Airenne KJ., and , Ylä-Herttuala S. Generation of lentivirus vectors using recombinant baculoviruses. Gene Ther. 2008;15:1280–1286. doi: 10.1038/gt.2008.76. [DOI] [PubMed] [Google Scholar]
- Huang K-S, Lo W-H, Chung Y-C, Lai Y-K, Chen C-Y, Chou S-T, et al. Combination of baculovirus-mediated gene delivery and packed-bed reactor for scalable production of adeno-associated virus. Hum Gene Ther. 2007;18:1161–1170. doi: 10.1089/hum.2007.107. [DOI] [PubMed] [Google Scholar]
- Chen H-C, Sung L-Y, Lo W-H, Chuang C-K, Wang Y-H, Lin J-L, et al. Combination of baculovirus-mediated BMP-2 expression and rotating-shaft bioreactor culture synergistically enhances cartilage formation. Gene Ther. 2008;15:309–317. doi: 10.1038/sj.gt.3303087. [DOI] [PubMed] [Google Scholar]
- Chen H-C, Chang Y-H, Chuang C-K, Lin C-Y, Sung L-Y, Wang Y-H, et al. The repair of osteochondral defects using baculovirus-mediated gene transfer with de-differentiated chondrocytes in bioreactor culture. Biomaterials. 2009;30:674–681. doi: 10.1016/j.biomaterials.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Chuang C-K, Sung L-Y, Hwang S-M, Lo W-H, Chen H-C., and , Hu Y-C. Baculovirus as a new gene delivery vector for stem cells engineering and bone tissue engineering. Gene Ther. 2007;14:1417–1424. doi: 10.1038/sj.gt.3302996. [DOI] [PubMed] [Google Scholar]
- Ho Y-C, Chen H-C, Wang K-C., and , Hu Y-C. Highly efficient baculovirus-mediated gene transfer into rat chondrocytes. Biotechnol Bioeng. 2004;88:643–651. doi: 10.1002/bit.20239. [DOI] [PubMed] [Google Scholar]
- Buchholz F, Ringrose L, Angrand PO, Rossi F., and , Stewart AF. Different thermostabilities of FLP and Cre recombinases: implications for applied site-specific recombination. Nucleic Acids Res. 1996;24:4256–4262. doi: 10.1093/nar/24.21.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lufino MMP, Edser PAH., and , Wade-Martins R. Advances in high-capacity extrachromosomal vector technology: episomal maintenance, vector delivery, and transgene expression. Mol Ther. 2008;16:1525–1538. doi: 10.1038/mt.2008.156. [DOI] [PubMed] [Google Scholar]
- Ren CP, Zhao M, Yang XY, Li DS, Jiang XJ, Wang L, et al. Establishment and applications of Epstein-Barr virus-based episomal vectors in human embryonic stem cells. Stem Cells. 2006;24:1338–1347. doi: 10.1634/stemcells.2005-0338. [DOI] [PubMed] [Google Scholar]
- Black J., and , Vos JM. Establishment of an oriP/EBNA1-based episomal vector transcribing human genomic beta-globin in cultured murine fibroblasts. Gene Ther. 2002;9:1447–1454. doi: 10.1038/sj.gt.3301808. [DOI] [PubMed] [Google Scholar]
- Kramer OH, Gottlicher M., and , Heinzel T. Histone deacetylase as a therapeutic target. Trends Endocrinol Metabol. 2001;12:294–300. doi: 10.1016/s1043-2760(01)00438-6. [DOI] [PubMed] [Google Scholar]
- Sung L-Y, Lo W-H, Chiu H-Y, Chen H-C, Chuang C-K, Lee H-P, et al. Modulation of chondrocyte phenotype via baculovirus-mediated growth factor expression. Biomaterials. 2007;28:3437–3447. doi: 10.1016/j.biomaterials.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Ho Y-C, Chung Y-C, Hwang S-M, Wang K-C., and , Hu Y-C. Transgene expression and differentiation of baculovirus-transduced human mesenchymal stem cells. J Gene Med. 2005;7:860–868. doi: 10.1002/jgm.729. [DOI] [PubMed] [Google Scholar]
- Wang K-C, Wu J-C, Chung Y-C, Ho Y-C, Chang MD., and , Hu Y-C. Baculovirus as a highly efficient gene delivery vector for the expression of hepatitis delta virus antigens in mammalian cells. Biotechnol Bioeng. 2005;89:464–473. doi: 10.1002/bit.20385. [DOI] [PubMed] [Google Scholar]
- Transfiguracion J, Jorio H, Meghrous J, Jacob D., and , Kamen A. High yield purification of functional baculovirus vectors by size exclusion chromatography. J Virol Methods. 2007;142:21–28. doi: 10.1016/j.jviromet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Wickham TJ, Shuler ML, Hammer DA, Granados RR., and , Wood HA. Equilibrium and kinetic analysis of Autographa californica nuclear polyhedrosis virus attachment to different insect cell lines. J Gen Virol. 1992;73:3185–3194. doi: 10.1099/0022-1317-73-12-3185. [DOI] [PubMed] [Google Scholar]
- Palombo F, Monciotti A, Recchia A, Cortese R, Ciliberto G., and , La Monica N. Site-specific integration in mammalian cells mediated by a new hybrid baculovirus-adeno-associated virus vector. J Virol. 1998;72:5025–5034. doi: 10.1128/jvi.72.6.5025-5034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-Y., and , Wang S. Adeno-associated virus inverted terminal repeats improve neuronal transgene expression mediated by baculoviral vectors in rat brain. Hum Gene Ther. 2005;16:1219–1226. doi: 10.1089/hum.2005.16.1219. [DOI] [PubMed] [Google Scholar]
- Zeng J, Du J, Zhao Y, Palanisamy N., and , Wang S. Baculoviral vector-mediated transient and stable transgene expression in human embryonic stem cells. Stem Cells. 2007;25:1055–1061. doi: 10.1634/stemcells.2006-0616. [DOI] [PubMed] [Google Scholar]
- Shan L, Wang LY, Yin J, Zhong P., and , Zhong J. An OriP/EBNA-1-based baculovirus vector with prolonged and enhanced transgene expression. J Gene Med. 2006;8:1400–1406. doi: 10.1002/jgm.978. [DOI] [PubMed] [Google Scholar]
- Mei WH, Qian GQ, Zhang XQ, Zhang P., and , Lu J. Sustained expression of Epstein-Barr virus episomal vector mediated factor VIII in vivo following muscle electroporation. Haemophilia. 2006;12:271–279. doi: 10.1111/j.1365-2516.2006.01274.x. [DOI] [PubMed] [Google Scholar]
- Krepple F., and , Kochanek S. Long-term transgene expression in proliferating cells mediated by episomally maintained high capacity adenovirus vectors. J Virol. 2004;78:9–22. doi: 10.1128/JVI.78.1.9-22.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Takahashi Y, Shiozawa S, Ichise H, Yoshida N, Kanegae Y, et al. Efficient sequential gene regulation via FLP- and Cre-recombinase using adenovirus vector in mammalian cells including mouse ES cells. Microbiol Immunol. 2006;50:831–843. doi: 10.1111/j.1348-0421.2006.tb03850.x. [DOI] [PubMed] [Google Scholar]
- Piechaczek C, Fetzer C, Baiker A, Bode J., and , Lipps HJ. A vector based on the SV40 origin of replication and chromosomal S/MARs replicates episomally in CHO cells. Nucleic Acids Res. 1999;27:426–428. doi: 10.1093/nar/27.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzini S, Vargiolu A, Stehle IM, Bacci ML, Cerrito MG, Giovannoni R, et al. Genetically modified pigs produced with a nonviral episomal vector. Proc Natl Acad Sci USA. 2006;103:17672–17677. doi: 10.1073/pnas.0604938103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Shan L, Lo KW, Yin J, Zhang YY, Sun R, et al. Inhibition of nasopharyngeal carcinoma growth by RTA-expressing baculovirus vectors containing oriP. J Gene Med. 2008;10:1124–1133. doi: 10.1002/jgm.1237. [DOI] [PubMed] [Google Scholar]
- Abdallah B., and , Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2008;15:109–116. doi: 10.1038/sj.gt.3303067. [DOI] [PubMed] [Google Scholar]
- Bushman F, Lewinski M, Ciuffi A, Barr S, Leipzig J, Hannenhalli S, et al. Genome wide analysis of retroviral DNA integration. Nat Rev Microbiol. 2005;3:848–858. doi: 10.1038/nrmicro1263. [DOI] [PubMed] [Google Scholar]
- Schroder ARW, Shinn P, Chen HM, Berry C, Ecker JR., and , Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- Ivics Z, Katzer A, Stuwe EE, Fiedler D, Knespel S., and , Izsvak Z. Targeted sleeping beauty transposition in human cells. Mol Ther. 2007;15:1137–1144. doi: 10.1038/sj.mt.6300169. [DOI] [PubMed] [Google Scholar]
- Wilson MH, Coates CJ., and , George AL., Jr PiggyBac transposon-mediated gene transfer in human cells. Mol Ther. 2007;15:139–145. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- Ehrhardt A, Yant SR, Giering JC, Xu H, Engler JA., and , Kay MA. Somatic integration from an adenoviral hybrid vector into a hot spot in mouse liver results in persistent transgene expression levels in vivo. Mol Ther. 2007;15:146–156. doi: 10.1038/sj.mt.6300011. [DOI] [PubMed] [Google Scholar]
- Wang S., and , Vos JM. A hybrid herpesvirus infectious vector based on Epstein-Barr virus and herpes simplex virus type 1 for gene trasnfer into human cells in vitro and in vivo. J Virol. 1996;70:8422–8430. doi: 10.1128/jvi.70.12.8422-8430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorigo O, Gil JS, Gallaher SD, Tan BT, Castro MG, Lowenstein PR, et al. Development of a novel helper-dependent adenovirus-Epstein-Barr virus hybrid system for the stable transformation of mammalian cells. J Virol. 2004;78:6556–6566. doi: 10.1128/JVI.78.12.6556-6566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshenko N, Krougliak N, Eisensmith RC., and , Krougliak VA. A novel system for the production of fully deleted adenovirus vectors that does not require helper adenovirus. Gene Ther. 2001;8:846–854. doi: 10.1038/sj.gt.3301459. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales F, Borges AC, Martinez K, Shanmugam KT., and , Ingram LO. Chromosomal integration of heterologous DNA in Escherichia coli with precise removal of markers and replicons used during construction. J Bacteriol. 1999;181:7143–7148. doi: 10.1128/jb.181.22.7143-7148.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y-C, Tsai C-T, Chang Y-J., and , Huang J-H. Enhancement and prolongation of baculovirus-mediated expression in mammalian cells: focuses on strategic infection and feeding. Biotechnol Prog. 2003;19:373–379. doi: 10.1021/bp025609d. [DOI] [PubMed] [Google Scholar]
- Shen H-C, Lee H-P, Lo W-H, Yang D-G., and , Hu Y-C. Baculovirus-mediated gene transfer is attenuated by sodium bicarbonate. J Gene Med. 2007;9:470–478. doi: 10.1002/jgm.1037. [DOI] [PubMed] [Google Scholar]