Abstract
Background
Amygdala-orbitofrontal cortical (OFC) functional connectivity (FC) to emotional stimuli and relationships with white matter remain little examined in bipolar disorder individuals (BD).
Methods
Thirty-one BD (type I; n = 17 remitted; n = 14 depressed) and 24 age- and gender-ratio-matched healthy individuals (HC) viewed neutral, mild, and intense happy or sad emotional faces in two experiments. The FC was computed as linear and nonlinear dependence measures between amygdala and OFC time series. Effects of group, laterality, and emotion intensity upon amygdala-OFC FC and amygdala-OFC FC white matter fractional anisotropy (FA) relationships were examined.
Results
The BD versus HC showed significantly greater right amygdala-OFC FC (p ≤ .001) in the sad experiment and significantly reduced bilateral amygdala-OFC FC (p = .007) in the happy experiment. Depressed but not remitted female BD versus female HC showed significantly greater left amygdala-OFC FC (p = .001) to all faces in the sad experiment and reduced bilateral amygdala-OFC FC to intense happy faces (p = .01). There was a significant nonlinear relationship (p = .001) between left amygdala-OFC FC to sad faces and FA in HC. In BD, antidepressants were associated with significantly reduced left amygdala-OFC FC to mild sad faces (p = .001).
Conclusions
In BD, abnormally elevated right amygdala-OFC FC to sad stimuli might represent a trait vulnerability for depression, whereas abnormally elevated left amygdala-OFC FC to sad stimuli and abnormally reduced amygdala-OFC FC to intense happy stimuli might represent a depression state marker. Abnormal FC measures might normalize with antidepressant medications in BD. Nonlinear amygdala-OFC FC–FA relationships in BD and HC require further study.
Keywords: Amygdala, bipolar disorder, emotional processing, functional connectivity, neuroimaging, OFC
Bipolar disorder is one of the most debilitating illnesses (1), characterized by severe emotion dysregulation that might persist even during remission (2–4). Understanding patho-physiological mechanisms of emotion dysregulation is a first step toward identifying biological targets for future treatment development for bipolar disorder. Neuroimaging studies of bipolar disorder individuals (BD) therefore examined functional abnormalities in amygdala and orbitofrontal cortex (OFC), highly interconnected regions that are well-known from animal and human lesion (5–14), and human neuroimaging (15–18) studies to support emotion processing and emotion regulation. These studies reported abnormal activity in amygdala and OFC in BD (19) to different emotional stimuli and during cognitive control tasks (19–21). More recently, studies examined white matter (WM) in emotion regulation neural circuitry in BD (22–26), with diffusion tensor imaging (DTI) and fractional anisotropy (FA), an index of the ratio of diffusional anisotropy in longitudinal versus radial directions in WM tracts. We demonstrated in BD versus healthy control participants (HC) reduced FA in one cluster in the region of right uncinate fasciculus (UF), a major tract connecting anterior temporal cortex—including the amygdala—with OFC and greater FA in three clusters of the region of left UF (27). Our findings parallel other studies reporting abnormalities in the UF in BD, including abnormal number of reconstructed fibers (22,25) or abnormal UF FA (23,25,26).
A main limitation of these studies in BD is that they did not examine how these key neural regions implicated in emotion processing and regulation are functionally integrated during emotion processing. Functional integration within a distributed network can be characterized in terms of “functional connectivity” (FC), referring to correlations over time between activities in different neural regions (28). This FC approach can be used to examine the functional integrity of neural systems supporting emotion processing and regulation to better understand biological mechanisms of emotion dysregulation in BD. One study reported decreased left amygdala-right OFC FC in manic BD relative to HC during fearful and angry face-matching and labeling (29). With a different connectivity methodology—effective connectivity, which refers to the impact that activity in one region exerts over that in another and can be used to estimate forward versus backward connectivity between regions—we previously showed abnormally reduced left OFC (Brodmann area 11)-left amygdala and right amygdala-right OFC effective connectivity during happy face emotion labeling in depressed BD (30). It remains unknown, however, whether changes in mood state impact amygdala-OFC FC to emotional stimuli in BD. Only one study examined FC–FA relationships in neural circuitry supporting emotion regulation in BD (31) and demonstrated reductions in FA and FC between amygdala and pericingulate gyrus during happy and fearful face processing in BD versus HC (31).
A well-known hemispheric lateralization of emotion processing theory emphasizes a left hemisphere specialization for approach-related (e.g., positive) and a right hemisphere specialization for withdrawal-related (e.g., negative) emotions (32). The left–right difference in abnormal amygdala-OFC WM that we previously demonstrated in BD might underlie abnormal amyg-dala-OFC FC to positive and negative emotional stimuli, but this remains unexamined. In the present study, we therefore first aimed to examine amygdala-OFC FC to positive and negative emotional faces in BD and HC. Our previous effective connectivity data in depressed BD allowed us to hypothesize that BD versus HC would show abnormally reduced bilateral OFC-amygdala FC to positive emotional faces but did not allow us to be more specific about the nature of left–right amygdala-OFC FC abnormalities to negative emotional faces in BD. We secondly aimed to examine whether mood state impacted left and right amygdala-OFC-amygdala FC in BD, by recruiting subgroups of remitted and depressed BD. Our previous findings (33) allowed us to hypothesize that depressed BD would show significantly reduced amygdala-OFC FC versus HC to happy faces in both hemispheres but did not allow us to have a specific hypothesis regarding the relative magnitude of amygdala-OFC FC abnormalities in depressed versus remitted BD. In exploratory analyses, we aimed to examine left and right amygdala-OFC FC–FA relationships in BD and HC to positive and negative emotional stimuli.
To examine these aims and hypotheses, we used a well-validated facial emotion labeling task that includes facial expressions displaying the range of emotion from neutral to mild and intense happiness or sadness displayed in everyday social interactions (30,34–36). We computed amygdala-OFC FC in each hemisphere with a methodology developed in our laboratory (37–39).
Methods and Materials
Participants
Fifty-six participants were recruited through local advertising and the Western Psychiatric Institute and Clinic, Mood Disorder Treatment and Research Program, and signed informed consent to participate in the study. Functional magnetic resonance imaging (fMRI) data were collected in 31 adult BD (type I; DSM-IV criteria) (40), with the Structured Clinical Interview for DSM-IV, Research Version, SCID-P (41), and in 24 of 25 HC (no previous psychiatric history, SCID-P criteria, or psychiatric history in first-degree relatives), alongside DTI data (27) in the same neuroimaging session. The fMRI data were not collected in one HC, because of scanner problems that day.
Fourteen BD were recruited in depressed episode on the basis of SCID-P criteria (at least 2 weeks of depressed mood); 17 euthymic BD were recruited in full remission on the basis of SCID-P criteria and had been euthymic for at least 2 months before the scanning session. All BD had a Young Mania Rating Scale (42) score ≤10; remitted and depressed BD had a 25-item Hamilton Rating Scale for Depression–25 (43) score ≤7 and ≥13, respectively. All BD had at least two episodes of depression or mania in the last 4 years. Ten BD had a lifetime history of alcohol/substance abuse/dependence. Remitted and depressed BD did not differ significantly in age, age of illness onset, illness duration, and history of alcohol/substance abuse but did differ in depression severity, gender ratio, and proportion of individuals taking anxiolytics but not other medications: there were more women than men, and more individuals taking anxiolytics in depressed than remitted BD (Table 1).
Table 1.
Demographic and Clinical Variables
| Group (n) | Mean [SD] | Statistics | df | p (2 tailed) | |
|---|---|---|---|---|---|
| Age at Scan (yr) | BD (31) | 35.9 [8.8] | t = 2.5 | 53 | .014a |
| HC (24) | 29.5 [9.6] | ||||
| dBD (14) | 38.0 [9.3] | t = 1.2 | 29 | .239 | |
| rBD (17) | 34.2 [8.4] | ||||
| Gender (M/F) | BD (11/20) | χ2 = 0.6 | 1 | .437 | |
| HC (11/13) | |||||
| dBD (2/12) | χ2 = 5.0 | 1 | .025 | ||
| rBD (9/8) | |||||
| Task Performance to SAD Emotional Labeling Taskb | BD (30) | 38.7 [8.9] | U = 293.5 | .355 | |
| HC (24) | 41.2 [9.1] | ||||
| dBD (14) | 36.8 [10.2] | U = 87.5 | .313 | ||
| rBD (17) | 40.4 [7.6] | ||||
| Task Performance to HAPPY Emotional Labeling Taskb | BD (31)c | 48.0 [7.9] | U = 209.0 | .019a | |
| HC (24) | 52.5 [5.2] | ||||
| dBD (14)c | 44.7 [9.6] | U = 60.0 | .088 | ||
| rBD (17)c | 50.9 [4.7] | ||||
| NART | BD (31) | 110.3 [8.30] | t = −1.5 | 52d | .128 |
| HC (23)d | 114.0 [9.2] | ||||
| Age of Illness Onset (y) | dBD (14) | 21.7 [7.0] | t = −1.2 | 29 | .247 |
| rBD (17) | 25.4 [10.8] | ||||
| Illness Duration (y) | dBD (14) | 11.8 [6.3] | t = −1.61 | 29 | .875 |
| rBD (17) | 12.3 [9.8] | ||||
| HRSD-25 | dBD (14) | 2.1 [2.6] | t = −5.8 | 29 | <.001 |
| rBD (17) | 15.2 [8.1] | ||||
| Mood Stabilizer (ON/OFF) | dBD (12/2) | χ2 = 2.7 | 1 | .101 | |
| rBD (10/7) | |||||
| Lithium Carbonate (ON/OFF) | dBD (4/9)e | χ2 = 1.3 | 2e | .516 | |
| rBD (6/11) | |||||
| Anti Psychotic Medications (ON/OFF) | dBD (9/5) | χ2 = 0.4 | 1 | .524 | |
| rBD (9/8) | |||||
| Anti Depressants (ON/OFF) | dBD (7/7) | χ2 = 0.3 | 1 | .87 | |
| rBD (8/9) | |||||
| Benzodiazepines (ON/OFF) | dBD (7/7) | χ2 = 3.7 | 1 | .055 | |
| rBD (3/14) | |||||
| Lifetime History of Alcohol/Substance | dBD (4/8)f | χ2 = 0.7 | 2f | .715 | |
| Abuse/Dependence (ON/OFF) | rBD (6/10)f |
All BD had a Young Mania Rating Scale (YMRS) score < 10; rBD and dBD had a 25-item Hamilton Rating Scale for Depression (HDRS-25) score <7 and >13, respectively, and diagnosis based on Structural Clinical Interview for DSM (SCID-P) criteria. Significance threshold was p ≤ .05 (2-tailed). Statistics refer to between group differences for either all BD vs. HC, or dBD vs. rBD.
BD, bipolar disorder individuals; dBD, depressed bipolar disorder individuals; rBD, remitted bipolar disorder individuals; HC, healthy control individuals.
BD were less accurate than HC or happy emotion labeling, resulting from greater mislabeling of intense happy faces as neutral in BD than HC (U = 244.0, p = .069).
Task performance was better in happy than sad experiment in BD (sad-happy: U = −4.0, p < .001) and HC (sad-happy: U = −4.1, p < .001).
Task performance was missing in 3 BD (2 dBD and 1 rBD) in the happy experiment.
The NART value was missing for one HC. HDRS-25 = Hamilton Depressive Rating Scale-25 items; missing information in one rBD (25 y male).
Accurate information was missing in one dBD.
Accurate information was missing in 3 BD (2 dBD, 1 rBD) regarding lifetime alcohol/substance abuse.
The HC and BD were gender-ratio- and premorbid IQ-matched. The HC were younger than BD. Age was therefore included as a covariate in between group analyses. All participants were right-handed (Annett criteria) (44).
Exclusion criteria for all participants included history of head injury (medical records and self-report), cardiovascular accident, epilepsy, dementia, neurodevelopmental/neurodegenerative disorder, loss of consciousness >10 min, systemic medical illness, cognitive impairment (score <24 Mini-Mental State Examination) (45), premorbid IQ estimate <85 (National Adult Reading Test) (46), Axis II borderline personality disorder, and exclusion criteria for MRI studies (presence/questionable history of body metallic objects, positive pregnancy test for women, and claustrophobia). Lifetime history and/or current alcohol and illicit substance abuse (determined by saliva and urine screen, respectively) were additional exclusion criteria for HC. The study was approved by the University of Pittsburgh Institutional Review Board.
Paradigm: Explicit Emotion Labeling Tasks
Participants completed two previously employed (35,36) 6-min fast event-related fMRI experiments displaying: 1) 20 intense happy, 20 mild happy, and 20 neutral facial expressions; and 2) 20 intense sad, 20 mild sad, and 20 neutral facial expressions (Methods and Materials in Supplement 1). The two experiments were presented in a counterbalanced order across groups and separated in time by structural data acquisition. Participants judged whether each face was emotional or neutral by pressing the index or middle finger in a touch-sensitive glove. Between-group difference in number of correct answers was performed with a Mann–Whitney U test. In three BD, data were unrecorded during the happy experiment because of technical difficulties. The experimenter verified that these participants performed the task by observing their responses made during task performance on a computer screen in the scanner control room. The FC data from these three BD were not included in analyses of relationships between FC and task performance in the happy experiment.
Data Acquisition
The fMRI and DTI data were acquired during the same scanning session with parameters previously employed (27,33, 47) (Methods and Materials in Supplement 1).
Data Analyses
The fMRI data were analyzed with SPM5 (Statistical Parametric Mapping; http://www.fil.ion.ucl.ac.uk/spm/software/spm5) software. The DTI data were analyzed as described previously (27) (Methods and Materials and Table S1 in Supplement 1).
We chose for FC analyses in each hemisphere two key regions connected by the UF that we included in our previous connectivity study in depressed BD (33): amygdala and OFC (Brodmann area 11). These regions were defined with the Wake Forest University PickAtlas software, the SPM atlas toolbox (http://fmri.wfubmc.edu/), on the basis of the Talairach Daemon database.
For FC analyses, eigen variates (weighted mean where atypical voxels are down-weighted) were extracted from the first-level maps of parameter estimates in each participant’s time series in both experiments in each voxel/region by setting p = .99 significance level, with volume of interest, in SPM5, and entered into FC analyses.
FC Analyses
We computed FC as the normalized partial mutual information, a measure of linear and nonlinear dependence in two time series (OFC and amygdala) in each hemisphere (37,38) (Figure S1 in Supplement 1). This methodology, employed previously (48), was implemented in MATLAB via a Functional Connectivity toolbox developed in our laboratory (39). Mutual information is a statistical measure of both linear and nonlinear dependence. It quantifies the shared information between two time series. This approach generally assumes stationarity of the time series. In the case of event-related designs, where the recurring effect of a stimulus always exists on two regions, it might overestimate true connectivity when calculating mutual information between the two regions. In this case, stimulus effects upon time series can be explicitly accounted for before computing connectivity via partial mutual information by entering a stimulus-related response as a covariate. We can assume that the effects of the stimulus are constant across trials. We therefore constructed a covariate waveform for each stimulus type (emotion intensity) in each experiment by convolving a canonical response with a δ function at the stimulus frequency.
BD Versus HC
To test our first main hypothesis comparing BD and HC for left and right amygdala-OFC FC, we examined main effects of group (BD–HC), FC laterality (left–right), emotion intensity (intense-mild-neutral), and interactions between these factors upon amygdala-OFC FC for each experiment, with a general linear model analysis of covariance, covarying for age.
Remitted and Depressed BD Versus HC
To examine our second aim, comparing each depressed and remitted BD subgroup with HC on left and right amygdala-OFC FC, we examined women only, because there were different gender ratios in each BD subgroup, with most BD women: 8 remitted women (7 for the sad experiment), and 12 depressed women. In view of the smaller numbers of women in each BD subgroup, we focused examination on the extent to which depressed and/or remitted BD showed abnormalities (vs. HC) on abnormal amygdala-OFC FC measures shown by all BD.
In post hoc analyses, examining significant main effects and interactions involving group, we used pairwise comparisons for estimated marginal means for both between- and within-subject factors, with p values corrected to control for multiple tests.
FC–FA Relationships
We applied “Best Fitting Curves” in SPSS (SPSS, Chicago, Illinois) to determine which linear or nonlinear curves were the best fit curves describing relationships between: 1) left amygdala-OFC FC in happy and sad experiments for all faces and FA in the three clusters in the region of left UF showing abnormal FA in BD relative to HC (27); and 2) right amygdala-OFC FC in happy and sad experiments for all faces and FA of the cluster in the region of right UF showing abnormal FA in BD relative to HC (27). We used a corrected statistical threshold of p < .05/8 = .006 to control for the eight multiple tests in each group between left amygdala-OFC FC to all faces in both experiments and the three clusters in the region of left UF (n = 6 tests/group) and between amygdala-OFC FC to all faces in both experiments and the cluster in the region of right UF (n = 2 tests/group).
Relationships Between Clinical Variables and FC
We examined relationships between age, age of illness onset, illness duration, depression severity, emotion labeling task performance, gender, the four individual psychotropic medication classes taken, and presence versus absence of lifetime comorbid substance abuse/dependence and FC in BD. We also examined relationships between age, task performance, and gender and FC in HC. We used Pearson correlations for the first six and Student t tests for the remaining six tests, with a statistical threshold of .05/12 = .004, to control for multiple tests.
Results
Emotion Labeling Accuracy
There was no significant difference between BD and HC in sad emotion labeling. The BD were less accurate than HC on happy emotion labeling (U = 209.0; p = .019), resulting from a trend for BD more than HC to mislabel intense happy faces as neutral (U = 244.0, p = .069). The HC and BD were more accurate on happy than sad emotion labeling (sad-happy: U = −4.1, p < .001 and U = −4.0, p < .001, respectively) there was no significant difference between depressed and remitted BD on emotion labeling for either experiment (Table 1).
Neuroimaging Data
Extraction of mean blood oxygen level dependent signal in a priori regions (amygdala and OFC) indicated that these regions were activated to stimuli (vs. baseline) in both experiments in BD and HC (Figure 1).
Figure 1.
Mean blood oxygen level dependent (BOLD) signal change in orbitomedial prefrontal cortex (red) and amygdala (green) a priori regions of interest in bipolar disorder (BD) and healthy control individuals (HC) to all faces (neutral, mild, and intense emotional) in each experiment shown, three orthogonal views (top-down: coronal, sagittal, and axial). Left panel: BOLD signal change in BD and HC in the happy experiment. Right panel: BOLD signal change in BD and HC in the sad experiment. A priori orbitofrontal cortex (OFC) and amygdala regions were defined with the Wake Forest University PickAtlas software in the SPM atlas toolbox, on the basis of the Talairach Daemon database. For OFC regions of interests, the voxelwise threshold was p ≤ .001. At a less stringent threshold (voxelwise p ≤ .05), BOLD signal was observed in bilateral amygdala in BD and HC in each experiment.
FC Analyses
BD Versus HC: Sad Experiment
Data were excluded for one remitted BD during the sad experiment, because of inability to tolerate the scanner. The FC data were therefore analyzed for 30 BD and 24 HC. There were significant main effects of group [F(1,51) = 13.55; p = .001], emotion intensity [F(2,50) = 4.61; p = .015], and a group × laterality interaction [F(1,51) = 6.47; p = .014] for amygdala-OFC FC. We focused post hoc tests upon examination of the main effect of group and group × laterality interaction to test our first hypothesis, with a Bonferroni-corrected statistical threshold of p = .05/4 = .013 (four post hoc tests comparing amygdala-OFC FC between two groups and two hemispheres). The BD showed significantly greater right amygdala-OFC FC overall emotion intensities than HC [F (1,51) = 17.98, p < .001] and greater left amygdala-OFC FC, which just missed our conservative significance threshold [F (1,51) = 6.28, p = .015]. The HC but not BD showed greater left than right amygdala-OFC FC overall emotion intensities, which also missed our conservative significance threshold [BD:F(1,28) = 2.82, p = .104; HC:F(1,22) = 4.59, p = .043] (Figure 2, Tables 2A and 3 for estimated marginal means for FC values in BD and HC).
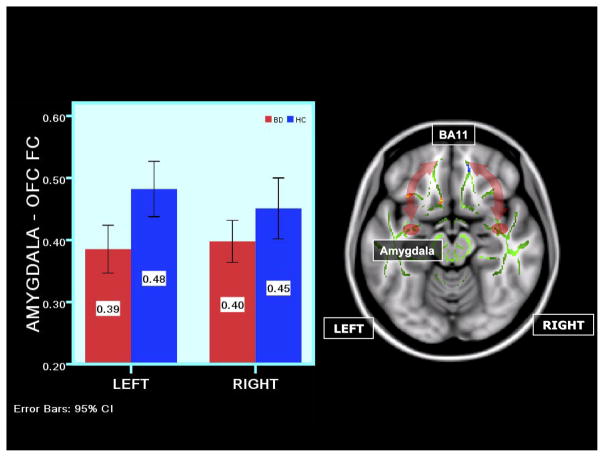
Figure 2.
Right panel: axial views showing a schematic representation of functional connectivity (FC) abnormalities in BD versus HC in the sad experiment to all faces. The arrows symbolize FC between amygdala and OFC. The template on which the FC data are depicted is the standard Montreal Neurological Institute 152 1-mm brain template showing the fractional anisotropy (FA) white matter skeleton used for tract-based spatial statistics analysis of diffusion tensor imaging data (shown in light-green) and between-group differences in FA in bilateral uncinate fasciculus (UF): decreased FA in a cluster in blue in the right UF, and increased FA in clusters in red-yellow in the left UF. The red ovoid is a representation of the amygdala region of interest. Left panel: a bar graph depicting significantly greater right amygdala-OFC FC to all faces in BD versus HC in the sad experiment (p < .001). Vertical axis: estimated marginal means of FC values. The error bars show SDs of FC in each group. BA, Brodmann area; other abbreviations as in Figure 1.
Table 2.
Amygdala-OFC Functional Connectivity (Estimated Marginal Mean and SD) in BD and HC
| FC |
||||
|---|---|---|---|---|
| Group | [n] | Mean | [SD] | |
| Sad Experiment | ||||
| Right | ||||
| Intense | BD | [30] | .36 | .02 |
| HC | [24] | .25 | .02 | |
| Mild | BD | [30] | .36 | .02 |
| HC | [24] | .24 | .02 | |
| Neutral | BD | [30] | .47 | .02 |
| HC | [24] | .36 | .03 | |
| Left | ||||
| Intense | BD | [30] | .33 | .02 |
| HC | [24] | .26 | .02 | |
| Mild | BD | [30] | .35 | .02 |
| HC | [24] | .28 | .03 | |
| Neutral | BD | [30] | .44 | .02 |
| HC | [24] | .40 | .02 | |
| Happy Experiment | ||||
| Right | ||||
| Intense | BD | [31] | .40 | .02 |
| HC | [24] | .45 | .02 | |
| Mild | BD | [31] | .35 | .02 |
| HC | [24] | .34 | .03 | |
| Neutral | BD | [31] | .38 | .02 |
| HC | [24] | .37 | .03 | |
| Left | ||||
| Intense | BD | [31] | .39 | .02 |
| HC | [24] | .48 | .02 | |
| Mild | BD | [31] | .33 | .02 |
| HC | [24] | .36 | .03 | |
| Neutral | BD | [31] | .38 | .02 |
| HC | [24] | .37 | .03 | |
| In Female Depressed, Remitted BD, and HC | ||||
| Sad Experiment | ||||
| Right | ||||
| Intense | dBD | [12] | .36 | .03 |
| rBD | [7] | .30 | .03 | |
| HC | [13] | .24 | .01 | |
| Mild | dBD | [12] | .38 | .04 |
| rBD | [7] | .32 | .03 | |
| HC | [13] | .22 | .01 | |
| Neutral | dBD | [12] | .51 | .02 |
| rBD | [7] | .45 | .04 | |
| HC | [13] | .38 | .02 | |
| Left | ||||
| Intense | dBD | [12] | .35 | .03 |
| rBD | [7] | .28 | .02 | |
| HC | [13] | .27 | .02 | |
| Mild | dBD | [12] | .37 | .03 |
| rBD | [7] | .28 | .02 | |
| HC | [13] | .25 | .02 | |
| Neutral | dBD | [12] | .44 | .02 |
| rBD | [7] | .47 | .03 | |
| HC | [13] | .38 | .02 | |
| Happy Experiment | ||||
| Right | ||||
| Intense | dBD | [12] | .40 | .02 |
| rBD | [8] | .42 | .02 | |
| HC | [13] | .46 | .02 | |
| Mild | dBD | [12] | .37 | .03 |
| rBD | [8] | .30 | .03 | |
| HC | [13] | .30 | .03 | |
| Neutral | dBD | [12] | .38 | .02 |
| rBD | [8] | .35 | .02 | |
| HC | [13] | .34 | .02 | |
| Left | ||||
| Intense | dBd | [12] | .40 | .02 |
| rBD | [8] | .42 | .04 | |
| HC | [13] | .49 | .02 | |
| Mild | dBD | [12] | .32 | .03 |
| rBD | [8] | .30 | .02 | |
| HC | [13] | .31 | .02 | |
| Neutral | dBD | [12] | .40 | .03 |
| rBD | [8] | .31 | .03 | |
| HC | [13] | .34 | .03 | |
BD, bipolar disorder individuals (evaluated at age = 36.0); HC, healthy central individuals (evaluated at age = 29.5); FC, functional connectivity; dBD, female depressed bipolar disorder individuals (mean age = 6.4); rBD, female remitted bipolar disorder individuals (mean age = 33.5); HC, female healthy control individuals (mean age = 28.8).
Table 3.
Amygdala-OFC Functional Connectivity in the Sad Experiment: Analysis of Covariance and Post Hoc Analyses Comparing BD and HC
| Main Effects | |||||
|---|---|---|---|---|---|
| Group |
Laterality |
Intensity of Emotion |
|||
| F [1,51] | p | F [1,51] | p | F [2,50] | p |
| 13.55 | .001a | .20 | .654 | 4.61 | .015a |
| Interactions | |||||||
|---|---|---|---|---|---|---|---|
| Group × Laterality |
Group × Intensity |
Laterality × Intensity |
Group × Laterality ×Intensity |
||||
| F [1,51] | p | F[2,50] | p | F [2,50] | p | F [2,50] | p |
| 6.47 | .014a | .08 | .921 | .43 | .653 | .223 | .801 |
| Post Hoc Analyses | |||
|---|---|---|---|
| BD |
HC |
||
| Left vs. Right |
Left vs. Right |
||
| F [1,28] | p | F [1,22] | p |
| 2.82 | .104 | 4.59 | 043b,c |
| Left |
Right |
||
| BD vs. HC |
BD vs. HC |
||
| F (1,51) | p | F [1,51] | p |
| 6.28 | .015e,f | 17.98 | <.001d,g |
| dBD vs. HC |
dBD vs. HC |
||
| F [1,22] | p | F [1,22] | p |
| 16.13 | .001d,h | 20.37 | <.001d,i |
| rBD vs. HC |
rBD vs. HC |
||
| F [1,17] | p | F [1,17] | p |
| 3.26 | .090 | 16.57 | .001d,i |
Age, covariate; BD, bipolar disorder individuals; dBD, subgroup of female depressed bipolar individuals; rBD, subgroup of female remitted bipolar disorder individuals; HC, subgroup of female healthy control individuals.
In BD versus HC: we used a Bonferroni-corrected statistical threshold of p = .05/4 ≤ .013 (four post-hoc tests comparing amygdala orbital frontal cortex (OFC) FC between two groups and two hemispheres.
Left amygdala-OFC FC >right amygdala-OFC FC in HC: right = .29 [.02], left = .32 [.02].
Trend range .013 < p ≤ .05.
In female dBD/rBD versus female HC: we used a Bonferroni-corrected statistical threshold of of p = .0134/4 ≤ .003 to control for the additional four post hoc tests comparing depressed BD versus HC and remitted BD versus HC on left and right amygdala-OFC FC over all intensities.
Trend range: .003 < p ≤ .05.
BD > HC: estimated marginal means for left amygdala-OFC FC [SD]: BD = .38 [.02]; HC = .31 [.02].
BD > HC: estimated marginal means for right amygdala-OFC FC [SD]: BD = .40 [.02]; HC = .28 [.02].
dBD > HC: estimated marginal means for right amygdala-OFC FC [SD]: dBD = .42 [.03]; HC = .28 [.01].
dBD > HC: estimated marginal means for left amygdala-OFC FC [SD]: dBD = .39 [.02]; HC = .30 [.01] and rBD = .35 [.02]; HC = .30 [.01]; rBD > HC: estimated marginal means for left amygdala-OFC FC [SD]: rBD = .35 [.02]; HC = .30 [.01] and HC = .30 [.01]; dBD vs rBD: trend greater left amygdala-OFC FC to mild sad faces in dBD relative to rBD [F [1,16] = 6.02, p = .026)].
BC Versus HC: Happy Experiment
There was a significant main effect of emotion intensity and a group × emotion intensity interaction upon amygdala-OFC FC [F(2,51) = 3.86, p = .028 and F(1,52) = 4.48, p = .016, respectively]. We focused post hoc tests upon examination of the group × emotion intensity interaction to test our first hypothesis comparing difference in amygdala-OFC between BD and HC, with a Bonferroni-corrected statistical threshold of p = .05/3 = .017 (three post hoc tests comparing amygdala-OFC FC between the two groups separately for each of the three emotion intensities). The BD showed significantly reduced amygdala-OFC FC to intense happy faces than HC over both hemispheres [F (1,52) = 7.95; p = .007] (Figure 3, Tables 2A and 4 for estimated marginal means for FC values in BD and HC).
Figure 3.
Right panel: axial views showing a schematic representation of FC abnormalities in BD versus HC in the happy experiment to intense faces. The arrows symbolize FC between amygdala and OFC. The template on which the FC data are depicted is the standard Montreal Neurological Institute 152 1-mm brain template showing the FA white matter skeleton used for tract based spatial statistics analysis of diffusion tensor imaging data (shown in light-green) and between-group differences in FA in bilateral UF: decreased FA in a cluster in blue in the right UF, and increased FA in clusters in red-yellow in the left UF. The red ovoid is a representation of the amygdala region of interest. Left panel: a bar graph depicting significantly reduced bilateral amygdala-OFC FC to intense faces in BD versus HC in the happy experiment (p < .007). Vertical axis: estimated marginal means of FC values. The error bars show SDs of FC in each group. Abbreviations as in Figure 1.
Table 4.
Amygdala-OFC Functional Connectivity in the Happy Experiment: Analysis of Covariance and Post Hoc Analyses Comparing BD and HC
| Main Effects | |||||
|---|---|---|---|---|---|
| Group |
Laterality |
Intensity of Emotion |
|||
| F [1,52] | p | F [1,52] | p | F [2,51] | p |
| .74 | .394 | .34 | .561 | 3.86 | .028a |
| Interactions | |||||||
|---|---|---|---|---|---|---|---|
| Group × Laterality |
Group × Intensity |
Laterality × Intensity |
Group × Laterality × Intensity |
||||
| F [1,52] | p | F[2,51] | p | F [2,51] | p | F [2,51] | p |
| 1.22 | .275 | 4.48 | .016a | .86 | .431 | .73 | .486 |
| Post Hoc Analyses | |||
|---|---|---|---|
| BD vs. HC |
|||
| F [1,52] | p | ||
| Intense | 7.95 | .007b | |
| Mild | .09 | .765 | |
| Neutral | .26 | .612 | |
| dBD vs. HC |
|||
| F [1,22] | p | ||
| Intense | 7.46 | .012c,d | |
| Mild | .76 | .393 | |
| Neutral | 2.30 | .144 | |
| rBD vs. HC |
|||
| F [1,18] | p | ||
| Intense | 3.11 | .095 | |
| Mild | .01 | .920 | |
| Neutral | .04 | .843 | |
Age, covariate; BD, bipolar disorder individuals; dBD, female depressed bipolar disorder individuals; rBD, female remitted bipolar disorder individuals; HC, female healthy control individuals.
In BD versus HC: we used a Bonferroni-corrected statistical threshold of p = .05/3 ≤ .017 (three post hoc tests comparing amygdala orbital frontal cortex (OFC) FC between the two groups separated for each of the three emotion intestines. Trend range: .017 < p ≤ .05.
In female dBD/rBD vs female HC: we used a Bonferroni-corrected statistical threshold of p = .0017/2 ≤ .008 to control for the additional two post-hoc tests compared dBD vs HC and dBD vs HC on amygdala-OFC FC to intense happy faces over both hemispheres.
BD <HC: estimated marginal means [SD]: BD = 39 [.02], HC = .46 [.02].
dBD < HC: estimated marginal means [SD]: dBD = .40 [.02]; HC = .49 [.02] (rBD = .42 [.04]).
Trend range: .008 < p ≤ .05.
Depressed and Remitted Female BD Versus Female HC: Sad Experiment
All BD showed significantly greater right (and to a lesser extent left) amygdala-OFC FC overall emotion intensities than HC (p = .013; see preceding text). We therefore performed separate analyses in depressed and remitted female BD versus female HC, with the statistical threshold of p = .013/4 = .003 to control for the additional four post hoc tests comparing depressed BD versus HC and remitted BD versus HC on left and right amygdala-OFC FC overall intensities. Both depressed and remitted BD showed significantly greater right amygdala-OFC FC than HC overall intensities [F(1,22) = 20.37; p < .001, and F(1,17) = 16.57; p = .001, respectively], whereas only depressed BD showed significantly greater left amygdala-OFC FC than HC overall intensities [F(1,22) = 16.13; p = .001] (Tables 2B and 3 for estimated marginal means for FC values in depressed and remitted BD and HC).
Depressed and Remitted Female BD Versus Female HC: Happy Experiment
All BD showed significantly reduced amygdala-OFC FC to intense happy faces than HC over both hemispheres (p = .017, see preceding text). We therefore performed separate analyses in depressed and remitted female BD versus female HC, with a statistical threshold of p = .0017/2 = .008 to control for the additional two post hoc tests comparing depressed BD versus HC and remitted BD versus HC on amygdala-OFC FC to intense happy faces over both hemispheres. Depressed but not remitted BD showed significantly reduced amygdala-OFC FC, compared with HC, to intense happy faces that only just missed the stringent significance threshold [F(1,22) = 7.46; p = .012] (Tables 2B and 4 for estimated marginal means for FC values in depressed and remitted BD and HC).
FC–FA Relationships
One cubic relationship (R2 = .6; p = .001) between left UF FA (Montreal Neurological Institute [−33, 21, −17]) and left amygdala-OFC FC to all sad faces in HC survived our criteria for statistical significance after controlling for multiple tests (Table S2 in Supplement 1). We did not examine FC–FA relationships separately in remitted and depressed BD, because these groups did not differ in right or left UF FA (27).
Demographic and Clinical Variables, Task Performance, and FC
The BD not taking antidepressants had greater left amygdala-OFC FC to mild sad faces than BD taking antidepressants (t = 3.99, p = .001) but did not differ from HC (t = −.02, p = .981). In BD, there were normalizing effects of aging and greater age of illness onset upon amygdala-OFC FC to faces in the sad but not happy experiment that did not survive correction for multiple tests (Tables S3 and S4 in Supplement 1 for all relationships).
Discussion
The goal of the present study was to examine amygdala-OFC FC to emotional faces in BD and HC. Our main finding, in support of our first hypothesis, was that BD showed abnormal amygdala-OFC FC to sad and happy faces. All BD showed significantly greater right amygdala-OFC FC than HC to all faces in the sad experiment. Although both depressed and remitted female BD showed significantly greater right amygdala-OFC FC than female HC to these faces, only depressed female BD showed significantly greater left amygdala-OFC FC than female HC to these faces. The BD showed significantly reduced amyg-dala-OFC FC over both hemispheres, compared with HC, to intense happy faces that was evident in depressed but not remitted female BD versus female HC, in support of our second hypothesis.
Our finding of significantly greater right amygdala-OFC FC to all faces in the sad experiment in BD versus HC is consistent with previous data implicating abnormal right frontal cortical activity in mood-disordered and anxiety-prone individuals (49,50) and greater right OFC activity to sad versus neutral distractors in manic BD versus HC (51).
We recently highlighted a role of ventrolateral prefrontal cortex in voluntary emotion regulatory subprocesses, including attentional control and reappraisal that might be mediated via OFC (18). In depressed and remitted individuals with unipolar depression, previous reports indicate positive relationships between activity in right ventrolateral prefrontal cortex during sad mood induction and depression severity in depressed individuals (52) and between increased regional cerebral blood flow in this region and depressive ruminations (53). We can speculate that our finding of greater right amygdala-OFC FC in all BD, compared with HC, to all faces in the sad experiment might be associated with abnormal “over-appraisal” of these faces, which might reflect a predisposition to negative ruminations in depressed and remitted BD (54).
Depressed but not remitted female BD showed significantly greater left amygdala-OFC FC, compared with female HC, to all faces in the sad experiment. This finding is consistent with previous studies showing significantly greater left OFC activity to negative emotional faces (55) and significantly greater left amygdala activity to sad faces (56) in depressed BD versus HC. All BD versus HC and depressed female BD versus female HC showed reduced amygdala-OFC FC over both hemispheres to intense happy faces, consistent with our previous finding of significantly reduced left “top-down” OFC-amygdala and right “bottom-up” amygdala-OFC effective connectivity to happy faces in depressed BD versus HC (33). Abnormally elevated right amygdala-OFC FC to sad faces that we observed in both remitted and depressed BD might represent a predisposition to depression in BD, abnormally elevated amygdala-OFC FC to sad faces, and abnormally reduced amygdala-OFC FC to happy faces, a state marker of depression in BD. These findings are in contrast to the abnormally elevated inverse left but not right OFC-amygdala effective connectivity to happy faces (33), which we recently reported in unipolar depressed adults; this suggests that, unlike unipolar depression, bipolar depression is associated with abnormal amygdala-OFC FC (and effective connectivity) to emotional stimuli in both hemispheres.
Left-sided prefrontal cortical dysfunction was previously associated with proneness to hypomania (57). We found no significant group × laterality interaction in the happy experiment but only a group × emotion interaction. It is possible that our study was not powered to detect a three-way interaction between group × intensity laterality. We were therefore unable to determine whether the significantly reduced amygdala-OFC FC in depressed BD versus HC was more evident in the left than in the right hemisphere. This can be a focus of future studies.
Only in HC did one nonlinear cubic relationship between left amygdala-OFC FC to sad faces and left UF FA meet our stringent significance threshold after controlling for multiple tests. A previous report indicated a positive linear relationship between pericingulate gyral-amygdala FC and UF FA in BD during happy and fearful facial expression processing but did not examine left and right FC–FA relationships separately in either BD or HC and did not include a sad face emotion labeling task condition (31). Direct comparison of these previous data and our present data are therefore difficult. Further studies are needed to elucidate nonlinear amygdala-OFC FC–FA relationships in BD and HC.
Although both HC and BD were more accurate on happy than on sad emotion labeling, BD were less accurate than HC on happy but not sad labeling. Here, BD showed a trend for mislabeling intense happy faces as neutral more than HC, which in turn might relate to the significantly decreased amygdala-OFC FC to intense happy faces in BD (driven by depressed BD) versus HC. These relationships need further exploration in future studies. Our findings from exploratory analyses suggest normalizing effects of aging and greater age of illness onset upon amygdala-OFC FC to faces in the sad but not happy experiment in BD but did not survive correction for multiple tests.
There were no differential patterns of between-group differences for all BD versus HC in right amygdala-OFC FC and for depressed female BD versus female HC in left amygdala-OFC FC, for the different facial emotion intensities in the sad experiment. This might reflect a tendency in BD for all faces in the sad experiment to be processed as negative emotional displays. In contrast, our finding of reduced amygdala-OFC FC in the happy experiment in depressed female BD was restricted to intense happy faces, suggesting that processing of intense but not mild happy or neutral faces was associated with abnormally reduced amygdala-OFC FC in this BD subgroup.
There are limitations to the study. Our findings should be replicated in future studies of BD. Our analyses of depressed and remitted BD subgroups versus HC were restricted to women only because of different gender ratios across BD subgroups. Future studies could include similar gender ratios across BD subgroups and HC. We recruited medicated BD adults, as in most neuroimaging studies of BD (18). Although depressed female BD had significantly greater left amygdala-OFC FC than female HC to all faces in the sad experiment, antidepressant medications were associated with reduced and not greater left amygdala-OFC FC to mild sad faces in all BD, and there was no significant difference in the proportion of individuals taking versus not taking antidepressant medications in depressed and remitted female BD. These findings suggest that antidepressants were associated with a normalization of abnormal amygdala-OFC FC to sad faces rather than being a potential confounding factor upon these neuroimaging measures in BD. Although we covaried for age in our main analyses, future studies could match age across BD and HC. Future studies could also employ more difficult paradigms including larger numbers of events for each stimulus to allow examination of between-group differences in FC to correct and incorrect behavioral responses and measures of electrodermal activity to examine emotional responses to different emotional stimuli in BD and HC. Although we showed no significant relationships between amygdala-OFC FC and lifetime comorbid substance abuse/dependence, it is possible that other related lifestyle factors in BD (e.g., disrupted sleep) might have impacted amygdala-OFC FC.
We show that abnormally elevated right amygdala-OFC FC to sad stimuli might reflect a predisposition to depression in BD, whereas abnormally elevated left amygdala-OFC FC to sad stimuli, together with abnormally reduced amygdala-OFC FC to intense happy stimuli, might represent a state marker of depression in BD. This pattern of abnormal amygdala-OFC FC might be specific to bipolar rather than shared with unipolar depression. In BD, abnormal FC measures might normalize with antidepressant and anxiolytic medication and aging. The nature of nonlinear relationships between amygdala-OFC FC and FA during emotion labeling in HC and BD requires further study. Future studies can determine the extent to which this pattern of abnormal amygdala-OFC FC to emotional stimuli represents a vulnerability marker of BD in individuals at future risk of BD.
Supplementary Material
Acknowledgments
This study was supported in part by National Institutes of Health Grants 1 R01 MH076971-01 (MLP) and K25 MH076981-01 (WKT), National Alliance for Research on Schizophrenia and Depression Independent Investigator Award (Nellie Blumenthal Investigator) (MLP), DMS-0806106 from the National Science Foundation (WKT), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)-Brazil 190105-2 (JRCA).
Footnotes
The authors reported no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Murray CJL, Lopez AD. Evidence-based health policy—lessons from the global burden of disease study. Science. 1996;274:740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- 2.Brooks JO, Hoblyn JC, Woodard SA, Rosen AC, Ketter TA. Corticolimbic metabolic dysregulation in euthymic older adults with bipolar disorder. Biol Psychiatry. 2008;63:181S–181S. doi: 10.1016/j.jpsychires.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodwin FK, Jamison KR, Ghaemi SN. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. New York: Oxford University Press; 2007. [Google Scholar]
- 4.Robinson JL, Monkul ES, Tordesillas-Gutierrez D, Franklin C, Bearden CE, Fox PT, et al. Frontolimbic circuitry in euthymic bipolar disorder: Evidence for prefrontal hyperactivation. Psychiatry Res Neuroimaging. 2008;164:106–113. doi: 10.1016/j.pscychresns.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Adolphs R, Tranel D. Impaired judgments of sadness but not happiness following bilateral amygdala damage. J Cogn Neurosci. 2004;16:453–462. doi: 10.1162/089892904322926782. [DOI] [PubMed] [Google Scholar]
- 6.Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- 7.Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, et al. Brain activation during facial emotion processing. Neuroimage. 2002;16:651–662. doi: 10.1006/nimg.2002.1097. [DOI] [PubMed] [Google Scholar]
- 11.Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Schoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J Neurosci. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delamater AR. The role of the orbitofrontal cortex in sensory-specific encoding of associations in Pavlovian and instrumental conditioning. In: Schoenbaum G, Gottfried JA, Murray EA, Ramus SJ, editors. Linking Affect to Action: Critical Contributions of the Orbitofrontal Cortex. New York: Wiley; 2007. pp. 152–173. [Google Scholar]
- 14.Highley JR, Walker MA, Esiri MM, Crow TJ, Harrison PJ. Asymmetry of the uncinate fasciculus: A post-mortem study of normal subjects and patients with schizophrenia. Cereb Cortex. 2002;12:1218–1224. doi: 10.1093/cercor/12.11.1218. [DOI] [PubMed] [Google Scholar]
- 15.Dannlowski U, Ohrmann P, Konrad C, Bauer J, Kugel H, Schoning S, et al. Reduced amygdala-prefrontal connectivity is associated with symptom severity in major depression. Pharmacopsychiatry. 2007;40:206–206. [Google Scholar]
- 16.Habel U, Windischberger C, Derntl B, Robinson S, Kryspin-Exner I, Gur RC, et al. Amygdala activation and facial expressions: Explicit emotion discrimination versus implicit emotion processing. Neuropsychologia. 2007;45:2369–2377. doi: 10.1016/j.neuropsychologia.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 17.Todorov A, Engell AD. The role of the amygdala in implicit evaluation of emotionally neutral faces. Soc Cogn Affect Neurosci. 2008;3:303–312. doi: 10.1093/scan/nsn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips M, Ladouceur C, Drevets W. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips ML, Vieta E. Identifying functional neuroimaging biomarkers of bipolar disorder: toward DSM-V. Schizophr Bull. 2007;33:893–904. doi: 10.1093/schbul/sbm060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abler B, Greenhouse I, Ongur D, Walter H, Heckers S. Abnormal reward system activation in mania. Neuropsychopharmacology. 2008;33:2217–2227. doi: 10.1038/sj.npp.1301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green MJ, Cahill CM, Malhi GS. The cognitive and neurophysiological basis of emotion dysregulation in bipolar disorder. J Affect Disord. 2007;103:29–42. doi: 10.1016/j.jad.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Houenou J, Wessa M, Douaud G, Leboyer M, Chanraud S, Perrin M, et al. Increased white matter connectivity in euthymic bipolar patients: Diffusion tensor tractography between the subgenual cingulate and the amygdalo-hippocampal complex. Mol Psychiatry. 2007;12:1001–1010. doi: 10.1038/sj.mp.4002010. [DOI] [PubMed] [Google Scholar]
- 23.Kafantaris V, Kingsley P, Ardekant B, Saito E, Lencz T, Lim K, et al. Lower orbital frontal white matter integrity in adolescents with bipolar I disorder. J Am Acad Child Adolesc Psychiatry. 2009;48:79–86. doi: 10.1097/CHI.0b013e3181900421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahon K, Wu J, Malhotra AK, Burdick KE, Derosse P, Ardekani BA, Szeszko PR. A voxel-based diffusion tensor imaging study of white matter in bipolar disorder. Neuropsychopharmacology. 2009;34:1590–1600. doi: 10.1038/npp.2008.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McIntosh AM, Maniega SM, Lymer GKS, McKirdy J, Hall J, Sussmann JED, et al. White matter tractography in bipolar disorder and schizophrenia. Biol Psychiatry. 2008;64:1088–1092. doi: 10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 26.Sussmann JE, Lymer GKS, McKirdy J, Moorhead TWJ, Maniega SM, Job D, et al. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disord. 2009;11:11–18. doi: 10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 27.Versace A, Almeida JRC, Hassel S, Walsh ND, Novelli M, Klein CR, et al. Elevated left and reduced right orbitomedial prefrontal fractional anisotropy in adults with bipolar disorder revealed by tract-based spatial statistics. Arch Gen Psychiatry. 2008;65:1041–1052. doi: 10.1001/archpsyc.65.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friston KJ, Frith CD, Fletcher P, Liddle PF, Frackowiak RSJ. Functional topography: Multidimensional scaling and functional connectivity in the brain. Cereb Cortex. 1996;6:156–164. doi: 10.1093/cercor/6.2.156. [DOI] [PubMed] [Google Scholar]
- 29.Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res Neuroimaging. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almeida JRC, Mechelli A, Hassel S, Versace A, Walsh N, Kupfer DJ, et al. Increased functional connectivity in the paralimbic-ventral pre-frontal cortical system during emotion processing in bipolar disorder: A dynamic causal modelling approach. Biol Psychiatry. 2008;63:148S–148S. [Google Scholar]
- 31.Wang F, Kalmar JH, He Y, Jackowski M, Chepenik LG, Edmiston EE, et al. Functional and structural connectivity between the Perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry. 2009;66:516–521. doi: 10.1016/j.biopsych.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irwin W, Anderle MJ, Abercrombie HC, Schaefer SM, Kalin NH, Davidson RJ. Amygdalar interhemispheric functional connectivity differs between the non-depressed and depressed human brain. Neuroimage. 2004;21:674–686. doi: 10.1016/j.neuroimage.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 33.Almeida JR, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, et al. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lange K, Williams LM, Young AW, Bullmore ET, Brammer MJ, Williams SCR, et al. Task instructions modulate neural responses to fearful facial expressions 1. Biol Psychiatry. 2003;53:226–232. doi: 10.1016/s0006-3223(02)01455-5. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 36.Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 37.Granger CWJ, Lin JL. Causality in the long-run. Econ Theory. 1995;11:530–536. [Google Scholar]
- 38.Salvador R, Suckling J, Schwarzbauer C, Bullmore E. Undirected graphs of frequency-dependent functional connectivity in whole brain networks. Philos Trans R Soc Lond B Biol Sci. 2005;360:937–946. doi: 10.1098/rstb.2005.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou D, Thompson WK, Siegle G. MATLAB toolbox for functional connectivity. Neuroimage. 2009;47:1590–1607. doi: 10.1016/j.neuroimage.2009.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 41.First MB, Spitzer RL, Gibbon ML, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research. New York State Psychiatric Institute; 2002. [Google Scholar]
- 42.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Annett M. A classification of hand preference by association analysis. Br J Psychol. 1970;61:303–321. doi: 10.1111/j.2044-8295.1970.tb01248.x. [DOI] [PubMed] [Google Scholar]
- 45.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 46.Nelson HE, Willison JR. The Revised National Adult Reading Test—Test Manual. Windsor, United Kingdom: Nfer-Nelson; 1991. [Google Scholar]
- 47.Hassel S, Almeida JRC, Kerr N, Nau S, Ladouceur CD, Fissell K, et al. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: No associations with psychotropic medication load. Bipolar Disord. 2008;10:916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun FT, Miller LM, D’Esposito M. Measuring interregional functional connectivity using coherence and partial coherence analyses of fMRI data. Neuroimage. 2004;21:647–658. doi: 10.1016/j.neuroimage.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 49.Blackhart GC, Minnix JA, Kline JP. Can EEG asymmetry patterns predict future development of anxiety and depression? A preliminary study. Biol Psychol. 2006;72:46–50. doi: 10.1016/j.biopsycho.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 50.Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. J Abnorm Psychol. 1990;99:22–31. doi: 10.1037//0021-843x.99.1.22. [DOI] [PubMed] [Google Scholar]
- 51.Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry. 2002;59:597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- 52.Keedwell PA, Andrew C, Williams SCR, Brammer MJ, Phillips ML. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol Psychiatry. 2005;58:495–503. doi: 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 53.Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: Mood challenge in patients with remitted unipolar depression. Am J Psychiatry. 2002;159:1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- 54.Van der Gucht E, Morriss R, Lancaster G, Kinderman P, Bentall RP. Psychological processes in bipolar affective disorder: Negative cognitive style and reward processing. Br J Psychiatry. 2009;194:146–151. doi: 10.1192/bjp.bp.107.047894. [DOI] [PubMed] [Google Scholar]
- 55.Altshuler L, Bookheimer S, Townsend J, Proenza MA, Sabb F, Mintz J, et al. Regional brain changes in bipolar I depression: A functional magnetic resonance imaging study. Bipolar Disord. 2008;10:708–717. doi: 10.1111/j.1399-5618.2008.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Almeida JRC, Versace A, Hassel S, Kupfer DJ, Phillips ML. Elevated amygdala activity to sad facial expressions: A state marker of bipolar but not unipolar depression [published online ahead of print November 20] Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peterson CK, Harmon-Jones E. Proneness to hypomania predicts EEG coherence between left motor cortex and left prefrontal cortex. Biol Psychol. 2008;78:216–219. doi: 10.1016/j.biopsycho.2008.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.