Abstract
Research on stem cells has progressed at a rapid pace and, as might be anticipated, the results have generated several controversies, a few myths and a change in a major paradigm. Some of these issues will be reviewed in this study with special emphasis on how they can be applied to the adult stem/progenitor cells from bone marrow, referred to as MSCs.
The field of the adult stem/progenitor cells, referred to as MSCs, has progressed so rapidly and covered so many disciplines of basic research and medical therapeutics that it is impossible to present a brief review, but rather only a comprehensive one. More detailed reviews have recently been published.1,2,3,4,5,6,7,8 I apologize to those whose important work has not been included or adequately discussed.
One Controversy: What To Call The Cells?
Since the first reports on MSCs, a series of different names has been assigned to the cells (Table 1). Friedenstein, who is generally credited with the discovery of MSCs, isolated the cells by their tight adherence to tissue culture surfaces and demonstrated their multipotential for differentiation both in culture and in vivo.9,10 Friedenstein et al. were impressed with the spindle-like shapes of the cells in culture and the ease with which they generated single cell–derived colonies. They, therefore, referred to them as “colony forming units-fibroblastic” (CFUs-F). Hematologists who discovered the usefulness of confluent cultures of MSCs as feeder layers for hematopoietic stem cells named them “marrow stromal cells.”11 Caplan focused on their known potential for multilineage differentiation and named them “mesenchymal stem cells.”12,13 A committee of the International Society for Cytotherapy tried to resolve the confusion by suggesting the name “multipotent mesenchymal stromal cells.”14 Some authors have referred to them as simply MSCs (Table 1). Unfortunately, many publications use one name for the cells to the exclusion of others. Therefore, searches of the literature frequently miss many publications, and thereby valuable information is lost. The difficulty in tracking the literature on MSCs is further confounded by the recent evidence that MSCs are either identical to or derived from pericytes.15,16,17
Table 1.
Citations in PubMed as of 5 December 2008
A Related Controversy: Are MSCs Stem Cells?
There is continuing disagreement as to whether MSCs can be called stem cells or perhaps adult stem cells. Unfortunately, the term stem cell has been embedded in the literature with varying degrees of rigor. A newly fertilized egg is the cleanest example of a stem cell that meets the classical criteria: it can divide asymmetrically, and differentiate into all cellular phenotypes. But, as has been repeatedly pointed out, we quickly move down a slippery slope in using the term for similar cells. Embryonic stem cells require a slight loosening of the definition, as they rarely differentiate into the trophoblasts of the placenta.18 Hematopoietic stem cells, epithelial stem cells, and neural stem cells further stretch the definition, because, with a few dissenting opinions, they are generally believed to differentiate only into restricted lineages. MSCs obviously require a similar loosening of the definition of stem cells, as the term is applied to the large family of nonhematopoietic stem-like cells that have been isolated from most mesenchymal tissues such as bone marrow, fat, and blood vessels. The debate on whether MSCs can be called stem cells in part revolves around the question of whether they can be differentiated into nonmesenchymal cells. Some of the earlier observations on differentiation of the cells were probably flawed because of technical limitations such as unreliable labels for the cells and inadequate experience with potential artifacts.19 But, numerous investigators are continuing to report that MSCs or related cells from bone marrow and other tissues can be differentiated into epithelial, endothelial, and neural cells.5,20,21,22,23 At the same time, the definition of a stem cell is further confused with the recognition that the properties of a stem cell depend as much on the niche in which it resides as on the inherent “stemness” of the cell. As concluded in a recent review on hematopoietic stem cells,24 the concept that stem cells differentiate in a hierarchical manner dictated by the inherent properties of the cell is a “seductive over-simplification.” It ignores the dynamic interaction between cellular niches that determine the fates of stem cells. In effect, the emphasis on hierarchical differentiation of hematopoietic stem cells overlooked the critical role of niches that was clearly demonstrated by earlier studies in simpler systems, such as oogenesis and spermatogenesis in Drosophila.25
Of course, the definition of a stem cell is blurred still further by nuclear transfer experiments and the more recent experiments with induced pluripotent stem cells in which even a transient exposure to the appropriate transcription factors is sufficient to reprogram the genome to a stem-like state.26 The results suggest that differences between a stem cell and a fully differentiated cell are primarily a question of the ease with which external or internal signals can change the microenvironment of the nucleus sufficiently to redirect the phenotype of the cell.
How can we resolve the current confusion concerning the definition of a stem cell? Unfortunately, we cannot yet fully define the state of any cell in terms of all its transcripts, its epigenetic status, and especially its proteomics (see recent review in ref. 27). Also, a static picture in time of a cell is probably not sufficient. The essence of a stem cell is not its status at a given point in time. It is the potential for change in an almost Aristotelian sense. Unfortunately, again, we do not have the means of defining the potential of a cell in a quantitative manner. The only practical solution to the current confusion is apparently in trying to convey the context of each type of cell with qualifiers such as hematopoietic stem cells or adult stem/progenitor cells.
A Myth: All Cultures Of MSCs Are The Same
Confluent cultures of MSCs have the uniform appearance of fibroblast-like cells. They retain the uniform appearance if passed many times as high density cultures. Therefore, it has frequently been assumed that all cultures of MSCs are the same and that they are homogeneous. The assumption overlooks the dynamic nature of MSC cultures that was clearly documented by early investigators in the field. For example, Mets and Verdonk28 examined early passage, low density cultures. They demonstrated that the cells that initially adhere to tissue culture surfaces are spindle-shaped and very rapidly proliferating. Mets and Verdonk referred to the cells as type 1 cells; we more recently referred to them as rapidly self-renewing MSCs (RS-MSCs).29 Mets and Verdonk noted that the type 1 cells give rise to larger, slowly replicating type 2 cells or slowly replicating MSCs (SR-MSCs). They were impressed by the dramatic transition of the cells and suggested that cultures of MSCs were a model for aging in vitro. Numerous subsequent publications documented the dramatic differences between type 1 or RS-MSCs and the type 2 or SR-MSCs, including the patterns of expressed genes, expression of surface epitopes, clonogenicity, potential to differentiate, and tendency to generate lethal pulmonary emboli after intravenous infusion.30,31 Remarkably, and as also recognized by early investigators (for review, see ref. 32), MSCs from early passage cultures can be reprogrammed to generate type 1 or RS-MSCs, if they are lifted before confluence and replated at low density. The assumption that all MSCs are the same also ignores the old observation that human MSCs (hMSCs) senesce after ≥25 population doublings in culture, particularly if expanded as high density cultures.33,34 As the cells senesce, they propagate slowly, decrease their clonogenicity, and lose most of their potential to differentiate. In effect, they lose the plasticity characteristic of the early passage, low density MSCs. Therefore, it is apparent that MSCs isolated and expanded under different conditions in culture differ dramatically in their properties and probably in their therapeutic potentials.
In addition, the frequent assumption that MSCs from different tissues such as fat are the same as MSCs from bone marrow probably ignores important differences in biology and therapeutic potentials of the cells that our technology is still too crude to define (see review in ref. 1). As one example of the differences, MSCs from biopsies of synovial tissue were found to be more effective in repair of cartilage defects of the knee of rabbits than MSCs from bone marrow, muscle, or fat tissue.35
At the same time, there is considerable confusion as to the inherent differences between MSCs and similar plastic adherent cells that are isolated from marrow with different protocols and assigned different names. The list of such cells includes marrow-isolated adult multilineage inducible (MIAMI) cells that were isolated from marrow of vertebral bodies under low oxygen and that appear to be particularly efficient at differentiation into neural cells.36 It includes very small embryonic-like (VSEL) stem cells that were isolated from murine bone marrow by selection of cells positive for the chemokine receptor CXCR4 and that express markers of embryonic cells.6 They also include multipotent adult progenitor cells (MAPCs) that were isolated from bone marrow under low oxygen and low serum and that differentiate into multiple lineages.37 In addition, they include hMSCs that were subjected to serum deprivation and that demonstrated enhanced expression of Oct-4 and other genes characteristic of embryonic cells.38 Each of these apparently distinct cells may be earlier progenitors in the hierarchy of MSCs. Alternatively, the cells such as MAPCs that do not appear until the cultures are extensively expanded may be products of culture-induced changes in MSC-like cells.
How can we resolve the confusion generated by the use of different preparations of MSCs and MSC-like cells? More detailed comparisons of the cells in the same laboratories will be of some help. The most critical question is probably which cell preparations will be the most effective for therapy of specific diseases. This question probably cannot be answered until reproducible in vivo models are developed that can become assays for the cells that are as effective as the marrow-ablated mouse is for assays of hematopoietic stem cells. (Note: To facilitate comparison among different cell preparations, we have established an NIH/NCRR funded center for distribution of MSCs prepared with standardized protocols that enrich cultures for early progenitor cells. The center has distributed the cells to over 250 laboratories in this country and abroad. Contact: msc@medicine.tamhsc.edu)
A Paradigm For The Expansion of MSCs In Culture: They Create Their Own In Vitro Niches
The dramatic changes of MSCs during expansion in culture can be explained by the ability of the cells to generate their own microenvironments or in vitro niches as they are plated at low density to generate single cell–derived colonies. In the colonies, subpopulations of the cells serve as nurse cells for other subpopulations. After early passage MSCs are plated at low density, they pass through a lag phase followed by a rapid exponential growth phase in which they double in <12 hours.39 The exponential growth phase of MSCs in culture is too rapid to be sustainable for any length of time in vivo. It is also more rapid than the proliferation of fibroblasts and most other cells cultured in serum. Consequently, it must reflect the release of the cells from the microenvironments or niches that limit their proliferation in vivo. The rapid growth phase is driven by some cells in the clonal colonies secreting Dkk-1, an inhibitor of the canonical Wnt signaling pathway, and thereby creating an in vitro niche at this stage.40 During the rapid growth phase, the cells are spindle-shaped type 1 or RS-MSCs. The cells express surface proteins that are anti-cell adhesion and linked to cell motility (Figure 1), proteins such as α6-integrin and podocalyxin-like protein (PODXL) that is a member of the CD34 family of sialomucins.31 Therefore, the colonies are loose41 and the cells are highly motile.42 As the colonies expand, secretion of Dkk-1 decreases and expression of PODXL and the related proteins are lost. The colonies then enter a near stationary or plateau phase, as the colonies become more tightly packed and develop distinct inner and outer regions.41 The inner regions form a distinct in vitro niche from the outer regions (Figure 2) in that the cells in the inner regions proliferate slowly and they begin to express proteins associated with differentiation.39,41 If the colonies are transferred to adipogenic medium, cells in the other regions that begin to differentiate move toward the inner regions.39 The commitment of the inner regions to differentiation is readily reversible at this stage in that replating either the inner or outer regions generates single cell–derived colonies with the same characteristics as the initial colonies.41 At each replating of a colony at clonal density, the daughter colonies are heterogeneous both in size and their potential to differentiate.34,43 Therefore, the clones and the in vitro niches are created in a stochastic manner, and they are easily dispersed and recreated (Figure 3). The reversibility of the cultures, however, markedly decreases if the colonies are allowed to expand to confluency in that there is a dramatic decrease in clonogenicity (from 90% CFUs-F to <20%), a decrease in the potential for multilineage differentiation, and increased expression of epitopes such as STRO-1 and GD-2 (ref. 31). However, even in confluent cultures that are passed several times, a fraction of the cells remain clonogenic, suggesting that there is persistence of one or more in vitro niches containing different subpopulations.
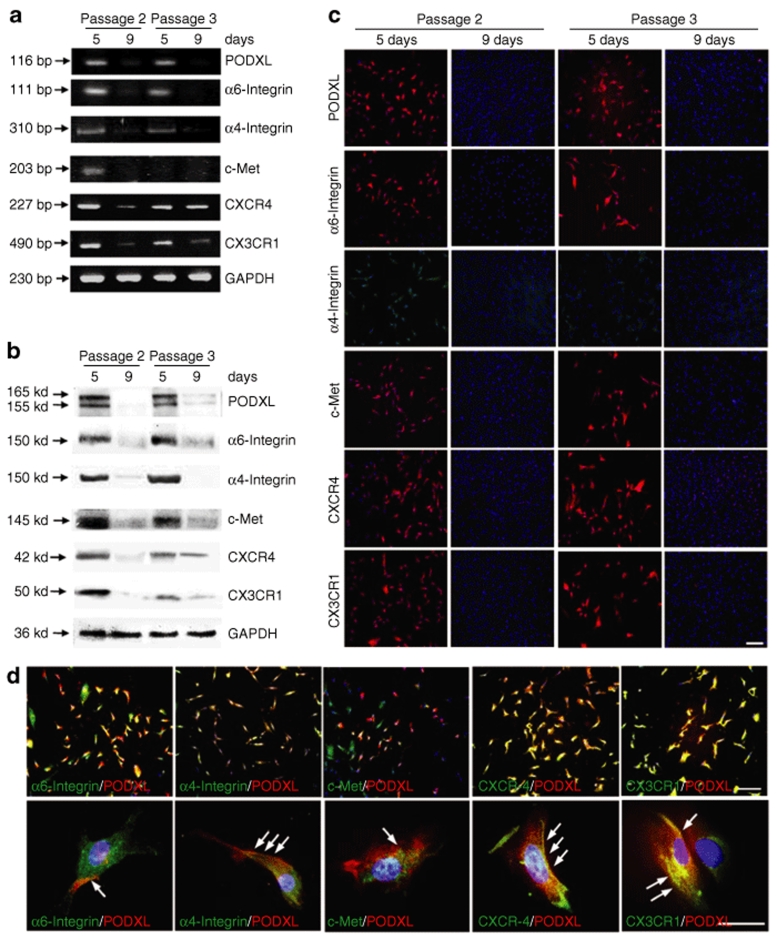
Figure 1.
Surface epitopes reversibly present in low density cultures enriched for rapidly self-renewing MSCs (RS-MSCs), but not as the cells were expanded toward confluency. Human MSCs (hMSCs) passage 1 that were plated at 100 cells/cm2 and incubated for 5 days or 9 days to generate passage 2 MSCs. To prepare passage 3 MSCs, 9-day cultures were lifted with trypsin/EDTA and replated at 100 cells/cm2 for incubation for 5 or 9 days. (a) RT-PCR assays. (b) Western blot assays. (c) Assays by immunocytochemistry. Bar = 200 µm. (d) Double-immunostaining for podocalyxin-like protein (PODXL) (red) and the five other surface proteins (green). Nuclei were labeled with DAPI (blue). Upper panels: bar = 200 µm. Lower panels: bar = 50 µm. Arrows: regions of the cells in which PODXL and other proteins are colocalized. With permission from ref. 31.
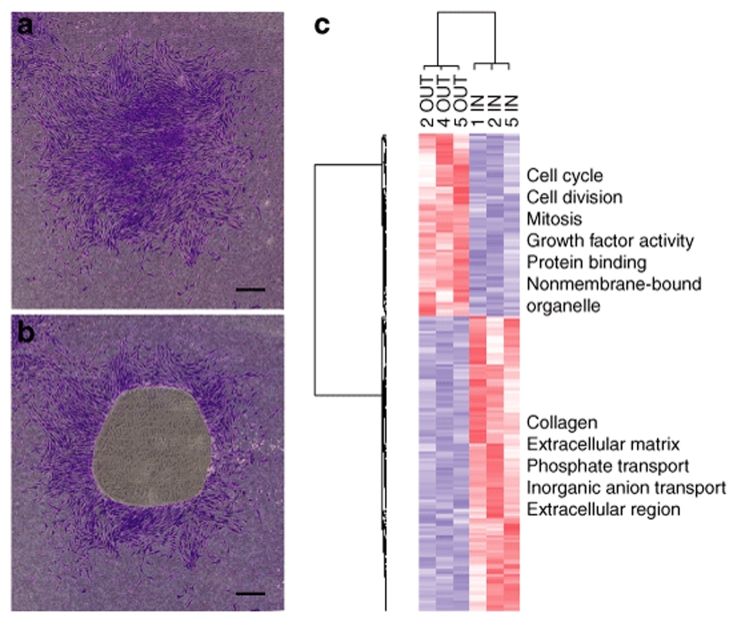
Figure 2.
Demonstration of an in vitro niche found in single cell–generated colony of human MSCs (hMSCs). hMSCs were plated on slides for laser microdissection at 2 cells/cm2 and incubated for 12 days without medium change. The colonies in the figure were stained with crystal violet for illustrative purposes only. (a) Intact colony. (b) Colony after IN region was captured by laser microdissection. (c) Heat map and gene ontologies from microarray data from IN and OUT samples from four colonies (numbers 1, 2, 4, and 5) using 199 differentially expressed genes. Bar in a, and b = 500 µm.
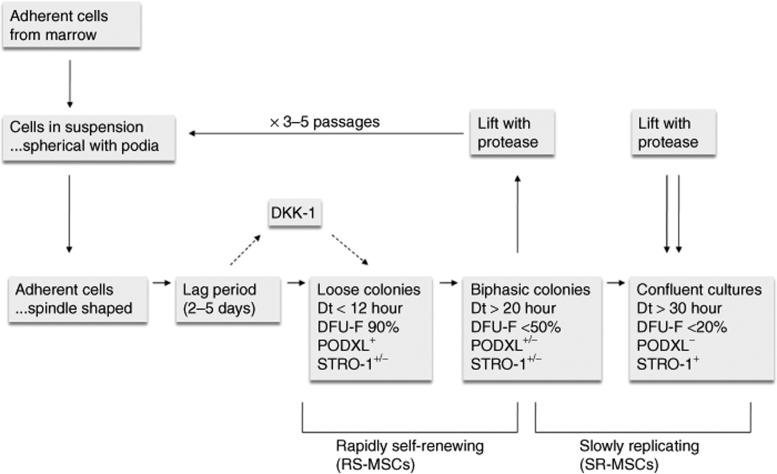
Figure 3.
Schematic summarizing changing properties of human MSCs (hMSCs) expanded in culture. MSCs in suspension are spherical with multiple podia94 but become spindle-shaped after adherence to culture surfaces. The cells then undergo a lag period followed by a rapid growth with doubling time (Dt) of <12 hour that is driven by expression of the Wnt inhibitor Dkk-1. During the rapid growth period, the small rapidly self-renewing MSCs (RS-MSCs) express a characteristic set of surface epitopes, including podocalyxin-like protein (PODXL) (Figure 1). They are weakly positive to STRO-1 and are highly clonogenic.31 As single cell–derived colonies expand, they develop distinct inner and outer regions that define in vitro niches. The colonies reach a near stationary phase with Dt of over 20 hour. The cells begin to lose expression of PODXL, express higher levels of STRO-1, and decrease in clonogenicity. If lifted before confluency and replated at low density, the sequence is repeated through four to seven passages.
A Distracting Controversy: “Adult Stem Cells Can Only Fuse”
A heated and probably unnecessary controversy was generated several years ago by a dramatic publication suggesting that hematopoietic stem cells and neural stem cells could differentiate into multiple cell lineages.44 Some of the most dramatic results were subsequently shown to arise from cell fusion under the strong selective pressure that was a key component of the experiments.45 Unfortunately, the results prompted several prominent scientists to conclude that cell fusion accounted for all previous observations on differentiation by stem/progenitor cells from bone marrow and other tissues of adults. The contention was unfortunate because it was advanced without examination of extensive data on the differentiation of MSCs under conditions in which fusion with differentiated cells was not possible. Multiple investigators repeated Friedenstein's experiments of the 1970s and demonstrated that the cells could be readily differentiated in culture and in vivo into osteoblasts, adipocytes, and chondrocytes without any contact with differentiated cells of the lineage, see ref. 32. Also, it was easy to demonstrate by light microscopy that the cells formed single cell–derived colonies and most of them were capable of trilineage differentiation.34,43,46 Perhaps we and the many others working with MSCs should have confronted the issue more directly,47 but graduate and postdoctrate students studying MSCs were not easily persuaded that they could publish data demonstrating the obvious multipotentiality of clonal colonies of MSCs. To a lesser extent, we were concerned that the results might fan the political debate, which at that time was threatening the cutoff of all research on human embryonic stem cells in the United States and several other countries.
Another Myth: MSCs Can Be Expanded Indefinitely In Culture
Stem/progenitor cells that remained stable as they were expanded indefinitely in culture would be extremely valuable as reagents for experimental manipulations and for therapy. Unfortunately, as has long been recognized, DNA replication is not an error-free process.48,49,50 Therefore, every division of a cell has a small but not infinitesimal chance of producing mutations, some of which can be carcinogenic. Unfortunately, current methods for detecting malignant mutations are highly insensitive. Karyotyping can detect only large chromosomal alterations and provides data on only a small fraction of the cells in a preparation. Tests for tumorigenicity in immunodeficient mice are also insensitive, as many human cancers do not produce tumors in mice without extensive manipulations.51,52,53 Microchip assays for mutations are becoming more sensitive, but they are still limited to spatial regions of several kilobases (http://www.dnavision.be/pharmacogenetics/agilent). In addition, as the assays of genome for mutations become more sensitive, a very large database will have to be developed to distinguish neutral polymorphisms from tumorigenic mutations.
Therefore, it is apparent that expansion of cells in culture can produce genomic alterations that affect the experimental properties of the cells. In addition, they pose a danger to patients that must be balanced by the potential benefits.54 Perhaps the most reliable test is still the demonstration that the cells are not immortal in culture. Fortunately, hMSCs are readily observed to senesce in culture. Also, they have not been found to develop significant genomic instability or become tumorigenic unless extensively expanded over many weeks under stressful culture conditions.55,56 The risks of expansion in culture can probably be avoided by isolating MSCs from adipose tissue, because the initial yields are so large that expansion in culture is not necessary for most applications.57,58 However, it is not yet clear whether MSCs from adipose tissue share all the properties and therapeutic benefits of the MSCs from bone marrow.
A Major Experimental Barrier: Mouse MSCs Readily Undergo Multistage Carcinogenesis In Culture
MSCs can be isolated from mouse bone marrow by plating the marrow to recover adherent cells. However, mouse MSCs differ from MSCs from most other species in that the initial cultures are heavily contaminated by hematopoietic cells.59 Also, the cells rapidly synthesize large amounts of extracellular matrix, which makes them very difficult to lift for passage even with extensive treatment with proteases and chelators. In addition, MSCs from different inbred strains have different media requirements for optimal expansion and have different potentials for differentiation.60 But the most troubling feature of cultures of mouse MSCs is that they grow slowly for many weeks, after which they suddenly proliferate rapidly and become tumorigenic as their genomes become unstable.61,62 In effect, they recapitulate the properties of mouse fibroblasts that first expand slowly in culture, pass through “crisis” phase in which most cells die, and then the remaining cells develop genomic instability that allows them to proliferate and become tumorigenic—a process that has been referred to as “multistage carcinogenesis in cell culture.”63 Unfortunately, the problems are not resolved by cloning the expanded murine MSCs. The genomes are highly unstable and continue to undergo further changes. As a consequence, it is very difficult to harness the power of mouse genomics in studying MSCs. MSCs from rats may show the same tendencies to transform in culture, but they have not been extensively examined.64
New Paradigm: Repair Of Tissues With Limited Engraftment
The ability of MSCs to expand in culture and differentiate into multiple cellular phenotypes suggested that they were potentially useful as therapeutic agents for the repair of tissues by engrafting and differentiating to replace necrotic or apoptotic cells.12,32 The suggestion was supported by observations in experimental animals in which the cells homed to injured tissues and appeared to differentiate to replace injured cells. Engraftment and differentiation of the cells was particularly apparent in rapidly growing neonates, embryos, fetuses, or adult animals with severely injured tissues.65,66,67,68,69,70 Many of the early experiments were handicapped by the lack of appropriate labels and assays to follow MSCs after in vivo administration, particularly as the cells proliferated and differentiated.19 But many of the observations have now been confirmed and extended with more refined techniques in continuing reports that MSCs improve the repair of tissues by differentiation and even transdifferentiation.69,70 However, it became apparent that in many situations the cells produce repair and functional improvement in injured tissues without significant engraftment or differentiation.71,71,72,73 Obviously, there was a need for a new paradigm: tissue repair by MSCs through paracrine secretions and cell-to-cell contacts.
In retrospect, the new paradigm of repair without engraftment was foreshadowed by many earlier observations including the ability of confluent cultures of MSCs to serve as feeder layers for hematopoietic stem cells,11 and the observation that MSCs in culture secreted a large number of cytokines, chemokines, and other factors.74,75 It was also foreshadowed by the observations that MSCs suppressed the mixed lymphocyte reaction,76 reduced immune rejection of skin grafts,77 improved some patients with severe graft-versus-host disease,78 and had therapeutic effects in a EAE mouse model for multiple sclerosis.79 There is now an overwhelming evidence that although MSCs can contribute to tissue repair in some circumstances by differentiation and transdifferentiation, functional improvements are observed in many animal models and a few patients by transient appearances of the cells. In effect, MSCs can repair tissues through “touch and go” effects.2
A Critical Feature Of The New Paradigm: Mscs Are Activated By Their Microenvironments
A critical feature of the new paradigm is that repair of tissues by MSCs is not solely dependent on the rich mixture soluble factors produced by MSCs in isolated cultures. Instead, the MSCs are activated by cross talk with the microenvironment generated by injured tissues to express factors that seem to be specifically tailored to the immediate needs of the tissue.
The responsiveness of MSCs to microenvironments is amply illustrated by the rich literature on the effects of the cells on the immune system.2,8 MSCs were demonstrated to interact with a broad spectrum of cells, including several types of T cells, natural killer cells, dendritic cells, monocytes, and neutrophils. Most, but not all, of the experiments indicated that MSCs decreased proliferation of target cells either through cell-to-cell contacts, secretion of factors, or a combination of cell-to-cell contacts and secretion of factors such as prostaglandins, indoleamine 2,3-dioxygenase, soluble HLA-G5, IL-6, IL-10, TGB-β1, HGF, BiNOS, and heme oxygenase-1. The data were generated primarily by experiments in culture and the in vivo outcomes of the interactions are difficult to predict.2
One specific example of activation of MSCs in an immune setting was the report that MSCs were activated by interferon-γ together with one of three other cytokines to express nitrous oxide and several cytokines.80 The cytokines drove migration of T cells to the MSCs and the nitrous oxide then inhibited T cell proliferation. The effects were not observed with MSCs from transgenic mice that were null for iNOS and interferon-γ.
As another example, hMSCs injected into the hippocampus of mice following transient global ischemia upregulated expression of 170 human genes that were either not expressed or expressed at lower levels by the same cells in culture.81 The upregulated genes were largely involved in anti-inflammatory or anti-immune genes.
As still another example, fibroblasts made apoptotic by ultraviolet irradiation activated hMSCs to suppress apoptosis by secretion of stanniocalcin-1, a calcium metabolizing hormone.82
The cross talk that activates MSCs to produce therapeutic agents in the microenvironment of injured tissues supports the suggestion currently pursued by several investigators that one of the most useful therapeutic strategies will be to inject the cells locally to enhance tissue repair.
Under some circumstances, the cross talk that activates MSCs is sometimes deleterious. Coincubation of hMSCs with multiple myeloma cells initiated a cross talk whereby the hMSCs increased expression of IL-6 and the IL-6 increased expression of Dkk-1 by the myeloma cells.73,83 As a result, the myeloma cells were stimulated to proliferate. At the same time the hMSCs were fixed in cell cycle and inhibited from differentiating to osteoblasts. The net effect was a vicious cycle that could prevent the repair of the lytic bone lesions seen in patients. Similar kinds of cross talk probably accounts for the observations that MSCs home to and increase the growth of some but not all cancers.84,85,86 Also, the cross talk that modulates immune responses may be harmful under some circumstances.
A Remaining Puzzle: How Do Intravenously Infused MSCs Repair Distal Tissues?
Secreted factors and cell contact mediated effects of MSCs do not in any simple way explain one of the puzzling observations made by many investigators: intravenous infusions of MSCs improved repair of multiple organs such as bone,66,87 ischemic brain,88 heart,73 and pancreas89 and modulated the immune system.2
The observations are puzzling because it was conclusively demonstrated that essentially all intravenously infused MSCs are trapped in the lung90,91 where they appear as emboli in afferent blood vessels.31 We recently infused hMSCs intravenously into mice and followed the fate of the human cells with quantitative PCR assays for the human-specific Alu sequences and human-specific mRNA for GAPDH,31 (R.-H. Lee, A.A. Pulin, M.J. Seo, D.J. Kota, B.L. Larson, L. Semprun-Prieto et al., manuscript submitted). The results confirmed the previous observations: over 95% of the infused cells were cleared from the blood within 5 minutes and most were trapped in the lungs. The hMSCs disappeared from the lungs with a half-life of about 24 hours, but <5% of the cells appeared in seven other tissues that were assayed (R.-H. Lee, A.A. Pulin, M.J. Seo, D.J. Kota, B.L. Larson, L. Semprun-Prieto et al., manuscript submitted).
How can intravenously infused MSCs repair distal organs or modulate the systemic immune system (Figure 4)? One possibility is that the cells trapped in the lung secrete soluble factors into the blood stream that enhance repair of the other tissues by suppressing inflammatory and immune reactions or perhaps by stimulating propagation and differentiation of tissue-endogenous stem cells.92 However, the soluble factors would have to be secreted in a transient burst and, probably, at high concentrations. There are no ready candidates for such soluble factors because most cytokines, chemokines, and related molecules produce toxic effects if infused in high concentrations. An alternative possibility is that the effects are produced by the small number of MSCs that escape trapping in the lung and that home to the injured tissues. After MSCs were intravenously infused into mice with permanent ligations of the coronary arteries, there was an increase in MSCs recovered in heart (R.-H. Lee, A.A. Pulin, M.J. Seo, D.J. Kota, B.L. Larson, L. Semprun-Prieto et al., manuscript submitted). However, the number was small and <0.2 % of the 1–2 million infused cells for a total of <2,000 MSCs per heart. Also, the cells disappeared from the heart in 24 hours. It seems unlikely that such transient engraftment of a small number of MSCs can explain the marked improvement in heart function and infarct size observed 3 weeks later.73 Also, the secretion of soluble factors from the lung or the low levels of engraftment in injured tissues do not offer a ready explanation for how intravenously infused MSCs can modulate the systemic immune responses to skin grafts or the EAE model for multiple sclerosis.2 The lung contains lymphatics and immune reactions are prominent in pulmonary diseases such as asthma and chronic obstructive pulmonary disease.93 However, there is no obvious means by which MSCs trapped in the lung or the small number of cells that appear in lymph nodes2 can transmit their immune modulatory effects to other tissues, particularly the effects that require cell-to-cell contacts.
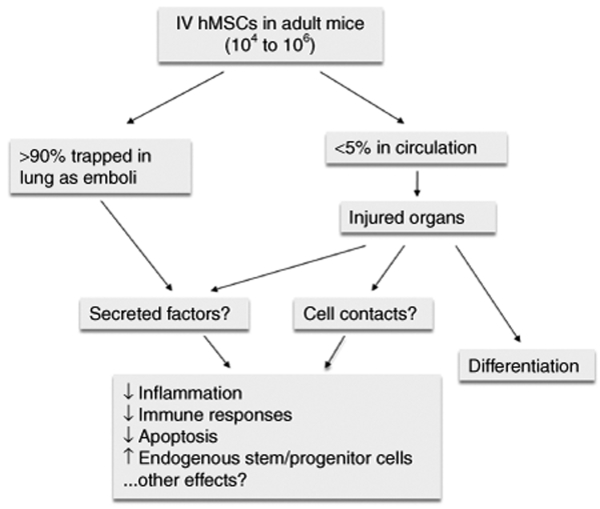
Figure 4.
Schematic summarizing the incompletely explained effects of MSCs on tissue repair after intravenous infusion into mice. As indicated, there are several unanswered questions as to how intravenously infused MSCs can modulate systemic immune responses or repair distal tissues.
Summary
Research on MSCs was initially stimulated by the ease with which they were isolated by their adherence to tissue culture surfaces and their ability to differentiate. In spite of intensive research by hundreds of investigators, there are still major limitations in our protocols for isolating and characterizing the cells, particularly as the cells are expanded in culture. Also, we have a limited understanding of their normal biological functions and how they participate in the repair of tissues. Therefore, we are largely at a loss to explain the beneficial effects that have been repeatedly observed after administration of the cells in animal models for disease and in some patients. In effect, we are forced to work in a reverse direction from most research, i.e., from the beneficial effects observed in vivo to the cellular and molecular mechanisms to explain them. The end of the story is difficult to predict. We still do not know the limits of the usefulness of MSCs in clinical medicine. It is possible that other types of cells such as endothelial cells, or alternatively activated macrophages, mast cells, or dendritic cells may produce the same or greater therapeutic benefits as MSCs. It is also possible that therapies with the proteins or cytokines produced by activated MSCs or related cells may be more practical than cell therapies. However, they may be singularly powerful therapeutic agents. We cannot exclude the possibility that MSCs may have a unique ability to monitor the microenvironment of injured tissues and respond appropriately.
REFERENCES
- Bianco P, Robey PG., and , Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelli A, Moretta L., and , Pistoia V.Mesenchymal stem cells in health and disease Nat Rev Immunol 2008. epub ahead of print [DOI] [PubMed]
- Parr AM, Tator CH., and , Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40:609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- Chen FH., and , Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther. 2008;10:223. doi: 10.1186/ar2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezawa M. Systematic neuronal and muscle induction systems in bone marrow stromal cells: the potential for tissue reconstruction in neurodegenerative and muscle degenerative diseases. Med Mol Morphol. 2008;41:14–19. doi: 10.1007/s00795-007-0389-0. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Zuba-Surma EK, Machalinski B, Ratajczak J., and , Kucia M. Very small embryonic-like (VSEL) stem cells: purification from adult organs, characterization, and biological significance. Stem Cell Rev. 2008;4:89–99. doi: 10.1007/s12015-008-9018-0. [DOI] [PubMed] [Google Scholar]
- Stagg J., and , Galipeau J. Immune plasticity of bone marrow-derived mesenchymal stromal cells. Handb Exp Pharmacol. 2007. pp. 45–66. [DOI] [PubMed]
- Nauta AJ., and , Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- Owen M., and , Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Gorskaja JF., and , Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- Eaves CJ, Cashman JD, Sutherland HJ, Otsuka T, Humphries RK, Hogge DE, et al. Molecular analysis of primitive hematopoietic cell proliferation control mechanisms. Ann NY Acad Sci. 1991;628:298–306. doi: 10.1111/j.1749-6632.1991.tb17260.x. [DOI] [PubMed] [Google Scholar]
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Ahn MY, Zhang ZG, Tsang W., and , Chopp M. Endogenous plasminogen activator expression after embolic focal cerebral ischemia in mice. Brain Res. 1999;837:169–176. doi: 10.1016/s0006-8993(99)01645-5. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L, Caplan AI., and , Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287–2299. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- He S, Pant D, Schiffmacher A, Meece A., and , Keefer CL. Lymphoid enhancer factor 1-mediated Wnt signaling promotes the initiation of trophoblast lineage differentiation in mouse embryonic stem cells. Stem Cells. 2008;26:842–849. doi: 10.1634/stemcells.2007-0356. [DOI] [PubMed] [Google Scholar]
- Phinney DG., and , Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- Greco SJ., and , Rameshwar P. Enhancing effect of IL-1α on neurogenesis from adult human mesenchymal stem cells: implication for inflammatory mediators in regenerative medicine. J Immunol. 2007;179:3342–3350. doi: 10.4049/jimmunol.179.5.3342. [DOI] [PubMed] [Google Scholar]
- Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, et al. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 2008;177:701–711. doi: 10.1164/rccm.200706-859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue WM, Liu W, Bi YW, He XP, Sun WY, Pang XY, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, reduce neointimal formation, and enhance endothelial function in a rat vein grafting model. Stem Cells Dev. 2008;17:785–793. doi: 10.1089/scd.2007.0243. [DOI] [PubMed] [Google Scholar]
- Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, et al. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci USA. 2003;100:2397–2402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin SH., and , Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MT., and , Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Nagaya M, Utikal J, Weir G., and , Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Service RF. Proteomics. Proteomics ponders prime time. Science. 2008;321:1758–1761. doi: 10.1126/science.321.5897.1758. [DOI] [PubMed] [Google Scholar]
- Mets T., and , Verdonk G. In vitro aging of human bone marrow derived stromal cells. Mech Ageing Dev. 1981;16:81–89. doi: 10.1016/0047-6374(81)90035-x. [DOI] [PubMed] [Google Scholar]
- Colter DC, Class R, DiGirolamo CM., and , Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory CA, Ylostalo J., and , Prockop DJ. Adult bone marrow stem/progenitor cells (MSCs) are preconditioned by microenvironmental “niches” in culture: a two-stage hypothesis for regulation of MSC fate. Sci STKE. 2005;2005:pe37. doi: 10.1126/stke.2942005pe37. [DOI] [PubMed] [Google Scholar]
- Lee RH, Seo MJ, Pulin AA, Gregory CA, Ylostalo J., and , Prockop DJ. The CD34-like protein PODXL and α6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood. 2009;113:816–826. doi: 10.1182/blood-2007-12-128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R., and , Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107:275–281. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- Koga H, Muneta T, Nagase T, Nimura A, Ju YJ, Mochizuki T, et al. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell Tissue Res. 2008;333:207–215. doi: 10.1007/s00441-008-0633-5. [DOI] [PubMed] [Google Scholar]
- Tatard VM, D'Ippolito G, Diabira S, Valeyev A, Hackman J, McCarthy M, et al. Neurotrophin-directed differentiation of human adult marrow stromal cells to dopaminergic-like neurons. Bone. 2007;40:360–373. doi: 10.1016/j.bone.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Aranguren XL, McCue JD, Hendrickx B, Zhu XH, Du F, Chen E, et al. Multipotent adult progenitor cells sustain function of ischemic limbs in mice. J Clin Invest. 2008;118:505–514. doi: 10.1172/JCI31153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochampally RR, Smith JR, Ylostalo J., and , Proctop DJ. Serum deprivation of human marrow stromal cells (hMSCs) selects for a subpopulation of early progenitor cells with enhanced expression of OCT-4 and other embryonic genes. Blood. 2004;103:1647–1652. doi: 10.1182/blood-2003-06-1967. [DOI] [PubMed] [Google Scholar]
- Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG., and , Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- Gregory CA, Singh H, Perry AS., and , Prockop DJ. The Wnt signaling inhibitor dickkopf-1 is required for reentry into the cell cycle of human adult stem cells from bone marrow. J Biol Chem. 2003;278:28067–28078. doi: 10.1074/jbc.M300373200. [DOI] [PubMed] [Google Scholar]
- Ylostalo J, Bazhanov N., and , Prockop DJ. Reversible commitment to differentiation by human multipotent stromal cells in single-cell-derived colonies. Exp Hematol. 2008;36:1390–1402. doi: 10.1016/j.exphem.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Hsu SC, Munoz J, Jung JS, Lee NR, Pochampally R, et al. A subset of human rapidly self-renewing marrow stromal cells preferentially engraft in mice. Blood. 2006;107:2153–2161. doi: 10.1182/blood-2005-07-2701. [DOI] [PubMed] [Google Scholar]
- Kuznetsov SA, Krebsbach PH, Satomura K, Kerr J, Riminucci M, Benayahu D, et al. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–1347. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlstrom H, et al. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- Wagers AJ., and , Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- Smith JR, Pochampally R, Perry A, Hsu SC., and , Prockop DJ. Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells. 2004;22:823–831. doi: 10.1634/stemcells.22-5-823. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Stem cell research has only just begun. Science. 2001;293:211–212. doi: 10.1126/science.293.5528.211c. [DOI] [PubMed] [Google Scholar]
- Battula N., and , Loeb LA. On the fidelity of DNA replication. Characterization of polynucleotides with errors in base-pairing synthesized by avian myeloblastosis virus deoxyribonucleic acid polymerase. J Biol Chem. 1975;250:4405–4409. [PubMed] [Google Scholar]
- Burgers PM. Polymerase dynamics at the eukaryotic DNA replication fork. J Biol Chem. 2009;284:4041–4045. doi: 10.1074/jbc.R800062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzankina Y, Asare A., and , Brown EJ. Replicative stress, stem cells and aging. Mech Ageing Dev. 2008;129:460–466. doi: 10.1016/j.mad.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- Engel LW., and , Young NA.Human breast carcinoma cells in continuous culture: a review Cancer Res 1978384327–4339.11 Pt 2 [PubMed] [Google Scholar]
- Berman SD, Calo E, Landman AS, Danielian PS, Miller ES, West JC, et al. Metastatic osteosarcoma induced by inactivation of Rb and p53 in the osteoblast lineage. Proc Natl Acad Sci USA. 2008;105:11851–11856. doi: 10.1073/pnas.0805462105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop DJ., and , Olson SD. Clinical trials with adult stem/progenitor cells for tissue repair: let's not overlook some essential precautions. Blood. 2007;109:3147–3151. doi: 10.1182/blood-2006-03-013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio D, Garcia-Castro J, Martin MC, de la Fuente R, Cigudosa JC, Lloyd AC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- Dahl JA, Duggal S, Coulston N, Millar D, Melki J, Shahdadfar A, et al. Genetic and epigenetic instability of human bone marrow mesenchymal stem cells expanded in autologous serum or fetal bovine serum. Int J Dev Biol. 2008;52:1033–1042. doi: 10.1387/ijdb.082663jd. [DOI] [PubMed] [Google Scholar]
- Bunnell BA, Flaat M, Gagliardi C, Patel B., and , Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115–120.. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk PA. Tissue engineering craniofacial defects with adult stem cells? Are we ready yet. Pediatr Res. 2008;63:478–486.. doi: 10.1203/PDR.0b013e31816bdf36. [DOI] [PubMed] [Google Scholar]
- Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, et al. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003;89:1235–1249. doi: 10.1002/jcb.10594. [DOI] [PubMed] [Google Scholar]
- Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF., and , Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis MA, McElmurry RT, Bell S, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- Li H, Fan X, Kovi RC, Jo Y, Moquin B, Konz R, et al. Spontaneous expression of embryonic factors and p53 point mutations in aged mesenchymal stem cells: a model of age-related tumorigenesis in mice. Cancer Res. 2007;67:10889–10898. doi: 10.1158/0008-5472.CAN-07-2665. [DOI] [PubMed] [Google Scholar]
- Rubin H.Multistage carcinogenesis in cell culture Dev Biol (Basel) 20011066761, 143–66.discussion [PubMed] [Google Scholar]
- Javazon EH, Colter DC, Schwarz EJ., and , Prockop DJ. Rat marrow stromal cells are more sensitive to plating density and expand more rapidly from single-cell-derived colonies than human marrow stromal cells. Stem Cells. 2001;19:219–225. doi: 10.1634/stemcells.19-3-219. [DOI] [PubMed] [Google Scholar]
- Kopen GC, Prockop DJ., and , Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, et al. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci USA. 1998;95:1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Elias G, Marcus AJ, Coyne TM, Woodbury D., and , Black IB. Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long-term survival. J Neurosci. 2004;24:4585–4595. doi: 10.1523/JNEUROSCI.5060-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochampally RR, Neville BT, Schwarz EJ, Li MM., and , Prockop DJ. Rat adult stem cells (marrow stromal cells) engraft and differentiate in chick embryos without evidence of cell fusion. Proc Natl Acad Sci USA. 2004;101:9282–9285. doi: 10.1073/pnas.0401558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Chen L, Scott PG., and , Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D., and , Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togel F, Weiss K, Yang Y, Hu Z, Zhang P., and , Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, et al. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007;354:700–706. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI., and , Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. “Stemness” does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs) Clin Pharmacol Ther. 2007;82:241–243. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- Le Blanc K., and , Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005;7:36–45. doi: 10.1080/14653240510018118. [DOI] [PubMed] [Google Scholar]
- Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci USA. 2008;105:14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block GJ, Ohkouchi S, Fung F, Frenkel J, Gregory C, Pochampally R, et al. Stem Cells. epub ahead of print; 2008. Multipotent Stromal Cells (MSCs) are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1 (STC-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ., and , Gregory CA. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24:986–991. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- Kidd S, Spaeth E, Klopp A, Andreeff M, Hall B., and , Marini FC. The (in) auspicious role of mesenchymal stromal cells in cancer: be it friend or foe. Cytotherapy. 2008;10:657–667. doi: 10.1080/14653240802486517. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D., and , Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21:304–310. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Honma T, Honmou O, Iihoshi S, Harada K, Houkin K, Hamada H, et al. Intravenous infusion of immortalized human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat. Exp Neurol. 2006;199:56–66. doi: 10.1016/j.expneurol.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yanez AJ., and , Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant. 2008;14:631–640. doi: 10.1016/j.bbmt.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Gao J, Dennis JE, Muzic RF, Lundberg M., and , Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC., and , Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Munoz JR, Stoutenger BR, Robinson AP, Spees JL., and , Prockop DJ. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci USA. 2005;102:18171–18176. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- Ruster B, Gottig S, Ludwig RJ, Bistrian R, Muller S, Seifried E, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]