Abstract
Immunotherapy against infectious agents and malignant tumors requires efficient priming of effector cells through direct expression and/or efficient cross-presentation of antigens by antigen-presenting cells. Electroporation is a new procedure aimed at transiently increasing cell membrane permeability and direct delivery of antigen or antigen-encoding nucleic acids inside targeted cells. We evaluated the tolerability including compliance with repeated electroporation treatments using MedPulser DDS in 24 healthy adults. Pain severity was evaluated at time of electroporation treatment, and at 1, 5, 10, and 20 minutes, and 24 hours thereafter, using two clinically validated questionnaires: McGill Pain Questionnaire (MPQ) (Present Pain Intensity) and Brief Pain Inventory (BPI). Electroporation treatments were generally well tolerated. Twenty-two out of 24 subjects returned for the second electroporation treatment 14 days after first treatment. Only two subjects reported a treatment-related systemic adverse experience following either electroporation application. For both pain assessment tools, maximum pain and/or discomfort were mostly reported immediately (within 5 minutes) after electroporation; Furthermore, no difference was observed when comparing peak-pain scores after first and second electroporation treatments. This study supports the clinical application of MedPulser DDS for the improvement of antigen-induced immune responses for prophylactic or therapeutic vaccines, especially in gene-based therapies for cancer.
Introduction
Despite the successful development of many prophylactic vaccines against numerous infectious diseases,1,2 the development of vaccines against new targets has been hampered by the difficulty to achieve optimal attenuation and genetic stability of viral agents or to identify potent immunogens. In particular, the majority of investigational cancer vaccines have been unable to consistently show clinical benefits for a variety of reasons, such as the lack of suitable immunogens, the similarity of the antigens to host proteins with its associated difficulty to break tolerance, poor antigenic stimulation of adequate immune responses, and tumor evasion mechanisms.3 DNA immunization is sought to stimulate both arms of the immune system (humoral and cell-mediated immune responses) or to interfere with tumor growth.4,5 DNA immunization has several advantages to justify its consideration for cancer vaccines. It can be delivered multiple times without the induction of antivector neutralization responses (typical for viral vectors), an important factor when repeated doses are required to overcome tolerance to self-antigens. In addition, potent adjuvants can be directly encoded by the DNA to ensure coexpression of antigen and adjuvants and optimal induction of immune responses.6,7 This type of immunization is generally viewed as a safe strategy but has failed to consistently achieve levels of antibody and cell-mediated immune responses measured after immunization with conventional vaccines, suggesting the need for improved design and delivery systems. Unaided uptake of DNA plasmids has been shown to be inefficient, and only a small proportion of the genetic material is internalized.8,9
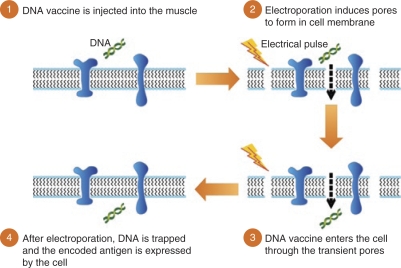
Development of new tools for DNA delivery, capable of inducing optimal, effective, and long-lasting immune responses or capable of reducing tumor cell differentiation and/or multiplication is needed. As cellular uptake of injected DNA material was found to be very inefficient, incorporation of DNA molecules or drug delivery inside target cells are thought to require physical delivery technology such as electroporation (EP). Over the past decades, EP technology has remained a reliable laboratory tool for the delivery of gene-encoded nucleic acid molecules into target cells. This approach uses brief electrical pulses that create transient “pores” in the cell membrane, thus allowing large molecules such as DNA or RNA to enter the cell's cytoplasm10 (Figure 1). Immediately following cessation of the electrical field, these pores would close, and the molecules would be trapped in the cytoplasm without causing cell death11 (Figure 2). In addition to the increased permeability of target cells, EP may also enhance immune responses through increased protein expression, secretion of inflammatory chemokines and cytokines, and recruitment of antigen-presenting cells (i.e., macrophages, dendritic cells) at the EP site.12,13 As a result, both antigen-specific humoral and cellular immune responses are increased by EP-augmented DNA vaccination in comparison to levels achieved with intramuscular injection of DNA alone. Studies in rodents, pigs, and nonhuman primates have shown that an intramuscular injection of plasmid DNA encoding an active anti-infective antigen followed by electric pulse application could increase DNA delivery and transgene expression by at least twofold, contributing to a marked enhancement of both humoral and cellular immune responses as compared to conventional intramuscular injection.14,15,16,17,18 Despite very encouraging results in small animals, plasmid DNA-based vaccines have long been hampered by the relatively poor immunogenicity when applied to larger animals. In contrast, the addition of in vivo electroporation has significantly enhanced the potency of DNA plasmids with antibody titers and functional activities comparable to those achieved by 100-fold higher dose of DNA plasmid given without EP. Furthermore, the addition of EP has been associated with a marked and consistent enhancement of cell-mediated and humoral immune responses in small and large animals, supporting its use in humans.19,20 The ability of EP treatment to maintain similar advantages and effectiveness when translated from small to large animals supports its potential value in the development of potent human prophylactic and therapeutic vaccines, including plasmid-based DNA vaccines.
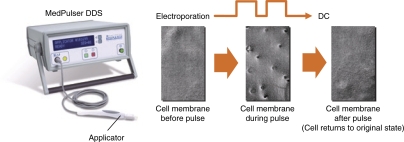
Figure 1.
MedPulser DDS device and formation of transient pores in cell membrane following electroporation.
Figure 2.
DNA vaccination and schematic representation of the effect of electroporation at the cell membrane level.
MedPulser DDS is an investigational EP device developed and manufactured by Inovio, BioMedical, San Diego, CA. aimed at delivering square-wave electrical pulses of a specified voltage intramuscularly via an applicator with a four-needle array (Figure 1). As the charge used could dissolve certain metals (i.e., iron, nickel, chromium) and may thus cause local and/or systemic side effects, only gold-coated electrodes are used for MedPulser DDS. An earlier version of the device, called the MedPulser electroporation therapy system, is currently being used in cancer patients for the intratumoral delivery of chemotherapy agents (e.g., bleomycin).21 Before its use for prophylactic or therapeutic vaccines without the use of anesthetics, a pilot study was conducted in rhesus macaques to assess the effects of the following parameters on peak gene expression: total injection volume (0.5 ml versus 1.0 ml), number of application sites (one versus two), and diameter of the needle array (5 mm versus 10 mm). In all cases, the level of gene expression as measured by the level of serum alkaline phosphatase was not significantly different across these different test conditions. Therefore, the least intrusive set of parameters (0.5 ml, 1 application site, and 5 mm diameter) were selected for the future human clinical trials using MedPulser DDS.22,23
The present study evaluates the tolerability of repeated treatments with the MedPulser DDS in healthy adults. Assessment of pain experience was based on patient self-report using two clinically validated tools, namely, the McGill Pain Questionnaire (MPQ) and the Brief Pain Inventory (BPI). Despite the development of several variants, the original MPQ and BPI are still the most widely used tools for the measurement of pain experienced by patients following surgery, during child delivery, and for conditions such as diabetic neuropathy, postherpetic neuralgia, leg ulcer, and cancer.24,25,26,27,28,29
Results
Study population
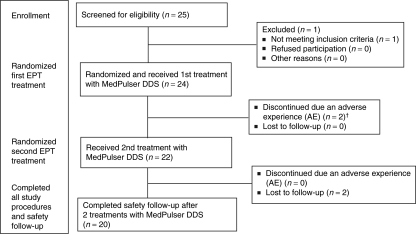
A total of 25 subjects were screened of which 24 met the study eligibility criteria and were enrolled into the study. The mean age was 26.3 years (range 20–35 years) at study entry and both genders were equally represented, with 14 (58.3%) of them being males. All 24 study subjects received at least one intramuscular injection of phosphate-buffered saline solution followed by a short electrostimulation (two pulses of 60 ms each) with the MedPulser DDS. Following the first treatment with the EP device, two subjects dropped out of the study due to an adverse experience, one due to prolonged EP-related mild injection-site pain lasting 5 days, and the other subject experienced a vasovagal reaction immediately after receiving the first EP treatment. Both patients recovered without sequelae. The remaining 22 subjects returned for their second treatment with the MedPulser DDS device ~14 days later and 20 out of 24 completed the 28 days study follow-up. Two subjects were lost to follow-up following the receipt of their second treatment. The subjects' disposition during the clinical trial is shown in the CONSORT chart (Figure 3).
Figure 3.
CONSORT chart: subject disposition. †One subject experienced a vasovagal reaction and one subject reported prolonged local pain after treatment with MedPulser DDS.
Injection-site reactions following treatment with MedPulser DDS
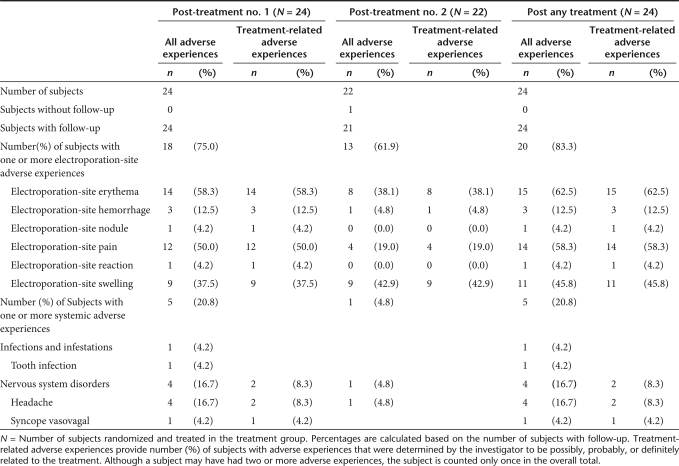
Subjects recorded injection-site reactions and temperatures for 5 days following each course of EP treatment as well as all systemic adverse experiences through day 14 postelectroporation. Electroporation-site adverse experiences reported on days 1 through 5 following Treatment Visit 1, Treatment Visit 2, and any treatment visit are summarized in Table 1. A total of 20 out of the 24 subjects (83.3% with 95% CI = 64.1%, 93.3%) reported a treatment-related electroporation-site adverse experiences following either application with the MedPulser DDS. Except for swelling at the site of electroporation, fewer electroporation-site adverse experiences were reported following the second treatment. No electroporation-site adverse experiences were reported after day 5 following Visits 1 or 2. Of the electroporation-site adverse experiences reported, most episodes were of mild intensity and of a maximum size ≤1 inch (2.5 cm).
Table 1.
Number (%) of subjects with electroporation-site and systemic adverse experiences (incidence >0%) after the first, second, or any treatment with PBS/MedPulser DDS
Systemic adverse experiences following treatment with MedPulser DDS
No serious adverse events were reported during the course of the study. Systemic clinical adverse experiences reported days 1 through 14 are summarized in Table 1 for all study subjects, following Treatment Visit 1, Treatment Visit 2, and any treatment visit. During the 14 days following the first EP treatment, 5 out of 24 (20.8%) subjects reported a systemic adverse experience, including tooth infection (one subject), headache (four subjects), and vasovagal reaction (one subject); only one subject experienced headache following the second EP treatment, but the event was determined not to be related to the EP treatment by the study investigator. In fact, only two subjects reported a treatment-related systemic adverse experience following either application with the MedPulser DDS [8.3% with 95% CI = (2.3%, 25.8%)]. Both treatment-related adverse experiences occurred after the first EP treatment and consisted of mild- to-moderate headache and a mild, transient vasovagal reaction. Overall, EP was well tolerated and was associated with transient local reaction, mostly local pain of short duration, in the majority of subjects. Also, the majority of subjects, 22 of 24 (91.7%), returned for the second EP treatment administered 14 days after the first treatment.
Pain assessment using the MPQ and BPI
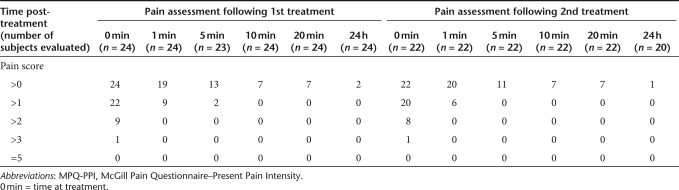
On a scale of 1 (Mild) to 5 (Excruciating), all 24 subjects reported some level of pain on the MPQ after each treatment with the MedPulser DDS (Table 2). Following either treatment, peak-pain scores were reported at 0 minutes (time of the electrostimulation) but subsided very rapidly with only scattered reports of mild pain beyond 5 minutes post-treatment. After the first EP treatment, peak-pain scores were highest at the time of the EP treatment, ranging from 1 (mild) to 4 (horrible) with the majority (15/24; 62.5%) of subjects reporting a peak-pain score ≤2 (discomforting). Only one subject reported a pain score of 4 (horrible) at the time of EP treatment that quickly decreased to 2 (discomforting) within 1 minute. In addition, 8 (33.3%) study subjects experienced a peak-pain score of 3 (distressing) at the time of EP treatment that subsequently decreased to 2 (discomforting) or 1 (mild) within 1 minute post-treatment. No subject reported a pain score ≥3 at 1 minute following the EP treatment with the majority (15/24; 62.5%) of them reporting no pain or mild pain and the remaining 9 (37.5%) reporting a pain score of 2. Following the second treatment, no significant change was observed in the level of pain reported by the study subjects with 14 out of 22 (63.6%) reporting a peak-pain score ≤2 (discomforting) at the time of EP treatment. To illustrate the transient nature of the pain associated with the treatment, the majority of study subjects [15/24 (62.5%) after the first treatment and 16/22 (72.7%) after the second treatment] reported mild to no pain at 1 minute following treatment with the MedPulser DDS. The mean peak-pain score was 2.3 following either the first or the second treatment and no statistically significant difference was observed between consecutive treatments with MedPulser DDS (P = 0.95).
Table 2.
Summary of present pain intensity scores by time using the MPQ-PPI
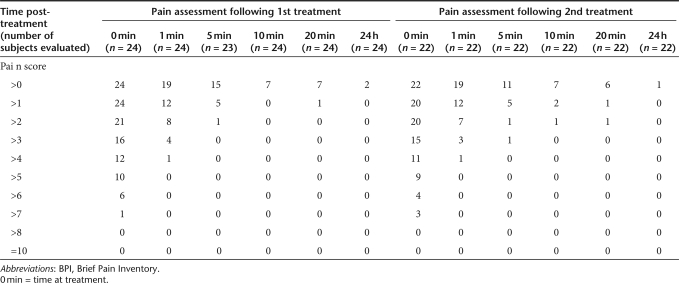
Similar trends were observed when evaluating the proportion of subjects reporting pain using the BPI (Table 3), a more sensitive pain assessment tool that utilizes a rating scale of 0–10 (0 = No pain to 10 = Pain as bad as you can imagine). As previously shown with the MPQ, peak-pain scores were mostly reported at the time of EP treatment, ranging from 2 to 8, with 14 out of 24 (58.3%) and 13 out of 22 (59.1%) subjects reporting a peak-pain score ≤5 (mild pain) after the first and second treatment with the MedPulser DDS, respectively. Similarly, peak-pain scores measured with BPI also decreased very rapidly with the majority of subjects [16/24 (66.7%) and 15/22 (68.2%) after the first and second treatment, respectively] reporting a pain score ≤2 (barely noticeable pain) at 1 minute following the treatment. The mean peak-pain score was 4.8 and 4.7 following the first and second treatment, respectively, and no statistically significant difference was observed between consecutive treatments with MedPulser DDS (P = 0.92).
Table 3.
Summary of pain intensity scores by time using the BPI assessment tool
Discussion
This study demonstrates that repeated EP treatments with the MedPulser DDS are well tolerated in healthy adults based on patient self-report of pain using two clinically validated tools, namely, the MPQ and the BPI.
During the past two decades, several techniques have been developed for the safe and effective delivery of genetic material into target cells. The use of these new techniques in humans has been hampered by practicality, safety, and/or tolerability issues. For example, gene-gun immunization was thought to be an efficient method for the administration of DNA vaccines through direct transfection of antigen-presenting cells or cross-presentation of exogenous antigens from transfected nonimmune cells, enabling activation of antigen-specific CD8+ cytotoxic T-lymphocytes and induction of antibodies. The technique uses DNA-coated gold microparticles that are propelled onto the skin by a short pulse of pressurized gas and showed great promise for a variety of infectious and noninfectious diseases. However, its use in humans was generally associated with severe local pain, erythema lasting 2–4 weeks postdelivery, and skin discoloration lasting up to 6 months.30 In some cases, skin necrosis was reported. New and improved DNA delivery systems were greatly needed.
Several studies have demonstrated the added benefit of applying an electrical field on living tissue to improve the direct delivery of chemotherapeutic agents into cancer cells and thus achieve antitumor effects.21,31,32,33 However, these techniques delivered relatively high amounts of electrical energy into the tumor and were performed under local or general anesthesia. More recently, the development of the MedPulser DDS as well as other EP-based delivery devices has provided a simple and easy DNA delivery tool that may be used at the physician's office without the need of anesthetics. More important, the improved tolerability and reproducibility of EP lends support for a possible use of this technology for therapeutic and prophylactic interventions.34
Because pain is a common concern for individuals subjected to devices penetrating through the skin for physical delivery of drugs or vaccines, patient self-reporting and adequate assessment of pain is essential for the acceptance of technology such as EP. MPQ and BPI are clinically validated tools widely used for the description and mean intensity of pain associated with several therapeutic procedures. MPQ has been used to assess pain reported by patients in postsurgical, obstetrical, dental, and physiotherapy wards and was shown to be sufficiently sensitive to demonstrate differences between various therapeutic interventions.
In the present study, peak-pain scores reported using MPQ were highest at the time of EP treatment and a similar mean peak score (2.3) was observed after the first and second EP treatments. The observed peak-pain score is comparable to levels previously reported for musculoskeletal pain but lower than those reported for postsurgical or delivery labor pains, using a similar pain assessment tool.24 The significant decrease in peak-pain score reported at 1 minute following each EP treatment (peak-pain scores of 1.16 and 1.18 after the first and second EP treatment, respectively) was comparable to levels (1.3 and 1.4, respectively) reported after physiological therapy (transcutaneous electrical nerve stimulation) in patients with musculoskeletal pain, or treatment with analgesics in patients with postsurgical pain.
Several studies have used BPI for the assessment of pain due to diabetic peripheral neuropathy, postherpetic neuralgia, and cancer. Although several variants of BPI have been developed, little is known about the correlation between pain scores measured using BPI and the commonly used classification for severity of clinical symptoms (absence, mild, moderate, and severe). Based on interference with daily function, Zelman et al.27 have proposed cut-points in BPI scores for mild, moderate, and severe pain due to diabetic neuropathy, assigning mild for scores 0–3, moderate for scores 4–6, and severe for scores 7 and higher. The proposed categories encompassed several factors including patients' ratings of their pain, interference with daily functions, patient outcomes, and medical utilization. Peak-pain scores reported using BPI in the present study were highest at the time of EP treatment and similar mean peak scores (4.8 and 4.7, respectively) were observed after the first and second EP treatments. Using the scale proposed by Zelman et al.,27 the observed peak-pain scores can be considered as mild to moderate in intensity. The significant decrease in peak-pain score reported at 1 minute following each EP treatment (mean peak-pain scores of 1.8 and 1.9 after the first and second EP treatment, respectively) provides further evidence of the transient and mild nature of the pain experienced by the majority of study subjects.
In addition, the rates of systemic adverse experiences and treatment-related adverse experiences observed in this study are comparable to rates observed in other vaccine clinical trials evaluated in healthy adults.35,36,37 More important, almost all study participants agreed to return for a second EP treatment administered 14 days after the first electrostimulation with MedPulser DDS, which indirectly lends support to a conclusion of an acceptable tolerability profile for EP treatment with the device. The very short duration of the pain, the lack of increased pain intensity, and EP-related systemic adverse experiences following the second EP treatment were important findings, suggesting that several rounds of treatment with the MedPulser DDS could be tolerated by healthy adult subjects. It is, therefore, conceivable that such repeated EP treatments would also be tolerated by the majority of cancer patients or patients suffering from a variety of chronic viral infections in which several applications with MedPulser DDS might be required to achieve optimal benefit. Finally, the small size of the device and its user-friendly features only require basic medical equipment and supplies, supporting its use in a physician's office or at the patient's bedside. The present study results provide clear evidence that EP treatment is a safe, tolerable, and clinically acceptable delivery method when administered with phosphate-buffered saline, a common solvent for DNA vaccines. As such, the investigation of the EP treatment as a clinically acceptable antigen or DNA delivery method, a technique that was shown to generate a more robust antigen-specific immune response than conventional intramuscular injections, is warranted.
Materials and Methods
Study subjects. This study was conducted at a single research center in the United States and was approved by the Institutional Review Board of that center. Declaration of Helsinki protocols were followed during the conduct of this clinical trial and written consent was obtained from each subject before enrollment. To be eligible for study enrollment, subjects were to be healthy, 18–35 years of age, with no history of hemophilia, impaired venous access, muscular atrophy, neurodegenerative or neuromuscular disorders, and no use of electronic devices (e.g., pacemaker). In addition, female subjects had to have a negative pregnancy test and not be currently breast-feeding to be enrolled in the study.
Electroporation treatment with the MedPulser DDS. The MedPulser DDS uses relatively low–field strength pulses (~200 V/cm for 60 ms) applied to a rectangular array delimited by four 1.5 cm long 26-gauge gold-plated needles. The needles are spaced such that the opposing corners of the rectangle are 5 mm apart. All four needles of the applicator tip are to be inserted into the skin simultaneously, centered around the injection site, and reach the deltoid muscle before delivering two EP pulses of 60 milliseconds each, separated by a pause of ~190 milliseconds (Figure 1). These clinical electroporation parameters were selected based on preclinical studies that quantitavely evaluated gene expression and antibody levels in rodents (including rabbits), based on the number of needles and distance between needles; the number, polarity, and duration of pulses; and the applied voltage. Effects of needle distance, pulse duration, injection volume, and number of injection sites on gene expression were also determined in rhesus macaques22 with a prototype device functionally equivalent to the MedPulser DDS used in this clinical trial.
In the present study, subjects received a 0.5 ml dose of phosphate-buffered saline solution intramuscularly in the deltoid muscle of the right arm followed within 5 minutes by the EP treatment with the MedPulser DDS on day 1. Subjects returned 14 days later for a second administration of phosphate-buffered saline solution followed by a second course of EP treatment in the left arm.
Safety surveillance. Subjects were monitored for immediate reaction for 30 minutes following each EP treatment. They were asked to complete a treatment report card and to record injection-site reactions and temperatures for 5 days following each EP course. Subjects were also instructed to record all systemic adverse experiences through day 14 following each EP treatment. All injection site and systemic adverse experiences, regardless of intensity, were recorded.
Pain severity was assessed at the time of treatment, at 1, 5, 10, and 20 minutes, and 24 hours after each EP treatment using two clinically validated questionnaires: the MPQ—Present Pain Intensity and the BPI.24,25,26,27,28,29 The MPQ measured pain on a link descriptor numerical scale of 0–5: 0 = “no pain,” 1 = “mild,” 2 = “discomforting,” 3 = “distressing,” 4 = “horrible,” 5 = “excruciating.” The BPI contained a question phrased as “Please rate your pain by circling the one number that tells how much pain you have right now” with a rating scale from 0 (no pain) to 10 (pain as bad as you can imagine).
Statistical methods. The incidence of EP-related serious adverse experiences and the proportion of subjects who received both scheduled courses of EP treatment were summarized using 95% confidence intervals. Summary statistics (mean, median, minimum, maximum, and standard deviation) were provided for the McGill pain scores and BPI at each time point after each EP treatment. The peak McGill pain scores after the 1st and 2nd EP treatments were formally compared using a repeated measures mixed-effects model. A similar model was used to compare the peak BPI scores after the 1st and 2nd EP treatments.
Acknowledgments
We thank all the study participants for their dedication to this investigational study. We are also particularly grateful to Terri Ludwig and Jill A. Williams (Merck & Co., Inc.) for their technical assistance; Kimberly Durski (Merck & Co., Inc.) for her help in data management; Alain Luxembourg, Xiaoming Li, and Nicola La Monica (Merck & Co., Inc.) for their help in the design of the clinical study and review of the manuscript. Some of the authors are present or former employees of Merck & Co., Inc. or Inovio Biomedical Corp. as indicated by their affiliations. This clinical trial conducted at the University of California, San Diego, CA, USA.
REFERENCES
- Arvin AM., and , Greenberg HB. New viral vaccines. Virology. 2006;344:240–249. doi: 10.1016/j.virol.2005.09.057. [DOI] [PubMed] [Google Scholar]
- Grijalva CG., and , Griffin MR. Population-based impact of routine infant immunization with pneumococcal conjugate vaccine in the USA. Expert Rev Vaccines. 2008;7:83–95. doi: 10.1586/14760584.7.1.83. [DOI] [PubMed] [Google Scholar]
- Lowe DB, Shearer MH., and , Kennedy RC. DNA vaccines: successes and limitations in cancer and infectious disease. J Cell Biochem. 2006;98:235–242. doi: 10.1002/jcb.20775. [DOI] [PubMed] [Google Scholar]
- Chestnut RW, Sette A, Celis E, Wentworth P, Kubo RT, Alexander J, et al. Design and testing of peptide-based cytotoxic T-cell-mediated immunotherapeutics to treat infectious diseases and cancer Vaccine Design: The Subunit and Adjuvant Approach 1995Plenum Press: New York; 847–874.In: Powell MF, Newman MJ (eds) [DOI] [PubMed] [Google Scholar]
- Facciabene A, Aurisicchio L, Elia L, Palombo F, Mennuni C, Ciliberto G, et al. DNA and adenoviral vectors encoding carcinoembryonic antigen fused to immunoenhancing sequences augment antigen-specific immune response and confer tumor protection. Hum Gene Ther. 2006;17:81–92. doi: 10.1089/hum.2006.17.81. [DOI] [PubMed] [Google Scholar]
- Ugen KE, Kutzler MA, Marrero B, Westover J, Coppola D, Weiner DB, et al. Regression of subcutaneous B16 melanoma tumors after intratumoral delivery of an IL-15-expressing plasmid followed by in vivo electroporation. Cancer Gene Ther. 2006;13:969–974. doi: 10.1038/sj.cgt.7700973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao LA, Wu L, Khan AS, Hokey DA, Yan J, Dai A, et al. Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques. Vaccine. 2008;26:3112–3120. doi: 10.1016/j.vaccine.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis M, Denis-Mize K, Woo C, Goldbeck C, Selby MJ, Chen M, et al. Distribution of DNA vaccines determines their immunogenicity after intramuscular injection in mice. J Immunol. 2000;165:2850–2858. doi: 10.4049/jimmunol.165.5.2850. [DOI] [PubMed] [Google Scholar]
- Ulmer JB, Wahren B., and , Liu MA. Gene-based vaccines: recent technical and clinical advances. Trends Mol Med. 2006;12:216–222. doi: 10.1016/j.molmed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Neumann E, Kakorin S., and , Toensing K. Fundamentals of electroporative delivery of drugs and genes. Bioelectrochem Bioenerg. 1999;48:3–16. doi: 10.1016/s0302-4598(99)00008-2. [DOI] [PubMed] [Google Scholar]
- Zhang L, Widera G., and , Rabussay D. Enhancement of the effectiveness of electroporation-augmented cutaneous DNA vaccination by particulate adjuvant. Bioelectrochemistry. 2004;63:369–373. doi: 10.1016/j.bioelechem.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Ahlén G, Soderhölm J, Tjelle T, Kjeken R, Frelin L, Höglund U, et al. In vivo electroporation enhances the immunogenicity of hepatitis C virus nonstructural 3/4A DNA by increased local DNA uptake, protein expression, inflammation, and infiltration of CD3+ T cells. J Immunol. 2007;179:4741–4753. doi: 10.4049/jimmunol.179.7.4741. [DOI] [PubMed] [Google Scholar]
- Chiarella P, Massi E, De Robertis M, Sibilio A, Parrella P, Fazio VM, et al. Electroporation of skeletal muscle induces danger and signal release and antigen-presenting cell recruitment independently of DNA vaccine administration. Expert Opin Biol Ther. 2008;8:1645–1657. doi: 10.1517/14712598.8.11.1645. [DOI] [PubMed] [Google Scholar]
- Otten G, Schaefer M, Doe B, Liu H, Srivastava I, zur Megede J, et al. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine. 2004;22:2489–2493. doi: 10.1016/j.vaccine.2003.11.073. [DOI] [PubMed] [Google Scholar]
- Widera G, Austin M, Rabussay D, Goldbeck C, Barnett SW, Chen M, et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J Immunol. 2000;164:4635–4640. doi: 10.4049/jimmunol.164.9.4635. [DOI] [PubMed] [Google Scholar]
- Zucchelli S, Capone S, Fattori E, Folgori A, Di Marco A, Casimiro D, et al. Enhancing B- and T-cell immune response to a Hepatitis C virus E2 DNA vaccine by intramuscular electrical gene transfer. J Virol. 2000;74:11598–11607. doi: 10.1128/jvi.74.24.11598-11607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon A, Casimiro DR, Finnefrock AC, Davies ME, Tang A, Chen M, et al. Enhanced in vivo transgene expression and immunogenicity from plasmid vectors following electrostimulation in rodents and primates. Vaccine. 2008;26:5202–5209. doi: 10.1016/j.vaccine.2008.03.058. [DOI] [PubMed] [Google Scholar]
- Babiuk S, Baca-Estrada ME, Foldvari M, Storms M, Rabussay D, Widera G, et al. Electroporation improves the efficacy of DNA vaccines in large animals. Vaccine. 2002;20:3399–3408. doi: 10.1016/s0264-410x(02)00269-4. [DOI] [PubMed] [Google Scholar]
- LeBlanc R, Vasquez Y, Hannaman D., and , Kumar N. Markedly enhanced immunogenicity of a Pfs25 DNA-based malaria transmission-blocking vaccine by in vivo electroporation. Vaccine. 2008;26:185–192. doi: 10.1016/j.vaccine.2007.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drunen Little-van den Hurk S, Luxembourg A, Ellefsen B, Wilson D, Ubach A, Hannaman, et al. Electroporation-based DNA transfer enhances gene expression and immune responses to DNA vaccines in cattle. Vaccine. 2008;26:5503–5509. doi: 10.1016/j.vaccine.2008.07.093. [DOI] [PubMed] [Google Scholar]
- Glass LF, Pepine ML, Fenske NA, Jaroszeski M, Reintgen DS., and , Heller R. Bleomycin-mediated electrochemotherapy of metastatic melanoma. Arch Dermatol. 1996;132:1353–1357. [PubMed] [Google Scholar]
- Rabussay D. Applicator and electrode design for in vivo DNA delivery by electroporation. Methods Mol Biol. 2008;423:35–59. doi: 10.1007/978-1-59745-194-9_3. [DOI] [PubMed] [Google Scholar]
- Zhang L, Finnefrock AC, Casimiro DR., and , Rabussay D. 11th Annual Meeting of the ASGT, Boston, MA. Abstract #396; 2008. DNA vaccination using the MedPulser DNA Delivery System in rhesus macaques: Development of clinical electroporation parameters. [Google Scholar]
- Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Cleeland CS., and , Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- Coplan PM, Schmader K, Nikas A, Chan IS, Choo P, Levin MJ, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain. 2004;5:344–356. doi: 10.1016/j.jpain.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Zelman DC, Dukes E, Brandenburg N, Bostrom A., and , Gore M. Identification of cut-points for mild, moderate and severe pain due to diabetic peripheral neuropathy. Pain. 2005;115:29–36. doi: 10.1016/j.pain.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Nemeth KA, Graham ID., and , Harrisson MB. The measurement of leg ulcer pain: identification and appraisal of pain assessment tools. Adv Skin Wound Care. 2003;16:260–267. doi: 10.1097/00129334-200309000-00017. [DOI] [PubMed] [Google Scholar]
- Hwang SS, Chang VT., and , Kasimis B. Dynamic cancer pain management outcomes: the relationship between pain severity, pain relief, functional interference, satisfaction and global quality of life over time. J Pain Symptom Manage. 2002;23:190–200. doi: 10.1016/s0885-3924(01)00418-3. [DOI] [PubMed] [Google Scholar]
- Fuller DH, Loudon P., and , Schmaljohn C. Preclinical and clinical progress of particle-mediated DNA vaccines for infectious diseases. Methods. 2006;40:86–97. doi: 10.1016/j.ymeth.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Orlowski S, Belehrader J, Jr, Paoletti C., and , Mir LM. Transient electropermeabilization of cells in culture. Increase of the cytotoxicity of anticancer drugs. Biochem Pharmacol. 1988;37:4727–4733. doi: 10.1016/0006-2952(88)90344-9. [DOI] [PubMed] [Google Scholar]
- Rabussay DP, Nanda GS., and , Goldfarb PM. Enhancing the effectiveness of drug-based cancer therapy by electroporation (electropermeabilization) Technol Cancer Res Treat. 2002;1:71–82. doi: 10.1177/153303460200100110. [DOI] [PubMed] [Google Scholar]
- Gothelf A, Mir LM., and , Gehl J. Electrochemotherapy: results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat Rev. 2003;29:371–387. doi: 10.1016/s0305-7372(03)00073-2. [DOI] [PubMed] [Google Scholar]
- Luxembourg A, Evans CF., and , Hannaman D. Electroporation-based DNA immunization: translation to the clinic. Expert Opin Biol Ther. 2007;7:1647–1664. doi: 10.1517/14712598.7.11.1647. [DOI] [PubMed] [Google Scholar]
- Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomized controlled trial. Lancet. 2004;364:1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- Halperin SA, Langley JM, Smith B, Wunderli P, Kaufman L, Kimura A, et al. Phase I first-in-human studies of the reactogenicity and immunogenicity of a recombinant meningococcal NspA vaccine in healthy adults. Vaccine. 2007;25:450–457. doi: 10.1016/j.vaccine.2006.08.001. [DOI] [PubMed] [Google Scholar]
- McNeil SA, Noya F, Dionne M, Predy G, Meekison W, Ojah C, et al. Comparison of the safety and immunogenicity of an adult formulation tetanus and diphtheria toxoids adsorbed combined with acellular pertussis (Tdap) vaccine and trivalent inactivated influenza vaccine in adults. Vaccine. 2007;25:3464–3474. doi: 10.1016/j.vaccine.2006.12.047. [DOI] [PubMed] [Google Scholar]