Abstract
Human adenovirus (Ad) is a ubiquitous pathogen causing a wide range of diseases. Although the interactions of human Ad serotype 5 (Ad5) with susceptible cells in vitro are known in great detail, host factors controlling the tissue specificity of Ad5 infection in vivo remain poorly understood. Here, we analyzed the mechanisms of sequestration by the liver for blood-born human Ads and Ad5-based vectors. Our data suggest that several known mechanisms that lead to Ad5 sequestration by the liver become engaged in a redundant, sequential, and synergistic manner to ensure the rapid clearance of circulating virus particles from the blood. These mechanisms include (i) trapping of the virus by liver residential macrophages, Kupffer cells; (ii) Ad5 hepatocyte infection via blood factor–hexon interactions; and (iii) Ad5 penton RGD motif–mediated interactions with liver endothelial cells and hepatocytes, mediating virus retention in the space of Disse. More important, we show that when all of these mechanisms are simultaneously inactivated via mutations of Ad5 capsid proteins and pharmacological interventions, virus sequestration by the liver is markedly reduced. Therefore, our study is the first demonstration of the principal possibility of ablating the sequestration of blood-born Ad in the liver via specific inactivation of a defined set of mechanisms that control this process.
Introduction
Gene delivery vectors based on human species C adenovirus serotype 5 (Ad5) are the most frequently used in clinical studies, which aim to correct human genetic and acquired diseases. The extreme propensity of the virus for hepatocyte infection following its intravascular delivery has made Ad5 the vector of choice for applications requiring high-level transgene expression in hepatocytes in vivo.1 However, the efficient interaction between Ad5 and liver, which sequester >99% of the intravenously delivered vector dose,2,3 represents a major hindrance if gene delivery to extrahepatic cells and tissues, such as disseminated metastatic tumors, is required.
In vitro studies demonstrated that Ad infection starts with the virus binding to a high-affinity primary attachment receptor on the cell surface. The fiber protein mediates this interaction when its distal knob domain binds to a specific cellular receptor. For binding to cells, species A, C, D, E, and F human Ads may utilize the coxsackievirus and Ad receptor (CAR); however, the majority of human species B Ads utilize CD46 as a high-affinity cellular attachment receptor.4,5,6,7 Fiber-mediated binding of Ad to cells is followed by RGD motif–mediated binding of the viral penton base protein to cellular integrins.8 This interaction induces integrin activation and cytoskeleton rearrangement that facilitates internalization of the virus particle into the cell.9,10
Ad entry into cells is a remarkably efficient process (reviewed in refs. 11,12). However, in vivo analyses of Ad vector interactions with a host revealed that when the virus is introduced at high doses and/or via entry routes that are distinct from those used upon its natural infection, the observed Ad infectivity and biodistribution cannot be explained solely by the levels of Ad fiber–specific receptor expression.13
Our earlier studies suggest that the transduction of hepatocytes and the sequestration of Ad in the liver tissue after intravascular virus injection are governed by distinct molecular mechanisms.14 The liver residential macrophages, Kupffer cells, were thought to play the dominant role among factors contributing to the sequestration of a blood-born Ad in the liver.2,3 Indeed, when Ad is injected into mice intravenously, Kupffer cells rapidly accumulate large amounts of virus particles.2,15,16 Moreover, elimination of Kupffer cells from the liver after treatment of mice with ether clodronate liposomes or gadolinium chloride results in a marked increase in the levels of hepatocyte-specific Ad-mediated gene transfer, suggesting that significant amounts of infectious virus particles might be trapped by Kupffer cells.15,17,18,19 Our earlier data suggested that Ad5 binding to blood factors is the primary pathway mediating efficient virus entry into hepatocytes after intravenous administration.20 Most recently, it was found that the binding of blood coagulation factor X (FX) to Ad5 hexon leads to efficient hepatocyte transduction.21,22 However, the study by Waddington et al. showed that liver trapping of Ad5 vectors is comparable in wild-type mice, and mice treated with warfarin, a drug that blocks the activity of all vitamin K–dependent coagulation factors,23 thus implying that FX-facilitated entry of Ad into hepatocytes may not be solely responsible for Ad5 trapping in the liver.
In this study, we systematically analyzed the contribution of each of the known mechanisms in mediating the sequestration of blood-born Ad in the liver. Our data suggest the existence of the previously underappreciated redundancy and synergism of molecular pathways that control the sequestration of blood-born Ad in the liver. We also show for the first time the principal possibility of ablating the sequestration of blood-born Ad in the liver via specific inactivation of a defined set of mechanisms that control this process.
Results
Blood-born Ad is efficiently sequestered in the liver independently of the virus fiber structure, RGD motif–mediated penton binding to integrins, or hexon binding to FX
The previously obtained data provide strong evidence that Ad vectors with modified fibers transduce liver cells with efficiency that varies greatly depending on the fiber length and structure.24,25,26,27,28 However, in few studies where accumulation of fiber-modified vectors in the liver has been tested within first 3 hours after their intravascular delivery, it was found that physical Ad particles were trapped in the liver efficiently independently of the fiber structure.14,24 Using a set of the previously constructed Ad5-based vectors which possessed mutated fibers or RGD motif deletion within the penton base, we confirmed by Southern blot analysis that Ad5-based vectors accumulate in the liver efficiently 1 hour after intravenous injection independently of the fiber structure or the presence of an RGD motif within the penton (Supplementary Figure S1).
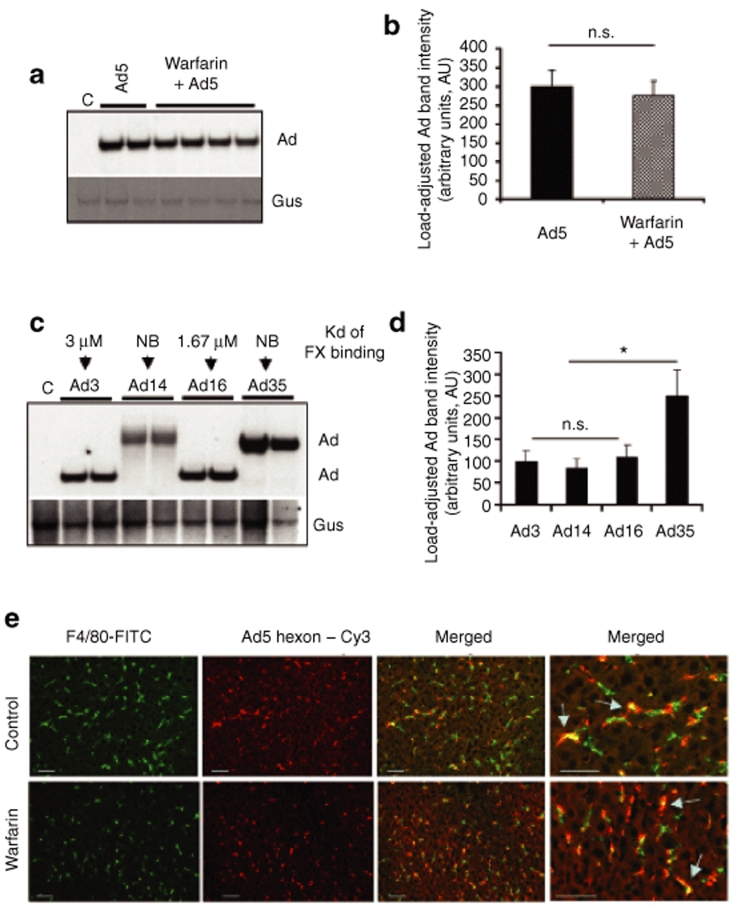
Recent evidence suggested that when Ad5 is injected intravenously, virus hexon protein binds coagulation FX with very high affinity and that this interaction leads to efficient virus entry into hepatocytes and hepatocyte transduction in vivo.21,22 To better understand how inactivation of this in vivo pathway of Ad entry into hepatocytes affects virus interactions with the liver, we administered unmodified Ad5 virus into control untreated mice and mice treated with warfarin, which inactivates all vitamin K–dependent blood coagulation factors.29 Analysis of Ad DNA trapped in the liver 1 hour after intravenous virus injection showed that warfarin treatment did not result in a significant reduction of Ad sequestration in the liver at this time point (Figure 1a,b). Our earlier analyses demonstrated that different wild-type human Ad serotypes show high variability in dissociation constant (Kds) of FX binding to their capsids.21 To further evaluate whether variations in amino acid composition of the exposed regions of hexon and Kds of FX binding could affect the efficacy of Ad sequestration in the liver, we injected mice intravenously with wild-type human Ad serotypes Ad3, Ad14, Ad16, and Ad35. Our earlier analysis showed that Ad16 has 1.67 µM Kd of FX binding, whereas Ad3 has Kd of FX binding equal 3 µM and Ad35 did not demonstrate any FX binding at all.21 The evaluation of sequestration of these viruses in the liver after intravenous administration showed no difference in the amounts of Ad3 and Ad16 and higher levels of Ad35 DNA (Figure 1c,d), suggesting that virus binding to FX cannot be the only important pathway mediating the sequestration of the blood-born Ad in the liver. Collectively, using Southern blot analysis of Ad DNA trapping in the liver for unmodified Ad5, fiber- and penton-modified Ad5-based vectors, and various wild-type Ad serotypes, our data confirm previous observations that the sequestration of the high doses of blood-born Ad occurs independently of the virus fiber structure, the presence of RGD motifs in the penton base, or virus hexon binding to blood coagulation factors.
Figure 1.
Sequestration of blood-born adenovirus (Ad) in the liver tissue after intravenous virus injection occurs independently of virus binding to blood coagulation factors. (a) Ad5 vector was injected into mice mock treated (Ad5 lanes) or pretreated with warfarin (warfarin lanes), and livers were harvested 1 hour after virus injection and processed for Southern blotting as described in Materials and Methods. Each lane represents liver samples harvested from a mouse individually injected with Ad (biological replicates). (b) Quantitative representation of vector accumulation in livers determined by PhosphorImager analysis of Ad-specific bands shown in a after adjustment of Ad DNA signal intensities for the Gus gene signal intensities for corresponding vectors using ImageQuant software. N = 4. n.s.—not statistically significant. (c) Southern blot analysis of genomes of wild-type human Ad present in the liver 1 hour after intravenous virus injection. Affinity of factor X (FX) binding for indicated serotypes is shown. Gus—mouse β-glucuronidase gene. C—Control liver DNA from a mouse injected with virus dilution buffer (phosphate-buffered saline) only. NB—not binding. The affinity of Ad3, Ad16, and Ad35 for FX was previously reported in ref. 49. The lack of Ad14 binding to FX is a personal communication of A. Lieber (University of Washington, 20 November 2008). Conservative species B Ad–specific DNA probe corresponding to E4 region was used for detection of species B genomic DNA in hybridization reaction. (d) Quantitative representation of vector accumulation in livers determined by PhosphorImager analysis of Ad-specific bands shown in c after adjustment of Ad DNA signal intensities for the Gus gene signal intensities for corresponding vectors using ImageQuant software. N = 3. n.s.—not statistically significant. *P < 0.05. (e) Association of Ad vectors with Kupffer cells of mice treated with warfarin or mock-treated controls. Ads were injected into the tail vein of C57Bl/6 mice. One hour later, livers were recovered and immediately frozen in an optimal cutting temperature compound. To visualize Kupffer cells (KCs), fixed liver sections were stained with anti-F4/80 antibody conjugated with fluorescein isothiocyanate (FITC) (green). Ad particles were visualized after staining the liver sections with Cy3-labeled anti-hexon polyclonal antibodies (red). Images of representative fields were taken with red and green filters and were then superimposed to reveal Ad association with KCs (yellow, merged). The overlapping of Kupffer cell–specific staining and Ad-specific staining is indicated by arrows.
Inactivation of the blood factor pathway of hepatocyte transduction does not prevent virus accumulation in Kupffer cells
After intravenous injection, large amounts of Ad are known to be trapped by liver residential macrophages, Kupffer cells.2 To assess whether the Kupffer cell capacity to trap high-dose blood-born Ad is changed in mice treated with warfarin, we administered Ad5 intravenously and 1 hour later analyzed the colocalization of virus particles with Kupffer cells by immunostaining and fluorescent microscopy. This analysis revealed that in the control and warfarin-treated mice, Kupffer cells were present in the liver (Figure 1e). More important, when liver sections of mice were costained with Ad5 hexon–specific antibody and F4/80 antibodies, we observed a clear colocalization of Ad hexon–specific staining with Kupffer cells (Figure 1e), suggesting that warfarin treatment of mice did not ablate the Kupffer cell capacity to trap blood-born Ad.
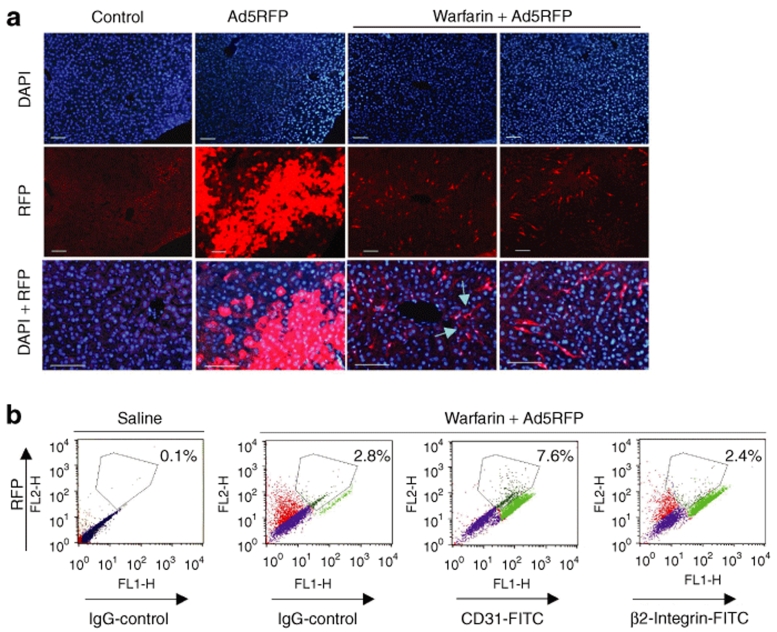
To analyze whether the treatment of mice with warfarin had any effect on the Ad-mediated hepatic cell transduction, we administered control mice and warfarin-treated mice with Ad5RFP vector, possessing unmodified wild-type Ad5 capsid, but expressing red fluorescent protein (RFP) under the control of cytomegalovirus promoter.30 Histological evaluation of liver sections for RFP expression showed that Ad5RFP transduced hepatocytes with very high efficiency in mock-treated mice (Figure 2a). Also, in agreement with previous data,29 we found that the treatment of mice treated with warfarin completely abrogated Ad5-mediated hepatocyte transduction. Surprisingly, detailed evaluation of liver sections also revealed that in the warfarin-treated group, Ad5RFP also transduced sinusoid endothelial cells (Figure 2a, lower panels, and b). Although the level of RFP expression in these cells was quite low, RFP expression was detected in 7.6% of all analyzed cells. RFP-positive cells represented 17% of all CD31-positive endothelial cells, suggesting that at least 17% of endothelial cells in the liver were transduced by Ad5RFP under the used conditions. In contrast, RFP expression was not observed in β2-integrin-positive cells of hematopoietic origin, including circulating and residential monocytes and macrophages (Figure 2b). Collectively, our data suggest that although warfarin-mediated inactivation of the blood factor pathway of hepatocyte infection completely abrogates Ad hepatocyte transduction, it does not prevent Ad accumulation in Kupffer cells; however, under the used conditions, Ad5RFP could infect hepatic sinusoid endothelial cells.
Figure 2.
Inactivation of blood coagulation factors leads to adenovirus (Ad) transduction of liver sinusoid endothelial cells. (a) In vivo transduction of hepatocytes with Ad5RFP after systemic vector application in control, mock-treated, and warfarin-treated mice. Twenty-four hours after intravenous Ad injection, livers were recovered and serial sections of formalin-fixed tissues were prepared. To visualize red fluorescent protein (RFP) fluorescence, images of sections were taken under ultraviolet light. Representative fields are shown. Magnification, ×200 on the two left sets of panels and ×400 on the right sets of panels. Note that the treatment of mice with warfarin completely eliminates hepatocyte transduction. However, sinusoid endothelial cells express RFP (indicated by arrows) in warfarin-treated mice. Two representative fields are shown. N = 4. (b) Analysis of surface markers of RFP expressing cells in warfarin-treated mice using flow cytometry (N = 5, the average percentage of RFP-positive cells is shown on the dot plots). Note that the RFP expressing cells are stained positive with antibody for CD31, endothelial cell marker, and not with antibody specific for β2-integrin, which is the marker for hematopoietic cells. DAPI, 4′,6-diamidino-2-phenylindole; FITC, fluorescein isothiocyanate; IgG, immunoglobulin G.
The depletion of Kupffer cells has no effect on Ad sequestration in the liver
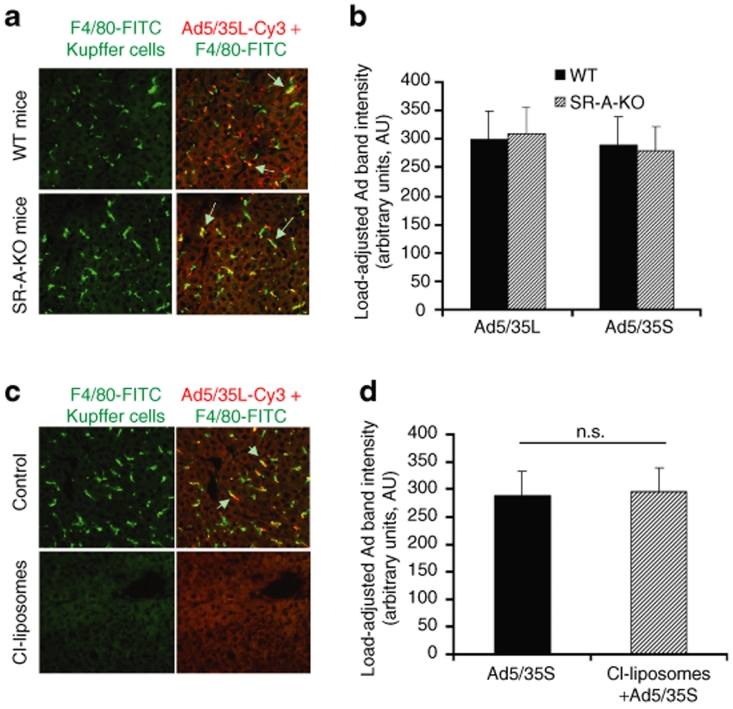
Kupffer cells are known to accumulate large amounts of Ad particles shortly after intravenous virus administration.15,18,31 Recently, it was shown that polyinosinic acid, poly(I), administration into mice prior to Ad injection drastically reduced the capacity of Kupffer cells to trap blood-born Ad in vivo.31,32 This data imply that a poly(I)-specific receptor, scavenger receptor A, might be involved in sequestering Ad from the blood after its intravenous injection. To evaluate this possibility, we administered Ad5-based vectors into wild-type or scavenger receptor A knockout (SR-A-KO) mice and analyzed both virus DNA deposition in the liver by Southern blotting and virus trapping in Kupffer cells by fluorescent microscopy. These analyses showed that in both control wild-type mice and SR-A-KO mice, Kupffer cells retained the capacity to accumulate Ad particles after intravenous virus administration (Figure 3a). We also found that the amount of Ad vector DNA that could be recovered from the liver after intravenous virus injection was virtually identical in wild-type and SR-A-KO mice (Figure 3b, and Supplementary Figure S2A), suggesting that SR-A cannot be the only receptor responsible for the Kupffer cells capacity to trap blood-born Ad in vivo. This finding is consistent with recent studies by Xu et al. who showed that multiple mechanisms mediate Ad interactions with Kupffer cells.33
Figure 3.
Kupffer cell elimination does not prevent the sequestration of blood-born adenovirus (Ad) in the liver. (a) Association of Ad vectors with Kupffer cells of wild-type (WT) mice or mice knockout for scavenger receptor A (SR-A-KO) gene. One hour after intravenous virus injection, livers were recovered and immediately frozen in optimal cutting temperature compound. To visualize KCs, fixed liver sections were stained with anti-F4/80 antibody (green). Ad particles were visualized after staining liver sections with Cy3-labeled anti-hexon polyclonal antibodies (red). Images of representative fields were taken with red and green filters and were then superimposed to reveal Ad association with KCs (yellow). The overlapping of Kupffer cell–specific staining and Ad-specific staining is indicated by arrows. (b) Quantitative representation of vector accumulation in livers determined by Southern blot analysis followed by ImageQuant data processing of Ad-specific bands are shown in Supplementary Figure S2A after adjustment of Ad DNA signal intensities for the Gus gene signal intensities. N = 4. (c) Wild-type mice were treated with clodronate liposomes as described in Materials and Methods. Furthermore, 24 hours after the treatment of mice with clodronate liposomes and 1 hour after intravenous virus injection, livers were recovered and immediately frozen in optimal cutting temperature compound. To visualize KCs, fixed liver sections were stained with anti-F4/80 antibody (green). Note that the treatment of mice with clodronate liposomes completely eliminates Kupffer cells from the liver. (d) Quantitative representation of the Southern blot analysis of Ad vector genomes' association with livers of wild-type mice treated with clodronate liposomes 1 hour after intravenous virus injection. Following hybridizations, the membranes were exposed to PhophorImager screens and the intensity volumes of Ad-specific and Gus-specific bands were recorded and processed using ImageQuant software. N = 4. n.s.—not statistically significant.
Next, we analyzed whether the depletion of these phagocytic cells could reduce the liver's capacity to sequester the blood-born Ad. To deplete Kupffer cells, we used the previously established and optimized techniques of macrophage elimination by a single-dose clodronate liposome injection.34 The clodronate liposomes are ingested by the macrophages that are then killed following phospholipase-mediated disruption of the liposomal bilayers and release of the clodronate.34 In agreement with the earlier data, intravenous administration of clodronate liposomes into mice resulted in complete elimination of Kupffer cells from the liver within 24 hours of injection (Figure 3c). However, the Southern blot analysis of Ad DNA amounts in the livers of clodronate liposome–treated mice or control mice treated only with phosphate-buffered saline 24 hours prior to virus administration, showed no difference in the amounts of Ad trapped in the liver independently of the presence of Kupffer cells in liver parenchyma (Figure 3d and Supplementary Figure S2B).
Simultaneous treatment of mice with warfarin and clodronate liposomes results in an only partial reduction in liver capacity to sequester blood-born Ad
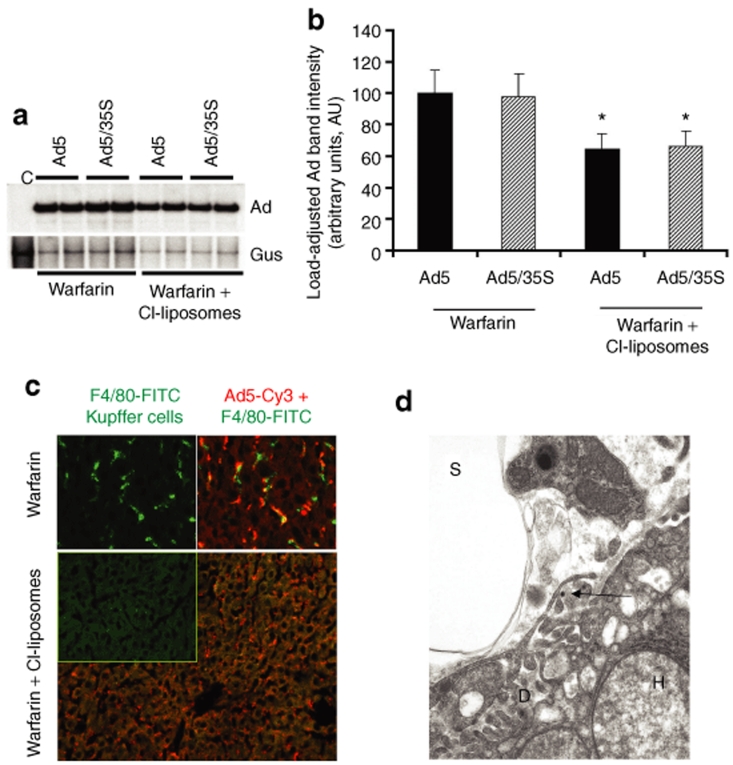
Our findings that independent inactivation of either blood factor pathway or depletion of Kupffer cells results in no measurable reduction in the amounts of Ad DNA trapped in the liver after intravenous virus administration may indicate that a dynamic balance might exist between the mechanisms of the Ad sequestration by Kupffer cells and hepatocytes in vivo. This data may also suggest that the mechanisms of sequestration of blood-born Ad may work in a redundant and synergistic manner. To evaluate this possibility, we analyzed the sequestration of Ad DNA in the livers of mice treated with both the warfarin and clodronate liposomes prior to Ad administration. This analysis revealed that when harvested at 1 hour after intravenous Ad injection, the amount of virus DNA trapped in the liver was 35% lower in mice treated with both warfarin and clodronate liposomes, compared to mice treated with warfarin only (Figure 4a,b). Although the difference between these two experimental groups was statistically significant, it was not dramatic. Close evaluation of Ad distribution in the livers of mice treated with warfarin and clodronate liposomes showed abundant punctuate Ad-specific staining localized to liver sinusoids (Figure 4c). Because we did not observe this type of Ad distribution in the livers of mice treated with either warfarin or clodronate liposomes independently (Figures 1e and 3c), our data suggest that yet another mechanism of Ad sequestration became engaged when both Kupffer cells and the blood factor pathway are inactivated.
Figure 4.
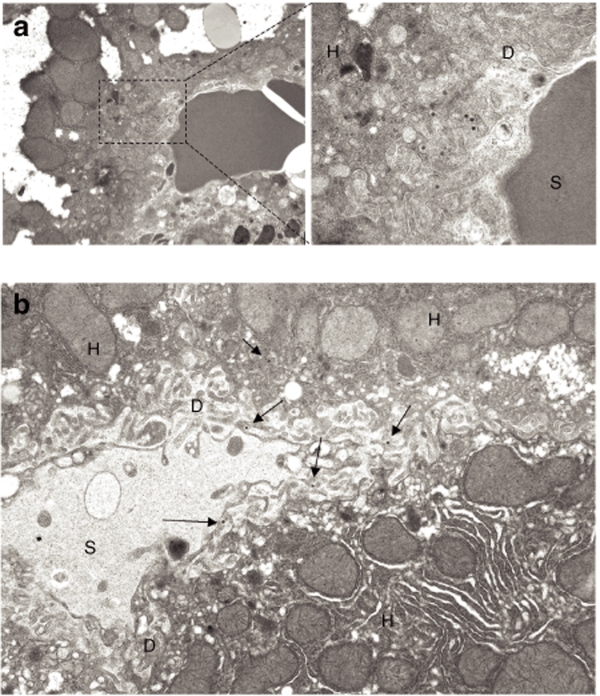
The treatment of mice with warfarin and clodronate liposomes allows for a partial reduction of the levels of adenovirus (Ad) DNA sequestered by the liver after intravenous virus administration. (a) Southern blot analysis for Ad vector genomes associated with livers of wild-type mice 1 hour after intravenous virus injection. Mice were treated with warfarin only or with a combination of warfarin and clodronate liposomes. Duplicate samples for each group are shown. Gus—mouse β-glucuronidase gene. Control—liver DNA from a mouse injected with virus dilution buffer (phosphate-buffered saline) only. (b) Quantitative representation of Ad accumulation in livers determined by PhosphorImager analysis of Ad-specific bands shown in a after adjustment of Ad DNA signal intensities for the Gus gene signal intensities for corresponding vectors. *P < 0.05. (c) Distribution of Ad particles in the livers of mice treated with warfarin or with a combination of warfarin and clodronate liposomes 1 hour after intravenous virus injection. Liver sections were stained with anti-F4/80 antibody to detect Kupffer cells (green) and with anti-Ad hexon antibody to detect Ad particles (red). Note that the large number of Ad particles was colocalized with Kupffer cells in warfarin-treated animals, whereas Ad particles were associated with liver sinusoids in mice treated with both of drugs. (d) Ad particles (shown by the arrow) localize to a subendothelial Disse space in the livers of mice treated with both warfarin and clodronate liposomes. Electron microscopy analysis was done on ultrathin sections of livers harvested 1 hour after intravenous virus injection. D—Disse space; H—hepatocyte; S—liver sinusoidal space. Magnification ×21,000.
To better define the localization of Ad particles in the liver sinusoids of mice treated with warfarin and clodronate liposomes, we employed transmission electron microscopy. One hour after virus injection, liver tissue was harvested, fixed, and processed for ultrathin sectioning. Electron microscopy analysis revealed that cytoplasmic organization of hepatocytes was highly distorted in warfarin-treated mice, suggesting a major cytotoxic effect of this drug on hepatic cells. Ad particles were easily identifiable on thin sections and were localized to the Disse space, the anatomical area underneath and between sinusoid endothelial cells and the hepatocyte surface (Figures 4d and 5a). As expected, Ad particles were present in liver sinusoids as free particles (Figure 5b).
Figure 5.
Visualization of adenovirus (Ad) distribution in liver tissue 1 hour after intravenous virus injection. (a) Ad particles present in hepatic sinusoids in a space of Disse; magnification of main image: ×4,400; enlarged image ×21,000. (b) Representative image showing distribution of free Ad particles in the Disse space (indicated by arrows). Magnification × 7,500. D—Disse space; H—hepatocyte; S—liver sinusoidal space.
Collectively, this data suggest that under the conditions when sequestration mechanisms mediated by Kupffer cells and blood coagulation factors are not functioning, Ad trapping in the Disse space between liver sinusoids endothelial cells and hepatocytes may become the major mechanism responsible for trapping large amounts of blood-born Ad in the liver.
RGD motif–mediated Ad interactions play a major role in sequestering blood-born Ad in the liver when other virus clearance mechanisms have been inactivated
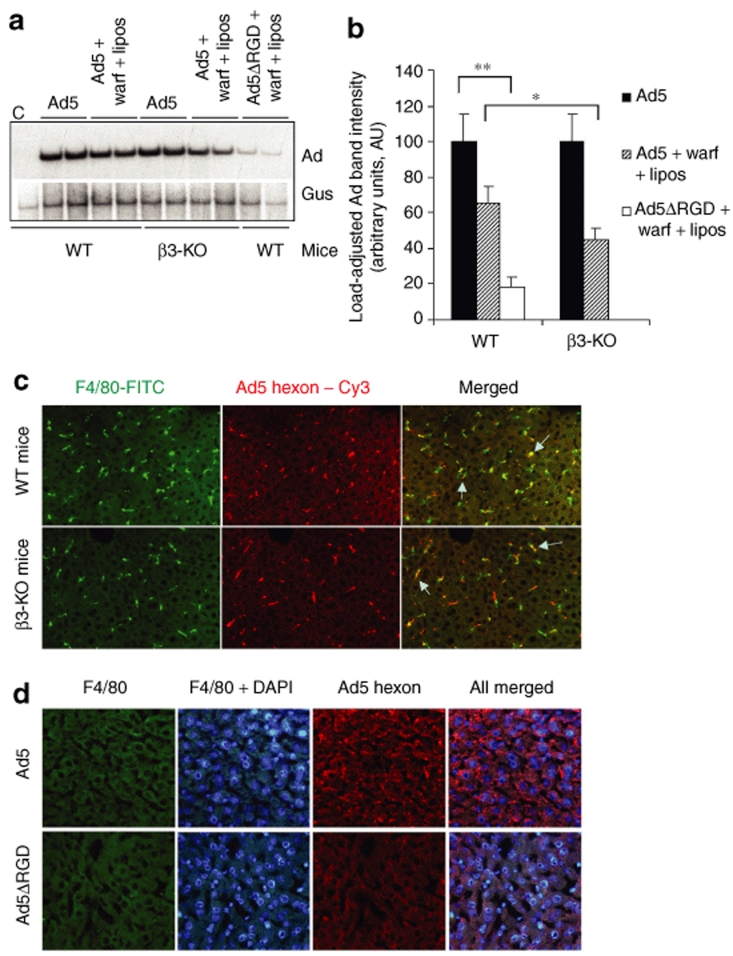
The Ad internalization into cells is facilitated by the penton base RGD motif–mediated binding to cellular integrins.8 Many RGD motif–interacting integrins were shown to serve as functional coreceptors, capable of facilitating Ad internalization into different kinds of cells.8,35 Moreover, it was shown that Ad interaction with integrins can mediate both the virus attachment to and internalization into cells.36 Because RGD motif–interacting β3-integrin is a subunit αvβ3-integrin expressed on endothelial cells, we speculated that sequestration of blood-born Ad in the liver might be reduced in mice that are knockout for β3-integrin gene. To evaluate this possibility we administered β3-integrin knockout (β3-KO) mice with Ad5 with or without treatment with warfarin and clodronate liposomes and analyzed the virus DNA deposition in the liver by Southern blotting. This analysis revealed that although the accumulation of Ad5 in the livers of wild-type control and β3-KO mice was comparable, the treatment of β3-KO mice with warfarin and clodronate liposomes resulted in a significantly greater reduction in levels of Ad trapped in the liver after intravenous virus injection, compared to wild-type animals (Figure 6a,b). The analysis of Ad trapping in Kupffer cells after intravenous virus administration showed that in both strains of mice, Kupffer cells were capable of trapping blood-born Ad (Figure 6c), suggesting that β3-integrin is unlikely to contribute significantly to the capacity of Kupffer cells to trap blood-born Ad particles.
Figure 6.
Adenovirus (Ad) penton RGD motifs play a critical role in supporting the sequestration of the blood-born Ad in the liver. (a) Wild-type (WT) or β3-integrin knockout mice (β3-KO) were treated with a combination of warfarin and liposomes and injected intravenously with Ad5 vector. In addition to drug treatment, WT mice were also injected with Ad5ΔRGD vector. One hour after virus injection, livers were harvested, and total liver DNA was purified and then subjected to a Southern blot analysis as described in Figure 1. Duplicate samples for each group are shown. Gus—mouse β-glucuronidase gene. C—Control liver DNA from a mouse injected with virus dilution buffer (phosphate-buffered saline) only. (b) Quantitative representation of Ad accumulation in livers determined by PhosphorImager analysis of Ad-specific bands shown in a after adjustment of Ad DNA signal intensities for the Gus gene signal intensities for corresponding vectors. *P < 0.05. **P < 0.01. (c) Distribution of Ad particles in the livers of wild-type or β3-KO mice 1 hour after intravenous virus injection. Liver sections were stained with anti-F4/80 antibody to detect Kupffer cells (green) and with anti-Ad hexon antibody to detect Ad particles (red). Colocalization of Ad-specific staining with Kupffer cell staining appears in yellow (merged) and is indicated by arrows. (d) Distribution of Ad and Ad5ΔRGD particles in the livers of mice treated with a combination of warfarin and clodronate liposomes 1 hour after intravenous injection. Liver sections were stained with anti-F4/80 antibody to ensure complete elimination of Kupffer cells with clodronate liposomes (green) as well as with 4′,6-diamidino-2-phenylindole (DAPI) (blue) and anti-Ad hexon antibody to detect Ad particles (red).
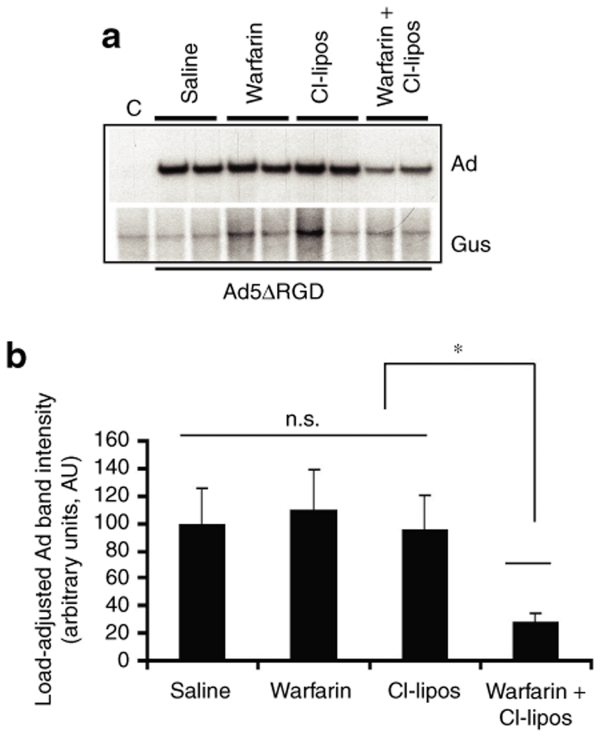
To further evaluate whether Ad penton RGD motif–mediated interactions might be responsible for Ad retention in the Disse space, wild-type mice were injected with an Ad5ΔRGD vector, possessing a three amino acid deletion in the penton base.37 Using Southern blotting, we observed a major reduction in levels of Ad DNA recovered from the livers of wild-type mice treated with warfarin and clodronate liposomes that were injected with Ad5ΔRGD vector (Figure 6a). The levels of Ad5ΔRGD DNA associated with the liver were 30%, compared to the levels of Ad5 DNA in mice treated with both warfarin and clodronate liposomes, and were 18% compared to Ad5 DNA levels in the mock-treated control group (Figure 6b). The analysis of Ad5ΔRGD distribution in the livers of mice treated with warfarin and clodronate liposomes using fluorescent microscopy showed a complete lack of Ad-specific staining (Figure 6d). To further verify the role of the Ad penton RGD motif in mediating the trapping of blood-born Ad in the liver and to assess the engagement order of pathways governing this process, we administered Ad5ΔRGD into mice treated with either warfarin or clodronate liposomes alone. The analysis of Ad DNA deposition in the livers of these mice showed that, similar to mice injected with control-unmodified Ad5, treatment with either warfarin or clodronate liposomes alone did not result in a measurable reduction of vector DNA trapped in the liver after intravenous injection (Figure 7). However, the combination of warfarin and clodronate liposome treatments and the ablation of RGD motif–mediated interactions resulted in major reduction in the amount of vector DNA associated with liver tissue after intravenous Ad injection.
Figure 7.
Sequestration of blood-born Ad5ΔRGD vector in the liver is reduced only in mice treated with both warfarin and clodronate liposomes. (a) Wild-type mice were individually treated with a saline, warfarin, clodronate liposomes, or a combination of warfarin and liposomes and injected intravenously with Ad5ΔRGD vector. One hour after virus injection, livers were harvested, and total liver DNA was purified and subjected to Southern blot analysis as described in Figure 1. Duplicate samples for each group are shown. Gus—mouse β-glucuronidase gene. C—Control liver DNA from a mouse injected with virus dilution buffer (phosphate-buffered saline) only. (b) Quantitative representation of Ad5ΔRGD accumulation in livers determined by PhosphorImager analysis of adenovirus (Ad)-specific bands shown in a after adjustment of Ad DNA signal intensities for the Gus gene signal intensities for corresponding vectors. *P < 0.05. n.s.—not statistically significant.
Discussion
Different Ad serotypes infect a variety of cells in vitro and in vivo with high efficiency. Based on this property of Ad, numerous Ad-based vectors have been developed for gene transfer and vaccination studies. However, when Ad vectors were delivered at high doses or via routes not associated with natural Ad infection, the infectivity and tissue biodistribution did not correlate with the levels of the fiber attachment receptors.13 In particular, after intravenous injection of human Ad5–based vectors, 99% of the delivered vector dose is rapidly sequestered in the liver.3,18 Moreover, ablation of both CAR and integrin interactions through mutation of Ad fiber and penton proteins did not prevent virus accumulation in the liver and efficient hepatocyte transduction.38,39 The efficient interaction between Ad and liver cells causes clinically significant hepatotoxicity and represents a major hindrance if gene delivery to extrahepatic cells and tissues, such as disseminated metastatic tumors, is required.
The liver residential macrophages, Kupffer cells, are among the most studied factors contributing to rapid sequestration of blood-born Ad in the liver. After intravenous virus injection, Kupffer cells trap large amounts of virus particles.2,18 This accumulation of Ad particles in Kupffer cells causes a nonlinear dose response for Ad vector–mediated transgene delivery into hepatocytes.16,19 It was shown that depletion of Kupffer cells from the liver by clodronate liposomes drastically increases the levels of transgene expression and vector DNA stability in hepatocytes.17,18 Ad interactions with Kupffer cells induce activation of a variety of proinflammatory responses.15,40,41,42 Recent data suggest that high-dose intravenous Ad administration can induce rapid Kupffer cell death that might also contribute to activation of proinflammatory host responses.43 On the other hand, it was shown that inactivation of Kupffer cells prior to intravenous Ad administration significantly increases the levels of circulating virus and transduction of hepatic and extrahepatic cells.2,44,45,46 Although Kupffer cells can accumulate large amounts of blood-born Ad, virus entry into Kupffer cells does not lead to their transduction in vivo, suggesting that Kupffer cells represent a poor niche for Ad propagation.47 Collectively, these data strongly suggest that Ad trapping by Kupffer cells is the first in line of the potential mechanisms mediating sequestration of blood-born Ad in the liver.
The data obtained in this study suggest, however, that the Ad trapping by Kupffer cells is not the only mechanism responsible for the sequestration of blood-born Ad in the liver. Specifically, complete elimination of Kupffer cells from the liver parenchyma after clodronate liposome administration led to no reduction in Ad DNA sequestered in the liver after intravenous virus injection (Figure 3 and Supplementary Figure S2B). Because elimination of Kupffer cells leads to marked increase in the level of Ad-mediated hepatocyte transduction, one could speculate that the vector particles that escaped sequestration by Kupffer cells are now efficiently entered hepatocytes, resulting in greatly increased levels of transgene expression.15,16,17,18
Our earlier data suggested that the entry of Ad5 into hepatocytes is mediated by blood factors and does not depend on virus interactions with CAR or integrins.20 Although warfarin treatment of mice completely abrogated hepatocytes transduction with Ad5 vectors in this study (Figure 2), in agreement with previous findings,23 we found that the amount of Ad DNA sequestered in the liver was similar in warfarin-treated and control mice (Figure 1). Our earlier studies showed that when liver tissue is perfused with Ad5 in situ in blood-free conditions, Kupffer cells can accumulate Ad particles in a blood factor (FIX)–dependent manner.20 Here, we found that in warfarin-treated mice, Kupffer cells retain the capacity to trap blood-born Ad, suggesting that the blood factor–mediated pathway of Ad entry in Kupffer cells may not be the only one to control Ad–Kupffer cell interactions. This observation is consistent with recent report suggesting that Kupffer cells may bind Ad via multiple mechanisms (including SR-A) and complement receptors or natural antibodies.33
The lack of a significant reduction in the amounts of Ad DNA sequestered in the livers of Kupffer cell–depleted or warfarin-treated mice, when compared to control animals, implies that a previously unknown redundancy and synergism between different mechanisms controlling sequestration of blood-born Ad in the liver might exist. To evaluate this possibility, we treated mice with both warfarin and clodronate liposomes and analyzed Ad sequestration in the liver after intravenous virus injection. Our studies unexpectedly revealed only a modest reduction in the amounts of Ad DNA sequestered in the livers of mice treated with the combination of drugs. While 35% less Ad DNA was trapped in livers of mice treated with both warfarin and clodronate liposomes, 65% of Ad DNA still remained trapped in livers of mice that lacked Kupffer cells and possessed no coagulation factors in blood (Figure 4). Although this data confirmed our hypothesis that Ad sequestration mechanisms operate in a redundant and synergistic manner, it also highlighted a previously unknown sequential order of engagement of these mechanisms to ensure efficient clearance of blood-born Ad from circulation. The detailed analyses of Ad particle distribution in the liver parenchyma using fluorescent microscopy and electron microscopy revealed abundant dispersed Ad particles localized to liver sinusoids. Because this type of virus distribution was not observed in livers of mice treated with each drug independently, or in mock-treated animals, this data suggest that new interactions of Ad with liver cells become activated only under the condition when other sequestration mechanisms have been inactivated.
Previous evidence suggests that penton base RGD motif binding to cellular integrins is essential for Ad internalization into cells.8 In this study, we demonstrate that Ad penton RGD motifs also contribute to virus trapping in the space of Disse that separates liver sinusoid endothelial cells from hepatocytes. Although it is currently unclear with which cells in the Disse space Ad interacts via RGD motifs, and which particular integrin type(s) mediates this interaction, our analysis of Ad deposition in the livers of β3-integrin knockout mice suggests that β3-integrin, at least in part, contributes to this interaction. Because αvβ3-integrin is expressed on endothelial cells, it is plausible to speculate that when Ad particles cannot enter hepatocytes, they may bind RGD-interacting integrins with β3-integrin subunit on sinusoid endothelial and thus become retained in the Disse space, leading to eventual internalization into endothelial cells or hepatocytes, and virus clearance from circulation. Our data, demonstrating low-level sinusoid endothelial cell transduction with Ad in mice treated with warfarin, support this idea (Figure 2).
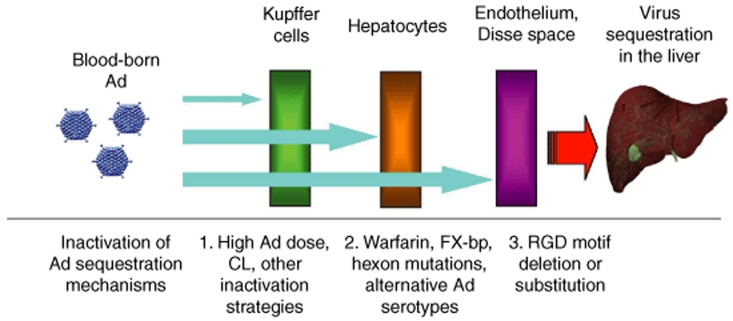
Collectively, our data uncovered a previously unknown redundancy and synergism in mechanisms mediating the sequestration of blood-born Ad in the liver. Based on earlier published data and data obtained in this study, we propose a model for sequestration of blood-born Ad in the liver (Figure 8). This model suggests that a defined set of specific molecular mechanisms become engaged in a redundant, synergistic, and orderly manner to ensure the clearance of blood-born Ad from circulation. When small amounts of Ad particles appear in blood, the virus trapping by Kupffer cells works as a first dominant mechanism, mediating Ad sequestration in the liver. When the Ad dose exceeds the capacity of Kupffer cells to trap the virus, hepatocytes absorb blood-born Ad particles in a blood factor–dependent manner, serving as a second dominant mechanism mediating sequestration of blood-born Ad. However, when the Ad dose is high and both the Kupffer cells and blood factor pathways are inactivated, sinusoid endothelial cells and the anatomical architecture of liver sinusoids become the third line of defense that sequesters Ad particles in an RGD motif–dependent manner.
Figure 8.
Schematic representation of mechanisms mediating the sequestration of blood-born adenovirus (Ad) in the liver and approaches to inactivate them. Upon entry of Ad into a bloodstream, a defined set of specific molecular mechanisms become engaged in a redundant, synergistic, and orderly manner to ensure the clearance of blood-born Ad from circulation. If small amounts of Ad particles appear in blood, the virus trapping by Kupffer cells works as a first dominant mechanism, mediating Ad sequestration in the liver. When the Ad dose exceeds the capacity of Kupffer cells to trap the virus, hepatocytes absorb blood-born Ad particles in a blood factor–dependent manner, serving as a second dominant mechanism mediating sequestration of blood-born Ad. However, when the Ad dose is high and both the Kupffer cells and blood factor pathways are inactivated, sinusoid endothelial cells and the anatomical architecture of liver sinusoids become the third line of defense that sequesters Ad particles in an RGD motif–dependent manner. CL—clodronate liposomes; FX-bp—FX binding protein.
To our knowledge, our study is the first demonstration of the principal possibility of ablating the sequestration of blood-born Ad in the liver via specific inactivation of a defined set of mechanisms that control this process. Because the molecular details mediating each of the mechanisms involved are emerging rapidly or are already known, our model may provide the basis for the design of novel, targeted, Ad-based vectors that will escape sequestration in the liver after delivery via an intravascular route.
Materials and Methods
Cells and viruses. A total of 293 (human embryonic kidney; Microbix, Toronto, ON, Canada) cells for Ad propagation were grown in Dulbecco's modified Eagle's medium, supplemented with 10% fetal calf serum, 2 mmol/l L-glutamine, and 1× penicillin/streptomycin solution (Invitrogen, Carlsbad, CA). Wild-type human Ad serotypes Ad3 (GB strain, VR3), Ad14 (de Wit, VR15), Ad16 (Ch.79, VR17), and Ad35 (Holden strain, VR-718) were purchased from the American Type Culture Collection (Manassas, VA). The human Ad5, possessing intact wild-type Ad5 capsid, was constructed previously and described in detail as Ad5GFP in ref. 48. The Ad5-based vector Ad5/35S, with Ad35-derived fiber shaft and knob domains, was previously constructed and described as Ad5GFP/F35 in ref. 48. The Ad5/35L, possessing Ad35-derived fiber knob domain, was previously constructed and described in ref. 49. Ad5ΔRGD and Ad5/35ΔRGD are identical to Ad5 and Ad5/35L, but possess an RGD motif deletion in the penton base protein. These vectors were previously constructed and described in ref. 37. Infectivity of these vectors (virus particles-to-PFU ratios) was also reported earlier for all analyzed vectors. Ad5*F and Ad5*FΔRGD were kindly provided by Dr. Ramon Alemany (Barcelona, Spain). Both of these Ad5-based vectors possess a single point Y477A amino acid mutation within the fiber knob domain that abrogates virus binding to CAR.50 Ad5*FΔRGD also possesses an RGD motif deletion within its penton base protein. Ad5 vector, Ad5RFP, expressing RFP was kindly provided by Dr. Michael Barry (Rochester, MN).30 All viruses were amplified in 293 cells under conditions preventing cross contamination. Viruses were banded in CsCl gradients; viral bands were collected, dialyzed, and aliquoted as described elsewhere.49 Ad particle concentrations were determined spectrophotometrically by measuring the optical density at 260 nm, using the extinction coefficient for wild-type Ad5, A260 = 9.09 × 1013 optical density ml cm virion−1.
Ad infection in vivo. All experimental procedures involving animals were conducted in accordance with the institutional guidelines set forth by the University of Washington. C57Bl/6 mice were purchased from Charles River Laboratories (Wilmington, MA). β3-integrin knockout mice, β3-KO (stock #4669), and SR-A-KO mice (Msr1−/−, stock #6096) were purchased from Jackson Laboratory (Bar Harbor, ME). All mice were housed in specific pathogen-free facilities. All wild-type viruses or Ad vectors were injected into the tail vein of mice at a dose of 1011 Ad particles (corresponds to 5 × 109 PFU of Ad5 vector determined on 293 cells) in 200 µl of phosphate-buffered saline. For in vivo transduction studies, mice were sacrificed 1 or 48 hours post virus infusion and livers were processed for histological analyses. For analysis of Ad genome accumulation in the liver tissue 1 and 24 hours after Ad vector administration into the tail vein, blood was flushed from the liver by cardiac saline perfusion, livers were harvested, and total DNA was purified as described earlier.14 To inactivate vitamin K–dependent blood coagulation factors, mice were injected with warfarin twice, 72 and 24 hours before Ad administration. Warfarin was resuspended in peanut oil and 150 µg of warfarin per mouse per injection were used as described elsewhere.29 To eliminate Kupffer cells from the liver, mice were injected with 200 µl of clodronate liposomes (clodronateliposomes.org/) 48 hours before virus administration.34 To analyze Ad liver cell transduction by flow cytometry, liver cells were purified with collagenase perfusion and plated on Primaria 6-well plates 24 hours after intravenous virus injection. Next day, cells were detached from the plate using 2 mmol/l EDTA solution and stained with anti-CD31 (endothelial cells), anti- β2-integrin (cells of hematopoietic origin, including Kupffer cells and circulating monocytes), or isotype control antibodies. Positive staining of cells was analyzed by flow cytometry.
Analysis of Ad–Kupffer cell interaction in vivo. To analyze Ad interactions with Kupffer cells, 1011 virus particles were injected into the tail vein, and 60 minutes later, livers were flushed with saline via cardiac perfusion, harvested and immediately frozen in an optimal cutting temperature compound. Frozen liver sections were fixed and stained with rat anti-mouse F4/80 primary antibody (BD Biosciences, San Diego, CA) to detect Kupffer cells. Specific binding of primary antibodies was visualized with secondary anti-rat Alexa Fluor 488 antibody (green) (Molecular Probes, Eugene, OR). To detect Ad particles, liver sections were stained with anti-hexon polyclonal antibody (Abcam, Cambridge, MA). The staining with biotinylated anti-hexon primary antibody was developed with secondary Cy3-labeled streptavidin which appears in red. To detect platelets and endothelial cells on liver sections, antibodies to CD41 (platelets, clone MWReg30) or CD31-FITC endothelial cells, clone MEC13.3 (both from BD Biosciences) were used. Cell nuclei were counterstained with 1 µg/ml 4′,6-diamidino-2-phenylindole (Sigma, St. Louis, MO).
Southern blot analyses. For analysis of Ad genomic DNA deposition and persistence in mouse livers, isolation of total liver DNA and Southern analysis were performed as described elsewhere.14 Briefly, at the indicated time points, livers were harvested and total liver DNA was isolated using DNA salting-out protocol, followed by phenol/chloroform and chloroform/isoamyl alcohol purifications. Next, 10 µg of purified total liver DNA was digested with HindIII endonuclease overnight and loaded onto 1% agarose gel. After completion of electrophoretic separation of the DNA fragments, they were transferred onto a Hybond-XL membrane (GE Healthcare, Piscataway, NJ) and the membrane was hybridized with a 32P-labeled mouse β-glucuronidase (Gus) gene–specific probe to ensure equivalent DNA loads. Gus-specific DNA for labeling was obtained by cutting mouse Gus gene–containing plasmid with BamHI and EcoRI exonucleases to generate 680-bp DNA fragment. The image of Gus-specific hybridization was obtained by exposing the membrane to both a PhosphorImager screen and an X-ray film. Next, the membrane was stripped off the Gus-specific probe and rehybridized with an Ad-specific 32P-labeled probe (8 kb HindIII, A-fragment, corresponding to the E2 region of the Ad genome). To analyze deposition of genomic DNA in the liver for various human species B Ad serotypes, we generated a E4 region–specific probe by amplifying a 200-bp-long fragment of E4 region that is highly conserved at the DNA level among analyzed serotypes using PCR and Ad35 genomic DNA as a template. The image of the hybridization reaction was obtained by exposing the membrane to a PhosphorImager screen and an X-ray film. The intensity of Gus- and Ad-specific signals in PhosphorImager-collected images were analyzed using manufacturer's software.
Statistical analysis. All statistical analyses were done using an unpaired two-sided Student's t-test on Instat software. The data are expressed as means ± SD. The number of animals used in the experiments for each individual condition varied from three to five and indicated in the figure legends.
Supplementary MaterialFigure S1. Sequestration of blood-born Ad in the liver tissue after intravenous virus injection. (A) The variations in efficacy of Ad interaction with primary attachment receptors and integrins do not affect Ad sequestration in the liver after intravenous injection. One hour after intravenous injection of indicated Ad vectors, livers were recovered from mice and the total DNA was purified as described in Materials and Methods. Ten μg of total DNA, digested with HindIII enzyme, were loaded onto the agarose gel. Following transfer to a Nybond-N+ membrane, filters were hybridized with a mouse β-glucuronidase-specific probe to confirm equivalent loads (Gus). Subsequently, the membranes were stripped and rehybridized with an Ad-specific probe (Ad). Control – DNA purified from livers of mice injected with PBS only. Ad5 virus possesses intact wild type human Ad5 capsid. The Ad5/35L vector possesses Ad5 capsid and Ad35-derived fiber knob domain. Ad5ΔRGD and Ad5/35ΔRGD are identical to Ad5 and Ad5/35L, but possess an RGD motif deletion in the penton base protein. Ad5*F and Ad5*FΔRGD are Ad5-based vectors possessing a single point Y477A amino acid mutation within the fiber knob domain that abrogates virus binding to CAR.1 Ad5*FΔRGD also possesses an RGD motif deletion within its penton base protein. The capacity of indicated Ad vectors to bind CAR is shown. (B) Quantitative representation of vector accumulation in livers determined by PhosphorImager analysis of Ad-specific bands shown in (A) after adjustment of Ad DNA signal intensities for the Gus gene signal intensities for corresponding vectors using ImageQuant software. N=3. (C) The length of the Ad fiber shaft domain does not affect Ad sequestration in the liver after intravenous injection. The Ad5-based vector Ad5/35S possesses short Ad35-derived fiber shaft and knob domains. Experiments were done exactly as described in (A). Biological duplicate are shown for each individual vector. (D) Quantitative representation of vector accumulation in livers determined by PhosphorImager analysis of Ad-specific bands shown in (C) after adjustment of Ad DNA signal intensities for the Gus gene signal intensities for corresponding vectors using ImageQuant software. N=4. n.s. – not statistically significant.Figure S2. Kupffer cell elimination does not prevent the sequestration of blood-born Ad in the liver. (A) – Southern blot analysis for Ad vector genomes associated with livers of wild type and SR-A-KO mice 1 h after intravenous virus injection. Biological duplicates are shown. Gus - mouse β-glucuronidase gene. C - Control liver DNA from a mouse injected with virus dilution buffer (PBS) only. (B) – Southern blot analysis for Ad vector genomes associated with livers 1 hour post virus injection in wild type mice treated with clodronate liposomes 24 h prior to intravenous virus administration. Biological duplicates are shown. Gus - mouse β-glucuronidase gene. C - Control liver DNA from a mouse injected with virus dilution buffer (PBS) only. N=4.
Supplementary Material
Sequestration of blood-born Ad in the liver tissue after intravenous virus injection. (A) The variations in efficacy of Ad interaction with primary attachment receptors and integrins do not affect Ad sequestration in the liver after intravenous injection. One hour after intravenous injection of indicated Ad vectors, livers were recovered from mice and the total DNA was purified as described in Materials and Methods. Ten μg of total DNA, digested with HindIII enzyme, were loaded onto the agarose gel. Following transfer to a Nybond-N+ membrane, filters were hybridized with a mouse β-glucuronidase-specific probe to confirm equivalent loads (Gus). Subsequently, the membranes were stripped and rehybridized with an Ad-specific probe (Ad). Control – DNA purified from livers of mice injected with PBS only. Ad5 virus possesses intact wild type human Ad5 capsid. The Ad5/35L vector possesses Ad5 capsid and Ad35-derived fiber knob domain. Ad5ΔRGD and Ad5/35ΔRGD are identical to Ad5 and Ad5/35L, but possess an RGD motif deletion in the penton base protein. Ad5*F and Ad5*FΔRGD are Ad5-based vectors possessing a single point Y477A amino acid mutation within the fiber knob domain that abrogates virus binding to CAR.1 Ad5*FΔRGD also possesses an RGD motif deletion within its penton base protein. The capacity of indicated Ad vectors to bind CAR is shown. (B) Quantitative representation of vector accumulation in livers determined by PhosphorImager analysis of Ad-specific bands shown in (A) after adjustment of Ad DNA signal intensities for the Gus gene signal intensities for corresponding vectors using ImageQuant software. N=3. (C) The length of the Ad fiber shaft domain does not affect Ad sequestration in the liver after intravenous injection. The Ad5-based vector Ad5/35S possesses short Ad35-derived fiber shaft and knob domains. Experiments were done exactly as described in (A). Biological duplicate are shown for each individual vector. (D) Quantitative representation of vector accumulation in livers determined by PhosphorImager analysis of Ad-specific bands shown in (C) after adjustment of Ad DNA signal intensities for the Gus gene signal intensities for corresponding vectors using ImageQuant software. N=4. n.s. – not statistically significant.
Kupffer cell elimination does not prevent the sequestration of blood-born Ad in the liver. (A) – Southern blot analysis for Ad vector genomes associated with livers of wild type and SR-A-KO mice 1 h after intravenous virus injection. Biological duplicates are shown. Gus - mouse β-glucuronidase gene. C - Control liver DNA from a mouse injected with virus dilution buffer (PBS) only. (B) – Southern blot analysis for Ad vector genomes associated with livers 1 hour post virus injection in wild type mice treated with clodronate liposomes 24 h prior to intravenous virus administration. Biological duplicates are shown. Gus - mouse β-glucuronidase gene. C - Control liver DNA from a mouse injected with virus dilution buffer (PBS) only. N=4.
Acknowledgments
This study was supported by funding from the National Institutes of Health (grants AI062853, AI064882, CA141439, and AI065429 to D.M.S.). We are thankful to Allyson Goldstein for manuscript editing. Clodronate was a gift of Roche Diagnostics GmbH, Mannheim, Germany.
REFERENCES
- Thomas CE, Ehrhardt A., and , Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Gen. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- Alemany R, Suzuki K., and , Curiel DT. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol. 2000;81:2605–2609. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- Worgall S, Wolff G, Falck-Pedersen E., and , Crystal RG. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum Gene Ther. 1997;8:37–44. doi: 10.1089/hum.1997.8.1-37. [DOI] [PubMed] [Google Scholar]
- Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Gaggar A, Shayakhmetov D., and , Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- Roelvink PW, Mi Lee G, Einfeld DA, Kovesdi I., and , Wickham TJ. Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science. 1999;286:1568–1571. doi: 10.1126/science.286.5444.1568. [DOI] [PubMed] [Google Scholar]
- Segerman A, Atkinson JP, Marttila M, Dennerquist V, Wadell G., and , Arnberg N. Adenovirus type 11 uses CD46 as a cellular receptor. J Virol. 2003;77:9183–9191. doi: 10.1128/JVI.77.17.9183-9191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham TJ, Mathias P, Cheresh DA., and , Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Suomalainen M, Nakano MY, Boucke K, Keller S., and , Greber UF. Adenovirus-activated PKA and p38/MAPK pathways boost microtubule-mediated nuclear targeting of virus. EMBO J. 2001;20:1310–1319. doi: 10.1093/emboj/20.6.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupack ELD, Bokoch GM., and , Nemerow GR. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J Virol. 1998;72:8806–8812. doi: 10.1128/jvi.72.11.8806-8812.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier O., and , Greber UF. Adenovirus endocytosis. J Gene Med. 2003;5:451–462. doi: 10.1002/jgm.409. [DOI] [PubMed] [Google Scholar]
- Nemerow GR., and , Stewart PL. Role of alpha(v) integrins in adenovirus cell entry and gene delivery. Microbiol Mol Biol Rev. 1999;63:725–734. doi: 10.1128/mmbr.63.3.725-734.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fechner H, Haack A, Wang H, Wang X, Eizema K, Pauschinger M, et al. Expression of coxsackie adenovirus receptor and alphav-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther. 1999;6:1520–1535. doi: 10.1038/sj.gt.3301030. [DOI] [PubMed] [Google Scholar]
- Shayakhmetov DM, Li ZY, Ni S., and , Lieber A. Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J Virol. 2004;78:5368–5381. doi: 10.1128/JVI.78.10.5368-5381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber A, He CY, Meuse L, Schowalter D, Kirillova I, Winther B, et al. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol. 1997;71:8798–8807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao N, Gao GP, Parr M, Johnston J, Baradet T, Wilson JM, et al. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol Ther. 2001;3:28–35. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- Schiedner G, Hertel S, Johnston M, Dries V, van Rooijen N., and , Kochanek S. Selective depletion or blockade of Kupffer cells leads to enhanced and prolonged hepatic transgene expression using high-capacity adenoviral vectors. Mol Ther. 2003;7:35–43. doi: 10.1016/s1525-0016(02)00017-5. [DOI] [PubMed] [Google Scholar]
- Wolff G, Worgall S, van Rooijen N, Song WR, Harvey BG., and , Crystal RG. Enhancement of in vivo adenovirus-mediated gene transfer and expression by prior depletion of tissue macrophages in the target organ. J Virol. 1997;71:624–629. doi: 10.1128/jvi.71.1.624-629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler RJ, Li C, Cherry M, Zhu YX, Hempel D, van Rooijen N, et al. Correction of the nonlinear dose response improves the viability of adenoviral vectors for gene therapy of Fabry disease. Hum Gene Ther. 2002;13:935–945. doi: 10.1089/10430340252939041. [DOI] [PubMed] [Google Scholar]
- Shayakhmetov DM, Gaggar A, Ni SH, Li ZY., and , Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL, et al. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci USA. 2008;105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington SN, Mcvey JH, Bhella D, Parker AL, Barker K, Atoda H, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Waddington SN, Parker AL, Havenga M, Nicklin SA, Buckley SMK, Mcvey JH, et al. Targeting of adenovirus serotype 5 (Ad5) and 5/47 pseudotyped vectors in vivo: fundamental involvement of coagulation factors and redundancy of CAR binding by Ad5. J Virol. 2007;81:9568–9571. doi: 10.1128/JVI.00663-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Sato K., and , Hamada H. Reduction of natural adenovirus tropism to the liver by both ablation of fiber-coxsackievirus and adenovirus receptor interaction and use of replaceable short fiber. J Virol. 2003;77:2512–2521. doi: 10.1128/JVI.77.4.2512-2521.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol CG, Graham D, Miller WH, White SJ, Smith TAG., and , Nicklin SA, et al. Effect of adenovirus serotype 5 fiber and penton modifications on in vivo tropism in rats. Mol Ther. 2004;10:344–354. doi: 10.1016/j.ymthe.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Shayakhmetov DM, Li ZY, Ni S., and , Lieber A. Targeting of adenovirus vectors to tumor cells does not enable efficient transduction of breast cancer metastases. Cancer Res. 2002;62:1063–1068. [PubMed] [Google Scholar]
- Smith TA, Idamakanti N, Rollence ML, Marshall-Neff J, Kim J, Mulgrew K, et al. Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice. Hum Gene Ther. 2003;14:777–787. doi: 10.1089/104303403765255165. [DOI] [PubMed] [Google Scholar]
- Vigne E, Dedieu JF, Brie A, Gillardeaux A, Briot D, Benihoud K, et al. Genetic manipulations of adenovirus type 5 fiber resulting in liver tropism attenuation. Gene Ther. 2003;10:153–162. doi: 10.1038/sj.gt.3301845. [DOI] [PubMed] [Google Scholar]
- Parker AL, Waddington SN, Nicol CG, Shayakhmetov DM, Buckley SM, Denby L, et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood. 2006;108:2554–2561. doi: 10.1182/blood-2006-04-008532. [DOI] [PubMed] [Google Scholar]
- Campos SK., and , Barry MA. Comparison of adenovirus fiber, protein IX, and hexon capsomeres as scaffolds for vector purification and cell targeting. Virology. 2006;349:453–462. doi: 10.1016/j.virol.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Smith JS, Xu Z., and , Byrnes AP. A quantitative assay for measuring clearance of adenovirus vectors by Kupffer cells. J Virol Methods. 2008;147:54–60. doi: 10.1016/j.jviromet.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Haisma HJ, Kamps JA, Kamps GK., and , Bellu AR. Poly I prevents adenoviral vector clearance by Kupffer cells and increases efficiency in gene therapy applications. Hum Gene Ther. 2007;18:981–982. [Google Scholar]
- Xu Z, Tian J, Smith JS., and , Byrnes AP. Clearance of adenovirus by Kupffer cells is mediated by scavenger receptors, natural antibodies, and complement. J Virol. 2008;82:11705–11713. doi: 10.1128/JVI.01320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooijen N., and , van Kesteren-Hendrikx E. “In vivo” depletion of macrophages by liposome-mediated “suicide”. Methods Enzymol. 2003;373:3–16. doi: 10.1016/s0076-6879(03)73001-8. [DOI] [PubMed] [Google Scholar]
- Davison E, Diaz RM, Hart IR, Santis G., and , Marshall JF. Integrin alpha5beta1-mediated adenovirus infection is enhanced by the integrin-activating antibody TS2/16. J Virol. 1997;71:6204–6207. doi: 10.1128/jvi.71.8.6204-6207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Kamata T, Takada Y, Ruggeri ZM., and , Nemerow GR. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J Virol. 1996;70:4502–4508. doi: 10.1128/jvi.70.7.4502-4508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayakhmetov DM, Eberly AM, Li ZY., and , Lieber A. Deletion of penton RGD motifs affects the efficiency of both the internalization and the endosome escape of viral particles containing adenovirus serotype 5 or 35 fiber knobs. J Virol. 2005;79:1053–1061. doi: 10.1128/JVI.79.2.1053-1061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi H, Koizumi N, Hosono T, Ishii-Watabe A, Uchida E, Utoguchi N, et al. CAR- or alphav integrin-binding ablated adenovirus vectors, but not fiber-modified vectors containing RGD peptide, do not change the systemic gene transfer properties in mice. Gene Ther. 2002;9:769–776. doi: 10.1038/sj.gt.3301701. [DOI] [PubMed] [Google Scholar]
- Smith T, Idamakanti N, Kylefjord H, Rollence M, King L, Kaloss M, et al. In vivo hepatic adenoviral gene delivery occurs independently of the coxsackievirus-adenovirus receptor. Mol Ther. 2002;5:770–779. doi: 10.1006/mthe.2002.0613. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zaiss AK, Colarusso P, Patel K, Haljan G, Wickham TJ, et al. The role of capsid-endothelial interactions in the innate immune response to adenovirus vectors. Hum Gene Ther. 2003;14:627–643. doi: 10.1089/104303403321618146. [DOI] [PubMed] [Google Scholar]
- Schiedner G, Bloch W, Hertel S, Johnston M, Molojavyi A, Dries V, et al. A hemodynamic response to intravenous adenovirus vector particles is caused by systemic Kupffer cell-mediated activation of endothelial cells. Hum Gene Ther. 2003;14:1631–1641. doi: 10.1089/104303403322542275. [DOI] [PubMed] [Google Scholar]
- Schnell MA, Zhang Y, Tazelaar J, Gao GP, Yu QC, Qian R, et al. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol Ther. 2001;3:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- Manickan E, Smith JS, Tian J, Eggerman TL, Lozier JN, Muller J, et al. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol Ther. 2006;13:108–117. doi: 10.1016/j.ymthe.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Machemer T, Engler H, Tsai V, Lee S, Cannon-Carlson S, Voloch M, et al. Characterization of hemodynamic events following intravascular infusion of recombinant adenovirus reveals possible solutions for mitigating cardiovascular responses. Mol Ther. 2005;12:254–263. doi: 10.1016/j.ymthe.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Shashkova EV, Doronin K, Senac JS., and , Barry MA. Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer Res. 2008;68:5896–5904. doi: 10.1158/0008-5472.CAN-08-0488. [DOI] [PubMed] [Google Scholar]
- Snoeys J, Mertens G, Lievens J, van Berkel T, Collen N, Biessen EA, et al. Lipid emulsions potently increase transgene expression in hepatocytes after adenoviral transfer. Mol Ther. 2006;13:98–107. doi: 10.1016/j.ymthe.2005.06.477. [DOI] [PubMed] [Google Scholar]
- Wheeler MD, Yamashina S, Froh M, Rusyn I., and , Thurman RG. Adenoviral gene delivery can inactivate Kupffer cells: role of oxidants in NF-kappa B activation and cytokine production. J Leukoc Biol. 2001;69:622–630. [PubMed] [Google Scholar]
- Shayakhmetov DM, Papayannopoulou T, Stamatoyannopoulos G., and , Lieber A. Efficient gene transfer into human CD34(+) cells by a retargeted adenovirus vector. J Virol. 2000;74:2567–2583. doi: 10.1128/jvi.74.6.2567-2583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayakhmetov DM., and , Lieber A. Dependence of adenovirus infectivity on length of the fiber shaft domain. J Virol. 2000;74:10274–10286. doi: 10.1128/jvi.74.22.10274-10286.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemany R., and , Curiel DT. CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther. 2001;8:1347–1353. doi: 10.1038/sj.gt.3301515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequestration of blood-born Ad in the liver tissue after intravenous virus injection. (A) The variations in efficacy of Ad interaction with primary attachment receptors and integrins do not affect Ad sequestration in the liver after intravenous injection. One hour after intravenous injection of indicated Ad vectors, livers were recovered from mice and the total DNA was purified as described in Materials and Methods. Ten μg of total DNA, digested with HindIII enzyme, were loaded onto the agarose gel. Following transfer to a Nybond-N+ membrane, filters were hybridized with a mouse β-glucuronidase-specific probe to confirm equivalent loads (Gus). Subsequently, the membranes were stripped and rehybridized with an Ad-specific probe (Ad). Control – DNA purified from livers of mice injected with PBS only. Ad5 virus possesses intact wild type human Ad5 capsid. The Ad5/35L vector possesses Ad5 capsid and Ad35-derived fiber knob domain. Ad5ΔRGD and Ad5/35ΔRGD are identical to Ad5 and Ad5/35L, but possess an RGD motif deletion in the penton base protein. Ad5*F and Ad5*FΔRGD are Ad5-based vectors possessing a single point Y477A amino acid mutation within the fiber knob domain that abrogates virus binding to CAR.1 Ad5*FΔRGD also possesses an RGD motif deletion within its penton base protein. The capacity of indicated Ad vectors to bind CAR is shown. (B) Quantitative representation of vector accumulation in livers determined by PhosphorImager analysis of Ad-specific bands shown in (A) after adjustment of Ad DNA signal intensities for the Gus gene signal intensities for corresponding vectors using ImageQuant software. N=3. (C) The length of the Ad fiber shaft domain does not affect Ad sequestration in the liver after intravenous injection. The Ad5-based vector Ad5/35S possesses short Ad35-derived fiber shaft and knob domains. Experiments were done exactly as described in (A). Biological duplicate are shown for each individual vector. (D) Quantitative representation of vector accumulation in livers determined by PhosphorImager analysis of Ad-specific bands shown in (C) after adjustment of Ad DNA signal intensities for the Gus gene signal intensities for corresponding vectors using ImageQuant software. N=4. n.s. – not statistically significant.
Kupffer cell elimination does not prevent the sequestration of blood-born Ad in the liver. (A) – Southern blot analysis for Ad vector genomes associated with livers of wild type and SR-A-KO mice 1 h after intravenous virus injection. Biological duplicates are shown. Gus - mouse β-glucuronidase gene. C - Control liver DNA from a mouse injected with virus dilution buffer (PBS) only. (B) – Southern blot analysis for Ad vector genomes associated with livers 1 hour post virus injection in wild type mice treated with clodronate liposomes 24 h prior to intravenous virus administration. Biological duplicates are shown. Gus - mouse β-glucuronidase gene. C - Control liver DNA from a mouse injected with virus dilution buffer (PBS) only. N=4.