Abstract
Acute lung inflammation is a potentially life-threatening complication of infections due to community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA), a worldwide emerging pathogen, which causes necrotizing pneumonia and acute respiratory distress syndrome (ARDS). MRSA virulence factors encompass immunotoxins termed superantigens that contribute to lung inflammation. In this study, we demonstrate that staphylococcal enterotoxin B (SEB)-induced lung inflammation is attenuated by a cell-penetrating peptide nuclear import inhibitor of nuclear factor (NF)-κB and other stress-responsive transcription factors (SRTFs). This inhibitor suppressed production of a wide spectrum of cytokines and chemokines induced by direct SEB airway exposure. Consequently, trafficking of neutrophils, monocytes/macrophages, and lymphocytes to the bronchoalveolar space was significantly reduced while vascular injury, manifested by increased permeability and protein leakage, was attenuated. Moreover, induction of systemic proinflammatory cytokines and chemokines in response to direct SEB airway exposure was reduced. Thus, intracellular delivery of a nuclear import inhibitory peptide suppresses respiratory and systemic expression of key mediators of lung inflammation evoked by SEB.
Introduction
Staphylococcus aureus is one of the most prominent bacterial pathogens in the community and hospital setting.1 Its global spread is alarming due to the rapidly emerging community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA). In 2005, these highly virulent staphylococci were responsible for more estimated deaths in the United States than HIV/AIDS.2 CA-MRSA can carry genetic elements encoding Panton-Valentine leukocidin and other immunotoxins known as superantigens. One of them, staphylococcal enterotoxin B (SEB), is capable of inducing fatal acute respiratory distress syndrome (ARDS) in nonhuman primates on airborne exposure, suggesting its use as a potential bioweapon.3,4,5,6,7,8,9 In humans, CA-MRSA infection encompasses a potentially fatal necrotizing pneumonia that complicates the seasonal outbreaks of influenza.10 Thus, necrotizing pneumonia caused by CA-MRSA and ventilator-associated pneumonia caused by hospital-acquired MRSA represent an increasing risk for acute lung injury (ALI) and its more severe form, ARDS.3,4,7,11,12 Unfortunately, worldwide attempts to reduce the spread of MRSA have been met with mixed success.13
SEB, as a virulence factor, induces robust proliferation of T cells.14 SEB targets antigen-presenting cells that express major histocompatibility complex class II and inflammatory CD4+ T cells that express the T-cell receptor Vβ8.2 in mice and T-cell receptor Vβ3, 12, 14, and 17 in humans.15 SEB clamps the antigen-presenting cells and T cells together, forming a tight signaling synapse that is responsible for robust production of proinflammatory cytokines and chemokines.16,17,18 In turn, the ensuing cytokine storm induces fever, endothelial injury, ALI/ARDS, multiple organ dysfunction, disseminated intravascular coagulation, vascular collapse (shock), and possibly death.19,20
The SEB-triggered cytokine storm consists of uncontrolled production of proinflammatory cytokines and chemokines, including tumor necrosis factor-α, interferon- (IFN-γ), and interleukins (IL)-1, 2, 6, 8, and 12.21,22,23 The genes that encode these cytokines are under the control of nuclear factor (NF)-κB and other stress-responsive transcription factors (SRTFs), including, activator protein-1, NF of activated T cells, and signal transducer and activator of transcription 1.24 Following their import from the cytoplasm to the nuclear compartment, NF-κB, along with other SRTFs, acts in concert to stimulate transcription of multiple genes encoding cytokines, chemokines, and other mediators of inflammation.25,26,27 We hypothesized that targeting the nuclear import machinery would attenuate SEB-induced production of inflammatory mediators in the lungs. To test this hypothesis, we used a cell-penetrating peptide inhibitor of nuclear import in a murine model of ALI induced by direct airway exposure to SEB. This model facilitates monitoring of inflammatory mediators in the bronchoalveolar space, including direct analysis of nuclear translocation of NF-κB in lung-derived inflammatory cells.
We report that intracellular delivery of a nuclear import inhibitor attenuates: (i) the induction of proinflammatory cytokines and chemokines in the bronchoalveolar space, (ii) trafficking of inflammatory cells therein in response to direct airway exposure to SEB, and (iii) pulmonary vascular injury. Thus, nuclear import inhibitory peptide can avert ALI mediated by a wide range of proinflammatory cytokines/chemokines in response to direct SEB airway exposure.
Results
Intracellular delivery of nuclear import inhibitor, cSN50, ex vivo and in vivo attenuates cytoplasmic/nuclear transport of NF-κB induced by SEB in bronchoalveolar leukocytes/lymphocytes
After having demonstrated previously that the cell penetrating nuclear import inhibitory peptide, cSN50, is delivered to blood leukocytes/lymphocytes, spleen, and liver to suppress acute liver inflammation and apoptosis;28,29 we hypothesized that cSN50 might suppress acute lung inflammation induced by direct SEB airway exposure. We tested this hypothesis by determining first whether fluorescein isothiocyanate (FITC)-labeled cSN50 peptide is delivered to the murine lungs after a single intraperitoneal (IP) injection. After 30 minutes, lungs were perfused with saline followed by analysis of cryosections by fluorescence microscopy. As shown in Figure 1, FITC-labeled cSN50 efficiently reached the lung parenchyma while the alveolar air space was free of fluorescence. FITC-labeled noncell-penetrating cN50 peptide, which contains the same cargo (cyclized NLS) but lacks the membrane translocating motif, produced a weak background of fluorescence, likely due to its nonspecific binding to cellular surfaces. Results obtained in mice treated with saline were similar to those treated with FITC only (data not shown). These results indicate that the lungs are efficiently targeted by cSN50, which depends on its membrane-translocating motif for rapid intracellular delivery after IP injection.
Figure 1.
Intracellular delivery of cell-penetrating peptide cSN50 to lungs. Cryosections (10 µm) of lungs were analyzed with fluorescence microscope. The image shows fluorescein isothiocyanate (FITC)-labeled cSN50 peptide distributed in lung parenchyma after intraperitoneal injection. Noncell-penetrating FITC-labeled cN50 peptide, which displayed the equivalent fluorescence units, and saline, as control, do not produce fluorescent signal in lung cells comparable to that of FITC-labeled cSN50. Bar = 18 µm.
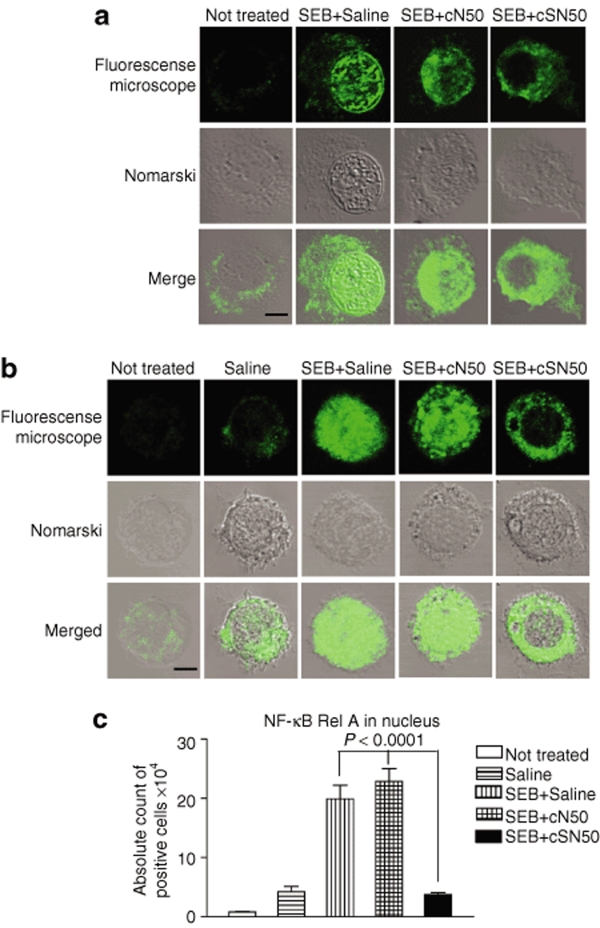
Subsequently, we examined the effect of cSN50 on cytoplasmic/nuclear transport of NF-κB, as a prototypical SRTF involved in SEB-induced inflammation.28 We conducted these experiments ex vivo in primary cells isolated from bronchoalveolar lavage (BAL) and stimulated with SEB. BAL cells were treated with cSN50 or the noncell penetrating cN50 as a control. We monitored nuclear translocation of NF-κB complexes using a monoclonal antibody directed to the Rel A subunit of NF-κB by confocal microscopy. Among 200 analyzed SEB-stimulated lymphocytes/leukocytes treated with control noncell-penetrating cN50 peptide, 32% were positive for nuclear translocation of NF-κB Rel A by displaying a strong fluorescence signal in the nuclei, which represents immunoreactive NF-κB Rel A. Likewise, 37% of SEB-stimulated cells treated with saline were positive, In contrast, treatment with cSN50 reduced positive nuclear fluorescence to only 10% of SEB-stimulated cells (Figure 2a). This result is similar to 15% of control nonstimulated cells, which displayed a nuclear fluorescence signal. As documented in Figure 1 and determined previously in our earlier studies,30,31 the transduction efficiency of cell penetrating peptides cSN50 and SN50 is >90% in cultured primary cells and in vivo. These peptides, which contain a membrane translocating motif derived from signal sequence hydrophobic region, penetrate plasma membrane of all cell types studied by us so far. Hence the difference in percentage of positive cells that display nuclear fluorescence due to translocation of NF-κB Rel A indicate that this SEB-induced process occurs in about one-third of cells in BAL fluid. In other words, not all cells are SEB responsive whereas almost all BAL cells are transduced with cSN50 peptide. In contrast, control peptide cN50 is not cell penetrating thereby it has no effect on SEB-induced nuclear translocation.
Figure 2.
SEB-induced nuclear translocation of NF-κB in BAL cells is inhibited by cSN50 peptide but not cN50 peptide. (a) The BAL cells were collected and pretreated ex vivo with peptides or saline, before stimulation with SEB (10 µg/ml) for 3 hours. (b) Mice were treated in vivo with peptides cSN50 (0.7 mg), cN50 (0.4 mg), or saline injected intraperitoneal 30 minutes before SEB direct airway exposure (intranasal) and thereafter (30, 90, and 150 minutes). Mice were killed 3 hours after SEB and the BAL cells were collected. After fixation and staining with a specific anti-Rel A NF-κB antibody, the cells were analyzed by fluorescence microscopy (see Materials and Methods for details). Pictures are representative of cells from three independent experiments. Fluorescence signal indicates intracellular localization of NF-κB Rel A (top). Nomarski image of the same cells and merged image are shown in the middle and bottom rows, respectively. Bar = 4.5 µm. (c) The absolute number of positive cells showing nuclear translocation of NF-κB Rel A = total cells in BAL × percentage of positive cells. Error bars indicate ± SEM of the mean value in three to six mice that are represented by each group. The P values (one-way analysis of variance) represent the significance of the difference between the control peptide cN50 or saline and cSN50 peptide-treated groups. BAL, bronchoalveolar lavage; NF, nuclear factor; SEB, staphylococcal enterotoxin B.
We extended this analysis to the in vivo system by demonstrating the effectiveness of systemically administered cSN50 in blocking nuclear import of NF-κB Rel A in BAL cells after direct airway exposure with SEB. As illustrated in Figure 2b, IP-administered cSN50, but not cN50, inhibited nuclear translocation of NF-κB in BAL cells in response to intranasal (IN) administration of SEB. As quantitatively recapitulated in Figure 2c, in vivo treatment with cSN50 reduced the number of BAL cells displaying nuclear translocation of NF-κB by 85%. Cumulatively, these ex vivo and in vivo results with lung-derived inflammatory cells responding to SEB document that intracellular delivery of cSN50 is effective in targeting the nuclear import machinery thereby preventing cytoplasmic/nuclear transport of NF-κB. Moreover, these results are consistent with inhibition of nuclear import of NF-κB and other SRTFs by a prototypical cell-penetrating SN50 peptide in cultured human T cells.32
cSN50 peptide inhibits cytokine/chemokine expression in the lung in response to SEB
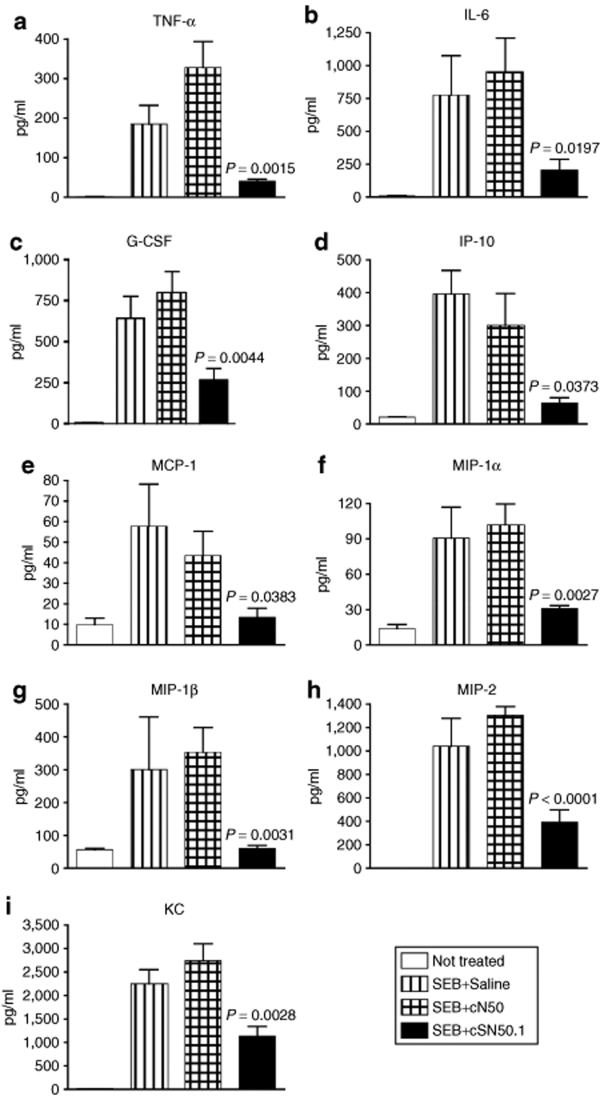
Airway exposure to aerosolized SEB in nonhuman primates causes a fatal ARDS that is associated with uncontrolled production of proinflammatory cytokines.9 We hypothesized that the inflammatory response initiated by direct airway exposure of SEB can be blocked by targeted disruption of SRTF nuclear import. Utilizing a murine model of SEB-induced ALI,33,34 we tested the ability of cell-penetrating peptide, cSN50, to suppress this process as compared to the noncell-penetrating cN50 control. Therefore, we monitored production of proinflammatory cytokines and chemokines in BAL fluid after challenging mice intranasally with SEB. Four hours after SEB challenge, mice were anesthetized and BAL was performed. As shown in Figure 3, mice treated with cSN50 had markedly reduced level of a wide spectrum of cytokines and chemokines. For example, cSN50 reduced expression of tumor necrosis factor-α (P < 0.002), IL-6 (P < 0.02), macrophage-inflammatory protein-2 (P < 0.001), monocyte chemotactic protein-1 (P = 0.04), keratinocyte-derived chemokine (P = 0.003), and IP-10 (P = 0.04) as compared to mice treated with the control peptide. Suppression of six chemokines by cSN50 was of particular significance due to their role in the induction of inflammatory cell migration to the bronchoalveolar space. Moreover, significant suppression of granulocyte colony stimulating factor production, which is responsible for emergency granulocytosis, adds additional anti-inflammatory mechanism to the action of cSN50.
Figure 3.
SEB-induced burst of inflammatory cytokine/chemokines in bronchoalveolar space is suppressed in animals treated with a cell-penetrating nuclear import inhibitor cSN50 peptide. Wild-type BALB/c mice were treated with equimolar doses of cSN50 peptide (0.7 mg) or cN50 (0.4 mg), or diluent in five intraperitoneal injections before and after administration of SEB (5 µg in 50 µl of saline) by intranasal instillation (see Materials and Methods for details). Mice were killed and bronchoalveolar lavage fluid was collected 4 hours after SEB instillation. (a,b) The cytokines, (c) G-CSF, and (d–i) chemokines were measured. Error bars indicate ± SEM of the mean value in six mice that are represented by each data point. The P values shown represent the significance of the difference between the control peptide cN50 and cSN50 peptide treated groups. G-CSF, granulocyte colony stimulating factor; IL, interleukin; IP, intraperitoneal; MCP-1, monocyte chemotactic protein-1; MIP-1, macrophage-inflammatory protein-1; SEB, staphylococcal enterotoxin B.
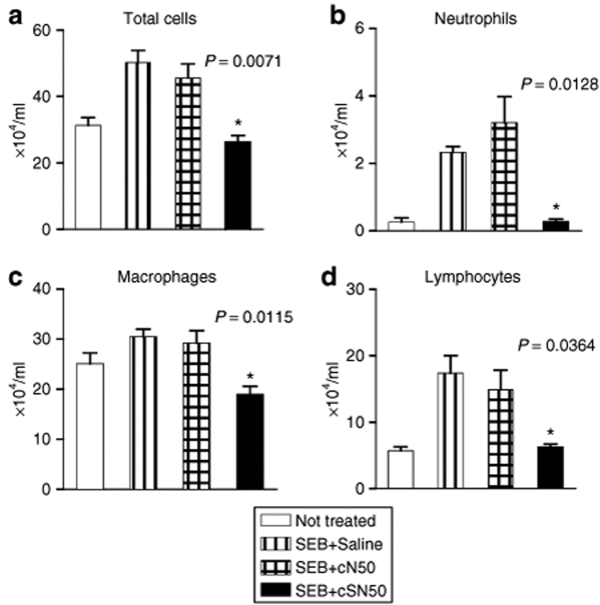
Inflammatory cell trafficking to the bronchoalveolar space in response to SEB is attenuated by cSN50
Consistent with suppression of a wide spectrum of chemokines by cSN50, the migration of inflammatory cells to the bronchoalveolar space was also inhibited. In response to direct airway exposure of SEB, the total cell count in BAL fluid increased 2.5-fold (Figure 4). In terms of individual cell types, the number of neutrophils in BAL fluid increased most dramatically (10- to 12-fold). The monocyte/macrophage count in BAL was moderately increased and lymphocyte influx was 2.5-fold higher in SEB-challenged mice as compared to control animals. Treatment with cSN50, but not with the control cN50 peptide, significantly reduced the trafficking of three types of inflammatory cells, neutrophils (P = 0.01), macrophages (P = 0.01), and lymphocytes (P = 0.04). The capacity of cSN50 to potently inhibit the migration of neutrophils is noteworthy given that these phagocytes represent a primary source of oxidant injury to the lungs.35
Figure 4.
Suppression of SEB-induced migration of leukocytes/lymphocytes to bronchoalveolar space by a cell-penetrating nuclear import inhibitor cSN50. Wild-type BALB/c mice were treated with cSN50 peptide (0.7 mg), cN50 (0.4 mg) or diluent in five intraperitoneal injections before and after administration of SEB (5 µg in 50 µl of saline) by intranasal instillation. Mice were killed and BAL fluid was collected 4 hours after SEB challenge. The total number of (a) leukocytes/lymphocytes, (b) neutrophils, (c) monocytes/macrophages, and (d) lymphocytes, were determined in BAL fluid (see Materials and Methods for details). Error bars indicate ± SEM of the mean value in six mice that are represented by each data point. The P values shown represent the significance of the difference between the control cN50 peptide and cSN50 peptide treated groups. BAL, bronchoalveolar lavage; SEB, staphylococcal enterotoxin B.
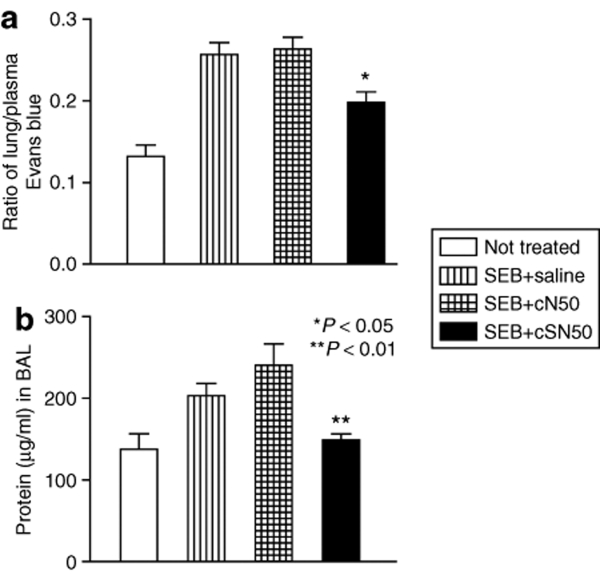
SEB-induced lung vascular injury is attenuated by cSN50 treatment
In response to direct airway exposure of SEB, the burst of cytokines/chemokines has the potential to alter the delicate architecture of the respiratory epithelium and vascular endothelium required for air/blood exchange. For example, increased vascular permeability caused by IL-236 opens up intercellular spaces in vascular endothelium for leakage of plasma proteins into the lungs. Indeed, IN installation of SEB results in increased vascular permeability of the lung parenchyma that includes an increase in the protein content of BAL fluid (Figure 5). These two indicators of injury to respiratory epithelial and endothelial barriers were significantly improved after administration of cSN50 (P < 0.03 and <0.01, respectively). Thus, endothelial and epithelial injury after respiratory exposure of SEB was attenuated by intracellular delivery of cSN50.
Figure 5.
Suppression of SEB-induced lung vascular permeability and protein leakage. Wild-type BALB/c mice were treated with cSN50 or cN50 peptides or saline before and after administration of SEB. (a) Each animal received 60 mg/kg Evans blue in saline by tail vein injection 1 hour before killing. Mice were killed and lungs were removed 4 hours after SEB instillation. Evans blue in lung homogenates was quantified by a dual wavelength spectrophotometric method (see Materials and Methods for details). (b) The protein concentration in cell-free BAL fluid was measured. Error bars indicate ± SEM of the mean value in five mice that are represented by each data point. The P values shown represent the significance of the difference between the control peptide cN50 and cSN50 peptide-treated groups. BAL, bronchoalveolar lavage; SEB, staphylococcal enterotoxin B.
Direct airway exposure to SEB evokes systemic cytokine/chemokine production suppressed by cSN50 peptide delivery
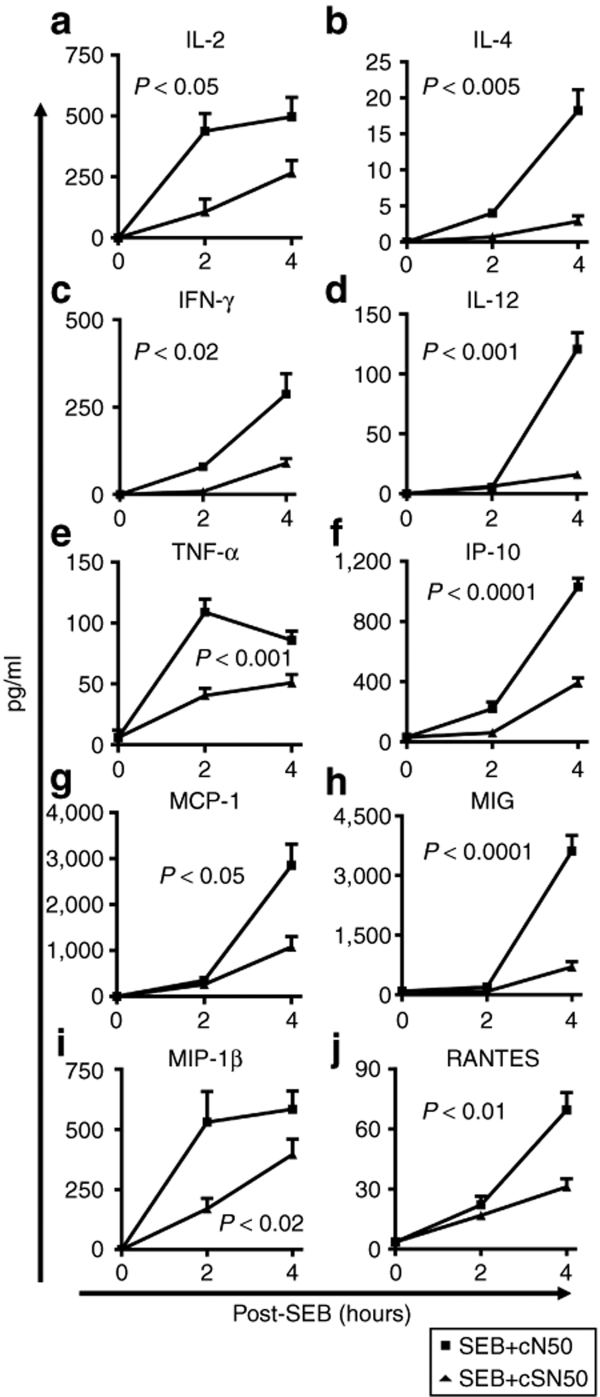
Consistent with the previous findings in nonhuman primates,9 we noted a significant production of blood proinflammatory cytokines and chemokines following direct airway exposure of SEB in control mice (Figure 6). Within this broad spectrum of cytokines and chemokines, their expression peaked rapidly at 2 hours (tumor necrosis factor-α) or increased progressively during the 4-hour time course (IL-2, IFN-γ, IL-12, IP-10, monocyte chemotactic protein-1, monokine-induced by IFN-γ, macrophage-inflammatory protein-1β, and regulated upon activation, normal T-cell expressed and secreted). Strikingly, the wide range response of five blood cytokines and five blood chemokines to respiratory exposure of SEB was significantly attenuated by the cell-penetrating cSN50 peptide. Plasma levels of IL-2 (P < 0.05), IFN-γ (P < 0.02), IL-12 (P < 0.001), tumor necrosis factor-α (P < 0.001), IP-10 (P < 0.0001), monocyte chemotactic protein-1 (P < 0.05), monokine-induced by IFN-γ (P < 0.0001), macrophage-inflammatory protein-1β (P < 0.02), and regulated upon activation, normal T-cell expressed and secreted (P < 0.01) were suppressed. Thus, direct airway exposure of SEB evoked a wide-ranging systemic proinflammatory cytokine and chemokine response that was significantly suppressed by intracellular delivery of cell-penetrating cSN50 peptide. Taken together, the cSN50 peptide-directed inhibition of nuclear import of NF-κB and other SRTFs attenuates the pulmonary and systemic inflammatory response to SEB.
Figure 6.
Inflammatory cytokine/chemokine production in blood, due to direct airway exposure of SEB, is suppressed in mice treated with cSN50. Wild-type BALB/c mice were treated with cSN50 (0.7 mg) or cN50 (0.4 mg) in five intraperitoneal injections before and after administration of SEB (5 µg in 50 µl of saline) by intranasal instillation (see Materials and Methods for details). Blood plasma levels of the indicated (a–e) cytokines and (f–j) chemokines in cSN50-treated mice (triangles) were reduced in comparison to mice treated with the control, cN50 (squares). Error bars indicate ± SEM of the mean value in six mice that are represented by each data point. The P values represent the significance of the difference between cN50 and cSN50 peptide-treated groups. IFN-γ, interferon-γ; IL, interleukin; IP, intraperitoneal; MCP-1, monocyte chemotactic protein-1; MIP-1, macrophage-inflammatory protein-1; SEB, staphylococcal enterotoxin B.
Discussion
We demonstrated that intracellular peptide delivery of a nuclear import inhibitor effectively attenuated acute lung inflammation induced by SEB, a virulence factor of MRSA. Our analysis of the mechanism of cSN50 peptide delivery and action in ALI, offers a new approach to counteracting excessive lung inflammation caused by microbial agents. Endothelial and epithelial injury by the uncontrolled production of proinflammatory chemokines and cytokines constitutes a cardinal feature of necrotizing pneumonia and ARDS caused by MRSA. As documented in this study, the collateral damage to delicate epithelial and endothelial lung compartments caused by lung inflammation can be reduced by rapid delivery of the cell-penetrating peptide that targets nuclear importation of NF-κB and other SRTFs. Thus, reduction of a broad spectrum of proinflammatory chemokines and cytokines induced by direct airway exposure to SEB may have a salutary effect on other lung-associated inflammatory cells. For example, intracellular delivery of cSN50 significantly reduced trafficking of neutrophils and other inflammatory cells to the bronchoalveolar space and the attendant vascular permeability and protein leakage. In terms of the mechanism of action, cSN50 peptide suppressed production of key cytokines elaborated by CD4+ T cells, which are primary targets of SEB. At least two such cytokines, IL-2 and IFN-γ37,38,39 through their localized action, contribute to the disruption of lung endothelial and epithelial barriers36 manifested by migration of leukocytes to the bronchoalveolar space, leakiness of proteins therein, and impairment of respiratory function. Suppression of the chemokines IP-10, monocyte chemotactic protein-1, monokine-induced by IFN-γ, macrophage-inflammatory protein-1β and regulated upon activation, normal T-cell expressed and secreted by cSN50 is of particular significance due to their role in induction of inflammatory cell migration to the bronchoalveolar space.40,41 Consistent with localized suppression of these chemokines by cSN50, a significant migration of neutrophils and other inflammatory cells to the bronchoalveolar space was attenuated, thereby reducing potential oxidant injury to the respiratory epithelium and vascular endothelium. Moreover, systemic production of proinflammatory cytokines and chemokines, which contribute to the subsequent development of toxic shock, was also attenuated.
Direct ex vivo and in vivo demonstration of the striking paucity of NF-κB in the nuclei of SEB-challenged leukocytes and lymphocytes in the bronchoalveolar space, following intracellular delivery of cSN50, provides the mechanistic basis for its effective action in inflamed lungs. By targeting the nuclear import shuttle that ferries NF-κB and other SRTFs from the cytoplasm to the nucleus in leukocytes and lymphocytes, we attenuated expression of multiple cytokines, chemokines, and other mediators of lung inflammation. This lung inflammation–directed intracellular peptide therapy follows our previous studies of cSN50 or SN50 peptides inhibitory effect on inflammation induced by other causative agents. They encompassed the experimental model of endotoxic lipopolysaccharide–induced acute liver inflammation and apoptosis29 and Shiga toxin-induced endothelial cell injury.42 Thus, our novel cell-penetrating inhibitor of SRTFs, including NF-κB, has a broad anti-inflammatory spectrum encompassing multiple innate immunity-triggered proinflammatory pathways. Due to excessive production of inflammatory cytokines/chemokines and subsequent neutrophils involvement, these pathways may lead to secondary organ injury after ischemia reperfusion.43 Our results demonstrating effective suppression of a wide spectrum of cytokines/chemokines and subsequent reduction of neutrophils trafficking in acute lung inflammation may have therapeutic implication for disease states causing secondary organ injury.
The intracellular delivery of cSN50 peptide is swift and its concentration is controlled. In comparison, prior efforts to use gene transfer technology based on adenoviral vector to deliver genes encoding IκBα or A20 to mice challenged with lipopolysaccharide and D-galactosamine reduced inflammation and apoptosis of the liver and increased survival.44,45 Nevertheless, optimal expression of IκBα or A20 was observed 5 days after injection of adenoviral vector and consequently lipopolysaccharide and D-galactosamine were administered after 5 days of waiting for an optimal level of IκBα or A20. This delay and other complications of gene therapy impede broader application of viral vectors for acute inflammatory states.46,47,48 In contrast, due to limited half-life of cell-penetrating peptides, the intracellular peptide therapy can be stopped and resumed as needed to provide a more facile approach to treat acute inflammation and ensuing organ injury.
Likewise, the prospect for an effective countermeasure based on a single cytokine/chemokine target for monoclonal antibodies or soluble cytokine receptor antagonists is dimmed by the potential for significant redundancy in cytokine signaling. Our findings suggest the potential utility of targeting nuclear import machinery with cell-penetrating peptides to suppress production of multiple cytokines and chemokines. Such novel peptides that target nuclear import of SRTFs may provide more complete attenuation of lung inflammation than inhibition of a single cytokine or chemokine. Therefore, it will be important, at both the fundamental and translational level, to expand preclinical testing of cell-penetrating peptides that target pivotal checkpoints in the noxious signaling pathways. Given the efficient delivery to the lungs and rapid action, a cell-penetrating peptide inhibitor which, targets nuclear import of NF-κB and other SRTFs, may represent a much-needed adjunctive therapy to complement antistaphylococcal antibiotics in ALI/ARDS caused by CA-MRSA. Such a combined treatment would limit the proliferation of staphylococci that express SEB and other immunotoxins and reduce the serious and costly consequences of uncontrolled inflammation and lung injury due to immunotoxins produced by highly pathogenic strains of CA-MRSA.
In conclusion, we have demonstrated the effectiveness of intracellular delivery of cSN50 peptide, which targets nuclear import of NF-κB and other SRTFs as a novel approach to combat SEB-induced acute lung inflammation.
Materials and Methods
Peptide synthesis, purification, and labeling. Membrane translocating motif–containing peptide (cSN50, MW = 3,149), and membrane translocating motif–deficient peptide (cN50, MW = 1,651), were synthesized, purified, filter-sterilized, and analyzed as described elsewhere.28,32 To monitor the intracellular delivery of peptides to cells and organs, the cSN50 and cN50 peptides were coupled with FITC (Pierce, Rockford, IL) according to the manufacturer's protocol. After extensive dialysis against water while excess of FITC was removed, labeled peptides were concentrated and used immediately. Relative fluorescence of peptide solutions was determined using the Fusion Universal Microplate Analyzer (PerkinElmer Life Sciences, Waltham, MA). Peptide solutions with equivalent fluorescence units were used in experiments to track peptide distribution in the lungs.
In vivo delivery and distribution of cell-penetrating peptide. For in vivo detection of FITC-labeled peptides in the lungs, BALB/c mice were killed 30 minutes after IP injection of peptides containing equivalent fluorescence units (~0.4 mg of cN50/mouse and 0.7 mg of cSN50/mouse). The lungs were perfused with saline and prepared for cryosections (10 µm thickness).
Animal treatment protocol. Wild-type BALB/c mice were purchased from the Charles River Laboratories (Chicago, IL). All mice were female (8–12 weeks old) with an average weight of 20 g. Mice were anesthetized by Nembutal with the dose of 50 mg/kg (200 µl of a 5 mg/ml saline solution, IP) and 5 µg of SEB (Toxin Technology, Sarasota FL) in 50 µl of saline was intranasally instilled into the anterior part of nose.33,34 The peptides, cSN50 (0.7 mg) and equimolar amount of cN50 (0.4 mg), were dissolved in saline and injected IP in 200 µl aliquots 30 minutes before SEB challenge and thereafter (30, 90, 150, and 210 minutes).
BAL. According to previously published protocol,49 animals were killed 4 hours after SEB IN instillation and the BAL performed via the tracheostomy by two consecutive lavages with 0.8 ml of cold saline. BAL fluid from each animal was pooled and total cell number was determined. Mean recovery volume was 1.4 ± 0.1 ml and no significant difference was detected between the study groups. Cytospin samples were prepared by centrifugation of 100 µl of BAL fluid (100g, 3 minutes). After air-drying at room temperature, cytospin preparations were stained with periodic acid Schiff. Differential cell count was done twice with 300 cells counted each time. The cells were classified as neutrophils, lymphocytes or monocytes/macrophages using standard morphological criteria. Cell-free BAL fluids were stored at −80 °C and subsequently analyzed for cytokines/chemokines level and for protein concentration by using the Bio-Rad dye reagent (Bio-RAD, Hercules, CA), according to the manufacture's instructions.
NF-κB nuclear translocation assay. The effect of cell-penetrating cSN50 and noncell-penetrating cN50 peptide on cytoplasmic/nuclear translocation of NF-κB p65 was analyzed by immunofluorescence microscopy.50 For ex vivo assays, BALB/c mice were pretreated with by IN instillation of saline for 3 hours, then the cells in BAL were collected and cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum containing no detectable lipopolysaccharide (≤0.006 ng/ml as determined by the manufacturer, Atlanta Biologicals, Norcross, GA), 2 mmol/l L-glutamine, 55 µmol/l 2-mercaptoethanol, streptomycin (100 µg/ml), and penicillin (100 U/ml). Cells (5 × 105/ml) were treated with peptides cSN50 (15 µmol/l), cN50 (15 µmol/l), or diluent for 30 minutes at 37 °C, 5% CO2, then incubated with SEB (10 µg/ml) for 3 hours and attached to slide by cytospin. For in vivo assay, 3 hours after pretreatment with saline (IN) mice were challenged with 5 µg of SEB (Toxin Technology) in 50 µl of saline (IN) at zero time. Peptides cSN50 (0.7 mg) and an equimolar amount of cN50 (0.4 mg) in saline, and saline as diluent were injected IP into mice 30 minutes before SEB challenge and thereafter (30, 90, and 150 minutes). Mice were killed 3 hours after SEB and the cells in BAL were collected and attached to slide by cytospin. After fixation and permeabilization, the cells were incubated with rabbit anti-NF-κB p65 (Rel A) polyclonal primary antibodies (eBioscience, 10 µg/ml in phosphate buffered saline/0.5% bovine serum albumin) for 1 hour at room temperature, washed with phosphate buffered saline, and incubated with Alexa 488-labeled goat antirabbit immunoglobulin G secondary antibodies (20 µg/ml in phosphate buffered saline/0.5% bovine serum albumin; Invitrogen, Carlsbad, CA). The slides were mounted and visualized with a fluorescence microscope using ×40 or ×100 oil immersion lenses. We counted as positive the cells with fluorescence signal in the nucleus indicating nuclear localization of NF-κB p65 (Rel A), with a total of 200 cells counted for each treatment group to determine percentage of positive cells. Absolute number of positive cells = total cells in BAL × percentage of positive cells.
Cytokine/chemokine assays of cell-free BAL fluids and blood. Blood samples (40 µl) taken from the saphenous vein were collected in heparinized tubes before and after SEB challenge at 2 and 4 hours. The levels of the cytokines/chemokines in plasma and BAL fluids were measured using flow cytometry-based cytometric bead array (BD PharMingen, San Jose, CA) or by using the Milliplex mouse cytokine/chemokine kit (Millipore, Danvers, MA) according to the manufacturer's instructions.
Microvascular permeability using Evans blue. According to the previously published protocol,34 each animal received 60 mg/kg Evans blue in saline by tail vein injection 1 hour before killing. Evans blue binds to serum albumin and its distribution was used as a marker for the transcapillary flux of macromolecules. At the time of killing, a heparinized sample of blood was collected, and plasma was removed by centrifugation. The lungs from each group were perfused free of blood with 3 ml of saline via the spontaneously beating heart right ventricle and then removed from the thoracic cavity. The trachea, main stem bronchi, and surrounding mediastinal structures were removed. Evans blue was extracted from the remaining lung tissue after homogenization in 1 ml of saline. This volume was added to two volume of deionized formamice and incubated at 50 °C for 20 hours. The supernatant was separated by centrifugation at 2,000g for 30 minutes. Evans blue in the plasma and lung tissue was quantified by dual-wavelength spectrophotometric analysis at 620 and 740 nm. This method corrects the specimen's absorbance at 620 nm for the absorbance of contaminating heme pigments, using the following formula: corrected absorbance at 620 nm = actual absorbance at 620 nm − (1.426 × absorbance at 740 nm + 0.03). We calculated a permeability index by dividing the corrected lung by plasma Evans blue absorbance at 620 nm; this index reflects the degree of extravasations of Evans blue into the extravascular lung compartment.
Statistical analysis. All in vivo and ex vivo experimental data were expressed as mean ± SE. A two-way repeated measure analysis of variance was used to determine the significance of the difference in cytokine/chemokine production. Student's t-test was used to determine the significance of the difference in cell count, Evans blue assay, and protein concentration of BAL fluid. A one-way analysis of variance was used to determine significance of peptide effect on nuclear translocation of NF-κB in BAL leukocytes/lymphocytes.
Acknowledgments
We thank Robert Collins and Martha Hutchens for advice and assistance with analysis of BAL cells, Dean Ballard and Daniel Moore for critical reading of the manuscript, Susanna Richards and Vicky Abney for assistance in its preparation. This work was supported by the USPHS National Institutes of Health grants RO1 HL069452, PO1 HL68744, T32 HL069765, and F32 HL087531. We acknowledge the Cell Imaging Core Services performed through Vanderbilt University Medical Center's Digestive Disease Research Center supported by NIH grant P30DK058404 and the Vanderbilt Ingram Cancer Center supported by NIH grant 2P30 CA 68485.
REFERENCES
- Stuber F, Petersen M, Bokelmann F., and , Schade U. A genomic polymorphism within the tumor necrosis factor locus influences plasma tumor necrosis factor-α concentrations and outcome of patients with severe sepsis. Crit Care Med. 1996;24:381–384. doi: 10.1097/00003246-199603000-00004. [DOI] [PubMed] [Google Scholar]
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- Moellering RC., Jr The growing menace of community-acquired methicillin-resistant Staphylococcus aureus. Ann Intern Med. 2006;144:368–370. doi: 10.7326/0003-4819-144-5-200603070-00014. [DOI] [PubMed] [Google Scholar]
- Ferry T, Bes M, Dauwalder O, Meugnier H, Lina G, Forey F, et al. Toxin gene content of the Lyon methicillin-resistant Staphylococcus aureus clone compared with that of other pandemic clones. J Clin Microbiol. 2006;44:2642–2644. doi: 10.1128/JCM.00430-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink M. Infectious diseases. Resistant staph finds new niches. Science. 2003;299:1639–1641. doi: 10.1126/science.299.5613.1639. [DOI] [PubMed] [Google Scholar]
- Clark NM, Hershberger E, Zervosc MJ, Lynch JP., 3rd Antimicrobial resistance among gram-positive organisms in the intensive care unit. Curr Opin Crit Care. 2003;9:403–412. doi: 10.1097/00075198-200310000-00011. [DOI] [PubMed] [Google Scholar]
- Fey PD, Said-Salim B, Rupp ME, Hinrichs SH, Boxrud DJ, Davis CC, et al. Comparative molecular analysis of community- or hospital-acquired methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:196–203. doi: 10.1128/AAC.47.1.196-203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen JM. Toxins as weapons of mass destruction. A comparison and contrast with biological-warfare and chemical-warfare agents. Clin Lab Med. 2001;21:593–605. [PubMed] [Google Scholar]
- Mattix ME, Hunt RE, Wilhelmsen CL, Johnson AJ., and , Baze WB. Aerosolized staphylococcal enterotoxin B-induced pulmonary lesions in rhesus monkeys (Macaca mulatta) Toxicol Pathol. 1995;23:262–268. doi: 10.1177/019262339502300304. [DOI] [PubMed] [Google Scholar]
- Francis JS, Doherty MC, Lopatin U, Johnston CP, Sinha G, Ross T, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40:100–107. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- Park DR.The microbiology of ventilator-associated pneumonia Respir Care 200550742, 763–763.discussion [PubMed] [Google Scholar]
- Turnidge J. Impact of antibiotic resistance on the treatment of sepsis. Scand J Infect Dis. 2003;35:677–682. doi: 10.1080/00365540310015953. [DOI] [PubMed] [Google Scholar]
- Cooper BS, Medley GF, Stone SP, Kibbler CC, Cookson BD, Roberts JA, et al. Methicillin-resistant Staphylococcus aureus in hospitals and the community: stealth dynamics and control catastrophes. Proc Natl Acad Sci USA. 2004;101:10223–10228. doi: 10.1073/pnas.0401324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinges MM, Orwin PM., and , Schlievert PM.Exotoxins of Staphylococcus aureus Clin Microbiol Rev 20001316–34.table of contents [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llewelyn M, Sriskandan S, Terrazzini N, Cohen J., and , Altmann DM. The TCR Vβ signature of bacterial superantigens spreads with stimulus strength. Int Immunol. 2006;18:1433–1441. doi: 10.1093/intimm/dxl076. [DOI] [PubMed] [Google Scholar]
- Scherer MT, Ignatowicz L, Winslow GM, Kappler JW., and , Marrack P. Superantigens: bacterial and viral proteins that manipulate the immune system. Annu Rev Cell Biol. 1993;9:101–128. doi: 10.1146/annurev.cb.09.110193.000533. [DOI] [PubMed] [Google Scholar]
- Schlievert PM. Will therapeutic peptides be kryptonite for superantigens. Nat Med. 2000;6:378–379. doi: 10.1038/74630. [DOI] [PubMed] [Google Scholar]
- Bueno C, Lemke CD, Criado G, Baroja ML, Ferguson SS, Rahman AK, et al. Bacterial superantigens bypass Lck-dependent T cell receptor signaling by activating a Gα11-dependent, PLC-β-mediated pathway. Immunity. 2006;25:67–78. doi: 10.1016/j.immuni.2006.04.012. [DOI] [PubMed] [Google Scholar]
- MacDonald KL, Osterholm MT, Hedberg CW, Schrock CG, Peterson GF, Jentzen JM, et al. Toxic shock syndrome. A newly recognized complication of influenza and influenzalike illness. JAMA. 1987;257:1053–1058. doi: 10.1001/jama.257.8.1053. [DOI] [PubMed] [Google Scholar]
- Kravitz GR, Dries DJ, Peterson ML., and , Schlievert PM. Purpura fulminans due to Staphylococcus aureus. Clin Infect Dis. 2005;40:941–947. doi: 10.1086/428573. [DOI] [PubMed] [Google Scholar]
- Miethke T, Wahl C, Heeg K, Echtenacher B, Krammer PH., and , Wagner H. T cell-mediated lethal shock triggered in mice by the superantigen staphylococcal enterotoxin B: critical role of tumor necrosis factor. J Exp Med. 1992;175:91–98. doi: 10.1084/jem.175.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer K, Matsuyama T, Kundig TM, Wakeham A, Kishihara K, Shahinian A, et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Car BD, Eng VM, Schnyder B, Ozmen L, Huang S, Gallay P, et al. Interferon γ receptor deficient mice are resistant to endotoxic shock. J Exp Med. 1994;179:1437–1444. doi: 10.1084/jem.179.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawiger J. Innate immunity and inflammation: a transcriptional paradigm. Immunol Res. 2001;23:99–109. doi: 10.1385/IR:23:2-3:099. [DOI] [PubMed] [Google Scholar]
- Goldfeld AE, McCaffrey PG, Strominger JL., and , Rao A. Identification of a novel cyclosporin-sensitive element in the human tumor necrosis factor α gene promoter. J Exp Med. 1993;178:1365–1379. doi: 10.1084/jem.178.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Dorman L, Viggiano V, Cippitelli M, Ghosh P, Rice N, et al. Interaction of NF-κB and NFAT with the interferon-γ promoter. J Biol Chem. 1997;272:30412–30420. doi: 10.1074/jbc.272.48.30412. [DOI] [PubMed] [Google Scholar]
- Tsai EY, Yie J, Thanos D., and , Goldfeld AE. Cell-type-specific regulation of the human tumor necrosis factor α gene in B cells and T cells by NFATp and ATF-2/JUN. Mol Cell Biol. 1996;16:5232–5244. doi: 10.1128/mcb.16.10.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Liu XY, Robinson D, Burnett C, Jackson C, Seele L, et al. Suppression of Staphylococcal Enterotoxin B-induced Toxicity by a Nuclear Import Inhibitor. J Biol Chem. 2004;279:19239–19246. doi: 10.1074/jbc.M313442200. [DOI] [PubMed] [Google Scholar]
- Liu D, Li C, Chen Y, Burnett C, Liu XY, Downs S, et al. Nuclear import of proinflammatory transcription factors is required for massive liver apoptosis induced by bacterial lipopolysaccharide. J Biol Chem. 2004;279:48434–48442. doi: 10.1074/jbc.M407190200. [DOI] [PubMed] [Google Scholar]
- Hawiger J. Noninvasive intracellular delivery of functional peptides and proteins. Curr Opin Chem Biol. 1999;3:89–94. doi: 10.1016/s1367-5931(99)80016-7. [DOI] [PubMed] [Google Scholar]
- Veach RA, Liu D, Yao S, Chen Y, Liu XY, Downs S, et al. Receptor/transporter-independent targeting of functional peptides across the plasma membrane. J Biol Chem. 2004;279:11425–11431. doi: 10.1074/jbc.M311089200. [DOI] [PubMed] [Google Scholar]
- Torgerson TR, Colosia AD, Donahue JP, Lin YZ., and , Hawiger J. Regulation of NF-kappa B, AP-1, NFAT, and STAT1 nuclear import in T lymphocytes by noninvasive delivery of peptide carrying the nuclear localization sequence of NF-kappa B p50. J Immunol. 1998;161:6084–6092. [PubMed] [Google Scholar]
- Herz U, Ruckert R, Wollenhaupt K, Tschernig T, Neuhaus-Steinmetz U, Pabst R, et al. Airway exposure to bacterial superantigen (SEB) induces lymphocyte-dependent airway inflammation associated with increased airway responsiveness—a model for non-allergic asthma. Eur J Immunol. 1999;29:1021–1031. doi: 10.1002/(SICI)1521-4141(199903)29:03<1021::AID-IMMU1021>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Neumann B, Engelhardt B, Wagner H., and , Holzmann B. Induction of acute inflammatory lung injury by staphylococcal enterotoxin B. J Immunol. 1997;158:1862–1871. [PubMed] [Google Scholar]
- Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:337–349. doi: 10.1055/s-2006-948288. [DOI] [PubMed] [Google Scholar]
- Vial T., and , Descotes J. Immune-mediated side-effects of cytokines in humans. Toxicology. 1995;105:31–57. doi: 10.1016/0300-483x(95)03124-x. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N, Young JW, Nisanian AJ, Baggers J., and , Steinman RM. Small amounts of superantigen, when presented on dendritic cells, are sufficient to initiate T cell responses. J Exp Med. 1993;178:633–642. doi: 10.1084/jem.178.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A, Stern LJ, Ottenhoff TH, Engel I, Owen MJ, Lamb JR, et al. Binary and ternary complexes between T-cell receptor, class II MHC and superantigen in vitro. Nature. 1994;369:324–327. doi: 10.1038/369324a0. [DOI] [PubMed] [Google Scholar]
- White J, Herman A, Pullen AM, Kubo R, Kappler JW., and , Marrack P. The V β-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989;56:27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- Raghavendran K, Davidson BA, Mullan BA, Hutson AD, Russo TA, Manderscheid PA, et al. Acid and particulate-induced aspiration lung injury in mice: importance of MCP-1. Am J Physiol Lung Cell Mol Physiol. 2005;289:L134–143. doi: 10.1152/ajplung.00390.2004. [DOI] [PubMed] [Google Scholar]
- Reutershan J, Morris MA, Burcin TL, Smith DF, Chang D, Saprito MS, et al. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J Clin Invest. 2006;116:695–702. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanchi C, Zoja C, Morigi M, Valsecchi F, Liu XY, Rottoli D, et al. Fractalkine and CX3CR1 mediate leukocyte capture by endothelium in response to Shiga toxin. J Immunol. 2008;181:1460–1469. doi: 10.4049/jimmunol.181.2.1460. [DOI] [PubMed] [Google Scholar]
- Qiu FH, Wada K, Stahl GL., and , Serhan CN. IMP and AMP deaminase in reperfusion injury down-regulates neutrophil recruitment. Proc Natl Acad Sci USA. 2000;97:4267–4272. doi: 10.1073/pnas.97.8.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohrer H, Qiu F, Zimmermann T, Zhang Y, Jllmer T, Mannel D, et al. Role of NFkappaB in the mortality of sepsis. J Clin Invest. 1997;100:972–985. doi: 10.1172/JCI119648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvelo MB, Cooper JT, Longo C, Daniel S, Grey ST, Mahiou J, et al. A20 protects mice from D-galactosamine/lipopolysaccharide acute toxic lethal hepatitis. Hepatology. 2002;35:535–543. doi: 10.1053/jhep.2002.31309. [DOI] [PubMed] [Google Scholar]
- Doerschug K, Sanlioglu S, Flaherty DM, Wilson RL, Yarovinsky T, Monick MM, et al. First-generation adenovirus vectors shorten survival time in a murine model of sepsis. J Immunol. 2002;169:6539–6545. doi: 10.4049/jimmunol.169.11.6539. [DOI] [PubMed] [Google Scholar]
- Engel BC, Kohn DB., and , Podsakoff GM. Update on gene therapy of inherited immune deficiencies. Curr Opin Mol Ther. 2003;5:503–507. [PubMed] [Google Scholar]
- Shayakhmetov DM, Li ZY, Ni S., and , Lieber A. Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J Virol. 2004;78:5368–5381. doi: 10.1128/JVI.78.10.5368-5381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz U, Braun A, Ruckert R., and , Renz H. Various immunological phenotypes are associated with increased airway responsiveness. Clin Exp Allergy. 1998;28:625–634. doi: 10.1046/j.1365-2222.1998.00280.x. [DOI] [PubMed] [Google Scholar]
- Powers KA, Zurawska J, Szaszi K, Khadaroo RG, Kapus A., and , Rotstein OD. Hypertonic resuscitation of hemorrhagic shock prevents alveolar macrophage activation by preventing systemic oxidative stress due to gut ischemia/reperfusion. Surgery. 2005;137:66–74. doi: 10.1016/j.surg.2004.05.051. [DOI] [PubMed] [Google Scholar]