Abstract
Baculovirus holds great promise for the genetic modification of mesenchymal stem cells (MSCs). However, whether baculovirus transduction provokes undesired MSCs responses that might compromise their in vivo applications has yet to be examined. Hereby, we unraveled that baculovirus transduction of human MSCs upregulated the transcription of interleukin (IL)-1β, interferon (IFN)-α and IL-6, but not tumor necrosis factor (TNF)-α and IFN-γ. However, only IL-6 secretion was detectable by enzyme-linked immunosorbent assay (ELISA). Baculovirus transduction also stimulated transient, low level upregulation of human leukocyte antigen I (HLA-I) on the human MSCs surface, yet it did not either altered the HLA-II expression or impaired the MSCs ability to inhibit lymphocyte proliferation. After transplantation into allogeneic rats, the transduced rat MSCs elicited transient, mild macrophage responses, but the cells remained tolerant as judged by the persistence of transplanted cells and absence of CD8+ T cells infiltration. Besides, transplantation of the transduced MSCs did not provoke systemic induction of monocytes and CD8+ T cells. This study, for the first time, explores the responses of MSCs to virus transduction and confirms the safety of transplanting baculovirus-engineered MSCs into immunocompetent animals for cell-based gene therapy.
Introduction
Bone marrow mesenchymal stem cells (MSCs) are capable of self-renewal and multilineage differentiation into various cell types including adipocytes, chondrocytes and osteoblasts1,2 under appropriate environmental cues. Additionally, MSCs are immunoprivileged and can suppress lymphocyte alloreactivity in mixed lymphocyte cultures.3 These characteristics have captured interests to exploit MSCs as a promising cell therapy source. Moreover, MSCs can be engineered by gene delivery vectors and serve as a platform for cell-based gene therapy.1 By genetic modification, MSCs can express therapeutic proteins that promote or modulate the MSCs differentiation and accelerate tissue/organ regeneration in vivo. However, the gene delivery vectors in common use possess various drawbacks of their own (for review see ref. 4). For example, retrovirus and lentivirus are prone to integration into coding or regulatory regions of transcriptionally active genes,5 raising concerns about gene silencing and insertional mutagenesis. Adenovirus mounts strong immune responses, which may compromise its therapeutic effects in vivo. Adeno-associated virus is successfully employed for MSCs engineering, yet its cloning capacity is limited. Moreover, hepatocellular carcinoma resulting from adeno-associated virus-mediated gene therapy has been reported,6 thus calling the safety of adeno-associated virus vector into question.
Aside from these vectors, baculovirus (Autographa californica multiple nucleopolyhedrovirus) has emerged as a novel gene delivery vector in recent years. As a DNA virus that infects insect as its natural host, baculovirus does not either replicate or is not toxic inside the transduced cells (for review, see refs. 7,8,9,10) and baculoviral DNA degrades in the mammalian cells over time,11,12 thus easing the safety concerns about the use of baculovirus for gene therapy. Moreover, baculovirus can transduce human bone marrow–derived MSCs at efficiencies >95% under optimized conditions,13 and the transduced MSCs remain capable of differentiation into adipogenic, osteogenic, and chondrogenic lineages.11,14 Transduction of human MSCs ex vivo with a recombinant baculovirus that expresses the osteoinductive bone morphogenetic protein-2 triggers in vitro differentiation of MSCs into osteoblasts, and transplantation of the transduced cells into the back subcutis of immunodeficient nude mice results in ectopic bone formation.15 These data collectively implicate the potential of baculovirus for ex vivo–genetic modification of MSCs and bone-tissue engineering.
Although baculovirus holds great promise for MSCs engineering, it has been uncovered that baculovirus transduction of differentiated mammalian cells [e.g., hepatocytes, macrophages, and dendritic cells (DCs)] triggers antiviral effects and innate immunity, as evidenced by the upregulation of a panel of cytokines including interleukin (IL)-6 and interferons (IFNs) (e.g., IFN-α and IFN-β).16,17,18,19,20 Moreover, in vivo administration of baculovirus provokes cell-mediated immune responses including the activation of DCs, natural killer cells, and antigen-specific CD4+ and CD8+ T cells.21,22,23 These findings raise the question regarding whether baculovirus transduction of the undifferentiated MSCs provokes the same responses, and how the responses may influence the applications of baculovirus in the context of MSCs-based gene therapy. To address these questions, in this study, we investigated whether baculovirus transduction triggered cytokine responses and altered the immunological properties of MSCs. Whether transplantation of the baculovirus-transduced MSCs into immunocompetent animals elicited undesired rejection responses was also investigated.
Results
Cytokine expression by baculovirus-transduced MSCs
Since we have demonstrated the potential of human MSCs in tissue engineering, we first sought to determine whether human MSCs express inflammatory cytokines in response to baculovirus transduction. To avoid the responses elicited by the transgene, human MSCs were transduced with a baculovirus that expressed no transgene. As controls, the cells were either mock-transduced or incubated with the virus-free culture supernatant (supernatant). The gene transcription levels were measured by quantitative real-time reverse transcription PCR at different times and normalized against that of the mock-transduction control at 12 hours post-transduction (hpt).
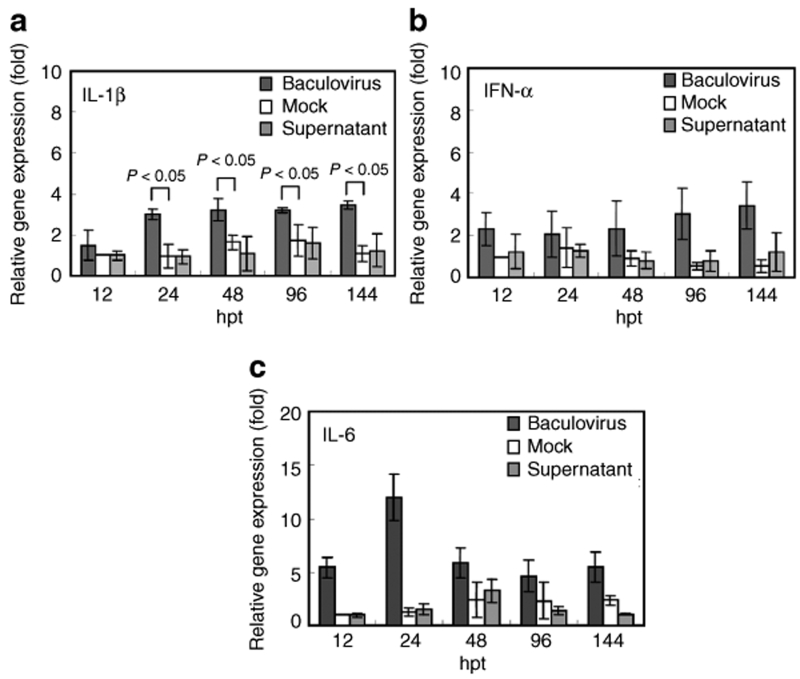
When compared with the mock-transduction control, incubation of MSCs with the insect cell culture supernatant did not provoke the upregulation of IL-1β, IFN-α and IL-6 at all time points (Figure 1a–c), but baculovirus transduction led to approximately two- to fourfold increases (P < 0.05) in the IL-1β and IFN-α transcription from 12 to 144 hpt (Figure 1a,b). Baculovirus transduction also gave rise to ≈12-fold upregulation of IL-6 at 24 hpt, which gradually waned thereafter (Figure 1c). However, neither baculovirus nor the supernatant significantly elicited the tumor necrosis factor (TNF)-α and IFN-γ transcription (data not shown).
Figure 1.
Cytokine expression by baculovirus-transduced MSCs. (a) IL-1β; (b) IFN-α; (c) IL-6. Human MSCs were transduced by a baculovirus that expressed no transgene at multiplication of infection 100. As controls, MSCs were mock-transduced (Mock) or treated with virus-free insect cell culture supernatant (Supernatant). The transcription levels of IL-1β, IFN-α, IL-6, TNF-α, and IFN-γ were measured by quantitative real-time reverse transcription-PCR at different times and normalized against that of the mock-transduction control at 12 hpt. The IFN-γ and TNF-α transcription levels were not significantly higher than the baseline levels and are not shown. IFN, interferon; IL, interleukin; MSCs, mesenchymal stem cells; TNF, tumor necrosis factor.
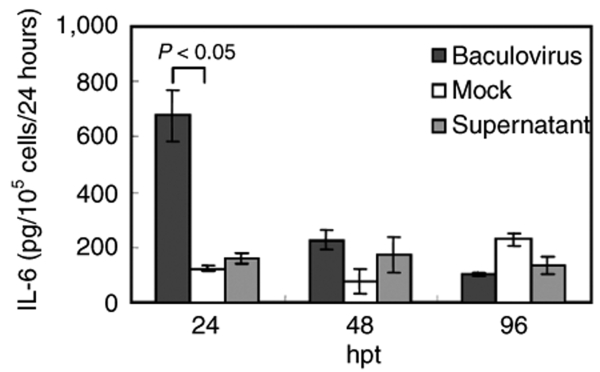
In line with the transcription data, the enzyme-linked immunosorbent assay (ELISA) data revealed that baculovirus transduction of MSCs provoked IL-6 secretion (Figure 2), which culminated at 24 hpt (≈676.5 pg/105 cells/24 hours) and then decreased precipitously thereafter (Figure 2). However, the supernatant did not trigger IL-6 secretion at levels significantly higher than that of mock-transduction controls. In contrast, the secretion of IL-1β and IFN-α by baculovirus-transduced MSCs fell below the detection limits of ELISA kits (<3.9 pg/ml for IL-1β and <8 pg/ml for IFN-α, data not shown), probably because the upregulation of these genes was not pronounced enough.
Figure 2.
Secretion of inflammatory cytokine (IL-6) by baculovirus-transduced MSCs. The MSCs were transduced as in Figure 1. After transduction, the spent media from the MSCs culture were completely replaced with fresh media at 24, 48, and 96 hpt. The concentrations of IL-1β, IFN-α, and IL-6 in the spent media were measured by ELISA. The IL-1β and IFN-α concentrations are not shown because they are lower than the detection limits of ELISA kits. ELISA, enzyme-linked immunosorbent assay; IFN, interferon; IL, interleukin; MSCs, mesenchymal stem cells.
Human leukocyte antigen expression on the baculovirus-transduced MSCs
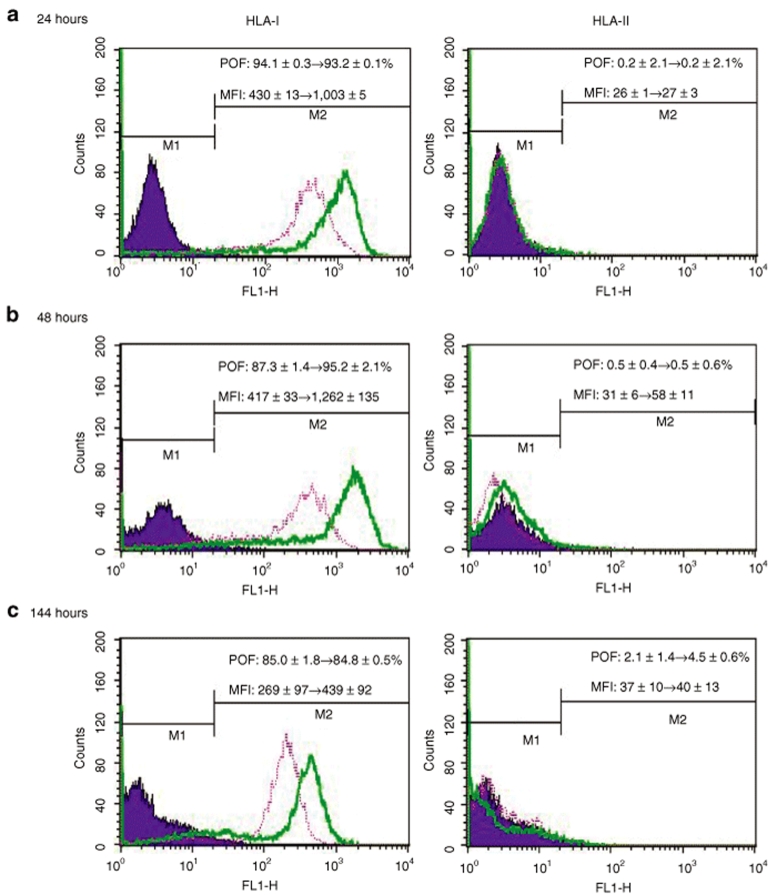
Human MSCs are immunoprivileged at least partly because they express moderate levels of class I human leukocyte antigen (HLA-I) and low levels of HLA-II,24 which play pivotal roles in determining the compatibility after tissue transplantation. To examine whether baculovirus transduction altered the immunological characteristics, we quantified the surface HLA by immunofluorescence labeling and flow cytometry. Prior to transduction, MSCs expressed moderate levels of HLA-I (percentage of fluorescing cells ≈91%, mean fluorescence intensity (MFI) ≈442) and low levels of HLA-II (percentage of fluorescing cells ≈0.2%, MFI ≈29, data not shown). Compared with the HLA-I expression on the mock-transduction controls (pink lines), the HLA-I expression levels on the surface of baculovirus-transduced cells (green lines) were elevated at 24 hpt (Figure 3a) and 48 hpt (Figure 3b), as evidenced by the significantly (P < 0.01) elevated MFI (from ≈430 to ≈1,003 at 24 hpt and from ≈417 to ≈1,262 at 48 hpt). Nonetheless, at 144 hpt (Figure 3c) the HLA-I expression considerably decreased as the MFI dropped (≈439) and approached that of the mock-transduction control. In contrast to HLA-I, the surface levels of HLA-II remained similarly low for the transduced and mock-transduced cells at all time points, indicating negligible induction of HLA-II by baculovirus transduction.
Figure 3.
Surface expression of HLA-I and HLA-II on the baculovirus-transduced MSCs. The transduced MSCs were detached at (a) 24, (b) 48, and (c) 144 hpt for immunofluorescence labeling and flow cytometry analysis (green lines). The mock-transduced cells were treated similarly as the control (pink lines). HLA, human leukocyte antigen; MFI, mean fluorescence intensity; MSCs, mesenchymal stem cells; POF, percentage of fluorescing cells.
Baculovirus-transduced MSCs remained capable of inhibiting lymphocyte proliferation
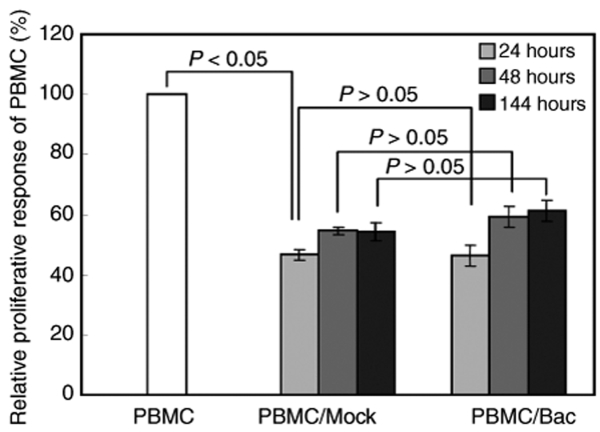
Human MSCs suppress lymphocyte proliferation in vitro in mixed lymphocyte cultures.3,25 This immunosuppressive property is essential for MSCs, allowing them to repress the severe graft-versus-host disease after transplantation into patients.26 To investigate whether baculovirus-transduced MSCs still retained the immunosuppressive property, the transduced and mock-transduced MSCs were harvested at different times (24, 48, or 144 hpt) for coculture with peripheral blood mononuclear cells (PBMC). The PBMC were stimulated with phytohaemagglutinin-L and their proliferation was measured 3 days after coculture by a bromodeoxyuridine (BrdU) proliferation kit.27 In comparison with the PBMC that were cultured alone (Figure 4), the PBMC proliferation was significantly inhibited (P < 0.05) when cocultured with either the mock-transduced or transduced MSCs. The levels of inhibition were statistically similar between the transduction and mock-transduction groups (P > 0.05), regardless of when (24, 48, or 144 hours) the MSCs were collected for coculture.
Figure 4.
Baculovirus (Bac)-transduced MSCs remained capable of inhibiting lymphocyte proliferation. MSCs were transduced or mock-transduced as in Figure 1 and the cells were harvested 24, 48, or 144 hours later for mixed lymphocyte culture. The PBMC (2 × 105 cells/well) were stimulated with phytohaemagglutanin-L and cocultured with the MSCs (2 × 104 cells/well) in the 96-well plates. The PBMC proliferation was measured 3 days after coculture by a bromodeoxyuridine proliferation kit. MSCs, mesenchymal stem cells; PBMC, peripheral blood mononuclear cells.
Distribution and survival of the transplanted MSCs
MSCs can escape immune recognition and suppress T cells activation in vivo.28 To explore whether baculovirus transduction of MSCs resulted in the loss of the immunomodulatory properties in vivo, we have transplanted human MSCs into the quadriceps of LEW/SsNNarl rats. However, these xenogeneic cells were seriously rejected as evidenced by rapid cell clearance and acute infiltration of macrophages and CD8+ T cells (data not shown). To circumvent the xenorejection, allogeneic bone marrow–derived MSCs were isolated from LEW/SsNNarl rats, transduced as described above, prelabeled with BrdU, trypsinized at 24 hpt, and then co-injected with carbon black (to track the location of the transplanted cells) into the quadriceps of LEW/SsNNarl rats. For comparison, the mock-transduced rat MSCs were treated and injected in a similar fashion.
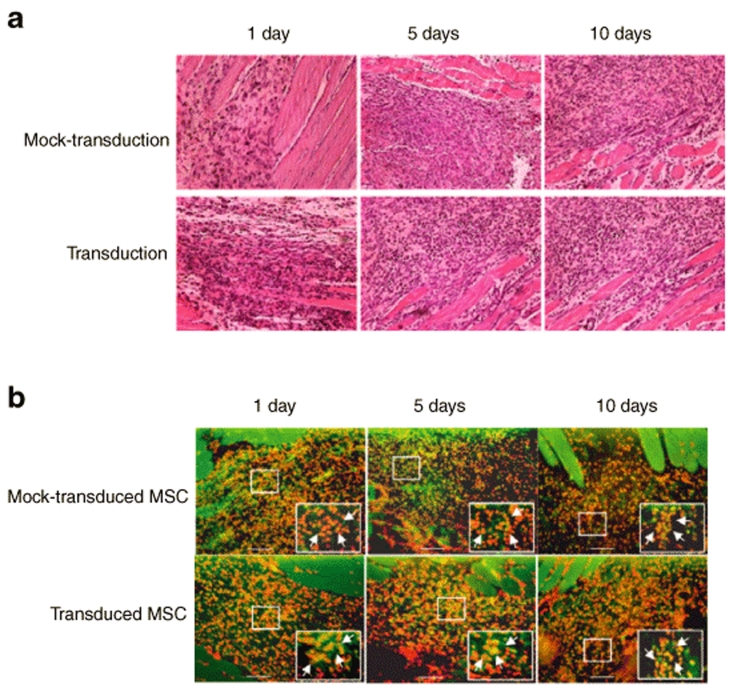
The tissue specimens colocalizing with the carbon black were removed at different time post-transplantation and examined by Hematoxylin and Eosin staining (Figure 5a) and immunohistochemical staining specific for BrdU (Figure 5b). Figure 5a shows that the injected cells were distributed near the muscle tissues and there was no apparent sign of immune cell infiltration. Similarly, Figure 5b reveals that the transduced and mock-transduced MSCs (orange cells) persisted near the muscle tissues and a large portion of the cells survived at 10 days post-transplantation. There appeared to be no manifest decrease in the number of transplanted MSCs over time, and there was no difference in the number of transplanted cells between the mock-transduction and the transduction groups.
Figure 5.
Distribution and survival of the transplanted MSCs. (a) Hematoxylin and Eosin staining. (b) Immunohistochemical staining. The transduced and mock-transduced rat MSCs were prelabeled with bromodeoxyuridine (BrdU) and co-injected with carbon black into the quadriceps of LEW/SsNNarl rats. The location of the transplanted cells was identified with the aid of the carbon black, and the tissue specimens were removed at 1, 5, or 10 days post-transplantation. The prelabeled MSCs were probed with the anti-BrdU monoclonal antibody and visualized with the Alexa 488-conjugated secondary antibody (green), while all nucleated cells were stained by 4′,6-diamidino-2-phenylindole (red), therefore the transplanted cells appear orange after superimposing the images. n = 3 for each time point. The original magnifications were ×200 (scale bars =100 µm). The enlarged images (magnification, ×1,000) are shown in the insets. MSCs, mesenchymal stem cells.
In vivo immune responses
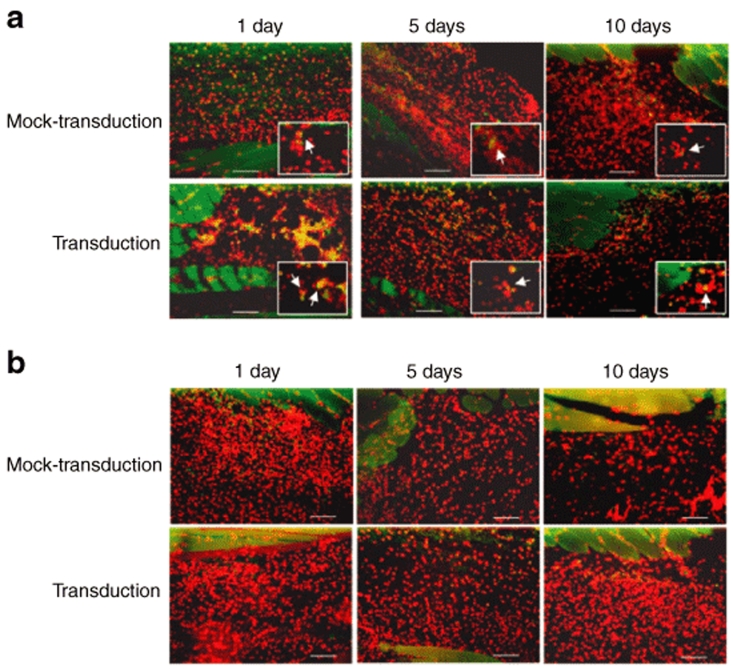
To assess the local immune responses triggered by the baculovirus-transduced MSCs, the infiltration of cells representative of the innate immunity (macrophages) and adaptive immunity (CD8+ T cells) was detected by immunohistochemical staining. CD4+ T cells were not examined as their activation requires HLA-II-mediated peptide presentation, which was minimal in MSCs (Figure 3). Figure 6a illustrates scarce distribution of macrophages (cells that stained green on the membrane) at the injection site of the mock-transduction group at day 1, and no signs of increased macrophage infiltration were observed through day 10. Conversely, at day 1, markedly more macrophages infiltrated into the injection site of the transduction group. The macrophage response subsided with time and nearly vanished at day 10. In contrast to the macrophage response, very few CD8+ T cells (cells that stained green on the membrane) were detected in both the mock-transduction and transduction groups (Figure 6b) from day 1 to day 10. These data indicated that transplantation of baculovirus-transduced MSCs only triggered local transient innate immune responses but not the adaptive immune responses.
Figure 6.
Infiltration of macrophages and CD8+ T cells into the transplantation site. (a) Immunohistochemical staining for macrophages. (b) Immunohistochemical staining for CD8+ T cells. The immune cells were visualized with Alexa 488-conjugated secondary antibody and appeared green on the cell membrane. All nucleated cells were stained by 4′,6-diamidino-2-phenylindole and appeared red. n = 3 for each time point. The original magnifications were ×200 (scale bars =100 µm). The enlarged images (magnification, ×1,000) are shown in the insets.
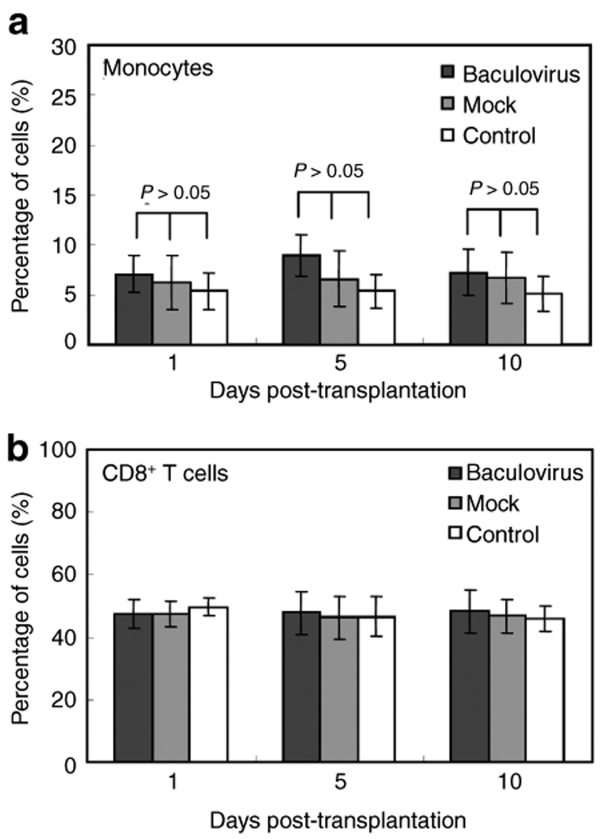
To quantify the systemic immune responses, the animals transplanted with the transduced or mock-transduced MSCs were sacrificed at 1, 5, and 10 days post-transplantation and the PBMC were collected. In parallel, the PBMC were collected from healthy animals without transplantation to serve as the control. The percentages of monocytes and CD8+ T cells in the PBMC population were measured by flow cytometry analysis after immunofluorescence labeling. Figure 7a shows slightly higher percentages of monocytes in the baculovirus-transduction group than in the mock-transduction group at days 1, 5, and 10. However, the differences were not statistically significant at all time points (P > 0.05). Likewise, the percentage of CD8+ T cells was unchanged in the baculovirus-transduction group at all times as compared with the mock-transduction group (Figure 7b).
Figure 7.
Quantification of monocytes and CD8+ T cells in the PBMC population. The percentages of (a) monocytes and (b) CD8+ T cells in the PBMC population were measured by immunofluorescence labeling/flow cytometry. The PBMC collected from healthy animals without transplantation serve as the control. n = 3 for each time point. Statistical analysis was performed by analysis of variance in conjunction with post hoc tests. PBMC, peripheral blood mononuclear cells.
Discussion
Baculovirus is a promising tool for gene delivery into MSCs, thankfully due to its high transduction efficiency (>95%), and the recombinant baculovirus expressing bone morphogenetic protein-2 has been harnessed to accelerate the osteogenic differentiation of MSCs and promote ectopic bone formation in immunodeficient animals.15 Given the numerous applications of genetically engineered MSCs1,2 (e.g., repair of cartilages and bones; repair of infracted myocardium; cancer therapy), baculovirus-engineered MSCs potentially have a wide array of applications worth of exploration. However, whether baculovirus transduction evokes unwanted cellular responses that might compromise the use of MSCs in immunocompetent animals has yet to be examined. It has been uncovered that the toll-like receptors of MSCs can be stimulated by pathogen-associated molecular patterns (e.g., lipopolysaccharide and Pam3Cys), leading to the induction of various cytokines and alteration of the MSCs migration and immunomodulatory responses.29,30 Furthermore, MSCs may influence such effector cells as T cells, B cells and DCs via the release of cytokines.31 As such, gaining insight into how MSCs respond to baculovirus transduction (e.g., by secretion of cytokines) is crucial to the safe use of baculovirus-transduced MSCs in vivo.
By this means, we unraveled that baculovirus transduction of MSCs upregulated the expression of pro-inflammatory genes including IL-1β and IFN-α, but not TNF-α and IFN-γ (Figure 1). Nonetheless, the secretion levels of IL-1β and IFN-α were too low to be detected by ELISA. This expression profile contradicted the profiles in other differentiated, specialized cell types such as macrophages and DCs, which secrete IFNs (IFN-α, IFN-β, and IFN-γ) and TNF-α in response to baculovirus transduction,16,17,18 and the disparity likely reflected the differences in the primitiveness of these cell types. IL-1β is a major mediator of inflammation and can stimulate the MSCs to secrete IL-6.32 IFN-α, IFN-γ, and TNF-α are important immunomodulatory cytokines that can activate T cells and recruit other immune cells to potentiate the immune responses. Also, MSCs acquire antigen-presenting functions following IFN-γ stimulation.28 As such, the absence of these cytokine responses reduces the possibility of mounting strong immune responses after transplantation.
In contrast to the aforementioned cytokines, IL-6 expression was markedly induced by baculovirus transduction as confirmed by quantitative real-time reverse transcription PCR and ELISA (Figures 1c and 2). It is known that IL-6 expression can be provoked as a result of virus infection and induction of toll-like receptor 3. In agreement with this notion, we found that baculovirus transduction stimulated the expression of toll-like receptor 3 (see Supplementary Figure S1), suggesting that baculovirus transduction may activate toll-like receptor 3. However, more experiments are required to confirm this finding. IL-6 is a common pro-inflammatory cytokine expressed by immune cells, and its overproduction is associated with the pathology of autoimmune diseases such as rheumatoid arthritis.29 Meanwhile, by nature, IL-6 is expressed abundantly by MSCs derived from bone marrow and cord blood32,33 and its expression is implicated in the regulation of development, differentiation, proliferation, and migration of different stem cells.34 For instance, continuous exposure of MSCs to IL-6 for 3 weeks impedes MSC differentiation.29 These findings suggest that IL-6 induction may impact on MSCs differentiation and potentiate the inflammatory response after transplantation. Fortunately, baculovirus transduction only elicited transient production of IL-6, which culminated at 24 hpt but subsided after 48 hpt. The transient response explains why baculovirus transduction does not impair MSCs proliferation11 and differentiation14 in vitro. These data, however, suggest that the MSCs should be transplanted after IL-6 responses cease, in order not to disturb the MSCs functions or provoke the immune systems in vivo.
Aside from cytokines, baculovirus transduction stimulated transient, low level upregulation of HLA-I on the MSCs surface, but barely influenced the HLA-II expression (Figure 3). Again, such response contradicted the DCs response to baculovirus transduction, which led to a striking upregulation of a number of surface molecules, including HLA-I and HLA-II.21,35 The discrepancy might arise from the fact that the undifferentiated MSCs only express moderate levels of HLA-I and low levels of HLA-II, hence baculovirus transduction probably was insufficient to trigger dramatic changes in the surface expression of HLA. Since HLA-I and HLA-II play pivotal roles in presenting endogenously synthesized and phagocytosed peptides to CD8+ and CD4+ T cells, the negligible induction of HLA-II as well as transient, low level induction of HLA-I are desired with regard to minimizing the presentation of peptides derived from the baculovirus or the transgene to T cells. Additionally, baculovirus-transduced and mock-transduced MSCs inhibited similar degrees of PBMC proliferation (Figure 4), attesting that baculovirus transduction does not impair the immunosuppressive property of MSCs.
Very importantly, after transplantation into allogeneic rats the mock-transduced rat MSCs were uniformly distributed and resided near the transplantation site without appreciable signs of cell clearance (Figure 5). The transduced MSCs were similarly tolerant in the recipient rats without signs of acute rejection, although the cells were transplanted at a time point when the responses culminated (at 24 hpt when IL-6 and HLA-I reached the maximum). Although the transduced MSCs resulted in the recruitment of macrophages into the injection site at day 1 post-transplantation, such inflammation response subsided at day 5 and virtually disappeared at day 10 (Figure 6a). Furthermore, CD8+ T cells were barely observed at the transplantation site throughout the experiment (Figure 6b), indicating the absence of adaptive immune response. Besides the local response, transplantation of the transduced MSCs did not provoke significant systemic induction of monocytes and CD8+ T cells (Figure 7). These data collectively attest that transplanting baculovirus-transduced MSCs into allogeneic immunocompetent animals only elicits inflammation responses that are mild, transient, and local, but provokes no rejection responses. These mild responses could be attributed to the low levels of cytokine induction and negligible perturbation of immunological characteristics of MSCs.
To our best knowledge, this is the first study exploring the responses of MSCs to virus transduction, although genetic modification of MSCs using various virus vectors has been reported.36,37,38,39,40,41 Our data unveils that baculovirus transduction of undifferentiated MSCs elicits distinct cytokine expression profile as compared with differentiated immune cells, and triggers transient upregulation of HLA-I expression, but does not compromise other immunological properties of MSCs. Furthermore, the baculovirus-transduced MSCs retained the immunoprivileged properties as they can evade immune recognition in allogeneic rats. These findings confirm the safety of transplanting baculovirus-transduced MSCs into immunocompetent animals.
Materials and Methods
Preparation and culture of bone marrow–derived MSCs. Human bone marrow–derived MSCs were obtained from Cambrex (Walkersville, MD) and the subsequent MSCs selection, enrichment, and culture were performed as described.11 Rat MSCs were obtained from the bone marrow of 6-week-old male LEW/SsNNarl rats. The femoral and tibial midshaft bone marrow was flushed into α-minimum essential medium (α-MEM) containing 20% fetal bovine serum (FBS; GIBCO, Grand Island, NY), 100 U/ml penicillin, 100 mg/ml streptomycin, and 0.25 µg/ml amphotericine (Sigma, St Louis, MO). A single-cell suspension was obtained by sequentially drawing the marrow into syringes through needles of decreasing size (gauge 18, 20, 22, respectively) and primary cultures of MSCs were seeded to 10-cm dishes (5 × 107 cells/dish). After 4 days of culture in a 37 °C, 5% CO2 incubator, the medium was replaced with fresh medium to remove nonadherent cells. MSCs were subcultured to passage 3 for transduction and transplantation.
Baculovirus preparation and transduction. A recombinant baculovirus that expressed no transgenes in mammalian cells was used throughout the study. The virus was amplified by infecting insect cells (Sf-9) cultured in TNM-FH medium containing 10% FBS, harvested by low speed centrifugation (1,000g for 5 minutes) and stored at 4 °C until use. The virus titer was determined by end-point dilution method.42 Alternatively, the infected culture supernatant was ultracentrifuged (80,000g for 90 minutes) to yield the virus-free supernatant.
Human and rat MSCs were transduced as described14 with minor modifications. In brief, MSCs were seeded onto six-well plates (2 × 105 cells/well) and cultured overnight. A certain volume of virus supernatant was mixed with phosphate-buffered saline (PBS) (pH 7.4) to adjust the final volume to 500 µl (per well). The transduction was initiated by directly adding the virus mixture to the cells and continued by gently shaking the six-well plates on a rocking plate for 4 hours at 25–27 °C. As controls, MSCs were incubated under the same conditions with the solution consisting of 400 µl PBS and 100 µl virus-free culture supernatant (supernatant) or TNM-FH medium (mock-transduction). After the incubation period, the cells were again washed with PBS and replenished with 2 ml α-MEM containing 20% FBS for culture at 37 °C.
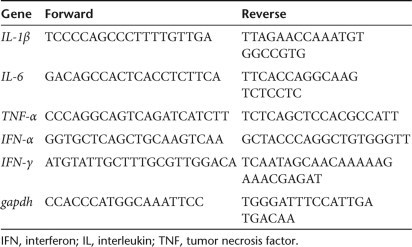
Quantitative real-time reverse transcription PCR. Total mRNA was isolated using RNeasy Mini Kit (Qiagen, Falencia, CA) and reverse transcribed to cDNA for 60 minutes at 37 °C using the RevertAid H Minus First Strand cDNA Synthesis kit (Fermentas, Hanover, MD). The cDNA was mixed with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) and subjected to real-time PCR using the primer pairs summarized in Table 1. The real-time PCR was performed using ABI 7300 (Applied Biosystems) under the following conditions: 2 minutes at 50 °C, 10 minutes at 95 °C, and then 40 cycles of 15 seconds at 95 °C and 1 minute at 60 °C. For each reaction, a no-template reaction was included as the negative control. The threshold cycle values of the target genes were normalized against that of the internal control gapdh. The data obtained at different time points were normalized against those of the mock-transduction control at 12 hpt.
Table 1.
The primer sequences used for quantitative real-time reverse transcription-PCR
ELISA. The spent media from the MSCs culture were collected at 24, 48, and 96 hpt and completely replaced with fresh media. The concentrations of IL-1β, IL-6, and IFN-α in the media were measured using Module set ELISA kits (Bender Medsystems, Vienna, Austria).
Flow cytometry for HLA expression. The transduced cells were trypsinized at 24, 48, and 144 hpt, washed and resuspended in PBS. Approximately 2 × 105 cells were incubated at 4 °C for 20 minutes with fluorescein isothiocyanate-conjugated monoclonal antibody (MAb) specific for HLA-I (antihuman HLA-ABC; eBioscience, San Diego, CA) or phycoerythrin -conjugated MAb specific for HLA-II (antihuman HLA-DR; eBioscience), and then analyzed by a flow cytometer (FACSCalibur; BD Biosciences, San Diego, CA). The mock-transduced cells were treated similarly to serve as controls. The percentage of fluorescing cells and MFI of each sample were measured three times by counting 10,000 cells in each measurement.
Mixed lymphocyte culture. The transduced and mock-transduced MSCs were harvested at 24, 48, or 144 hpt, treated with mitomycin-C (40 µg/ml; Sigma) for 30 minutes to stop MSCs proliferation and then seeded onto 96-well plates (2 × 104 cells/well). The PBMC were obtained by means of Histopaque-1077 (Sigma) gradient separation of peripheral blood from healthy donors. Purified PBMC were suspended in RPMI-1640 medium (Sigma) containing 5 µg/ml phytohaemagglutanin-L (PHA-L; Sigma) and 10% FBS, and added to the wells containing MSCs at a ratio of 1:10 (MSCs: PBMC). After 48 hours of incubation, 20 µl of 10 µmol/l BrdU (Merck, Darmstadt, Germany) was added to each well for 24 hours, and the proliferation of PBMC was measured using the BrdU cell proliferation assay kit (Merck) as described.27 In parallel, the PBMC were cultured in the presence of PHA-L for 3 days and their proliferation was defined as 100%.
Transplantation of baculovirus-transduced MSCs. Bone marrow–derived MSCs isolated from LEW/SsNNarl rats were transduced or mock-transduced and then cultured in α-MEM containing 10 µmol/l BrdU for prelabeling. At 24 hpt, the cells were trypsinized and resuspended in α-MEM (4 × 106 cells per 300 µl). To indentify the transplantation site after injection, 300 µl cell suspensions were mixed with 200 µl carbon black dispersion solution (Faber-Castell, Newark, NJ). The cell suspensions were injected into LEW/SsNNarl rats in the lateral regions of quadriceps using 1 ml syringes and 23 G needles.
Immunohistochemical staining. The rats were sacrificed at different times post-transplantation (n = 3 for each time point) in compliance with the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animals Resources, National Science Council. The transplantation site was identified by the carbon black. The tissue specimens were removed, fixed, and sectioned, and the sections were deparaffinized and rehydrated using xylene and a gradient of ethanol (100, 90, 70, 50, and 0%, respectively). The sections were treated with Target Retrieval solution (Dako, Hamburg, Germany) for 10 minutes at 90 °C and then blocked with PBS containing 10% FBS for 30 minutes at room temperature. Depending on the target, the sections were incubated at 4 °C overnight with mouse anti-BrdU MAb (1:100 dilution; Abcam, Cambridge, UK), mouse antimacrophage MAb (1:100 dilution; Abcam), or mouse anti-CD8 MAb (1:100 dilution; GeneTex, San Antonio, TX). After washing, the sections were incubated with goat antimouse IgG conjugated with Alexa Fluor 488 (Invitrogen, Carlsbad, CA) for 1 hour in the dark. Finally, the sections were mounted with the mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Labs, Burlingame, CA). These were observed using a fluorescence microscope and photographed.
Quantification of systemic immune response. The rats were sacrificed at 1, 5, and 10 days post-transplantation and the PBMC were collected from the hearts. In parallel, the PBMC were collected from healthy animals without transplantation to serve as the control. The purified PBMC (5 × 105 cells/ml) were incubated with mouse antimonocyte MAb (1:100 dilution; Abcam) or mouse anti-CD8 MAb for 20 minutes at 4 °C in the dark. After washing, the PBMC were incubated with goat antimouse IgG conjugated with Alexa Fluor 488 for 20 minutes at 4 °C in the dark. The percentage of fluorescing cells and MFI were measured three times by flow cytometry.
Statistical analysis. Statistical analyses for gene transcription, IL-6 secretion and PBMC proliferation were performed using independent samples t-tests. Statistical analyses for monocyte and CD8+ T cells in the blood were performed by analysis of variance in conjunction with post hoc analyses. The data are expressed as mean ± SD or mean values of three independent experiments. P < 0.05 was considered significant.
Supplementary Material Figure S1. Baculovirus transduction of MSCs stimulates the TLR3 expression. Baculovirus transduction of MSCs stimulates the TLR3 expression. The cells were transduced by baculovirus (a) or treated with poly (I:C) (b). Thirty minutes after treatment, the cells were probed with primary antibodies specific for TLR3 and then labeled with secondary antibodies conjugated with Alexa 488, followed by flow cytometry analysis. The cells treated with poly (I:C) served as the positive control.
Supplementary Material
Baculovirus transduction of MSCs stimulates the TLR3 expression. Baculovirus transduction of MSCs stimulates the TLR3 expression. The cells were transduced by baculovirus (a) or treated with poly (I:C) (b). Thirty minutes after treatment, the cells were probed with primary antibodies specific for TLR3 and then labeled with secondary antibodies conjugated with Alexa 488, followed by flow cytometry analysis. The cells treated with poly (I:C) served as the positive control.
Acknowledgments
We acknowledge the support from the National Tsing Hua University Booster Program (97N2511E1), VTY Joint Research Program, Tsou's Foundation (VGHUST97-P5-15), National Tsing Hua University-Chang Gung Memorial Hospital Joint Research Program (96N2425E1 and CMRPG361041), National Science Council (NSC 97-2627-B-007-014, NSC 97-2622-E-007-009-CC3) and Ministry of Economic Affairs (98-EC-17-A-17-R7-0525), Taiwan.
REFERENCES
- Kumar S, Chanda D., and , Ponnazhagan S. Therapeutic potential of genetically modified mesenchymal stem cells. Gene Ther. 2008;15:711–715. doi: 10.1038/gt.2008.35. [DOI] [PubMed] [Google Scholar]
- Abdallah B., and , Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2008;15:109–116. doi: 10.1038/sj.gt.3303067. [DOI] [PubMed] [Google Scholar]
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- Phillips JE, Gersbach CA., and , Garcia AJ. Virus-based gene therapy strategies for bone regeneration. Biomaterials. 2007;28:211–229. doi: 10.1016/j.biomaterials.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Bushman F, Lewinski M, Ciuffi A, Barr S, Leipzig J, Hannenhalli S, et al. Genome wide analysis of retroviral DNA integration. Nat Rev Microbiol. 2005;3:848–858. doi: 10.1038/nrmicro1263. [DOI] [PubMed] [Google Scholar]
- Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477–477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- Hu Y-C. Baculovirus vectors for gene therapy. Adv Virus Res. 2006;68:287–320. doi: 10.1016/S0065-3527(06)68008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y-C. Baculoviral vectors for gene delivery: a review. Curr Gene Ther. 2008;8:54–65. doi: 10.2174/156652308783688509. [DOI] [PubMed] [Google Scholar]
- Kost TA, Condreay JP, Ames RS, Rees S., and , Romanos MA. Implementation of BacMam virus gene delivery technology in a drug discovery setting. Drug Discov Today. 2007;12:396–403. doi: 10.1016/j.drudis.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Tani H, Abe T, Matsunaga TM, Moiihi K., and , Matsuura Y. Baculovirus vector for gene delivery and vaccine development. Future Virol. 2008;3:35–43. [Google Scholar]
- Ho Y-C, Chung Y-C, Hwang S-M, Wang K-C., and , Hu Y-C. Transgene expression and differentiation of baculovirus-transduced human mesenchymal stem cells. J Gene Med. 2005;7:860–868. doi: 10.1002/jgm.729. [DOI] [PubMed] [Google Scholar]
- Wang K-C, Wu J-C, Chung Y-C, Ho Y-C, Chang MD., and , Hu Y-C. Baculovirus as a highly efficient gene delivery vector for the expression of hepatitis delta virus antigens in mammalian cells. Biotechnol Bioeng. 2005;89:464–473. doi: 10.1002/bit.20385. [DOI] [PubMed] [Google Scholar]
- Lo W-H, Hwang S-M, Chuang C-K, Chen C-Y., and , Hu Y-C.Development of a hybrid baculoviral vector for sustained transgene expression Mol Ther 2009. in the press [DOI] [PMC free article] [PubMed]
- Ho Y-C, Lee H-P, Hwang S-M, Lo W-H, Chen H-C, Chung C-K, et al. Baculovirus transduction of human mesenchymal stem cell-derived progenitor cells: variation of transgene expression with cellular differentiation states. Gene Ther. 2006;13:1471–1479. doi: 10.1038/sj.gt.3302796. [DOI] [PubMed] [Google Scholar]
- Chuang C-K, Sung L-Y, Hwang S-M, Lo W-H, Chen H-C., and , Hu Y-C. Baculovirus as a new gene delivery vector for stem cells engineering and bone tissue engineering. Gene Ther. 2007;14:1417–1424. doi: 10.1038/sj.gt.3302996. [DOI] [PubMed] [Google Scholar]
- Hervas-Stubbs S, Rueda P, Lopez L., and , Leclerc C. Insect baculoviruses strongly potentiate adaptive immune responses by inducing type I IFN. J Immunol. 2007;178:2361–2369. doi: 10.4049/jimmunol.178.4.2361. [DOI] [PubMed] [Google Scholar]
- Abe T, Takahashi H, Hamazaki H, Miyano-Kurosaki N, Matsuura Y., and , Takaku H. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J Immunol. 2003;171:1133–1139. doi: 10.4049/jimmunol.171.3.1133. [DOI] [PubMed] [Google Scholar]
- Abe T, Hemmi H, Miyamoto H, Moriishi K, Tamura S, Takaku H, et al. Involvement of the toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus. J Virol. 2005;79:2847–2858. doi: 10.1128/JVI.79.5.2847-2858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowski AM, Hilbert DM, Sheehan KCF, Garotta G., and , Schreiber RD. Baculovirus stimulates antiviral effects in mammalian cells. J Virol. 1999;73:9944–9951. doi: 10.1128/jvi.73.12.9944-9951.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck NB, Sidhu JS., and , Omiecinski CJ. Baculovirus vectors repress phenobarbital-mediated gene induction and stimulate cytokine expression in primary cultures of rat hepatocytes. Gene Ther. 2000;7:1274–1283. doi: 10.1038/sj.gt.3301246. [DOI] [PubMed] [Google Scholar]
- Strauss R, Hüser A, Ni S, Tuve S, Kiviat N, Sow PS, et al. Baculovirus-based vaccination vectors allow for efficient induction of immune responses against Plasmodium falciparum circumsporozoite protein. Mol Ther. 2007;15:193–202. doi: 10.1038/sj.mt.6300008. [DOI] [PubMed] [Google Scholar]
- Facciabene A, Aurisicchio L., and , La Monica N. Baculovirus vectors elicit antigen-specific immune responses in mice. J Virol. 2004;78:8663–8672. doi: 10.1128/JVI.78.16.8663-8672.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C-H, Yoon J-S, Sohn H-J, Kim C-K, Paik S-Y, Hong Y-K, et al. Direct vaccination with pseudotype baculovirus expressing murine telomerase induces anti-tumor immunity comparable with RNA-electroporated dendritic cells in a murine glioma model. Cancer Lett. 2007;250:276–283. doi: 10.1016/j.canlet.2006.10.022. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Tammik C, Rosendahl K, Zetterberg E., and , Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- Oh W, Kim D-S, Yang Y-S., and , Lee J-K. Immunological properties of unbilical cord blood-derived mesenchymal stromal cells. Cell Immunol. 2008;251:116–123. doi: 10.1016/j.cellimm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Stagg J. Immune regulation by mesenchymal stem cells: two sides to the coin. Tissue Antigens. 2007;69:1–9. doi: 10.1111/j.1399-0039.2006.00739.x. [DOI] [PubMed] [Google Scholar]
- Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, et al. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109:1422–1432. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- Tomchuck SL, Zwezdaryk KJ, Coffelt SB, Waterman RS, Danka ES., and , Scandurro AB. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem Cells. 2008;26:99–107. doi: 10.1634/stemcells.2007-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta F, Angeli R, Cosmi L, Fili L, Manuelli C, Frosali F, et al. Toll-like receptors 3 and 4 are expressed by human bone marrow-derived mesenchymal stem cells and can inhibit their T-cell modulatory activity by impairing Notch signaling. Stem Cells. 2008;26:279–289. doi: 10.1634/stemcells.2007-0454. [DOI] [PubMed] [Google Scholar]
- Liu CH., and , Hwang SM. Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine. 2005;32:270–279. doi: 10.1016/j.cyto.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Wagner W, Roderburg C, Wein F, Diehlmann A, Frankhauser M, Schubert R, et al. Molecular and secretory profiles of human mesenchymal stromal cells and their abilities to maintain primitive hematopoietic progenitors. Stem Cells. 2007;25:2638–2647. doi: 10.1634/stemcells.2007-0280. [DOI] [PubMed] [Google Scholar]
- Klassen HJ, Imfeld KL, Kirov II, Tai LQ, Gage FH, Young MJ, et al. Expression of cytokines by multipotent neural progenitor cells. Cytokine. 2003;22:101–106. doi: 10.1016/s1043-4666(03)00120-0. [DOI] [PubMed] [Google Scholar]
- Schutz A, Scheller N, Breinig T., and , Meyerhans A. The Autographa californica nuclear polyhedrosis virus AcNPV induces functional maturation of human monocyte-derived dendritic cells. Vaccine. 2006;24:7190–7196. doi: 10.1016/j.vaccine.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Olmsted-Davis EA, Gugala Z, Gannon FH, Yotnda P, McAlhany RE, Lindsey RW, et al. Use of a chimeric adenovirus vector enhances BMP2 production and bone formation. Hum Gene Ther. 2002;13:1337–1347. doi: 10.1089/104303402760128568. [DOI] [PubMed] [Google Scholar]
- Meinel L, Hofmann S, Betz O, Fajardo R, Merkle HP, Langer R, et al. Osteogenesis by human mesenchymal stem cells cultured on silk biomaterials: Comparison of adenovirus mediated gene transfer and protein delivery of BMP-2. Biomaterials. 2006;27:4993–5002. doi: 10.1016/j.biomaterials.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Kumar S, Mahendra G, Nagy TR., and , Ponnazhagan S. Osteogenic differentiation of recombinant adeno-associated virus 2-transduced murine mesenchymal stem cells and development of an immunocompetent mouse model for ex vivo osteoporosis gene therapy. Hum Gene Ther. 2004;15:1197–1206. doi: 10.1089/hum.2004.15.1197. [DOI] [PubMed] [Google Scholar]
- Engstrand T, Daluiski A, Bahamonde ME, Melhus H., and , Lyons KM. Transient production of bone morphogenetic protein 2 by allogeneic transplanted transduced cells induces bone formation. Hum Gene Ther. 2000;11:205–211. doi: 10.1089/10430340050016274. [DOI] [PubMed] [Google Scholar]
- Sugiyama O, An DS, Kung SPK, Feeley BT, Gamradt S, Liu NQ, et al. Lentivirus-mediated gene transfer induces long-term transgene expression of BMP-2 in vitro and new bone formation in vivo. Mol Ther. 2005;11:390–398. doi: 10.1016/j.ymthe.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Palmer GD, Steinert A, Pascher A, Gouze E, Gouze JN, Betz O, et al. Gene-induced chondrogenesis of primary mesenchymal stem cells in vitro. Mol Ther. 2005;12:219–228. doi: 10.1016/j.ymthe.2005.03.024. [DOI] [PubMed] [Google Scholar]
- O'Reilly D, Miller L., and , Luckow V. Baculovirus Expression Vectors: A Laboratory Manual. W.H. Freeman: New York; 1992. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baculovirus transduction of MSCs stimulates the TLR3 expression. Baculovirus transduction of MSCs stimulates the TLR3 expression. The cells were transduced by baculovirus (a) or treated with poly (I:C) (b). Thirty minutes after treatment, the cells were probed with primary antibodies specific for TLR3 and then labeled with secondary antibodies conjugated with Alexa 488, followed by flow cytometry analysis. The cells treated with poly (I:C) served as the positive control.