Abstract
The treatment of rheumatoid arthritis remains suboptimal; thus there is considerable interest in the development of strategies that mediate tolerance to autoantigens. Using lentiviral gene transfer in vivo, we expressed the immunodominant epitope of collagen type II (CII) on major histocompatibility complex class II molecules (MHC II) in a mouse model of destructive arthritis. A sequence corresponding to amino acids 259–270 of CII was fused into the class II–associated invariant chain peptide (CLIP) position of the invariant chain to achieve efficient binding to MHC II. Transduction of cloned cells and primary antigen-presenting cells (APCs) in vitro demonstrated successful presentation of the peptide on MHC II, and a physiological glycosylation pattern. Compared with controls, mice intravenously injected with lentiviral vectors encoding this epitope displayed significantly less frequent, less severe, and less destructive arthritis, decreased lymphocyte proliferation in response to restimulation with CII, and lower CII–specific antibody levels. This was associated with an increased production of transforming growth factor-β (TGF-β) in vitro. We suggest that overexpression of the immunodominant CII epitope on MHC II induces T cell production of TGF-β and leads to inhibition of arthritis by means of both antigen-specific and bystander mechanisms. Thus, antigen-specific tolerance induction using lentiviral gene delivery can ameliorate arthritis.
Introduction
Rheumatoid arthritis (RA) is a chronic and destructive autoimmune disease, which affects about 0.5–1% of the world population. It is not clear what causes this disease, but susceptibility to RA is highly associated with certain major histocompatibility complex class II (MHC II) molecules that share a peptide-binding pocket,1 suggesting that they present specific autoantigenic peptides to T cells, which validates a pathogenic role of autoreactive MHC II-restricted T cells. T cell autoreactivity in RA has been detected against a range of epitopes such as binding protein, RA33, glycosylphosphatidylinositol, and collagen type II (CII).2,3,4,5 Indeed, CII is recognized as one of the major autoantigens in RA because of the high levels of anti-CII antibodies and T cell responses to CII seen in patients with RA.6,7,8 Furthermore, exposure to CII triggers polyarthritis in several species including primates.8,9 CII has been studied extensively in a collagen-induced arthritis (CIA) model in mice.10
During the past few years, the development of new drugs for RA has greatly improved the disease outcome.11 However, these therapies are ineffective in 30–50% of cases and occasionally have severe side-effects.11 A potential new treatment strategy is to induce antigen-specific tolerance. Recent evidence in mice shows that CII is expressed in the thymus and mediates protection against CIA during homeostasis by inducing central tolerance.12 Several attempts using oral CII as a tolerogen for the treatment of RA have met with, at best, limited success,13,14,15 and one of the major problems with this strategy is optimizing the efficient delivery of the tolerogen. However, tolerance to CIA in mice can be temporarily achieved by exposure to CII via different mucosal routes.16,17
In both RA and CIA, there are autoreactive T cells directed against the same CII epitope, namely, the CII amino acid (aa) sequence 259–270 (refs. 2,18,19,20,21,22,23,24), where the lysine at position 264 (K264) can be hydroxylated and further glycosylated with mono- or disaccharides. We hypothesized that an endogenously increased expression of CII aa259–270 on resting antigen-presenting cells (APCs) would promote tolerance, and lead to amelioration of CIA. We inserted nucleotides encoding CII aa259–270 in the class II–associated invariant chain peptide (CLIP)-encoding position of the invariant chain (Ii) gene25,26 using a lentiviral vector as the delivery system to increase endogenous loading of MHC II with the CII peptide. Intravenously injected lentiviral vectors result in stable chromosomal integration in the transduced cells, have a high transduction efficiency, and do not cause an inflammatory response by themselves.27 We observed significant amelioration of the severity of CIA, and increased production of transforming growth factor-β (TGF-β) in vitro, indicating both antigen-specific and bystander tolerance mechanisms were involved.
Results
The tolerization constructs
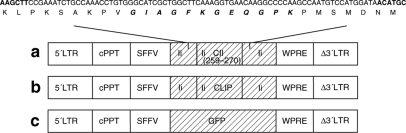
We generated lentiviral constructs (LNT) containing the spleen focus forming virus (SFFV) long terminal repeat (LTR) promoter, and a woodchuck post-transcriptional regulatory element for expression of the following cassettes: (i) LNT-Ii-CII, in which nucleotides encoding CII aa259–270 were inserted in the CLIP-encoding position of the Ii gene (Figure 1a); (ii) LNT-Ii-CLIP, a control construct in which the original Ii with CLIP was intact (Figure 1b); (iii) LNT-GFP, a second control construct in which enhanced green fluorescent protein (eGFP) driven by the SFFV promoter was used (Figure 1c).
Figure 1.
The lentivirus constructs used in this study. (a) LNT-Ii-CII, (b) LNT-Ii-CLIP, and (c) LNT-GFP. LNT (lentivirus), Ii (invariant chain), CII (collagen type II amino acids 259–270), CLIP (class II–associated invariant chain peptide). WPRE, woodchuck post-transcriptional regulatory element; cPPT, central polypurine tract; LTR, long terminal repeat.
Glycosylation pattern of the CII peptide in the CLIP position
To determine whether the CII aa259–270 peptide was expressed on MHC II, we transduced primary and cloned cells with LNT-Ii-CLIP and LNT-Ii-CII viral particles, and investigated whether they induced interleukin-2 (IL-2) production in vitro from four T cell hybridomas specific for different glycosylation patterns of the CII peptide.28 To confirm that we could measure appropriate hybridoma responses, we pulsed nontransduced peritoneal cells (B cells and macrophages) with denatured CII. We demonstrated IL-2 production from Hcq3 and Hcq4 hybridomas (data not shown), indicating, as expected, that the denatured CII is present in two forms: either glycosylated/galactosylated or naked. Nonpulsed peritoneal cells did not induce any IL-2 response from the hybridomas. Peritoneal cells and bone marrow–derived dendritic cells transduced with LNT-Ii-CII presented naked/hydroxylated peptides, but did not present glycosylated/galactosylated forms of the CII peptide (Supplementary Table S1). Aap/Abq+ NIH/3T3 cells transduced with LNT-Ii-CII presented both forms of the peptide (Supplementary Table S1). None of the cells transduced with LNT-Ii-CLIP induced IL-2 production from any of the T cell hybridomas, demonstrating that they did not present any form of the CII peptide (data not shown).
In vivo detection of the lentiviral vectors
We used flow cytometry to analyze cells in the bone marrow, spleen, peripheral lymph nodes, and blood from mice 28 days after injection with LNT-Ii-CLIP and LNT-Ii-CII viral particles, and showed that the leukocyte distribution and cell number did not differ between these groups of mice (data not shown).
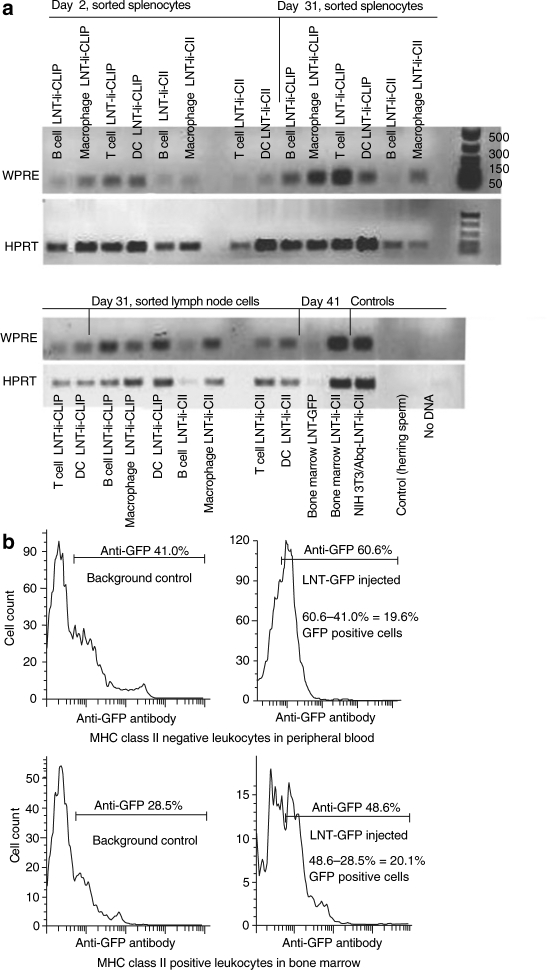
To determine whether lentivirus integration could be detected in vivo in different cell types over a period of several weeks, we removed spleen, lymph nodes, and bone marrow cells at days 2, 31, and 41 after CII immunization from mice injected with LNT-GFP, LNT-Ii-CLIP, and LNT-Ii-CII viral particles 28 days before CII immunization. Cells from the spleen and lymph node were sorted by flow cytometry into B cells (typically 90% pure), T cells (typically 90% pure), dendritic cells (typically 70% pure), and macrophages (typically 70% pure). PCR of the post-transcriptional regulatory element in these cell populations demonstrated integration of the vector (100 bp band) in all cell types examined and at every timepoint (Figure 2a). The amount of PCR product varied between wells, even for the control HPRT. However, we did not correct for these differences as our aim was to demonstrate lentivirus integration and not to compare absolute amounts of product between the different cell populations.
Figure 2.
In vivo detection of the lentiviral vectors. (a) PCR analysis of the woodchuck post-transcriptional regulatory element (WPRE) element in the lentiviral vector in cells sorted from the spleen and lymph node [B cells, T cells, macrophages, dendritic cells (DC)] and in bone marrow cells (not sorted) from mice injected with LNT-Ii-CLIP, LNT-Ii-CII, and LNT-GFP at the indicated times after CII immunization. Aap/Abq+ NIH/3T3 cells stably transfected with LNT-Ii-CII were used as positive controls, and herring sperm DNA (Invitrogen, Sweden), and no DNA were used as negative controls. (b) Flow cytometry analysis of GFP expression in leukocytes in peripheral blood (gated on MHC class II negative cells) and bone marrow (gated on MHC class II positive cells) from control mice (injected with nonvirus) and mice injected with LNT-GFP viral particles 28 days after injection.
We also used flow cytometry to determine transduction efficiency in mice 28 days after injection with LNT-GFP viral particles, and demonstrated that ~20% of both peripheral (MHC class II negative) and bone marrow leukocytes (MHC class II positive) from these mice were positive for GFP (Figure 2b). It is possible that these cells consist partly of dividing repopulating bone marrow–derived cells. No GFP-positive cells were observed in the liver or spleen of these mice (data not shown).
Together, these data show that intravenous injection of lentivirus transduces both bone marrow and peripheral leukocytes. Furthermore, the integrated gene can be detected in a number of cell populations and organs for several weeks.
In vivo gene therapy using LNT-Ii-CII delays the onset and ameliorates the severity and destructivity of arthritis
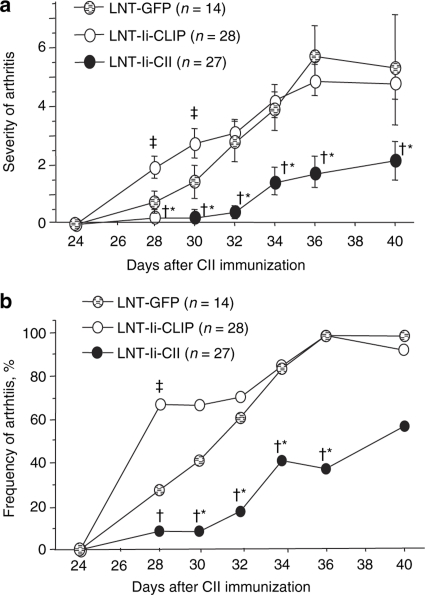
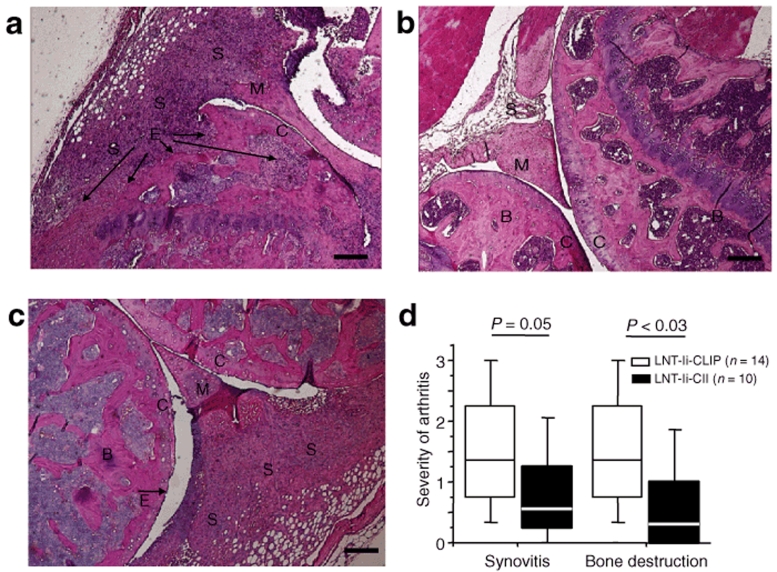
Mice injected with LNT-Ii-CII viral particles 28 days before CII immunization showed a delayed onset of arthritis, which was significantly less severe and less frequent than in mice injected with LNT-Ii-CLIP or LNT-GFP (Figure 3). Histological examination revealed a trend toward less synovitis and significantly less bone destruction in mice injected with LNT-Ii-CII than in mice injected with LNT-Ii-CLIP (Figure 4).
Figure 3.
Severity and frequency of CIA in lentivirus-treated mice. (a) The severity and (b) frequency of arthritis in mice injected with LNT-GFP, LNT-Ii-CLIP, and LNT-Ii-CII viral particles 28 days before CII immunization. Data in a are presented as mean score ± SEM. *P < 0.05 LNT-Ii-CII versus LNT-Ii-CLIP; †P < 0.05 LNT-Ii-CII versus LNT-GFP; ‡P < 0.05 LNT-Ii-CLIP versus LNT-GFP.
Figure 4.
Histology of joints from lentivirus-treated mice with CIA. (a) Destructive arthritis in a knee joint from a mouse injected with LNT-Ii-CLIP viral particles 28 days before CII immunization and killed at day 40 after CII. (b) Nonarthritic and (c) arthritic knee joints from mice injected with LNT-Ii-CII viral particles 28 days before CII immunization and killed at day 40 after CII. (d) Quantification of histological examinations. Box plots represent median and 75th and 95th centile. S = synovia, C = cartilage, B = bone, M = menicus, E = erosions. Scale bar, 50 µm.
Transplantation of LNT-Ii-CII-transduced bone marrow reduces the severity and frequency of arthritis in recipient mice
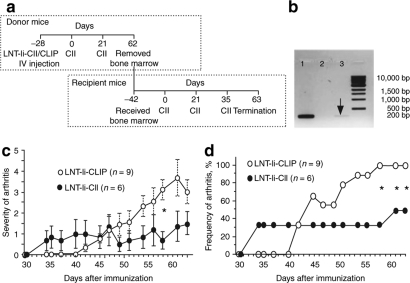
To investigate whether the tolerization effect of LNT-Ii-CII was mediated by bone marrow–derived cells, naive, irradiated mice received bone marrow taken 62 days after CII immunization from mice injected with LNT-Ii-CLIP or LNT-Ii-CII viral particles 28 days before CII immunization (Figure 5a). We first assessed whether LNT-Ii-CII integration could be detected in the donor bone marrow by performing PCR of the Ii-CII fragment and observed a band of the expected size (200 bp) in cells from mice injected with LNT-Ii-CII viral particles, but not from mice injected with LNT-Ii-CLIP (Figure 5b). The recipient mice were immunized with CII 42 days after bone marrow transplantation (Figure 5a). The severity and frequency of arthritis at day 58 post-transplantation CII immunization in mice that received bone marrow from mice injected with LNT-Ii-CII viral particles were reduced compared with mice that received bone marrow from mice injected with LNT-Ii-CLIP (Figure 5c,d).
Figure 5.
Severity and frequency of CIA in mice that received bone marrow cells from lentivirus-treated mice. (a) Bone marrow was taken 62 days after the first CII immunization from mice injected with LNT-Ii-CLIP and LNT-Ii-CII viral particles 28 days before CII immunization. The recipient mice were irradiated 24 hours before intravenous injection with the donor bone marrow and were immunized with CII 42 days following transplantation. They received boosts of CII after a further 21 and 35 days. (b) PCR analysis of the Ii-CII fragment in NIH/3T3 cells stably transduced with LNT-Ii-CII (lane 1) and in bone marrow cells from donor mice injected with LNT-Ii-CLIP (lane 2) and LNT-Ii-CII (lane 3) viral particles at day 62 after CII immunization. (c) The severity and (d) frequency of arthritis in mice that received bone marrow taken 62 days after the first CII immunization from mice injected with LNT-Ii-CLIP and LNT-Ii-CII viral particles 28 days before CII. The experiment has been performed once. Data in (c) are presented as mean score ± SEM. *P < 0.05 LNT-Ii-CII versus LNT-Ii-CLIP.
Anti-CII antibodies are decreased in LNT-Ii-CII-treated mice
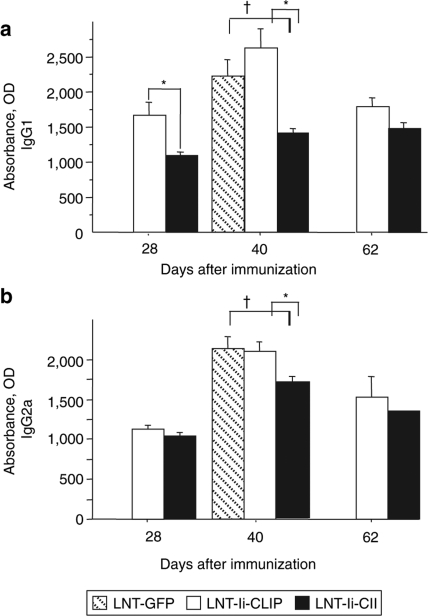
To evaluate the effect of LNT-Ii-CII transduction on the levels of CII-specific antibodies, we analyzed serum at the days indicated from mice injected with LNT-GFP, LNT-Ii-CLIP, or LNT-Ii-CII viral particles 28 days before CII immunization (Figure 6). We observed reduced levels of anti-CII IgG1 in serum from mice injected with LNT-Ii-CII compared with LNT-Ii-CLIP at days 28 and 40, but not at day 62 after CII immunization (Figure 6a). The anti-CII IgG2a levels in serum from mice injected with LNT-Ii-CII were significantly lower compared with LNT-Ii-CLIP at day 40, but not at days 28 or 60 after CII immunization (Figure 6b). Both the anti-IgG1 and anti-IgG2a levels in mice injected with LNT-Ii-CII were also significantly lower compared with LNT-GFP at day 40 (Figure 6a,b).
Figure 6.
Anti–CII-specific antibody levels in serum from lentivirus-treated mice. (a) IgG1 and (b) IgG2a isotype CII–specific antibodies in serum obtained at days 28, 40, and 62 after immunization from mice injected with LNT-Ii-CLIP and LNT-Ii-CII viral particles 28 days before CII immunization. Statistical analyses were performed using Student's t-test. Data are expressed as mean ± SEM. Day 28: LNT-Ii-CLIP (n = 9), LNT-Ii-CII (n = 10); Day 40: LNT-GFP (n = 8), LNT-Ii-CLIP (n = 13), LNT-Ii-CII (n = 11); Day 62: LNT-Ii-CLIP (n = 5), LNT-Ii-CII (n = 5). *P < 0.05 LNT-Ii-CII versus LNT-Ii-CLIP; †P < 0.05 LNT-Ii-CII versus LNT-GFP.
Splenocytes from LNT-Ii-CII-treated mice display decreased proliferation
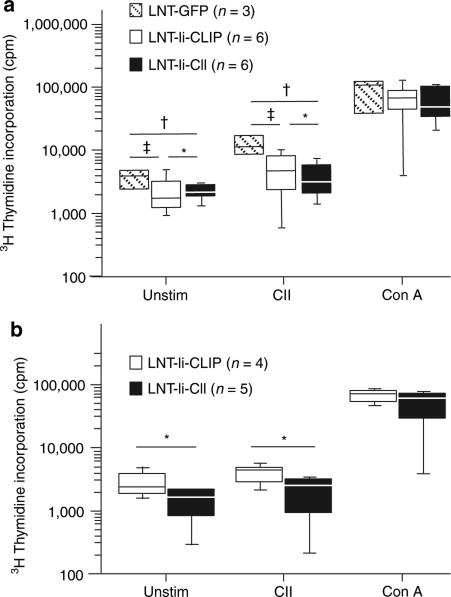
To evaluate the effect of LNT-Ii-CII transduction on antigen specificity, splenocytes were obtained at day 40 after CII immunization from mice injected with LNT-GFP, LNT-Ii-CLIP, or LNT-Ii-CII viral particles 28 days before CII immunization. Mice injected with LNT-Ii-CII displayed lower proliferation of both unstimulated splenocytes and splenocytes stimulated with denatured CII compared with LNT-GFP-injected mice (Figure 7a). Mice injected with LNT-Ii-CLIP also displayed lower proliferation of both unstimulated splenocytes and splenocytes stimulated with denatured CII compared with LNT-GFP-injected mice, suggesting that LNT-Ii-CLIP might have an effect on its own. However, the proliferation in response to denatured CII was lower in mice injected with LNT-Ii-CII compared with LNT-Ii-CLIP-injected mice (Figure 7a).
Figure 7.
Proliferation of splenocytes from lentivirus-treated mice and from mice that received bone marrow cells from lentivirus-treated mice. Proliferation of splenocytes taken (a) 40 days after CII immunization from mice injected with LNT-GFP, LNT-Ii-CLIP, and LNT-Ii-CII viral particles and (b) 63 days after CII immunization from mice that received bone marrow from mice injected with LNT-Ii-CLIP and LNT-Ii-CII viral particles. Splenocytes were cultured without any stimuli or in the presence of denatured CII (50 µg/ml) or, as a positive control, Con A (1.25 µg/ml) for 72 hours. Box plots represent median and 75th and 95th centile. Statistical analyses were performed using Student's t-test. *P < 0.05 LNT-Ii-CII versus LNT-Ii-CLIP; †P < 0.05 LNT-Ii-CII versus LNT-GFP; ‡P < 0.05 LNT-Ii-CLIP versus LNT-GFP.
We also transplanted bone marrow from mice injected with LNT-Ii-CLIP and LNT-Ii-CII viral particles into naive, irradiated recipient mice, and observed that the proliferation of both unstimulated splenocytes and splenocytes stimulated with denatured CII was lower in mice that received LNT-Ii-CII-transduced bone marrow compared with mice that received LNT-Ii-CLIP-transduced bone marrow (Figure 7b).
Overexpression of the CII epitope skews the immune response toward a regulatory T cell phenotype
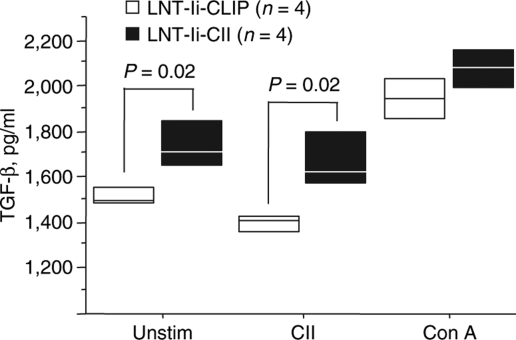
We then investigated whether the amelioration of arthritis seen in the LNT-Ii-CII–transduced mice could be caused by a changed cytokine pattern or induction of natural regulatory T cells. Splenocytes were obtained at day 40 after CII immunization from mice injected with LNT-Ii-CLIP or LNT-Ii-CII viral particles 28 days before CII immunization. We observed a significantly higher production of TGF-β from both unstimulated splenocytes and splenocytes stimulated with denatured CII from mice injected with LNT-Ii-CII compared with LNT-Ii-CLIP-injected mice (Figure 8). There were no differences between the groups with respect to interferon-γ (IFN-γ), IL-12, and IL-17 production in supernatants measured by enzyme-linked immunosorbent assay (data not shown). We did not detect IL-4 production, and we measured IL-10 levels that were barely over detection limit and, therefore, difficult to interpret (data not shown). Mice injected with LNT-Ii-CII viral particles and those that received bone marrow from mice previously injected with LNT-Ii-CII viral particles displayed a trend toward increased numbers of CD4+Foxp3+ T cells in spleen and lymph nodes compared with control mice, but the differences were not statistically significant (data not shown).
Figure 8.
TGF-β production from splenocytes from lentivirus-treated mice. Splenocytes were taken 40 days after CII immunization from mice injected with LNT-Ii-CLIP and LNT-Ii-CII viral particles and cultured without any stimuli or in the presence of denatured CII (50 µg/ml) or, as a positive control, Con A (1.25 µg/ml) for 72 hours. Box plots represent median and 75th centile. Statistical analyses were performed using Student's t-test.
Discussion
This report represents the first successful attempt to use lentivirus technology to induce tolerance as a rheumatic disease treatment. We used a lentiviral construct to mediate expression of the immunodominant CII peptide inserted in the Ii chain, and demonstrated in vitro expression of various post-translational forms of the peptide presented on the MHC class II molecule. We showed integration of the vectors in vivo in different cell populations over a time period of several weeks. Importantly, in vivo expression of LNT-Ii-CII led to reduction of arthritis, measured both by evaluation of erythema and joint swelling in mice in vivo and by histological observation of cartilage/bone destruction. The reduction of arthritis may, at least partly, be caused by a decreased auto-antigen–specific response as we demonstrated decreased splenocyte reactivity to CII as well as lower levels of CII-specific antibodies in serum from mice injected with LNT-Ii-CII viral particles. Interestingly, the immune responses were skewed toward a regulatory phenotype with increased levels of TGF-β.
It is well known that pretreatment with soluble CII or with its peptides suppresses the development of arthritis.29,30,31,32,33,34 One problem is that injected peptides are rapidly degraded in vivo and thereby become less effective with time. To overcome this hurdle, we used a lentiviral approach, which provides permanent integration and long-lasting expression. In our study, CII was presented on MHC II primary cells (B cells, dendritic cells, and macrophages) in a naked or hydroxylated form, but not in a glycosylated or galactosylated form. A previous study by Dzhambazov et al. has shown that glycosylation of CII is required to maintain tolerance.35 Thus, it is possible that the absence of CII glycosylation seen in our study partly explains why the effect of the LNT-Ii-CII treatment on the development of arthritis was reduced at later time points.
Gene therapy has been performed in animal models of RA using a range of viral vectors, which have mainly encoded anti-cytokine proteins and been administrated intra-articularly.36,37 Systemic administration of lentiviral vectors to induce antigen-specific tolerance in RA has not been described earlier. It has been shown previously that transgene expression remains high in peripheral bone marrow–derived leukocytes 40 days after intravenous administration of lentivirus.38 By amplifying part of the lentiviral backbone that integrates, we showed vector integration in B cells, T cells, dendritic cells, and macrophages in spleen and lymph nodes at up to 69 days after injection with the lentivirus. Unfortunately, there is no available antibody for the CII peptide in the MHC II pocket, and thus it was not possible to detect the lentiviral constructs at the protein level. Instead, we used GFP driven by the SFFV promoter and injected intravenously as a surrogate marker. We demonstrated expression of GFP in peripheral blood and bone marrow by flow cytometry 28 days after injection of the virus (i.e., the same time as the first CII immunization). Although we observed integration of the vector in the spleen using PCR, we did not show GFP expression in the spleen. This apparent contradiction may be explained by the fact that the sensitivity of analysis by flow cytometry is considerably lower than that for PCR, and the low dose of virus that we used to achieve tolerization was too low to be detected by the less sensitive method.
Our data indicate that there was a sustained expression of the transgenes over a considerable period of time. Thus, the reduced effect of LNT-Ii-CII treatment on the development of arthritis at later time points cannot be explained by reduced vector expression over the time period used in our study. Furthermore, by amplifying the Ii-CII fragment, we showed integration of LNT-Ii-CII in bone marrow cells from mice 90 days after injection with the lentivirus. We also showed that transplantation of these bone marrow cells to lethally irradiated recipient mice mediated a tolerogenic response both in vivo, shown by reduced severity and frequency of arthritis, and in vitro, shown by decreased splenocyte proliferation both in the absence of stimulus and in response to CII.
The aim of this study was to achieve CII tolerization rather than CII immunization. Thus, our goal was to express the CII peptide on APCs before the CII immunization, thereby bypassing simultaneous expression of costimulatory molecules. Overexpression of a peptide in this way has the capacity to induce TGF-β-producing T cells.39,40 TGF-β is a regulatory cytokine that actively participates in the development and maintenance of tolerance.39,40 In our study, we found increased production of TGF-β from both unstimulated and CII-stimulated splenocytes from mice injected with LNT-Ii-CII viral particles. These data are supported by previous findings by Thorbecke et al., demonstrating that systemic administration of TGF-β inhibits CIA.41 Furthermore, LNT-Ii-CII treatment led to significantly decreased proliferation of CII-stimulated splenocytes and levels of CII-specific antibodies, which might at least partly be mediated by the increased TGF-β levels. Mice injected with LNT-Ii-CLIP also showed a decreased proliferation of both unstimulated and CII-stimulated splenocytes compared with LNT-GFP-injected mice, suggesting that LNT-Ii-CLIP might have an immune effect on its own. However, this immune effect did not translate into a beneficial tolerogenic effect as the severity and frequency of arthritis were no different in LNT-Ii-CLIP- and LNT-GFP–treated mice, except at the earliest time points when we in fact observed a greater extent of arthritis in the LNT-Ii-CLIP–treated mice.
The effect of any vaccination will be heavily dependent on the environment in vivo during the administration of the specific antigen. The expression of lentivirus is known to give limited stress to the cells and thereby only minimally expose them to the immune system, in comparison with many other gene therapy protocols including adenoviruses or naked DNA injections.36,37 Indeed, gene therapy using lentiviral vectors has been successfully used in clinical settings.42 The minimal impact of the transgene on non-antigen–specific immune activation is a very important requirement concerning future human gene therapy, especially in the case of inflammatory diseases such as RA. In this regard, lentivirus-mediated delivery of genes has the potential to be an ideal way of inducing tolerance in autoimmune diseases.
Materials and Methods
Generation of constructs. Generation of pHR'SIN-cPPT-SEW (LNT-GFP) has been previously described.43 The cDNA-encoding mouse Ii (p31; provided by Lars Karlsson, Johnson Pharmaceutical Research Institute, San Diego, CA) was amplified using primers 5′-GTT CTC GAG TCT TCA CAG GGT GAC TTG and 3′-AGG AAT TCA CTA GAG GCT AGA GCC AT and subcloned into a Topo Zero-blunt vector (Invitrogen, Loughborough, UK). Ii was digested using EcoRI and XhoI and further subcloned into pBS (SK+) where the pre-existing NspI site had been destroyed by inserting a synthetic oligo 5′-GTA GAT CTG TCA TG (Invitrogen). To generate the construct Ii-CII, CLIP in Ii was replaced with oligonucleotides encoding aa259–270 from rat CII (5′-AGC TTC CGA AAT CTG CCA AAC CTG TGG GCA TCG CTG TCA AAG GTG AAC AAG GCC CCA AGC CAA TGT CCA TGG ATA ACA TG, Invitrogen) by digestion with NspI and HindIII. The original Ii-CLIP was used as a control construct. Ii-CLIP and Ii-CII were liberated by digestion with BamHI and XhoI, ligated into BamHI/XhoI-digested pENTR1A (Gateway, Invitrogen), and inserted into the HIV-1–based lentivirus destination vector pHR'SIN-Gateway (which contains the same controlling elements as pHR'SINcPPT SEW but the Gateway recombination cassette replaces GFP) by Gateway LR clonase reaction (Invitrogen). The final products are termed LNT-GFP, LNT-Ii-CLIP, and LNT-Ii-CII, and contain the SFFV LTR promoter (Figure 1).
Lentivirus production. See Supplementary Materials and Methods.
Testing of lentiviral particles in vitro. See Supplementary Materials and Methods.
Intravenous injection of lentivirus and induction of arthritis. For the in vivo experiments, we used male DBA/1 mice at 7–10 weeks of age, which were bred and maintained (10 per cage) under standard conditions of temperature and light. LNT-Ii-CLIP, LNT-Ii-CII, and LNT-GFP viral particles (5 × 106 particles) were injected into the tail vein of DBA/1 mice in a total volume of 200 µl, and the mice were either killed or immunized with 100 µg rat CII emulsified in 0.1 mol/l HAc and complete Freund's adjuvant (Sigma-Aldrich, Stockholm, Sweden) CII after 28 days. Boosts of 100 µg rat CII in incomplete Freund's adjuvant were given 21 days after the primary immunization. Mice were assessed for severity of arthritis (see below) and killed on the indicated day after the first CII immunization. Blood, bone marrow, spleen, lymph nodes, and paws were taken for analysis.
For bone marrow transfer, recipient mice were irradiated (10 Gy) 24 hours before intravenous injection with bone marrow cells (6 × 106) taken at day 62 after CII immunization from mice injected with LNT-Ii-CII and LNT-Ii-CLIP viral particles. Both groups of recipient mice were immunized with CII 42 days after bone marrow transplantation, and received boosts of CII 21 and 35 days after the primary immunization (Figure 5a). Mice were assessed for severity of arthritis (see below) on the indicated days after the first CII immunization.
Permission from the local animal research ethics committee, in accordance with national animal welfare legislation, was obtained for all the mice experiments.
Analysis of leukocyte distribution and cell number. Single-cell suspensions from bone marrow, spleen, peripheral lymph nodes, and blood taken from mice 28 days after injection with LNT-Ii-CII and LNT-Ii-CLIP viral particles were stained with PE-I-A/I-E (MHC II), PerCP-CD3 (T cells), and APC-CD19 (B cells) (BD, Pharmingen, Sweden) and analyzed by flow cytometry (FACSCalibur) using FlowJo Software (Tree Star, Ashland, OR).
Assessment of in vivo gene integration by PCR. To detect vector integration in different cell populations and at different timepoints, bone marrow, spleen, and lymph nodes were taken from mice injected with LNT-Ii-CLIP, LNT-Ii-CII, and LNT-GFP at days 2, 31, and 41 after CII immunization. Cell populations in spleen and lymph nodes were sorted by flow cytometry (FACSAria II, BD). Cell suspensions were prepared at a concentration of 20 × 106 cells/ml in PBS and 5% FCS. Fc-receptors were blocked using Fc-block, and the cells were stained with antibodies detecting FITC-CD3, PE-CD19, APC-CD11c (dendritic cells) (BD), and Pacific blue-F4/80 (macrophages) (eBioscience, Stockholm, Sweden). DNA from the sorted populations and nonsorted bone marrow cells was purified using standard procedures, and 1 ng/µl was used in all reactions. The woodchuck post-transcriptional regulatory element sequence in the integrating part of the lentiviral backbone was amplified using the forward primer 5′ GGC ACT GAC AAT TCC GTG GT and the reverse primer 5′AGG GAC GTA GCA GAA GGA CG (Invitrogen). Aap/Abq+ NIH/3T3 cells stably transduced with LNT-Ii-CII were used as positive controls and herring sperm DNA (Invitrogen), and no DNA were used as negative controls.
To confirm the presence of genomic DNA from each picked colony and to determine transduction efficiency, we also performed a PCR of exon 9 of the murine housekeeping gene hypoxanthine phosphoribosyl transferase using the forward primer 5′ TCC CCA GAC TTT TGA TTT GC 3′ and the reverse primer 5′ GGA AAA TAC AGC CAA CAC TGC 3′. The reaction was performed at an annealing temperature of 58 °C and amplified as a 324 bp product.
Assessment of in vivo gene expression by flow cytometry. To determine expression of GFP, single-cell suspensions from bone marrow and blood taken from mice 28 days after injection with LNT-GFP viral particles and nonvirus were stained with PE-anti-I-A/I-E, PerCP anti-CD3, APC-anti-CD19 (BD), and anti-GFP (Invitrogen, Taastrup, Denmark). To determine Foxp3 expression in spleen and lymph node T cells, single-cell suspensions were stained with anti-CD4 (BD), washed in FACS buffer, and fixed in 2% paraformaldehyde in 4 °C overnight. The following morning, the cells were permeabilized with a permeabilization kit (eBioscience) and stained with anti-Foxp3 (eBioscience). All samples were analyzed by flow cytometry (FACSCalibur) and FlowJo Software.
Assessment of in vivo gene integration in donor bone marrow by PCR. To detect lentivirus integration in donor bone marrow used in the transplantation study (Figure 5a), bone marrow was taken at day 62 after CII immunization from mice injected with LNT-Ii-CII and LNT-Ii-CLIP viral particles. DNA from 3 × 106 bone marrow cells was extracted by standard procedures. The Ii-CII fragment was amplified using the forward primer 5′-GGC AAC CGC CCT AGA GAG C-3′ and the reverse primer 5′-ATG GAC ATT GGC TTG GG -3′ (MWG Biotech AG), which cover the junction between the CII and Ii sequences and ensure specific amplification of the inserted DNA. NIH/3T3 cells stably transduced with LNT-Ii-CII were used as a positive control.
Arthritis evaluation. Arthritis severity and frequency were graded blindly at the indicated times after CII immunization in mice injected with LNT-GFP, LNT-Ii-CLIP, and LNT-Ii-CII viral particles. All the mice were followed up individually and arthritis (defined as visible erythema and/or joint swelling) was evaluated by inspection of finger/toe and ankle/wrist joints. Severity of arthritis was evaluated by macroscopic inspection of each limb, and a score was assigned for each limb: 0, neither swelling nor erythema; 1, mild swelling and/or erythema; 2, moderate swelling and erythema; and 3, marked swelling and erythema. The total score for each mouse was calculated by adding up the scores for each limb.
Histological examination of inflamed joints. Histopathologic examination of the joints was performed after routine fixation, decalcification, and paraffin embedding. Tissue sections from fore and hind paws were cut and stained with hematoxylin–eosin. All the slides were coded and evaluated by two blinded observers. The specimens were evaluated with regard to synovial hypertrophy, pannus formation, and cartilage/subchondral bone destruction. The degree of synovitis and destruction in every joint concerning finger/toes, wrists/ankles, elbows, and knees was assigned a score from 0 to 3. Occasionally one paw was missing in the histological sections, or embedded in such a way that it was impossible to evaluate the degree of synovitis and bone/cartilage destruction. Therefore, the total score per mouse was divided by the number of joints evaluated.
CII-specific antibodies in serum. Levels of CII-specific IgG1 and IgG2a in serum taken at the indicated times after CII immunization from mice injected with LNT-GFP, LNT-Ii-CLIP, and LNT-Ii-CII viral particles were determined as described earlier44 using biotinylated rat antimouse IgG1 or biotinylated rat antimouse IgG2a both at 0.5 µg/ml (Serotec, Oxford, UK).
In vitro splenocyte proliferation. Proliferation was measured in splenocytes taken (i) 40 days after CII immunization from mice injected with LNT-GFP, LNT-Ii-CLIP, and LNT-Ii-CII viral particles; and (ii) 63 days after CII immunization from mice that received bone marrow from mice injected with LNT-Ii-CLIP and LNT-Ii-CII viral particles (Figure 5a). A single-cell suspension was made in complete Iscoves medium at 106 cells/ml and plated in flat-bottomed tissue culture plates. The cells were cultured without any stimuli or in the presence of CII (50 µg/ml) or concanavalin A (Con A, 1.25 µg/ml) for 72 hours. 1 µCi [3H]-thymidine was then added to each well and the plates cultured for another 16–20 hours before harvest. Each sample was tested in triplicate.
Cytokine assay. Cytokine activity was measured in splenocytes taken 40 days after CII immunization from mice injected with LNT-Ii-CLIP and LNT-Ii-CII viral particles and cultured without any stimuli or in the presence of CII (50 µg/ml) or Con A (1.25 µg/ml) for 72 hours (as above). The supernatant was harvested at 72 hours, frozen at −20 °C, and cytokines (TGF-β, IL-4, IL-10, IL-12, IL-17, and IFN-γ) were measured by sandwich enzyme-linked immunosorbent assay (R&D Systems, Abingdon, UK) according to the manufacturer's recommendation.
Statistical analyses. Arthritis frequency was evaluated by Fisher's exact test. The remaining data comparing two groups were analyzed by Mann–Whitney U-test, unless otherwise stated. A P value <0.05 was considered to be significant.
Supplementary MaterialTable S1. Glycosylation patterns in cells transduced with LNT-Ii-CII viral particles.Supplementary Materials and Methods.
Supplementary Material
Glycosylation patterns in cells transduced with LNT-Ii-CII viral particles.
Acknowledgments
We thank Dr Rosie Perkins for editing the manuscript. This study was supported by grants from the Swedish Medical Research Council, Göteborgs Läkaresällskap, King Gustav V's 80 Year Foundation, Reumatikerförbundet i Göteborg, Professor Nanna Svartz Foundation, Thölen and Kristler Foundation, the Swedish Foundation for Strategic Research, the 6th Framework Program of the European Union, NeuroproMiSe, LSHM-CT-2005-01863, AUTOCURE, LSHM-CT-2005-018661, and Arthritis Research Campaign (ARC) UK. Lentiviral packaging plasmids were produced by the Plasmid Factory.
REFERENCES
- Fernando MM, Stevens CR, Walsh EC, De Jager PL, Goyette P, Plenge RM, et al. Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genet. 2008;4:e1000024. doi: 10.1371/journal.pgen.1000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backlund J, Carlsen S, Hoger T, Holm B, Fugger L, Kihlberg J, et al. Predominant selection of T cells specific for the glycosylated collagen type II epitope (263-270) in humanized transgenic mice and in rheumatoid arthritis. Proc Natl Acad Sci USA. 2002;99:9960–9965. doi: 10.1073/pnas.132254199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook AD, Rowley MJ, Mackay IR, Gough A., and , Emery P. Antibodies to type II collagen in early rheumatoid arthritis. Correlation with disease progression. Arthritis Rheum. 1996;39:1720–1727. doi: 10.1002/art.1780391015. [DOI] [PubMed] [Google Scholar]
- Corrigall VM, Bodman-Smith MD, Fife MS, Canas B, Myers LK, Wooley P, et al. The human endoplasmic reticulum molecular chaperone BiP is an autoantigen for rheumatoid arthritis and prevents the induction of experimental arthritis. J Immunol. 2001;166:1492–1498. doi: 10.4049/jimmunol.166.3.1492. [DOI] [PubMed] [Google Scholar]
- Fritsch R, Eselbock D, Skriner K, Jahn-Schmid B, Scheinecker C, Bohle B, et al. Characterization of autoreactive T cells to the autoantigens heterogeneous nuclear ribonucleoprotein A2 (RA33) and filaggrin in patients with rheumatoid arthritis. J Immunol. 2002;169:1068–1076. doi: 10.4049/jimmunol.169.2.1068. [DOI] [PubMed] [Google Scholar]
- Boissier MC, Chiocchia G, Texier B., and , Fournier C. Pattern of humoral reactivity to type II collagen in rheumatoid arthritis. Clin Exp Immunol. 1989;78:177–183. [PMC free article] [PubMed] [Google Scholar]
- Corrigall VM., and , Panayi GS. Autoantigens and immune pathways in rheumatoid arthritis. Crit Rev Immunol. 2002;22:281–293. [PubMed] [Google Scholar]
- Kim WU, Cho ML, Jung YO, Min SY, Park SW, Min DJ, et al. Type II collagen autoimmunity in rheumatoid arthritis. Am J Med Sci. 2004;327:202–211. doi: 10.1097/00000441-200404000-00006. [DOI] [PubMed] [Google Scholar]
- Wang D, Hill JA, Cairns E., and , Bell DA. The influence of HLA-DR4 (0401) on the immune response to type II collagen and the development of collagen induced arthritis in mice. J Autoimmun. 2002;18:95–103. doi: 10.1006/jaut.2001.0569. [DOI] [PubMed] [Google Scholar]
- Cho YG, Cho ML, Min SY., and , Kim HY. Type II collagen autoimmunity in a mouse model of human rheumatoid arthritis. Autoimmun Rev. 2007;7:65–70. doi: 10.1016/j.autrev.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Scheinecker C, Redlich K., and , Smolen JS. Cytokines as therapeutic targets: advances and limitations. Immunity. 2008;28:440–444. doi: 10.1016/j.immuni.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Chin RK, Zhu M, Christiansen PA, Liu W, Ware C, Peltonen L, et al. Lymphotoxin pathway-directed, autoimmune regulator-independent central tolerance to arthritogenic collagen. J Immunol. 2006;177:290–297. doi: 10.4049/jimmunol.177.1.290. [DOI] [PubMed] [Google Scholar]
- Trentham DE, Dynesius-Trentham RA, Orav EJ, Combitchi D, Lorenzo C, Sewell KL, et al. Effects of oral administration of type II collagen on rheumatoid arthritis. Science. 1993;261:1727–1730. doi: 10.1126/science.8378772. [DOI] [PubMed] [Google Scholar]
- Barnett ML, Kremer JM, St Clair EW, Clegg DO, Furst D, Weisman M, et al. Treatment of rheumatoid arthritis with oral type II collagen. Results of a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 1998;41:290–297. doi: 10.1002/1529-0131(199802)41:2<290::AID-ART13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Choy EH, Scott DL, Kingsley GH, Thomas S, Murphy AG, Staines N, et al. Control of rheumatoid arthritis by oral tolerance. Arthritis Rheum. 2001;44:1993–1997. doi: 10.1002/1529-0131(200109)44:9<1993::AID-ART347>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Nagler-Anderson C, Bober LA, Robinson ME, Siskind GW., and , Thorbecke GJ. Suppression of type II collagen-induced arthritis by intragastric administration of soluble type II collagen. Proc Natl Acad Sci USA. 1986;83:7443–7446. doi: 10.1073/pnas.83.19.7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkowski A, Sun JB, Holmdahl R, Holmgren J., and , Czerkinsky C. Treatment of experimental autoimmune arthritis by nasal administration of a type II collagen-cholera toxoid conjugate vaccine. Arthritis Rheum. 1999;42:1628–1634. doi: 10.1002/1529-0131(199908)42:8<1628::AID-ANR10>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Rosloniec EF, Whittington KB, Zaller DM., and , Kang AH. HLA-DR1 (DRB1*0101) and DR4 (DRB1*0401) use the same anchor residues for binding an immunodominant peptide derived from human type II collagen. J Immunol. 2002;168:253–259. doi: 10.4049/jimmunol.168.1.253. [DOI] [PubMed] [Google Scholar]
- Andersson EC, Hansen BE, Jacobsen H, Madsen LS, Andersen CB, Engberg J, et al. Definition of MHC and T cell receptor contacts in the HLA-DR4 restricted immunodominant epitope in type II collagen and characterization of collagen-induced arthritis in HLA-DR4 and human CD4 transgenic mice. Proc Natl Acad Sci USA. 1998;95:7574–7579. doi: 10.1073/pnas.95.13.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessen A, Lawrence CM, Cupo S, Zaller DM., and , Wiley DC. X-ray crystal structure of HLA-DR4 (DRA*0101, DRB1*0401) complexed with a peptide from human collagen II. Immunity. 1997;7:473–481. doi: 10.1016/s1074-7613(00)80369-6. [DOI] [PubMed] [Google Scholar]
- Diab BY, Lambert NC, L'Faqihi FE, Loubet-Lescoulie P, de Preval C., and , Coppin H. Human collagen II peptide 256-271 preferentially binds to HLA-DR molecules associated with susceptibility to rheumatoid arthritis. Immunogenetics. 1999;49:36–44. doi: 10.1007/s002510050461. [DOI] [PubMed] [Google Scholar]
- Kjellen P, Brunsberg U, Broddefalk J, Hansen B, Vestberg M, Ivarsson I, et al. The structural basis of MHC control of collagen-induced arthritis; binding of the immunodominant type II collagen 256-270 glycopeptide to H-2Aq and H-2Ap molecules. Eur J Immunol. 1998;28:755–767. doi: 10.1002/(SICI)1521-4141(199802)28:02<755::AID-IMMU755>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Michaelsson E, Andersson M, Engstrom A., and , Holmdahl R. Identification of an immunodominant type-II collagen peptide recognized by T cells in H-2q mice: self tolerance at the level of determinant selection. Eur J Immunol. 1992;22:1819–1825. doi: 10.1002/eji.1830220722. [DOI] [PubMed] [Google Scholar]
- Rosloniec EF, Whittington KB, Brand DD, Myers LK., and , Stuart JM. Identification of MHC class II and TCR binding residues in the type II collagen immunodominant determinant mediating collagen-induced arthritis. Cell Immunol. 1996;172:21–28. doi: 10.1006/cimm.1996.0210. [DOI] [PubMed] [Google Scholar]
- Koch N, van Driel IR., and , Gleeson PA. Hijacking a chaperone: manipulation of the MHC class II presentation pathway. Immunol Today. 2000;21:546–550. doi: 10.1016/s0167-5699(00)01717-5. [DOI] [PubMed] [Google Scholar]
- Fujii S, Senju S, Chen YZ, Ando M, Matsushita S., and , Nishimura Y. The CLIP-substituted invariant chain efficiently targets an antigenic peptide to HLA class II pathway in L cells. Hum Immunol. 1998;59:607–614. doi: 10.1016/s0198-8859(98)00058-5. [DOI] [PubMed] [Google Scholar]
- Srinivasakumar N. HIV-1 vector systems. Somat Cell Mol Genet. 2001;26:51–81. doi: 10.1023/a:1021074613196. [DOI] [PubMed] [Google Scholar]
- Corthay A, Backlund J, Broddefalk J, Michaelsson E, Goldschmidt TJ, Kihlberg J, et al. Epitope glycosylation plays a critical role for T cell recognition of type II collagen in collagen-induced arthritis. Eur J Immunol. 1998;28:2580–2590. doi: 10.1002/(SICI)1521-4141(199808)28:08<2580::AID-IMMU2580>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Garcia G, Komagata Y, Slavin AJ, Maron R., and , Weiner HL. Suppression of collagen-induced arthritis by oral or nasal administration of type II collagen. J Autoimmun. 1999;13:315–324. doi: 10.1006/jaut.1999.0320. [DOI] [PubMed] [Google Scholar]
- Higuchi K, Kweon MN, Fujihashi K, McGhee JR., and , Kiyono H. Comparison of nasal and oral tolerance for the prevention of collagen induced murine arthritis. J Rheumatol. 2000;27:1038–1044. [PubMed] [Google Scholar]
- Honda A, Ametani A, Matsumoto T, Iwaya A, Kano H, Hachimura S, et al. Vaccination with an immunodominant peptide of bovine type II collagen induces an anti-TCR response, and modulates the onset and severity of collagen-induced arthritis. Int Immunol. 2004;16:737–745. doi: 10.1093/intimm/dxh075. [DOI] [PubMed] [Google Scholar]
- Khare SD, Krco CJ, Griffiths MM, Luthra HS., and , David CS. Oral administration of an immunodominant human collagen peptide modulates collagen-induced arthritis. J Immunol. 1995;155:3653–3659. [PubMed] [Google Scholar]
- Staines NA, Harper N, Ward FJ, Malmstrom V, Holmdahl R., and , Bansal S. Mucosal tolerance and suppression of collagen-induced arthritis (CIA) induced by nasal inhalation of synthetic peptide 184-198 of bovine type II collagen (CII) expressing a dominant T cell epitope. Clin Exp Immunol. 1996;103:368–375. doi: 10.1111/j.1365-2249.1996.tb08289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Li XY, Wang HK, Jia JF, Zheng ZH, Ding J, et al. Oral administration of type-II collagen peptide 250-270 suppresses specific cellular and humoral immune response in collagen-induced arthritis. Clin Immunol. 2007;122:75–84. doi: 10.1016/j.clim.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Dzhambazov B, Nandakumar KS, Kihlberg J, Fugger L, Holmdahl R., and , Vestberg M. Therapeutic vaccination of active arthritis with a glycosylated collagen type II peptide in complex with MHC class II molecules. J Immunol. 2006;176:1525–1533. doi: 10.4049/jimmunol.176.3.1525. [DOI] [PubMed] [Google Scholar]
- Evans CH, Ghivizzani SC., and , Robbins PD. Gene therapy for arthritis: what next. Arthritis Rheum. 2006;54:1714–1729. doi: 10.1002/art.21886. [DOI] [PubMed] [Google Scholar]
- Adriaansen J, Vervoordeldonk MJ., and , Tak PP. Gene therapy as a therapeutic approach for the treatment of rheumatoid arthritis: innovative vectors and therapeutic genes. Rheumatology (Oxford) 2006;45:656–668. doi: 10.1093/rheumatology/kel047. [DOI] [PubMed] [Google Scholar]
- Pan D, Gunther R, Duan W, Wendell S, Kaemmerer W, Kafri T, et al. Biodistribution and toxicity studies of VSVG-pseudotyped lentiviral vector after intravenous administration in mice with the observation of in vivo transduction of bone marrow. Mol Ther. 2002;6:19–29. doi: 10.1006/mthe.2002.0630. [DOI] [PubMed] [Google Scholar]
- Lan RY, Ansari AA, Lian ZX., and , Gershwin ME. Regulatory T cells: development, function and role in autoimmunity. Autoimmun Rev. 2005;4:351–363. doi: 10.1016/j.autrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Sanjabi S, Robertson AK., and , Flavell RA. Transforming growth factor-β regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- Thorbecke GJ, Shah R, Leu CH, Kuruvilla AP, Hardison AM., and , Palladino MA. Involvement of endogenous tumor necrosis factor alpha and transforming growth factor β during induction of collagen type II arthritis in mice. Proc Natl Acad Sci USA. 1992;89:7375–7379. doi: 10.1073/pnas.89.16.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci USA. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaison C, Parsley K, Brouns G, Scherr M, Battmer K, Kinnon C, et al. High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther. 2002;13:803–813. doi: 10.1089/10430340252898984. [DOI] [PubMed] [Google Scholar]
- Verdrengh M, Jonsson IM, Holmdahl R., and , Tarkowski A. Genistein as an anti-inflammatory agent. Inflamm Res. 2003;52:341–346. doi: 10.1007/s00011-003-1182-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Glycosylation patterns in cells transduced with LNT-Ii-CII viral particles.