Ever since Briggs and King first reported nuclear transfer in Rana pipiens,1 somatic cell reprogramming has been actively studied both for its therapeutic potential and to advance the frontiers of developmental, cell, and molecular biology. Recent efforts in somatic cell reprogramming have culminated in the generation of induced pluripotent stem (iPS) cells, wherein defined transcription factors are used to reprogram fibroblasts into embryonic stem (ES)-like cells.2 iPS cells, like ES cells, have the potential to serve as a source of cells for different tissues but evade problems related to allogeneic rejection. Although extraordinary, iPS cells, like ES cells, face the same hurdles concerning efficacy and applicability for transplantation therapy and other in vivo applications. All in all, though, these studies underscore the crucial role of transcription factors in the molecular control of nuclear reprogramming. So, why not go straight to another cell type instead of going through an ES cell–like pluripotent state? Yechoor et al. demonstrated this in their recent report, but with a twist.3 They show that administration of the endocrine transcription factor neurogenin 3 (Ngn3) and betacellulin (Btc, an islet growth factor) results in a long-term reversal of streptozotocin (STZ)-induced diabetes in mice, via formation of insulin-producing cells in the liver. Furthermore, they present convincing evidence that the cells that are receptive to long-term islet-lineage reprogramming are progenitor cells, not differentiated cells in the liver, i.e., the hepatocytes. They liken this phenomenon to a transdetermination process, one that is similar to cell-fate switching of imaginal disc cells in Drosophila.4
Transdetermination is defined as a switch in lineage commitment in a stem or progenitor cell to a closely related cell type. This is in contrast to transdifferentiation, which occurs when a differentiated cell switches to another differentiated lineage without an intermediate stage of dedifferentiation.5 It is well established that both the liver and pancreas develop from the endoderm and can be considered part of the same organ system.6 Hepatocytes have been shown to develop in the pancreas of rodents fed a copper-deficient diet.7 This is considered a model for transdifferentiation. The conversion of liver to pancreas has also been described in recent reports.8,9,10,11 Ferber et al. were the first to report that the adenoviral delivery of the pancreatic transcription factor Pdx1 to the liver resulted in ectopic expression of insulin and the reversal of STZ-induced diabetes.9 This same phenomenon was documented with the delivery of NeuroD, a transcription factor downstream of Pdx1, and Btc.8 However, although these studies establish an important proof of concept, the lack of lineage-tracing data precludes determining the origin of insulin-producing cells. Toward this end, Zhou et al. attempted the transdifferentiation of exocrine tissue to endocrine islets and traced the cells receptive to change.12 The authors succeeded in forming β cells from exocrine acinar tissue via direct adenoviral injection of Pdx1, Ngn3, and MafA directly into pancreata. Furthermore, by using an inducible system of Cre recombinase, they demonstrated that the induced β cells originated from mature acinar tissue. To our knowledge, these data provide the first definitive evidence of in vivo transdifferentiation within the pancreas.
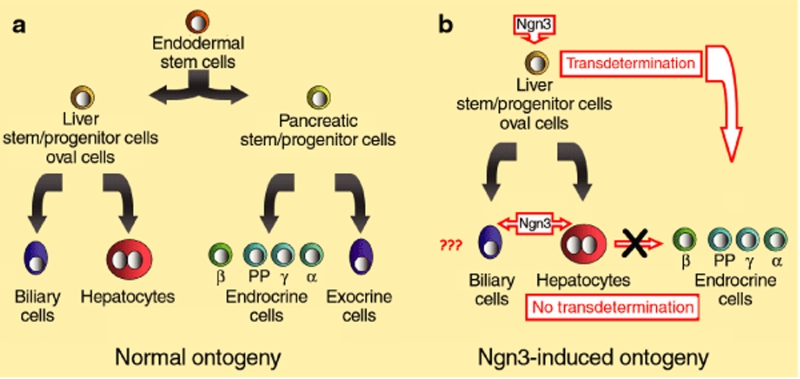
In counterpoint, Yechoor et al. show transdetermination to be the mechanistic process whereby insulin-producing cells are generated in the liver.3 Using a helper-dependent adenoviral vector, the authors deliver Ngn3-Btc to the pancreas and observe an immediate and long-term reversal in STZ-induced diabetes. Contrary to prior studies, this reversal occurs in two waves of insulin expression. The first, occurring in hepatocytes, is transient and lasts from the time of injection to 6 weeks after injection. Insulin expression then decreases in the hepatocytes, while concomitantly increasing in a periportal cell population. This second wave of insulin expression in the periportal cells persists beyond 6 months after injection, corroborating past studies in which long-term insulin-producing cells were identified around the portal area.8,10 The periportal population is glycemia-responsive and expresses a host of transcription factors that collectively specify the endocrine lineage. The authors trace the cells and determine that they express albumin in their lineage history. However, they lose albumin expression once they acquire the ability to express insulin. Therefore, the insulin-producing cells do not originate from hepatocytes. Intriguingly, these cells also express Pax4, an embryonic transcription factor not expressed in mature islets, and CK19, a ductal marker expressed in islet progenitors.13 So it seems that they resemble embryological or immature islets rather than mature islets. The authors consequently name these “neo-islets.” Finally, the authors note that the neo-islets resemble oval cells in their size, morphology, nuclear-to-cytoplasmic ratio, and expression of the oval cell marker A6.
Oval cells, or hepatic progenitor cells, are thought to expand during chronic liver injury. Their existence has been documented in human liver disease and a variety of animal models, notably when hepatocyte proliferation is blocked.14,15,16 Furthermore, they are considered bipotent, capable of differentiation into biliary cells and hepatocytes. Yechoor et al. note in their study that the neo-islets appear only in livers of mice treated with Ngn3-Btc or Ngn3 vectors, but not the empty vector. This implies that they develop as a result of a deficiency in insulin secretion rather than as a host response to the vector. Furthermore, the neo-islets appear roughly 3 weeks after injection with Ngn3-Btc but do not begin to express insulin until after 6 weeks. Therefore, the neo-islets, like oval cells, emerge because of tissue injury. However, the novel finding is that the injury is outside the liver—in this case, the endocrine pancreas. For that reason, one could imagine that the neo-islets arise because of β-cell ablation and begin to express insulin when the hepatocytes cannot, potentially maintaining insulin homeostasis.
In a recent study, Kuwahara et al. used label retention assays to identify four candidate niches for hepatic stem cells.17 All of these niches were either in or around the intrahepatic bile duct system. The observation by Yechoor et al. that the neo-islets grow from the periportal area substantiates the bile duct system as a stem cell niche. It is appealing to imagine that the extrahepatic biliary system could be a niche as well. Interestingly, Dutton et al. have demonstrated that extrahepatic bile duct cells in the hilar region of adult liver normally express endocrine proteins,18 implying that the extrahepatic bile duct cells and endocrine islets have a similar transcriptome. Furthermore, the extrahepatic bile ducts in Hes1–/– mice ectopically express Ngn3 and differentiate into endocrine cells.19 Therefore, all the studies indicate that the biliary tree seems to contain or be associated with a resident stem cell population that is capable of differentiation not only into biliary cells and hepatocytes but also into endocrine cells (Figure 1). It follows that these cells would be “permissive” to transdetermination and would have tremendous clinical significance. However, this tale requires a cautionary note. Definitive markers for oval cells do not yet exist, and these cells cannot be prospectively isolated, or even defined in the steady state, using currently available protocols. These are hurdles that must be overcome if these cells are to have therapeutic significance.
Figure 1.
Induced neurogenin 3 (Ngn3) in the hepatic lineage. (a) Normal ontogeny. Both liver and pancreas develop from the endoderm. The endodermal stem cell divides to form the hepatic and pancreatic stem cells during development. The liver stem cell divides to form biliary cells and hepatocytes. (b) Ngn3-induced ontogeny. Ngn3 induction results in the aborted transdifferentiation of hepatocytes into endocrine cells but successful transdetermination of the liver stem–progenitor cell population. The effects of Ngn3 on the biliary cell population remain to be assessed. PP, pancreatic polypeptide.
A hallmark of the study by Yechoor et al. is the demonstration that a single transcription factor can specify the entire endocrine lineage. The authors observe that the neo-islets express the complete endocrine transcription profile, containing cells that produce either insulin or other endocrine hormones. Similar results were obtained with NeuroD-Btc.8 These data are in sharp contrast to the transdifferentiation of exocrine acini to endocrine islets demonstrated by Zhou et al.,12 wherein three transcription factors were necessary. It is therefore possible that transdetermination might be more feasible than transdifferentiation—a clinically significant difference. However, these studies make use of adenoviral-mediated delivery of transcription factors, and one of the major limitations thus far to a clinical setting is that this type of reprogramming requires the adenovirus particle itself.20
It is difficult to predict the course or timeline for the implementation of clinical cellular reprogramming. The work by Yechoor et al. may represent an elegant solution to the need for functional islet cells. The rapidly growing field of small molecules mimicking transcription factors and extracellular signals may help bring this work to clinical reality. It is noteworthy that the use of such an approach, as with iPS cells, would circumvent the hurdles of allogeneic transplantation. Meanwhile, the transdetermination of a stem or progenitor cell into a related lineage using a single transcription factor has not been demonstrated outside of the hepato-pancreatic system. In conclusion, the study by Yechoor et al. highlights a new trend in cellular reprogramming (Figure 2). It underscores the ability of a primitive cell to become something other than what its physiological lineage commitment dictates, and postulates that this reprogramming might be possible with a single exogenous factor. If transdetermination proves to be clinically feasible, the ramifications in the field of therapeutic tissue replacement would be far-reaching.
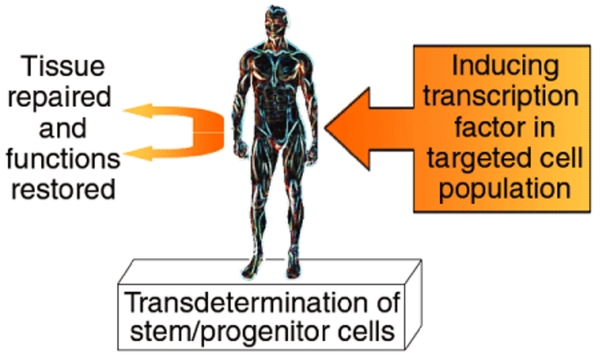
Figure 2.
In vivo cellular reprogramming for tissue replacement. Resident stem and/or progenitor cells within an organ system could be targeted in vivo with a single transcription factor. Transdetermination would result in functional tissue replacement and recovery. The added feasibility of this approach lies in its being carried out in vivo, with a single transcription factor being activated.
REFERENCES
- Briggs R., and , King TJ. Transplantation of living nuclei from blastula cells into enucleated frogs' eggs. Proc Natl Acad Sci USA. 1952;38:455–463. doi: 10.1073/pnas.38.5.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1:39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Yechoor V, Liu V, Espiritu C, Paul A, Oka K, Kojima H.Neurogenin 3 is sufficient for transdetermination of hepatic progenitor cells into neo-islets in vivo but not transdifferentiation of hepatocytes Dev Cell 200916358–373.et al [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadorn E. Transdetermination in cells. Sci Am. 1968;219:110–114. doi: 10.1038/scientificamerican1168-110. [DOI] [PubMed] [Google Scholar]
- Wagers AJ., and , Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- Zaret KS., and , Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Dwivedi RS, Subbarao V, Usman MI, Scarpelli DG, Nemali MR.Almost total conversion of pancreas to liver in the adult rat: a reliable model to study transdifferentiation Biochem Biophys Res Commun 1988156131–136.et al [DOI] [PubMed] [Google Scholar]
- Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M.NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice Nat Med 20039596–603.et al [DOI] [PubMed] [Google Scholar]
- Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I.Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia Nat Med 20006568–572.et al [DOI] [PubMed] [Google Scholar]
- Ber I, Shternhall K, Perl S, Ohanuna Z, Goldberg I, Barshack I.Functional, persistent, and extended liver to pancreas transdifferentiation J Biol Chem 200327831950–31957.et al [DOI] [PubMed] [Google Scholar]
- Kaneto H, Matsuoka TA, Nakatani Y, Miyatsuka T, Matsuhisa M, Hori M.A crucial role of MafA as a novel therapeutic target for diabetes J Biol Chem 200528015047–15052.et al [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J., and , Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, D'Hoker J, Stangé G, Bonné S, De Leu N, Xiao X.Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas Cell 2008132197–207.et al [DOI] [PubMed] [Google Scholar]
- Dorrell C, Erker L, Lanxon-Cookson KM, Abraham SL, Victoroff T, Ro S. Surface markers for the murine oval cell response. Hepatology. 2008;48:1282–1291. doi: 10.1002/hep.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird TG, Lorenzini S., and , Forbes SJ. Activation of stem cells in hepatic diseases. Cell Tissue Res. 2008;331:283–300. doi: 10.1007/s00441-007-0542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N., and , Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117–130. doi: 10.1016/s0925-4773(02)00338-6. [DOI] [PubMed] [Google Scholar]
- Kuwahara R, Kofman AV, Landis CS, Swenson ES, Barendswaard E., and , Theise ND. The hepatic stem cell niche: identification by label-retaining cell assay. Hepatology. 2008;47:1994–2002. doi: 10.1002/hep.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton JR, Chillingworth NL, Eberhard D, Brannon CR, Hornsey MA, Tosh D.Beta cells occur naturally in extrahepatic bile ducts of mice J Cell Sci 2007120239–245.et al [DOI] [PubMed] [Google Scholar]
- Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M.Conversion of biliary system to pancreatic tissue in Hes1-deficient mice Nat Genet 20043683–87.et al [DOI] [PubMed] [Google Scholar]
- Wang AY, Ehrhardt A, Xu H., and , Kay MA. Adenovirus transduction is required for the correction of diabetes using Pdx1 or Neurogenin 3 in the liver. Mol Ther. 2007;15:255–263. doi: 10.1038/sj.mt.6300032. [DOI] [PubMed] [Google Scholar]