Abstract
Over the past decade, cell therapy has emerged as a potential new treatment of a variety of cardiac diseases, including acute myocardial infarction, refractory angina, and chronic heart failure. A myriad of cell types have been tested experimentally, each of them being usually credited by its advocates of a high “regeneration” potential. This has led to a flurry of clinical trials entailing the use of skeletal myoblasts or bone marrow–derived cells either unfractionated or enriched in progenitor subpopulations. As often in medicine, the hype generated by the early uncontrolled and small-sized studies has been dampened by the marginally successful outcomes of the subsequent, more rigorously conducted randomized trials. Although they may have failed to achieve their primary end points, these trials have been positive in the sense that they have allowed to identify some key issues and it is reasonable to speculate that if these issues can now be addressed by appropriately focused benchwork, the outcomes of the second generation of cell-transplantation studies would likely be upgraded. It, thus, appears that not “one cell fits all” but that the selection of the cell type should be tailored to the primary clinical indication. On the one hand, it does not make sense to develop an “ideal” cell in a culture dish, if we remain unable to deliver it appropriately and to keep it alive, at least for a while, which requires to improve on the delivery techniques and to provide cells along with the vascular and extracellular matrix type of support necessary for their survival and patterning. On the other hand, the persisting mechanistic uncertainties about cell therapy should not preclude continuing clinical trials, which often provide the unique opportunity of identifying issues missed by our suboptimal preclinical models. Finally, regardless of whether cells are expected to act paracrinally or by physically replacing lost cardiomyocytes and, thus, effecting a true myocardial regeneration, safety remains a primary concern. It is, thus, important that clinical development programs be shaped in a way that allows the final cell-therapy product to be manufactured from fully traceable materials, phenotypically well characterized, consistent, scalable, sterile, and genetically stable as these characteristics are those that will be required by the ultimate gatekeeper, i.e., the regulator, and are thus unbypassable prerequisites for an effective and streamlined leap from bench to bedside.
Over the past decade, stem cells have been the subject of intense experimental and clinical research in virtually all fields of medicine. In the specific setting of cardiac diseases, this interest has been largely driven by two major considerations: the improved survival rate of patients with acute myocardial infarction due to revascularization therapies has put more of them at risk of developing heart failure1 and despite the advances in drug therapy and resynchronization devices, the proportion of cardiovascular deaths in the group of heart failure patients with depressed left ventricular (LV) function has not substantially improved over time.2 Put together, these observations account for the continued search for new option treatments among which cell therapy has gained a growing interest. Although the early wave of clinical trials has generated marginally successful results, it has also provided a huge amount of data that can now be used as a building block to move the field forward. This review will thus highlight some of the lessons learned from these initial clinical studies and discuss how these clinical findings, along with the most recent basic data on stem-cell biology, open attractive perspectives for cardiac regenerative therapy.
Skeletal Myoblasts
After almost a decade of experimental studies, clinical trials of myoblast transplantation started in June 2000, when we performed the first human transplantation of autologous myoblasts in a patient with severe ischemic heart failure.3 This case initiated a series of 10 patients with a severe LV dysfunction (reflected by an ejection fraction ≤35%), a postinfarction nonviable scar and an indication for coronary artery bypass grafting (CABG) in ischemic but viable areas remote from the transplanted ones (which were thus not revascularized). The reassessment of these patients at an average follow-up of 52 months (18–58) has basically shown a symptomatic improvement, a relatively low incidence of hospitalizations for heart failure (0.13/patient-years) and a stabilization of echocardiographically measured LV ejection fraction (LVEF) and volumes.4 In one patient who died 18 months postoperatively from a stroke, some engrafted myotubes could still be identified embedded in scar tissue.
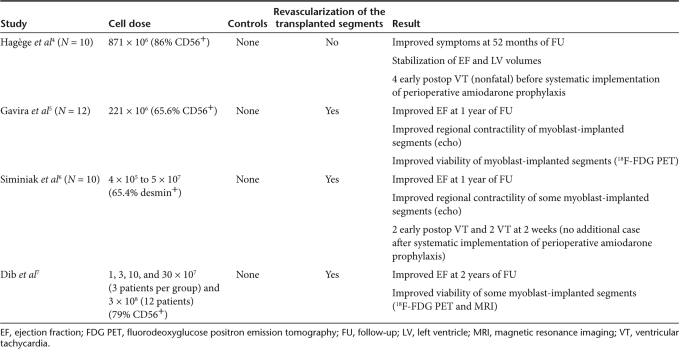
Three other adjunct-to-CABG transplantation studies were then performed.5,6,7 Whereas the patient profile and technique of open-chest multiple injections were very similar to those used in our study, the number of transplanted myoblasts was highly variable (221 × 106 in the study of Gavira et al.5 from 4 × 105 to 5 × 107 in the study of Siminiak et al.6 1, 3, 10, 30 × 107 and 3 × 108 in the dose-escalating study of Dib et al.7). Importantly, the protocol of these three studies also differed from ours in that it systematically entailed a concomitant revascularization of the myoblast-injected areas (Table 1).
Table 1.
Summary of phase I adjunct-to-bypass surgical trials of skeletal myoblast transplantation
Put together, these studies have primarily demonstrated the feasibility of the procedure as well as the safety of multiple needle punctures in the postinfarction scar and along its borders. Likewise, none of the myoblast-injected patients have developed a cardiac tumor (our longest survivor was operated on in December 2000). Indeed, the only safety concern has been an increased risk of arrhythmias after some of these early-phase trials reported postoperative episodes of sustained ventricular tachycardia.4,6 The currently prevailing hypothesis is that differentiated myotubes fail to express gap junction proteins and, as such, feature islet-like clusters electrically isolated from the surrounding cardiomyocytes;8 this, in turn, could slow the conduction velocity of electrical impulses and consequently predispose to reentry circuits.9 This hypothesis is largely based on coculture experimental data showing that myoblast transfection with connexin 43 decreases arrhythmogenicity.9 In vivo data have been more conflicting10,11,12 but overall they support an increased risk of myoblast-related arrhythmias, possibly worsened by needle-induced disruption of myocardial tissue and the associated inflammatory damage.13 Clinically, however, the assessment of this risk is complicated by the interplay of several factors including concurrent medications, graft size,9 the location of myoblast injections (those performed in the core of the scar seem less arrhythmogenic than those lining the border zone)14 and the intrinsically arrhythmogenic nature of the underlying heart failure.
Although these initial studies were neither designed nor powered to provide efficacy data, the functional effects of myoblast injections were nevertheless assessed up to 4 years7 and even later (58 months in our trial).4 Outcomes were found to range from stabilization of LVEF and volumes4 to improvements in regional and global LV function from baseline values3,4 and, occasionally, in metabolic viability of transplanted areas, as assessed by positron emission tomography and magnetic resonance imaging (MRI).5,7 It is clear, however, that the small size of these series, their open-label type of design and the lack of controls made these data inconclusive.
For this reason, we implemented a randomized, double blind, placebo-controlled trial (MAGIC, an acronym for Myoblast Autologous Grafting in Ischemic Cardiomyopathy), which involved 21 centers in Europe and included patients meeting the same inclusion criteria as in the phase I studies (severe LV dysfunction, postinfarction nonviable scar, and indication for CABG). Muscular biopsies were shipped to two core laboratories where they were cultured and 3 weeks later, either cells (at two different doses: 400 and 800 million) or a placebo solution were injected in ~30 sites encompassing the core and the margins of the infarct area during the bypass surgery. Notably, an internal cardioverter-defibrillator was implanted in every patient before hospital discharge. Out of 120 randomized patients, 97 were effectively treated and the major outcomes, at the 6-month study point, can be summarized as follows: (i) the proportion of patients who experienced arrhythmias was not significantly different between the myoblast-treated and the placebo-injected groups. However, analysis of the time course of events showed that arrhythmias tended to be clustered in the early postoperative period in the myoblast-treated groups, whereas they were more evenly distributed over time in the placebo arm of the trial. Not unexpectedly, these findings make a strong case for the benefits of internal cardioverter-defibrillator implantation in this high-risk patient population irrespective of any cell therapy; (ii) neither regional nor global LV function, as assessed blindly by echocardiography in a core laboratory, were significantly improved by myoblast injections, regardless of the dose, compared with controls, which sharply contrasts with the encouraging results of the above-mentioned phase I trials and once again highlight the importance of randomization, blind assessment, and adequate controls to draw meaningful conclusions; (iii) however, the highest dose of cells resulted in a significant reversal of remodeling, evidenced by a decrease in LV enddiastolic and endsystolic volumes (a prespecified secondary end point) compared with the placebo group.15 Although this encouraging signal tended to validate the concept that cells can exert some beneficial effects, it is clear that under the conditions of the trial, these effects were not powerful enough to translate into meaningful improvements in contractile function.
In parallel to these surgical trials, three phase I catheter–based studies have been reported. One has entailed administration of myoblasts through the coronary sinus with a dedicated catheter, which allows direct cell injections into the target area under endovascular ultrasound guidance.16 Experimentally, this system has resulted in a successful engraftment of myoblasts17 and the 10-patient clinical study, conducted by Siminiak et al.16 has confirmed both the feasibility and safety of this approach. However, this route of cell transfer may be technically challenging, particularly in patients who have previously undergone lead implantation for cardiac-resynchronization therapy. The other two percutaneous trials have entailed endoventricular injections of myoblasts under electromechanical guidance18,19 and are clearly fraught with the same methodologic limitations as those of the phase I surgical trials. These hurdles have been partly overcome by another percutaneous study, which has randomized 23 patients with LVEF <40% and old (>10 years) infarction to endoventricular myoblast injections or optimal medical management alone.20 Although the 6-month interim results look encouraging (no major safety concern and a trend toward smaller LV dimensions in the myoblast-treated patients), they are still limited and in contradiction with those of the randomized SEISMIC trial reported by Serruys at the 2008 American College of Cardiology meeting; in this study, which allocated 31 patients to myoblast injections while 16 patients received “optimal medical therapy,” there was no added benefit of cell therapy on LV function measured at 6 months after the procedure. An upcoming larger-scale randomized controlled trial will hopefully help in clarifying this issue but one has to admit that currently available data have not provided conclusive evidence for a favorable shift of the risk-to-benefit ratio following skeletal myoblast transplantation.
Bone Marrow Cells
Acute myocardial infarction
In this setting, mononuclear cells (MNCs) derived from bone marrow or peripheral blood, CD133+ progenitors and mesenchymal stem cells (MSCs) have undergone clinical testing.
Most studies have focused on MNC extemporaneously processed from bone marrow aspirates and reinjected into the infarct-related artery a few days after its recanalization by balloon angioplasty and stenting. Not unexpectedly, the enthusiasm raised by the consistently positive results of early-phase uncontrolled and usually small-sized studies has been dampened by the data yielded by the following wave of randomized trials. The most recent meta-analysis has compiled the data of 811 patients included in 13 randomized trials.21 Overall, stem-cell therapy was found to improve LVEF by 2.99% (95% confidence interval, 1.26–4.72%, P = 0.0007), despite a considerable degree of heterogeneity in LVEF comparisons, and reduce LV endsystolic volume by 4.74 ml (95% confidence interval, −7.84 to −1.64 ml, P = 0.003) and myocardial lesion area by 3.51% (95% confidence interval, −5.91 to −1.11%, P = 0.004) compared with controls. Subgroup analysis revealed that the benefit of cell therapy was greater when cells were infused within 7 days following infarction and when the dose administered was higher than 108. However, bone marrow–cell therapy failed to alter postinfarction remodeling, which is a major predictor of late adverse outcomes22 and should, thus, be logically the primary target of interventions adjunctive to current percutaneous revascularization procedures. Indeed, a more focused analysis of the large randomized controlled trials shows that the 204-patient REPAIR-AMI study23 is the only one to have unequivocally shown the benefits of intracoronary infusions of bone marrow–derived MNC, particularly in the subgroup of patients with an LVEF at or below the median value of 49% (absolute improvement in LVEF, 5.0; 95% confidence interval, 2.0–8.1). Furthermore, in this trial, cell therapy was reported to have reduced the 1-year prespecified combined clinical endpoint of death, recurrence of myocardial infarction, and any revascularization procedure (P = 0.01). In the other large studies, the alleged benefits of stem-cell therapy have been less straightforward, totally negative (ASTAMI),24 mixed like in the Belgium25 trial, where EF failed to improve despite a reduction in infarct size (similar to what has been observed in a swine model duplicating the clinical scenario)26 or transient, like in the BOOST trial, where the better outcomes seen at 4 months after cell infusions were not apparent 14 months later because of a gradual improvement in the control group.27 The interpretation of results also needs to be cautious; for example, in the recently published FINCELL trial,28 which randomized 80 patients to intracoronary infusions of MNC or placebo, a “positive” outcome is inferred from the finding that LVEF increased to a greater extent in the treated group compared with controls. However, a head-to-head comparison of absolute LVEF values at 6 months shows similar values in the two groups. Finally, at the November 2008 American Heart Association Scientific Sessions meeting, two additional randomized trials (BONAMI and HEBE, presented by Mouquet et al. and Piek et al., respectively) were reported to have missed their primary endpoints.
The reasons for these mixed and discrepant results are not really surprising if one takes into consideration the differences in patient- and cell-related factors across all these studies. Among the former, the predictive value of pro-NT-BNP serum levels has been identified in the REPAIR-MI trial (pretransplantation values ≥735 pg/ml being associated with improved outcomes).29 Among cell-related factors, dosing and timing of delivery probably play a role, as mentioned earlier, but the technique of cell processing30 as well as cell functionality are equally important as shown by the predictive value of the colony forming unit capacity of the infused cells.29 This issue of cell function is critical since coronary artery disease is known to affect it.31,32 The method used for assessing the results also needs to be considered and, for example, the gain in EF reported after bone marrow–cell therapy, when measurements are based on angiocardiography, are in the order of magnitude of those yielded by MRI after successful infarct reperfusion in the absence of any cell therapy.33 Clearly, additional larger-scale trials are still required to assess whether, to what extent and under which conditions, intracoronary infusions of bone marrow cells in patients with acutely reperfused myocardial infarcts are clinically beneficial.
Using a similar intracoronary route of cell transfer, the group of Aalst in Belgium has focused on the specific population of CD133+ progenitors.34 However, despite some evidence for improvement in LV function, they stopped the trial prematurely because of the worrisome observation of a higher-than-expected rate of in-stent restenosis associated with luminal loss and decreased pressure-derived fractional flow reserve. Whether these findings reflect the Janus phenomenon whereby the downside effect of proangiogenic cells is to also stimulate atherogenesis35 or are only due to bad luck in a small group of patients, remains currently uncertain but should be elucidated by an ongoing randomized trial.
MSC comprise the third cell type which has been investigated. Chen et al.36 have conducted a randomized placebo-controlled trial that has tested the effects of intracoronary infusions of large doses of autologous MSC. Three months after the procedure, LV function and perfusion were found significantly improved in treated patients compared with those infused with saline, but to this point, these results have not been duplicated. Indeed, the major advantage of MSC in this acute infarction setting would be their alleged immune privilege37 allowing to use them as an allogeneic off-the-shelf readily available cell product functionally unaffected by the patient's coronary artery disease. A company-sponsored randomized controlled double-blind trial has recently tested this concept by assessing the effects of injecting intravenously allogeneic MSC in patients with acute myocardial infarction. Six months later, LV function was found significantly improved compared with baseline values in treated patients but these outcome measurements did not differ significantly from those obtained in control patients (Hare, Late-Breaking sessions of the 2007 American College of Cardiology meeting). The latter result highlights the major problem of the optimal route for MSC delivery as each of them is associated with specific issues. Thus, systemic intravenous infusion, which is obviously simple and safe is unlikely to be functionally effective because extracardiac trapping of MSC prevents them to home in the target myocardium.38 A direct intramyocardial transfer of MSC through an endoventricular catheter would be likely more effective, but it can be risky to puncture a freshly infracted myocardium. From a practical standpoint, intracoronary infusions, as used in the study of Chen et al.36 are the most appealing but raise a safety concern related to distal capillary plugging due to the large size of MSC and subsequent microinfarctions.39 However, it seems possible to overcome this problem by an appropriate cell dosing and a bracketing of the procedure by a robust antithrombotic therapy.40 In line with this route for delivery, a clinical study (APOLLO) has been initiated to assess the effects of autologous stem cells, derived from fat tissue,41,42 extemporaneously processed by a dedicated device and intracoronarily reinjected. Notably, however, the resulting cell yield, at this point, is a heterogeneous mix of different populations and cannot probably be assimilated to bone marrow–derived MSC. Additional studies are thus warranted to better characterize the mechanisms of the immune tolerance to MSC and validate the intracoronary route for ensuring their safe and effective delivery to the myocardium. A positive answer to these two conditions could have a major clinical impact by allowing one to move closer to a consistent and well-characterized, readily available cell-therapy product.
Aside from the direct intracoronary infusion of bone marrow–derived cells, the assumption that their intramyocardial engraftment could contribute to repair infarcted tissue has led to test an alternate strategy based on cytokine-induced mobilization to increase the circulating pool of progenitors and also enhance their subsequent homing in sites of injury. Out of three randomized studies that entailed administration of granulocyte colony-stimulating factor (G-CSF) early after successful percutaneous reperfusion, two failed to show any benefit.43,44 The reasons for the discrepancy between these negative outcomes and the positive ones of the third trial45 are unclear but could be due to differences in timing of treatment, lack of true controls in the positive study, and the use of a more sensitive tool for assessing outcomes (MRI) in the negative trials while the positive one was only based on echocardiography. Nevertheless, there is currently no evidence that G-CSF provides any additional benefit over best-of-care management of patients with acute myocardial infarction. To move the field forward, it could be worth looking at agents more effective than G-CSF for mobilizing stem cells while avoiding an excessive release of unwanted proinflammatory cells.46
Refractory angina
Although the continuous improvements in revascularization procedures and drug therapies may have restricted the relevance of this indication, there is still a substantial number of patients who may yet exhaust conventional treatments and continue to experience angina. Initial studies by Fuchs et al.47 have shown a symptomatic improvement in some of these “no option” patients following transendocardial delivery of autologous MNC. However, the subjective nature of the primary endpoint, i.e., angina relief, the open-label design of this study, and the lack of controls made these findings difficult to interpret. Hence, the interest of the randomized safety trial conducted by Losordo et al.48 to test the effects of an endoventricular transfer of CD34+ progenitors sorted following G-CSF-induced cell mobilization and apheresis. The choice of this cell population looks sound, in that it is based on its expectedly high angiogenic potential that is critical in a setting where improved blood flow is the primary target. The encouraging results yielded by the pilot study have led these investigators to set a larger efficacy trial, which is currently under way.
In the perspective of cell-based angiogenesis, another trial (PRECISE) has recently started to test the endoventricular delivery of adipose tissue–derived stem cells.
Chronic heart failure
A limited number of studies have yet looked at the effects of bone marrow cell transplantation in patients with heart failure (reviewed in ref. 49). In the surgical setting, cells have been injected epicardially into the target areas, except for one study in which they were also infused directly into the coronary artery through the bypass graft.50 Overall, the results have been disappointing. The study by Mocini et al.51 (36 patients) has reported a 3-month significant improvement in regional and global LV function compared with baseline, but these outcome measures did not significantly differ from those obtained in control patients in whom, surprisingly, there was no functional benefit from bypass surgery. The study by Hendrikx et al.52 (20 patients) showed that after 4 months, there was a significantly greater wall thickening in cell-transplanted patients (as assessed by MRI) but no improvement in EF or perfusion defects beyond values was seen in control patients. Finally, the trial in which cells were injected both in the myocardium and in the bypass grafts also failed to meet its primary endpoint (Galinanes, presentation at the Late-breaking clinical trials of the 2007 Scientific Sessions of the American Heart Association).53 Altogether, these results are consistent with previous experimental data showing that transplantation of unfractionated bone marrow in chronically infarcted myocardium does not provide a functional benefit.54
Assuming that inducing myocardial regeneration through bone marrow–cell implantation was still highly problematic, other investigators have more pragmatically chosen to rather build on the better documented angiogenic properties of some progenitor subpopulations of these cells. In this setting, the most encouraging results have been reported by Stamm et al.55 with the use of CD133+ cells epicardially injected during CABG. Although this trial was not strictly randomized, it has been rigorously conducted and provides encouraging hints in favor of the capacity of the CD133+ population to safely improve LV function and perfusion at 6 months postoperatively, particularly in patients with the poorest preoperative LV function.
Catheter-based studies of bone marrow cells in patients with heart failure are also limited. The way has been opened by an open-label nonrandomized trial56 in which 11 patients experienced a 1-year improvement in exercise capacity following endoventricular injections of MNC. In two other studies, MNCs have been infused directly into the coronary arteries. The IACT study57 has reported strikingly improved outcomes supposed to reflect “regeneration” of the infarcted muscle but these results have to be interpreted very cautiously because of the multiple confounders (particularly the small sample size, lack of true randomization and heterogeneity of baseline LV function). Using a more elaborate crossover type of design in a 75-patient study, Assmus et al.58 have similarly reported, at a follow-up of 3 months, the benefits of intracoronary infusions of either circulating progenitor cells or bone marrow–derived MNC. These data open interesting perspectives for the catheter-based treatment of chronic heart failure by bone marrow cells, but they still need validation by large-scale randomized controlled trials.
Finally, two studies are currently registered on the clinicaltrials.org website for the assessment of MSC intramyocardial injections in conjunction with CABG in patients with ischemic heart failure. Their endpoints are efficacy and safety, which is particularly critical in view of the experimental report that because the differentiation patterns of MSC are very sensitive to the physical nature of the substrate, their implantation into postinfarction stiff scars could drive their fate toward an osteogenic phenotype and result in intramyocardial calcifications.59
Limitations and Remaining Hurdles
In a clinically oriented perspective, they can be stratified into three main categories.
Issues related to cells
A consistent finding of cell-therapy studies is the very low rate of sustained cell engraftment, regardless of the route of cell transfer. More than 90% of injected cells disappear over the first days and <1% of donor cells can still be identified 4 weeks after transplantation.60 In the case of skeletal myoblasts, the fraction surviving cell injections may proliferate until exiting the cell cycle but this phenomenon is far from catching up the initial attrition rate,61 which accounts for the scarcity of myoblast-derived myotubes found at autopsy in the long term.62 This is likely to hamper the functional efficacy of the procedure, because the benefits of myoblast transplantation have been linked to graft volume.63 Likewise, as previously mentioned, increased cell dosing appears to be one of the factors of the efficacy of bone marrow cell-intracoronary infusions.21 A major lesson from preclinical and early clinical trials is thus that enhancement of cell engraftment is mandatory for optimizing the therapeutic benefits of the procedure. This scalability issue is particularly relevant to heart failure, where the cardiomyocyte deficit has been estimated to be on the range of one billion cells.64
This low rate of engraftment is actually due to two sequential events: an early mechanical loss followed by cell death caused by biological processes. The mechanical leakage of cells at the time of injections has been found to represent ~30% of the injectate when cells are delivered into an arrested heart and it increases more than twofold in the beating heart (as occurs during off-pump bypass surgery or catheter-based cell transfer).65 Another recent study has reported still more pessimistic data in that, in a porcine model of cardiopulmonary bypass, only 10% of intramyocardially injected microspheres approximating the size of MSC were retained within the sites of injection after 30 minutes, and this rate was still lower when the heart was beating compared with the arrested state.66 Intracoronary delivery of cells is also associated with a low efficiency rate as human imaging studies have shown that only 2–5% of unselected MNC infused by this route were retained in the myocardium after a few hours67 but the rate of homing has recently been shown to be time-dependent, being greater when cells are delivered shortly after the acute ischemic event.68 This delivery-associated mechanical leakage not only decreases the amount of cell engraftment in the target area but also leads to a systemic dissemination of the injected cells,69 which may raise safety issues depending on the cell type. Improvements in cell-transfer techniques are thus eagerly awaited. If graft delivery is planned through the coronary arteries, preimplantation treatment of the cells (for example, by nitric oxide synthase enhancers) or manipulation of the host tissue (for example, by low-energy shock wave to enhance tissue expression of stroma-derived factor 1) could be helpful for increasing homing.70 However, some of these biologically oriented interventions might be difficult to implement in daily practice because of regulatory constraints, and improvements in device design might thus offer a more pragmatic way of increasing cell retention; such is the case of a recently described intracoronary catheter, which features an expandable microneedle that punctures the arterial wall and thus allows a perivascular delivery of the cells. If cells are to be delivered directly into the myocardium during a cardiac surgery procedure, one approach is the development of more accurate computer-driven or even robot-assisted71 injection devices allowing a tighter control of the various parameters of delivery than the hand-held syringe that has been used so far. However, one of the major lessons learnt from this first decade of cell therapy is the recognition of the multiple limitations of multiple needle-based intramyocardial injections: high rate of leakage, inhomogeneous distribution, poor reproducibility, disruption of the extracellular matrix, and subsequent loss of signals modulating cell survival, differentiation and patterning, and formation of potentially arrhythmogenic clusters.13 For these reasons, an appealing alternative specifically relevant to open-chest procedures is the replacement of the injection technique by the epicardial deposition of cell-seeded bioresorbable patches, commonly made of collagen72,73 or even of scaffold-free cell sheets cultured on temperature-sensitive films, stacked, and overlaid onto the infarct area.74 From a practical standpoint, this technique clearly makes cell delivery both less invasive and more reproducible.
The second event that decreases engraftment is the death of cells that have been initially retained and results from the interplay of multiple factors, including ischemia due to the poor vascularization of the injected areas, inflammation, and apoptosis subsequent to detachment of anchorage-dependent cells from their extracellular matrix (anoikis). For this reason, time has probably come to move from “cell therapy” to “tissue therapy” whereby cells are delivered along with some form of vascular and matrix support. In this setting, a variety of gene-, cell-, or protein-based angiogenic strategies have been tested experimentally and shown to improve graft vascularization (reviewed in ref. 75). Boosting of cell-survival pathways can also be accomplished by a pretransplantation engineering of cells with genes encoding antiapoptotic factors like bcl2 or Akt, although a simpler approach has consisted of preconditioning cells either physically by heat shock or pharmacologically by potassium channel agonists.76 Embedding cells into biomaterials (i.e., fibrin glue, peptide nanofibers) is another means of enhancing graft retention (because of increased injectate viscosity) and viability through creation of a three-dimensional environment although, from this perspective, cell sheets are probably the best means for preserving cell cohesion and thus survival signals linked to cell-to-cell and cell-to-matrix contacts. Finally, encapsulation of cells is another approach for protecting them from inflammatory (and immune) damage inflicted by host cells but it implies that one exclusively relies on the paracrine effects of the cells (see in the later part) as, by definition, encaging cells into particles precludes any functional integration of the graft.
Clearly, the development of these retention- and survival-enhancing strategies cannot be dissociated from that of techniques of cell tracking, allowing a comparative quantification of engraftment rates. Those based on radionuclide, MRI, and reporter genes are currently the most extensively used, at least in the laboratory, although none of them optimally combines resolution, sensitivity, safety, accuracy of cell quantification, and clinical applicability.77 A word of caution, in particular, has been raised about the use of cell loading with iron nanoparticles and subsequent MRI-based imaging because of the potential for false positives when iron released by dead stem cells is engulfed by host macrophages.78 The amount of resources currently devoted to this area should hopefully allow to successfully address most of the issues still associated with cell-imaging techniques.
Issues related to adult cells
A consistent finding of experimental cell-therapy studies has been the discrepancy between the scarcity of cell engraftment and the improvement in heart function, which has led to the postulation that the beneficial effects of the grafted cells were not due to the physical replacement of lost cardiomyocytes, but rather to the release of cytokines and growth factors able to trigger host-associated endogenous cytoprotective pathways. This paracrine hypothesis is now documented by several studies, which have screened the composition of conditioned media derived from bone marrow cells79,80 and skeletal myoblasts;81 and have shown that these media alone, without the physical presence of the cells, were able to effect some cardioprotection.82 Possible targets of these paracrine mediators include stimulation of angiogenesis, limitation of apoptosis, extracellular matrix remodeling leading to increased scar elasticity and decreased fibrosis, and, more hypothetically, recruitment of cardiac stem cells.
The basic question is then to determine which should be the primary objective assigned to cell therapy; two can be considered.
The paracrine objective implies that the transplant has primarily to supply a missing mediator like insulin or dopamine in the case of diabetes and Parkinson's disease, respectively. In these settings, the transplanted cells must not necessarily adopt the phenotype of the diseased cells they are supposed to rescue, as long as the missing substance is appropriately secreted. Thus, if the clinical indication is refractory angina and the ultimate objective, an increase in angiogenesis, donor cells are not required to convert into endothelial cells (which, in anyway, is unlikely to occur), but only to release the appropriate signalling molecules for triggering the formation of a new vasculature. As such, bone marrow–derived MNC and, possibly to a greater extent, the CD34+ fraction, appear as sound candidates.83
Conversely, the structural objective implies that the grafted cells ensure the true regeneration of a dead tissue, in which case they should be phenotypically identical to the diseased host cells that they must replace. This is best exemplified by the ability of transplanted autologous skeletal myoblasts and fibroblasts to improve the symptoms of urinary incompetence by substituting for the same cell populations that were defective.84 This requirement for donor-recipient cell matching is also relevant to heart failure, where improvement of LV function requires that large areas of nonfunctioning myocardium (see as mentioned previously) be repopulated by new contractile cells able to electromechanically couple with the host cardiomyocytes and thus allow the graft to beat in synchrony with the remainder of the heart. Unfortunately, skeletal myoblasts85 or bone marrow cells86 cannot convert into cardiomyocytes, and a recent study using two-photon laser fluorescence microscopy has elegantly demonstrated the inability of engrafted bone marrow cells to respond to a depolarizing current by a cyclic calcium transient (which is a fundamental attribute of cardiomyocytes).87 Not unexpectedly, this remuscularization is best achieved by cells that recapitulate the developmental cardiomyogenic pathway. In this setting, the limited availability and poor scalability potential of fetal cardiomyocytes88 along with the more than doubtful persistence of cardiac stem cells in adulthood89,90 highlight the potential interest of human embryonic stem cells (ESCs). Because of their pluripotency, ESC can be specified in vitro toward a cardiac lineage and differentiate into cardiomyocytes following engraftment in postinfarction scars.91,92 More recently, we have shown that sorting of cardiac-specified cells from human and nonhuman primate ESC on the basis of their expression of a surface maker (CD15)93 was effective for selectively retaining a population of cardiac progenitors; when these purified progenitors were transplanted allogeneically into infarcted areas in Rhesus monkeys, they differentiated into cardiomyocytes without causing teratomas (G. Blin, D. Nury, S. Stefanoric, O. Guillevic, B. Brinon, V. Bellamy et al., unpublished results). Apart from ethical and technical issues, the potential clinical use of ESC is plagued with the immune response that they induce.94,95 Studies are warranted to better characterize the time course, patterns, and extent of the alloreactivity of ESC-derived differentiated cells and develop strategies of immunotolerance or immunosuppression allowing to mitigate rejection at the expense of minimal side-effects.
The generation of inducible pluripotent stem cells (iPSs) from human skin fibroblasts is the latest major advance in stem-cell biology and the field has made tremendous progress within a short time frame, as the four retroviruses initially required for reprogramming (and which precluded any clinical application) have already been successfully replaced by nonviral vectors.96 Cardiomyocytes have also been derived from these iPS,97 and some investigators already view them as the elective cells for replacement therapy as they share with ESC a cardiac lineage commitment without posing the ethical and immune problems associated with the latter. Several basic and experimental studies, however, still need to be done before we can think of a safe leap to clinical applications.98 Notably, although the fact that iPS are harvested from the patient himself is legitimately recognized as a major advantage over ESC, in clinical practice, this benefit needs to be weighted against the limitations inherent in autologous cell products, which are discussed in the following paragraph.
Issues related to autologous cells
Clearly, one of the strong arguments favoring the use of skeletal myoblasts and bone marrow–derived cells has been their autologous origin. However, with accumulated clinical experience, the limitations of patient-specific products have become increasingly evident. They include (i) the naturally occurring individual variability between patients that makes it difficult to end up with a reproducible and well characterized cell-therapy product.29 The common impairment of the functionality of bone marrow MNC and endothelial progenitors in patients with ischemic cardiomyopathy31 and diabetes32, respectively, makes unpredictable the therapeutic efficacy of customized cell-therapy batches. The approach consisting of screening patient-specific cells in the perspective of fixing pharmacologically or genetically a potential defect is conceptually appealing, experimentally feasible,99 but likely difficult to implement in practice when large numbers of patients have to be treated; (ii) the cost of quality controls that need to be repeated for each patient-specific batch, and (iii) the logistical complexity related to back-and-forth shipments of the cellular products when the processing is centralized in a core laboratory. In the case of skeletal myoblasts, an additional constraint is the delay in treatment corresponding to the expansion of the muscular biopsy. Thus, in the perspective of a widely available therapy, the ideal approach would be to have cell banks able to supply a readily available “off-the-shelf,” consistent, controlled, and accurately characterized product. It is clear that the major drawback of such an allogeneic product would be its immunogenicity (except, maybe, in the case of MSC37). As it remains uncertain whether this problem might be satisfactorily solved in the future by the relentless improvements in immunomodulatory therapies, additional studies remain warranted to thoroughly compare the risk-benefit and cost-effectiveness ratios of autologous vs. allogeneic cell-therapy products.
Because there is no animal model that can fully duplicate the complex situation of patients with coronary artery disease, we believe that it is legitimate to continue in undertaking adequately designed and powered clinical trials provided that their experimental grounds are robust and consistent. The cell type should be selected in relation with the clinical indication and the primary objective assigned to the cells, i.e., angiogenesis or myogenesis. Efforts are required to improve the consistency of cell yields by methods combining reliability, practicality of implementation, and realistic approvability by the regulatory authorities. The timing of cell therapy may also need to be revisited to more closely approximate the time course of myocardial healing and homing signals and, along with dosing and route for delivery, repeating administrations of the cells could even be considered.100,101 As it is likely premature to launch large-scale mortality trials, surrogate endpoints and imaging modalities should be selected so as to confirm the proof of principle, unravel potential medium- or long-term safety issues, and provide further mechanistic insights. In this perspective, the assessment of cell-related morphological changes such as infarct size, LV remodeling, or regional wall thickness areas might be as informative as the commonly used measurements of global LV function.
Acknowledgments
This study was supported in part by a grant from the LeDucq Foundation (Cardiac Progenitors Transatlantic Alliance network 04 CVD 04).
REFERENCES
- Velagaleti RS, Pencina MJ, Murabito JM, Wang TJ, Parikh NI, D'Agostino RB, et al. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118:2057–2062. doi: 10.1161/CIRCULATIONAHA.108.784215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel DM, Redfield MM, Weston SA, Gerber Y., and , Roger VL. Death in heart failure. A community perspective. Circ Heart Fail. 2008;1:91–97. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasche P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, et al. Myoblast transplantation for heart failure. Lancet. 2001;357:279–280. doi: 10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- Hagege AA, Carrion C, Menasche P, Vilquin JT, Duboc D, Marolleau JP, et al. Skeletal myoblast transplantation in ischemic heart failure: long-term follow-up of the first phase I cohort of patients Circulation 2006114I108–I113.suppl. 1 [DOI] [PubMed] [Google Scholar]
- Gavira JJ, Herreros J, Perez A, Garcia-Velloso MJ, Barba J, Martin-Herrero F, et al. Autologous skeletal myoblast transplantation in patients with nonacute myocardial infarction: 1-year follow-up. J Thorac Cardiovasc Surg. 2006;131:799–804. doi: 10.1016/j.jtcvs.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Siminiak T, Kalawski R, Fiszer D, Jerzykowska O, Rzezniczak J, Rozwadowska N, et al. Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: phase I clinical study with 12 months of follow-up. Am Heart J. 2004;148:531–537. doi: 10.1016/j.ahj.2004.03.043. [DOI] [PubMed] [Google Scholar]
- Dib N, Michler RE, Pagani FD, Wright S, Kereiakes DJ, Lengerich R, et al. Safety and feasibility of autologous myoblast transplantation in patients with ischemic cardiomyopathy: four-year follow-up. Circulation. 2005;112:1748–1755. doi: 10.1161/CIRCULATIONAHA.105.547810. [DOI] [PubMed] [Google Scholar]
- Leobon B, Garcin I, Menasche P, Vilquin JT, Audinat E., and , Charpak S. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci USA. 2003;100:7808–7811. doi: 10.1073/pnas.1232447100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham MR, Henrikson CA, Tung L, Chang MG, Aon M, Xue T, et al. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ Res. 2005;97:159–167. doi: 10.1161/01.RES.0000174794.22491.a0. [DOI] [PubMed] [Google Scholar]
- Fernandes S, Amirault JC, Lande G, Nguyen JM, Forest V, Bignolais O, et al. Autologous myoblast transplantation after myocardial infarction increases the inducibility of ventricular arrhythmias. Cardiovasc Res. 2006;69:348–358. doi: 10.1016/j.cardiores.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Fouts K, Fernandes B, Mal N, Liu J., and , Laurit KR. Electrophysiological consequence of skeletal myoblast transplantation in normal and infarcted canine myocardium. Heart Rhythm. 2006;3:452–461. doi: 10.1016/j.hrthm.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Mills WR, Mal N, Kiedrowski MJ, Unger R, Forudi F, Popovic ZB, et al. Stem cell therapy enhances electrical viability in myocardial infarction. J Mol Cell Cardiol. 2007;42:304–314. doi: 10.1016/j.yjmcc.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Fukushima S, Varela-Carver A, Coppen SR, Yamahara K, Felkin LE, Lee J, et al. Direct intramyocardial but not intracoronary injection of bone marrow cells induces ventricular arrhythmias in a rat chronic ischemic heart failure model. Circulation. 2007;115:2254–2261. doi: 10.1161/CIRCULATIONAHA.106.662577. [DOI] [PubMed] [Google Scholar]
- Soliman AM, Krucoff MW, Crater S, Morimoto Y., and , Taylor DA. Cell location may be a primary determinant of safety after myoblast transplantation into the infarcted heart. J Am Coll Cardiol. 2004;43:15A. [Google Scholar]
- Menasché Ph, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) Trial. First Randomized Placebo-Controlled Study of Myoblast Transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- Siminiak T, Fiszer D, Jerzykowska O, Grygielska B, Rozwadowska N, Kalmucki P, et al. Percutaneous trans-coronary-venous transplantation of autologous skeletal myoblasts in the treatment of post-infarction myocardial contractility impairment: the POZNAN trial. Eur Heart J. 2005;26:1188–1195. doi: 10.1093/eurheartj/ehi159. [DOI] [PubMed] [Google Scholar]
- Brasselet C, Morichetti MC, Messas E, Carrion C, Bissery A, Bruneval P, et al. Skeletal myoblast transplantation through a catheter-based coronary sinus approach: an effective means of improving function of infarcted myocardium. Eur Heart J. 2005;26:1551–1556. doi: 10.1093/eurheartj/ehi151. [DOI] [PubMed] [Google Scholar]
- Biagini E, Valgimigli M, Smits PC, Poldermans D, Schinkel AF, Rizzello V, et al. Stress and tissue Doppler echocardiographic evidence of effectiveness of myoblast transplantation in patients with ischaemic heart failure. Eur J Heart Fail. 2006;8:641–648. doi: 10.1016/j.ejheart.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Ince H, Petzsch M, Rehders TC, Chatterjee T., and , Nienaber CA. Transcatheter transplantation of autologous skeletal myoblasts in postinfarction patients with severe left ventricular dysfunction. J Endovasc Ther. 2004;11:695–704. doi: 10.1583/04-1386R.1. [DOI] [PubMed] [Google Scholar]
- Dib N, Dinsmore J, Mozak R, White B, Moravec S., and , Diethrich EB.Safety and feasability of percutaneous autologous skeletal myoblast transplantation for ischemic cardiomyopathy: six-month interim analysis Circulation 2006114II88suppl. II [Google Scholar]
- Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A., and , Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29:1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- Udelson JE., and , Konstam MA.Relation between left ventricular remodeling and clinical outcomes in heart failure patients with left ventricular systolic dysfunction J Card Fail 20028S465–S470.suppl. 6 [DOI] [PubMed] [Google Scholar]
- Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27:2775–2783. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- Moelker AD, Baks T, van den Bos EJ, van Geuns RJ, de Feyter PJ, Duncker DJ, et al. Reduction in infarct size, but no functional improvement after bone marrow cell administration in a porcine model of reperfused myocardial infarction. Eur Heart J. 2006;27:3057–3064. doi: 10.1093/eurheartj/ehl401. [DOI] [PubMed] [Google Scholar]
- Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrow transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- Huikuri HV, Kervinen K, Niemelä M, Ylitalo K, Säily M, Koistinen P, et al. Effects of intracoronary injection of mononuclear bone marrow cells on left Ventricular function, arrhythmia risk profile, and restenosis after thrombolytic therapy of acute myocardial infarction. Eur Heart. 2008;29:2723–2732. doi: 10.1093/eurheartj/ehn436. [DOI] [PubMed] [Google Scholar]
- Assmus B, Fischer-Rasokat U, Honold J, Seeger FH, Fichtlscherer S, Tonn T, et al. TOPCARE-CHD Registry. Transcoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOPCARE-CHD Registry. Circ Res. 2007;100:1234–1241. doi: 10.1161/01.RES.0000264508.47717.6b. [DOI] [PubMed] [Google Scholar]
- Seeger FH, Tonn T, Krzossok N, Zeiher AM., and , Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766–772. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- Kissel CK, Lehmann R, Assmus B, Aicher A, Honold J, Fischer-Rasokat U, et al. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J Am Coll Cardio. 2007;49:2341–2349. doi: 10.1016/j.jacc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- Sorrentino SA, Bahlmann FH, Besler C, Muller M, Schulz S, Kirchhoff N, et al. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2007;116:163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- Baks T, van Geuns RJ, Biagini E, Wielopolski P, Mollet NR, Cademartiri F, et al. Recovery of left ventricular function after primary angioplasty for acute myocardial infarction. Eur Heart J. 2005;26:1070–1077. doi: 10.1093/eurheartj/ehi131. [DOI] [PubMed] [Google Scholar]
- Mansour S, Vanderheyden M, De Bruyne B, Vandekerckhove B, Delrue L, Van Haute I, et al. Intracoronary delivery of hematopoietic bone marrow stem cells and luminal loss of the infarct-related artery in patients with recent myocardial infarction. J Am Coll Cardiol. 2006;47:1727–1730. doi: 10.1016/j.jacc.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Epstein SE, Stabile E, Kinnaird T, Lee CW, Clavijo L., and , Burnett MS. Janus phenomenon: the interrelated tradeoffs inherent in therapies designed to enhance collateral formation and those designed to inhibit atherogenesis. Circulation. 2004;109:2826–2831. doi: 10.1161/01.CIR.0000132468.82942.F5. [DOI] [PubMed] [Google Scholar]
- Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Ryan JM, Barry FP, Murphy JM., and , Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- Vulliet PR, Greeley M, Halloran SM, MacDonald KA., and , Kittleson MD. Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet. 2004;363:783–784. doi: 10.1016/S0140-6736(04)15695-X. [DOI] [PubMed] [Google Scholar]
- Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, et al. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667–2677. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ., and , Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffler A., and , Buchler C. Concise review: adipose tissue-derived stromal cells--basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- Ripa RS, Jorgensen E, Wang Y, Thune JJ, Nilsson JC, Sondergaard L, et al. Stem cell mobilization induced by subcutaneous granulocyte-colony stimulating factor to improve cardiac regeneration after acute ST-elevation myocardial infarction: result of the double-blind, randomized, placebo-controlled stem cells in myocardial infarction (STEMMI) trial. Circulation. 2006;113:1983–1992. doi: 10.1161/CIRCULATIONAHA.105.610469. [DOI] [PubMed] [Google Scholar]
- Zohlnhofer D, Ott I, Mehilli J, Schomig K, Michalk F, Ibrahim T, et al. REVIVAL-2 Investigators. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. JAMA. 2006;295:1003–1010. doi: 10.1001/jama.295.9.1003. [DOI] [PubMed] [Google Scholar]
- Ince H, Petzsch M, Kleine HD, Schmidt H, Rehders T, Korber T, et al. Preservation from left ventricular remodeling by front-integrated revascularization and stem cell liberation in evolving acute myocardial infarction by use of granulocyte-colony-stimulating factor (FIRSTLINE-AMI) Circulation. 2005;112:3097–3106. doi: 10.1161/CIRCULATIONAHA.105.541433. [DOI] [PubMed] [Google Scholar]
- Larochelle A, Krouse A, Metzger M, Orlic D, Donahue RE, Fricker S, et al. AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. Blood. 2006;107:3772–3778. doi: 10.1182/blood-2005-09-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs S, Kornowski R, Weisz G, Satler LF, Smits PC, Okubagzi P, et al. Epstein SE. Safety and feasibility of transendocardial autologous bone marrow cell transplantation in patients with advanced heart disease. Am J Cardiol. 2006;97:823–829. doi: 10.1016/j.amjcard.2005.09.132. [DOI] [PubMed] [Google Scholar]
- Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- Ang KL, Shenje LT, Srinivasan L., and , Galinanes M. Repair of the damaged heart by bone marrow cells: from experimental evidence to clinical hope. Ann Thorac Surg. 2006;82:1549–1558. doi: 10.1016/j.athoracsur.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Galinanes M, Loubani M, Davies J, Chin D, Pasi J., and , Bell PR. Autotransplantation of unmanipulated bone marrow into scarred myocardium is safe and enhances cardiac function in humans. Cell Transplant. 2004;13:7–13. doi: 10.3727/000000004772664842. [DOI] [PubMed] [Google Scholar]
- Mocini D, Staibano M, Mele L, Giannantoni P, Menichella G, Colivicchi F, et al. Autologous bone marrow mononuclear cell transplantation in patients undergoing coronary artery bypass grafting. Am Heart J. 2006;151:192–207. doi: 10.1016/j.ahj.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Hendrikx M, Hensen K, Clijsters C, Jongen H, Koninckx R, Bijnens E, et al. Recovery of regional but not global contractile function by the direct intramyocardial autologous bone marrow transplantation: results from a randomized controlled clinical trial Circulation 2006114I101–I107.suppl. 1 [DOI] [PubMed] [Google Scholar]
- Ang KL, Chin D, Leyva F, Foley P, Kubal C, Chalil S, et al. Randomized, controlled trial of intramuscular or intracoronary injection of autologous bone marrow cells into scarred myocardium during CABG versus CABG alone. Nat Clin Pract Cardiovasc Med. 2008;5:663–670. doi: 10.1038/ncpcardio1321. [DOI] [PubMed] [Google Scholar]
- Bel A, Messas E, Agbulut O, Richard P, Samuel JL, Bruneval P, et al. Transplantation of autologous fresh bone marrow into infarcted myocardium: a word of caution Circulation 2003108II247–II252.suppl. 1 [DOI] [PubMed] [Google Scholar]
- Stamm C, Kleine HD, Choi YH, Dunkelmann S, Lauffs JA, Lorenzen B, et al. Intramyocardial delivery of CD133+ bone marrow cells and coronary artery bypass grafting for chronic ischemic heart disease: safety and efficacy studies. J Thorac Cardiovasc Surg. 2007;133:717–725. doi: 10.1016/j.jtcvs.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Silva GV, et al. Improved exercise capacity and ischemia 6 and 12 months after transendocardial injection of autologous bone marrow mononuclear cells for ischemic cardiomyopathy Circulation 2004110II213–II218.11 suppl. 1 [DOI] [PubMed] [Google Scholar]
- Strauer BE, Brehm M, Zeus T, Bartsch T, Schannwell C, Antke C, et al. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: the IACT Study. J Am Coll Cardiol. 2005;46:1651–1658. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–1369. doi: 10.1182/blood-2006-12-063412. [DOI] [PubMed] [Google Scholar]
- Zeng L, Hu Q, Wang X, Mansoor A, Lee J, Feygin J, et al. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation. 2007;115:1866–1875. doi: 10.1161/CIRCULATIONAHA.106.659730. [DOI] [PubMed] [Google Scholar]
- Maurel A, Azarnoush K, Sabbah L, Vignier N, Le Lorc'h M, Mandet C, et al. Can cold or heat shock improve skeletal myoblast engraftment in infracted myocardium. Transplantation. 2005;80:660–665. doi: 10.1097/01.tp.0000172178.35488.31. [DOI] [PubMed] [Google Scholar]
- Hagege AA, Carrion C, Menasche P, Vilquin JT, Duboc D, Marolleau JP, et al. Viability and differentiation of autologous skeletal myoblast grafts in ischaemic cardiomyopathy. Lancet. 2003;361:491–502. doi: 10.1016/S0140-6736(03)12458-0. [DOI] [PubMed] [Google Scholar]
- Tambara K, Sakakibara Y, Sakaguchi G, Lu F, Premaratne GU, Lin X, et al. Transplanted skeletal myoblasts can fully replace the infarcted myocardium when they survive in the host in large numbers Circulation 2003108II259–II263.suppl. 1 [DOI] [PubMed] [Google Scholar]
- Murry CE, Reinecke H., and , Pabon LM. Regeneration gaps. Observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006;47:1777–1785. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Teng CJ, Luo J, Chiu RC., and , Shum-Tim D. Massive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: implications for cellular cardiomyoplasty. J Thorac Cardiovasc Surg. 2006;132:628–632. doi: 10.1016/j.jtcvs.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Hudson W, Collins MC, deFreitas D, Sun YS, Muller-Borer B., and , Kypson AP. Beating and arrested intramyocardial injections are associated with significant mechanical loss: implications for cardiac cell transplantation. J Surg Res. 2007;142:263–267. doi: 10.1016/j.jss.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- Schächinger V, Aicher A, Döbert N, Röver R, Diener J, Fichtlscherer S, et al. Pilot trial on determinants of progenitor cell recruitment to the infarcted human myocardium. Circulation. 2008;118:1425–1432. doi: 10.1161/CIRCULATIONAHA.108.777102. [DOI] [PubMed] [Google Scholar]
- Dow J, Simkhovich BZ, Kedes L., and , Kloner RA. Washout of transplanted cells from the heart: a potential new hurdle for cell transplantation therapy. Cardiovasc Res. 2005;67:301–307. doi: 10.1016/j.cardiores.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Chavakis E, Urbich C., and , Dimmeler S. Homing and engraftment of progenitor cells: A prerequisite for cell therapy. J Mol Cell Cardiol. 2008;45:514–522. doi: 10.1016/j.yjmcc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Ota T, Patronik NA, Schwartzman D, Riviere CN., and , Zenati MA.Minimally invasive epicardial injections using a novel semiautonomous robotic device Circulation 2008118S115–S120.suppl. 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschka I, Chen IY, Kofidis T, Arai T, von Degenfeld G, Sheik AY, et al. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts Circulation 2006114I167–I173.suppl. 1 [DOI] [PubMed] [Google Scholar]
- Simpson D, Liu H, Fan TH, Nerem R., and , Dudley SC. A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells. 2007;25:2350–2357. doi: 10.1634/stemcells.2007-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memon IA, Sawa Y, Fukushima N, Matsumiya G, Miyagawa S, Taketani S, et al. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg. 2005;130:1333–1341. doi: 10.1016/j.jtcvs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Menasche P. Skeletal myoblasts as a therapeutic agent. Prog Cardiovasc Dis. 2007;50:7–17. doi: 10.1016/j.pcad.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Niagara MI, Haider HKh, Jiang S., and , Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100:545–555. doi: 10.1161/01.RES.0000258460.41160.ef. [DOI] [PubMed] [Google Scholar]
- Chang GY, Xie X., and , Wu JC. Overview of stem cells and imaging modalities for cardiovascular diseases. J Nucl Cardiol. 2006;13:554–569. doi: 10.1016/j.nuclcard.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Terrovitis J, Stuber M, Youssef A, Preece S, Leppo M, Kizana E, et al. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation. 2008;117:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.732073. [DOI] [PubMed] [Google Scholar]
- Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- Perez-Ilzarbe M, Agbulut O, Pelacho B, Ciorba C, San Jose-Eneriz E, Desnos M, et al. Characterization of the paracrine effects of human skeletal myoblasts transplanted in infarcted myocardium. Eur J Heart Fail. 2008;10:1065–1072. doi: 10.1016/j.ejheart.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Research. 2008;1:129–137. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, et al. CD34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- Strasser H, Marksteiner R, Margreiter E, Pinggera GM, Mitterberger M, Frauscher F, et al. Autologous myoblasts and fibroblasts versus collagen for treatment of stress urinary incontinence in women: a randomised controlled trial. Lancet. 2007;369:2179–2186. doi: 10.1016/S0140-6736(07)61014-9. [DOI] [PubMed] [Google Scholar]
- Reinecke H, Poppa V., and , Murry CE. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J Mol Cell Cardiol. 2002;34:241–249. doi: 10.1006/jmcc.2001.1507. [DOI] [PubMed] [Google Scholar]
- Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- Scherschel JA, Soonpaa MH, Srour EF, Field LJ., and , Rubart M. Adult bone marrow-derived cells do not acquire functional attributes of cardiomyocytes when transplanted into peri-infarct myocardium. Mol Ther. 2008;16:1129–1137. doi: 10.1038/mt.2008.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leor J, Patterson M, Quinones MJ, Kedes LH., and , Kloner RA.Transplantation of fetal myocardial tissue into the infarcted myocardium of rat. A potential method for repair of infarcted myocardium Circulation 199694II332–II336.suppl. 9 [PubMed] [Google Scholar]
- Pouly J, Bruneval P, Mandet C, Proksch S, Peyrard S, Amrein C, et al. Cardiac stem cells in the real world. J Thorac Cardiovasc Surg. 2008;135:673–678. doi: 10.1016/j.jtcvs.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Amir G, Ma X, Reddy VM, Hanley FL, Reinhartz O, Ramamoorthy C, et al. Dynamics of human myocardial progenitor cell populations in the neonatal period. Ann Thorac Surg. 2008;86:1311–1319. doi: 10.1016/j.athoracsur.2008.06.058. [DOI] [PubMed] [Google Scholar]
- Menard C, Hagege AA, Agbulut O, Barro M, Morichetti MC, Brasselet C, et al. Transplantation of cardiac-committed mouse embryonic stem cells to infracted sheep myocardium:a preclinical study. Lancet. 2005;366:1005–1012. doi: 10.1016/S0140-6736(05)67380-1. [DOI] [PubMed] [Google Scholar]
- Tomescot A, Leschik J, Bellamy V, Dubois G, Messas E, Bruneval P, et al. Differentiation in vivo of cardiac committed human embryonic stem cells in post-myocardial infarcted rats. Stem Cells. 2007;25:2200–2205. doi: 10.1634/stemcells.2007-0133. [DOI] [PubMed] [Google Scholar]
- Leschik J, Stefanovic S, Brinon B., and , Pucéat M. Cardiac commitment of primate embryonic stem cells. Nat Protoc. 2008;3:1381–1387. doi: 10.1038/nprot.2008.116. [DOI] [PubMed] [Google Scholar]
- Swijnenburg RJ, Schrepfer S, Govaert JA, Cao F, Ransohoff K, Sheikh AY, et al. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci USA. 2008;105:12991–12996. doi: 10.1073/pnas.0805802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidgey AP., and , Boyd RL. Immune privilege for stem cells: not as simple as it looked. Cell Stem Cell. 2008;3:357–358. doi: 10.1016/j.stem.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T., and , Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- Narazaki G, Uosaki H, Teranishi M, Okita K, Kim B, Matsuoka S, et al. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118:498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- Tulloch NL, Pabon L., and , Murry CE. Get with the (re)program: cardiovascular potential of skin-derived induced pluripotent stem cells. Circulation. 2008;118:472–475. doi: 10.1161/CIRCULATIONAHA.108.791442. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Heeschen C, Aicher A, Ziebart T, Honold J, Urbich C, et al. Ex vivo pretreatment of bone marrow mononuclear cells with endothelial NO synthase enhancer AVE9488 enhances their functional activity for cell therapy. Proc Natl Acad Sci USA. 2006;103:14537–14541. doi: 10.1073/pnas.0604144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premaratne GU, Tambara K, Fujita M, Lin X, Kanemitsu N, Tomita S, et al. Repeated implantation is a more effective cell delivery method in skeletal myoblast transplantation for rat myocardial infarction. Circ J. 2006;70:1184–1189. doi: 10.1253/circj.70.1184. [DOI] [PubMed] [Google Scholar]
- Poh KK, Sperry E, Young RG, Freyman T, Barringhaus KG., and , Thompson CA. Repeated direct endomyocardial transplantation of allogeneic mesenchymal stem cells: safety of a high dose, “off-the-shelf”, cellular cardiomyoplasty strategy. Int J Cardiol. 2007;117:360–364. doi: 10.1016/j.ijcard.2006.04.092. [DOI] [PubMed] [Google Scholar]