Abstract
NV1FGF is an expression plasmid encoding sp.FGF-121–154 currently under investigation for therapeutic angiogenesis in clinical trials. NV1FGF plasmid distribution and transgene expression following intramuscular (IM) injection in patients is unknown. The study involved six patients with chronic critical limb ischemia (CLI) planned to undergo amputation. A total dose of 0.5, 2, or 4 mg NV1FGF was administered as eight IM injections (0.006, 0.25, or 0.5 mg per injection) 3–5 days before amputation. Injected sites (30 cm3) were divided into equally sized smaller pieces to assess spatial distribution of NV1FGF sequences (PCR), NV1FGF mRNA (reverse transcriptase-PCR), and fibroblast growth factor-1 (FGF-1)-expressing cells (immunohistochemistry). Data indicated gene expression at all doses. The distribution area was within 5–12 cm for NV1FGF sequences containing the expression cassette, up to 5 cm for NV1FGF mRNA, and up to 3 cm for FGF-1-expressing myofibers. All FGF receptors were detected indicating robust potential for bioactivity after NV1FGF gene transfer. Circulating levels of NV1FGF sequences were shown to decrease within days after injection. Data support demonstration of plasmid-mediated gene transfer and expression in muscles from patients with CLI. FGF-1 expression was shown to be limited to injection sites, which supports the concept of multiple-site injection for therapeutic use.
Introduction
NV1FGF is an expression plasmid encoding sp.FGF-121–154 currently in clinical phase III for treatment of patients with critical limb ischemia (CLI).1 Intramuscular (IM) administration of NV1FGF has been shown to express fibroblast growth factor-1 (FGF-1) and promote collateral vessel growth in a rabbit ischemic hindlimb model2 and in a hypercholesterolemic hamster displaying impaired neovascularization after experimental ischemia.3 In a phase I clinical trial, IM administration of NV1FGF to limbs of patients with CLI was shown to be well tolerated.4 In a double blind, randomized, placebo-controlled phase II trial in 125 patients with CLI unsuitable for revasuclarization, NV1FGF significantly reduced by twofold the risk of major amputations.5 Patients received four administrations, at 2-week interval, of eight IM injections of placebo or 0.5 mg NV1FGF per injection.
Clinical trials have sought to establish the “proof of concept” for therapeutic angiogenesis using IM gene transfer mediated by plasmids or viral vectors in patients with peripheral arterial disease, though results have been inconsistent.4,5,6,7,8,9,10,11,12 Possible reasons include efficiency of gene transfer and/or distribution of the therapeutic angiogenic growth factor, which may not have been optimal to generate neovascularization in large ischemic areas of an affected limb.13 Due to the difficulty to collect tissue samples from transfected muscle parts in patients, clinical trials were initiated without precise knowledge of transfection efficiency and related protein production required to result in clinically relevant improvement of vascularization.14 In addition, it is not known whether skeletal muscles from patients with CLI still display the receptors that mediate the cellular response after being activated by their respective ligand.
This study was conducted in patients with advanced chronic limb ischemia who underwent major limb amputation within 5 days of IM injection of NV1FGF. As a result of precise identification of injection sites and intraoperative harvesting of the injected tissue, the goals of this study were made possible. The specific goals were to: (i) document muscle NV1FGF gene transfer; (ii) document the spatial distribution of plasmid sequences and mRNA; (iii) document the distribution of NV1FGF-expressing cells; and (iv) examine whether severely ischemic muscle cells still express FGF receptors. An additional goal was to analyze the short-term kinetics of NV1FGF sequences by the appropriate collection of blood samples.
Results
NV1FGF gene transfer was well tolerated by all patients. No serious adverse event was related to the study treatment. Neither systemic complication nor abnormal laboratory values were observed after dosing.
Detection of NV1FGF DNA in muscle tissue
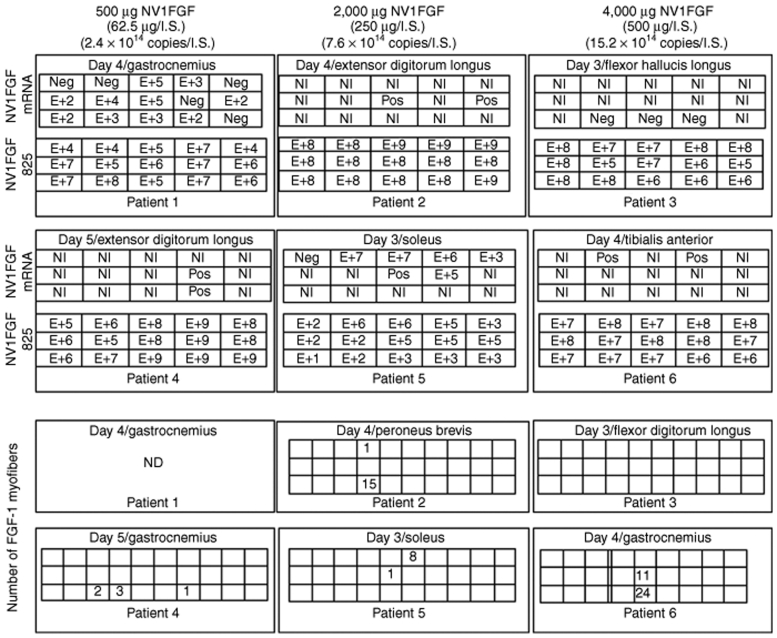
NV1FGF sequences were detected in all injection sites and over the entire volume of the muscle samples. Levels of NV1FGF 69-bp and 825-bp sequences were found above the limit of quantification (LOQ) in all 135 pieces and in 134/135 pieces, respectively. Maximum levels of NV1FGF 69-bp sequences per injected site ranged between 109 and 1010 copies/µg DNA. Maximum levels of the NV1FGF 825-bp sequences were roughly one order of magnitude below. Figure 1 illustrates the levels of NV1FGF 825-bp sequences assessed in each piece of the injection site investigated per patient. There was no obvious relation between levels of NV1FGF sequences and the three doses of NV1FGF (0.06, 0.25, and 0.5 mg/site) in the six patients. For a few samples, the distribution map pictured a bell-shaped profile around the peak level at the injection point (Figure 2a,b).
Figure 1.
Spatial distribution of NV1FGF mRNA levels, NV1FGF 825-bp sequences levels, FGF-1-expressing myofibers in each series of 15 or 30 pieces reconstituted for muscle samples at the injection-site. Levels (E+) are given as number (in logarithmic value) of NV1FGF mRNA copies/6 µg total RNA, and number of NV1FGF DNA copies/µg of DNA; e.g., a figure with E + 2 indicates 102 NV1FGF sequence per µg total DNA or 102 NV1FGF mRNA copies per 6 µg total RNA. FGF-1, fibroblast growth factor-1; ND, not done; Neg, negative value; NI, noninformative (poor RNA preservation); Pos, positive value but below limit of quantification or poor RNA preservation but reverse transcriptase-PCR value above limit of detection.
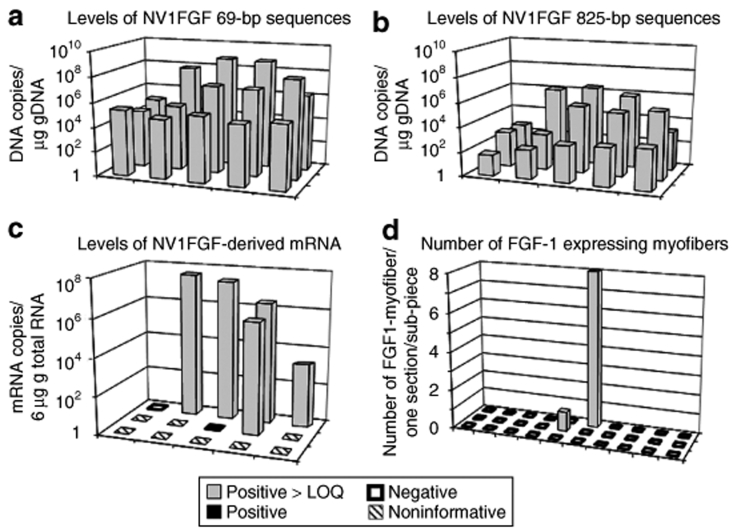
Figure 2.
NV1FGF plasmid distribution and transgene expression following intramuscular injection. Spatial distribution of levels of (a) NV1FGF 69-bp sequences, (b) NV1FGF 825-bp sequences, (c) NV1FGF mRNA, and (d) number of FGF-1-expressing myofibers in a representative patient (250 µg/injection site, patient 5). Levels are given as number of NV1FGF DNA copies/µg of DNA and as number of NV1FGF mRNA copies/6 µg of RNA for each piece. NV1FGF sequences and NV1FGF mRNA were assessed in 15 pieces from the same injected muscle samples. FGF-1-expressing myofibers were assessed in 30 pieces from another injected site. Values are given as number per section for one histological section per piece. FGF-1, fibroblast growth factor-1; LOQ, limit of quantification.
The samples from noninjected sites were collected at a distance of 2–12 cm from the nearest injected site (data not shown). Levels of NV1FGF 69-bp sequences were found above the LOQ in all pieces from all samples. NV1FGF 825-bp sequences were detected in one-third of the pieces from all samples.
Detection of NV1FGF mRNA expression in muscle tissue samples
NV1FGF transcription was demonstrated in five of six patients treated and for each dose group.
Figure 1 illustrates individual data obtained in each piece of the injection site investigated per patient. Despite prompt intraoperative sample collection and freezing process, many pieces contained degraded RNA that did not allow reliable quantification. Although there was no apparent correlation between the number of poorly preserved samples and the time delay of tissue retrieval that ranged between 10 and 26 minutes, a shorter interval of muscle retrieval in the operative theatre could have partially influenced the yield of nondegraded mRNA. Quantitative analysis conducted in two samples (from patients 1 and 5) with less degraded RNA showed maximum levels up to ~107 in vitro transcript equivalent copies/6 µg total RNA. In patient 1 (0.06 mg/injection), 10/15 pieces tested positive for NV1FGF mRNA. In patients 1 and 5 showing numerous pieces displaying well preserved RNA, NV1FGF mRNA distributed by a maximum of 5 and 4 cm, respectively (Figures 1 and 2c). The injected sites that scored positive for NV1FGF mRNA originated from different muscles, namely peroneus brevis and longus, extensor digitorum longus, medial-head and lateral-head of gastrocnemius, extensor digitorum longus, and soleus. The injected sample that did not test positive for NV1FGF mRNA was collected from flexor hallucis longus (patient 3): out of the 30 pieces, only three were interpretable and truly negative; the other pieces were not exploitable due to RNA degradation (Figure 1).
NV1FGF mRNA was not detected in noninjected sites: testing outcomes were negative (7/27) or not exploitable (20/27) due to RNA degradation. In patient 5, a noninjected site and an injected site were collected in the same muscle (m. soleus). The injected site was tested positive for NV1FGF mRNA whereas the noninjected site, collected at 3 cm from the injected site and displaying well preserved RNA, was tested negative for NV1FGF mRNA (data not shown).
FGF-1 expression in muscle tissue
As the immunohistochemical assay cannot differentiate exogenous FGF-1 (related to NV1FGF expression) from endogenous FGF-1, muscles biopsies from patients with CLI not treated with NV1FGF were investigated for FGF-1 immunoreactivity. Data demonstrated FGF-1 immunostaining in satellites cells spared through the muscle mass, which indicated expression of endogenous FGF-1 in satellite cells within the muscles from patients with CLI. FGF-1 immunoreactivity was not observed in other cell types, including myofibers (data not shown).
In the samples from the patients treated with NV1FGF, FGF-1 immunostaining was shown in two cell types: myofibers and satellite cells (Figure 3a,b).
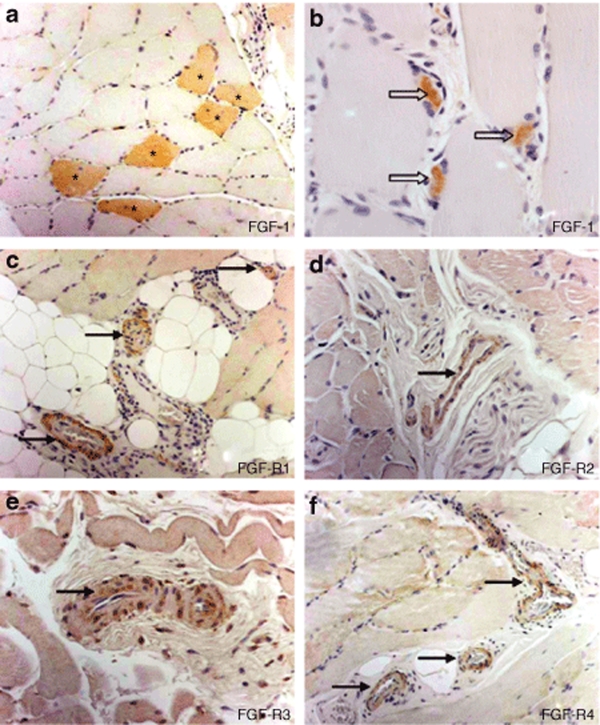
Figure 3.
Histological sections taken from muscle tissue of a representative patient injected with NV1FGF. Anti-FGF-1 immunohistochemistry demonstrated (a) FGF-1 labeling of myofibers (asterisks) only in injected sites. It showed FGF-1 labeling of (b) some skeletal muscle satellite cells (arrow heads) both in injected and distant noninjected sites. (c–f) Presence of FGF receptors (R1, R2, R3, R4) was shown in the microvasculature. FGF-1, fibroblast growth factor-1.
FGF-1 immunoreactive myofibers (Figure 3a) were detected only within the samples from the injected sites. In one case (patient 3), FGF-1 immunoreactivity was not observed in the sections from an injected site (Figure 1). A maximum of 24 FGF-1 immunoreactive myofibers observed in one histological section was found in the injected site from patient 6. All pieces with FGF-1 immunoreactive myofibers were located at or close to center of the sample, expected as the injection point. The FGF-1- expressing myofibers were distributed by a maximum of 3 cm (Figure 1). Figure 2d illustrates the spatial distribution of positive myofibers in an injected site of patient 5.
FGF receptors expression in ischemic muscle
Immunohistochemistry analysis demonstrated the presence of all four FGF receptors (R1, R2, R3, R4) in the microvasculature of both injected and noninjected sites in five patients tested (Figure 3c–f), with the exception of one patient, for whom FGFR3 was not detected in the noninjected site. FGFR1 and FGFR4 were detected in myofibers from all injected and noninjected sites. FGFR2 and FGFR3 were detected in myofibers from some samples in injected sites (two patients) and noninjected sites (three patients).
Levels of NV1FGF 69-bp and 825-bp sequences in plasma
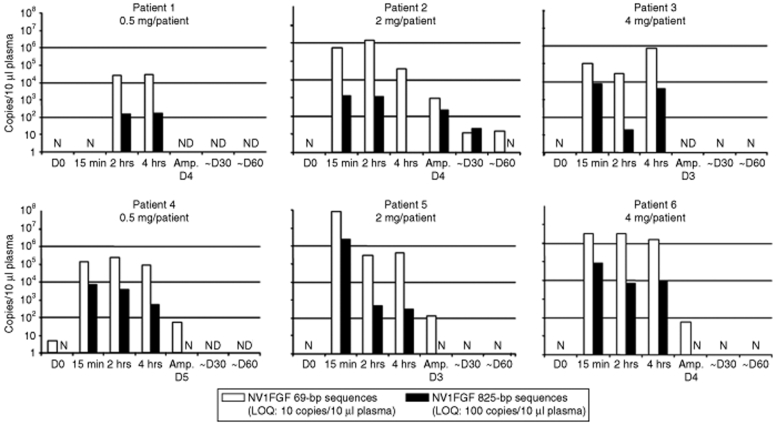
Circulating NV1FGF levels were noticed within a narrow time window following administration (Figure 4). Both NV1FGF 69-bp and 825-bp sequences were detected at 15 minutes. Their levels decreased rapidly over time. NV1FGF 825-bp sequences were one to three orders of magnitude lower than that of the NV1FGF 69-bp sequences even at the earliest time point assessed. At the time of amputation (i.e., 3–5 days after administration), NV1FGF 825-bp sequences were no longer detectable in three of four patients tested. The fourth patient tested positive at the time of amputation showed circulating levels of NV1FGF 825-bp sequences at a level equal or below LOQ at the time of amputation (day 4). Samples from four patients were tested at day 30 and 60 after administration. All values were negative at day 60 except in patient 2 displaying levels of NV1FGF 69-bp sequences close to LOQ (Figure 4).
Figure 4.
Kinetic of NV1FGF sequences in plasma using two PCR assays to amplify sequences of different sizes. The short amplicon of 69 bp spans the junction between the CMV promoter and beginning of sp.FGF-1 transgene. The long amplicon of 825 bp encompasses the minimal FGF-1 expression cassette with the potential to express FGF-1. Amp., actual day of amputation (3–5 days after NV1FGF administration); D, day postadministration; D0, baseline; LOQ, limit of quantification; N, negative; ND, not done.
Discussion
This study protocol was developed for patients with CLI requiring amputation to objectively establish transgene expression and plasmid distribution within 3–5 days in patients treated for gene transfer–mediated therapeutic angiogenesis. The procedure identified the injection sites and intraoperatively harvested them at the time of amputation. This enabled the investigation of NV1FGF gene transfer and expression in muscles from patients with severe limb ischemia.
Efficiency of plasmid-mediated gene transfer has been well documented in skeletal muscle from various animal models.3,15,16 Following injection of angiogenic growth factors, development of collateral vessels and improved blood flow was observed in experimental ischemia.17,18 Two clinical studies have shown that direct IM administration of a plasmid DNA can lead to transgene expression in humans. Transient increase in serum levels of vascular endothelial growth factor was reported after IM plasmid-mediated gene transfer of vascular endothelial growth factor165 in patients with CLI.6 Dystrophin was shown to be expressed in muscle from six of nine patients treated with plasmid DNA for Duchenne/Becker muscular dystrophy.19 In another clinical study, eight patients were treated with catheter-mediated adenoviral gene transfer in peripheral arteries with success reported in six of the eight patients in this study.20 We have demonstrated successful NV1FGF transgene delivery and expression in ischemic muscle from patients with severe CLI. A single IM NV1FGF administration (0.06, 0.25, and 0.5 mg NV1FGF per site) resulted in local FGF-1 transgene expression. NV1FGF-related FGF-1 expression was detected only in the myofibers of the injected sites. On the contrary, FGF-1 expression was also detected in satellite cells located in both injected and noninjected muscles from NV1FGF-treated patients. This FGF-1 expression in satellite cells was likely due to expression of endogenous FGF-1 because similar FGF-1 immunoreactivity had been observed, using the same immunohistochemical assay, in satellite cells in muscle biopsies from nontreated patients with CLI (data not shown).
In addition, our data demonstrate that the four FGF receptors are present on microvasculature cells within ischemic muscle tissue and on myofibers in patients with severe limb ischemia suggesting potential for biological activity of FGF-1 protein. Indeed, FGFs mediate their cellular responses by binding to and activating a family of four receptor tyrosine kinases designated the high-affinity FGF receptors FGFR1-FGFR4.21
Three NV1FGF doses were tested for gene expression at injection site: 0.06, 0.25, and 0.5 mg per site, the highest dose per injection being that injected in patients with CLI in the phase II trial.5 All dose resulted in NV1FGF gene expression. However, a clear dose effect could not be evidenced due to the restricted group size and the large variability of NV1FGF gene transfer. The numbers of FGF-1-expressing myofibers per sample may appear low, and were lower than that observed in ischemic animal models.3 It has to be noted that the FGF-1 immunohistochemistry assay was not used to provide a semiquantitative assessment. It was used to identify the NV1FGF-expressing cells and localize them in one section for each of the 30 pieces per injection site. In addition, muscles were collected at the time of amputation, which was 3–5 days after IM injection. This postinjection time might have been too short to achieve maximal levels of protein expression in transfected myofibers.22,23 In animal models, peak gene expression is reached within 1–2 weeks after a single IM administration.24 This may represent the best-case scenario and might be substantially longer in humans with advanced chronic limb ischemia, as were the subjects in this study. Therefore, the number of FGF-1-expressing myofibers reported in this study may be significantly underestimated and does not reflect the level of protein expression.
There is an ongoing debate as to whether production of stable mature collateral vessels can be induced by a short burst of high doses as observed using adenovirus or whether moderate, but more sustained levels, as observed by plasmid delivery with the potential of repeated administrations might serve as the better option to induce therapeutic angiogenesis.25,26 Negative results from RAVE and DELTA-1 trials have dampened enthusiasm for therapeutic angiogenesis.8,11,27 However, interest in vascular gene therapy has returned, and in a large placebo-controlled phase II gene therapy trial it was shown that the level of transgene FGF-1 expression obtained following four repeated IM administrations of NV1FGF in patients with CLI is sufficient to establish functional arterial collateral networks and restore tissue oxygenation to prevent major amputation. NV1FGF was injected at a dose of 0.5 mg per site in four sites above the knee and four sites below the knee.5 The presented pharmacokinetic information substantiate the evidence that IM injection of NV1FGF plasmid DNA in patients with severe limb ischemia results in clinically relevant transfection of striated muscle fibers and measurable NV1FGF transgene expression.
NV1FGF is a new generation plasmid vector with improved properties in terms of biosafety while achieving more efficient gene expression in vivo.2 Longer time points would be desirable to study both long-term expression levels as well as local biological effects of FGF-1 gene transfer in the ischemic limb of patients, but such a study is difficult, if not impossible, to achieve in humans with the same vigor as the present study. In our animal studies, FGF-1-expressing myofibers have been detected within weeks after a single IM administration of NV1FGF in rats.28 IM administration of NV1FGF in two animal models of hindlimb ischemia have demonstrated the statistical improvement of formation of new blood vessels as well as the improvement of reperfusion in the NV1FGF-treated ischemic hindlimb when compared to vehicle or plasmid controls.2,3 FGF-1 activates proliferation, migration, and differentiation of endothelial cells required for sprouting of pre-existing vessels and formation of new capillaries, but FGF-1 also induces migration and proliferation of smooth muscle cells, mural cells, and pericytes required for arterioles maturation and vessel remodeling.29
Our data of NV1FGF gene transfer to ischemic muscle in patients with CLI demonstrate for the first time a spatial distribution of plasmid DNA and its transgenic protein. The NV1FGF 825 bp sequences that contain the minimal transcription sequence able to drive FGF-1 expression was distributed over the entire volume (30 cm3) of all the injected muscle samples. In some cases, quantitative data indicated a bell-shaped spatial distribution of NV1FGF plasmid sequences with decreasing gradient in sequence levels from the piece at the injection site to more distal pieces. Despite prompt intraoperative sample collection and freezing process, many pieces contained degraded RNA. This technical issue may mitigate the findings regarding NV1FGF mRNA expression. Nevertheless, it appears clear from the data collected in well-preserved samples that NV1FGF mRNA could be distributed to 5 cm in 20/30 cm3 and was detected only at the injected sites. NV1FGF mRNA was not detected in the muscle areas distant by a few centimeters from the injection sites. Expression of FGF-1 showed a more restricted spatial distribution. FGF-1-expressing myofibers were observed only in the injected sites and were located in the center of the assessed surface area, expected to be the injection point. The gene transfer restricted to the injection site has to be related to the restricted diffusion of plasmid solution within the muscle tissue. Using ultrasound imaging in patients with peripheral arterial disease, IM injection of 2.5 ml plasmid solution has been demonstrated to be distributed by a maximum of 6 cm in calf muscle.30 New formation of capillaries and arterioles was not recognized within 3–5 days after NV1FGF injection.
Although it remains speculative, needle injury might have played a role. Including a control group would have answered this question, however, this also would have meant that these patients were forced to undergo a rather strenuous screening process and delay their amputation. Due to ethical consideration, the steering committee decided not to include a control group in this pharmacokinetic phase I trial.
Following IM injection of plasmid, DNA sequences appear to enter the blood stream at early-time points, with levels declining over time. Lew et al. reported that intact plasmid DNA complexed with cationic lipids has a half-life in blood of <5 minutes when assessed by Southern blot and has become undetectable at 1 hour after intravenous injection in mice.31 Plasmid distribution and degradation have not been systematically documented in blood samples from patients after IM administration. We thus developed two PCR assays, one amplifying a short 69-bp sequence, the other amplifying an 825-bp sequence corresponding to the minimal expression cassette. We have used these assays to discriminate between plasmid sequences retaining the potential to drive sp.FGF-121–154 expression from small plasmid degradation products. Our results indicate that NV1FGF plasmid present in the circulation undergoes progressive degradation. The 825-bp sequence corresponding to the minimal expression cassette was detected up to 2 hours in all and up to day 30 in one patient in this study. Animal studies have shown that systemic and transient tissue distribution of NV1FGF sequences was not associated with NV1FGF expression in organs other than the injected muscles.28 Verification of this finding in patients was not feasible in this trial.
In conclusion, successful NV1FGF gene transfer and expression were demonstrated in skeletal muscles from patients with CLI planned to be amputated. Together with the detection of the four FGF receptors, these data indicate that even severely ischemic muscles display the potential for response to NV1FGF-mediated therapeutic angiogenesis. The spatial distribution of NV1FGF expression was shown to be restricted to the injection site supporting the NV1FGF being a local treatment. We concluded that IM administration of NV1FGF in patients with CLI translated into localized expression and transient systemic distribution of the plasmid support the use of repeated, multiple injections performed at different sites of the limb, which is safe, to achieve a broad distribution of FGF-1 transgene protein.
Materials and Methods
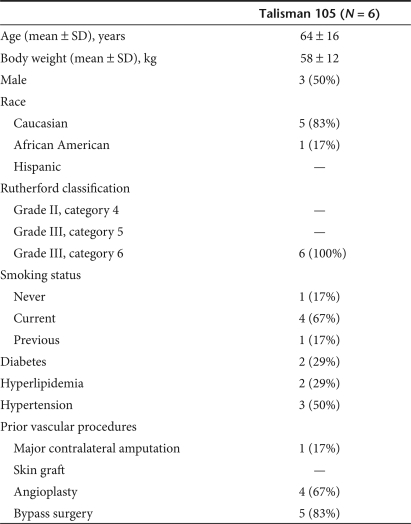
Patients. Patients with CLI scheduled for major lower limb amputation were screened for enrollment into this study according to criteria previously described.4,5 Patients with active proliferative retinopathy, history or evidence of cancer, or serum creatinine >2.0 mg/dl (176 µmol/l) were not eligible. Qualified subjects were randomized to one of the three tested doses (0.5, 2, or 4 mg NV1FGF). Study design allowed administration of the study drug in a time period of 3–5 days before amputation. Table 1 summarizes patient demographics and baseline characteristics. The study was approved by the Institutional Review Board of each study center, by the Swiss Agency for the Environment, Forests and Landscape, by the Swiss Federal Office of Public Health, the Recombinant DNA Advisory Committee of the National Institutes of Health, and the Food and Drug Administration. All patients gave written informed consent. Study procedures were conducted according to guidelines of Good Clinical Practices.
Table 1.
Subjects demographics and baseline characteristics
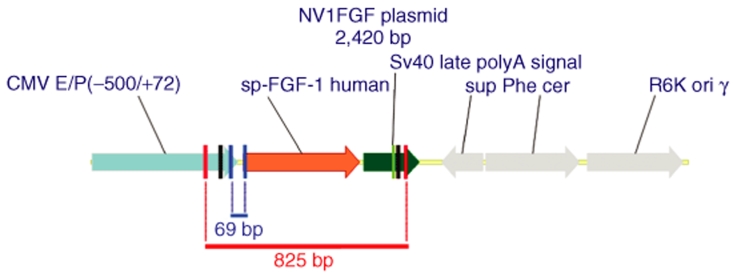
Plasmid. NV1FGF (Figure 5) is a 2.4 kb plasmid consisting of an expression cassette for sp.FGF-121–154 inserted in a plasmid backbone displaying a conditional origin of replication and no antibiotic resistance marker.1 The sequence encoding FGF-121–154 is a fusion between the sequences encoding the secretion signal peptide (sp) from human fibroblast interferon and the human FGF-1 from amino acids 21 to 154. Expression of sp.FGF-121–154 is driven by the human cytomegalovirus immediate early enhancer/promoter (from nucleotide −522 to +72). The late polyadenylation signal from simian virus 40 (nucleotides 2,538–2,759 from SV40 genome, Genbank locus SV4CG) is inserted downstream of the sp.FGF-121–154 fusion to ensure proper and efficient transcription termination and subsequent polyadenylation of the transcript.
Figure 5.
Expression cassette. The plasmid contains an expression cassette inserted into an original backbone creating a closed circular plasmid of 2.4 kb. The sequence encoding sp.FGF-1 is a fusion between the sequences encoding the secretion signal peptide (sp) from human fibroblast interferon and the human FGF-1 from amino acids 21 to 154. Expression of sp.FGF-1 is driven by the human cytomegalovirus (CMV) immediate early enhancer/promoter. The late polyadenylation signal from simian virus 40 is inserted downstream of the sp.FGF-1 fusion. Amplicon 69 bp. A short sequence of 69 bp spanning the junction between immediate early CMV 5′-UTR and the first nucleotides of the transgenic sp.FGF-1-coding sequence. Amplicon 825 bp. A long amplicon of 825 bp encompassing the minimal sp.FGF-1 expression cassette.
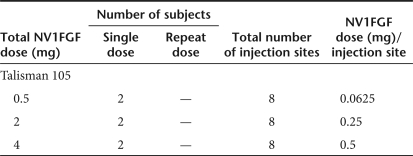
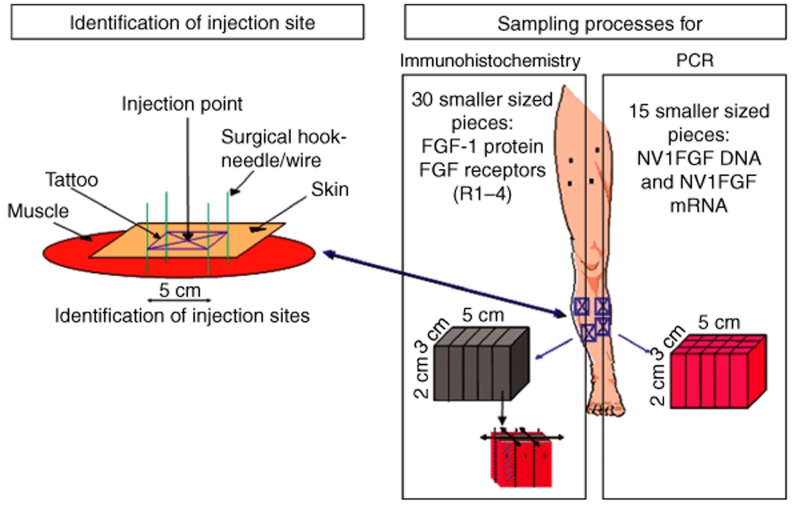
Treatment and specimen collection schedule. Six patients were randomized to receive 0.5, 2, or 4 mg of NV1FGF administered as eight IM injections of 2.5 ml each: four injections above the knee and four below the knee of the leg planned to be amputated (Table 2). Injection sites were arbitrarily selected by the clinical operator outside of areas of damaged tissue according to available muscle mass (at least 2 cm thick, except for anterior muscles). Injections in the nonamputated part of the limb were performed to enable potential therapeutic benefit to the nonamputated limb. All patients were planned for a major amputation; however, only the four injection sites below the knee were marked with a tattoo pen to accurately retrieve the injected tissue at the time of amputation (Figure 6). NV1FGF was injected in various calf muscles: peroneus brevis and longus, extensor digitorum longus, gastrocnemius, tibialis anterior, flexor hallucis longus, flexor digitorum longus, and soleus.4,5 Muscle samples were collected intraoperatively from injected and noninjected sites of the amputated limb according to a carefully defined procedure. After anesthesia before amputation, marking hook needles or suture stitches were positioned at least 2-cm deep through each of the four rectangle tattoos to secure the correlation between skin and injected muscle tissue thereby avoiding differential tissue retraction which occurs at the time of amputation. Biopsies were intraoperatively collected as rapidly as possible to best preserve the quality of nucleic acids. The 24 samples (four per patient) that corresponded to injected sites comprised blocks of 5 cm long, 3 cm wide, and 2 cm deep (30 cm3), centered on the injection point, i.e., the needle track. Twenty-four small muscles samples (four per patient) were collected from a location as far as possible from any injection site. Half of the collected samples were processed for further analysis. The remaining samples were backup in case of technical issues.
Table 2.
Subjects enrollment by dose of NV1FGF
Figure 6.
Method of tissue sampling for the assessment of NV1FGF sequences and NV1FGF-derived mRNA using real-time PCR assays as well as FGF-1 protein and FGF receptors (FGF-R1, -R2, -R3, -R4) using immunohistochemistry. Before NV1FGF administration, injection sites from the part of the limb to be amputated were marked using a tattoo pen. After anesthesia before major amputation, marking hook needles or suture stitches were positioned at least 2-cm deep through each of the four rectangle tattoos to secure the correlation between skin and injected muscle tissue by avoiding differential tissue retraction after amputation. Muscle tissue was collected from injected sites for PCR and for immunohistochemistry. The muscle samples were divided into 15 equally sized pieces for PCR analyses and in 30 equally sized pieces for immunhistochemistry. FGF-1, fibroblast growth factor-1.
For analysis of NV1FGF DNA sequences and NV1FGF-mRNA, the samples (30 cm3) from injected sites (two per patients) were further divided into 15 equal pieces. Samples (6 cm3) from noninjected sites (two per patients) were subdivided in three equal pieces. Muscles samples were collected by the surgeon in a time frame ranging from 10 to 30 minutes after amputation. They were immediately snap frozen and stored at −80 °C.
Samples (30 cm3) from other injected sites (two per patient) were collected to be processed for histological investigation using immunohistochemistry for expression of FGF-1 and FGF receptors (R1, R2, R3, R4). They were divided into 30 equal pieces. Samples (18 cm3) from noninjected sites (two per patient) were subdivided into 18 equal pieces. Muscles samples were collected by the surgeon in a time frame ranging from 2 to 47 minutes. Samples were immediately fixed in a formaldehyde fixative solution.
Blood samples were collected at baseline, 15 minutes, 2 hours, 4 hours, and day of amputation, 30 and 60 days after NV1FGF administration. Blood samples were stored at −80 °C.
NV1FGF DNA and NV1FGF mRNA levels in plasma and muscle tissue samples. Real-Time (TaqMan; Life Technology, Foster City, CA) PCR assays were used for quantitative assessment of NV1FGF DNA levels in plasma and muscle tissue samples. Frozen muscle samples were processed for combined RNA and DNA extraction using the Trizol method to compare levels of NV1FGF mRNA and NV1FGF DNA from the same samples. Genomic DNA was quantified using ultraviolet spectrophotometry and mRNA was purified using oligo-dt columns. Two PCR assays were used to amplify NV1FGF sequences of different length (Figure 5). A short 69-bp sequence spanning the junction between the immediate early cytomegalovirus 5′-untranslated region and the first nucleotides of the signal peptide (sp.) sequence. A long 825-bp sequence encompassing the minimal expression cassette displaying the potential to express FGF-1 transgene. The LOQ of the 69-bp PCR assay was 10 copies/10 µl plasma, and 10 copies/µg of genomic DNA from muscle tissue. The LOQ for the 825-bp PCR assay was 100 copies/10 µl plasma and 100 copies/µg of genomic DNA from muscle tissue. A sample was considered positive and reproducibly quantifiable for values greater than or equal to the LOQ.
Reverse transcribed mRNA were submitted to the 69-bp PCR assay. The standard curve was obtained from sp.FGF-121–154 transcripts obtained by in vitro transcription from a suitable plasmid. Limit of detection and LOQ were 10 and 6,000 copies of in vitro transcripts/6 µg of total RNA, respectively. Quantitative analysis of NV1FGF mRNA was carried out for samples displaying an electrophoretic profile (obtained either by 1% agarose gel electrophoresis or using the Agilent 2100 Bioanalyser; Agilent Technology, Foster City, CA) showing no degradation of total RNA preparation.
PCR amplification and fluorescence detection were performed using an ABI Prism 7700 sequence detection system (Life Technology, Foster City, CA). Analysis was conducted by Althea Technologies (San Diego, CA) for plasma samples, and Centelion SAS (Vitry sur Seine, France) for muscle samples. Nested PCR analysis was not performed.
FGF-1-expressing myofibers and FGF receptors in muscle tissue. Formaldehyde-fixed tissue samples were processed for immunohistochemistry. Each piece was oriented to reconstitute the original muscle samples. For each oriented piece, only the five first sections were processed for FGF-1 protein and the four FGF receptors (R1, R2, R3, and R4) immunohistostaining. FGF-1 and its receptors were detected using rabbit polyclonal antibodies (anti-FGF-1: R&D Systems, Minneapolis, MN; anti-FGF receptors: Santa Cruz, Santa Cruz, CA) in a standard streptavidin-biotin assay. The number of FGF-1-expressing myofibers and the presence of FGF receptors were assessed in 30 sections (1 section/piece/assay) per injected site and 18 sections per noninjected site (Figure 6). Analysis was conducted by Centelion SAS.
Acknowledgments
We are grateful to E. Senechal, O. Tejedor, A. Ginesty, and A.H. Casse for there valuable technical assistance.
REFERENCES
- Soubrier F, Cameron B, Manse B, Somarriba S, Dubertet C, Jaslin G, et al. pCOR: a new design of plasmid vectors for nonviral gene therapy. Gene Ther. 1999;6:1482–1488. doi: 10.1038/sj.gt.3300968. [DOI] [PubMed] [Google Scholar]
- Witzenbichler B, Mahfoudi A, Soubrier F, Le Roux A, Branellec D, Schultheiss H-P, et al. Intramuscular gene transfer of fibroblast growth factor-1 using improved pCOR plasmid design stimulates collateral formation in a rabbit ischemic hindlimb model. J Mol Med. 2006;84:491–502. doi: 10.1007/s00109-005-0031-3. [DOI] [PubMed] [Google Scholar]
- Caron A, Michelet S, Caron A, Sordello S, Ivanov MA, Delaère P, et al. Human FGF-1 gene transfer promotes the formation of collateral vessels and arterioles in ischemic muscles of hypercholesterolemic hamsters. J Gene Med. 2004;6:1033–1045. doi: 10.1002/jgm.594. [DOI] [PubMed] [Google Scholar]
- Comerota AJ, Throm RC, Miller K, Henry T, Chronos NA, Laird J, et al. Naked plasmid DNA encoding fibroblast growth factor type 1 for the treatment of end-stage unreconstructable lower extremity ischemia: preliminary results of a phase I trial. J Vasc Surg. 2002;35:930–936. doi: 10.1067/mva.2002.123677. [DOI] [PubMed] [Google Scholar]
- Nikol S, Baumgartner I, Van Belle E, Diehm C, Visoná A, Capogrossi M, et al. Therapeutic angiogenesis with intramuscular NV1FGF improves amputation-free survival in patients with critical limb ischemia. Mol Ther. 2008;16:972–978. doi: 10.1038/mt.2008.33. [DOI] [PubMed] [Google Scholar]
- Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K, et al. Constitutive expression of phVEGF165 following intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97:1114–1123. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Trachtenberg J, Mohler E, Olin J, McBride S, Pak R, et al. Phase I study of direct administration of a replication deficient adenovirus vector containing the vascular endothelial growth factor cDNA (CI-1023) to patients with claudication. Am J Cardiol. 2002;90:512–516. doi: 10.1016/s0002-9149(02)02524-9. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Mohler ER, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: A phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation. 2003;108:1933–1938. doi: 10.1161/01.CIR.0000093398.16124.29. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., and , Alitalo K. Gene transfer as a tool to induce therapeutic vascular growth. Nature Med. 2003;9:694–701. doi: 10.1038/nm0603-694. [DOI] [PubMed] [Google Scholar]
- Collinson DJ., and , Donnelly R. Therapeutic angiogenesis in peripheral artery disease: can biotechnology produce an effective collateral circulation. Eur J Vasc Endovasc Surg. 2004;28:9–23. doi: 10.1016/j.ejvs.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Grossman PM, Mendelsohn F, Henry TD, Hermiller JB, Litt M, Saucedo JF, et al. Results from a phase II multicenter, double-blind placebo-controlled study of Del-1 (VLTS-589) for intermittent claudication in subjects with peripheral arterial disease. Am Heart J. 2007;153:874–880. doi: 10.1016/j.ahj.2007.01.038. [DOI] [PubMed] [Google Scholar]
- Powell RJ, Simons M, Mendelsohn ME, Daniel G, Henry TD, Koga M, et al. Results of a double-blind, placebo-controlled study to assess the safety of intramuscular injection of hepatocyte growth factor plasmid to improve limb perfusion in patients with critical limb ischemia. Circulation. 2008;118:58–65. doi: 10.1161/CIRCULATIONAHA.107.727347. [DOI] [PubMed] [Google Scholar]
- Ruponen M, Hyvönen Z, Urtti A., and , Ylä-Herttuala S. Nonviral gene delivery methods in cardiovascular diseases. Methods Mol Med. 2005;108:315–328. doi: 10.1385/1-59259-850-1:315. [DOI] [PubMed] [Google Scholar]
- Pislaru S, Janssens SP, Gersh BJ., and , Simar RD. Defining gene transfer before expecting gene therapy—putting the horse before the cart. Circulation. 2002;106:631–636. doi: 10.1161/01.cir.0000019621.18368.b7. [DOI] [PubMed] [Google Scholar]
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G., and , Jani A. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Isshiki T., and , Satd T. Increased expression of direct gene transfer into skeletal muscle observed after acute ischemic injury in rats. Lab Invest. 1996;74:1061–1065. [PubMed] [Google Scholar]
- Vincent KA, Shyu KG, Luo Y, Magner M, Tio RA., and , Jiang C. Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding an HIF-1 α/VP16 hybrid transcription factor. Circulation. 2000;102:2255–2261. doi: 10.1161/01.cir.102.18.2255. [DOI] [PubMed] [Google Scholar]
- Taniyama Y, Morishita R, Hiraoka K, Aoki M., and , Nakagami H. Therapeutic angiogenesis induced by human hepatocyte growth factor gene in rat diabetic hindlimb ischaemia model. Circulation. 2001;104:2344–2350. doi: 10.1161/hc4401.098470. [DOI] [PubMed] [Google Scholar]
- Romero N, Braun S, Benveniste O, Leturcq F, Hogrel J., and , Morris G. Phase I study of dystrophin plasmid-based gene therapy in Duchenne/Becker muscular dystrophy. Hum Gene Ther. 2004;15:1065–1076. doi: 10.1089/hum.2004.15.1065. [DOI] [PubMed] [Google Scholar]
- Laitinen M, Mäkinen K, Manninen H, Matsi P, Kossila M, Agrawal RS, et al. Adenovirus-mediated gene transfer to lower limb artery of patients with chronic critical leg ischemia. Hum Gene Ther. 1998;1:1481–1486. doi: 10.1089/hum.1998.9.10-1481. [DOI] [PubMed] [Google Scholar]
- Eswarakumar P, Lax I., and , Schlessinge J. Cellular signaling by fibroblast growth factors. Cytokine Growth Factor Rev. 2005;16:149–198. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Bekeredjian R, Chen S, Frenkel P, Grayburn P., and , Shohet R. Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation. 2003;108:1022–1026. doi: 10.1161/01.CIR.0000084535.35435.AE. [DOI] [PubMed] [Google Scholar]
- Leong-Poi H, Kuliszewski MA, Lekas M, Sibbald M, Teichert-Kuliszewska K., and , Klibanov AL. Therapeutic arteriogenesis by ultrasound-mediated VEGF165 plasmid gene delivery to chronically ischemic skeletal muscle. Circ Res. 2007;101:295–303. doi: 10.1161/CIRCRESAHA.107.148676. [DOI] [PubMed] [Google Scholar]
- Doh SG, Vahlsing HL, Hartikka J, Liang X., and , Manthorpe M. Spatial-temporal patterns of gene expression in mouse skeletal muscle after injection of lacZ plasmid DNA. Gene Ther. 1997;4:648–663. doi: 10.1038/sj.gt.3300460. [DOI] [PubMed] [Google Scholar]
- Gounis M, Spiga M-G, Graham R, Wilson A, Haliko S., and , Liebe B. Angiogenesis is confined to the transient period of VEGF expression that follows adenoviral gene delivery to ischemic muscle. Gene Ther. 2005;12:762–771. doi: 10.1038/sj.gt.3302481. [DOI] [PubMed] [Google Scholar]
- Wolff JA, Ludtke JJ, Acsadi G, Williams P., and , Jani A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum Mol Genet. 1992;1:363–369. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- Tongers J, Roncalli JG., and , Losordo DW. Therapeutic angiogenesis for critical limb ischemia: microvascular therapies coming of age. Circulation. 2008;118:9–16. doi: 10.1161/CIRCULATIONAHA.108.784371. [DOI] [PubMed] [Google Scholar]
- Caron A, Finiels F, Caron A, Senechal E, Tejedor O., and , Vigne EPD. Local expression of human FGF61 in the rat skeletal muscle following intramuscular administration of NV1FGF, a plasmid DNA for the treatment of peripheral artery disease. Eur Heart J. 2005;26 suppl. 1:586. [Google Scholar]
- Presta M, Dell'era P, Mitola S, Moroni E, Ronca R., and , Rusnati M. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Rauh G, Pieczek A, Irwin W, Schainfeld R., and , Isner JM. In vivo analysis of intramuscular gene transfer in human subjects studied by on-line ultrasound imaging. Hum Gene Ther. 2001;12:1543–1549. doi: 10.1089/10430340152480267. [DOI] [PubMed] [Google Scholar]
- Lew D, Parker SE, Latimer T, Abai AM, Kuwahara-Rundell A, Doh SG, et al. Cancer gene therapy using plasmid DNA: pharmakokinetic study of DNA following injection in mice. Hum Gene Ther. 1995;6:553–564. doi: 10.1089/hum.1995.6.5-553. [DOI] [PubMed] [Google Scholar]