Abstract
Hematopoietic cell gene therapy using retroviral vectors has achieved success in clinical trials. However, safety issues regarding vector insertional mutagenesis have emerged. In two different trials, vector insertion resulted in the transcriptional activation of proto-oncogenes. One strategy for potentially diminishing vector insertional mutagenesis is through the use of self-inactivating lentiviral vectors containing the 1.2-kb insulator element derived from the chicken β-globin locus. However, use of this element can dramatically decrease both vector titer and transgene expression, thereby compromising its practical use. Here, we studied lentiviral vectors containing either the full-length 1.2-kb insulator or the smaller 0.25-kb core element in both orientations in the partially deleted long-terminal repeat. We show that use of the 0.25-kb core insulator rescued vector titer by alleviating a postentry block to reverse transcription associated with the 1.2-kb element. In addition, in an orientation-dependent manner, the 0.25-kb core element significantly increased transgene expression from an internal promoter due to improved transcriptional termination. This element also demonstrated barrier activity, reducing variability of expression due to position effects. As it is known that the 0.25-kb core insulator has enhancer-blocking activity, this particular insulated lentiviral vector design may be useful for clinical application.
Introduction
Hematopoietic stem cell–targeted gene therapy using retroviral vectors has the potential to cure hematological disorders. Compared to other gene therapy strategies, it has several beneficial aspects, including long-term persistence of transgene expression, central tolerance for the foreign gene product, and the potential delivery of the therapeutic gene product to nonhematopoietic organs. Recently, two different severe combined immunodeficiency diseases have been cured by γ-retroviral gene delivery to patient autologous hematopoietic cells.1,2,3,4 Unfortunately, in the X-linked, common γ-chain deficiency-severe, combined immunodeficiency disease trials, several patients developed leukemia, caused in part by vector insertional mutagenesis, raising concern about this approach.5,6 Vector insertional proto-oncogene activation was also observed in a recent gene therapy trial for chronic granulomatous disease.7 These patients developed clonal myeloid outgrowths due to proto-oncogene activation by the inserted vector.
To improve the risk–benefit balance associated with retroviral-mediated gene therapy, several approaches have been proposed to reduce the potential of vector insertional mutagenesis. One approach to enhance safety of retroviral vectors involves the use of a self-inactivating (SIN) design.8,9 SIN γ-retroviral and lentiviral vectors lack the enhancer element(s) contained within the U3 region of the long-terminal repeat (LTR). The enhancer elements are thought to have caused activation of the proto-oncogenes in the above noted clinical trials, which both utilized γ-retroviral vectors.9,10 However, SIN vectors still rely on the use of an internal promoter to drive expression of the therapeutic transgene. Depending on the regulatory elements chosen to drive transgene expression, there may be residual capacity to alter the regulation of genes near the insertion site. Therefore, it has been proposed that use of a 1.2-kb insulator element of the 5′ hypersensitive site 4 (5′HS4) of the chicken β-globin gene (cHS4 insulator) may provide an additional safety feature to the SIN design.9,11
The cHS4 insulator element has two major activities: (i) enhancer blocking when placed between an enhancer element and a promoter12 and (ii) barrier activity that enhances the probability of transgene expression by protecting the transgene from encroaching chromosomal condensation and heterochromatinization.13,14,15 Felsenfeld and colleagues have shown that the bulk of the insulating activity of the 1.2-kb fragment resides in a 0.25-kb core element, which contains a CTCF-binding site.12,15 The CTCF element contains the enhancer-blocking activity, while the barrier activity maps to other unique sites within the 0.25-kb core.15 Transfection studies using cell lines indicate that while one copy of the 0.25-kb core is modestly inferior in enhancer-blocking activity to the full 1.2-kb fragment, two copies of the 0.25-kb element exceed the enhancer-blocking activity of the 1.2-kb fragment, while also providing equivalent protection against position effect.15,16,17 Investigators have studied the effect of this insulator element in retroviral vectors by inserting it into the U3 region of the vector 3′LTR, where it can subsequently be copied over to the U3 of the 5′LTR during reverse transcription (RT). This results in a sandwich configuration of the vector genome with the element at both ends of the integrated provirus.
Incorporation of the 1.2-kb element into γ-retroviral and lentiviral vectors improves the probability and consistency of transgene expression by protecting against position effects through its barrier activity.18,19,20,21 The enhancer-blocking activity is also potentially of great importance, as it might block or reduce insertional gene activation of proto-oncogenes or growth-regulatory genes lying near a vector insertion. A recent study from our laboratory showed that the 1.2-kb insulator or the 0.25-kb core element in duplicate can diminish insertional gene activation in an in vitro cell genotoxicity assay.22 In addition, the 1.2-kb insulator element was found to reduce the incidence of clonal dominance in a cell line assay when it was included in a lentiviral vector containing an internal murine stem cell virus–driven expression cassette.23 Further studies are needed to document that inclusion of the insulator reduces the possibility of insertional gene activation in primary cells in vivo.
Despite these beneficial effects, the 1.2-kb element has two major disadvantages. The first is that its presence can significantly diminish the overall level of transgene expression per cell in some instances.11,20 Second, its presence in vectors often decreases titer.11,19,20,24 In this article, we show that transgene expression from an internal promoter in the SIN lentiviral format can be significantly enhanced, compared to the parental vector, when the 1.2-kb cHS4 insulator or a single copy of the 0.25-kb core insulator element is placed into the partially deleted U3 region in a specific orientation. Surprisingly, we find that the mechanism of this effect is through enhancement of transgene mRNA levels. In addition, we demonstrate that the mechanism of reduction in vector titer caused by inclusion of the 1.2-kb cHS4 insulator is due to a postentry failure in the target cell to complete the process of RT. This defect can be circumvented by the use of a single copy of the 0.25-kb core insulator element. Together, these data argue for use of a single copy of the 0.25-kb insulator in SIN lentiviral vectors.
Results
Reverse orientation of insulator elements in the HIV1 SIN-LTR increases transgene expression
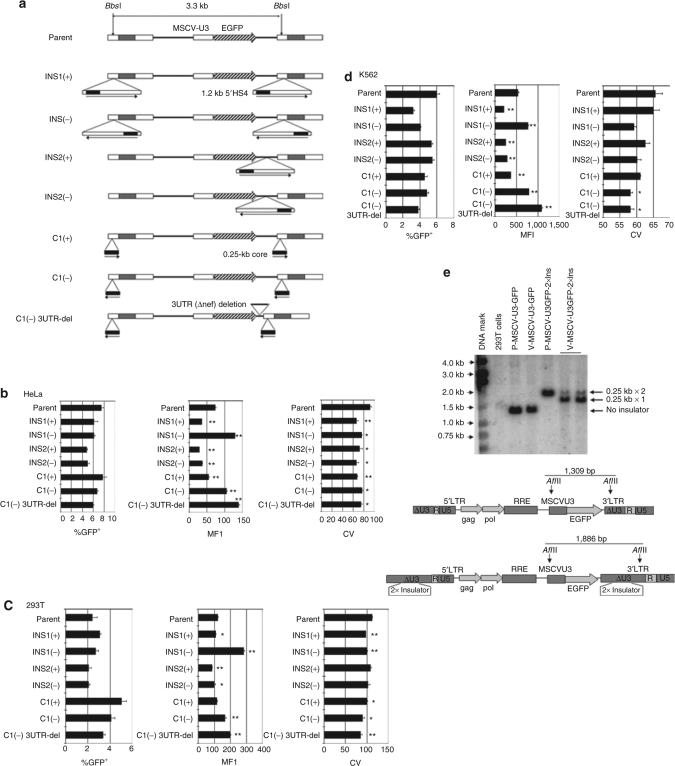
Due to its ability to reduce position-effect variegation and stabilize transgene expression, the 1.2-kb cHS4 insulator element is particularly useful when incorporated into retroviral vectors.15,20,21,24 However, its inclusion in vectors can also cause the unwanted effects of decreased levels of cellular expression of the integrated, vector-encoded transgene,11,20 and diminished vector titer.11,19,24 In our studies, we sought to identify vector modifications that would overcome these two detrimental effects of the 1.2-kb cHS4 insulator in the context of a human immunodeficiency virus (HIV)-based, third-generation SIN lentiviral vector. When we inserted the 1.2-kb insulator into the partially deleted U3 region of the 3′LTR of our SIN–HIV1 vector (SJ1)25 in the forward orientation [Figure 1a; INS1(+)], which is the natural direction of the element in the chicken β-globin locus control region, transgene expression from the internal murine stem cell virus U3 enhancer/promoter decreased significantly as assessed by the mean fluorescence intensity (MFI) of the transduced cells (Figure 1b–d). This effect was reproducibly observed in three different target cell populations (HeLa, 293T, and K562). These results are consistent with those observed by the Hawley group.11
Figure 1.
Schematic diagrams and gene expression levels of lentiviral vectors containing various cHS4 1.2- or 0.25-kb core insulator configurations. (a) Schematic diagrams of the parental and cHS4 insulator vectors. The 1.2-kb cHS4 insulator was inserted into the SIN-LTR [INS1(+) and INS1(−)] or between enhanced green fluorescent protein (EGFP) cDNA and the 3′LTR [INS2(+) and INS2(−)]. The horizontal arrows indicate the original direction of the insulator elements relative to the chicken β-globin genes. (b–d) HeLa and 293T cells were transduced at a multiplicity of infection (MOI) of 0.1, and K562 cells were transduced with the various vectors at an MOI of 0.4 in triplicate. Mean fluorescence intensity (MFI) and coefficient of variation (CV) values of the EGFP-positive fraction of each cell line are shown with the indicated vectors. The error bar indicates SEM. *P < 0.05, **P < 0.005. (e) Southern blot analysis of DNA from 293T cells transduced with the parental vector or the parental vector containing the 0.25 kb × 2 insulator element, as indicated. P = plasmid, V = vector. DNA was digested with AflII as shown, and probe with an EGFP probe. LTR, long-terminal repeat; MSCV, murine stem cell virus; RRE, rev-responsive element; SIN, self-inactivating.
Through the testing of various designs of insulator-containing lentiviral vectors, we were surprised by the finding that when the 1.2-kb insulator element was inserted into SIN-LTR in reverse orientation [Figure 1a; INS1(−)], transgene expression was significantly enhanced (Figure 1b–d). To our knowledge, this orientation has not been studied previously. This unique observation was reproducible in all three different cell lines (Figure 1b–d). These differences were not due to differences in vector copy number of the transduced cell populations because the percentages of green fluorescent protein (GFP)-positive cells in the populations were quite low for all of the vectors tested in all three cell lines tested (none >8%), consistent with transduced cells containing single proviral inserts.26 The coefficient of variation values for cells transduced with the vector containing the insulator in both the forward or reverse orientation was significantly reduced compared to that of the parental vector, demonstrating orientation-independent barrier activity in the way of protection from position effects by the full-length 1.2-kb insulator (Figure 1b–d).
To further evaluate these observations, additional vectors were generated for testing (Figure 1a). We found that two copies of the 0.25-kb core element in the 3′LTR of the SIN design could not be transmitted to target cells, and the Southern blot analysis revealed that most of the integrated vector demonstrated deletion of one of the 0.25-kb copies (Figure 1e). Thus, we decided to test vectors containing only one copy of the 0.25-kb core. Interestingly, the same significant increase of gene expression in transduced cells was observed when the 0.25-kb core insulator, a subfragment of the 1.2-kb element that retains insulator function,15 was inserted into the SIN-LTR in the reverse orientation [C1(−); Figure 1a–d]. In contrast, transgene expression was diminished when it was placed in the forward orientation [C1(+)]. When the full-length 1.2-kb element was removed from the LTR and inserted into the vector backbone between the enhanced green fluorescent protein (EGFP) expression cassette and the SIN-LTR [Figure 1a; INS2(+) and INS2(−)], a decrease in gene expression was observed, regardless of orientation. These results indicated that transgene expression from the internal promoter in these vectors containing the insulator elements is dependent on both the orientation of the insulator element and its positioning in the 3′LTR. Finally, we observed similar coefficient of variation values for the vectors containing the 0.25-kb core element, relative to those carrying the full 1.2-kb insulator, indicating equivalent barrier protection against position effects (Figure 1c).
Reverse-oriented 1.2- or 0.25-kb core insulator elements in the SIN-HIV1 3′LTR increase gene expression by enhancing transgene mRNA
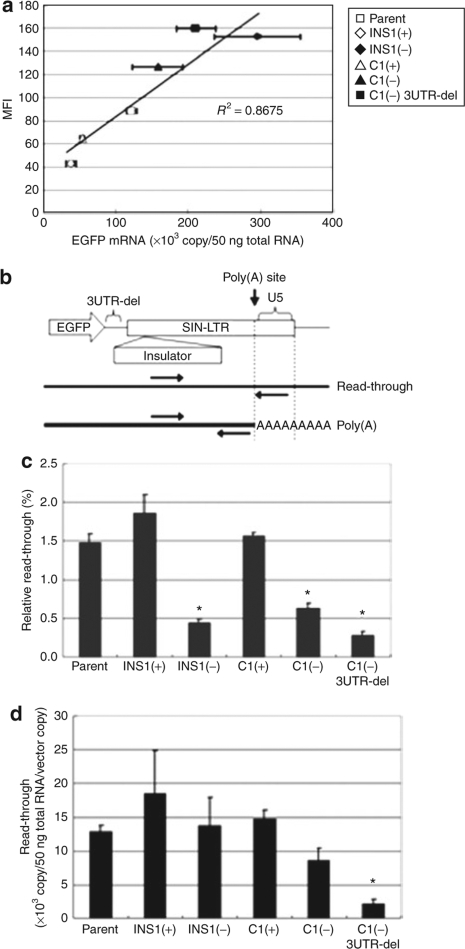
To examine the mechanism of the effect that the orientation of the insulator elements had on vector-encoded transgene expression, we first measured the amount of EGFP mRNA, as assessed by quantitative RT-PCR (qRT-PCR), and EGFP levels, as assessed by the MFI in transduced cells. HeLa cells were transduced with the various vectors at a low multiplicity of infection of 0.1 to obtain populations with similar fractions of EGFP-positive cells and similar vector copy numbers. The amount of EGFP mRNA (normalized by the vector copy number) and the MFI of the EGFP expressing cells were ascertained for each of the populations 4 days after transduction. We observed a dose–response relationship between the amount of cellular EGFP mRNA and EGFP expression (MFI) (Figure 2a). Compared to the forward orientation [INS1(+)] of the 1.2-kb HS4-containing vector, INS1(−), with the reverse orientation, showed higher transgene mRNA levels and protein. Likewise, the reverse-oriented 0.25-kb core insulator, C1(−), when compared to its opposite orientation counterpart C1(+), also showed elevated levels of EGFP mRNA and protein (Figure 2a). The removal of residual HIV backbone nef sequences between EGFP and the LTR from the C1(−) vector, resulting in vector C1(−) 3UTR-del, further augmented EGFP mRNA and protein to a level comparable to that of the INS1(−) vector (Figure 2a).
Figure 2.
The “reverse-oriented” 0.25-kb core cHS4 insulator coupled with removal of the residual nef sequences increases transgene mRNA. (a) HeLa cells were transduced with low multiplicity of infection (MOI = 0.1) and DNA and total RNA were extracted from the cells. Enhanced green fluorescent protein (EGFP) mRNA was quantified using quantitative reverse transcription–PCR (qRT-PCR) and values, normalized to DNA copy number, are shown plotted against mean fluorescence intensity (MFI). The experiments were performed in triplicate and the error bar indicates the SEM. (b) Schematic diagram of the modified insulated vectors and the positions of the PCR primers used in this experiment. (c,d) The efficacy of the 3′end processing was assessed by qRT-PCR as described in Materials and Methods. The relative and absolute amount of read-through is indicated. The relative read-through value was calculated by dividing the U3-U5 cDNA copy number by the U3-R cDNA copy number. The experiments were performed in triplicate and the error bar indicates the SEM. *P < 0.005.
We hypothesized that a potential mechanism responsible for the differing transgene mRNA levels among the various vectors might be differential cleavage and/or polyadenylation efficiency of the expressed RNA transcript encoding EGFP. SIN HIV vectors, due to the U3 deletion, have diminished proper transcriptional termination, and this might be further compromised by inclusion of heterologous sequences in the deleted U3.27,28,29 To test this hypothesis, we quantified read-through mRNA species using qRT-PCR (Figure 2b and Materials and Methods). The relative read-through of the vectors, INS1(−) and C1(−), was significantly lower (Figure 2c), compared to the vectors with the insulators in the forward orientation, as well as the parent. However, the absolute read-through of all these vectors was similar to parent (Figure 2d), suggesting that the cleavage step of RNA processing was not enhanced. Thus, the increased amounts of polyadenylated EGFP mRNA might rather be due to enhancement of the poly(A) addition reaction following cleavage, an increase in the cytoplasmic export, or an enhanced mRNA stability. In contrast, the vector C1(−) 3UTR-del did show a reduction in the levels of both the relative and absolute read-through. Absolute read-through mRNA levels with this construct were 17% of that of parental vector (P < 0.005) or to 25% of C1(−) (P < 0.05) (Figure 2d), suggesting that removal of the disposable residual nef sequences did enhance both cleavage and subsequent polyadenylation.
Insertion of the 1.2-kb cHS4 insulator in either orientation into the partially deleted U3 region of the lentiviral 3′LTR reduces the apparent transducing titer
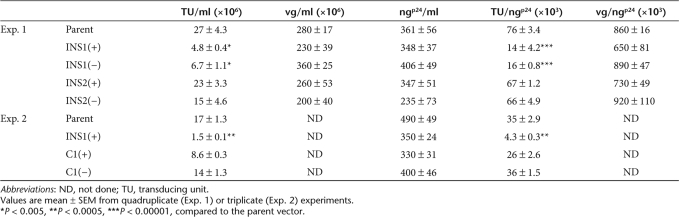
When the 1.2-kb cHS4 insulator was inserted into the partially deleted U3 region of the LTR of the SIN-HIV1 vector in either orientation [INS1(+) and INS1(−)], we consistently observed a significant reduction in vector titer [transducing unit (TU)/ml], compared to the parental vector, as measured by fluorescence-activated cell sorting analysis for GFP expression in target HeLa cells. This phenomenon has also been observed by other investigators.11,19,24 These differences in titer were even more significant when titers were normalized to the p24 antigen (Ag) level contained in the vector preparation (TU/ngp24) (Table 1, Exp. 1). More important, the numbers of viral RNA genomes per milliliter, a surrogate marker for infectious particles, were similar for all of the vectors containing the insulator in the 3′LTR, compared to the parental vector preparation. Together, these data strongly argue that all the vector preparations had similar viral particle content. However, only 20% of the INS1(+) or INS1(−) vector particles were competent to complete transmission of the vector genome into target cells compared to the parental vector. Interestingly, when the insulator was inserted between EGFP and LTR [INS2(+) and INS2(−)], there was almost no decrease in titer (TU/ml) (Table 1, Exp. 1), establishing that the larger vector genome size of the insulated vectors was not responsible for the reduction in titer. These data suggested that the position of the insulator element in the 3′LTR significantly reduced vector infectivity but not particle formation.
Table 1.
Titer of the vectors with various insulators
The 0.25-kb core insulator preserves transducing ability
In the next set of experiments, we examined the titer of the vectors containing the 0.25-kb core insulator. The preparations of vector containing the 0.25-kb insulator had similar viral particle content to the vectors containing the 1.2-kb insulator as well as the parental vector (Table 1, Exp. 2). However, unlike the 1.2-kb insulator vectors, the vectors containing the 0.25-kb insulator [C1(+) and C1(−)] had no loss of transducing titer, relative to the parental vector.
The 1.2-kb cHS4 insulator element, but not the 0.25-kb core, inhibits transduction in the target cell at the RT step
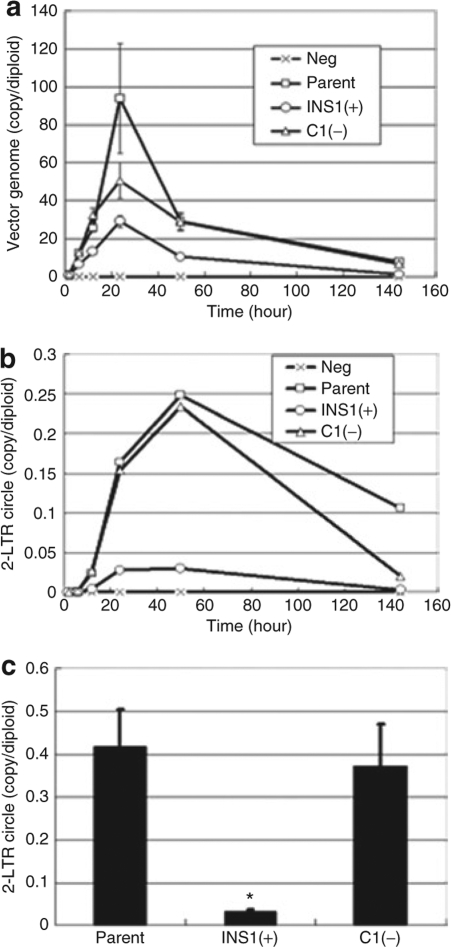
To determine the mechanism by which the 1.2-kb cHS4 insulator element diminishes apparent vector titer, we performed a RT kinetic assay following cell transduction with the different vectors. We hypothesized that the 0.95 kb of sequences contained in the 1.2-kb insulator but not present in the 0.25-kb core element might be problematic for RT. To test this possibility, we transduced cells using the same amount of p24 Ag (and similar amounts of RNA genomes) for each of the vectors, and 24 hours after initiation of transduction, we began measuring the total cellular cDNA product. At the 24-hour time point, a peak in the total RT cDNA product level was observed, which by day 6 decreased to a steady-state level that was in the expected range, given the multiplicity of infection used for the transduction (Figure 3a). The vector with the 1.2-kb insulator INS1(+) in the 3′LTR showed greatly diminished amounts of peak total RT cDNA product, compared to the parental vector, indicating inefficient RT of the viral RNA genome. However, there was much less diminishment in cDNA product with the 0.25-kb insulator vector C1(−). To further verify that there was a postentry block to RT of the genomic RNA of the INS1(+) vector, the kinetics of 2-LTR circle formation were determined, as a surrogate marker for successful nuclear translocation of the preintegration complex. Furthermore, 2-LTR circles are exclusively formed in the nucleus after successful RT of the vector genome in the cytoplasm. While C1(−) showed kinetics similar to those of the parental vector, INS1(+) showed severely diminished 2-LTR circle formation, consistent with a significant reduction in the quantity of vector genomes of INS1(+) successfully imported into the nucleus and subsequently integrated. Together these data suggest a deficiency in the RT process of the vector RNA genome.
Figure 3.
Reverse transcription kinetic assay for vector cDNA in target cells transduced with the parental and cHS4-insulated lentiviral vectors. 293T cells were transduced with equal amounts (80 ng of p24 Ag) of each vector stock, and DNA, including low-molecular-weight DNA, was subsequently extracted at various time points. (a) The total amount of the reverse-transcribed vector genome was measured in triplicate using a primer-probe set targeted at the human immunodeficiency virus 1 (HIV1) packaging signal sequence. Reverse-transcribed vector genome copy numbers of cells transduced with INS1(+) were significantly lower (P < 0.05), compared to cells transduced with the parental vector at 6, 12, 50, and 144 hours after transduction. (b) 2-LTR circle formation was measured using quantitative PCR. (c) The peak 2-LTR circle formation at 50 hours after transduction. All experiments were performed in triplicate. *P = 0.01. LTR, long-terminal repeat.
Discussion
Incorporation of the cHS4 insulator element into γ-retroviral and lentiviral vectors may provide an enhancement of vector safety due to its enhancer-blocking activity, which might reduce vector insertional gene activation.22,23,30 Another major beneficial effect of this insulator is its ability to reduce position-effect variegation and silencing of vector-encoded transgene expression.11,18,19,20,21,31 Thus, it can be argued that the cHS4 insulator should be considered a highly desirable component in the design of therapeutic vectors. Unfortunately, we and other researchers have observed a consistent reduction in titer of lentiviral vectors containing the 1.2-kb cHS4 insulator, which significantly hampers its practical use. This effect on titer was previously attributed to the increase in vector genome size by the addition of the insulator.11 In addition, reduction in the absolute transgene expression level per cell, despite diminishment of position-effect variegation and silencing of cHS4-insulated vectors, has been noted by several laboratories,11,20 including our own. This study sought to identify an optimized SIN lentiviral vector design containing a functional insulator element that minimized its impact on titer and cellular-expression level, while retaining beneficial effects.
We consistently observed reduced transgene expression from an internal promoter in our SIN-HIV1-based vector system SJ1 when the 1.2-kb cHS4 insulator element was inserted in a “forward” orientation, that is, the natural 5′ to 3′ direction of the cHS4 element in the β-globin locus, relative to the sense orientation of the lentiviral vector genome (Figure 1a–d). However, when we placed the element in the opposite, “reverse” orientation, vector transgene expression level actually increased to a level higher than that of the parental vector. This orientation-dependent effect was also retained when the 0.25-kb core element was used in place of the larger fragment. This observation has not previously been reported. In addition, we found that the 0.25-kb insulator equally decreased the variability of expression, as judged by the coefficient of variation of EGFP MFI in transduced cells, compared to that observed with the 1.2-kb fragment (Figure 1b–d). Recent work by the Emery laboratory suggested that the 0.25-kb element was inferior in this regard to the 1.2-kb element or an extended 0.4-kb fragment.31 The 0.4-kb fragment used in this study contained the 0.25-kb core plus an additional 3′ adjacent 0.15 kb of sequence from the cHS4 1.2-kb fragment. However, unlike our experiments, these studies were performed using non-SIN γ-retroviral vectors. Of significance, the experiments were performed using drug selection to identify and obtain vector-transduced cells. We believe that this approach has the potential to minimize differences due to selection for integration sites permissive for vector expression. It would be interesting in the future to compare these two elements in an unbiased context without drug selection.
Mechanistic investigation of our observations regarding vector transgene expression showed that the “reverse” orientation of either the 1.2- or 0.25-kb elements augmented the levels of EGFP mRNA and protein expression level (Figure 2a). Additional experiments showed that this effect on mRNA level occurred at a postcleavage step of mRNA processing, involving either enhanced polyadenylation of the cleaved mRNA or enhanced stability of the mRNA or enhanced nuclear-to-cytoplasmic transport (Figure 2c,d). Because we had previously found that the removal of the residual nef sequences located immediately 5′ of the 3′LTR in the vector backbone led to increased transgene expression32 (H. Hanawa, unpublished results), we combined the “reverse” 0.25-kb core insulator and the removal of the residual nef sequence. This combination further increased the transgene expression in K562 cells up to 2-fold compared to the parent and 5.2-fold compared to INS1(+) (Figure 1b). Similar results were observed with HeLa and 293T target cells. The additional increase in expression with this vector was observed to be due to increased transcriptional termination, as both the relative and absolute read-through transcripts were significantly decreased with this construct. In addition to providing enhanced transgene expression, increased transcriptional termination also is beneficial in reducing read-through transcription that could lead to abnormal expression of a neighboring, downstream gene.27 Further enhancement of transgene expression and safety might be obtained in our vector by the additional incorporation of two copies of the 45-bp upstream regulatory element from SV40 that has been recently shown to also reduce transcriptional read-through in γ-retroviral and lentiviral vectors.27
The other major negative attribute of the cHS4 insulator is its deleterious effects on vector titer which have been observed in the context of both γ-retroviral and lentiviral vectors. To date, no definitive mechanism for this effort has been elucidated. Here, we show that the reduction in lentiviral vector titer is due to a decreased particle-transducing ability rather than decreased production of vector particles. The cause of a low-vector titer can occur at any step from the vector production/packaging of the RNA genome into the virus particle to the integration of the vector into the cellular genome. We, therefore, evaluated this process from production to the final integrated vector genome using a series of molecular assays. Interestingly, we found that decreased RNA genome packaging, due to an increased vector genomic size by inclusion of the insulator element, is not responsible for the reduction of the titer (Table 1), as direct measurement of the RNA content of the vector preparations confirmed equal packaging of each vector genome into the vector particle. To identify the rate-limiting step, we performed a RT kinetic assay targeting the HIV1 packaging sequence to measure the total reverse-transcribed cDNA and 2-LTR circular-form levels in transduced cells. The results documented severely attenuated RT and subsequent nuclear transport with the 1.2-kb insulator-containing vector, while the 0.25-kb core element demonstrated only modestly reduced levels of the reverse-transcribed genome and 2-LTR circles. Interestingly, this phenomenon was not seen with the non-double, copy insulator vector, suggesting that placement within the U3-deleted LTR was necessary to disrupt the RT process. Pausing of RT can occur due to stable secondary structure in the viral genome.33,34 If a particular region of the RNA genome has sufficiently stable secondary structure, abortive RT may occur due to sustained pausing.35 When we compared the 1.2- and 0.25-kb fragments for potentially stable RNA secondary structures,36 we found that the two elements shared one potentially strong one; however, the 1.2-kb element had several additional ones. We do not know as yet whether these additional potential secondary structures underlie the gene-transfer defect, but our findings highlight that care must be used when modifying the viral LTR.
Directional independency of the cHS4 insulator function in terms of enhancer blocking and protection from chromosomal condensation has been shown in the context of γ-retroviral vectors in the past.20 However, the insulator element has been used primarily by most investigators in the “forward” orientation, following the initial observation, in the context of a γ-retroviral vector, that the “reverse” orientation diminished transcription from the LTR.20,37 This effect was attributed to the close proximity of the cHS4 core to the LTR enhancer/promoter. However, it is not necessary to avoid the “reverse” orientation in a SIN lentiviral vector. Indeed, the optimal orientation of the insulator in the lentiviral context has remained undefined until our current studies.
In conclusion, we have developed an optimized HIV1-based SIN vector design with an insulator element which provides high titer, a high level of transgene expression, and retains significant functional insulator activity. This vector design should be useful for consideration in future gene therapy clinical trials.
Materials and Methods
Vector construction. Details regarding the construction of the vector plasmids are available upon request. Briefly, lentiviral vector plasmids were constructed based on SJ1 HIV1 system.25 The transfer vector plasmids, pCL20c INS1(+) MpGFP [INS1(+)] and pCL20c INS1(−) MpGFP [INS1(−)], have the 1.2-kb 5′HS4 insulator element of chicken β-globin gene (from pJC13-1, kindly provided by Dr. Gary Felsenfeld) in the ΔU3 region of pCL20c MpGFP in the forward or reverse orientation, respectively, relative to its orientation in β-globin gene locus. The 3′LTR consists of parts of the U3 and R region (nt 9,086–9,120 and nt 9,521–9,621, respectively, Genebank accession number K03455). [INS2(+)] and pCL20c INS2(−) MpGFP [INS2(−)] have the same 1.2-kb insulator element between the EGFP cDNA and the 3′SIN-LTR in the forward or reverse orientation. Similarly, pCL20c C1(+) MpGFP [C1(+)] and pCL20c C1(−) MpGFP [C1(−)] have a single 0.25-kb core insulator from pNI-CD (kindly provided by Dr. Gary Felsenfeld) in the ΔU3. The pCL36 C1(−) MpGFP [C1(−) 3UTR-del] was derived from pCL20c C1(−) MpGFP by removal of the 46-bp residual nef sequence between the KpnI site and the 3′ ppt.
Vector production, titration, and transduction. All vectors were produced by transient transfection of 293T cells. The TU titer of the frozen vector stock was measured as previously described.38 The p24Ag concentration was measured by enzyme-linked immunosorbent assay (ZeptoMetrix, Buffalo, NY). To quantify the mRNA viral genome content of the vector preparation, genomic viral mRNA was first extracted from particles after polyethyleneglycol-mediated precipitation from conditioned medium. Viral mRNA genomes were then quantified by real-time qRT-PCR.39
In all, 100,000 HeLa cells, 293T cells, or K562 cells were transduced in a 6-well plate in a total volume of 2 ml in the presence of 8 µg/ml of polybrene. Flow cytometry analysis (FACSCalibur; BD Biosciences, San Jose, CA) for the detection of EGFP expression was done 3–4 days after transduction. Titers were calculated from transduced samples having <20% EGFP-positive cells to insure accuracy.26
Southern blot analysis of vector genome transmission. DNA was prepared from naïve 293T cells transduced with the various vector preparations according the manufacturer's specifications (Gentra PureGene Kit; Qiagen, Valencia, CA) were digested with AflII, which restricts the integrated provirus as shown in Figure 1e, liberating a diagnostic fragment that indicates the integrity of the 250 bp × 2 insulator core element repeat in the LTR (Figure 1e). A radiolabeled GFP DNA probe was hybridized with the blot, and the signal intensity of the hybridizing bands was visualized using a Molecular Dynamic Storm 860 Phosphorimager (Molecular Dynamics, Sunnyvale, CA) and its accompanying software. Equivalent loading of lanes was confirmed by ethidium bromide staining of gels prior to DNA transfer to the nylon filters.
RT kinetic assay. A total of 30,000 293T cells were seeded into 6-well plates 24 hours before transduction. Transduction was performed using 80 ng of p24 Ag per well in the presence of 8 µg/ml of polybrene. DNA was harvested 2, 6, 12, 24, and 50 hours after the initiation of transduction without splitting of the cultures. An additional well was cultured for a total of 6 days with splitting on days 3 and 5. DNA was harvested to recover low-molecular-weight molecules by lysing the cells in 0.5 ml of lysis buffer (10 mmol/l Tris–HCl pH 8.0, 0.5% sodium dodecyl sulfate, 100 mmol/l NaCl, 25 mmol/l EDTA, 0.5 mg/ml proteinase K). The lysate was incubated at 65 °C for 6 hours followed by phenol:chloroform extraction and ethanol precipitation. After one wash with 70% ethanol, the DNA pellet was dried and then hydrated with 1 × TE. The total amount of reverse-transcribed viral genome (nonintegrated forms) was quantified using a primer-probe set targeting the HIV1 packaging sequence.39 The primer sets used to detect the 2-LTR circular form have been previously described.40 The PCR and the detection of fluorescence were performed using the ABI PRISM 7700 sequence detection system (Applied Biosystems, Foster City, CA) under standard thermal cycling conditions. The amount of viral genome was determined using the standard curve method involving a series of diluted plasmids (vector plasmid or plasmid containing a synthetic 2-LTR junction). The viral genome copy number was normalized using the amount of genomic DNA measured with the rRNA kit (Applied Biosystems) and indicated as copies/diploid genome.
Quantification of gene expression and assessment of HIV1 3′LTR read-through by qRT-PCR. To quantify gene expression from the HIV1 vector with various 3′end modifications, ~1 × 105 HeLa cells were transduced with a vector multiplicity of infection of 0.1 in the presence of polybrene 8 µg/ml. Six days after the initiation of transduction, EGFP expression was determined using fluorescence-activated cell sorting analysis, and total RNA was extracted to quantify gene expression at the transcriptional level. The total RNA was extracted using the RNeasy Mini Kit (Qiagen), and plasmid DNA contaminant was digested on the column during the RNA extraction procedure according to the manufacturer's instructions. RT was performed using high-capacity cDNA reverse transcription kit (Applied Biosystems) under standard thermal cycling conditions. The amount of total EGFP cDNA obtained from 50 ng of total RNA was measured using primers 5′-CCC GAC AAC CAC TAC CTG AG-3′, 5′-GTC CAT GCC GAG AGT GAT CC-3′, and SYBR Premix Ex Taq (Takara, Otsu, Japan) and a series of diluted EGFP–containing plasmids as a standard. Amplification was done under standard thermal cycling conditions. The quality of PCR was assessed by both electrophoresis and the dissociation curve method, and a clean single band or peak was confirmed.
To assess polyadenylation efficiency, RT was performed using TaqMan Reverse Transcription Reagents (Applied Biosystems) and oligo(dT)16. To quantify the entire mRNA amount that was transcribed from the internal promoter, an upstream primer (HIV1 LTR-U3 forward): 5′-CTG CTT TTT GCC TGT ACT G-3′, and a downstream primer (HIV1 LTR-R reverse): 5′-TCA AGG CAA GCT TTA TTG AG-3′ were used. To quantify the RNA species that were not cleaved at the poly(A) site of HIV1 LTR, the same upstream primer and a different downstream primer (HIV1 LTR-U5 reverse), 5′-CTG AGG GAT CTC TAG TTA CC-3′, were used. Real-time PCR was done using each of the primer sets and the SYBR Premix Ex Taq (Takara) against cDNA obtained from 50 ng of total RNA. Relative read-through at the HIV1 vector 3′LTR was calculated by dividing the copy number of the RNA species that did not cleave at the poly(A) site by the copy number of the total RNA from the internal promoter.
Statistical analysis. For statistical analysis, the Student's t-test was used to evaluate for statistically significant differences between each modified vector and the parental vector.
Acknowledgments
We thank Arthur W. Nienhuis [St. Jude Children's Research Hospital (SJCRH), Memphis, TN] for encouragement and Gary Felsenfeld (National Institutes of Health, Bethesda, MD) for providing the chicken 5′HS4 insulator fragments. We thank John Gray for evaluating the insulator sequences for potential RNA secondary structure. This investigation was supported in part by the National Heart, Lung, and Blood Institute Program Project PO1HL053749, SJCRH Cancer Center Support (Core) Grant CA-21765, and the American Lebanese Associated Charities.
References
- Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- Gaspar HB, Parsley KL, Howe S, King D, Gilmour KC, Sinclair J, et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet. 2004;364:2181–2187. doi: 10.1016/S0140-6736(04)17590-9. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- Baum C, Dullmann J, Li Z, Fehse B, Meyer J, Williams DA, et al. Side effects of retroviral gene transfer into hematopoietic stem cells. Blood. 2003;101:2099–2114. doi: 10.1182/blood-2002-07-2314. [DOI] [PubMed] [Google Scholar]
- Nienhuis AW, Dunbar CE., and , Sorrentino BP. Genotoxicity of retroviral integration in hematopoietic cells. Mol Ther. 2006;13:1031–1049. doi: 10.1016/j.ymthe.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Kohn DB, Sadelain M., and , Glorioso JC. Occurrence of leukaemia following gene therapy of X-linked SCID. Nat Rev Cancer. 2003;3:477–488. doi: 10.1038/nrc1122. [DOI] [PubMed] [Google Scholar]
- Ramezani A, Hawley TS., and , Hawley RG. Performance- and safety-enhanced lentiviral vectors containing the human interferon-beta scaffold attachment region and the chicken beta-globin insulator. Blood. 2003;101:4717–4724. doi: 10.1182/blood-2002-09-2991. [DOI] [PubMed] [Google Scholar]
- Bell AC, West AG., and , Felsenfeld G. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell. 1999;98:387–396. doi: 10.1016/s0092-8674(00)81967-4. [DOI] [PubMed] [Google Scholar]
- Burgess-Beusse B, Farrell C, Gaszner M, Litt M, Mutskov V, Recillas-Targa F, et al. The insulation of genes from external enhancers and silencing chromatin Proc Natl Acad Sci USA 20029916433–16437.suppl. 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaszner M., and , Felsenfeld G. Insulators: exploiting transcriptional and epigenetic mechanisms. Nat Rev Genet. 2006;7:703–713. doi: 10.1038/nrg1925. [DOI] [PubMed] [Google Scholar]
- Recillas-Targa F, Pikaart MJ, Burgess-Beusse B, Bell AC, Litt MD, West AG, et al. Position-effect protection and enhancer blocking by the chicken beta-globin insulator are separable activities. Proc Natl Acad Sci USA. 2002;99:6883–6888. doi: 10.1073/pnas.102179399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JH, Bell AC., and , Felsenfeld G. Characterization of the chicken beta-globin insulator. Proc Natl Acad Sci USA. 1997;94:575–580. doi: 10.1073/pnas.94.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recillas-Targa F, Bell AC., and , Felsenfeld G. Positional enhancer-blocking activity of the chicken beta-globin insulator in transiently transfected cells. Proc Natl Acad Sci USA. 1999;96:14354–14359. doi: 10.1073/pnas.96.25.14354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam PI, Scholes J, Perelman N, Xia P, Yee JK., and , Malik P. Improved human beta-globin expression from self-inactivating lentiviral vectors carrying the chicken hypersensitive site-4 (cHS4) insulator element. Mol Ther. 2007;15:1863–1871. doi: 10.1038/sj.mt.6300259. [DOI] [PubMed] [Google Scholar]
- Emery DW, Yannaki E, Tubb J, Nishino T, Li Q., and , Stamatoyannopoulos G. Development of virus vectors for gene therapy of beta chain hemoglobinopathies: flanking with a chromatin insulator reduces gamma-globin gene silencing in vivo. Blood. 2002;100:2012–2019. doi: 10.1182/blood-2002-01-0219. [DOI] [PubMed] [Google Scholar]
- Emery DW, Yannaki E, Tubb J., and , Stamatoyannopoulos G. A chromatin insulator protects retrovirus vectors from chromosomal position effects. Proc Natl Acad Sci USA. 2000;97:9150–9155. doi: 10.1073/pnas.160159597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivella S, Callegari JA, May C, Tan CW., and , Sadelain M. The cHS4 insulator increases the probability of retroviral expression at random chromosomal integration sites. J Virol. 2000;74:4679–4687. doi: 10.1128/jvi.74.10.4679-4687.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu BY, Evans-Galea MV, Gray JT, Bodine DM, Persons DA., and , Nienhuis AW. An experimental system for the evaluation of retroviral vector design to diminish the risk for proto-oncogene activation. Blood. 2008;111:1866–1875. doi: 10.1182/blood-2007-04-085506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans-Galea MV, Wielgosz MM, Hanawa H, Srivastava DK., and , Nienhuis AW. Suppression of clonal dominance in cultured human lymphoid cells by addition of the cHS4 insulator to a lentiviral vector. Mol Ther. 2007;15:801–809. doi: 10.1038/sj.mt.6300103. [DOI] [PubMed] [Google Scholar]
- Yao S, Osborne CS, Bharadwaj RR, Pasceri P, Sukonnik T, Pannell D, et al. Retrovirus silencer blocking by the cHS4 insulator is CTCF independent. Nucleic Acids Res. 2003;31:5317–5323. doi: 10.1093/nar/gkg742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa H, Kelly PF, Nathwani AC, Persons DA, Vandergriff JA, Hargrove P, et al. Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol Ther. 2002;5:242–251. doi: 10.1006/mthe.2002.0549. [DOI] [PubMed] [Google Scholar]
- Kustikova OS, Wahlers A, Kuhlcke K, Stahle B, Zander AR, Baum C, et al. Dose finding with retroviral vectors: correlation of retroviral vector copy numbers in single cells with gene transfer efficiency in a cell population. Blood. 2003;102:3934–3937. doi: 10.1182/blood-2003-05-1424. [DOI] [PubMed] [Google Scholar]
- Schambach A, Galla M, Maetzig T, Loew R., and , Baum C. Improving transcriptional termination of self-inactivating gamma-retroviral and lentiviral vectors. Mol Ther. 2007;15:1167–1173. doi: 10.1038/sj.mt.6300152. [DOI] [PubMed] [Google Scholar]
- Yang Q, Lucas A, Son S., and , Chang LJ. Overlapping enhancer/promoter and transcriptional termination signals in the lentiviral long terminal repeat. Retrovirology. 2007;4:4. doi: 10.1186/1742-4690-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss AK, Son S., and , Chang LJ. RNA 3' readthrough of oncoretrovirus and lentivirus: implications for vector safety and efficacy. J Virol. 2002;76:7209–7219. doi: 10.1128/JVI.76.14.7209-7219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu BY, Persons DA, Evans-Galea MV, Gray JT., and , Nienhuis AW. A chromatin insulator blocks interactions between globin regulatory elements and cellular promoters in erythroid cells. Blood Cells Mol Dis. 2007;39:221–228. doi: 10.1016/j.bcmd.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Aker M, Tubb J, Groth AC, Bukovsky AA, Bell AC, Felsenfeld G, et al. Extended core sequences from the cHS4 insulator are necessary for protecting retroviral vectors from silencing position effects. Hum Gene Ther. 2007;18:333–343. doi: 10.1089/hum.2007.021. [DOI] [PubMed] [Google Scholar]
- Hanawa H, Persons DA., and , Nienhuis AW. High-level erythroid lineage-directed gene expression using globin gene regulatory elements after lentiviral vector-mediated gene transfer into primitive human and murine hematopoietic cells. Hum Gene Ther. 2002;13:2007–2016. doi: 10.1089/10430340260395866. [DOI] [PubMed] [Google Scholar]
- Harrison GP, Mayo MS, Hunter E., and , Lever AM. Pausing of reverse transcriptase on retroviral RNA templates is influenced by secondary structures both 5' and 3' of the catalytic site. Nucleic Acids Res. 1998;26:3433–3442. doi: 10.1093/nar/26.14.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasens BI, Huthoff HT, Das AT, Jeeninga RE., and , Berkhout B. The effect of template RNA structure on elongation by HIV-1 reverse transcriptase. Biochim Biophys Acta. 1999;1444:355–370. doi: 10.1016/s0167-4781(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Lanciault C., and , Champoux JJ. Pausing during reverse transcription increases the rate of retroviral recombination. J Virol. 2006;80:2483–2494. doi: 10.1128/JVI.80.5.2483-2494.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannaki E, Tubb J, Aker M, Stamatoyannopoulos G., and , Emery DW. Topological constraints governing the use of the chicken HS4 chromatin insulator in oncoretrovirus vectors. Mol Ther. 2002;5:589–598. doi: 10.1006/mthe.2002.0582. [DOI] [PubMed] [Google Scholar]
- Hanawa H, Hargrove PW, Kepes S, Srivastava DK, Nienhuis AW., and , Persons DA. Extended beta-globin locus control region elements promote consistent therapeutic expression of a gamma-globin lentiviral vector in murine beta-thalassemia. Blood. 2004;104:2281–2290. doi: 10.1182/blood-2004-03-0863. [DOI] [PubMed] [Google Scholar]
- Sastry L, Johnson T, Hobson MJ, Smucker B., and , Cornetta K. Titering lentiviral vectors: comparison of DNA, RNA and marker expression methods. Gene Ther. 2002;9:1155–1162. doi: 10.1038/sj.gt.3301731. [DOI] [PubMed] [Google Scholar]
- Van Maele B, De Rijck J, De Clercq E., and , Debyser Z. Impact of the central polypurine tract on the kinetics of human immunodeficiency virus type 1 vector transduction. J Virol. 2003;77:4685–4694. doi: 10.1128/JVI.77.8.4685-4694.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]