Abstract
Over the last decade many studies have demonstrated the importance of reactive oxygen species (ROS) production by NADPH oxidases in angiotensin II (Ang II) signaling, as well as a role for ROS in the development of different diseases in which Ang II is a central component. In this review, we summarize the mechanism of activation of NADPH oxidases by Ang II and describe the molecular targets of ROS in Ang II signaling in the vasculature, kidney and brain. We also discuss the effects of genetic manipulation of NADPH oxidase function on the physiology and pathophysiology of the renin angiotensin system.
Keywords: Angiotensin II, NADPH oxidases, reactive oxygen species, vascular, kidney, brain
Over a decade ago, it was observed that angiotensin II (Ang II) is able to activate a NADPH oxidase (Griendling et al., 1994) that mediates the hypertensive response induced by Ang II infusion (Rajagopalan et al., 1996). Since then, numerous studies have demonstrated that NADPH oxidases regulate several Ang II functions in different tissues, many of which contribute to hypertension. In this review, we will describe the consequences of Ang II-mediated NADPH oxidase activation, with a focus on the cardiovascular, renal and central nervous systems.
NADPH Oxidases
Structure and activity
All types of cells have the ability to produce reactive oxygen species (ROS), including superoxide anion (O2.-), hydrogen peroxide (H2O2) and peroxynitrite. Among ROS, H2O2 is the most stable and has been most often implicated as a second messenger. It is mainly derived from dismutation of O2.- by superoxide dismutase (SOD), which in turn can be produced by multiple enzyme systems, of which NADPH oxidases are major sources in non-phagocytic cells.
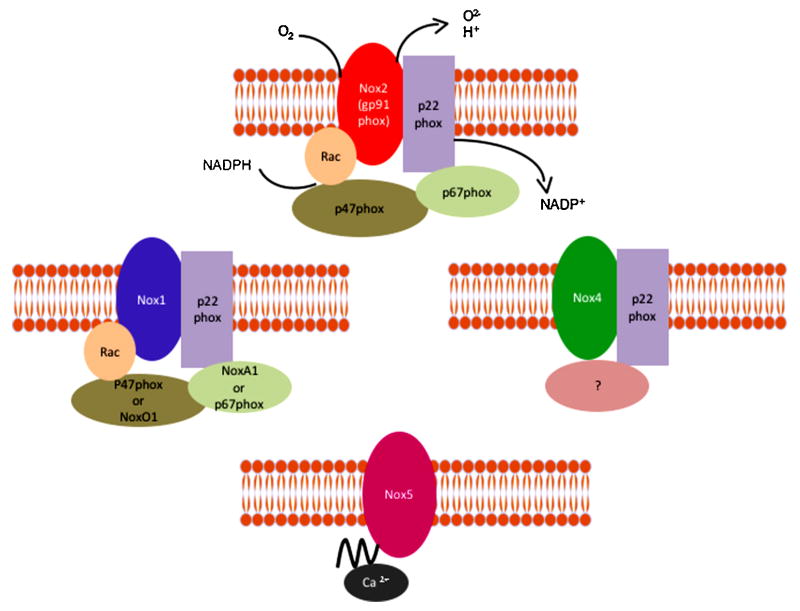
The classical NADPH oxidase is a multimeric enzyme that originally was described in neutrophils, where it plays an antimicrobial role. The phagocytic NADPH oxidase contains a heterodimeric membrane-bound cytochrome b558 complex, which consists of gp91phox (renamed Nox2) and p22phox, and 3 cytosolic subunits (p47phox, p67phox and the small G-protein Rac). Upon agonist stimulation, these cytosolic subunits translocate to the cytochrome complex, leading to an increase in enzymatic activity. In humans, there are five different NADPH oxidase homologues called Nox1 to Nox5, and two related oxidases, Duox1 and Duox2, as well as two additional homologues of the cytosolic units, NoxO1 and NoxA1 (Banfi et al., 2003; Geiszt et al., 2003; Takeya et al., 2003; Ueyama et al., 2007). Although all Nox enzymes are able to increase intracellular ROS, there are important differences regarding their activation, subunit composition, localization and expression (Figure 1). For more details please refer to the following comprehensive reviews (Bedard and Krause, 2007; Hordijk, 2006; Sumimoto, 2008). Ang II has been functionally linked to Nox1 (Dikalova et al., 2005; Lassegue et al., 2001; Matsuno et al., 2005), Nox2 (Bendall et al., 2002; Lavigne et al., 2001; Pagano et al., 1997) and variably to Nox4 (Wingler et al., 2001) in the vasculature, Nox2 and Nox4 in the kidney (Block et al., 2008; Gorin et al., 2003; Haque and Majid, 2004) and Nox2 in the brain (Wang et al., 2006).
Figure 1. Structure of the different vascular NADPH oxidases.
Different homologues of NADPH oxidases have been found in the vasculature. NoxA1 (NADPH oxidase activator 1) and NoxO1 (NADPH oxidase organizer 1).
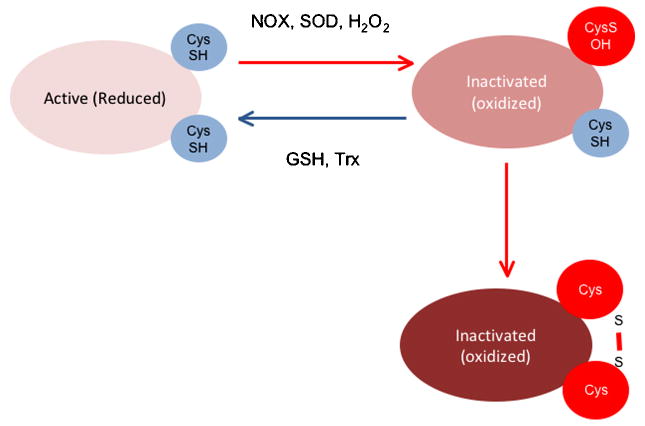
ROS derived from NADPH oxidases serve a signaling function by inducing specific biochemical changes in their molecular targets. H2O2 can oxidize the thiol group of protein (P) cysteines to sulfenic acid (P-SOH), disulfide (PSSP), sulfinic acid (P-SO2H), or sulfonic acid (P-SO3H), the latter of which is an irreversible state of thiol oxidation (Monteiro et al., 2008) (Figure 2). Thiol oxidation by H2O2 (Juarez et al., 2008) has been shown to inhibit protein tyrosine phosphatase (PTP) activity (Kwon et al., 2004; Mahadev et al., 2004; Tabet et al., 2008). Sensitivity to H2O2 is dictated largely by the pKa of the cysteines in the active site of these enzymes: they have an unusually low pKa, which renders them more susceptible to oxidation (Salmeen and Barford, 2005; Tonks, 2005). In contrast, superoxide reacts with iron-sulfur (Fe-S) centers of heme-containing molecules, resulting in altered activity. Two examples are inactivation of aconitase, leading to inhibition of mitochondrial function (Gardner et al., 1994), and activation of guanylate cyclase (Burke-Wolin et al., 1991; Burke and Wolin, 1987).
Figure 2. Inactivation of protein tyrosine phosphatases (PTP) by H2O2-induced thiol oxidation of cysteine residues.
H2O2 can inactivate PTPs either irreversibly (right side) or reversibly (left side). Reactivation of the PTPs requires reduction via thioredoxin (Trx) or glutathione (GSH).
NADPH oxidase activation by Ang II
The mechanism of NADPH oxidase activation by Ang II is complex and still incompletely understood. It has been shown that Ang II-mediated ROS production in vascular smooth muscle cells (VSMCs) from large arteries is mediated by Nox1 activation, as shown by loss of the ROS response after treatment with an adenovirus expressing antisense Nox1 (Lassegue et al., 2001), while Nox2 appears to be the Ang II-responsive Nox in resistance vessels, as demonstrated in human VSMCs transfected with gp91phox antisense oligonucleotides (Touyz et al., 2002), in the heart, as shown using small interference RNA (siRNA) against Nox2 (Hingtgen et al., 2006) and in the kidney, as described in gp91phox knockout mice (Haque and Majid, 2004). Typically, there are two different phases of ROS production: one is rapid and transient and the other is delayed and sustained. The first peak is due to an acute activation of NADPH oxidases by Ang II, while the second is dependent on the initial burst and is a consequence of upregulation of different NADPH oxidase subunits by Ang II. For example, in VSMC, the sustained phase of ROS production is preceded by upregulation of Nox1 mRNA (Lassegue et al., 2001; Wingler et al., 2001), p22phox mRNA (Fukui et al., 1997)(Fukui et al., 1997)(Fukui et al., 1997)and protein (Touyz et al., 2002) and p47phox protein (Ni et al., 2007; Touyz et al., 2002). There is also one report that Ang II can upregulate Nox4 mRNA in the vasculature (Wingler et al., 2001), although this is not observed universally (Mollnau et al., 2002).
In VSMCs, rapid ROS release requires p47phox via its phosphorylation by protein kinase C (PKC) and subsequent translocation to the membrane (Lavigne et al., 2001; Seshiah et al., 2002; Touyz et al., 2002), where it organizes the activating subunits NoxA1 and Rac (Ambasta et al., 2006; Hassanain et al., 2007). While each of these subunits has been implicated in the response to Ang II, actual formation of this complex in VSMCs remains to be shown biochemically. Ang II can stimulate PKC-dependent NADPH oxidase activity through three different phospholipases (PL): PLC (Karathanassis et al., 2002), PLD (Touyz and Schiffrin, 1999), and PLA2 (Zafari et al., 1999), with PLD likely predominating. Another important mediator of p47phox phosphorylation is Src (Touyz et al., 2003), although it is not clear whether it regulates p47phox directly or by its actions on the cytoskeleton through cortactin, a Src substrate that binds p47phox (Agerer et al., 2005; Sangrar et al., 2007; Touyz et al., 2005) and facilitates the proper assembly and function of NADPH oxidases by Ang II (Touyz et al., 2005).
Ang II activation of Nox1 and Nox2 also requires Rac1 (Cheng et al., 2006; Miyano et al., 2006; Ueyama et al., 2006). Stimulation of Rac1 by Ang II seems to be mediated by activation of the guanine nucleotide exchange factor (GEF), SOS-1 (Zuo et al., 2005), although a relationship between SOS-1 and Nox activation remains to be demonstrated. Activation of the Rac-GEF is dependent upon Src-mediated transactivation of the epidermal growth factor receptor (EGFR), which then serves as a binding site for phosphatidylinositol 3-kinase (PI-3K), an immediate activator of the Rac-GEF (Seshiah et al., 2002). This initial activation step appears to occur in caveolae (Ushio-Fukai et al., 2001), the site of Nox1 localization (Hilenski et al., 2004). Of importance, the H2O2 that is produced as a result of initial NADPH oxidase activation then activates Src, creating an amplification of oxidase activity (Seshiah et al., 2002). Prolonged signal generation requires upregulation of the Nox subunit, p47phox and p22phox (Fukui et al., 1997; Lassegue et al., 2001; Touyz et al., 2002).
ANG II Activation of NADPH Oxidases in the Cardiovascular System
Hypertension
Ang II is a major contributor to hypertension via its central, vascular and renal effects. It is clear that NADPH oxidase-derived ROS production plays a critical role in the hypertension induced by Ang II (Dikalova et al., 2005; Fukui et al., 1997; Landmesser et al., 2002; Rajagopalan et al., 1996; Weber et al., 2005). For example, our group demonstrated that p22phox is increased in hypertensive rats (Fukui et al., 1997), that smooth-muscle specific p22phox overexpressing mice have elevated blood pressure (Weber et al., 2005), and that smooth-muscle specific overexpression of Nox1 in mice induces a higher pressure response to Ang II (Dikalova et al., 2005). It should be noted that overexpression of one subunit is often accompanied by an increase in the others, and in most reported cases results in an increase in NADPH oxidase activity (Dikalova et al., 2005; Fukui et al., 1997; Laude et al., 2005; Weber et al., 2005). For example, overexpression of p22phox increases the expression of Nox1 protein and NADPH oxidase activity (Laude et al., 2005). In contrast, in p47phox- or Nox1–deficient mice, the Ang II effects on vascular tone and blood pressure are inhibited (Gavazzi et al., 2006; Landmesser et al., 2002; Matsuno et al., 2005). The infusion of Ang II does, however, induce hypertension in Nox2 deficient mice (Touyz et al., 2005; Wang et al., 2001). Other studies showed that different ROS scavengers, including catalase (Wilson, 1990; Yang et al., 2003) and SOD (Gongora et al., 2006; Wilson, 1990), blunt the response to Ang II. In seeming contrast to these results, a recent study demonstrated that in a model of chronic Ang II elevation, deletion of Nox1 has no effect on blood pressure, but does improve hypertension-related end-organ damage (Yogi et al., 2008). Similarly, Liu et al. (Liu et al., 2003) infusion of the Nox2 inhibitor gp91-dstat into rats co-infused with AngII for 1 week attenuated the increase in medial hypertrophy, but not blood pressure. It is thus possible that NADPH oxidases are necessary for the development, but not the maintenance, of hypertension by Ang II, and contribute to its pathophysiological effects.
With respect to the role of H2O2 and O2.- in the regulation of vascular tone per se, the observed response is tissue and concentration dependent (Barlow and White, 1998; Wei et al., 1996; Yang et al., 1999; Yang et al., 1998). For example, O2.- and H2O2 have vasodilator effects in cerebral arteries, while they have a vasoconstrictor effect in non-cerebral arteries (for more details, please refer to (Miller et al., 2006)). Superoxide exerts its effects mainly, but not exclusively, by inactivating nitric oxide, while H2O2 acts via upregulation of endothelial nitric oxide synthase (eNOS) (Drummond et al., 2000) and via direct effects on ion channels (Liu and Gutterman, 2002) or the contractile machinery (Jin and Rhoades, 1997). In coronary vessels, H2O2 is a potent vasodilator acting via modulation of K+ channels (Miura et al., 2003; Sato et al., 2003). The ROS produced by Ang II activation of NADPH oxidases thus modulate vascular tone by regulating both endothelial function and VSMC contraction.
Endothelial cells
Endothelial dysfunction is largely thought to be a consequence of hypertension, although recent studies suggest that endothelial dysfunction also may predispose to the development of hypertension (Schlaich et al., 2004; Taddei et al., 1996; Yang et al., 2007). Impaired endothelium-dependent relaxation results from a decrease of nitric oxide bioavailability, largely due to increased O2.-. The link between Ang II, ROS and endothelial dysfunction had been demonstrated in hypertensive animals. Acute infusion of Ang II results in impaired relaxation to acetylcholine, the calcium ionophore A23187, and nitroglycerin (Rajagopalan et al., 1996). Moreover, Ang II, through PKC activation, induces an upregulation of Nox1 (Lassegue et al., 2001) and uncouples eNOS (Mollnau et al., 2002), thus increasing O2.- production in a positive feedback fashion. Interestingly, these effects are accompanied by a decrease in soluble guanylate cyclase and a diminished activation of protein kinase G (Mollnau et al., 2002). In other models of hypertension such as the two kidney-one clip (2K-1C) model, it has been demonstrated that Ang II-induced NADPH oxidase activation is responsible for impaired vasodilator responses to endogenous and exogenous nitrovasodilators (Heitzer et al., 1999). Recently, Doughan et al. (Doughan et al., 2008) demonstrated that Ang II activation of NADPH oxidases leads to mitochondrial dysfunction in endothelial cells, amplifying the increase in ROS. This represents a new pathophysiological pathway of Ang II activation.
Vascular smooth muscle cells
Contraction
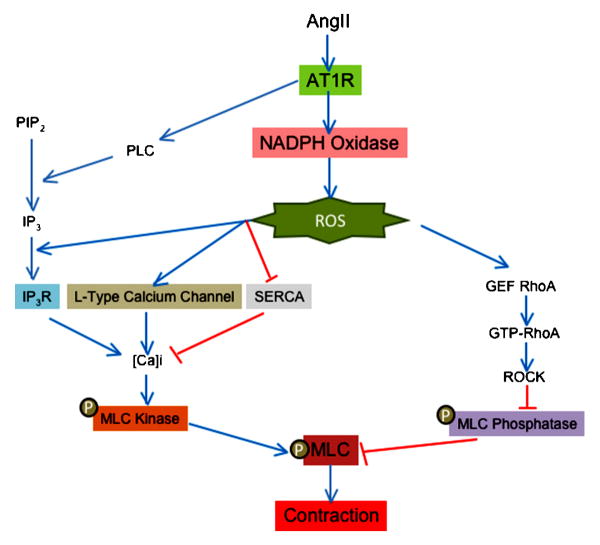
Ang II is one of the most powerful vasoconstrictor agents for vascular smooth muscle. As with other vasoconstrictors, phosphorylation of the myosin light chain (MLC) is a crucial step in force development to Ang II. The rate of MLC phosphorylation depends on a balance between Ca2+/calmodulin-dependent MLC kinase and myosin phosphatase activity (Somlyo and Somlyo, 2000). Thus, an increase in MLC kinase stimulation or a decrease in MLC phosphatase activity leads to VSMC contraction. ROS have been implicated in both of these pathways (Figure 3).
Figure 3. Role of ROS in contraction induced by angiotensin II in vascular smooth muscle cells.
ROS production is implicated in calcium dependent and independent induced by angiotensin II. Red lines show inhibitory effects and blue lines depict activation. ROS (reactive oxygen species), AT1R (angiotensin II receptor type 1), PLC (phospholipase C), PIP2 (phosphoinositol biphosphate), IP3 (inositol 1,4,5 triphosphate), IP3R (inositol 1,4,5 triphosphate receptor), SERCA (sarco/endoplasmic reticulum Ca2+-ATPase), GEF (guanine nucleotide exchange factor), GTP (guanosin triphosphate), ROCK (Rho-associate kinase) and MLC (myosin light chain).
One of the most important early signals in the initiation of contraction by Ang II is thus a PLC-mediated increase in intracellular calcium concentration ([Ca]i). Torrecillas et al. (Torrecillas et al., 2001) demonstrated that H2O2 production by Ang II in primary aortic VSMCs (Rodriguez-Puyol et al., 2002) mediates this increase in intracellular calcium concentration ([Ca]i), and therefore MLC phosphorylation, without modifying inositol 1,4,5-trisphosphate (IP3) production by PLC. This suggests that ROS production by Ang II may modify the affinity of the IP3 receptor for its ligand. In support of this idea, Redondo et al. (Redondo et al., 2004) observed that the H2O2-induced increase in [Ca]i in platelets is abolished by preincubation with Xetospongin C (an IP3 receptor inhibitor). However, other studies have shown that activation of Src (Touyz et al., 2001) and ERK (Touyz et al., 1999) by Ang II is upstream of IP3 production and the increase in [Ca]i, suggesting that they may be the actual targets of H2O2 because they have previously been shown to be redox sensitive (Touyz et al., 2003; Ushio-Fukai et al., 2001; Viedt et al., 2000). Because the activation of ERK by ROS is not universally observed (Pinzar et al., 2005; Sano et al., 2001; Touyz et al., 2003; Viedt et al., 2000) and Src activation is only partially ROS dependent (Seshiah et al., 2002), it is likely that ROS generation by Ang II may also be necessary for the correct release of calcium by IP3. In fact, [Ca]i is enhanced in arteries from spontaneously hypertensive rats, and this increase is both ROS- and Ang II-dependent (Tabet et al., 2004). Another mechanism responsible for the ROS-sensitivity of the Ang II-induced increase in [Ca]i is calcium channel activation. Thus, it has been shown that increased expression of the α1C subunit of the L-type calcium channel by Ang II is ROS-, PKC- and CREB-dependent (Tsai et al., 2007), and the function of this channel is regulated by NADPH oxidases (Wang et al., 2006). Finally, it has been demonstrated that H2O2 inhibits sarcoendoplasmic reticulum Ca-ATPase (SERCA), thereby blocking the reuptake of calcium from the cytosol to the internal stores and increasing [Ca]i. Inhibition of SERCA occurs through oxidation of specific sulphydryl groups (Redondo et al., 2004), although the source of the responsible ROS is unclear. Thus, it is likely that ROS regulate the increase in [Ca]i induced by Ang II at multiple levels.
As noted above, the other factor that regulates the levels of phosphorylated and therefore activated MLC is the activity of MLC phosphatase, which is inhibited by RhoA/RhoA kinase (ROCK) (Seko et al., 2003)-mediated phosphorylation on Thr696 of MYPT1, a myosin binding subunit of MLC phosphatase. Importantly, Uehata et al. (Uehata et al., 1997) observed that ROCK is implicated in the pathophysiology of hypertension induced by Ang II. Recently, it has been shown that the Ang II-stimulated ROCK-mediated phosphorylation of MLC (Seko et al., 2003) is dependent on NADPH oxidases (Higashi et al., 2003). The mechanism of activation of RhoA/ROCK by ROS is not clear, but may involve PDZ- (Hilgers et al., 2007) and leukemia-associated (Ying et al., 2006) GEFs. More studies are necessary to define the actual molecular target of ROS in this pathway.
Hypertrophy
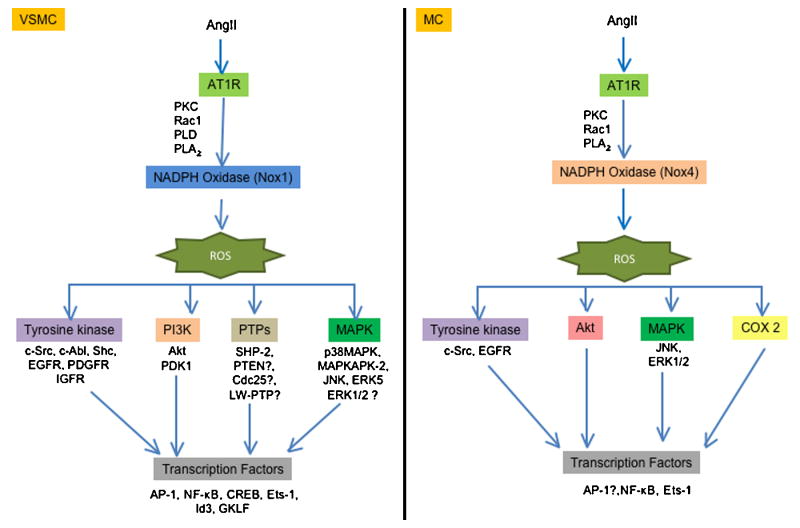
Many of the pathophysiological effects of Ang II are due to its pro-growth properties. In vivo proof that these events are ROS-dependent comes from studies in genetically modified animals. Thus, overexpression of Nox1 (Dikalova et al., 2005) or p22phox (Weber et al., 2005) potentiates the effect of Ang II on vascular hypertrophy, while knockout of Nox1 (Matsuno et al., 2005) or Nox2 (Bendall et al., 2002) inhibits it. Similarly, Ang II-induced cardiac hypertrophy requires the Nox activator Rac1 (Satoh et al., 2006). In both VSMCs and cardiomyocytes, multiple molecular targets that are activated by Ang II in a ROS-dependent manner contribute to hypertrophy, including tyrosine kinases, mitogen activated protein kinases (MAPK), the survival kinase Akt and redox-sensitive transcriptional factors (Taniyama et al., 2004; Touyz et al., 2003; Touyz et al., 2004; Ushio-Fukai et al., 1999) (Figure 4). In addition, ROS-mediated inactivation of protein tyrosine phosphatases (PTP) amplifies growth-related signaling. Classic PTPs include transmembrane receptor-like proteins (RPTPs) and non-transmembrane cytoplasmic PTPs. All members of the PTP family are characterized by the presence of a highly conserved sequence HCXXGXXRS/T, which contains a Cys residue that is necessary for catalysis. As mentioned earlier, the thiol oxidation in this Cys by addition of H2O2 leads to the inactivation of the PTP activity (Juarez et al., 2008). A recent study has shown that SOD1 is essential for PTP inactivation by growth factors, indicating that H2O2, but not O2.-, is implicated in PTP inactivation (Juarez et al., 2008). Tabet et al. (Tabet et al., 2008) demonstrated that Ang II oxidizes PTP/SHP-2 via Nox1 activation, and that PTP/SHP-2 inactivation regulates Ang II-induced activation of Akt, but not of ERK1/2 or p38 MAPK. Other studies have demonstrated that H2O2 inactivates phosphatase and tensin homolog (PTEN) (Lee et al., 2002; Leslie et al., 2003), thus increasing the concentration of PIP3 and activating Akt (Leslie et al., 2003), low molecular weight PTP (LMW-PTP) (Caselli et al., 1998), and Cdc25 (Savitsky and Finkel, 2002). However, whether Ang II similarly inactivates these phosphatases remains to be determined.
Fig 4. Role of ROS in the hypertrophy induced by angiotensin II in vascular smooth muscle cells (left pannel) or messangial cells (right pannel).
Vascular smooth muscle and messangial cells exhibit differences in the targets activated in the hypertrophy induced by angiotensin II. ROS (reactive oxygen species), AT1R (angiotensin II receptor type 1), PKC (protein kinase C), PLD (phospholipase D), PLA2 (phospholipase A2), EGFR (epidermal growth factor receptor), PDGFR (platelet derived growth factor receptor), IGFR (insulin like growth factor receptor) PI3K (phosphatidylinositol 3-kinase), PDK1 (phosphoinositide-dependent protein kinase 1), SHP-2 (Src homology 2 domain-containing protein tyrosine phosphatase), PTEN (phosphatase and tensin homolog), LMW-PTP (low molecular weight phosphatase), p38MAPK (p38 mitogen-activated protein kinase), MAPKAPK-2 (MAPK-activated protein kinase-2), JNK (C-Jun N-terminal kinases), ERK (extracellular signal-regulated kinases), AP-1 (activator protein 1), NF-κβ (nuclear factor-kappa β), CREB (cAMP response element-binding), Ets-1 (E26 transformation-specific sequence), GFLK (Gut-enriched Krüppel-like factor) and COX-2 (ciclooxigenase 2).
One of the early effects of Ang II stimulation is c-Src activation, which is inhibited by catalase overexpression or treatment with pharmacological antioxidants (Ushio-Fukai et al., 2001). Phosphorylated Src mediates the recruitment of the AT1R to caveolae, which is required for proper organization of ROS-dependent Ang II signaling. Ang II also transactivates the IGF-1R (Touyz et al., 2003) and PDGFR via ROS and Shc (Heeneman et al., 2000). Transactivation of these receptors leads to the recruitment and stimulation of different proteins, including phosphatidylinositol 3-kinase, which activates Akt (Ushio-Fukai et al., 1999). Whether the ROS sensitivity of this pathway is conferred by Src, or if there are redox modifications of these proteins or their associated phosphatases, remains to be determined. Because Akt is phosphorylated by MAPKAPK-2, a substrate of the ROS-sensitive p38MAPK (Taniyama et al., 2004), it is possible that it is p38MAPK activation that is the actual target of ROS. One intriguing hypothesis is that ROS mediate the dissociation of the p38MAPK kinase Ask-1 from redox-active thioredoxin (Hsieh and Papaconstantinou, 2006; Matsuzawa et al., 2005), although this remains to be investigated.
Activation of other MAPKs by Ang II also requires ROS derived from NADPH oxidases. Thus, c-Jun NH2 terminal kinase (JNK) activation in response to Ang II is blunted by different antioxidants (Kyaw et al., 2001) or by transfection with antisense against the p22phox subunit (Viedt et al., 2000). Moreover, Shc is implicated in the phosphorylation of JNK (Yoshizumi et al., 2001). In contrast to p38MAPK and JNK, whose redox sensitivity to Ang II is nearly universal in the vasculature, the sensitivity of ERK1/2 to ROS is controversial. Some studies found that activation of ERK1/2 is ROS independent (Kyaw et al., 2001; Touyz et al., 2003; Viedt et al., 2000), while others found it to be ROS sensitive (Frank et al., 2000; Pinzar et al., 2005; Sano et al., 2001). This suggests that ERK1/2 is not an actual target of ROS; rather, when redox-sensitivity is observed, it is likely conferred by an upstream kinase or phosphatase and may occur in a specific subcellular compartment.
A number of years ago, we suggested that some aspects of Ang II signaling may occur subsequent to internalization of the AT1R in endosomes (Griendling et al., 1986). The apparent role of caveolae in organizing redox signals (Zuo et al., 2005) suggests that ROS-dependent signaling is intimately linked to vesicular trafficking. A recent series of elegant papers by the Englehardt and Miller labs shows that Nox-dependent ROS generation in response to IL-1β or TNF-α occurs in the early endosome and is released into the cytosol by a pathway requiring activation of chloride channels (Miller et al., 2007; Mumbengegwi et al., 2008), although the origin of the vesicles is unknown. This provides a molecular explanation for the finding that ROS generation in nonphagocytic cells is intracellular, even though the Nox enzymes are oriented to the outside of the plasma membrane. Although this has not been studied for Ang II, it is likely that a similar mechanism explains the robust intracellular generation of H2O2 observed in VSMCs, and the apparent compartmentalization of redox signaling in these cells. Both caveolin-mediated and clathrin-dependent internalization have been shown for AT1R internalization (Gaborik et al., 2001; Ishizaka et al., 1998), but have not been studied with respect to Ang II stimulated ROS generation. However, Choi et al. (Choi et al., 2008) demonstrated that β-arrestins are not necessary for Nox1 activation by Ang II, which may indicate that activation occurs prior to internalization. Because this latter study was conducted entirely in a reconstituted system, these results must be interpreted with caution.
Activation of these ROS-sensitive signal transduction pathways ultimately leads to stimulation of specific transcription factors. One of the most well-studied is AP-1, a heterodimer of Fos and Jun. Viedt et al. (Viedt et al., 2004) and Wu et al. (Wu et al., 2005) demonstrated that activation of a p22phox- and gp91phox-containing oxidase is essential for Ang II-stimulated AP-1 binding to DNA in VSMCs and cardiomyocytes. Conversely, activation of AP-1 is necessary for increased transcription of p22phox by Ang II, suggesting a feed-forward mechanism (Manea et al., 2008). AP-1 is implicated in diverse biological functions, such as cell proliferation, protein synthesis, apoptosis and secretion of pro-fribotic factors via its induction of specific genes. With respect to Ang II, the increase in TGF-β, endothelin, MMP-1 and fibronectin mRNA expression is mediated by ROS- and AP-1 dependent mechanisms (Browatzki et al., 2005; Cheng et al., 2003; Moriguchi et al., 1999; Wenzel et al., 2001). Another important ROS-dependent transcription factor activated by Ang II is NFκB. The prototype of the NFκB family is the p50/p65 heterodimer expressed constitutively in most mammalian cells. It is well established that NFκB is complexed with and sequestered in the cytoplasm by inhibitory IκB (inhibitor of NFκB) proteins, and that many activating stimuli induce phosphorylation of IκB by the IκB-kinase complex (IKKα, IKKβ, and IKKγ), initiate IκB ubiquitination and degradation by means of the 26S proteasome, and allow translocation of NFκB to the nucleus (Chen et al., 1996; DiDonato et al., 1996; DiDonato et al., 1995; Ghosh and Karin, 2002). Although many investigators have shown that NFκB is redox sensitive, there are just a few studies that demonstrated the activation of this factor by ROS in VSMCs (Browatzki et al., 2005; Ortego et al., 1999; Shang et al., 2008). Among the different targets regulated by Ang II in a NFκB dependent manner is monocyte chemoattractant protein–1 (MCP-1) (Capers et al., 1997; Chen et al., 1998). Other Ang II- and ROS-sensitive transcription factors are cyclic AMP response element-binding protein (CREB) (Funakoshi et al., 2002; Ichiki et al., 2003; Tsai et al., 2007), hypoxia inducible factor 1-α (Gorlach et al., 2001), Id3 and GKLF (Nickenig et al., 2002). These latter two factors have opposite effects on cell cycle progression, suggesting that ROS must be compartmentalized. Recently, it has been shown that And II-induced activation of Ets-1, which is important in vascular remodeling (Zhan et al., 2005), is redox sensitive and its activation induces an increase in p47phox expression (Ni et al., 2007). Ets-1 is also an important regulator of the cyclin-dependent kinase inhibitor p21CIP(Zhang et al., 2003), plasminogen activator inhibitor–1 (PAI-1) and MCP-1 (Zhan et al., 2005).
ANG II Activation of NADPH Oxidases in the Kidney
The kidney also expresses NADPH oxidases, the most abundant of which is Nox4 (originally called Renal NADPH oxidase or Renox)(Geiszt et al., 2000). The effect of Ang II on NADPH oxidase activity in this organ is well described, although the molecular mechanisms are less clear and have been investigated mainly at the level of gene expression. Thus, infusion of Ang II upregulates the expression of p22phox and Nox1 and downregulates the expression of extracellular superoxide dismutase (EC-SOD) (Chabrashvili et al., 2003). The decrease of EC-SOD seems to participate in the upregulation of p22phox and Nox1. Moreover, the resulting increase in ROS production by Ang II in the kidney is important in the regulation of renal function (Chabrashvili et al., 2003; Jaimes et al., 2005; Lopez et al., 2003; Modlinger et al., 2006; Nouri et al., 2007). Thus, López et al. (Lopez et al., 2003) observed that renal infusion of Ang II in rats reduced renal blood flow (RBF), glomerular filtration (GF), sodium excretion, and NO levels, all of which were blunted by the AT1R antagonist valsartan, the superoxide scavenger tempol and the NADPH oxidase inhibitor apocynin. Furthermore, treatment of rats with siRNA against p22phox prevents the increase of renal cortical NADPH oxidase activity and increases the excretion of the lipid peroxidation marker 8-isoprostane PGF2α induced by perfusion with Ang II (Modlinger et al., 2006). The role of superoxide in regulating renal function during Ang II infusion has been confirmed in a number of studies (Kopkan et al., 2006; Mori and Cowley, 2003; Mori et al., 2007; Wang et al., 2003; Welch et al., 2005).
Similar to its effects in VSMCs, Ang II can stimulate protein synthesis in mesangial cells (MC) and tubular cells (Hannken et al., 1998; Jaimes et al., 1998), leading to hypertrophy (Figure 4) and synthesis of extracellular components. Both processes contribute to the pathogenesis of fibrosis of the glomerular microvascular bed (Ardaillou et al., 1999). However, whereas in VSMC Ang II activation of the NADPH oxidase has been studied extensively in renal cells, it is less well understood. Similar to VSMC, the activation of the NADPH oxidase is dependent on the production of arachidonic acid (Block et al., 2006; Gorin et al., 2001), PKC (Jaimes et al., 1998) and Rac1 (Gorin et al., 2003); however, the principal Nox that is coupled to Ang II is Nox4 (Gorin et al., 2003). Because Nox4 has been demonstrated to be Rac1 independent (Martyn et al., 2006), the significance of Rac1 in this system requires further study. As in VSMC, multiple targets are downstream of Nox activation by Ang II, including Akt (Gorin et al., 2003), EGFR (Ding et al., 2007), JNK (Ding et al., 2007), ERK1/2 (Yu et al., 2005) (Gorin et al., 2004), ETS-1 (Pearse et al., 2008), cyclooxigenase-2 (COX2) (Jaimes et al., 2005; Jaimes et al., 2008; Pearse et al., 2008) and PDK1 (Block et al., 2008). Some of the extracellular components regulated by ROS in Ang II-stimulated MC include fibronectin (Block et al., 2006; Pearse et al., 2008), collagen I (Zhao et al., 2008) and TGF-β (Lodha et al., 2002; Zhao et al., 2008), a profibrotic cytokine.
ANG II Activation of NADPH Oxidases in the Central Nervious System
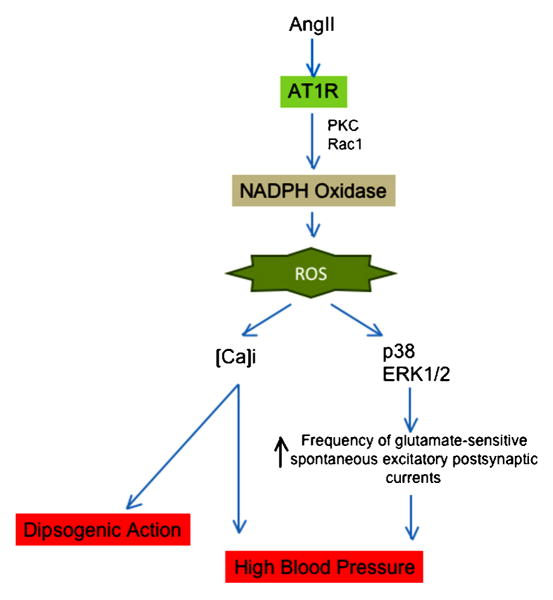
Ang II in the brain participates in regulation of blood pressure, sympathetic activity, vasopressin release, thirst, and sodium appetite (Phillips, 1987). In the last several years, numerous studies have demonstrated that ROS generation by Ang II modulates some of these effects in the CNS, acting in different regions of the brain. Zimmerman et al. observed that the acute intracerebroventricular injection (Zimmerman et al., 2002) or chronic systemic perfusion (Zimmerman et al., 2004) of Ang II induced O2.- production in the subfornical organ (SFO), leading to an increase in blood pressure and dipsogenesis. Notably, transfection with adenovirus that expresses Mn-SOD or Cu/Zn SOD blocked these effects of Ang II. Moreover, studies from Zucker's group (Gao et al., 2004) demonstrated that the Ang II-NADPH oxidase axis is regulated by sympathetic excitation in the rostral ventrolateral medulla (RVLM), which is implicated in chronic heart failure (Gao et al., 2005). Ang II increases gp91phox and p47phox expression in the RVLM as well (Gao et al., 2004).
The mechanism of NADPH activation by Ang II in the CNS is incompletely understood. Even so, it has been demonstrated that NADPH oxidase activation requires Rac1 (Zimmerman et al., 2004) and PKC (Wang et al., 2006). More information is available concerning the downstream consequences of NADPH oxidase-derived ROS in the CNS (Figure 5). Thus, Wang et al. (Wang et al., 2006) observed that inhibition of NADPH oxidases by apocynin or an inhibitory Nox2 peptide docking sequence decreased Ang II-stimulated increases in [Ca]i, and, more recently, it was observed that O2.- production by Ang II is implicated in the elevation of [Ca]i (Zimmerman et al., 2005) in neuroblastoma Neuro 2A cells. In addition, ROS production by Ang II has been shown to be critical for the inhibition of the delayed rectifier potassium current (I(Kv)), which contributes to neuronal activity (Sun et al., 2005). Other targets of Ang II stimulated NADPH oxidases in the CNS are p38 and ERK1/2. Chan et al. (Chan et al., 2005) demonstrated that Ang II injection in the RVLM increased the phosphorylation of ERK1/2 and p38MAPK, but not in JNK. These effects were attenuated by application into the RVLM of a flavin oxidase inhibitor, DPI, antisense oligonucleotides that target p22phox or p47phox mRNA, or Tempol. Moreover, they observed that DPI, Tempol and the p38MAPK inhibitor decreased the frequency of glutamate-sensitive spontaneous excitatory postsynaptic currents and the effects on the blood pressure induced by Ang II (Chan et al., 2005).
Fig 5. Role of ROS in modulation of angiotensin II signaling in the brain.
ROS are implicated in the dipsogenic action and blood pressure modulated by angiotensin II in the brain. ROS (reactive oxygen species), AT1R (angiotensin II receptor type 1), PKC (protein kinase C), p38MAPK (p38 mitogen-activated protein kinase) and ERK (extracellular signal-regulated kinases).
Conclusions and Future Directions
Multiple studies have demonstrated a role for NADPH oxidases in Ang II function. Although many downstream targets have been identified, the temporal sequence of events that lead to the proper activation and function of NADPH oxidase is not completely understood, and the compartmentalization of signaling remains to be fully investigated. In addition, more work is necessary to identify the precise molecular modifications of the putative signaling targets of ROS after Ang II stimulation, with particular attention to phosphatases. Understanding which NADPH oxidases are activated by Ang II in specific tissues, in normal physiology and in disease development is also important, and may help us to define better interventions aimed at blocking the deleterious effects of Ang II activation while supporting those necessary for survival.
Acknowledgments
This work was supported by NIH grants HL38206, HL075209, HL058000, and HL05863.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agerer F, Lux S, Michel A, Rohde M, Ohlsen K, Hauck CR. Cellular invasion by Staphylococcus aureus reveals a functional link between focal adhesion kinase and cortactin in integrin-mediated internalisation. J Cell Sci. 2005;118:2189–200. doi: 10.1242/jcs.02328. [DOI] [PubMed] [Google Scholar]
- Ambasta RK, Schreiber JG, Janiszewski M, Busse R, Brandes RP. Noxa1 is a central component of the smooth muscle NADPH oxidase in mice. Free Radic Biol Med. 2006;41:193–201. doi: 10.1016/j.freeradbiomed.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Ardaillou R, Chansel D, Chatziantoniou C, Dussaule JC. Mesangial AT1 receptors: expression, signaling, and regulation. J Am Soc Nephrol. 1999;10 11:S40–6. [PubMed] [Google Scholar]
- Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol. 1998;275:H1283–9. doi: 10.1152/ajpheart.1998.275.4.H1283. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–6. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- Block K, Assaad E, Griendling KK, Lee DY, Wittrant Y, Gorin Y. Nox4 NAD(P)H oxidase mediates Src-dependent tyrosine phosphorylation of PDK-1 in response to angiotensin II. Role in mesangial cell hypertrophy and fibronectin expression. J Biol Chem. 2008 doi: 10.1074/jbc.M803964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block K, Ricono JM, Lee DY, Bhandari B, Choudhury GG, Abboud HE, Gorin Y. Arachidonic acid-dependent activation of a p22(phox)-based NAD(P)H oxidase mediates angiotensin II-induced mesangial cell protein synthesis and fibronectin expression via Akt/PKB. Antioxid Redox Signal. 2006;8:1497–508. doi: 10.1089/ars.2006.8.1497. [DOI] [PubMed] [Google Scholar]
- Browatzki M, Larsen D, Pfeiffer CA, Gehrke SG, Schmidt J, Kranzhofer A, Katus HA, Kranzhofer R. Angiotensin II stimulates matrix metalloproteinase secretion in human vascular smooth muscle cells via nuclear factor-kappaB and activator protein 1 in a redox-sensitive manner. J Vasc Res. 2005;42:415–23. doi: 10.1159/000087451. [DOI] [PubMed] [Google Scholar]
- Burke-Wolin T, Abate CJ, Wolin MS, Gurtner GH. Hydrogen peroxide-induced pulmonary vasodilation: role of guanosine 3′,5′-cyclic monophosphate. Am J Physiol. 1991;261:L393–8. doi: 10.1152/ajplung.1991.261.6.L393. [DOI] [PubMed] [Google Scholar]
- Burke TM, Wolin MS. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am J Physiol. 1987;252:H721–32. doi: 10.1152/ajpheart.1987.252.4.H721. [DOI] [PubMed] [Google Scholar]
- Capers Qt, Alexander RW, Lou P, De Leon H, Wilcox JN, Ishizaka N, Howard AB, Taylor WR. Monocyte chemoattractant protein-1 expression in aortic tissues of hypertensive rats. Hypertension. 1997;30:1397–402. doi: 10.1161/01.hyp.30.6.1397. [DOI] [PubMed] [Google Scholar]
- Caselli A, Marzocchini R, Camici G, Manao G, Moneti G, Pieraccini G, Ramponi G. The inactivation mechanism of low molecular weight phosphotyrosine-protein phosphatase by H2O2. J Biol Chem. 1998;273:32554–60. doi: 10.1074/jbc.273.49.32554. [DOI] [PubMed] [Google Scholar]
- Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol Regul Integr Comp Physiol. 2003;285:R117–24. doi: 10.1152/ajpregu.00476.2002. [DOI] [PubMed] [Google Scholar]
- Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC, Chan JY. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res. 2005;97:772–80. doi: 10.1161/01.RES.0000185804.79157.C0. [DOI] [PubMed] [Google Scholar]
- Chen XL, Tummala PE, Olbrych MT, Alexander RW, Medford RM. Angiotensin II induces monocyte chemoattractant protein-1 gene expression in rat vascular smooth muscle cells. Circ Res. 1998;83:952–9. doi: 10.1161/01.res.83.9.952. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Parent L, Maniatis T. Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–62. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- Cheng G, Diebold BA, Hughes Y, Lambeth JD. Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem. 2006;281:17718–26. doi: 10.1074/jbc.M512751200. [DOI] [PubMed] [Google Scholar]
- Cheng TH, Cheng PY, Shih NL, Chen IB, Wang DL, Chen JJ. Involvement of reactive oxygen species in angiotensin II-induced endothelin-1 gene expression in rat cardiac fibroblasts. J Am Coll Cardiol. 2003;42:1845–54. doi: 10.1016/j.jacc.2003.06.010. [DOI] [PubMed] [Google Scholar]
- Choi H, Leto TL, Hunyady L, Catt KJ, Bae YS, Rhee SG. Mechanism of angiotensin II-induced superoxide production in cells reconstituted with angiotensin type 1 receptor and the components of NADPH oxidase. J Biol Chem. 2008;283:255–67. doi: 10.1074/jbc.M708000200. [DOI] [PubMed] [Google Scholar]
- DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDonato JA, Mercurio F, Karin M. Phosphorylation of I kappa B alpha precedes but is not sufficient for its dissociation from NF-kappa B. Mol Cell Biol. 1995;15:1302–11. doi: 10.1128/mcb.15.3.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–76. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- Ding G, Zhang A, Huang S, Pan X, Zhen G, Chen R, Yang T. ANG II induces c-Jun NH2-terminal kinase activation and proliferation of human mesangial cells via redox-sensitive transactivation of the EGFR. Am J Physiol Renal Physiol. 2007;293:F1889–97. doi: 10.1152/ajprenal.00112.2007. [DOI] [PubMed] [Google Scholar]
- Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–96. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- Drummond GR, Cai H, Davis ME, Ramasamy S, Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res. 2000;86:347–54. doi: 10.1161/01.res.86.3.347. [DOI] [PubMed] [Google Scholar]
- Frank GD, Eguchi S, Yamakawa T, Tanaka S, Inagami T, Motley ED. Involvement of reactive oxygen species in the activation of tyrosine kinase and extracellular signal-regulated kinase by angiotensin II. Endocrinology. 2000;141:3120–6. doi: 10.1210/endo.141.9.7630. [DOI] [PubMed] [Google Scholar]
- Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Qt, Taylor WR, Harrison DG, de Leon H, Wilcox JN, Griendling KK. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ Res. 1997;80:45–51. doi: 10.1161/01.res.80.1.45. [DOI] [PubMed] [Google Scholar]
- Funakoshi Y, Ichiki T, Takeda K, Tokuno T, Iino N, Takeshita A. Critical role of cAMP-response element-binding protein for angiotensin II-induced hypertrophy of vascular smooth muscle cells. J Biol Chem. 2002;277:18710–7. doi: 10.1074/jbc.M110430200. [DOI] [PubMed] [Google Scholar]
- Gaborik Z, Szaszak M, Szidonya L, Balla B, Paku S, Catt KJ, Clark AJ, Hunyady L. Beta-arrestin- and dynamin-dependent endocytosis of the AT1 angiotensin receptor. Mol Pharmacol. 2001;59:239–47. doi: 10.1124/mol.59.2.239. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res. 2004;95:937–44. doi: 10.1161/01.RES.0000146676.04359.64. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, Zucker IH. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol. 2005;288:H2271–9. doi: 10.1152/ajpheart.00949.2004. [DOI] [PubMed] [Google Scholar]
- Gardner PR, Nguyen DD, White CW. Aconitase is a sensitive and critical target of oxygen poisoning in cultured mammalian cells and in rat lungs. Proc Natl Acad Sci U S A. 1994;91:12248–52. doi: 10.1073/pnas.91.25.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, Krause KH. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580:497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A. 2000;97:8010–4. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiszt M, Lekstrom K, Witta J, Leto TL. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J Biol Chem. 2003;278:20006–12. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- Gongora MC, Qin Z, Laude K, Kim HW, McCann L, Folz JR, Dikalov S, Fukai T, Harrison DG. Role of extracellular superoxide dismutase in hypertension. Hypertension. 2006;48:473–81. doi: 10.1161/01.HYP.0000235682.47673.ab. [DOI] [PubMed] [Google Scholar]
- Gorin Y, Kim NH, Feliers D, Bhandari B, Choudhury GG, Abboud HE. Angiotensin II activates Akt/protein kinase B by an arachidonic acid/redox-dependent pathway and independent of phosphoinositide 3-kinase. Faseb J. 2001;15:1909–20. doi: 10.1096/fj..01-0165com. [DOI] [PubMed] [Google Scholar]
- Gorin Y, Ricono JM, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Nox4 mediates angiotensin II-induced activation of Akt/protein kinase B in mesangial cells. Am J Physiol Renal Physiol. 2003;285:F219–29. doi: 10.1152/ajprenal.00414.2002. [DOI] [PubMed] [Google Scholar]
- Gorin Y, Ricono JM, Wagner B, Kim NH, Bhandari B, Choudhury GG, Abboud HE. Angiotensin II-induced ERK1/ERK2 activation and protein synthesis are redox-dependent in glomerular mesangial cells. Biochem J. 2004;381:231–9. doi: 10.1042/BJ20031614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlach A, Diebold I, Schini-Kerth VB, Berchner-Pfannschmidt U, Roth U, Brandes RP, Kietzmann T, Busse R. Thrombin activates the hypoxia-inducible factor-1 signaling pathway in vascular smooth muscle cells: Role of the p22(phox)-containing NADPH oxidase. Circ Res. 2001;89:47–54. doi: 10.1161/hh1301.092678. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–8. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Rittenhouse SE, Brock TA, Ekstein LS, Gimbrone MA, Jr, Alexander RW. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J Biol Chem. 1986;261:5901–6. [PubMed] [Google Scholar]
- Hannken T, Schroeder R, Stahl RA, Wolf G. Angiotensin II-mediated expression of p27Kip1 and induction of cellular hypertrophy in renal tubular cells depend on the generation of oxygen radicals. Kidney Int. 1998;54:1923–33. doi: 10.1046/j.1523-1755.1998.00212.x. [DOI] [PubMed] [Google Scholar]
- Haque MZ, Majid DS. Assessment of renal functional phenotype in mice lacking gp91PHOX subunit of NAD(P)H oxidase. Hypertension. 2004;43:335–40. doi: 10.1161/01.HYP.0000111137.15873.4a. [DOI] [PubMed] [Google Scholar]
- Hassanain HH, Gregg D, Marcelo ML, Zweier JL, Souza HP, Selvakumar B, Ma Q, Moustafa-Bayoumi M, Binkley PF, Flavahan NA, Morris M, Dong C, Goldschmidt-Clermont PJ. Hypertension caused by transgenic overexpression of Rac1. Antioxid Redox Signal. 2007;9:91–100. doi: 10.1089/ars.2007.9.91. [DOI] [PubMed] [Google Scholar]
- Heeneman S, Haendeler J, Saito Y, Ishida M, Berk BC. Angiotensin II induces transactivation of two different populations of the platelet-derived growth factor beta receptor. Key role for the p66 adaptor protein Shc. J Biol Chem. 2000;275:15926–32. doi: 10.1074/jbc.M909616199. [DOI] [PubMed] [Google Scholar]
- Heitzer T, Wenzel U, Hink U, Krollner D, Skatchkov M, Stahl RA, MacHarzina R, Brasen JH, Meinertz T, Munzel T. Increased NAD(P)H oxidase-mediated superoxide production in renovascular hypertension: evidence for an involvement of protein kinase C. Kidney Int. 1999;55:252–60. doi: 10.1046/j.1523-1755.1999.00229.x. [DOI] [PubMed] [Google Scholar]
- Higashi M, Shimokawa H, Hattori T, Hiroki J, Mukai Y, Morikawa K, Ichiki T, Takahashi S, Takeshita A. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res. 2003;93:767–75. doi: 10.1161/01.RES.0000096650.91688.28. [DOI] [PubMed] [Google Scholar]
- Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–83. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- Hilgers RH, Todd J, Jr, Webb RC. Increased PDZ-RhoGEF/RhoA/Rho kinase signaling in small mesenteric arteries of angiotensin II-induced hypertensive rats. J Hypertens. 2007;25:1687–97. doi: 10.1097/HJH.0b013e32816f778d. [DOI] [PubMed] [Google Scholar]
- Hingtgen SD, Tian X, Yang J, Dunlay SM, Peek AS, Wu Y, Sharma RV, Engelhardt JF, Davisson RL. Nox2-containing NADPH oxidase and Akt activation play a key role in angiotensin II-induced cardiomyocyte hypertrophy. Physiol Genomics. 2006;26:180–91. doi: 10.1152/physiolgenomics.00029.2005. [DOI] [PubMed] [Google Scholar]
- Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res. 2006;98:453–62. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- Hsieh CC, Papaconstantinou J. Thioredoxin-ASK1 complex levels regulate ROS-mediated p38 MAPK pathway activity in livers of aged and long-lived Snell dwarf mice. Faseb J. 2006;20:259–68. doi: 10.1096/fj.05-4376com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiki T, Tokunou T, Fukuyama K, Iino N, Masuda S, Takeshita A. Cyclic AMP response element-binding protein mediates reactive oxygen species-induced c-fos expression. Hypertension. 2003;42:177–83. doi: 10.1161/01.HYP.0000079791.26014.04. [DOI] [PubMed] [Google Scholar]
- Ishizaka N, Griendling KK, Lassegue B, Alexander RW. Angiotensin II type 1 receptor: relationship with caveolae and caveolin after initial agonist stimulation. Hypertension. 1998;32:459–66. doi: 10.1161/01.hyp.32.3.459. [DOI] [PubMed] [Google Scholar]
- Jaimes EA, Galceran JM, Raij L. Angiotensin II induces superoxide anion production by mesangial cells. Kidney Int. 1998;54:775–84. doi: 10.1046/j.1523-1755.1998.00068.x. [DOI] [PubMed] [Google Scholar]
- Jaimes EA, Tian RX, Pearse D, Raij L. Up-regulation of glomerular COX-2 by angiotensin II: role of reactive oxygen species. Kidney Int. 2005;68:2143–53. doi: 10.1111/j.1523-1755.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- Jaimes EA, Zhou MS, Pearse DD, Puzis L, Raij L. Upregulation of cortical COX-2 in salt-sensitive hypertension: role of angiotensin II and reactive oxygen species. Am J Physiol Renal Physiol. 2008;294:F385–92. doi: 10.1152/ajprenal.00302.2007. [DOI] [PubMed] [Google Scholar]
- Jin N, Rhoades RA. Activation of tyrosine kinases in H2O2-induced contraction in pulmonary artery. Am J Physiol. 1997;272:H2686–92. doi: 10.1152/ajpheart.1997.272.6.H2686. [DOI] [PubMed] [Google Scholar]
- Juarez JC, Manuia M, Burnett ME, Betancourt O, Boivin B, Shaw DE, Tonks NK, Mazar AP, Donate F. Superoxide dismutase 1 (SOD1) is essential for H2O2-mediated oxidation and inactivation of phosphatases in growth factor signaling. Proc Natl Acad Sci U S A. 2008;105:7147–52. doi: 10.1073/pnas.0709451105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karathanassis D, Stahelin RV, Bravo J, Perisic O, Pacold CM, Cho W, Williams RL. Binding of the PX domain of p47(phox) to phosphatidylinositol 3,4-bisphosphate and phosphatidic acid is masked by an intramolecular interaction. Embo J. 2002;21:5057–68. doi: 10.1093/emboj/cdf519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopkan L, Castillo A, Navar LG, Majid DS. Enhanced superoxide generation modulates renal function in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol. 2006;290:F80–6. doi: 10.1152/ajprenal.00090.2005. [DOI] [PubMed] [Google Scholar]
- Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, Rhee SG. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci U S A. 2004;101:16419–24. doi: 10.1073/pnas.0407396101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyaw M, Yoshizumi M, Tsuchiya K, Kirima K, Tamaki T. Antioxidants inhibit JNK and p38 MAPK activation but not ERK 1/2 activation by angiotensin II in rat aortic smooth muscle cells. Hypertens Res. 2001;24:251–61. doi: 10.1291/hypres.24.251. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–5. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–94. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- Laude K, Cai H, Fink B, Hoch N, Weber DS, McCann L, Kojda G, Fukai T, Schmidt HH, Dikalov S, Ramasamy S, Gamez G, Griendling KK, Harrison DG. Hemodynamic and biochemical adaptations to vascular smooth muscle overexpression of p22phox in mice. Am J Physiol Heart Circ Physiol. 2005;288:H7–12. doi: 10.1152/ajpheart.00637.2004. [DOI] [PubMed] [Google Scholar]
- Lavigne MC, Malech HL, Holland SM, Leto TL. Genetic demonstration of p47phox-dependent superoxide anion production in murine vascular smooth muscle cells. Circulation. 2001;104:79–84. doi: 10.1161/01.cir.104.1.79. [DOI] [PubMed] [Google Scholar]
- Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–42. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. Embo J. 2003;22:5501–10. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yang F, Yang XP, Jankowski M, Pagano PJ. NAD(P)H oxidase mediates angiotensin II-induced vascular macrophage infiltration and medial hypertrophy. Arterioscler Thromb Vasc Biol. 2003;23:776–82. doi: 10.1161/01.ATV.0000066684.37829.16. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gutterman DD. Oxidative stress and potassium channel function. Clin Exp Pharmacol Physiol. 2002;29:305–11. doi: 10.1046/j.1440-1681.2002.03649.x. [DOI] [PubMed] [Google Scholar]
- Lodha S, Dani D, Mehta R, Bhaskaran M, Reddy K, Ding G, Singhal PC. Angiotensin II-induced mesangial cell apoptosis: role of oxidative stress. Mol Med. 2002;8:830–40. [PMC free article] [PubMed] [Google Scholar]
- Lopez B, Salom MG, Arregui B, Valero F, Fenoy FJ. Role of superoxide in modulating the renal effects of angiotensin II. Hypertension. 2003;42:1150–6. doi: 10.1161/01.HYP.0000101968.09376.79. [DOI] [PubMed] [Google Scholar]
- Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–54. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manea A, Manea SA, Gafencu AV, Raicu M, Simionescu M. AP-1-dependent transcriptional regulation of NADPH oxidase in human aortic smooth muscle cells: role of p22phox subunit. Arterioscler Thromb Vasc Biol. 2008;28:878–85. doi: 10.1161/ATVBAHA.108.163592. [DOI] [PubMed] [Google Scholar]
- Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112:2677–85. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K, Ichijo H. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005;6:587–92. doi: 10.1038/ni1200. [DOI] [PubMed] [Google Scholar]
- Miller AA, Drummond GR, Sobey CG. Novel isoforms of NADPH-oxidase in cerebral vascular control. Pharmacol Ther. 2006;111:928–48. doi: 10.1016/j.pharmthera.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Miller FJ, Jr, Filali M, Huss GJ, Stanic B, Chamseddine A, Barna TJ, Lamb FS. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101:663–71. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res. 2003;92:e31–40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- Miyano K, Ueno N, Takeya R, Sumimoto H. Direct involvement of the small GTPase Rac in activation of the superoxide-producing NADPH oxidase Nox1. J Biol Chem. 2006;281:21857–68. doi: 10.1074/jbc.M513665200. [DOI] [PubMed] [Google Scholar]
- Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, Li M, Raggio J, Wellstein A, Chen Y, Welch WJ, Wilcox CS. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertension. 2006;47:238–44. doi: 10.1161/01.HYP.0000200023.02195.73. [DOI] [PubMed] [Google Scholar]
- Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res. 2002;90:E58–65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- Monteiro HP, Arai RJ, Travassos LR. Protein tyrosine phosphorylation and protein tyrosine nitration in redox signaling. Antioxid Redox Signal. 2008;10:843–89. doi: 10.1089/ars.2007.1853. [DOI] [PubMed] [Google Scholar]
- Mori T, Cowley AW., Jr Angiotensin II-NAD(P)H oxidase-stimulated superoxide modifies tubulovascular nitric oxide cross-talk in renal outer medulla. Hypertension. 2003;42:588–93. doi: 10.1161/01.HYP.0000091821.39824.09. [DOI] [PubMed] [Google Scholar]
- Mori T, O'Connor PM, Abe M, Cowley AW., Jr Enhanced superoxide production in renal outer medulla of Dahl salt-sensitive rats reduces nitric oxide tubular-vascular cross-talk. Hypertension. 2007;49:1336–41. doi: 10.1161/HYPERTENSIONAHA.106.085811. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Matsubara H, Mori Y, Murasawa S, Masaki H, Maruyama K, Tsutsumi Y, Shibasaki Y, Tanaka Y, Nakajima T, Oda K, Iwasaka T. Angiotensin II-induced transactivation of epidermal growth factor receptor regulates fibronectin and transforming growth factor-beta synthesis via transcriptional and posttranscriptional mechanisms. Circ Res. 1999;84:1073–84. doi: 10.1161/01.res.84.9.1073. [DOI] [PubMed] [Google Scholar]
- Mumbengegwi DR, Li Q, Li C, Bear CE, Engelhardt JF. Evidence for a superoxide permeability pathway in endosomal membranes. Mol Cell Biol. 2008;28:3700–12. doi: 10.1128/MCB.02038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Zhan Y, He H, Maynard E, Balschi JA, Oettgen P. Ets-1 is a critical transcriptional regulator of reactive oxygen species and p47(phox) gene expression in response to angiotensin II. Circ Res. 2007;101:985–94. doi: 10.1161/CIRCRESAHA.107.152439. [DOI] [PubMed] [Google Scholar]
- Nickenig G, Baudler S, Muller C, Werner C, Werner N, Welzel H, Strehlow K, Bohm M. Redox-sensitive vascular smooth muscle cell proliferation is mediated by GKLF and Id3 in vitro and in vivo. Faseb J. 2002;16:1077–86. doi: 10.1096/fj.01-0570com. [DOI] [PubMed] [Google Scholar]
- Nouri P, Gill P, Li M, Wilcox CS, Welch WJ. p22phox in the macula densa regulates single nephron GFR during angiotensin II infusion in rats. Am J Physiol Heart Circ Physiol. 2007;292:H1685–9. doi: 10.1152/ajpheart.00976.2006. [DOI] [PubMed] [Google Scholar]
- Ortego M, Bustos C, Hernandez-Presa MA, Tunon J, Diaz C, Hernandez G, Egido J. Atorvastatin reduces NF-kappaB activation and chemokine expression in vascular smooth muscle cells and mononuclear cells. Atherosclerosis. 1999;147:253–61. doi: 10.1016/s0021-9150(99)00193-8. [DOI] [PubMed] [Google Scholar]
- Pagano PJ, Clark JK, Cifuentes-Pagano ME, Clark SM, Callis GM, Quinn MT. Localization of a constitutively active, phagocyte-like NADPH oxidase in rabbit aortic adventitia: enhancement by angiotensin II. Proc Natl Acad Sci U S A. 1997;94:14483–8. doi: 10.1073/pnas.94.26.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse DD, Tian RX, Nigro J, Iorgulescu JB, Puzis L, Jaimes EA. Angiotensin II increases the expression of the transcription factor ETS-1 in mesangial cells. Am J Physiol Renal Physiol. 2008;294:F1094–100. doi: 10.1152/ajprenal.00458.2007. [DOI] [PubMed] [Google Scholar]
- Phillips MI. Functions of angiotensin in the central nervous system. Annu Rev Physiol. 1987;49:413–35. doi: 10.1146/annurev.ph.49.030187.002213. [DOI] [PubMed] [Google Scholar]
- Pinzar E, Wang T, Garrido MR, Xu W, Levy P, Bottari SP. Angiotensin II induces tyrosine nitration and activation of ERK1/2 in vascular smooth muscle cells. FEBS Lett. 2005;579:5100–4. doi: 10.1016/j.febslet.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–23. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo PC, Salido GM, Rosado JA, Pariente JA. Effect of hydrogen peroxide on Ca2+ mobilisation in human platelets through sulphydryl oxidation dependent and independent mechanisms. Biochem Pharmacol. 2004;67:491–502. doi: 10.1016/j.bcp.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Puyol M, Griera-Merino M, Perez-Rivero G, Diez-Marques ML, Ruiz-Torres MP, Rodriguez-Puyol D. Angiotensin II induces a rapid and transient increase of reactive oxygen species. Antioxid Redox Signal. 2002;4:869–75. doi: 10.1089/152308602762197407. [DOI] [PubMed] [Google Scholar]
- Salmeen A, Barford D. Functions and mechanisms of redox regulation of cysteine-based phosphatases. Antioxid Redox Signal. 2005;7:560–77. doi: 10.1089/ars.2005.7.560. [DOI] [PubMed] [Google Scholar]
- Sangrar W, Gao Y, Scott M, Truesdell P, Greer PA. Fer-mediated cortactin phosphorylation is associated with efficient fibroblast migration and is dependent on reactive oxygen species generation during integrin-mediated cell adhesion. Mol Cell Biol. 2007;27:6140–52. doi: 10.1128/MCB.01744-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M, Fukuda K, Sato T, Kawaguchi H, Suematsu M, Matsuda S, Koyasu S, Matsui H, Yamauchi-Takihara K, Harada M, Saito Y, Ogawa S. ERK and p38 MAPK, but not NF-kappaB, are critically involved in reactive oxygen species-mediated induction of IL-6 by angiotensin II in cardiac fibroblasts. Circ Res. 2001;89:661–9. doi: 10.1161/hh2001.098873. [DOI] [PubMed] [Google Scholar]
- Sato A, Sakuma I, Gutterman DD. Mechanism of dilation to reactive oxygen species in human coronary arterioles. Am J Physiol Heart Circ Physiol. 2003;285:H2345–54. doi: 10.1152/ajpheart.00458.2003. [DOI] [PubMed] [Google Scholar]
- Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci U S A. 2006;103:7432–7. doi: 10.1073/pnas.0510444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitsky PA, Finkel T. Redox regulation of Cdc25C. J Biol Chem. 2002;277:20535–40. doi: 10.1074/jbc.M201589200. [DOI] [PubMed] [Google Scholar]
- Schlaich MP, Parnell MM, Ahlers BA, Finch S, Marshall T, Zhang WZ, Kaye DM. Impaired L-arginine transport and endothelial function in hypertensive and genetically predisposed normotensive subjects. Circulation. 2004;110:3680–6. doi: 10.1161/01.CIR.0000149748.79945.52. [DOI] [PubMed] [Google Scholar]
- Seko T, Ito M, Kureishi Y, Okamoto R, Moriki N, Onishi K, Isaka N, Hartshorne DJ, Nakano T. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res. 2003;92:411–8. doi: 10.1161/01.RES.0000059987.90200.44. [DOI] [PubMed] [Google Scholar]
- Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–13. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- Shang LL, Sanyal S, Pfahnl AE, Jiao Z, Allen J, Liu H, Dudley SC., Jr NF-kappaB-dependent transcriptional regulation of the cardiac scn5a sodium channel by angiotensin II. Am J Physiol Cell Physiol. 2008;294:C372–9. doi: 10.1152/ajpcell.00186.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522(Pt 2):177–85. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. Febs J. 2008;275:3249–77. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- Sun C, Sellers KW, Sumners C, Raizada MK. NAD(P)H oxidase inhibition attenuates neuronal chronotropic actions of angiotensin II. Circ Res. 2005;96:659–66. doi: 10.1161/01.RES.0000161257.02571.4b. [DOI] [PubMed] [Google Scholar]
- Tabet F, Savoia C, Schiffrin EL, Touyz RM. Differential calcium regulation by hydrogen peroxide and superoxide in vascular smooth muscle cells from spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2004;44:200–8. doi: 10.1097/00005344-200408000-00009. [DOI] [PubMed] [Google Scholar]
- Tabet F, Schiffrin EL, Callera G, He Y, Yao G, Ostman A, Kappert K, Tonks NK, Touyz RM. Redox-Sensitive Signaling by Angiotensin II Involves Oxidative Inactivation and Blunted Phosphorylation of Protein Tyrosine Phosphatase SHP-2 in Vascular Smooth Muscle Cells From SHR. Circ Res. 2008 doi: 10.1161/CIRCRESAHA.108.178608. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Sudano I, Salvetti A. Defective L-arginine-nitric oxide pathway in offspring of essential hypertensive patients. Circulation. 1996;94:1298–303. doi: 10.1161/01.cir.94.6.1298. [DOI] [PubMed] [Google Scholar]
- Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem. 2003;278:25234–46. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- Taniyama Y, Ushio-Fukai M, Hitomi H, Rocic P, Kingsley MJ, Pfahnl C, Weber DS, Alexander RW, Griendling KK. Role of p38 MAPK and MAPKAPK-2 in angiotensin II-induced Akt activation in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2004;287:C494–9. doi: 10.1152/ajpcell.00439.2003. [DOI] [PubMed] [Google Scholar]
- Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–70. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Torrecillas G, Boyano-Adanez MC, Medina J, Parra T, Griera M, Lopez-Ongil S, Arilla E, Rodriguez-Puyol M, Rodriguez-Puyol D. The role of hydrogen peroxide in the contractile response to angiotensin II. Mol Pharmacol. 2001;59:104–12. doi: 10.1124/mol.59.1.104. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Cruzado M, Tabet F, Yao G, Salomon S, Schiffrin EL. Redox-dependent MAP kinase signaling by Ang II in vascular smooth muscle cells: role of receptor tyrosine kinase transactivation. Can J Physiol Pharmacol. 2003;81:159–67. doi: 10.1139/y02-164. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res. 2002;90:1205–13. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- Touyz RM, He G, Deng LY, Schiffrin EL. Role of extracellular signal-regulated kinases in angiotensin II-stimulated contraction of smooth muscle cells from human resistance arteries. Circulation. 1999;99:392–9. doi: 10.1161/01.cir.99.3.392. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Mercure C, He Y, Javeshghani D, Yao G, Callera GE, Yogi A, Lochard N, Reudelhuber TL. Angiotensin II-dependent chronic hypertension and cardiac hypertrophy are unaffected by gp91phox-containing NADPH oxidase. Hypertension. 2005;45:530–7. doi: 10.1161/01.HYP.0000158845.49943.5e. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. Ang II-stimulated superoxide production is mediated via phospholipase D in human vascular smooth muscle cells. Hypertension. 1999;34:976–82. doi: 10.1161/01.hyp.34.4.976. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Wu XH, He G, Park JB, Chen X, Vacher J, Rajapurohitam V, Schiffrin EL. Role of c-Src in the regulation of vascular contraction and Ca2+ signaling by angiotensin II in human vascular smooth muscle cells. J Hypertens. 2001;19:441–9. doi: 10.1097/00004872-200103000-00012. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Yao G, Quinn MT, Pagano PJ, Schiffrin EL. p47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: role in NAD(P)H oxidase regulation by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:512–8. doi: 10.1161/01.ATV.0000154141.66879.98. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Yao G, Schiffrin EL. c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:981–7. doi: 10.1161/01.ATV.0000069236.27911.68. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Yao G, Viel E, Amiri F, Schiffrin EL. Angiotensin II and endothelin-1 regulate MAP kinases through different redox-dependent mechanisms in human vascular smooth muscle cells. J Hypertens. 2004;22:1141–9. doi: 10.1097/00004872-200406000-00015. [DOI] [PubMed] [Google Scholar]
- Tsai CT, Wang DL, Chen WP, Hwang JJ, Hsieh CS, Hsu KL, Tseng CD, Lai LP, Tseng YZ, Chiang FT, Lin JL. Angiotensin II increases expression of alpha1C subunit of L-type calcium channel through a reactive oxygen species and cAMP response element-binding protein-dependent pathway in HL-1 myocytes. Circ Res. 2007;100:1476–85. doi: 10.1161/01.RES.0000268497.93085.e1. [DOI] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–4. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Geiszt M, Leto TL. Involvement of Rac1 in activation of multicomponent Nox1- and Nox3-based NADPH oxidases. Mol Cell Biol. 2006;26:2160–74. doi: 10.1128/MCB.26.6.2160-2174.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama T, Lekstrom K, Tsujibe S, Saito N, Leto TL. Subcellular localization and function of alternatively spliced Noxo1 isoforms. Free Radic Biol Med. 2007;42:180–90. doi: 10.1016/j.freeradbiomed.2006.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, Griendling KK. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem. 1999;274:22699–704. doi: 10.1074/jbc.274.32.22699. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Griendling KK, Becker PL, Hilenski L, Halleran S, Alexander RW. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2001;21:489–95. doi: 10.1161/01.atv.21.4.489. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Hilenski L, Santanam N, Becker PL, Ma Y, Griendling KK, Alexander RW. Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J Biol Chem. 2001;276:48269–75. doi: 10.1074/jbc.M105901200. [DOI] [PubMed] [Google Scholar]
- Viedt C, Fei J, Krieger-Brauer HI, Brandes RP, Teupser D, Kamimura M, Katus HA, Kreuzer J. Role of p22phox in angiotensin II and platelet-derived growth factor AA induced activator protein 1 activation in vascular smooth muscle cells. J Mol Med. 2004;82:31–8. doi: 10.1007/s00109-003-0500-5. [DOI] [PubMed] [Google Scholar]
- Viedt C, Soto U, Krieger-Brauer HI, Fei J, Elsing C, Kubler W, Kreuzer J. Differential activation of mitogen-activated protein kinases in smooth muscle cells by angiotensin II: involvement of p22phox and reactive oxygen species. Arterioscler Thromb Vasc Biol. 2000;20:940–8. doi: 10.1161/01.atv.20.4.940. [DOI] [PubMed] [Google Scholar]
- Wang D, Chen Y, Chabrashvili T, Aslam S, Borrego Conde LJ, Umans JG, Wilcox CS. Role of oxidative stress in endothelial dysfunction and enhanced responses to angiotensin II of afferent arterioles from rabbits infused with angiotensin II. J Am Soc Nephrol. 2003;14:2783–9. doi: 10.1097/01.asn.0000090747.59919.d2. [DOI] [PubMed] [Google Scholar]
- Wang G, Anrather J, Glass MJ, Tarsitano MJ, Zhou P, Frys KA, Pickel VM, Iadecola C. Nox2, Ca2+, and protein kinase C play a role in angiotensin II-induced free radical production in nucleus tractus solitarius. Hypertension. 2006;48:482–9. doi: 10.1161/01.HYP.0000236647.55200.07. [DOI] [PubMed] [Google Scholar]
- Wang HD, Xu S, Johns DG, Du Y, Quinn MT, Cayatte AJ, Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res. 2001;88:947–53. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- Weber DS, Rocic P, Mellis AM, Laude K, Lyle AN, Harrison DG, Griendling KK. Angiotensin II-induced hypertrophy is potentiated in mice overexpressing p22phox in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2005;288:H37–42. doi: 10.1152/ajpheart.00638.2004. [DOI] [PubMed] [Google Scholar]
- Wei EP, Kontos HA, Beckman JS. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am J Physiol. 1996;271:H1262–6. doi: 10.1152/ajpheart.1996.271.3.H1262. [DOI] [PubMed] [Google Scholar]
- Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS. Angiotensin-induced defects in renal oxygenation: role of oxidative stress. Am J Physiol Heart Circ Physiol. 2005;288:H22–8. doi: 10.1152/ajpheart.00626.2004. [DOI] [PubMed] [Google Scholar]
- Wenzel S, Taimor G, Piper HM, Schluter KD. Redox-sensitive intermediates mediate angiotensin II-induced p38 MAP kinase activation, AP-1 binding activity, and TGF-beta expression in adult ventricular cardiomyocytes. Faseb J. 2001;15:2291–3. doi: 10.1096/fj.00-0827fje. [DOI] [PubMed] [Google Scholar]
- Wilson SK. Role of oxygen-derived free radicals in acute angiotensin II--induced hypertensive vascular disease in the rat. Circ Res. 1990;66:722–34. doi: 10.1161/01.res.66.3.722. [DOI] [PubMed] [Google Scholar]
- Wingler K, Wunsch S, Kreutz R, Rothermund L, Paul M, Schmidt HH. Upregulation of the vascular NAD(P)H-oxidase isoforms Nox1 and Nox4 by the renin-angiotensin system in vitro and in vivo. Free Radic Biol Med. 2001;31:1456–64. doi: 10.1016/s0891-5849(01)00727-4. [DOI] [PubMed] [Google Scholar]
- Wu S, Gao J, Ohlemeyer C, Roos D, Niessen H, Kottgen E, Gessner R. Activation of AP-1 through reactive oxygen species by angiotensin II in rat cardiomyocytes. Free Radic Biol Med. 2005;39:1601–10. doi: 10.1016/j.freeradbiomed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Yang H, Shi M, VanRemmen H, Chen X, Vijg J, Richardson A, Guo Z. Reduction of pressor response to vasoconstrictor agents by overexpression of catalase in mice. Am J Hypertens. 2003;16:1–5. doi: 10.1016/s0895-7061(02)03086-8. [DOI] [PubMed] [Google Scholar]
- Yang Z, Venardos K, Jones E, Morris BJ, Chin-Dusting J, Kaye DM. Identification of a novel polymorphism in the 3′UTR of the L-arginine transporter gene SLC7A1: contribution to hypertension and endothelial dysfunction. Circulation. 2007;115:1269–74. doi: 10.1161/CIRCULATIONAHA.106.665836. [DOI] [PubMed] [Google Scholar]
- Yang ZW, Zheng T, Wang J, Zhang A, Altura BT, Altura BM. Hydrogen peroxide induces contraction and raises [Ca2+]i in canine cerebral arterial smooth muscle: participation of cellular signaling pathways. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:646–53. doi: 10.1007/s002109900128. [DOI] [PubMed] [Google Scholar]
- Yang ZW, Zheng T, Zhang A, Altura BT, Altura BM. Mechanisms of hydrogen peroxide-induced contraction of rat aorta. Eur J Pharmacol. 1998;344:169–81. doi: 10.1016/s0014-2999(97)01576-8. [DOI] [PubMed] [Google Scholar]
- Ying Z, Jin L, Palmer T, Webb RC. Angiotensin II up-regulates the leukemia-associated Rho guanine nucleotide exchange factor (RhoGEF), a regulator of G protein signaling domain-containing RhoGEF, in vascular smooth muscle cells. Mol Pharmacol. 2006;69:932–40. doi: 10.1124/mol.105.017830. [DOI] [PubMed] [Google Scholar]
- Yogi A, Mercure C, Touyz J, Callera GE, Montezano AC, Aranha AB, Tostes RC, Reudelhuber T, Touyz RM. Renal redox-sensitive signaling, but not blood pressure, is attenuated by Nox1 knockout in angiotensin II-dependent chronic hypertension. Hypertension. 2008;51:500–6. doi: 10.1161/HYPERTENSIONAHA.107.103192. [DOI] [PubMed] [Google Scholar]
- Yoshizumi M, Tsuchiya K, Kirima K, Kyaw M, Suzaki Y, Tamaki T. Quercetin inhibits Shc- and phosphatidylinositol 3-kinase-mediated c-Jun N-terminal kinase activation by angiotensin II in cultured rat aortic smooth muscle cells. Mol Pharmacol. 2001;60:656–65. [PubMed] [Google Scholar]
- Yu HY, Inoguchi T, Nakayama M, Tsubouchi H, Sato N, Sonoda N, Sasaki S, Kobayashi K, Nawata H. Statin attenuates high glucose-induced and angiotensin II-induced MAP kinase activity through inhibition of NAD(P)H oxidase activity in cultured mesangial cells. Med Chem. 2005;1:461–6. doi: 10.2174/1573406054864052. [DOI] [PubMed] [Google Scholar]
- Zafari AM, Ushio-Fukai M, Minieri CA, Akers M, Lassegue B, Griendling KK. Arachidonic acid metabolites mediate angiotensin II-induced NADH/NADPH oxidase activity and hypertrophy in vascular smooth muscle cells. Antioxid Redox Signal. 1999;1:167–79. doi: 10.1089/ars.1999.1.2-167. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Brown C, Maynard E, Anshelevich A, Ni W, Ho IC, Oettgen P. Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J Clin Invest. 2005;115:2508–16. doi: 10.1172/JCI24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Kavurma MM, Lai A, Khachigian LM. Ets-1 protects vascular smooth muscle cells from undergoing apoptosis by activating p21WAF1/Cip1: ETS-1 regulates basal and and inducible p21WAF1/Cip: ETS-1 regulates basal and inducible p21WAF1/Cip1 transcription via distinct cis-acting elements in the p21WAF/Cip1 promoter. J Biol Chem. 2003;278:27903–9. doi: 10.1074/jbc.M304328200. [DOI] [PubMed] [Google Scholar]
- Zhao W, Chen SS, Chen Y, Ahokas RA, Sun Y. Kidney Fibrosis in Hypertensive Rats: Role of Oxidative Stress. Am J Nephrol. 2008;28:548–554. doi: 10.1159/000115289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman MC, Dunlay RP, Lazartigues E, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Requirement for Rac1-dependent NADPH oxidase in the cardiovascular and dipsogenic actions of angiotensin II in the brain. Circ Res. 2004;95:532–9. doi: 10.1161/01.RES.0000139957.22530.b9. [DOI] [PubMed] [Google Scholar]
- Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91:1038–45. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]
- Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–6. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- Zimmerman MC, Sharma RV, Davisson RL. Superoxide mediates angiotensin II-induced influx of extracellular calcium in neural cells. Hypertension. 2005;45:717–23. doi: 10.1161/01.HYP.0000153463.22621.5e. [DOI] [PubMed] [Google Scholar]
- Zuo L, Ushio-Fukai M, Ikeda S, Hilenski L, Patrushev N, Alexander RW. Caveolin-1 is essential for activation of Rac1 and NAD(P)H oxidase after angiotensin II type 1 receptor stimulation in vascular smooth muscle cells: role in redox signaling and vascular hypertrophy. Arterioscler Thromb Vasc Biol. 2005;25:1824–30. doi: 10.1161/01.ATV.0000175295.09607.18. [DOI] [PubMed] [Google Scholar]