Abstract
Improving the delivery of therapeutics to disease-affected tissues can increase their efficacy and safety. Here, we show that chemical conjugation of a synthetic oligosaccharide harboring mannose 6-phosphate (M6P) residues onto recombinant human acid α-glucosidase (rhGAA) via oxime chemistry significantly improved its affinity for the cation-independent mannose 6-phosphate receptor (CI-MPR) and subsequent uptake by muscle cells. Administration of the carbohydrate-remodeled enzyme (oxime-neo-rhGAA) into Pompe mice resulted in an approximately fivefold higher clearance of lysosomal glycogen in muscles when compared to the unmodified counterpart. Importantly, treatment of immunotolerized Pompe mice with oxime-neo-rhGAA translated to greater improvements in muscle function and strength. Treating older, symptomatic Pompe mice also reduced tissue glycogen levels but provided only modest improvements in motor function. Examination of the muscle pathology suggested that the poor response in the older animals might have been due to a reduced regenerative capacity of the skeletal muscles. These findings lend support to early therapeutic intervention with a targeted enzyme as important considerations in the management of Pompe disease.
Introduction
Manipulating the carbohydrate moieties on therapeutic drugs to improve their biological characteristics is gaining interest as a general strategy to increase their overall performance. Over the past several years, glycoengineering has been successfully deployed to enhance the pharmacokinetics and pharmacodynamics of several glycoprotein biologics.1 For example, the introduction of an additional site of N-linked glycosylation onto recombinant erythropoietin was effective in generating a modified biologic with increased circulating half-life and thereby extended biological activity.2 Analogous manipulation of tissue plasminogen activator to reduce the number of glycosylation sites was also successful in generating a longer-acting variant.3 Improved targeting of glycoproteins to disease-affected cells and organs could also be realized through alteration of their carbohydrate composition. A prototypical example would be the enzymatic remodeling of the carbohydrate on recombinant glucocerebrosidase to expose the core mannose residues on its oligosaccharide side chains. This maneuver enhanced its recognition and uptake by the mannose receptor on macrophages, the cells that are primarily affected in Gaucher disease.4,5 As an extension of these findings, we sought to evaluate the relative merits of engineering the carbohydrate on recombinant human acid α-glucosidase (rhGAA) as an approach to increase its delivery to the muscles of subjects with Pompe disease.
Acid α-glucosidase (GAA) is a lysosomal enzyme that is involved in the normal catabolism of glycogen, the deficiency of which causes Pompe disease.6,7 A reduction or loss of GAA activity results in the progressive accumulation of lysosomal glycogen in a variety of cells, with the myocytes of the cardiac, respiratory, and skeletal muscles being most severely affected. Depending on the extent of enzyme deficiency, subjects with Pompe disease present a broad spectrum of clinical symptoms ranging from severe hypotonia, generalized muscle weakness, and profound hypertrophic cardiomyopathy in infantile patients8,9 to minimal cardiac involvement in late-onset patients.10,11,12 Patients with the infantile form of the disease invariably die ~1 year of age from cardiac failure. In the late-onset patients, progressive deterioration of the respiratory and skeletal muscles leads to significant morbidity and in many instances early mortality, generally from respiratory failure. Significant efforts have been applied toward developing an enzyme replacement therapy for Pompe disease culminating with the recent regulatory approval of a rhGAA produced from Chinese hamster ovary cells for treating the infantile population.13,14,15 The clinical experience with enzyme replacement therapy indicated that administration of 20 mg/kg of rhGAA is effective at addressing several of the disease manifestations. However, correction of the pathology in the skeletal muscles appears to present a challenge, particularly in patients with more advanced disease that could not be resolved with higher doses or more frequent infusions of the enzyme. In part, this refractoriness may be due to the relatively low efficiency by which the enzyme can be translocated from the circulatory system across the endothelial cells and interstitial tissues to the affected muscles. The cation-independent mannose 6-phosphate receptor (CI-MPR) on skeletal muscle that is primarily involved in the cellular uptake of rhGAA is also reportedly present in low abundance in this tissue.16,17 To address this potential limitation of enzyme replacement therapy, we sought to improve the delivery of rhGAA to the affected muscles.
Uptake of exogenous rhGAA by the skeletal muscles is mediated primarily by the CI-MPR.18 However, analysis of rhGAA prepared from different sources including those from Chinese hamster ovary cells or the milk of transgenic rabbits indicated that they harbored only modest levels of the cognate ligand, mannose 6-phosphate (M6P).19 Introduction of additional M6P moieties onto rhGAA either by enzymatic engineering (HP-GAA) or chemical conjugation of synthetic oligosaccharides bearing M6P residues (neo-rhGAA) has been shown to improve their binding to the CI-MPR and subsequent uptake by cells in culture.19,20,21 Moreover, in the case of neo-rhGAA, administration of the modified enzyme to Pompe mice resulted in greater clearance of the aberrant accumulation of lysosomal glycogen when compared with the unmodified enzyme.21 We have extended these findings and report here our efforts to improve the chemical stability of neo-rhGAA and importantly, to ascertain the effects of this modification on its biological activity. We show that using oxime chemistry to conjugate synthetic oligosaccharides bearing M6P residues onto rhGAA generated a modified enzyme (oxime-neo-rhGAA) with higher stability in vivo and an improved ability to reduce the burden of glycogen storage in the skeletal and cardiac muscles of Pompe mice. Importantly, we show that associated with these biochemical improvements were significant enhancements in motor function in the treated Pompe mice.
Results
Generation of conjugates of rhGAA and synthetic M6P-bearing oligosaccharides with enhanced stability and affinity for the CI-MPR
Previously, we had reported the feasibility of conjugating synthetic oligosaccharides bearing M6P residues (Figure 1a) onto rhGAA using carbonyl-coupled hydrazone chemistry.20,21 Administration of the carbohydrate-modified enzyme (neo-rhGAA) into Pompe mice resulted in higher uptake by the affected muscles with subsequent greater clearance of the accumulated lysosomal glycogen compared to the unmodified, parent enzyme. Here, we evaluated an alternate carbonyl-coupled chemistry to generate an oxime bond using an aminooxy-derived glycan.
Figure 1.
Structure of the synthetic oligosaccharide ligand and scheme for its conjugation to rhGAA. (a) Design of the synthetic oligosaccharide used in the conjugations studies. (b) Scheme used to conjugate the synthetic glycan to rhGAA. The sialic acids on the enzyme were oxidized with periodate before reacting with the reactive group (aminooxy) on the synthetic glycan to generate oxime-neo-rhGAA. Symbols in diagram: open circle, mannose; open square, N-acetylglucosamine; open diamond, galactose; open triangle, sialic acid. GAA, acid α-glucosidase; M6P, mannose 6-phosphate; rhGAA, recombinant human acid α-glucosidase.
To assess the relative merits of using an oxime bond in the conjugate, the synthetic M6P-bearing oligosaccharide (Figure 1a) containing a hydrazide reactive group was first converted to an aminooxy reactive group through a single step reaction with N-(t-BOC)-aminooxyacetic acid tetrafluorophenyl ester, followed by deprotection in trifluoroacetic acid. The purified M6P-aminooxy ligand was then coupled to periodate-oxidized rhGAA as outlined in Figure 1b. The conjugation reaction was very efficient and proceeded to completion when 40–50 µmol/l (~5 mg/ml) oxidized rhGAA was reacted with 0.75 mmol/l (1 mg/ml) M6P-containing glycan. Consistent with the addition of M6P-bearing ligands, the resultant product, oxime-neo-rhGAA, demonstrated an increased molecular mass and a decreased isoelectric point due to the incorporation of additional phosphate groups (data not shown). Subsequent analysis by Dionex chromatography (Dionex, Sunnyvale, CA) indicated a significant increase in M6P content in the conjugated enzyme. Based on the number of M6P on oxime-neo-rhGAA and the number of sialic acids on rhGAA, it would suggest that synthetic glycans were appended onto all the sialic acids on rhGAA. The observed increase in molecular weight of oxime-neo-rhGAA (~8 kd when compared with the unmodified enzyme) measured using mass spectrometry or sodium dodecyl sulfate-gel electrophoresis (data not shown) was consistent with this assumption. Measurement of the enzymatic activity of oxime-neo-rhGAA showed an increased Km (~50%) presumably due to steric hindrance by the added oligosaccharide side chains. However, after the modified enzyme was delivered to the lysosomal compartment where the glycans were processed, its enzymatic activity was similar to that of unmodified rhGAA (data not shown). The Vmax of the modified enzyme was not affected. Although our preliminary studies showed that the antibody titers to administration of oxime-neo-rhGAA and unmodified enzyme were not different (data not shown), further analysis will be required to fully explore the repertoire of antibodies generated against the two enzymes.
Increasing the content of M6P residues on oxime-neo-rhGAA improved its affinity for the CI-MPR when compared to the unmodified enzyme. Greater than 95% of oxime-neo-rhGAA bound to a CI-MPR column as compared to 15–30% of the unmodified rhGAA (Figure 2a). The addition of M6P onto rhGAA also significantly improved enzyme uptake by L6 myoblasts in vitro (Figure 2b), similar to those reported previously for enzyme conjugates formed using hydrazone chemistry. Stability studies indicated that those formed using oxime chemistry had the added advantage of being significantly more stable (Figure 2c). No measurable dissociation of the synthetic glycan from oxime-neo-rhGAA was detected after incubation of the glycoconjugate at 25 °C for 14 days or 4 °C for 90 days. Together, these data indicate that oxime-neo-rhGAA has the desired characteristics that would support its adoption for preclinical development.
Figure 2.
Biochemical characteristics of the unmodified and glycoengineered enzymes. (a) Chromatography of oxime-neo-rhGAA (open circle) and rhGAA (closed circle) over a CI-MPR column. Approximately 5 µg of the different enzymes were loaded onto a 2 ml column. After washing the column with binding buffer, the bound material was eluted (starting at fraction 11) with binding buffer containing 5 mmol/l M6P. Fractions (2 ml) were collected and assayed for GAA activity. (b) Uptake of the enzymes by L6 myoblasts in culture. Increasing amounts of either oxime-neo-rhGAA (open circle) or rhGAA (closed circle) were added to L6 myoblasts and incubated at 37 °C for 18 hours. After washing, the cells were lysed and the enzyme activity in the lysates assayed using the fluorogenic substrate, 4-methylumbelliferyl-α-D-glucopyranoside. Enzyme activity was expressed in relative units (RU). (c) Stability of oxime-neo-rhGAA at 4 °C (open square) and 25 °C (closed triangle). The modified enzyme was incubated at the respective temperatures for the times indicated after which they were subjected to analysis to determine the level of M6P. CI-MPR, cation-independent mannose 6-phosphate receptor; GAA, acid α-glucosidase; M6P, mannose 6-phosphate; rhGAA, recombinant human acid α-glucosidase.
Oxime-neo-rhGAA demonstrates greater efficacy than unmodified rhGAA at reducing glycogen storage in muscles of Pompe mice
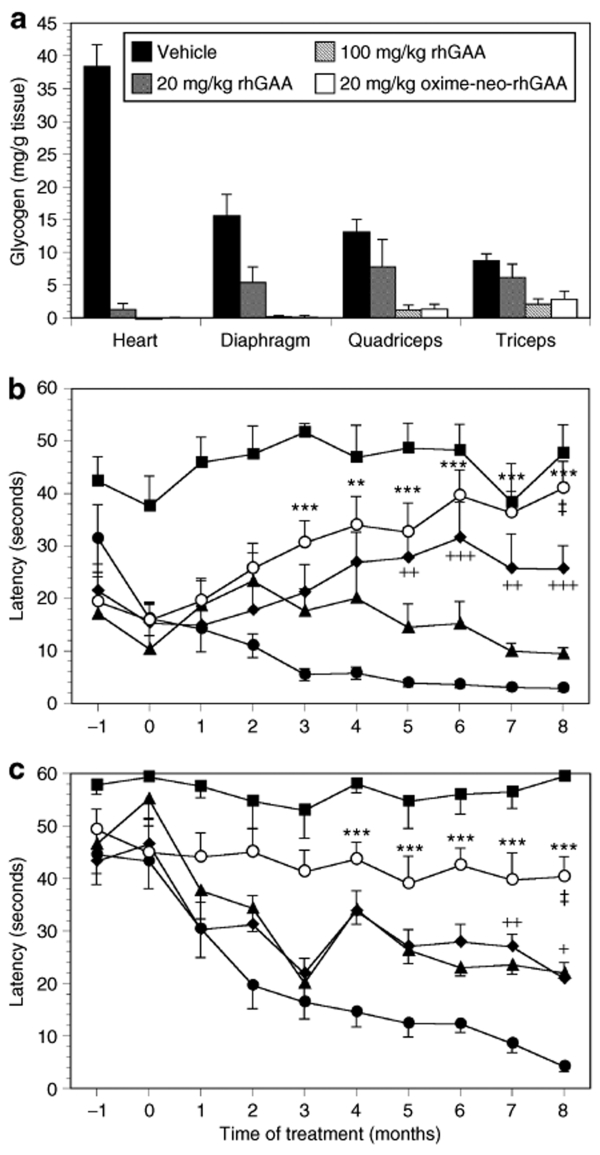
To ascertain whether the improved characteristics of oxime-neo-rhGAA observed in vitro translated to greater efficacy in vivo, 5-month-old Pompe mice were administered four weekly doses of either the modified or unmodified enzyme. The muscles of Pompe mice at 5 months of age exhibit significant lysosomal accumulation of glycogen. Analysis of the tissues 2 weeks after the last enzyme administration showed that both versions of rhGAA reduced the levels of glycogen in the heart, diaphragm, quadriceps, and triceps in a dose-dependent manner (Figure 3a). However, treatment with oxime-neo-rhGAA was more effective as illustrated by the observation that a comparable reduction in glycogen could be attained using a fivefold lower dose of the modified enzyme. For example, the extent of glycogen clearance achieved using 10 and 20 mg/kg of oxime-neo-rhGAA was comparable to that attained using 50 and 100 mg/kg of rhGAA, respectively (Figure 3a). The greater efficacy of oxime-neo-rhGAA at reducing tissue glycogen content could also be visualized by examining tissue sections stained for glycogen with periodic acid-Schiff reagent (Figure 3b, panels iii and iv). Analysis of the heart and quadriceps of animals administered 20 mg/kg rhGAA showed a significant decrease in periodic acid-Schiff–stained structures compared to these same tissues in untreated mice. However, mice treated with a similar dose of oxime-neo-rhGAA showed an even more complete clearance of glycogen-containing structures from both tissues (Figure 3b, panels v and vi).
Figure 3.
Relative abilities of unmodified and modified rhGAA to reduce tissue glycogen levels in young Pompe mice. (a) Cohorts of 5-month-old Pompe mice were administered increasing amounts of either rhGAA or oxime-neo-rhGAA. The mice (8 animals/group) were treated with four weekly doses of enzyme and killed 2 weeks after the last treatment. Tissues were collected and assayed for glycogen levels using the Amplex Red glucose assay. Data are expressed as means ± SD. (b) Sections of the heart and quadriceps were stained with PAS and then analyzed by high resolution light microscopy. Representative sections from the heart of Pompe mice treated with vehicle (i), 20 mg/kg rhGAA (iii) and 20 mg/kg oxime-neo-rhGAA (v) are shown, as are quadriceps of animals treated with vehicle (ii), 20 mg/kg rhGAA (iv) and 20 mg/kg oxime-neo-rhGAA (vi). Glycogen is visualized as purple-beaded structures within the myocytes. PAS, periodic acid-Schiff; rhGAA, recombinant human acid α-glucosidase.
Motor function improvement was significantly greater in oxime-neo-rhGAA-treated immunotolerant Pompe mice
To determine if the greater ability of oxime-neo-rhGAA to clear lysosomal glycogen deposits from the muscle translated to improved motor function, Pompe mice were also subjected to a variety of functional tests. Because improvements in muscle function could only be visualized over a period of months and because weekly administrations of rhGAA engendered a robust immune response to the human enzyme, Pompe mice were first immunotolerized to rhGAA. This was accomplished by treating the animals with a recombinant adeno-associated virus (AAV) vector encoding an inactive mutant of rhGAA (D404N) under the transcriptional control of a liver-restricted promoter.22 This approach of using AAV vectors to confer immunotolerance has been reported for a number of different transgene products including GAA.23,24,25 Intravenous administration of a recombinant AAV vector encoding the mutant enzyme (AAV8/DC190-GAAD404N) into Pompe mice resulted in high-level hepatic expression and secretion of mutant GAAD404N into the systemic circulation. However, as the specific activity of the mutant GAA was <1% of wild type GAA, there was no measurable decrease in glycogen levels in the Pompe-affected tissues (Figure 4a) compared to naive Pompe mice (data not shown). One month after dosing mice with AAV8/DC190-GAAD404N, subsequent periodic intravenous administrations of rhGAA did not elicit antibodies against the recombinant enzyme (data not shown). In contrast, administration of rhGAA into Pompe mice that had not received AAV8/DC190-GAAD404N developed high titers of antibody to the hydrolase.
Figure 4.
Assessment of motor coordination and muscle strength after enzyme therapy of young Pompe mice. Pompe mice (4.5 months old) were first administered 5E11 drp of AAV8/DC190-GAAD404N to induce immunotolerance to human GAA. One month after dosing with the viral vector, the mice were treated biweekly with injections of different doses of rhGAA or oxime-neo-rhGAA for 8 months. (a) At the end of the study, the mice were killed and their tissues analyzed for glycogen levels. Data are expressed as means ± SD (n = 10 animals per group). Throughout the study, the animals (both wild type and Pompe mice) were subjected to (b) rocking rotarod and (c) wire-hang tests. Age-matched wild type mice (closed square) and Pompe mice treated with vehicle (closed circle), 20 mg/kg rhGAA (closed triangle), 100 mg/kg rhGAA (closed diamond), and 20 mg/kg oxime-neo-rhGAA (open circle) were tested monthly. Statistical analyses were performed between vehicle and enzyme-treated groups (vehicle versus 20 mg/kg oxime-neo-rhGAA, *P < 0.05, **P < 0.01, and ***P < 0.001; vehicle versus 100 mg/kg rhGAA, +P < 0.05, ++P < 0.01, +++P < 0.001), as well as between oxime-neo-rhGAA- and rhGAA-treated groups (‡P < 0.01)) for each time point. Data are presented as means ± SEM. drp, DNase resistant particle; rhGAA, recombinant human acid α-glucosidase.
To determine the relative ability of the modified and unmodified rhGAA to correct the motor function deficits of Pompe mice, the animals were first administered AAV8/DC190-GAAD404N when they were 4.5 months old. One month later, after the animals were immunotolerized to the human enzyme, cohorts of 10 mice each were treated biweekly with 20 mg/kg rhGAA, 100 mg/kg rhGAA, 20 mg/kg oxime-neo-rhGAA, or vehicle for 8 months. To evaluate the impact of the different treatments on muscle strength and motor coordination, the animals were tasked with performing on a rotarod programmed to rock backwards and forwards (referred to as a rocking rotarod). In this assay, the rotarod was programmed to accelerate to 17.5 rpm within 2.5 seconds after which the direction of rotation was reversed and this repeated over a period of 60 seconds. In contrast to wild type mice, vehicle-treated Pompe mice displayed a deficit in this assay at the outset (4.5 months old) that became gradually worse as the animals aged (Figure 4b). This was indicated by the progressively shorter time the vehicle-treated Pompe mice were able to remain on the rotarod. Pompe mice treated biweekly with 20 mg/kg rhGAA showed a trend toward improvement but this did not reach statistical significance when compared with the vehicle-treated cohort (Figure 4b). Mice treated at the higher dose of 100 mg/kg rhGAA showed a marked improvement in motor function that reached statistical significance when compared to the vehicle-treated group after 5 months of enzyme therapy. The cohort administered 20 mg/kg oxime-neo-rhGAA fared the best, exhibiting statistically significant improvements over control animals after just 2 months on enzyme therapy and reaching levels of performance that were similar to wild type mice after 8 months of therapy (Figure 4b). At 8 months post-treatment, the performance of the cohort administered 20 mg/kg oxime-neo-rhGAA was also significantly greater than that treated with 100 mg/kg of unmodified enzyme. These improvements in motor function were consistent with the extent of glycogen clearance attained using the different enzyme entities (Figure 4a), and indicated that the modified oxime-neo-rhGAA was at least fivefold more efficacious than the unmodified version at addressing the motor function deficits in the Pompe mice.
The enhanced therapeutic effect observed with oxime-neo-rhGAA was further corroborated by the wire-hang assay that measured muscle strength. In this assay, the animals were placed on a wire mesh that was then inverted, and their latency to fall recorded. As with the rotarod assay, measurements were made monthly starting when the animals were 4.5 months old. At this age, all the Pompe mice showed a deficit in muscle strength when compared to wild type mice, as indicated by their decreased latency (Figure 4c). As expected, vehicle-treated mice demonstrated a continual decline in muscle strength as the disease progressed. Pompe mice dosed with either 20 or 100 mg/kg of the unmodified enzyme (starting at 5.5 months of age) initially demonstrated a similar decline in performance but appeared to stabilize after 3 months of treatment. However, these improvements did not reach statistical significance until after 7 months of treatment (Figure 4c). In contrast, the cohort of Pompe mice administered 20 mg/kg oxime-neo-rhGAA showed no diminution in muscle strength throughout the duration of the study. Although they did not improve to the level observed in wild type mice, their performance in this assay was significantly greater than that observed with both the untreated and rhGAA-treated groups (Figure 4c). This result suggests that animals treated with the modified enzyme responded more robustly than those treated with the unmodified counterpart, even when administered a fivefold lower dose.
Reducing lysosomal glycogen levels in older Pompe mice did not translate to significant improvements in motor function
To determine if the virtues shown associated with oxime-neo-rhGAA could be recapitulated in older, more symptomatic Pompe mice, a similar study was performed using mice starting at 10 months of age (instead of 5.5 months). Ten-month-old Pompe mice have higher levels of accumulation of lysosomal glycogen and demonstrate impaired motor function. As with the previous study design, 10-month-old Pompe mice were first treated with AAV8/DC190-GAAD404N to induce immunotolerance to the human enzyme; 1 month post-treatment with the viral vector, the animals were injected weekly with 40 mg/kg rhGAA, 100 mg/kg rhGAA, 40 mg/kg oxime-neo-rhGAA, or vehicle for 5 months. A higher (40 mg/kg) and more frequent (weekly) dosing regimen was used because older Pompe mice are reportedly more refractory to treatment.25,26 Analysis of tissue glycogen levels at the end of the study showed near-complete correction of the storage pathology in the heart and diaphragm of animals treated with either form of rhGAA (Figure 5a). In the quadriceps and triceps, treatment with 40 mg/kg oxime-neo-rhGAA was as effective as treatment with 100 mg/kg of the unmodified enzyme at reducing glycogen levels (Figure 5a), consistent with the observations noted earlier. The extent of reduction in storage material in the skeletal muscles of treated mice was significant but incomplete (~70–90% of stored glycogen was cleared with treatment).
Figure 5.
Assessment of motor coordination and muscle strength after enzyme therapy of older, symptomatic Pompe mice. Pompe mice (10 months old) were first immunotolerized to human GAA by administering 7E11 drp AAV8/DC190-GAAD404N and after 1 month they were subjected to weekly injections with varying doses of either rhGAA or oxime-neo-rhGAA for 5 months. (a) At the end of the study, the mice were killed and their tissues analyzed for glycogen levels. Data are expressed as means ± SD (n = 10 animals per group). Throughout the study, the animals were subjected to (b) rocking rotarod and (c) wire-hang tests. Pompe mice treated with vehicle (closed circle), 40 mg/kg rhGAA (closed triangle), 100 mg/kg rhGAA (closed diamond), and 40 mg/kg oxime-neo-rhGAA (open circle) were tested monthly. drp, DNase resistant particle; GAA, acid α-glucosidase; rhGAA, recombinant human acid α-glucosidase.
Interestingly, despite a significant reduction in glycogen levels in the skeletal muscles, measurements of motor function using the rotarod and wire-hang assays showed only marginal improvements over the vehicle-treated controls (Figure 5b,c). Treatment with either the modified or unmodified rhGAA resulted in some improvement over the control mice when assayed using the rocking rotarod that had been adjusted to spin at a lower velocity (rotarod was programmed to spin at a speed of 5 rpm/2.5 seconds instead of 17.5 rpm/second). However, we could not detect any differences in performance between the three enzyme-treated groups (Figure 5b). In the wire-hang test, the performance of the enzyme-treated mice was not different from that of the vehicle-treated cohort (Figure 5c). These results indicated that a reduction in glycogen storage levels per se in older Pompe mice did not necessarily translate to improvements in muscle function.
Early therapeutic intervention with oxime-neo-rhGAA abated the extent of degeneration and regeneration of muscle fibers in Pompe mice
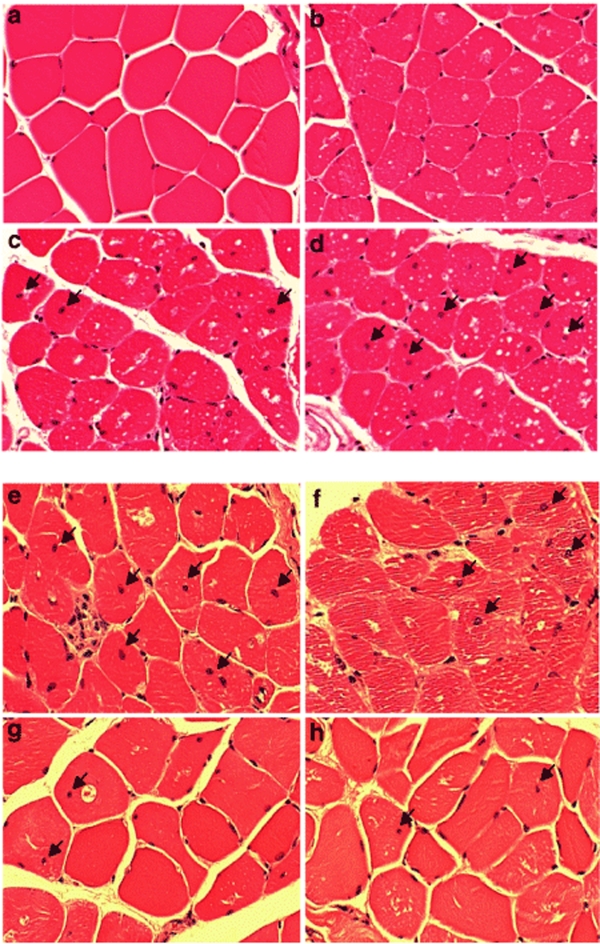
To examine the basis for the observed difference in functional response to enzyme therapy between animals that were treated earlier versus later, histopathological analysis of the muscles was performed. In healthy muscle fibers, as expected, the nuclei were primarily located at the periphery of the cells (Figure 6a). A centronuclear appearance is suggestive of a degenerative and regenerative event as may occur after injury or a serious myopathy. Examination of the quadriceps from 3-month-old Pompe mice showed a similar pattern of peripheral localization of the nuclei as observed in wild type mice (Figure 6b). However, when the Pompe animals reached 5 months (Figure 6c) or 10 months (Figure 6d) of age, progressively greater numbers of myofibers were observed to harbor centrally localized nuclei.
Figure 6.
Histopathological analysis of skeletal muscles of Pompe mice. Representative sections of quadriceps from Pompe mice of different ages and after enzyme therapy were fixed in 10% neutral buffer formalin and stained with hematoxylin and eosin. The extent of glycogen accumulation (visualized as white deposits) and centronucleation was noted in tissues obtained from representative tissues (×20 magnification) of (a) 3-month-old wild type mouse, (b) 3-month-old Pompe mouse, (c) 5-month-old Pompe mouse and (d) 10-month-old Pompe mouse. Panel (e) was sectioned from a 13.5-month-old Pompe mouse treated with vehicle for 8 months; (f) was from a 13.5-month-old Pompe mouse that received 20 mg/kg rhGAA beginning at 5.5 months of age for a total of 8 months; panel (g) was obtained from a Pompe mouse that received 100 mg/kg rhGAA for 8 months and panel (h) from one that received 20 mg/kg oxime-neo-rhGAA for 8 months. Arrows denote the location of myofibers with centronucleation. rhGAA, recombinant human acid α-glucosidase.
Quantitation of the number of muscle fibers with a centronuclear appearance (collated from ~2,000 myofibers from different sections of three Pompe mice at each time point) showed an increase in the number of cells with this phenotype as a function of age, reflecting the progressive nature of the disease (Figure 7a). The number would appear to stabilize after 10 months suggesting perhaps a loss of regenerative capacity. In addition to having a larger number of centrally localized nuclei, the sections from the older Pompe mice also displayed evidence of accumulation of glycogen in the cells (visualized as white, punctate spots).
Figure 7.
Extent of degeneration and regeneration in Pompe mouse muscles treated at different ages. Hematoxylin and eosin stained sections from three animals of each treatment group (at each time point) was scored for the number of myofibers harboring a centralized nuclei. For each animal, 1,000–3,500 muscle fibers from different fields were examined. The results illustrated in a were from Pompe mice that were treated with biweekly infusions of enzyme starting at 5.5 months of age, and those in (b) were from Pompe mice treated weekly with enzyme infusions starting when they were 11 months old. Data are presented as means ± SD. Dashed lines represent the time periods the animals were treated.
Skeletal muscles from Pompe mice treated with enzyme starting at 5.5 months of age and analyzed 8 months later showed evidence of qualitative and quantitative changes. Pompe mice treated with biweekly infusions of 20 mg/kg rhGAA showed a reduction in the number of glycogen deposits but no difference in the number muscle fibers with a centronuclear appearance (~20%) compared to those noted in untreated, age-matched Pompe mice (Figures 6e,f and 7a). However, those treated with 100 mg/kg rhGAA (Figure 6g) or 20 mg/kg oxime-neo-rhGAA (Figure 6h) showed a lower number of fibers (~12%) with central nuclei and greater clearance of glycogen deposits. The number of fibers with a centronuclear appearance in these animals at the conclusion of the study period (8 months post-treatment) was similar to that noted at the start of the therapy, when the animals were 5.5 months old (Figure 7a). This suggested that early treatment with either 100 mg/kg of the unmodified enzyme or 20 mg/kg of oxime-neo-rhGAA essentially prevented further degeneration and regeneration of the muscle fibers. These results were also consistent with the biochemical and motor function data showing that 20 mg/kg of unmodified rhGAA was less efficacious than 100 mg/kg of the same enzyme or 20 mg/kg of oxime-neo-rhGAA.
Analysis of the skeletal muscles of Pompe mice that received enzyme therapy starting at 10 months of age (when the disease was more advanced) showed no change in the frequency of myofibers with central nuclei when compared to vehicle-treated controls (Figure 7b). Irrespective of whether they were administered vehicle, unmodified or modified enzyme, the number of cells with central nuclei was the same in all the Pompe animals when their muscles were analyzed at 5 months post-treatment (Figure 7b). The high number of myofibers with the centronuclear phenotype (~20%) suggests that delayed therapeutic intervention was not effective at reversing muscle damage. Exposure of the muscles to a chronic insult from sustained accumulation of glycogen in the lysosomes was likely responsible for the development of the observed muscle pathology. Reducing the burden of glycogen accumulation in these more affected muscles was not sufficient to improve motor function, at least when tested after 5 months of enzyme therapy.
Discussion
The experience with enzyme replacement therapy for Pompe disease suggests that more efficient delivery of the recombinant enzyme, particularly to the skeletal muscle, may improve clinical response. Factors that may be contributing to this refractoriness include the relatively poor accessibility of the skeletal muscle to infused rhGAA, the large mass of this organ, the purported differential response of different muscle fiber types to therapy, and the low levels of the CI-MPR that is responsible for intracellular uptake of the enzyme in this tissue.16,27,28 To address this challenge, rhGAA was glycoengineered to enhance its recognition by the cell surface receptor and thereby increase its uptake by the affected tissues.
Manipulating the carbohydrate moieties of glycoproteins as an approach to modulate their properties has been used successfully for a variety of therapeutics. The growing adoption of this strategy is borne of the recognition that glycosylation impacts a number of functions, including a protein's pharmacokinetic and pharmacodynamic profile, as well as its solubility, stability, intracellular trafficking, and receptor binding activity.29,30,31 For example, hyperglycosylation of erythropoietin and follicle stimulating hormone through the introduction of additional sites for N-linked glycosylation increased their serum half-lives and enhanced their biological activities.32,33,34 Engineered cell lines that produce afucosylated monoclonal antibody therapeutics increased their antibody-dependent cell cytotoxic activities.35 These examples employed strategies that either altered the coding sequence of the protein such that they encoded for additional sites of glycosylation or involved re-engineering the machinery of glycosylation in expression systems to produce a more active biologic. Another approach to alter the carbohydrate moieties on therapeutic proteins is to effect what is sometimes referred to as synthetic post-translational modification. This modification is usually imposed after protein production is completed and frequently during downstream processing of the protein. The remodeling of the oligosaccharides on recombinant glucocerebrosidase to generate Cerezyme4 and the acylation of insulin to generate Levemir36 are examples of this approach. Yet another illustration of synthetic post-translational modification is the chemical or enzymatic conjugation of polyethylene glycol onto therapeutic proteins to reduce their immunogenicity or decrease their rate of clearance from the blood. Polyethylene glycolated biopharmaceuticals that have gained regulatory approval over the past few years include polyethylene glycolated interferon (Pegasys) and human growth hormone (Somavert).37,38 Our effort to alter the architecture of the oligosaccharides on GAA to present higher numbers of M6P residues represents a novel application of this concept.
We have evaluated two approaches to decorate the oligosaccharides on GAA with terminal M6P residues. One involves the stepwise enzymatic modification of rhGAA using N-acetylglucosamine-1-phosphotransferase and N-acetylglucosamine-1-phosphodiester α-N-acetylglucosaminidase to generate what is referred to as HP-GAA.19,39,40 However, testing of this hyper-mannose 6-phosphorylated variant showed that it was no more effective than unmodified enzyme at clearing tissue glycogens from Pompe mice. This result was due to HP-GAA presenting additional mannose residues that led to the nonproductive sequestration of the infused enzyme by mannose receptors on endothelial cells and macrophages.19 To overcome this impediment, we developed an alternate approach that involved chemically conjugating a synthetic oligosaccharide bearing M6P residues directly onto rhGAA to generate oxime-neo-rhGAA. The carbonyl-coupled oxime chemistry employed for conjugation could be accomplished under conditions that did not significantly affect the enzyme's activity. The reaction was also efficient and reproducible and the resultant product was stable and conducive to manufacturing at scale. In this regard, rhGAA containing the oxime bond represented an improvement over that containing the hydrazone bond reported previously.21
The resulting modified rhGAA (oxime-neo-rhGAA) displayed higher affinity for the CI-MPR with consequent greater receptor-mediated endocytosis into L6 myoblast cells in vitro and presumably into Pompe-affected muscles in vivo. This translated to an approximately fivefold higher efficacy in clearing the tissue glycogen, based on the ability of the oxime-neo-rhGAA to effect an equivalent reduction in substrate levels at one-fifth the dose of the unmodified enzyme. Importantly, this improved clearance of glycogen resulted in a corresponding increase in muscle strength and motor function in mice treated starting at 5–6 months of age. The observed improvement in motor function was not due to a reduction of glycogen in the CNS of Pompe mice.41 Although mannose 6-phosphorylated β-glucuronidase was reportedly able to traverse the blood–brain barrier of mucopolysaccharidosis VII mice,42 we were not able to detect either GAA activity or a reduction in glycogen in the CNS of oxime-neo-rhGAA-treated Pompe mice (data not shown).
Surprisingly, although comparable reductions in glycogen levels were observed in Pompe mice treated with 20 mg/kg oxime-neo-rhGAA and 100 mg/kg rhGAA, the improvement in muscle function was greater in animals administered the carbohydrate-engineered enzyme. It may be that oxime-neo-rhGAA effected a more rapid clearance of glycogen and thereby reduced the time the muscles were exposed to the insult. This in turn may have reduced or minimized the muscle damage of oxime-neo-rhGAA-treated mice compared to those administered rhGAA. Preventing the onset of muscle damage is an important consideration, as treatment of older Pompe mice with more established muscle pathology was largely ineffective at rescuing motor function. Clearance of glycogen from these older and more damaged muscles was insufficient to improve their performance on the rotarod or wire-hang tests. This suggests that other factors besides glycogen have a role in modulating muscle function. The importance of early intervention has also been noted in human clinical studies, in which a more robust outcome was observed in infantile Pompe subjects treated before they were 6 months old than in those treated between 6 and 36 months of age.13,43
The basis for this differential response in animals treated early and late is unclear but may be related to the reported decreased ability of older Pompe mice muscles to take up the enzyme.21,25,26 More damaged muscle fibers, particularly type II fibers, also reportedly exhibit altered autophagic activity that may affect normal trafficking of the endocytosed enzyme.44 Of additional relevance was the observation that mice treated earlier had fewer numbers of fibers with centralized nuclei, a marker of muscle degeneration and regeneration. Although both early and late therapeutic interventions prevented further increases in the numbers of fibers containing centralized nuclei, the basal numbers in the older animals were much higher. Hence, the inability to rescue muscle function in the older mice may have been due to a loss in their capacity to repair damaged fibers, perhaps due to exhaustion of satellite cells. This loss of regenerative capacity has been reported for other degenerative muscle diseases and severe fibrotic diseases, the latter due to an inability of satellite cells to fuse with the damaged muscle fibers.45 Irrespective of the mechanism, the results here strongly advocate the desire for early diagnosis and therapeutic intervention in the management of Pompe disease. Another consideration is the use of adjuvant therapies designed to improve muscle physiology such as by coadministering insulin-like growth factor-1 and antagonists of myostatin. Both agents have reportedly shown promise in improving muscle function in mouse models of Duchenne muscular dystrophy.46,47
In summary, we showed that the approach of chemically conjugating a ligand onto rhGAA to enhance its recognition by the CI-MPR significantly improved the enzyme's potency at clearing tissue glycogen levels from Pompe mice. Importantly, this translated to a concomitant delay in disease progression and an increase in motor function. The availability of this improved enzyme (oxime-neo-rhGAA) means that either a lower dose of this enzyme could be used for treatment or that a dose comparable with that of the unmodified enzyme could provide better efficacy. The use of lower doses could lessen the magnitude of the host immune response to the administered therapeutic which in turn could positively impact the efficacy of treatment. The carbonyl chemistry used for conjugation was relatively innocuous to the enzyme's activity and was conducive to scale up. Based on these observations, we contend that this approach to glycoengineering could be broadly applied to other therapeutics (besides lysosomal enzymes) bearing different synthetic ligands designed to target delivery via their respective cognate receptors to disease tissues. As such, this represents a general approach for optimizing therapeutic protein leads and thus can be applied to developing new and improved protein pharmaceuticals.
Materials and Methods
Chemicals. Sodium meta-periodate, M6P, β-glycerophosphate, 4- methylumbelliferyl-α-D-glucopyranoside, Aspergillus niger amyloglucosidase, bovine liver glycogen, and other common chemical reagents were purchased from Sigma Chemical (St. Louis, MO) unless otherwise stated. N-(t-BOC)-aminooxyacetic acid tetrafluorophenyl ester, fetal bovine serum, Dulbecco's modified Eagle's media and other cell culture reagents were from Invitrogen (Carlsbad, CA). Recombinant human rhGAA purified from Chinese hamster ovary cells was from Genzyme (Cambridge, MA). Dialysis cassettes of varying capacities and with a molecular weight cutoff of 10 kd were from Pierce (Rockford, IL). Diafiltration cartridges were obtained from GE Amersham Biosciences (Piscataway, NJ). The Amplex Red glucose assay kit was from Molecular Probes (Eugene, OR).
Modification of the glycan to contain an aminooxy group. The original synthetic oligosaccharide was derivatized with butyl hydrazide for coupling to rhGAA through a hydrazone bond.21 To convert the hydrazide group to an aminooxy group for oxime bond formation, N-(t-BOC)-aminooxyacetic acid tetrafluorophenyl ester (t-Boc-AOAA-TFP) was used. The synthetic glycan and t-Boc-AOAA-TFP were dissolved separately in DMSO and water (50:50, vol:vol) to a final concentration of 5.3 mmol/l and 39.33 mmol/l, respectively. Then two volumes of the synthetic glycan were mixed with 1 volume of t-Boc-AOAA-TFP such that the final molar ratio of glycan:t-Boc-AOAA-TFP was 1:3.7. Following mixing, 3-hydroxy-1,2,3-benzotriazin-4(3H)-one (Dhbt-OH) from a stock (196.6 mmol/l) was added such that the glycan:t-Boc-AOAA-TFP:Dhbt-OH ratio was 1:3.7:1.85. The reaction was allowed to proceed overnight at room temperature with gentle shaking. Completion of the reaction was verified by Dionex analysis. The glycan-aminooxy-t-Boc in the reaction mixture was purified by gel filtration over a Sephadex G-10 column (Sigma Chemical) using distilled H2O as eluent; the fractions containing the reaction product were collected and lyophilized. Deprotection of the lyophilized glycan-aminooxy-t-Boc was performed in 50% trifluoroacetic acid in dichlorormethane at room temperature for 30 minutes after which the solvent was removed under nitrogen. The final material was dissolved in 0.5 mol/l sodium acetate buffer, pH 3–4, and fractionated on a Sephadex G-10 column as describe above. Fractions containing the final product were collected and lyophilized. Overall yield was >90% of the starting material.
Conjugation of synthetic glycan onto rhGAA. The procedure for chemically conjugating the derivatized synthetic oligosaccharide onto rhGAA was essentially as described previously.21 Briefly, 500 mg to 1 g of periodate-oxidized rhGAA were conjugated to the aminooxy-derivatized synthetic oligosaccharide by mixing at a molar ratio of 1:15 and incubating at 37 °C for 6 hours with gentle shaking. Following conjugation, the preparations were diafiltrated using a dialfiltration cartridge (molecular weight cutoff of 30 kd) with 50 volumes of 25 mmol/l sodium phosphate buffer, pH 6.25. Following diafiltration, the oxime-neo-rhGAA was adjusted to contain 2% mannitol and 0.005% Tween-80 and then sterile filtered. The samples were aliquoted, snap-frozen on dry ice and stored at −80 °C until used. The number of M6P residues on rhGAA and oxime-neo-rhGAA was quantitated using the method described by Zhou et al.48
Characterization of oxime-neo-rhGAA. Methods to measure the binding of oxime-neo-rhGAA to a CI-MPR column and to assay the uptake of the enzyme into L6 myoblast cells have been described previously.20,21 To measure enzyme activity, cells were first lysed in assay buffer (0.2 mol/l sodium acetate, 0.4 mol/l potassium chloride, pH 4.3) containing 0.1% Triton X-100. Following centrifugation to remove cellular debris, the supernatants were assayed for enzymatic activity using the fluorogenic substrate 4-methylumbelliferyl-α-D-glucopyranoside. In studies to determine the stability of the conjugates, the different preparations were incubated at 4 °C or room temperature (25 °C) for various times. Samples were snap-frozen on dry ice and stored at −80 °C until all the time points were collected. They were then transferred into a DiaLyzer (Pierce) with a molecular weight cutoff of 30 kd and dialyzed overnight against 25 mmol/l phosphate buffer, pH 6.25 at 4 °C. Samples were collected and their M6P content then measured.
Generation of AAV8/DC190-GAAD404N. The previral plasmid pSP70/DC190-GAA containing the cDNA for human GAA and a liver-specific promoter (DC190) was generated as described in Ziegler et al.25 AAV8/DC190-GAAD404N encodes human GAA harboring a mutation (D404N) that is located in the active site.22 The terminology used is modified such that the D518N mutation referred to in the original publication is D404N as described herein. The expression cassette flanked by AAV2 ITRs was packaged into AAV8 capsids using a standard triple transfection protocol. AAV8 pseudotyped vectors were purified by iodixanol gradient centrifugation followed by ion exchange chromatography over Hi Trap Q HP columns. The concentration of DNase resistant particles was determined using realtime TaqMan PCR assay (ABI PRISM 7700; Applied Biosystems, Foster City, CA) with primers designed to amplify the vector-specific bovine growth hormone polyadenylation sequence.
Short-term efficacy studies. Animal experiments were conducted in accordance with the guidelines issued by the US Department of Health and Human Services (NIH Publication No. 86-23) and by Genzyme's IACUC committee. The mouse model of Pompe disease (6neo/6neo)25,28 that had been crossed onto a predominantly C57BL/6J genetic background recapitulates aspects of the human disease in that they harbor a mutated GAA gene, exhibit no measurable α-glucosidase activity, and lysosomal accumulation of glycogen in muscle. The short-term efficacy studies used young (~5–6 months old) Pompe mice. Groups of Pompe mice (n = 8) were injected via a tail vein with vehicle or varying doses of rhGAA or oxime-neo-rhGAA. The mice were administered 4 weekly doses and then sacrificed 2 weeks after the last treatment. To minimize the hypersensitivity reactions frequently observed after repeated administrations of rhGAA, mice were routinely pretreated with 5 mg/kg diphenhydramine (delivered intraperitoneally) 10 minutes before injection of the enzyme preparations. Various tissues including the heart, diaphragm, and skeletal muscles (quadriceps and triceps) were collected and stored at −80 °C until assayed.
Long-term efficacy studies. Longer-term studies were performed with either young (~4.5 months of age, n = 10) or older (~10 months of age, n = 10) Pompe mice. For these studies, animals were first immunotolerized by administering 5E11 (young mice) or 7E11 (older mice) DNase resistant particle of AAV8/DC190-GAAD404N via a tail vein. Starting 1 month later, animals were subjected to periodic injections with different enzyme preparations. Motor function was assessed using a variety of tests. A rotarod test in the rocking mode was used to examine motor function.25 The speed of the rotarod used to test the young Pompe mice was set to increase to 17.5 rpm within 2.5 seconds and the direction of spin then reversed to accelerate in the opposite direction. For the older Pompe mice, the speed was set to increase to 5 rpm within 2.5 seconds. Time was recorded as the latency of the animal to stay on the apparatus. Wire-hang tests were used to measure muscle strength. Individual animals were placed on a metal mesh grid that was then inverted; the time the animal was able to hold on the mesh in an up-side-down position was recorded. For each time point, three tests were typically performed for each animal and the average reading recorded.
Measurement of glycogen levels. The glycogen content in various muscles of the Pompe mice was assayed by measuring the difference in the amount of glucose released from a boiled tissue homogenate following digestion with or without A. niger amyloglucosidase, as described previously.20,21 The glucose levels from the digested and undigested sample sets were then assayed using the Amplex Red glucose assay kit according to the manufacturer's instructions. Bovine liver glycogen was used as standard.
Histopathology. Quadriceps from Pompe mice was fixed in 10% formalin and embedded in paraffin. They were then sectioned to 5-µm thickness and stained with hematoxylin and eosin for assessment of centralized nuclei. Tissue sections from three animals in each group were scored for the number of muscle fibers harboring centralized nuclei. On average, a total of 1,000–3,500 muscle fibers from different fields were examined for each animal. For periodic acid-Schiff staining, tissues were fixed in 3% glutaraldehyde, embedded in epon and then stained with periodic acid-Schiff.
Statistical analysis. Behavioral data are expressed as mean ± standard deviation. Data were analyzed by one-way analysis of variance followed by a Bonferroni's multiple comparison tests with GraphPad Prism 4 software (GraphPad Software, La Jolla, CA). A probability value of P < 0.05 was considered statistically significant.
Acknowledgments
We thank the scientific community at Genzyme for their valuable discussions and input into the designs of the studies. We are especially thankful to members of the Departments of Comparative Medicine, Bioengineering, Applied Discovery Research, Histology, Protein Therapy Research, Glycobiology, and Therapeutic Protein Development for providing reagents and technical support. We thank Ron Scheule for his help with editing and proof-reading the manuscript. We are very grateful to Nina Raben at NIH for providing the Pompe mice. Finally, we would like to thank Jonathan Fidler and Michael Zhao for their help with muscle function tests, and Qun Zhou and Josephine Kyazike for M6P analysis.
REFERENCES
- Koury MJ. Sugar coating extends half-lives and improves effectiveness of cytokine hormones. Trends Biotech. 2003;21:462–464. doi: 10.1016/j.tibtech.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Elliott S, Lorenzini T, Asher S, Aoki K, Brankow D, Buck L, et al. Enhancement of therapeutic protein in vivo activities through glycoengineering. Nat Biotechnol. 2003;21:414–421. doi: 10.1038/nbt799. [DOI] [PubMed] [Google Scholar]
- Lau D, Kuzma G, Wei CM, Livingston DJ., and , Hsiung N. A modified human tissue plasminogen activator with extended half-life in vivo. Nat Biotechnol. 1987;5:953–958. [Google Scholar]
- Furbish FS, Steer CJ, Krett NL., and , Barranger JA. Uptake and biodistribution of placental glucocerebrosidase in rat hepatic cells and effects of sequential deglycosylation. Biochim Biophys Acta. 1981;673:425–434. doi: 10.1016/0304-4165(81)90474-8. [DOI] [PubMed] [Google Scholar]
- Brady RO, Murray GJ., and , Barton NW. Modifying exogenous glucocerebrosidase for effective replacement therapy in Gaucher disease. J Inherit Metab Dis. 1994;17:510–519. doi: 10.1007/BF00711365. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R., and , Reuser AJJ.Glycogen storage disease type II: acid α-glucosidase (acid maltase) deficiency The Metabolic and Molecular Basis of Inherited Disease 2001McGraw-Hill: New York; 3389–3420.In: CR Scriver, AL Beaudet, WS Sly and D Valle (eds) [Google Scholar]
- Van der Ploeg AT., and , Reuser AJJ. Pompe's disease. Lancet. 2008;372:1342–1353. doi: 10.1016/S0140-6736(08)61555-X. [DOI] [PubMed] [Google Scholar]
- Van den Hout HM, Hop W, van Diggelen OP, Smeitink JA, Smit GP, Poll-The BT, et al. The natural course of infantile Pompe's disease: 20 original cases compared with 133 cases from the literature. Pediatrics. 2003;112:332–340. doi: 10.1542/peds.112.2.332. [DOI] [PubMed] [Google Scholar]
- Kishnani PS., and , Howell RR. Pompe disease in infants and children. J Pediatr. 2004;144:S35–S43. doi: 10.1016/j.jpeds.2004.01.053. [DOI] [PubMed] [Google Scholar]
- Felice KJ, Alessi AG., and , Grunnet ML. Clinical variability in adult-onset acid maltase deficiency; report of affected sibs and review of the literature. Medicine. 1995;74:131–135. doi: 10.1097/00005792-199505000-00002. [DOI] [PubMed] [Google Scholar]
- Vorgerd M, Burwinkel B, Reichmann H, Malin JP., and , Kilimann MW. Adult-onset glycogen storage disease type II: phenotypic and allelic heterogeneity in German patients. Neurogenetics. 1998;1:205–211. doi: 10.1007/s100480050030. [DOI] [PubMed] [Google Scholar]
- Kroos MA, Pomponio RJ, Hagemans ML, Keulemans JL, Phipps M, DeRiso M, et al. Broad spectrum of Pompe disease in patients with the same c.-32-13T→G haplotype. Neurology. 2007;68:110–115. doi: 10.1212/01.wnl.0000252798.25690.76. [DOI] [PubMed] [Google Scholar]
- Kishnani PS, Nicolino M, Voit T, Rogers RC, Tsai AC, Waterson J, et al. Chinese hamster ovary cell-derived recombinant human acid α-glucosidase in infantile-onset Pompe disease. J Pediatr. 2006;149:89–97. doi: 10.1016/j.jpeds.2006.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Parenti G, Della Casa R, Romano A, Mansi G, Agovino T, et al. Long-term enzyme replacement therapy for Pompe disease with recombinant human α-glucosidase derived from Chinese hamster oveary cells. J Child Neurol. 2007;22:565–573. doi: 10.1177/0883073807302598. [DOI] [PubMed] [Google Scholar]
- Van Capelle CI, Winkel LP, Hagemans ML, Shapira SK, Arts WF, van Doorn PA, et al. Eight years experience with enzyme replacement therapy in two children and one adult with Pompe disease. Neuromuscul Disord. 2008;18:447–452. doi: 10.1016/j.nmd.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Wenk J, Hille A., and , von Figura K. Quantitation of Mr 46000 and Mr 300000 mannose 6-phosphat receptors in human cells and tissues. Biochem Int. 1991;23:723–731. [PubMed] [Google Scholar]
- Funk B, Kessler U, Eisenmenger W, Hansmann A, Kolb HJ., and , Kiess W. Expression of the insulin-like growth factor-II/mannose 6-phosphate receptor in multiple human tissues during fetal life and early infancy. J Clin.Endocrinol Metab. 1992;75:424–431. doi: 10.1210/jcem.75.2.1379254. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Dahms S., and , Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat Rev. 2003;4:202–212. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- McVie-Wylie AJ, Lee KL, Qiu H, Jin X, Do H, Gotschall R, et al. Biochemical and pharmacological characterization of different recombinant acid α-glucosidase preparations evaluated for the treatment of Pompe disease. Mol Genet Metab. 2008;94:448–455. doi: 10.1016/j.ymgme.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Li X, Kyazike J, Zhou Q, Thurberg BL, Raben N, et al. Conjugation of mannose 6-phosphate-containing oligosaccharides to acid α-glucosidase improves the clearance of glycogen in Pompe mice. J Biol Chem. 2004;279:50336–50341. doi: 10.1074/jbc.M409676200. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Li X, McVie-Wylie AJ, Jiang C, Thurberg BL, Raben N, et al. Carbohydrate-remodelled acid α-glucosidase with higher affinity for the cation-independent mannose 6-phosphate receptor demonstrates improved delivery to muscles of Pompe mice. Biochem J. 2005;389:619–628. doi: 10.1042/BJ20050364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans MMP, Kroos MA, van Beeumen J, Oostra BA., and , Reuser AJJ. Human lysosomal α-glucosidase. Characterization of the catalytic site. J Biol Chem. 1991;266:13507–13512. [PubMed] [Google Scholar]
- Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, et al. Induction of immune tolerance to coagulation factor IX by in vivo hepatic gene transfer. J Clin Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler RJ, Cherry M, Barbon CM, Li C, Bercury SD, Armentano D, et al. Correction of the biochemical and functional deficits in Fabry mice following AAV8-mediated hepatic expression of α-galactosidase A. Mol Ther. 2007;15:492–500. doi: 10.1038/sj.mt.6300066. [DOI] [PubMed] [Google Scholar]
- Ziegler RJ, Bercury SD, Fidler J, Zhao MA, Foley J, Taksir T, et al. Ability of AAV8-mediated hepatic expression of acid α-glucosidase to correct the biochemical and motor function deficits of presymptomatic and symptomatic Pompe mice. Hum Gene Ther. 2008;6:609–621. doi: 10.1089/hum.2008.010. [DOI] [PubMed] [Google Scholar]
- Raben N, Fukuda T, Gilbert AL, de Jong D, Thurberg BL, Mattaliano RJ, et al. Replacing α-glucosidase in Pompe disease: recombinant and transgenic enzymes are equipotent, but neither completely clears glycogen from type II muscle fibers. Mol Ther. 2005;11:48–56. doi: 10.1016/j.ymthe.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Thurberg BL, Lynch Maloney C, Vaccaro C, Afonso K, Tsai AC, Bossen E, et al. Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease. Lab Invest. 2006;86:1208–1220. doi: 10.1038/labinvest.3700484. [DOI] [PubMed] [Google Scholar]
- Raben N, Jatkar T, Lee A, Lu N, Dwivedi S, Nagaraju K, et al. Glycogen stored in skeletal but not in cardiac muscle in acid α-glucosidase mutant (Pompe) mice is highly resistant to transgene-encoded human enzyme. Mol Ther. 2002;6:601–608. [PubMed] [Google Scholar]
- Walsh G., and , Jefferis R. Post-translational modifications in the context of therapeutic proteins. Nat Biotechnol. 2006;24:1241–1252. doi: 10.1038/nbt1252. [DOI] [PubMed] [Google Scholar]
- Campbell CT, Sampathkumar S., and , Yarema KJ. Metabolic oligosaccharide engineering: perspectives, applications, and future directions. Mol Biosyst. 2007;3:187–194. doi: 10.1039/b614939c. [DOI] [PubMed] [Google Scholar]
- Werner RG, Kopp K., and , Schlueter M. Glycosylation of therapeutic proteins in different production systems. Acta Paediatr. 2007;96:17–22. doi: 10.1111/j.1651-2227.2007.00199.x. [DOI] [PubMed] [Google Scholar]
- Egrie JC, Dwyer E, Browne JK, Hitz A., and , Lykos MA. Darbepoeitin alfa has a longer circulating half-life and greater in vivo potency than recombinant human erythropoietin. Exp Hematol. 2003;31:290–299. doi: 10.1016/s0301-472x(03)00006-7. [DOI] [PubMed] [Google Scholar]
- Perlman S, van den Hazel B, Christiansen J, Gram-Nielsen S, Jeppesen CB, Andersen KV, et al. Glycosylation of an N-terminal extension prolongs the half-life and increased the in vivo activity of follicle stimulating hormone. J Clin Endocrinol Metab. 2003;88:3227–3235. doi: 10.1210/jc.2002-021201. [DOI] [PubMed] [Google Scholar]
- Sinclair AM., and , Elliott S. Glycoengineering: the effect of glycosylation on the properties of therapeutic proteins. J Pharm Sci. 2005;94:1626–1635. doi: 10.1002/jps.20319. [DOI] [PubMed] [Google Scholar]
- Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, Kusunoki M, Iida S, Nakano R, et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 2004;87:614–622. doi: 10.1002/bit.20151. [DOI] [PubMed] [Google Scholar]
- Goldman-Levine JD., and , Lee KW. Insulin detemir—a new basal insulin analog. Ann Pharmacother. 2005;39:502–507. doi: 10.1345/aph.1E334. [DOI] [PubMed] [Google Scholar]
- Parkinson C., and , Trainer PJ. The place of pegvisomant in the management of acromegaly. Expert Opin Investig Drugs. 2001;10:1725–1735. doi: 10.1517/13543784.10.9.1725. [DOI] [PubMed] [Google Scholar]
- Foster GR. Pegylated interferons: chemical and clinical differences. Ailment Pharmacol Ther. 2004;20:825–830. doi: 10.1111/j.1365-2036.2004.02170.x. [DOI] [PubMed] [Google Scholar]
- Kornfeld R, Bao M, Brewer K, Noll C., and , Canfield WM. Purification and multimeric structure of bovine N-acetylglucosamine-1-phosphodiester α-N-acetylglucosaminidase. J Biol Chem. 1998;273:23203–23210. doi: 10.1074/jbc.273.36.23203. [DOI] [PubMed] [Google Scholar]
- Kudo M., and , Canfield WM. Structural requirements for efficient processing and activation of recombinant human UDP-N-acetylglucosamine:lysosomal-enzyme-N-acetylglucosamine-1-phosphotransferase. J Biol Chem. 2006;281:11761–11768. doi: 10.1074/jbc.M513717200. [DOI] [PubMed] [Google Scholar]
- Sidman RL, Taksir T, Fidler J, Zhao M, Dodge JC, Passini MA, et al. Temporal neuropathologic and behavioral phenotype of 6neo/6neo Pompe disease mice. J Neuropathol Exp Neurol. 2008;67:803–818. doi: 10.1097/NEN.0b013e3181815994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urayama A, Grubb JH, Sly WS., and , Banks WA. Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood-brain barrier. Proc Natl Acad Sci USA. 2004;101:12658–12663. doi: 10.1073/pnas.0405042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandal H, Hwu WL, et al. Recombinant human α-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. doi: 10.1212/01.wnl.0000251268.41188.04. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Ahearn M, Roberts A, Mattaliano RJ, Zaal K, Plotz PH, et al. Autophagy and mistargeting of therapeutic enzyme in skeletal muscle in Pompe disease. Mol Ther. 2006;14:831–839. doi: 10.1016/j.ymthe.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jejurika SS., and , Kuzon WM. Satellite cell depletion in degenerative skeletal muscle. Apoptosis. 2003;8:573–578. doi: 10.1023/A:1026127307457. [DOI] [PubMed] [Google Scholar]
- Patel K, Macharia R., and , Amthor H. Molecular mechanisms involving IGF-1 and myostatin to induce hypertrophy as a therapeutic strategy for Duchenne muscular dystrophy. Acta Myol. 2005;24:230–241. [PubMed] [Google Scholar]
- Nakatani M, Takehara Y, Sugino H, Matsumoto M, Hashimoto O, Hasegawa Y, et al. Transgenic expression of a myostatin inhibitor derived from follistatin increases skeletal muscle mass and ameliorates dystrophic pathology in mdx mice. FASEB J. 2008;22:477–487. doi: 10.1096/fj.07-8673com. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Kyazike J, Edmunds T., and , Higgins E. Mannose 6-phosphate quantitation in glycoproteins using high-pH anion-exchange chromatography with pulsed amperometric detection. Anal Biochem. 2002;306:163–170. doi: 10.1006/abio.2002.5703. [DOI] [PubMed] [Google Scholar]