Abstract
Prostate stem cell antigen (PSCA) is a cell surface antigen expressed in normal human prostate and over expressed in prostate cancer. Elevated levels of PSCA protein in prostate cancer correlate with increased tumor stage/grade, with androgen independence and have higher expression in bone metastases. In this study, the PSCA gene was isolated from the transgenic adenocarcinoma mouse prostate cell line (TRAMPC1), and a vaccine plasmid construct was generated. This plasmid PSCA (pmPSCA) was delivered by intramuscular electroporation (EP) and induced effective antitumor immune responses against subcutaneous TRAMPC1 tumors in male C57 BL/6 mice. The pmPSCA vaccination inhibited tumor growth, resulting in cure or prolongation in survival. Similarly, the vaccine inhibited metastases in PSCA expressing B16 F10 tumors. There was activation of Th-1 type immunity against PSCA, indicating the breaking of tolerance to a self-antigen. This immunity was tumor specific and was transferable by adoptive transfer of splenocytes. The mice remained healthy and there was no evidence of collateral autoimmune responses in normal tissues. EP-assisted delivery of the pmPSCA evoked strong specific responses and could, in neoadjuvant or adjuvant settings, provide a safe and effective immune control of prostate cancer, given that there is significant homology between human and mouse PSCA.
Introduction
There is renewed optimism that many cancers, including prostate, may be forestalled by immune-based therapies. Cancer cells escape the normal immune elimination by several mechanisms including poor antigen presentation, a failure to react to self-antigens, and active tolerogenic processes.1,2 However, immunologic therapies can be developed that prime the immune system, overcome tolerogenic mechanisms, and selectively eliminate cancer cells. We reported that an intratumoral immunogene therapy can induce effective control of growth and spread of previously nonimmunogenic cancers.3 Vaccination strategies induce humoral and cellular immune responses, which may include the breaking of tolerance and could be refined for tumor containment.4,5,6
The serum prostate-specific antigen test revolutionized the early detection of prostate cancer and energized the search for additional novel and increasingly specific markers,7 leading to identification of >20 genes with prostate specific/abundant expression such as prostate-specific membrane antigen,7 prostate stem cell antigen (PSCA),8 and six transmembrane epithelial antigen.9 Given their cancer/prostate-specific distributions, these genes theoretically encode potential immune targets but the development of such therapies has, until recently, been severely restricted by the absence of a suitable animal model. Yang et al.10 established that murine PSCA (mPSCA) from transgenic adenocarcinoma mouse prostate (TRAMP) cells shares major homologies to human PSCA (hPSCA) at both nucleotide and amino acid level. The PSCA is a glycosylphosphatidylinositol anchored 123-amino acid protein related to the Ly-6 family of cell surface proteins. The biological function of both hPSCA and mPSCA is not clear, but as PSCA is considered a member of the Ly-6 family, it may be involved in signal transduction and cell–cell adhesion.8 The mPSCA is expressed predominantly in normal prostate, with a low level of expression in testis, kidney, and colon. It is highly expressed in primary TRAMP cancer tissue, TRAMP cell lines, and metastatic samples from lymph nodes, liver, and viscera.10 Similarly, the hPSCA is found to be stably expressed in advanced prostate cancer tissue, and this expression is retained by metastases8,11,12 and to correlate with increased tumor stage, grade, and progression to a hormone refractory phase.12 Thus, the significant cell surface expression of PSCA in local and metastatic prostate cancer together with its restrictive expression in normal tissues12 makes PSCA a potential target for immunotherapy.
Vaccine-based immunotherapy may be primarily used to prevent progression of early stage disease or as an adjuvant therapy for containment of established bulky or metastatic disease. The prostate is not an essential organ and the abundant expression of PSCA in prostate tissues and cancers makes it an attractive target for vaccine therapy. Indeed, a recent study has shown that a combination of cutaneous gene gun and viral ballist delivery of a PSCA vaccine, established immunity, which inhibited the progression of the prostate in situ neoplasia in TRAMP mice.13 We were interested to know whether a plasmid-based DNA vaccine for PSCA delivered intramuscularly would provide durable immunologic responses, which would have tumor containing capability. The plasmid vectors would have the advantages of low cost easy production, nonintegration, and sustained gene expression when delivered intramuscularly.14 In vivo electroporation (EP) is known to be a potent method for plasmid-based DNA delivery and not only mediates high levels of transfection, but also enhances cellular immune responses.4,15,16,17 In this article, we show the delivery of mPSCA plasmid DNA vaccine, plasmid PSCA (pmPSCA), to the muscle tissue by EP, induced tumor-specific effector immune responses against PSCA, which inhibited primary tumor growth and metastases.
Results
EP-mediated muscle transfection
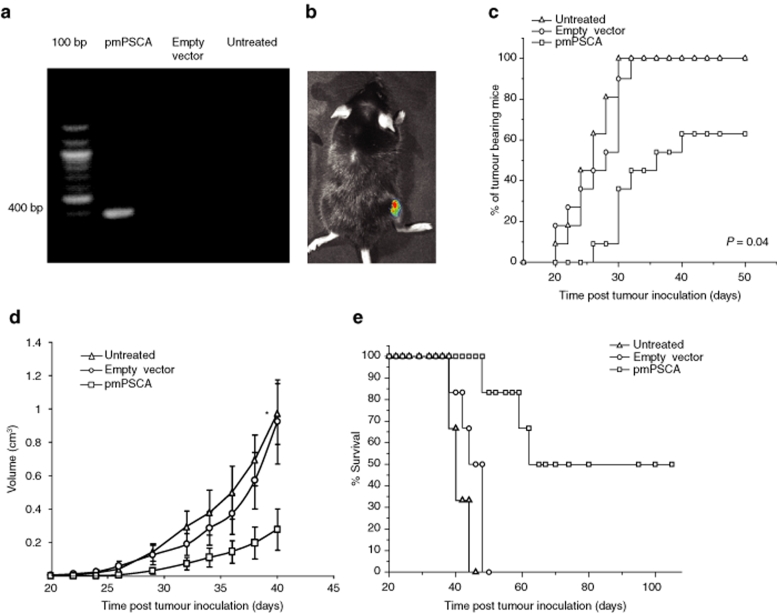
EP-driven gene delivery was successfully applied; the mPSCA mRNA was only present in the muscles administered pmPSCA, but not those receiving empty vector and untreated (Figure 1a). In vivo muscle transfection was also demonstrated by luciferase activity after 72-hour post-transfection (Figure 1b).
Figure 1.
EP-driven vaccination and tumor protective effects of pmPSCA vaccine. (a) RT-PCR analysis of mRNA expression of mPSCA in muscle. DNase treated total RNA from the muscle tissues was submitted to 32 cycles of RT-PCR amplification with mPSCA specific forward primer (5′-ACC ATG AAG ACA GTC TTC TTT C-3) and reverse primer (5′-TCT CCC AGA GCC TAC AGA C-3) to yield a 400 bp product (agarose/ethidium bromide gel electrophoresis). The mPSCA was only detected in muscles injected with pmPSCA. (b) In vivo intramuscular plasmid delivery. In vivo muscle transfection by electroporation was demonstrated by luciferase activity, analyzed 72 hours postintramuscular pCMVluc, plasmid transfection via electroporation and subsequent gene expression was assessed using whole body imaging of luciferase expression. Live anesthetized mice were imaged for 1 minute using an intensified CCD camera. A representative mouse is shown with successful muscle transfection. This image is comprised of a pseudocolor image representing the intensity of emitted light (red most intense and blue least intense) superimposed on a gray scale reference image. (c–e) Male C57 BL/6 mice were vaccinated for 4 weeks on weekly intervals, one week after the last vaccination s.c. tumor inoculation was done with 5 × 106 TRAMPC1 cells. (c) Time of tumor appearance. Data from two experiments showed that the pmPSCA vaccination resulted in total cure (4/11) and also the tumor bearing mice in this group remained tumor free for longer period of time as compared to both control groups (P = 0.04). (d) Representative tumor growth curve. Vaccinated mice showed retarded tumor growth (n = 6, *P: pmPSCA versus empty vector = 0.04, pmPSCA versus untreated = 0.01). Points mean tumor volumes of mice in representative experiment; bars, SE. (e) Representative Kaplan Meyer survival curve. The pmPSCA treated mice survived longer (n = 6, versus empty vector P < 0.01, versus untreated P < 0.01). Electroporation with empty vector resulted in a slight improvement in survival, but this did not approach significance. s.c., subcutaneous. pmPSCA, plasmid expressing prostate stem–cell antigen; TRAMPC1, transgenic adenocarcinoma mouse prostate cell line.
pmPSCA provided protection against tumor challenge
Male C57 BL/6 mice were randomly divided into three groups: pmPSCA, empty vector, and untreated (each experiment was performed twice with total n = 11/group). Tumor protection was observed in pmPSCA immunized mice; after tumor challenge, 100% mice developed tumors in empty vector and untreated groups, whereas in pmPSCA group, 63% mice (7/11) developed tumors. Additionally, in these 63% of immunized mice, tumor onset was significantly delayed, with median time of tumor appearance = 32.28 days (Figure 1c) and differ from both the empty vector (P = 0.04) and untreated groups (P < 0.01). The combined results of both independent experiments showed 37% relative risk reduction of tumor development in the pmPSCA group, but importantly vaccinated tumor bearing mice also had significantly less tumor burden than the control groups. Tumor growth kinetics indicated slower tumor growth in the pmPSCA treated group (versus empty vector P = 0.04, versus untreated P = 0.01) (Figure 1d). These results demonstrated that the pmPSCA could provide either complete protection or result in containment of the disease. There was a significant survival advantage with pmPSCA vaccination (versus empty vector P < 0.01, versus untreated P < 0.01) (Figure 1e). The tumor free mice (4/11) in pmPSCA groups displayed survival >100 days. Survival benefit was also observed in the remaining pmPSCA tumor bearing mice; average survival in tumor bearing pmPSCA vaccinated mice was 60 days compared with 47 days in empty vector and 40 days in untreated groups (versus empty vector P < 0.01, versus untreated P < 0.01). Tumor growth curves showed that the empty vector had a minimal protective effect on tumor growth (statistically insignificant), which may be explained by presence of CpG motifs on plasmid DNA that can stimulate the immune system. We tested the utility of the pmPSCA vaccine in the presence of established TRAMPC1 tumors (3–5 mm major diameter). The tumor growth was slightly slower (not significant) in the vaccinated mice (Supplementary Figure S1). In this model, the rapid growth of the TRAMPC1 tumors provided only a brief opportunity to test efficacy of a four vaccine schedule. The time interval between detection of palpable tumors and culling was usually 20–25 days and the completion of vaccine schedule required 21 days. In athymic nude mice, DNA vaccination with pmPSCA had no effect on TRAMPC1 tumor growth (data not shown). This indicates the necessity for T-lymphocyte function in immune response against tumors.
Neoadjuvant pmPSCA provided long-term tumor-specific protection
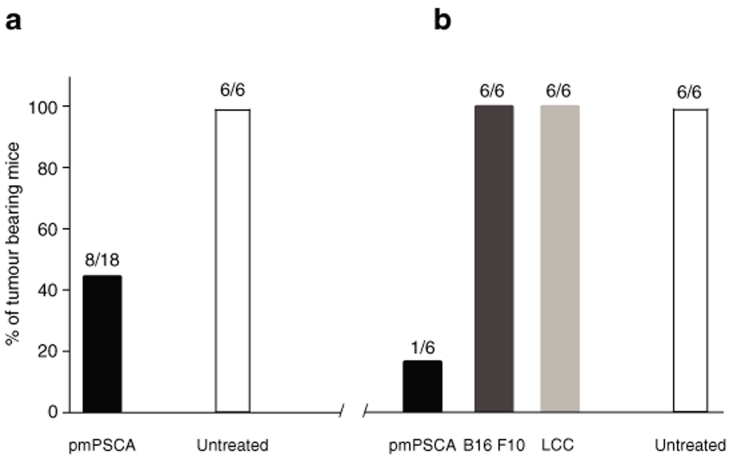
Long-term tumor-specific protection was only seen in pmPSCA treated mice. After first tumor challenge, 45% (8/18) mice developed tumors in pmPSCA group, whereas all (6/6) untreated mice succumbed to disease (Figure 2a). Although this difference was not statistically significant, importantly, there were protective immune responses in the pmPSCA group resulting in delay in onset of the tumors [mean 33 (n = 8) versus 23.3 (n = 6) days (P < 0.01)]. On rechallenge 30 days after tumor excision, all (6/6) mice in untreated group developed cancers. In contrast, tumors only occurred in 17% (1/6) of mice treated by neoadjuvant vaccine (Figure 2b). These data suggest that subcutaneous TRAMPC1 tumors without vaccination did not evoke protective antitumor immunity. The immune response was antigen specific, as tumor protection was limited to TRAMPC1 challenged and not to the previously unexposed tumors such as B16 F10 melanoma (6/6) and Lewis lung cancer (6/6) (Figure 2b). Taken together, these data suggest a durable response to the pmPSCA vaccination. Whether the combination of vaccination and exposure to TRAMPC1 antigens from the growing tumor prior to excision confers additional benefit requires a further study.
Figure 2.
Tumor rechallenge study. (a) Tumor development after first tumor inoculation. Vaccinated (n = 18) and untreated (n = 6) mice were challenged (s.c.) with 5 × 106 TRAMPC1 in the right flanks. These mice were observed for tumor development. In pmPSCA vaccinated group (8/18), 45% mice developed tumors, whereas 100% (6/6) in untreated group. The tumor bearing mice underwent surgical excision of the tumors when tumor size reached 5–7 mm in largest dimension. (b) Tumor developments after rechallenge. All mice were observed for another 30 days before tumor rechallenge was given subcutaneously into the left flank. The previously vaccinated mice were divided into three groups (n = 6/group) and challenged either with TRAMPC1, B16 F10 or Lewis lung cancer (LLC) cell lines. The untreated group was rechallenged with TRAMPC1. After rechallenge with TRAMPC1, 83% mice in the vaccinated group remained tumor free, whereas 100% mice in untreated group developed tumors. Similarly, after rechallenge with previously unexposed cell line (B16 F10 and LCC) no tumor protection was observed. s.c., subcutaneous.
In vitro cytotoxicity and adoptive transfer of lymphocytes
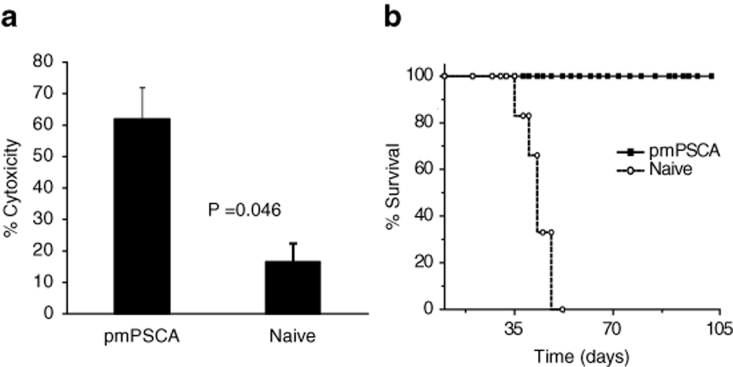
Using the MTT-based assay, as described in Materials and Methods, the cytolytic activity of splenic T lymphocytes against TRAMPC1 cells was significantly greater (P = 0.04) in the pmPSCA treated (mean 62%) than in the naive mice (mean 16.5%) (Figure 3a). These results correspond with the observed systemic enhanced immunity in vivo. The possible development of an immune-mediated antitumor activity following pmPSCA vaccination was also tested by a modified Winn assay as described in Materials and Methods, where groups of mice received subcutaneous inoculation of a mixture of TRAMPC1 cells and splenocytes from either pmPSCA treated or naive mice. All mice inoculated with splenocytes from naive groups developed tumors, whereas no tumor growth was observed in mice inoculated with splenocytes from cured pmPSCA groups—this tumor protective effect resulted in the prolonged survival (Figure 3b). This suggests adoptive transfer to naive mice of a specific antitumor immune response provided protection to tumor challenge.
Figure 3.
CTL assays. (a) In vitro augmentation of the cytolytic activities of the spleen. After pmPSCA immunization, the specific cytotoxicity was greatest at an effector target ratio of 20:1. The data shown represents one of two separate experiments with similar results. (b) Adoptive transfer of the lymphocytes. Mice (n = 6) received s.c., injections of a mixture of TRAMPC1 cells and splenocytes either from cured mice or naive mice. All mice receiving splenocytes from pmPSCA vaccinated cured mice failed to grow tumors, resulting in survival >100 days whereas tumors developed in all animals receiving splenocytes from naive mice. CTL, cytotoxic T lymphocyte assays; s.c., subcutaneous.
Immunization of C57 BL/6 mice with pmPSCA generated Th-1 biased immune response
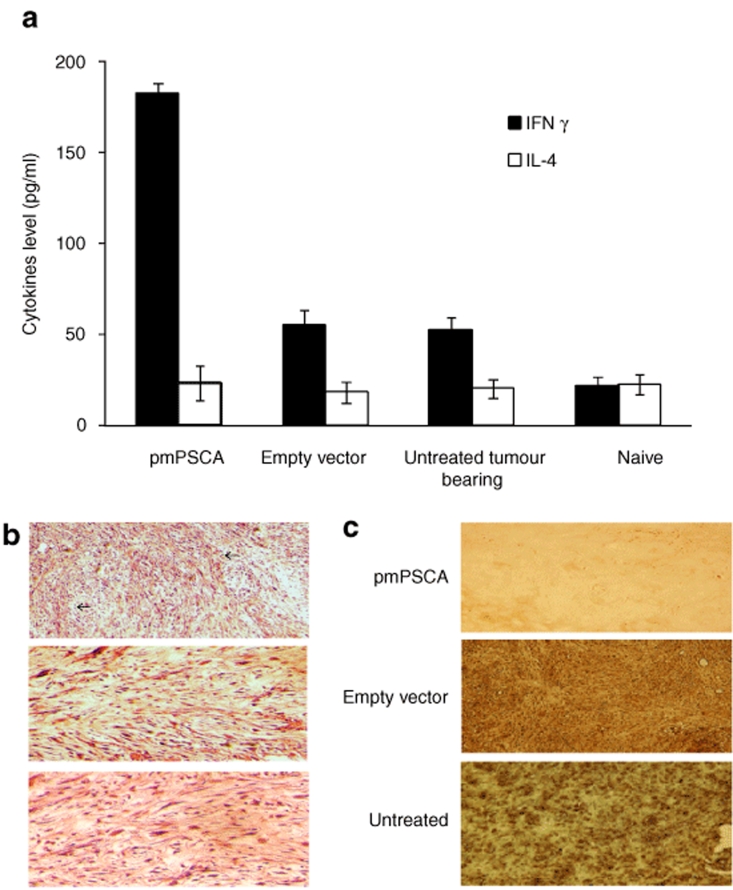
Interferon-γ (IFN-γ) is a prototypical Th-1 type cytokine involved in the regulation of T-cell mediated cytotoxic immune responses, whereas interleukin (IL)-4 is a Th-2 type cytokine.18 We observed significantly higher levels of IFN-γ from the group immunized with pmPSCA. Mean value of IFN-γ in immunized group was 182.5 pg/ml, over threefold higher than empty vector (mean 54.33 pg/ml) and over eight times higher than naive group (mean 21 pg/ml). It was also noted that untreated tumor bearing mice (mean 51.83 pg/ml), produced more IFN-γ as compared with naive nontumor bearing animals. This observation reflects the possibility of some degree of Th-1 activity in untreated mice against TRAMPC1 tumors. No significant difference in levels of IL-4 was observed in all groups (Figure 4a). Overall, the results indicate a high level of Th-1 T-cell stimulation in the vaccinated group and support the cellular nature of the immune response observed in the cytotoxic T lymphocyte assays.
Figure 4.
pmPSCA-induced tumor protective immunity. (a) Cytokine profile. Supernatants from stimulated splenocytes collected (48 hours) and tested for production of IFN-γ and IL-4. High levels of IFN-γ were produced by pmPSCA immunized mice. No difference in IL-4 levels found between experimental groups. The y-axis represents the concentration of cytokines in pg/ml of the supernatant from the stimulated splenocytes. Bars, SE. (b) Lymphocytes infiltration of tumors. TRAMPC1 tumors from study groups were excised from mice at necropsy; paraffin-embedded, H&E-stained sections examined microscopically (×100). Representative images show numerous lymphocytes (arrows) infiltration in tumors from pmPSCA immunized mice as compared to control groups. (c) Apoptosis in tumors. Tumors were removed from mice at necropsy, at various time points following treatment; paraffin embedded and stained sections were examined microscopically. TUNEL stained for evidence of apoptotic cells indicate significant apoptosis in the tumor vaccinated with pmPSCA but not from either empty vector or untreated groups. Brown TUNEL HRP-positive nuclei indicate apoptosis. TUNEL, transferase-mediated dUTPnick end-labeling.
Lymphocyte infiltration of tumors and transferase-mediated dUTPnick end-labeling analysis
Microscopic examination of tumors from pmPSCA vaccinated groups showed an increased infiltrate of lymphocytes as compared to other groups (Figure 4b). Similarly, apoptosis in tumors was evident in immunized mice, as evidenced when tumors of 100 mm3 volume were subjected to transferase-mediated dUTPnick end-labeling assay (Figure 4c). The immune mediated effects of the vaccine in tumor tissues resulted in infiltration of immune cells and increased cell lysis.
Absence of collateral autoimmune tissue injury
Vaccination with pmPSCA did not induce significant infiltration by inflammatory cells or cause damage to healthy organs including prostate gland. There were no histological evidences of tissue damage in other PSCA expressing organs, e.g., testis, kidney, and colon (Supplementary Figure S2). In addition, nine mice that had pmPSCA vaccination and tumor challenge were found to be healthy for >100 days. These findings suggest the safety of the pmPSCA vaccine.
pmPSCA vaccination provided protection against lung metastases
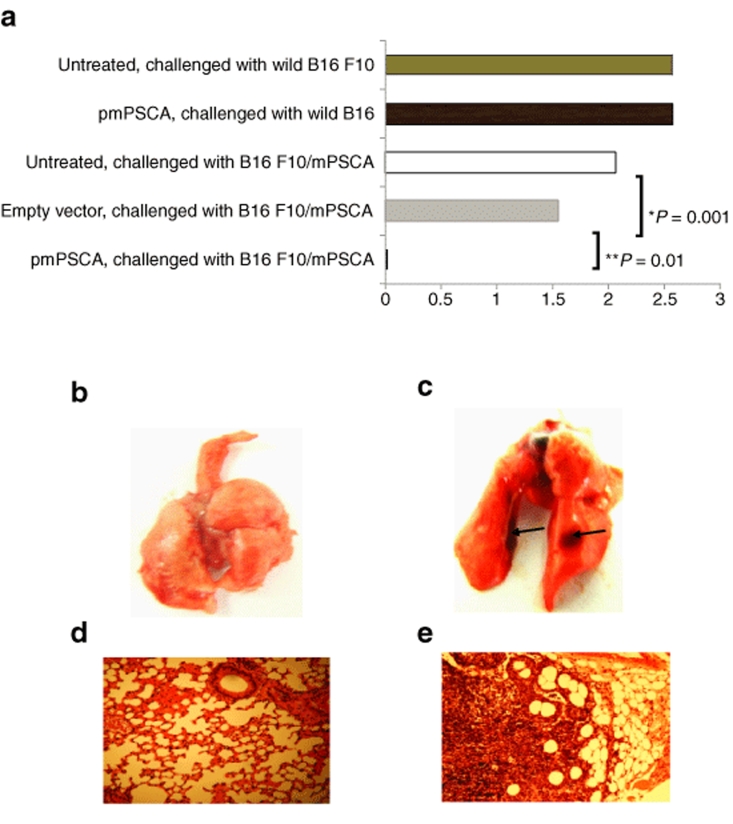
Wild-type B16 F10 and transfected (B16 F10/mPSCA) (Supplementary Materials and Methods) cells were inoculated subcutaneously in C57 BL/6 mice. Although statistically insignificant (P > 0.34), in pmPSCA vaccinated mice, primary tumor volumes were smaller compared with other groups (data not shown). However, immunization provided significant protection against lung metastases. At study end point (primary tumor size 1.5–2 cm in the major dimension), animals were culled and lungs were analyzed macroscopically and microscopically for the presence of metastatic deposits. The protective effects of the vaccine were limited to the B16 F10/mPSCA (only one mouse developed single metastatic deposit) (Figure 5a–e). There was no difference observed in incidence of the lung metastases in the mice challenged with wild B16 F10 cells with or without vaccination (P > 0.88). These findings not only confirmed the specificity of the pmPSCA but also highlighted the ability of the vaccine to prevent spontaneous metastatic development from the growing primary tumors.
Figure 5.
pmPSCA inhibited metastases development. pmPSCA vaccination significantly influences the metastatic capacity of B16 F10 melanoma cell line (stably transfected with mPSCA (B16 F10/mPSCA)). Various groups of mice (n = 6) were given s.c. tumor challenge with either wild type or B16 F10/mPSCA. The metastases were then determined by counting lung surface nodules at study endpoint. (a) Mean numbers of lung metastases. In various groups are shown. The effects pmPSCA are specific to B16 F10/mPSCA tumors and prevented lung metastases in immunized mice (pmPSCA versus untreated *P < 0.01 (both groups received B16 F10/mPSCA tumors), pmPSCA versus empty vector **P = 0.01). The transfected B16 F10 cells (B16F10/mPSCA) retained the metastatic capacity and there was no statistically significant difference in metastatic potential of wild type and transfected B16 F10 cells (P = 0.21). Representative images of the lungs from (b) pmPSCA immunized and (c) untreated mice. Black arrows indicate the lung metastases. Gross presence of metastases in lungs confirmed by H&E staining and analysis microscopically (×100) from (d) pmPSCA immunized and (e) untreated group.
Discussion
In this study, we have shown that the intramuscular delivery of a plasmid DNA vaccine against the PSCA provided significant immune containment of growing prostate cancers and PSCA expressing metastasizing tumors. Additionally, the immune responses were only demonstrable to the tumors. Thus, there is a breaking of tolerance by the vaccine to the PSCA without evident immune reactions to normal PSCA expressing tissues. Possible reasons for an absent immune reaction to the normal prostate may include the lower level expression of the antigen in normal tissues or perhaps because differences in post-translational processes such as glycosylation, may determine the availability of the epitope at cell membrane level.19 Similar results have been obtained in carcinoembryonic antigen transgenic mice models, where vaccination with a vaccinia bearing carcinoembryonic antigen vector resulted in inhibition of gastrointestinal polyp development without injury to carcinoembryonic antigen expressing normal tissues.20 It is encouraging that in this study there was no evidence of autoimmunity by either the vaccination or vaccine-induced tumor destruction for up to 100 days after tumor ablation—all mice were healthy and immune infiltrates were not evident in any of the organs, including the normal prostate.
The effectiveness of a DNA vaccine for any malignant disease including prostate cancer is related to immune responsiveness of the cancer, to the expression and immunogenicity of the candidate gene and to the method of gene delivery. The prostate is a unique nonessential accessory organ that until recently has been considered relatively inaccessible by the immune system. However, a number of studies have shown that prostate cancers responding to hormone therapy are infiltrated with immune cells indicating that at least the neoplastic tissues are accessible to immune traffic.21,22 It would appear that these immune infiltrating lymphocytes have tumor containing functions as their absence or low densities are independently predictive of a poor prognosis.23 The enhanced expression of the PSCA in the prostate cancers and the absence of collateral autoimmune responses by the pmPSCA vaccine suggest a level of immune specificity for the PSCA in prostate cancer that may be exploited for immunotherapy. We demonstrated that muscle EP with a PSCA coding plasmid has the potential to be a powerful vaccine, effective against growing and metastatic cancers. For cancer immunogene therapy/vaccination, a plasmid DNA vector is an attractive and safe alternative to viral-based systems. Plasmid vectors in themselves do not excite specific immune responses or systemic reactions, are thus less toxic than viral-based systems, and may be used repeatedly as in this study. In plasmid, unlike viral systems, the transgene is not integrated in the chromosome, has little risk of mutagenesis, but is episomally transcribed and yet can activate both cellular and humoral responses without adjuvant stimulation.24,25,26,27 In this study, for the in vivo DNA delivery, we used previously reported safe and nontoxic parameters of EP.3,17 Mir et al.14 and Kusumanto et al.28 have respectively demonstrated in vivo that EP significantly improves the transfection efficiency of plasmid DNA into the skeletal muscle above those achievable for naked DNA or ultrasound delivery. EP-assisted intracutaneous DNA vaccination has also been reported to be effective—presumed through antigen expression and presentation by the resident dendritic cells.29 It is possible that there is EP-induced transfection and antigen expression in local antigen-presenting cells within the muscle region. To ensure a continued gene expression and antigen presentation, we elected to give four separate vaccine treatments. However, Mir et al.14 have reported a continued transgene expression in muscle for at least 9 months after a single EP transfection with a plasmid vector containing a cytomegalovirus promoter. It is possible that additional benefits of repeat vaccination could be due to a transfection of new antigen presenting cells transiting the muscle or nonspecific stimulation of the antigen-presenting cells by CpG islands in the plasmid. Further studies are necessary to address the putative advantages, mechanisms, and schedule of repeat vaccination.
The transfection of the muscle with pmPSCA is expected to result in PSCA peptide presentation on the cell surface in the context of the major histocompatibility complex class I molecules. The ensuing CD8+ cytotoxic T cells have both direct cellular and cytokine-mediated antitumor effects. Cross presentation of the PSCA by the major histocompatibility complex class II pathway is also likely to occur from different sources; transfected antigen-presenting cells, shed antigen from the transfected muscles or from dying tumor cells. This cross presentation would elicit helper T-cells (CD4+) responses. Depending on the type of CD4+ cells that binds to the complex, B cells can be activated resulting in production of antibodies. This is the same manner in which traditional vaccines work.30
In preclinical and clinical trials, the Th-1 response has been established to be critical for immune base tumor control.25,31 IFN-γ is a glycoprotein produce primarily by T-helper cells and is intimately associated with the regulation of T-cell mediated cytotoxic immune responses.18 In our study, the postvaccination cytokines responses suggested a Th-1 biased response; the presence of plasmid CpG motifs would also promote these responses as the CpG motifs engage TRL9 on dendritic cells and induce a variety of cytokines, including IFN-γ that drive the immune response toward Th-1.32 This feature of a DNA vaccine may be important in enhancing effective tumor protective immunity.
Important mechanisms of immune escape by a cancer, which may have to be surmounted for maximum vaccine advantage, include tolerance to self-antigens and the acquisition of tolerogenic mechanisms that subjugate effector responses within the growing tumor mass. Although other investigators have explored DNA vaccines in mice using intramuscular immunization and showed responses similar to ours,33,34 the ability to provide tumor protection by breaking tolerance to a self-antigen was not tested. In this study and in a recent report,13 it has been shown that it is possible to break tolerance to the self-antigen, mPSCA, and to provide control of prostate cancer without deleterious consequences to normal tissues. Furthermore, we have shown that the induced-systemic responses inhibited metastatic growth of PSCA expressing cancers albeit without elimination of the “primary” tumor mass. Thus, there are differential responses between the disseminated malignant cells and the tumor mass. A proposed explanation for this is the selective accumulation of T-regulatory cells in the growing tumor mass and in many tumors, including prostate cancer, the presence of the enzyme indoleamine 2,3-dioxygenase, which degrades the essential amino acid and tryptophan. The T-regulatory cells accumulation, tryptophan degradation, and tryptophan degradation products are inhibitory of immune effector cells and compromise locally within the growing tumor the antitumor immune efficacy—in contrast systemic disease at the level of low volume tumorlets are amenable to elimination. However, Degl'Innocenti et al.35 have reported that in the TRAMP mice, the peripheral tolerance is independent from T-regulatory cells and also the inhibition of indoleamine 2,3-dioxygenase did not reverse the tolerance. Hence, further tolerogenic mechanisms are likely and additional strategies are needed to enhance the effectiveness of the vaccine in prostate cancer. The pmPSCA vaccination could be effective in neoadjuvant setting against minimal residual disease and would expand the potential of ablative treatment of the primary prostate cancer. In clinical settings, elimination of minimal residual disease after surgery would be a significant achievement.
The antitumor immune responses from the vaccination were durable, and even in tumor bearing mice conferred a survival advantage suggesting that worthwhile responses may be achievable without total tumor eradication. In tumor rechallenge experiments, we demonstrated that after excision of the tumors, the vaccination induced specific immune responses were also persistent and protected against tumor redevelopment; thus, supporting the concept of neoadjuvant vaccination. Immunotherapeutic approaches such as vaccination for malignant diseases are most likely to be effective in patients with early disease or following removal of primary disease36 when the immune system would not be compromised by immune suppressive effects of disease progression or anticancer cytotoxic drugs.
There is a recent interest in the role of immune base therapies as part of multimodal treatment for advanced cancers including prostate. Because testosterone modulates T- and B-cell immune responses,37 a rational for combining immune therapy with hormone deprivation emerged. Data from phase I and phase II clinical trials suggest that androgen ablation may boost immune responses to cell based and ex vivo primed dendritic cells and thus similar responses would be expected from the more convenient DNA vaccine.38,39 Encouraging antitumor and cytotoxic T-cell responses have been reported in phase II studies to a combination of viral-based vaccinations against prostate-specific antigen and the cytotoxic agent docetaxel40 or to a peptide vaccine with estramustine.41 Radiotherapies stimulate the expression of major histocompatibility molecules and of intercellular adhesion molecule 1,42 and in combination with neoadjuvant, hormonal therapy was found to elicit tumor specific autoantibody responses in nonmetastatic prostate cancer.43 Thus, a DNA-based vaccine to PSCA could be applicable at all stages of prostate cancer, permit repeated delivery, and could easily be incorporated into multimodal programs.
In summary, a mPSCA encoding a plasmid DNA vaccine was evaluated in mice for the development of antigen specific functional antitumor immune responses and tumor control in an experimental prostate cancer model. Tumor specific antitumor immune responses were induced which were active against primary and metastatic tumors. The immune response was Th-1 biased and adoptively transferable. This study suggests that a plasmid based vaccine against PSCA may have potential for clinical development. It would be applicable in both neoadjuvant and adjuvant disease settings and could be included in multimodal treatment programs of prostate cancer.
Materials and Methods
Plasmid construction. Plasmid for DNA vaccination was constructed by cloning the complete coding sequence of mPSCA into the pIRES2 DsRed2 vector (Clontech; Unitech, Dublin, Ireland). The mPSCA was extracted from TRAMPC1 cells; total RNA from TRAMPC1 was isolated using RNeasy mini kit (Qiagen, West Sussex, UK) according to manufacturer's instructions. For reverse transcription–PCR, first strand complementary DNA was synthesized using Omniscript reverse transcription kit (Qiagen). mPSCA complementary DNA was amplified by PCR using Pwo polymerase (Roche, West Sussex, UK) with the forward primer (5′-ACCATGAAGACAGTCTTCTTTC-3) and reverse primer (5′-TCTCCC AGAGCCTACAGAC-3). The PCR conditions included 15 minutes of initial denaturation at 96 °C followed by 32 cycles of 1 minute at 94 °C, 1 minute at 56 °C, and 1 minute at 72 °C. The mPSCA gene was inserted into pCR2.1-TOPO by using TOPO TA cloning kit (Invitrogen, BioSciences, Dublin, Ireland). A 372 bp mPSCA fragment was subcloned into the Ava1–EcoRI sites of pIRES2 DsRed2 downstream of the cytomegalovirus promoter. The resulting plasmid, pmPSCA (5.7 kb) was fully confirmed by restriction enzyme digestion and DNA sequencing (MWG, Munich, Germany). For in vivo vaccination, plasmid DNA was prepared using Endotoxin free mega kit (Qiagen).
In vivo EP-driven vaccine delivery. Mice were anesthetized during all treatments by intraperitoneal administration of 200 mg xylazine and 2 mg ketamine. For vaccine delivery, a custom designed applicator (Cliniporator; IGEA, Modena, Italy) with two needles 4 mm apart was used. Both needles were placed through the skin central to the quadriceps muscle. The muscle was injected between electrode needles with 50 µg plasmid DNA in 50 µl sterile injectable phosphate buffer saline. After 80 seconds, square wave pulses (1200 V/cm 100 ms × 1 and 120 V/cm 20 ms, 8 pulses) were administered in sequence using a custom designed pulse generator (Cliniporator; IGEA). The high voltage pulse was used to induce EP in the cell membrane and the ensuing small voltage pulses were used to create an electrophoretic field to assist movement of the negative charged DNA plasmid across the cells.44,45 In vivo EP has been shown by Mir et al. to be noninjurious to the muscle tissues.46
Cell tissue culture. The murine recycled prostate cancer cell line TRAMPC1 was provided by R.P. Ciavarra47 of Eastern Virginia Medical School, Norfolk, USA. This cell line was originally established by Greenberg et al., from TRAMP.48 TRAMP mice are transgenic C57 BL/6 mice that develop histological prostatic intraepithelial neoplasia by 8–12 weeks of age that progresses to adenocarcinoma with metastases by 24–30 weeks. These mice develop tumors as a result of prostate specific expression of SV40 T antigen driven by minimal probasin promoter. Grossmann et al.49 have demonstrated that TRAMP cells can be grown in an androgen independent manner, capable of expressing major histocompatibility complex class I and susceptible to specific lysis by cytotoxic T lymphocytes. The TRAMPC1 cells were grown in culture at 37 °C in a humidified 5% CO2 atmosphere, in Dulbecco's modified Eagle's medium high glucose with L-glutamine and without sodium pyruvate (Gibco, Paisley, Scotland) supplemented with 5% fetal calf serum, 5% Nu Serum IV (BD Biosciences, Oxford, UK), insulin from bovine pancreas (Sigma, Dublin, Ireland), 10‐8 mol/l 5a-Androstan-17b-ol-3-one (Sigma) and 25 U/ml penicillin and streptomycin.
Experimental animals, tumor induction, and vaccination schedule. Male C57 BL/6 mice (Harlan Laboratories, Oxfordshire, UK) were used in all experiments. The animal ethics committee of University College Cork approved all experiments. The mice were kept at a constant room temperature (RT) (22 °C) with a natural day/night light cycle in a conventional animal colony. All mice were maintained in a pathogen free animal facility for at least 2 weeks before the experiments. Male mice in good condition, without fungal or other infections, 6–8 weeks of age, were included in experiments. For routine tumor induction 5 × 106 TRAMPC1 cells were trypsinized, suspended in 200 ml of serum free Dulbecco's modified Eagle's medium and injected subcutaneously into the right flank of a mouse. The tumor inoculation (subcutaneous) was performed 1 week after the last vaccination. Following establishment, the tumors were monitored by alternate day measurements in two dimensions using a verniers calliper. Tumor volume was calculated according to the formula V = ab2II/6, where a is the longest diameter of the tumor and b is the longest diameter perpendicular to diameter a. From these volumes, tumor growth curves were constructed. A mouse was considered incurable and humanely euthanized when the tumor diameter was between 1.5 and 2 cm. In cases of successful treatment, 100 days subsequent observation without tumor development was considered as cure. All of the immunological data reported is representative of at least two independent studies. Each study was performed with five or six mice per group. In each experiment with a group of pmPSCA vaccinated mice, two control groups were included [one group received pIRES2 DsRed2 (empty vector) and the other group was untreated]. Anesthetized mice were vaccinated by intramuscular injections into alternate quadriceps on weekly interval for 4 weeks with 50 µg/immunization of pmPSCA. (We choose a four-vaccination schedule, as in separate studies using the prostate specific antigen, the immunological responses were superior to two vaccinations.) The mice in the empty vector group were mocked with 50 µg/immunization of pIRES2 DsRed2. EP, as described previously, used to enhance the muscle transfection with given plasmid. Post-transfection the gene expression was confirmed (Supplementary Materials and Methods). The same protocol of the vaccination and tumor inoculation was used in all experiments expect for the studies on established tumors.
Antigen specificity and long term tumor protection. Groups of mice, vaccinated and untreated were challenged with TRAMPC1. When tumors reached ~100 mm3 in size, these were surgically excised and animals were observed for 30 days. Tumor-free mice at that stage were rechallenged with the same tumourogenic dose of TRAMPC1 in the opposite flank. To assess the specificity of the pmPSCA vaccine restricted to TRAMPC1 cells, mice bearing two different tumor types, including B16 F10 melanoma and Lewis lung cancer, were also studied in the same vaccination protocol.
In vitro cytotoxicity assay and adoptive transfer of splenocytes. To assay cytotoxic T lymphocyte activity against TRAMPC1 cells, splenocytes were isolated from mice cured by pmPSCA immunization and from naive mice. The spleen was harvested and in order to induce tumor specific lymphocytes, 2 × 106 splenocytes were incubated with 2 × 105 mitomycin C-treated TRAMPC1 cells in the presence of 25 IU/ml rmIL-2 (Sigma) for 5 days. Lymphoid cells were then harvested, washed three times in serum-free medium and applied as effectors at various effector: target ratios (100:1, 20:1, 1:1), with 2 × 104 TRAMPC1 cells as targets. In vitro cytotoxicity measured as described previously.3 Results of representative experiments are given as the mean ± SD and of multiple experiments as the mean ± SE. The development of an immune mediated antitumor activity was also tested in vivo by a modified Winn assay.3,50
Histological analysis. Tumors from all the study groups were excised, H&E stained (using standard procedures), and analyzed for the presence of inflammatory infiltrate. Similarly, to examine any autoimmune effects on PSCA expressing organs (testis, kidney, and colon), these organs were excised, stained (H&E), and analyzed microscopically. The transferase-mediated dUTPnick end-labeling method3 was used to detect apoptotic cells in tumors.
Enzyme-linked immunosorbent assay for cytokines analysis. Supernatants from splenocytes stimulated similar as for in vitro cytotoxic T lymphocyte responses were collected and tested for cytokines profiles using mouse IFN-γ enzyme-linked immunosorbent assay kit II (BD) and mouse IL-4 enzyme-linked immunosorbent assay set (BD, Oxford, UK). For IFN-γ, briefly, plates were coated with 50 µl/well of enzyme-linked immunosorbent assay diluent. Serially diluted supernatants from splenocytes (stimulated for 3 days) or standard were added (50 µl/well) and incubated for 2 hours at RT. Mouse anti-IFN-γ antibodies at 2 µg/ml were added. After 2 hours of incubation at RT and washing, 100 µl prepared working detector was added and was incubated at RT for 1 hour. After seven washes, 100 µl substrate solution was added to each well and left at RT for 30 minute. Then 50 µl stop solution was added and the absorbance value of each well was measured by a Softmax-Pro microculture plate reader at a 450 nm wavelength. For IL-4 measurements, microwells were coated with 100 µl of captured antibody and diluted in coating buffer, and sealed plates were incubated at 4 °C overnight. After three washes, plates were blocked with 200 µl assay diluents and incubated at RT for 1 hour. After three washes 100 µl of samples or standard added and left at RT for 2 hour. Aspiration and five washes done, 100 µl of working detector (detection Ab+ SAv-HRP) was added to each well and incubated for 1 hour at RT. After washing for seven times (1 minute soaks), 100 µl substrate solution was added to each well and left at RT for 30 minutes in darkness. Finally, 50 µl stop solution was added and plates were read at 450 nm within 30 minutes.
Statistical analysis. The primary outcome variable of the statistical analyses was the tumor volume in each mouse measured at each time point. The principal explanatory variables were the different treatment groups. Tumor volume was analyzed continuously. Treatment groups were analyzed as categorical variables. At each time point, a two-sampled t-test was used to compare mean tumor volume within each treatment group. A P value <0.05 was interpreted as a significant difference. Microsoft Excel 10.0 (Microsoft) was used to manage and analyze data.
SUPPLEMENTARY MATERIALFigure S1. Effects of pmPSCA vaccination on established TRAMPC1 tumors.Figure S2. Absence of collateral autoimmune tissue damage by the pmPSCA vaccine.Materials and Methods.
Supplementary Material
Effects of pmPSCA vaccination on established TRAMPC1 tumors.
Absence of collateral autoimmune tissue damage by the pmPSCA vaccine.
Acknowledgments
We thank Richard P. Ciavarra of Eastern Virginia Medical School, Norfolk, USA, for providing us the TRAMPC1 recycled cell line used in these studies. We also thank Garry Lee, Consultant Histopathologist, Mercy University Hospital, Cork, Ireland for reviewing hisopathological slides. This work was funded by Cork Cancer Research Centre. The authors have no financial conflicts of interest related to the submitted manuscript.
REFERENCES
- Sanda MG, Restifo NP, Walsh JC, Kawakami Y, Nelson WG, Pardoll DM.Molecular characterization of defective antigen processing in human prostate cancer J Natl Cancer Inst 199587280–285.et al [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkord E. Immunology and immunotherapy approaches for prostate cancer. Prostate Cancer Prostatic Dis. 2007;10:224–236. doi: 10.1038/sj.pcan.4500964. [DOI] [PubMed] [Google Scholar]
- Collins CG, Tangney M, Larkin JO, Casey G, Whelan MC, Cashman J, et al. Local gene therapy of solid tumors with GM-CSF and B7-1 eradicates both treated and distal tumors. Cancer Gene Ther. 2006;13:1061–1071. doi: 10.1038/sj.cgt.7700976. [DOI] [PubMed] [Google Scholar]
- Roos AK, Moreno S, Leder C, Pavlenko M, King A., and , Pisa P. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol Ther. 2006;13:320–327. doi: 10.1016/j.ymthe.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Ulmer JB, Wahren B., and , Liu MA. Gene-based vaccines: recent technical and clinical advances. Trends Mol Med. 2006;12:216–222. doi: 10.1016/j.molmed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Ren J, Zheng L, Chen Q, Li H, Zhang L., and , Zhu H. Co-administration of a DNA vaccine encoding the prostate specific membrane antigen and CpG oligodeoxynucleotides suppresses tumor growth. J Transl Med. 2004;2:29. doi: 10.1186/1479-5876-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israeli RS, Powell CT, Fair WR., and , Heston WD. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res. 1993;53:227–230. [PubMed] [Google Scholar]
- Reiter RE, Gu Z, Watabe T, Thomas G, Szigeti K, Davis E, et al. Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci USA. 1998;95:1735–1740. doi: 10.1073/pnas.95.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert RS, Vivanco I, Chen E, Rastegar S, Leong K, Mitchell SC, et al. STEAP: a prostate-specific cell-surface antigen highly expressed in human prostate tumors. Proc Natl Acad Sci USA. 1999;96:14523–14528. doi: 10.1073/pnas.96.25.14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Holt GE, Velders MP, Kwon ED., and , Kast WM. Murine six-transmembrane epithelial antigen of the prostate, prostate stem cell antigen, and prostate-specific membrane antigen: prostate-specific cell-surface antigens highly expressed in prostate cancer of transgenic adenocarcinoma mouse prostate mice. Cancer Res. 2001;61:5857–5860. [PubMed] [Google Scholar]
- Dannull J, Diener PA, Prikler L, Fürstenberger G, Cerny T, Schmid U, et al. Prostate stem cell antigen is a promising candidate for immunotherapy of advanced prostate cancer. Cancer Res. 2000;60:5522–5528. [PubMed] [Google Scholar]
- Gu Z, Thomas G, Yamashiro J, Shintaku IP, Dorey F, Raitano A, et al. Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene. 2000;19:1288–1296. doi: 10.1038/sj.onc.1203426. [DOI] [PubMed] [Google Scholar]
- Garcia-Hernandez Mde L, Gray A, Hubby B, Klinger OJ., and , Kast WM. Prostate stem cell antigen vaccination induces a long-term protective immune response against prostate cancer in the absence of autoimmunity. Cancer Res. 2008;68:861–869. doi: 10.1158/0008-5472.CAN-07-0445. [DOI] [PubMed] [Google Scholar]
- Mir LM, Bureau MF, Rangara R, Schwartz B., and , Scherman D. Long-term, high level in vivo gene expression after electric pulse-mediated gene transfer into skeletal muscle. C R Acad Sci III, Sci Vie. 1998;321:893–899. doi: 10.1016/s0764-4469(99)80003-1. [DOI] [PubMed] [Google Scholar]
- Foldvari M, Babiuk S., and , Badea I. DNA delivery for vaccination and therapeutics through the skin. Curr Drug Deliv. 2006;3:17–28. doi: 10.2174/156720106775197493. [DOI] [PubMed] [Google Scholar]
- Tangney M, Casey G, Larkin JO, Collins CG, Soden D, Cashman J, et al. Non-viral in vivo immune gene therapy of cancer: combined strategies for treatment of systemic disease. Cancer Immunol Immunother. 2006;55:1443–1450. doi: 10.1007/s00262-006-0169-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soden DM, Larkin JO, Collins CG, Tangney M, Aarons S, Piggott J, et al. Successful application of targeted electrochemotherapy using novel flexible electrodes and low dose bleomycin to solid tumours. Cancer Lett. 2006;232:300–310. doi: 10.1016/j.canlet.2005.03.057. [DOI] [PubMed] [Google Scholar]
- Mogensen SC., and , Virelizier JL. The interferon-macrophage alliance. Interferon. 1987;8:55–84. [PubMed] [Google Scholar]
- Turner MS, Cohen PA., and , Finn OJ. Lack of effective MUC1 tumor antigen-specific immunity in MUC1-transgenic mice results from a Th/T regulatory cell imbalance that can be corrected by adoptive transfer of wild-type Th cells. J Immunol. 2007;178:2787–2793. doi: 10.4049/jimmunol.178.5.2787. [DOI] [PubMed] [Google Scholar]
- Hodge JW, Grosenbach DW, Aarts WM, Poole DJ., and , Schlom J. Vaccine therapy of established tumors in the absence of autoimmunity. Clin Cancer Res. 2003;9:1837–1849. [PubMed] [Google Scholar]
- Kelalis PP, Greene LF., and , Harrison EG. Granulomatous prostatitis. A mimic of carcinoma of the prostate. JAMA. 1965;191:287–289. doi: 10.1001/jama.191.4.287. [DOI] [PubMed] [Google Scholar]
- Neaves WB., and , Billingham RE. The lymphatic drainage of the rat prostate and its status as an immunologically privileged site. Transplantation. 1979;27:127–132. doi: 10.1097/00007890-197902000-00011. [DOI] [PubMed] [Google Scholar]
- Vesalainen S, Lipponen P, Talja M., and , Syrjänen K. Histological grade, perineural infiltration, tumour-infiltrating lymphocytes and apoptosis as determinants of long-term prognosis in prostatic adenocarcinoma. Eur J Cancer. 1994;30A:1797–1803. doi: 10.1016/0959-8049(94)e0159-2. [DOI] [PubMed] [Google Scholar]
- Moingeon P. Strategies for designing vaccines eliciting Th1 responses in humans. J Biotechnol. 2002;98:189–198. doi: 10.1016/s0168-1656(02)00131-1. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Nakui M, Sato M, Iwakabe K, Kitamura H, Sekimoto M, et al. The critical role of Th1-dominant immunity in tumor immunology Cancer Chemother Pharmacol 200046S52–S61.Suppl [DOI] [PubMed] [Google Scholar]
- Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- Gurunathan S, Klinman DM., and , Seder RA. DNA vaccines: immunology, application, and optimization*. Annu Rev Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- Kusumanto YH, Mulder NH, Dam WA, Losen M, Losen MH, De Baets MH, et al. Improvement of in vivo transfer of plasmid DNA in muscle: comparison of electroporation versus ultrasound. Drug Deliv. 2007;14:273–277. doi: 10.1080/10717540601098807. [DOI] [PubMed] [Google Scholar]
- Peachman KK, Rao M., and , Alving CR. Immunization with DNA through the skin. Methods. 2003;31:232–242. doi: 10.1016/s1046-2023(03)00137-3. [DOI] [PubMed] [Google Scholar]
- Schirmbeck R., and , Reimann J. Revealing the potential of DNA-based vaccination: lessons learned from the hepatitis B virus surface antigen. Biol Chem. 2001;382:543–552. doi: 10.1515/BC.2001.068. [DOI] [PubMed] [Google Scholar]
- Mosmann TR., and , Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Marshall DJ, San Mateo LR, Rudnick KA, McCarthy SG, Harris MC, McCauley C, et al. Induction of Th1-type immunity and tumor protection with a prostate-specific antigen DNA vaccine. Cancer Immunol Immunother. 2005;54:1082–1094. doi: 10.1007/s00262-005-0687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Trivedi NN, Wilson DM, Mahalingam S, Morrison L, Tsai A, et al. Molecular and immunological analysis of genetic prostate specific antigen (PSA) vaccine. Oncogene. 1998;17:3125–3135. doi: 10.1038/sj.onc.1201736. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Yang JS, Dang K, Manson KH., and , Weiner DB.Engineering enhancement of immune responses to DNA-based vaccines in a prostate cancer model in rhesus macaques through the use of cytokine gene adjuvants Clin Cancer Res 20017882s–889s.3 Suppl [PubMed] [Google Scholar]
- Degl'Innocenti E, Grioni M, Capuano G, Jachetti E, Freschi M, Bertilaccio MT, et al. Peripheral T-cell tolerance associated with prostate cancer is independent from CD4+CD25+ regulatory T cells. Cancer Res. 2008;68:292–300. doi: 10.1158/0008-5472.CAN-07-2429. [DOI] [PubMed] [Google Scholar]
- Rosenberg SA. Cancer vaccines based on the identification of genes encoding cancer regression antigens. Immunol Today. 1997;18:175–182. doi: 10.1016/s0167-5699(97)84664-6. [DOI] [PubMed] [Google Scholar]
- Hsueh EC, Gupta RK, Lefor A, Reyzin G, Ye W., and , Morton DL. Androgen blockade enhances response to melanoma vaccine. J Surg Res. 2003;110:393–398. doi: 10.1016/s0022-4804(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Aragon-Ching JB, Williams KM., and , Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci. 2007;12:4957–4971. doi: 10.2741/2441. [DOI] [PubMed] [Google Scholar]
- Sanda MG, Smith DC, Charles LG, Hwang C, Pienta KJ, Schlom J, et al. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology. 1999;53:260–266. doi: 10.1016/s0090-4295(98)00539-1. [DOI] [PubMed] [Google Scholar]
- Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi M, Itoh K, Yao A, Mine T, Yamada A, Obata Y, et al. Immunological evaluation of individualized peptide vaccination with a low dose of estramustine for HLA-A24+ HRPC patients. Prostate. 2005;63:1–12. doi: 10.1002/pros.20157. [DOI] [PubMed] [Google Scholar]
- Li CY, Huang Q., and , Kung HF. Cytokine and immuno-gene therapy for solid tumors. Cell Mol Immunol. 2005;2:81–91. [PubMed] [Google Scholar]
- Nesslinger NJ, Sahota RA, Stone B, Johnson K, Chima N, King C, et al. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin Cancer Res. 2007;13:1493–1502. doi: 10.1158/1078-0432.CCR-06-1772. [DOI] [PubMed] [Google Scholar]
- Satkauskas S, Bureau MF, Puc M, Mahfoudi A, Scherman D, Miklavcic D, et al. Mechanisms of in vivo DNA electrotransfer: respective contributions of cell electropermeabilization and DNA electrophoresis. Mol Ther. 2002;5:133–140. doi: 10.1006/mthe.2002.0526. [DOI] [PubMed] [Google Scholar]
- Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177:437–447. doi: 10.1046/j.1365-201X.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- Hojman P, Gissel H, Andre F, Cournil-Henrionnet C, Eriksen J, Gehl J, et al. Physiological effects of high and low voltage pulse combinations for gene electrotransfer in muscle Hum Gene Ther 2008. epub ahead of print [DOI] [PubMed]
- Somers KD, Brown RR, Holterman DA, Yousefieh N, Glass WF, Wright GL, et al. Orthotopic treatment model of prostate cancer and metastasis in the immunocompetent mouse: efficacy of flt3 ligand immunotherapy. Int J Cancer. 2003;107:773–780. doi: 10.1002/ijc.11464. [DOI] [PubMed] [Google Scholar]
- Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann ME, Wood M., and , Celis E. Expression, specificity and immunotherapy potential of prostate-associated genes in murine cell lines. World J Urol. 2001;19:365–370. doi: 10.1007/pl00007104. [DOI] [PubMed] [Google Scholar]
- Winn HJ. Immune mechanisms in homotransplantation. II. Quantitative assay of the immunologic activity of lymphoid cells stimulated by tumor homografts. J Immunol. 1961;86:228–239. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effects of pmPSCA vaccination on established TRAMPC1 tumors.
Absence of collateral autoimmune tissue damage by the pmPSCA vaccine.