Abstract
Direct intramuscular injection (IM) of adeno-associated virus (AAV) has been proven a safe and potentially efficient procedure for gene therapy of many genetic diseases including hemophilia B. It is, however, contentious whether high antigen level induces tolerance or immunity to coagulation factor IX (FIX) following IM of AAV. We recently reported induction of FIX-specific immune tolerance by IM of AAV serotype one (AAV1) vector in mice. We hypothesize that the expression of high levels of FIX is critical to induction of FIX tolerance. In this study, we investigated the correlation among AAV dose, FIX expression, and tolerance induction. We observed that induction of immune tolerance or immunity to FIX was dependent on the dose of AAV1–human FIX (hFIX) given and the level of FIX antigen expressed in both normal and hemophilia mice. We then defined the minimum AAV1–hFIX dose and the lowest level of FIX needed for FIX tolerance. Different from hepatic AAV–hFIX gene transfer, we found that FIX tolerance induced by IM of AAV1 was not driven by regulatory T cells. These results provided further insight into the mechanism(s) of FIX tolerance, contributing to development of hemophilia gene therapy, and optimization of FIX tolerance induction protocols.
Introduction
Although protein replacement remains the standard therapy for hemophilia B, gene therapy is emerging as a potentially effective alternative treatment. Intramuscular injection (IM) of adeno-associated virus (AAV) has advantages over other methods of gene delivery because of its safety and minimal toxicity. However, preclinical and clinical studies have found that subtherapeutic expression of coagulation factor IX (FIX) and development of anti-FIX antibodies are two of the major obstacles of IM of AAV for successful hemophilia gene transfer.1,2 The newly developed AAV serotype one (AAV1) vector displayed robust muscular transduction efficiency and FIX expression in preclinical studies.3,4,5 Nevertheless, high expression of FIX subsequent to IM of AAV1 has led to diverse observations of host immune responses to FIX in immune competent mice and controversy in the field.4,6,7,8 We recently reported that IM of AAV1–human FIX (hFIX) efficiently induced permanent antigen-specific immune tolerance to hFIX in immune competent, FIX knock out (FIXKO, hemophilia B) mice, regardless of their immunological or genetic backgrounds.7 These mice expressed therapeutic levels of hFIX, compared to AAV serotype two (AAV2)–hFIX vector-injected mice that expressed undetectable levels of FIX.7 We hypothesize that sustained expression of high levels of FIX following IM of AAV1–hFIX is a determining factor for induction of FIX tolerance.
In order to understand how levels of FIX following IM of AAV determine host immune responses to FIX, we examined levels of FIX antigen and formation of anti-FIX antibodies in immune competent mice given different doses of AAV vectors expressing hFIX. We found that the hFIX antigen level and formation of anti-hFIX antibodies is dependent on the dose of the AAV–hFIX vector injected. We further defined the minimum dose of AAV1–hFIX and the lowest level of hFIX critical for FIX tolerance induction. Further mechanistic investigations suggested that the FIX tolerance induced by IM of AAV1 is not driven and/or mediated via upregulation of regulatory T cells. It is different from that for the FIX tolerance in hepatic AAV–FIX gene transfer. Accordingly, we proposed a three-zone model of immune responses to FIX subsequent to intramuscular AAV gene transfer.
Results
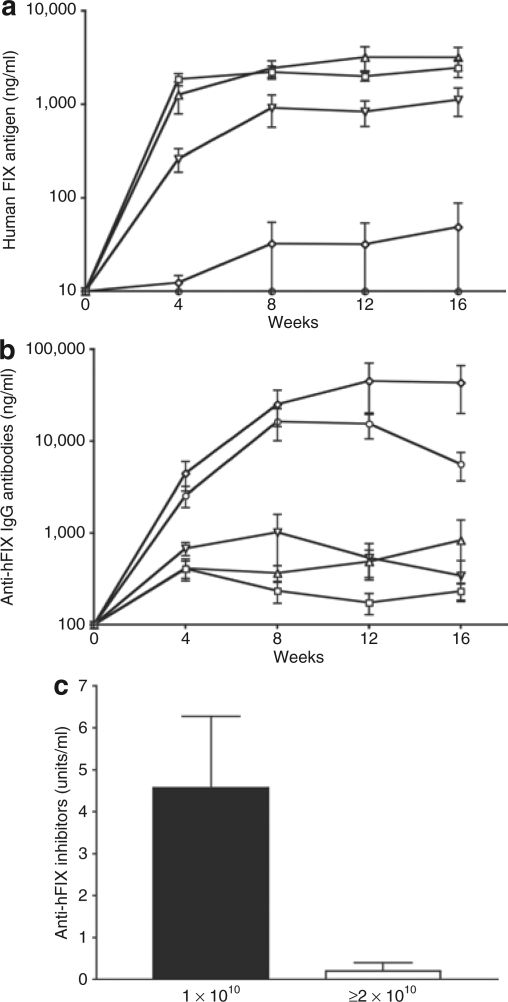
We previously reported probable dependence of FIX immune tolerance on the hFIX antigen level subsequent to intramuscular AAV gene transfer.7 In this study, we set to systemically investigate the correlation between hFIX antigen levels and the corresponding immune responses to hFIX after intramuscular AAV gene transfer. We first examined the correlation between hFIX antigen levels and AAV1 dose in normal C57BL/6 mice. Cohorts of 8- to 10-week-old C57BL/6 mice were injected with increasing doses of AAV1–hFIX (n = 10 per cohort). As illustrated in Figure 1a, hFIX antigen levels in mouse plasma directly correlate to the dose of AAV1–hFIX vector injected to the mice. This is consistent with our previous report of expression of canine FIX (cFIX) in immune deficient NOD/SCID mice following direct IM of AAV1–cFIX vector.4 However, similar levels of hFIX antigen were measured in the mice injected with 1 × 1011 and 5 × 1010 vector genomes (vg) per mouse of AAV1–hFIX (Figure 1a). Considering the robust muscular transduction efficiency of the AAV1 vector,3 it is likely that the maximum capacity of synthesis and secretion of hFIX of the injected muscle may be saturated with injection of 5 × 1010 vg of AAV1–hFIX vector.
Figure 1.
hFIX antigen levels are directly proportional to the dose of AAV1–hFIX vectors and a minimum dose of AAV1–hFIX is necessary to induce tolerance to FIX in immune competent C57BL/6 mice. Eight- to 10-week-old C57BL/6 normal mice were used for the studies. AAV vectors were injected at escalating doses (1 × 1010, 2 × 1010, 5 × 1010, and 1 × 1011 vector genome (vg) of AAV1–hFIX, or 6 × 1010 vg of AAV2–hFIX per mouse) into the gastrocnemius muscle of mice. Two separate sets of experiments were conducted, in which 10 mice in total were injected with each AAV dose. Graphs in this figure depict results of one set of the experiments, which are representative of all the mice analyzed. Results are the mean ± SEM for each time point (n = 5 for each dose group). Open square: 1 × 1011 vg AAV1–hFIX, open triangle: 5 × 1010 vg AAV1–hFIX, open inverted triangle: 2 × 1010 vg AAV1–hFIX, open diamond: 1 × 1010 vg AAV1–hFIX, open circle: 6 × 1010 vg AAV2–hFIX. (a) hFIX antigen. Difference of hFIX antigen level between the tolerant mice (≥2 × 1010 vg of AAV1-injected) and the FIX-immunized mice (AAV2 or 1 × 1010 vg of AAV1-injected) is significant (P = 0.0045 for comparison of the samples of 2 × 1010 and 1 × 1010 vg of AAV1-injected mice collected at the time point of 12 weeks after AAV injection). (b) Anti-hFIX IgG antibody. Difference of the level of anti-FIX antibodies between the tolerant mice (≥2 × 1010 vg of AAV1-injected) and the FIX-immunized mice (AAV2 or 1 × 1010 vg of AAV1-injected) is significant (P = 0.0156 for comparison of the samples of 2 × 1010 vg of AAV1 and 6 × 1010 vg of AAV2-injected mice collected at the time point of 12 week after AAV injection). (c) Inhibitory anti-hFIX antibodies measured by Bethesda inhibitor assay. Closed bar: Inhibitory anti-hFIX antibodies in mice that received 1 × 1010 vg of AAV1–hFIX (n = 3), Open bar: Inhibitory anti-hFIX antibodies in mice that received 2 × 1010 vg or higher dose of AAV1–hFIX (n = 4). The graph depicts results of samples that were collected 12 weeks after AAV injection. AAV, adeno-associated virus; AAV1, AAV serotype one; AAV2, AAV serotype two; FIX, coagulation factor IX; hFIX, human FIX.
We then investigated formation of anti-hFIX antibodies and the correlation with AAV1–hFIX dose and hFIX antigen levels. IM of AAV1–hFIX as low as 2 × 1010 vg per mouse resulted in sustained expression of therapeutic levels of hFIX antigen without detection of significant levels of anti-hFIX antibody (Figure 1b). However, high titers of anti-hFIX antibody and only background level of hFIX antigen were detected when the dose of AAV1–hFIX was reduced to 1 × 1010 vg per mouse or when AAV2–hFIX vectors were injected (Figure 1a and b). The titers of anti-hFIX antibodies in the cohort of 1 × 1010 vg of AAV1-injected mice were higher than that of AAV2-injected mice (Figure 1b). Expression of higher levels of FIX antigen in the 1 × 1010 vg of AAV1-injected mice than the 6 × 1010 vg of AAV2–hFIX-injected mice may account for the higher titer of anti-hFIX antibodies in these mice.3,4,9 These higher levels of FIX antigen still seem within the range of the intermediate levels of antigen that facilitate anti-FIX immunity, nevertheless below the minimal level needed for induction of tolerance (Figure 6). Challenging the mice with hFIX in adjuvant (CFA, Complete Freud's Adjuvant) confirmed immune tolerance to hFIX in the mice injected with high doses of AAV1 (data not shown). We detected low levels of anti-hFIX antibodies in the mice that were injected with 2 × 1010 vg or higher doses of AAV1–hFIX vectors by enzyme-linked immunosorbent assay (Figure 1b). These antibody levels are only slightly higher than the background of congenic naive mice (Figure 1). These mice concurrently expressed therapeutic levels of circulating hFIX antigen (Figure 1a). Bethesda inhibitor assay detected a considerable titer of inhibitory anti-hFIX activity in all the 1 × 1010 AAV1–hFIX or AAV2–hFIX-injected mice (Figure 1c and ref. 7). However, we detected a transient, lower titer of inhibitory anti-hFIX activity in one mouse that received 2 × 1010 vg of AAV1–hFIX, while detecting no inhibitory anti-hFIX activity in the remaining mice that received 2 × 1010 vg or higher doses of AAV1–hFIX vector (Figure 1c). Immunization of naive mice, by hFIX/CFA challenge, can elicit formation of high titer of anti-hFIX antibodies.7 As high as 31,000 ng/ml of anti-hFIX antibodies (mean ± SEM: 22,500 ± 3,398 ng/ml, n = 7) were detected in naive mice 2 weeks after hFIX/CFA immunization. No elevation in the titer of the anti-hFIX antibodies following hFIX/CFA challenge, in high dose AAV1–hFIX injected mice, further confirms induction of immune tolerance to hFIX in these mice (Figures 1 and 2). Taken together, the results we presented here establish an AAV-dose/FIX-level-dependent induction of immune tolerance to FIX following direct muscular AAV gene transfer.
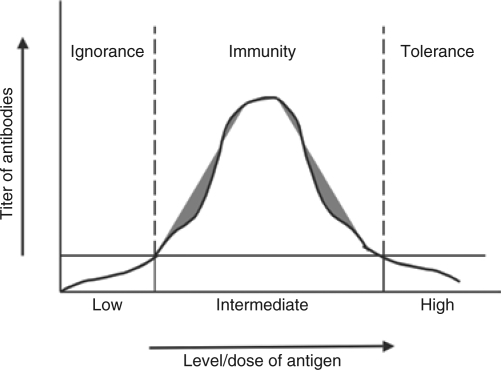
Figure 6.
Three-zone model of immune responses to FIX subsequent to intramuscular AAV gene transfer. AAV, adeno-associated virus; FIX, coagulation factor IX.
Figure 2.
AAV dose and FIX antigen level-dependent induction of immune tolerance to hFIX in FIXKO mice. Eight- to 10-week-old FIXKO mice on C57BL/6 background were given intramuscular injection of either 2 × 1010 vg (n = 7) or 1 × 1010 vg (n = 5) of AAV1–hFIX. hFIX antigen and anti-FIX IgG antibody levels were analyzed every 4 weeks after AAV injection. Results are the mean ± SEM for each time point. Closed inverted triangle: 2 × 1010 vg AAV1–hFIX, closed diamond: 1 × 1010 vg AAV1–hFIX. (a) hFIX antigen. Difference of hFIX antigen level between the two cohorts is significant (P = 0.0017 for comparison of the samples collected at the time point of 12 week after AAV injection). (b) Anti-hFIX IgG antibody. Difference of the level of anti-FIX antibodies between two cohorts is significant (P = 0.0015 for comparison of the samples collected at the time point of 12 week after AAV injection). AAV, adeno-associated virus; AAV1, AAV serotype one; FIX, coagulation factor IX; FIXKO, FIX knock out; hFIX, human FIX.
It was reported that formation of anti-FIX antibodies following gene transfer may be affected by the hemostatic status of the animal tested. A high risk of development of anti-FIX antibodies, is also observed, in hemophilia B animals with null mutation of FIX.10,11 We therefore extended to test whether IM of 2 × 1010 vg of AAV1–hFIX could induce immune tolerance to hFIX in FIXKO mice (with null FIX mutation), as it does in the hemostatically normal mice.
We injected 2 × 1010 and 1 × 1010 vg of AAV1–hFIX vectors per mouse to the skeletal muscle (gastrocnemius) of two cohorts of 8- to 10-week-old FIXKO mice. We observed that IM of 2 × 1010 vg of AAV1–hFIX expressed equivalent levels of hFIX antigen in the FIXKO mice compared to the normal C57BL/6 mice (Figures 1a and 2a). Immune tolerance to hFIX in the 2 × 1010 vg of AAV1–hFIX injected FIXKO mice with null FIX mutation was demonstrated by absence or low titer of anti-hFIX antibodies despite challenging the mice with hFIX/CFA (Figure 2b). Such titers of anti-hFIX antibodies detected by enzyme-linked immunosorbent assay are only slightly above the background antibody levels seen in congenic naive mice, and bear little inhibitory anti-hFIX activity when measured by the Bethesda inhibitor assay (data not shown). High titers of anti-hFIX antibodies and background level of hFIX antigen were measured in the 1 × 1010 vg of AAV1–hFIX injected FIXKO mice (n = 5, Figure 2b). High titers of anti-hFIX antibodies were detected in both normal and FIXKO C57BL/6 mice injected with 1 × 1010 vg/mouse of AAV1–hFIX. Therefore, AAV1-dose and FIX-level-dependent induction of immune tolerance to hFIX following IM of AAV1–hFIX is independent of the hemostatic status and FIX gene mutation of the animals. The AAV1-dose and FIX-level-dependent FIX tolerance in the C57BL/6 mice was consistent with our previous observation that a higher dose of AAV1–hFIX was essential to induce FIX tolerance while a lower dose of AAV1–hFIX elicited anti-hFIX immunity in Balb/c and C3H mice.7
Statistical analysis further validates the significant difference of the levels of hFIX antigen as well as the titers of anti-hFIX antibodies between cohorts of mice that received 2 × 1010 and 1 × 1010 vg of AAV1–hFIX vectors in normal and FIXKO mice (Figures 1 and 2).
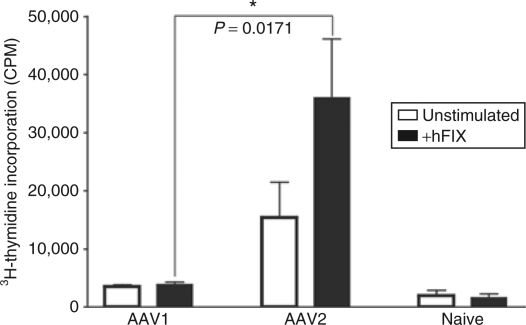
Development of anti-FIX antibodies is T-cell dependent. Absence of anti-FIX antibodies nevertheless can be T cell-independent, i.e., the result of B cell tolerance even without tolerance of the T cells. We thereby examined activity of the hFIX-specific T cells in AAV treated mice. As shown in Figure 3, we observed vigorous proliferation of hFIX-specific T cells upon stimulation with hFIX antigen in AAV2–hFIX treated mice. The same group also showed high titers of anti-FIX antibodies indicating that antibody formation may be CD4+ T-cell dependent. Lack or suppression of hFIX-specific CD4+ T-cell proliferation upon stimulation of hFIX antigen denotes induction of T-cell tolerance in the FIX-tolerant AAV1-treated mice (Figure 3).
Figure 3.
Absence of anti-hFIX-specific T cell response in AAV1-treated tolerant mice. Ten-week-old mice with C57BL/6 background were given IM of AAV1–hFIX (1 × 1011 vg, n = 3) or IM of AAV2–hFIX (6 × 1010 vg, n = 3). Six to eight months after AAV injection, the mice were challenged/boosted by subcutaneous injection of rhFIX in CFA as described. Two weeks later, tolerance or immunity to hFIX was further confirmed by examination of hFIX antigen and anti-hFIX IgG antibodies in the mice as described. CD4+ T cells were collected from spleens of the mice for T-cell proliferation assay as described. Cells from naive C57BL/6 mice with the same age were used as negative control (n = 3). This figure depicts results of 3H-thymidine incorporation (CPM) of a typical experiment out of 3 performed. Closed bars, stimulation with 10 µg/ml of rhFIX; Open bars, mock, absence of rhFIX. All the samples were performed in triplicate. Results are mean ± SEM. AAV, adeno-associated virus; AAV1, AAV serotype one; CFA, Complete Freud's Adjuvant; CPM, counts per minute; hFIX, human coagulation factor IX; IM, intramuscular injection; rhFIX, recombinant hFIX.
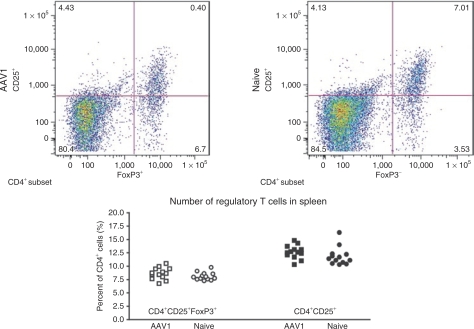
T-cell tolerance can be the result of clonal deletion, anergy of the antigen-specific T cells, or induction of regulatory T cells. It was reported that FIX tolerance subsequent to hepatic AAV–FIX gene transfer was mediated by induction of regulatory T cells.12,13 We thereby evaluated regulatory T cells in the mice that received IM of AAV. We first examined number of CD4+CD25+ and CD4+CD25+FoxP3+ regulatory T cells in the AAV1-injected, FIX-tolerant mice by flow cytometry, using naive, un-injected congenic mice as control. We observed no change in the number of regulatory T cells (CD4+CD25+ and CD4+CD25+FoxP3+ cells) in the AAV1-injected, FIX-tolerant mice (n = 13) when compared with naive, un-injected mice (n = 13, Figure 4). These results suggest that the upregulation of regulatory T cells may not be accountable for the FIX tolerance induced by IM of AAV1.
Figure 4.
hFIX-specific immune tolerance induced by IM of AAV1 is not accompanied by upregulation of regulatory T cells. Ten- to 12-week-old mice with C57BL/6 backgrounds (n = 13) were given IM of AAV1–hFIX (1 × 1011 vg). Lymphocytes were collected from the mice 3–4 months after AAV injection for antibody staining and flow cytometry analysis as described. Tolerance to hFIX in the mice was verified by examination of hFIX antigen and anti-hFIX antibody at the time of lymphocyte collection. Lymphocytes from naive mice with the same backgrounds and matched age (n = 13) were examined simultaneously. This figure depicts frequency (% of CD4+ cells) of CD4+CD25+ and CD4+CD25+FoxP3+ regulatory T cells in the spleen of AAV1-injected tolerant and naive (control) mice with C57BL/6 background. AAV, adeno-associated virus; AAV1, adeno-associated virus serotype one; FIX, coagulation factor IX; hFIX, human FIX; IM, intramuscular injection.
In addition to increase of number of regulatory T cells, enhancement of activity/quality or other types of regulatory T cells can also be induced to mediate or promote the FIX tolerance.14 Immune tolerance driven by regulatory T cells is a transferable, active tolerance. To further verify the role of regulatory T cells on the induction of the FIX tolerance by IM of AAV1, we performed adoptive T-cell transfer to test the transferability of the FIX tolerance induced by IM of AAV1. We collected CD4+ T cells from the spleens of AAV1-injected, FIX-tolerant mice as well as naive, un-injected mice as control. The cells were injected to congenic, naive recipient mice as described. The recipient mice were immunized with rhFIX/CFA 72 hours after cell transfer, and analyzed for anti-FIX antibodies and hFIX-specific T-cell proliferation. As shown in Figure 5, transfer of CD4+ T cells from AAV1-injected, FIX-tolerant mice did not inhibit immunization of the recipient congenic mice by FIX antigen. The results of the adoptive T-cell transfer experiments further substantiated the insignificance of regulatory T cells in the induction of FIX tolerance following IM of AAV1.
Figure 5.
Transfer of CD4+ T cells from AAV1-treated, FIX-tolerant mice does not suppress formation of anti-hFIX immunity in recipient mice. Adoptive T-cell transfer, mice immunization, anti-hFIX antibody enzyme-linked immunosorbent assay, and T-cell proliferation were performed as described. Naive, recipient mice of naive cells (n = 3); AAV1, recipient mice of cells from AAV1-injected, FIX-tolerant mice (n = 4); Control, naive mice without any treatment (n = 2). Results are mean ± SEM. Results are a representative of three experiments performed. (a) This figure depicts titer of anti-hFIX antibodies in the recipient mice. Closed bars, mice that received T cells from naive mice (n = 3). Open bars, mice that received T cells from AAV1-treated, tolerant mice (n = 4). All the samples were performed in duplicate. (b) This figure depicts results of hFIX-specific T-cell proliferation in the recipient mice. Closed bars, stimulation with 10 µg/ml of rhFIX; open bars, mock, absence of rhFIX. All the samples were performed in triplicate. AAV1, adeno-associated virus serotype one; CPM, counts per minute; FIX, coagulation factor IX; hFIX, human FIX; rhFIX, recombinant hFIX.
Discussion
Formation of inhibitory anti-FIX antibodies is a major complication in FIX replacement treatment for hemophilia B patients. Although gene therapy is being developed as a potentially effective treatment for hemophilia, formation of anti-FIX antibodies following gene transfer was observed and therefore needs to be addressed. Among the currently tested strategies for FIX gene transfer, direct IM of the nonpathogenic AAV vector is one of the safest and most convenient procedures. Although IM of AAV1 vector could overcome the subtherapeutic expression of FIX seen with the AAV2 vector, controversial results were reported regarding incidence of formation of anti-FIX antibodies subsequent to IM of AAV1.4,6,7,8
In our previous report, we found that insufficient expression of FIX following IM of lower dose of AAV1 vectors in Balb/c and C3H mice led to formation of anti-FIX antibodies. Immune tolerance to FIX, however, was induced upon sufficient expression of FIX with an increase of AAV1 dose injected to the mice. In this study, we examined the correlation between AAV dose, FIX expression and induction of FIX tolerance. We observed AAV1 dose and FIX antigen level-dependent FIX tolerance following IM of AAV1 in both normal and hemophilia B mice. We defined the minimum AAV1 dose and the lowest level of FIX needed for FIX tolerance. Thus, there exists a threshold level of AAV1 and FIX, above which tolerance is induced and below which immunity is elicited. These results evidently validate our hypothesis that induction of immune tolerance to FIX subsequent to muscular AAV gene transfer is dependent on expression of high levels of hFIX antigen.
We would like to point out that the defined minimum tolerizing dose of 2 × 1010 vg of AAV1 is specific for hFIX in mice with C57BL/6 background. Such threshold doses of AAV1 for induction of FIX tolerance in other strains of mice, such as Balb/c and C3H, are in fact different.7 The threshold level of antigen essential for induction of antigen-specific immune tolerance to other transgene products, as well as the corresponding dose of AAV1, may vary from transgene to transgene.
Plateau of expression of hFIX antigen in the mice upon injection of 5 × 1010 vg of AAV1–hFIX vectors (Figure 1a) may denote the maximum capacity of synthesis and secretion of hFIX of the injected muscle. Considering the robust muscular transduction efficiency of the AAV1 vector,3 more injection sites with a relatively low dose of AAV1 vector (not >5 × 1010 vg) per injection site may be beneficial for higher hFIX expression, especially in preclinical testing of AAV1 in large animal models and for clinical trials in patients.
The mechanism(s) accounting for formation of anti-FIX antibody in patients subsequent to FIX replacement is not well understood.15 One method for treatment of patients with anti-FIX antibodies is continuous infusion of high doses of FIX, which can successfully induce FIX tolerance.16 Because AAV1 vectors lead to prolonged expression of high levels of FIX, this type of gene therapy may induce tolerance via the mechanism that is the same as or similar to continuous infusion of high doses of FIX. In gene therapy, many factors, including characteristics of the gene transfer vector, promoter specificity, vector administration route, FIX cDNA species, expression levels of FIX, and nature of FIX mutation, can contribute to the formation of anti-FIX immune responses.10,11,17,18,19,20,21 For instance, following AAV-based hepatic FIX gene transfer, the nonpathogenic nature of AAV, the tolerogenic property of the liver and expression of high levels of FIX favor FIX tolerance.12 However, intramuscular AAV–FIX gene transfer appears to produce different results.4,6,7,8,10,19,20,21
IM of AAV1-induced FIX tolerance, although anti-FIX antibodies were elicited in AAV2-injected mice that expressed lower levels of FIX.4,7 The apparent distinction between AAV1 and AAV2 is the transduction efficiency in muscle, which leads to lower FIX antigen expression in AAV2 injected mice, compared to AAV1. Antigen signal strength, duration of antigen stimulation and maturation status of antigen presenting cells can determine the progression, differentiation and ultimate fate of antigen-specific T cells.22 The “high zone tolerance” concept proposed by Mitchison,23 which suggests that high levels of antigen promote tolerance, can explain the tolerance induced in AAV1-injected mice. Thus, we hypothesize that sustained expression of high levels of FIX is a determining factor for induction of FIX immune tolerance following IM of AAV1. The data presented in this report confirm that there is a threshold level of antigen needed to achieve immune tolerance. Along with these data, the nonpathogenic nature of the AAV vector and sustained expression of high levels of FIX conceivably determines FIX tolerance following IM of AAV1. This mechanism is also evident in patients who successfully achieve induction of FIX and coagulation factor VIII tolerance using high doses of protein.16 An independent report corroborates our hypothesis of high level of FIX antigen-dependent tolerance following IM of AAV1.8 Even data presented in the literature that suggested high dose AAV vector facilitates stronger anti-FIX immunity actually support our hypothesis of high level of FIX antigen-dependent tolerance by showing disappearance of anti-hFIX antibody and upregulation of hFIX after 7–12 weeks in the highest vector dose.24 Induction of immune tolerance to serum proteins such as human α1-antitrypsin and coagulation factor VIII, by expression of high-level of the antigen following gene transfer using recombinant adenoviral or retroviral vectors that was reported in two recent independent papers, further supports our hypothesis.25,26
Accordingly, we propose a three-zone FIX-level-dependent model of immune responses to FIX following AAV gene transfer: low zone ignorance, intermediate zone immunity and high zone tolerance, as illustrated in Figure 6. When low doses of AAV are injected and low levels of FIX antigen are consequently expressed, the low levels of FIX antigen are insufficient to result in anti-FIX immunity, i.e., ignored by the host immune system thus no antibody develops (ignorance zone).10,24 Upon injection of intermediate doses of AAV vectors there is expression of intermediate levels of FIX antigen, the anti-FIX T and B cells are activated to develop anti-FIX immunity (immunity zone, activated FIX-specific CD4+ T cells and formation of anti-FIX antibodies).7,10,24 Such intermediate doses of AAV and levels of FIX antigen nevertheless were defined as “high” doses in some reports.6,9,10,24 Within the range of intermediate zone, higher doses or a more transduction-efficient AAV vector such as 1 × 1010 vg of AAV1–hFIX, and the resultant elevated (local and systemic) levels of FIX antigen lead to stronger anti-FIX immunity (Figure 1a,b).6,9,10,24 Immune tolerance, however, is induced when vector dose (and efficiency of vector) and FIX antigen level increases to a threshold (Figures 1 and 2).4,7,8,23,24 The principle of this three-zone model of immune responses may also be applicable in protein replacement, as well as gene transfer of a variety of secretory proteins using different gene transfer approaches.
Upregulation of CD4+CD25+ regulatory T cells may be responsible for FIX tolerance following hepatic delivery of AAV vector.12,13 However, the muscle is not as tolerogenic as liver.27 In fact, IM is frequently used for vaccination inoculation, when an efficient and strong immune response is desired. The mechanism of tolerance following gene transfer in the nontolerance-favoring muscle may not parallel the tolerogenic liver. High doses of antigen usually induce immune tolerance to the antigen by T cell clone deletion through activation-induced cell death.22,28 Prolonged exposure to low doses of antigen, however, results in tolerance mediated by regulatory T cells.29 In this study, we investigated the potential role of regulatory T cells in induction of FIX tolerance subsequent to IM of AAV. Our results strongly suggested mechanisms other than upregulation of regulatory T cells to be responsible for the FIX tolerance induced by IM of AAV1 (Figures 4 and 5). It is different from the mechanism of FIX tolerance by induction of regulatory T cells following hepatic AAV gene transfer.12,13 We are continuing our effort in exploring the specific mechanism that mediates the FIX tolerance induced by IM of AAV1. Our preliminary results suggest in vivo T-cell anergy is likely to be the major mechanism underlying induction of FIX tolerance following IM of AAV1. However, more experimental evidence is needed to conclude the actual mechanism. We are also investigating other factors that may contribute to FIX tolerance, such as the characteristics and activation/differentiation status of antigen presenting cells subsequent to administration of AAV, as well as the persistent expression of the FIX by AAV gene transfer.
High doses of clotting factor are considered critical for successful FIX and FVIII tolerance induction through continuous infusion of the factor protein.16 To our knowledge, no one has analyzed the quantitative correlation of the factor dose/level and tolerance, or reported an evidence-based minimal “high dose” of factor essential for tolerance. Our results in this report indicate that sustained, but “modest” levels of FIX can efficiently induce FIX tolerance. These results imply that high doses of FIX and FVIII that are routinely used in current tolerance protocols may be adjusted to reduce cost and complications without affecting the success of the tolerance outcome.
Materials And Methods
AAV-hFIX vector production, animal care, and procedures. AAV1 and AAV2 vectors expressing hFIX were produced as previously described.7 C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). FIXKO mice were gifts from Paul Monahan (University of North Carolina at Chapel Hill, Chapel Hill, NC). All the mice were maintained in pathogen-free animal facilities at Mount Sinai School of Medicine, and treated in accordance with the guidelines of the Institutional Animal Care and Use Committee of Mount Sinai School of Medicine. AAV-hFIX vectors were injected into the gastrocnemius muscle of 8- to 10-week-old mice as described previously.7 Blood collection and preparation of mouse plasma were conducted as previously described.7 All injections and blood drawings were preceded by Forane (Baxter, Deerfield, IL) inhaled anesthesia.
Measurement of hFIX antigen and detection of anti-hFIX antibodies. An enzyme-linked immunosorbent assay was employed for hFIX antigen measurement as described previously.7 The hFIX levels were calculated from a standard curve derived from serial dilutions of pooled normal human plasma (Universal Coagulation Reference Plasma; Fisher Scientific, Pittsburgh, PA) diluted in normal isogenic mouse plasma.
Enzyme-linked immunosorbent assay and Modified Bethesda inhibitor assay were conducted to detect and measure anti-hFIX antibodies as previously described.7 Antibody levels were calculated based on standard curves of successive dilutions of the relevant mouse antibody (mouse total IgG antibodies from BD Biosciences, San Jose, CA).
Lymphocyte preparation and purification. Lymphocytes were isolated from spleen and/or lymph nodes of the pertinent mice by mechanic disintegration followed by red blood cell lysis (BD Pharmingen, San Diego, CA). The cells were counted and then maintained in RPMI 1640 media (Invitrogen, Carlsbad, CA) at 37 °C. CD4+ T cells were purified and enriched by negative selection using MACS CD4+T cell Isolation Kit (Miltenyi Biotec, Auburn, CA), according to the manufacturer's instructions. Analysis by flow cytometry showed ~87.5% purity of the enriched CD4+ T cells with ~2% of CD8+ T cells and 2% of B cells. Irradiated splenocytes (3,000 rad) from congenic naive mice were used as APCs for T-cell proliferation assay.
Flow cytometry analysis. Single cell suspensions were obtained from spleen and/or lymph nodes of the pertinent mice as described above. The cells were suspended in 100 µl of fluorescence-activated cell sorting staining buffer (5 ml phosphate buffered saline, 100 µl each of normal mouse, rabbit and human serum, 333 µl 30% bovine serum albumin, 5 ml HBSS complete with bovine serum albumin and 100 mmol/l EDTA), and then stained with the following fluorochrome conjugated antibodies: CD4-APC (1:300), FoxP3-PE (1:200) (eBiosciences, San Diego, CA), CD25-PerCP-Cy5.5 (1:300) (BD Pharmingen). The cells were incubated with the antibody mixture (cell surface antibodies) for 30 minutes on ice. The cells were washed twice by the addition of 1-ml fluorescence-activated cell sorting buffer. The cell pellet was resuspended in 1 ml freshly prepared fixation/permeabilization reagent (FoxP3 staining buffer set; eBioscience) and incubated for 30 minutes on ice. Cells were washed twice by addition of 1 ml of 1× permeabilization buffer followed by incubation with the intracellular antibody (FoxP3-PE) for 30 minutes on ice. They were washed twice with 1× permeabilization buffer, and then were stored in 1× formalin buffer before analysis. Data was acquired on BD LSRII flow cytometer equipped with the BD FACSDiva software (Becton Dickinson, San Jose, CA). The data were analyzed using FlowJo analysis software (TreeStar, Ashland, OR).
In vitro culture and stimulation of T cell and T-cell proliferation assays. Freshly isolated CD4+ T cells (2 × 105/well) plus APCs (1 × 105/well) were cultured in 96-well plates in 200 µl/well of RPMI medium, with or without stimulation of immunogen (10 µg/ml rhFIX; Genetics Institute, Cambridge, MA) at 37 °C for 4 days. For T-cell proliferation assay, 1 µCi 3H-thymidine (GE Healthcare, Piscataway, NJ) was added to each well 18 hours before harvest. Lymphocyte (T cell) proliferation was measured by scintillation count of 3H-thymidine incorporation using Beta Reader (1450 Microbeta Plus; Wallac, Turku, Finland).
Adoptive T-cell transfer. CD4+ T cells isolated from naive or AAV1-injected C57BL/6 mice (1.4–2 × 107 per mouse) were collected and adoptively transferred to 7-week-old congenic naive mice by retro-orbital injection. Recipient mice were challenged by hFIX in CFA as previously described 7 3 days after adoptive T-cell transfer. Anti-hFIX T cell responses and antibody formation were examined 14 days after immunization.
Statistical analysis. The data was analyzed using GraphPad Prism (GraphPad Software, La Jolla, CA). Statistical differences between the various experimental groups were evaluated by t test. P < 0.05 was considered statistically significant.
Acknowledgments
The authors declare no conflict of interest. We thank G. Randolph, F. Tacke, and C. Qu for advice and technical assistance, Y. Sun and W. Zhang for advice and assistance in statistical analyses of the data. This work was supported by grant NIH R01-HL076699 to H.J.C. H.J.C. is an NHLBI/NHF researcher. H.J.C. designed the study, analyzed the data and wrote the paper. M.E.K., J.Z., and A.B. performed the experiments. M.E.K. and A.B. also contributed to data summarization, analysis, and manuscript preparation.
REFERENCES
- Chao H., and , Walsh CE. AAV vectors for hemophilia B gene therapy. Mt Sinai J Med. 2004;71:305–313. [PubMed] [Google Scholar]
- Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- Chao H, Liu Y, Rabinowitz J, Li C, Samulski RJ., and , Walsh CE. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol Ther. 2000;2:619–623. doi: 10.1006/mthe.2000.0219. [DOI] [PubMed] [Google Scholar]
- Chao H, Monahan P, Liu Y, Samulski R., and , Walsh C. Sustained and complete phenotype correction of hemophilia B mice following intramuscular injection of AAV1 serotype vectors. Mol Ther. 2001;4:217–222. doi: 10.1006/mthe.2001.0449. [DOI] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J., and , Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YL, Mingozzi F, Rodriguez-Colon SM, Joseph S, Dobrzynski E, Suzuki T, et al. Therapeutic levels of factor IX expression using a muscle-specific promoter and adeno-associated virus serotype 1 vector. Hum Gene Ther. 2004;15:783–792. doi: 10.1089/1043034041648453. [DOI] [PubMed] [Google Scholar]
- Cohn EF, Zhuo J, Kelly ME., and , Chao HJ. Efficient induction of immune tolerance to coagulation factor IX following direct intramuscular gene transfer. J Thromb Haemost. 2007;5:1227–1236. doi: 10.1111/j.1538-7836.2007.02522.x. [DOI] [PubMed] [Google Scholar]
- Zhang TP, Jin DY, Wardrop RM 3rd, Gui T, Maile R, Frelinger JA, et al. Transgene expression levels and kinetics determine risk of humoral immune response modeled in factor IX knockout and missense mutant mice. Gene Ther. 2007;14:429–440. doi: 10.1038/sj.gt.3302881. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Fields PA, Arruda VR, Brubaker JO, Armstrong E, McClintock D, et al. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum Gene Ther. 2002;13:1281–1291. doi: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- Fields PA, Arruda VR, Armstrong E, Chu K, Mingozzi F, Hagstrom JN, et al. Risk and prevention of anti-factor IX formation in AAV-mediated gene transfer in the context of a large deletion of F9. Mol Ther. 2001;4:201–210. doi: 10.1006/mthe.2001.0441. [DOI] [PubMed] [Google Scholar]
- Herzog RW, Mount JD, Arruda VR, High KA., and , Lothrop CD., Jr Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001;4:192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C, et al. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar AN, Vukmanovic-Stejic M, Taams LS., and , Macallan DC. The dynamic co-evolution of memory and regulatory CD4+ T cells in the periphery. Nat Rev Immunol. 2007;7:231–237. doi: 10.1038/nri2037. [DOI] [PubMed] [Google Scholar]
- Key NS. Inhibitors in congenital coagulation disorders. Br J Haematol. 2004;127:379–391. doi: 10.1111/j.1365-2141.2004.05168.x. [DOI] [PubMed] [Google Scholar]
- Dimichele DM. Immune tolerance: critical issues of factor dose, purity and treatment complications. Haemophilia. 2006;126 Suppl:81–86. doi: 10.1111/j.1365-2516.2006.01376.x. [DOI] [PubMed] [Google Scholar]
- Brown BD., and , Lillicrap D. Dangerous liaisons: the role of “danger” signals in the immune response to gene therapy. Blood. 2002;100:1133–1140. doi: 10.1182/blood-2001-11-0067. [DOI] [PubMed] [Google Scholar]
- Follenzi A, Battaglia M, Lombardo A, Annoni A, Roncarolo MG., and , Naldini L. Targeting lentiviral vector expression to hepatocytes limits transgene-specific immune response and establishes long-term expression of human antihemophilic factor IX in mice. Blood. 2004;103:3700–3709. doi: 10.1182/blood-2003-09-3217. [DOI] [PubMed] [Google Scholar]
- Ge Y, Powell S, Van Roey M., and , McArthur JG. Factors influencing the development of an anti-factor IX (FIX) immune response following administration of adeno-associated virus-FIX. Blood. 2001;97:3733–3737. doi: 10.1182/blood.v97.12.3733. [DOI] [PubMed] [Google Scholar]
- Monahan PE, Samulski RJ, Tazelaar J, Xiao X, Nichols TC, Bellinger DA, et al. Direct intramuscular injection with recombinant AAV vectors results in sustained expression in a dog model of hemophilia. Gene Ther. 1998;5:40–49. doi: 10.1038/sj.gt.3300548. [DOI] [PubMed] [Google Scholar]
- Chao H, Samulski R, Bellinger D, Monahan P, Nichols T., and , Walsh C. Persistent expression of canine factor IX in hemophilia B canines. Gene Ther. 1999;6:1695–1704. doi: 10.1038/sj.gt.3301024. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A., and , Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- Mitchison NA. Induction of immunological paralysis in two zones of dosage. Proc R Soc Lond B Biol Sci. 1964;161:275–292. doi: 10.1098/rspb.1964.0093. [DOI] [PubMed] [Google Scholar]
- Wang L, Cao O, Swalm B, Dobrzynski E, Mingozzi F., and , Herzog RW. Major role of local immune responses in antibody formation to factor IX in AAV gene transfer. Gene Ther. 2005;12:1453–1464. doi: 10.1038/sj.gt.3302539. [DOI] [PubMed] [Google Scholar]
- Cerullo V, McCormack W, Seiler M, Mane V, Cela R, Clarke C, et al. Antigen-specific tolerance of human alpha1-antitrypsin induced by helper-dependent adenovirus. Hum Gene Ther. 2007;18:1215–1224. doi: 10.1089/hum.2006.036. [DOI] [PubMed] [Google Scholar]
- Xu L, Mei M, Ma X., and , Ponder KP. High expression reduces an antibody response after neonatal gene therapy with B domain-deleted human factor VIII in mice. J Thromb Haemost. 2007;5:1805–1812. doi: 10.1111/j.1538-7836.2007.02629.x. [DOI] [PubMed] [Google Scholar]
- Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- Green DR, Droin N., and , Pinkoski M. Activation-induced cell death in T cells. Immunol Rev. 2003;193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Apostolou I., and , von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]