Abstract
The emergence of leukemia following gene transfer to restore common cytokine receptor γ chain (γC) function in X-linked severe combined immunodeficiency (SCID-X1) has raised important questions with respect to gene therapy safety. To explore the risk factors involved, we tested the oncogenic potential of human γC in new strains of transgenic mice expressing the gene under the control of the CD2 promoter and locus control region (LCR). These mice demonstrated mildly perturbed T-cell development, with an increased proportion of thymic CD8 cells, but showed no predisposition to tumor development even on highly tumor prone backgrounds or after γ-retrovirus infection. The human CD2-γC transgene rescued T and B-cell development in γC−/− mice but with an age-related delay, mimicking postnatal reconstitution in SCID-X1 gene therapy subjects. However, we noted that γC−/− mice are acutely susceptible to murine leukemia virus (MLV) leukemogenesis, and that this trait was not corrected by the γC transgene. We conclude that the SCID-X1 phenotype can be corrected safely by stable ectopic expression of γC and that the transgene is not significantly oncogenic when expressed in this context. However, an underlying predisposition conferred by the SCID-X1 background appears to collaborate with insertional mutagenesis to increase the risk of tumor development.
Introduction
Although correction of X-linked severe combined immunodeficiency (SCID-X1) was an early success for the gene therapy field,1 this advance was compromised by the development of leukemia (T-lineage acute lymphoblastic leukemia) in trial subjects, with 5 of 19 patients from two similar trials developing this complication to date.2,3,4 As a matter of further concern, the therapeutic vector was implicated as a causal agent in these leukemias and in four cases was found to have integrated into the Lim-only protein 2 (LMO2) gene, a known target for oncogenic activation in human T-lineage acute lymphoblastic leukemia.5
This outcome was not anticipated as it was assumed that a single hit of vector integration would be insufficient to initiate oncogenic transformation of a normal cell.6 However, it is conceivable that factors specific to the trial led to increased leukemia susceptibility. One of the features that distinguishes gene therapy for SCID-X1 from most other trials is the marked selective advantage conferred on corrected cells.7 It has also been suggested that the therapeutic gene itself has oncogenic properties, particularly when expression is controlled by heterologous retroviral regulatory elements rather than endogenous sequences. The common cytokine receptor γ chain (γC) is a component of receptors for multiple cytokines, including IL-2, 4, 7, 9, 15, and 21.8 Increased responsiveness to any of these cytokines is likely to have biological effects which may include aberrant proliferation and differentiation.9 Although no neoplastic disease was observed in preclinical trials of vectors expressing γC in mice,10 these studies were of short duration and did not test the effects of combination with other leukemogenic factors. Circumstantial evidence in support of the cooperating oncogene hypothesis was provided by the finding of insertions near murine γC as well as LMO2 in a case of murine T-cell leukemia.11 However, there are conflicting findings on the leukemogenicity of vector-driven expression of γC in mouse models. A recent study reported that, γC null (γC−/−)mice reconstituted with a lentivirus vector expressing murine γC at high level succumbed to lymphomas at a significant rate.12 In contrast, another study reported that vector-driven LMO2 expression perturbed hematopoietic development of human CD34 cells whereas γC had no obvious effects.13
For this study, we derived new lines of mice expressing high levels of human γC under the control of the human CD2 promoter and locus control region (LCR). The CD2 element was chosen for its reliability and potency in driving transgene expression in the T-cell compartment, which has revealed the oncogenic potential of a diverse range of genes.14,15,16,17,18,19,20 Transgenic systems provide a powerful approach to testing the oncogenic potential of weak oncogenes, which can often be revealed in combination with a more powerful initiating oncogene or an inactive tumor suppressor gene in the germline.18,21,22,23 Such systems also afford the opportunity to test for synergy with long terminal repeat-driven insertional mutagenesis by Moloney murine leukemia virus (MoMLV) following neonatal infection.23,24 MoMLV is a potent inducer of T-cell lymphoma, with tissue-specificity mapping principally to its long terminal repeat enhancer elements.25
We followed γC transgenic mice closely for signs of developmental abnormalities, for predisposition to spontaneous tumors, and for evidence that the transgene can accelerate tumor development in combination with other oncogenic factors. Although the transgene can reconstitute lymphoid development on a γC−/− background, we find no evidence of an increase in tumor development. However, infection of γC−/− mice reveals that these animals are highly susceptible to murine leukemia virus (MLV) leukemogenesis, and this underlying susceptibility remains uncorrected by the γC transgene. The significance of these observations for SCID-X1 therapy is discussed.
Results
Generation of transgenic lines ectopically expressing human γC in the T-cell compartment
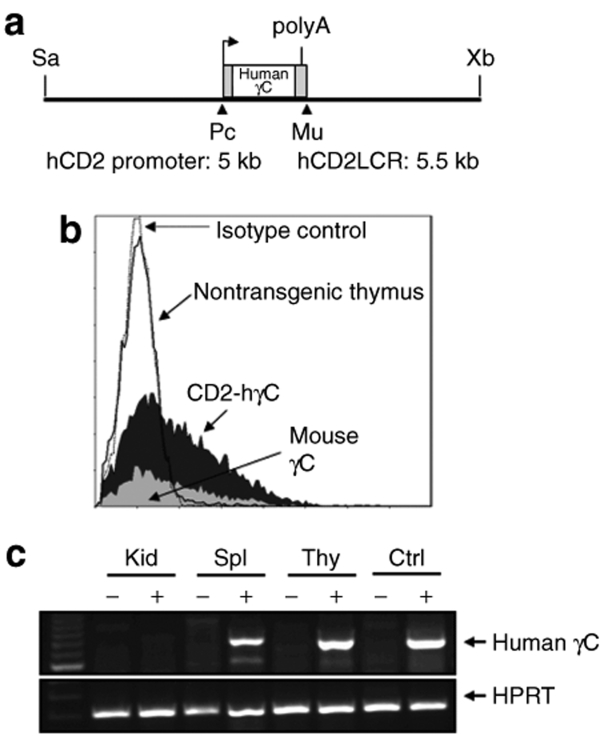
To study the effects of ectopic γC expression on hematopoietic development and tumor susceptibility, we generated a construct containing an LCR from the human CD2 locus linked 5′ to the coding region to generate CD2-hγC (Figure 1a). This construct was used to generate two transgenic lines, gCA and gCB, ectopically expressing human γC. Presence of the transgene was confirmed by both Southern blot and PCR analysis in positive mice (data not shown). Flow cytometry was carried out to confirm the expression of human γC in T cells present in thymocytes and splenocytes from transgenic mice using antihuman CD132 (Figure 1b). Reverse transcriptase-PCR specific to the human transgene was also used to verify expression of γC transcripts in the expected tissue compartments (Figure 1c).
Figure 1.
Generation and analysis of animals transgenic for human γC. (a) Plasmid construct illustrating the CD2 promoter driven human γC transgene (Sa – Sal1, Xb – XbaI, Pc – PciI, Mu – MluI). (b) Expression of the human γC from the CD2 promoter was confirmed in the transgenic mice by flow cytometry analysis of human γC surface expression in thymocytes from a CD2-hγC transgenic and control mouse. Expression of mouse γC is also shown for comparison. (c) RT-PCR in tissue from spleen (Spl), kidney (Kid), and thymus (Thy) from either a CD2-hγC transgenic animal (lanes indicated by +) or a nontransgenic littermate (lanes indicated by −). Control RNA isolated from PG-13 cells without (ctrl−) or with human γC vector (ctrl+).
CD2-hγC mice show a mild perturbation of T-cell development but no predisposition to spontaneous tumors
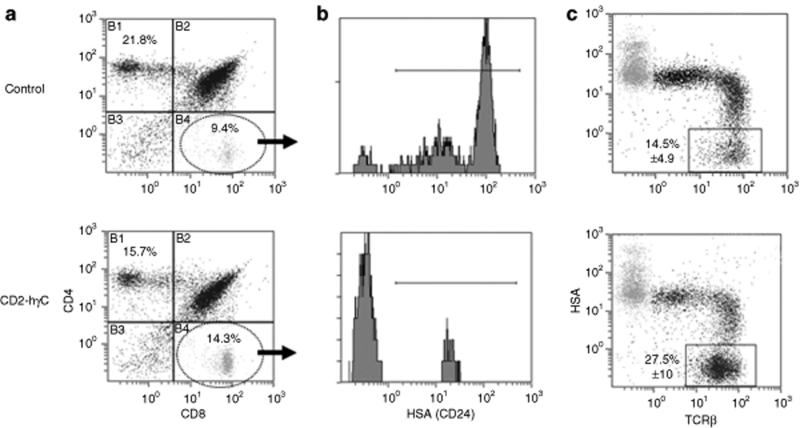
As the CD2 LCR directs expression mainly to the T-cell compartment,14,26 thymic development in the transgenic mice was examined in detail. Although there was no evidence for changes in total thymocyte number or gross phenotypic alteration, cell surface marker expression analysis of thymocytes by flow cytometry revealed a mild perturbation in the CD4:CD8 T-cell ratio. Specifically, transgenic mice from the higher expressing line (gCB) had significantly lower levels of CD4 single positive cells and higher levels of CD8 single positive cells than their nontransgenic counterparts (Table 1 and Figure 2a). A similar trend, though less pronounced, was seen in the gCA line. To establish the nature of the expanded CD8+ population we examined expression of functional markers including CD24 (heat stable antigen) and T-cell receptor β. Transgenic CD8+ thymocytes displayed lower expression of heat stable antigen when compared to nontransgenic controls consistent with a shift in the maturity of the T-cell population (Figure 2b), whereas expression of T-cell receptor β was also consistent with a more mature phenotype (Figure 2c). There was no difference between transgenics and littermate controls in the level of heat stable antigen by flow cytometry when gated on CD4+ cells (data not shown).
Table 1.
Average population values for T-cell markers in both control and transgenic mice
Figure 2.
Thymus explanted from transgenic and control animals were analyzed for cell surface marker expression to characterize the cell phenotype. (a) Perturbation of the CD4:CD8 T-cell ratio was observed in the total cell population for the CD2-hγC transgenic mice (1:1) (n = 15) compared with (2:1) in littermate controls (n = 22). Data representative of a transgenic profile and littermate control is shown. Average population values are indicated in Table 1. (b) Transgenic CD8+ thymocytes displayed lower expression of HSA (CD24) when compared to nontransgenic controls, illustrating a more mature T-cell phenotype. These histograms were gated on the CD8SP population (quadrant B4 in a). (c) Total thymocyte populations were stained for HSA and TCRβ to show that CD2-hγC transgenic mice appear to have an increased percentage of mature lymphoid cells as represented by TCRβ+HSA−. Average mean ± s.d. values are given.
A cohort of transgenic animals was maintained for a period of 2 years to determine whether expression of human γC in the T-cell compartment predisposed to the development of lymphoma. Transgenic lines gCA (n = 31) and gCB (n = 35) remained tumor free up to 18 months of age. All of the littermate controls (n = 48) remained tumor free over the observation period.
Lack of tumor acceleration by human γC in mice infected with MoMLV or genetically predisposed to T-cell lymphoma
In transgenic mouse systems, the action of weak oncogenes that are inefficient initiators of tumor development can often be revealed by neonatal infection with MLVs. The rationale for this observation is that these viruses are potent insertional mutagens that insert essentially randomly and cause gene activation or inactivation events that act in concert with the weak initiator delivered through the germline. The rapid onset of tumors in transgenic compared to wild-type control mice is an indication of this type of synergistic relationship.27,28 To test the oncogenic potential of ectopic human γC by this criterion, transgenic mice and littermate controls were infected with MoMLV, a potent inducer of T-cell lymphomas. However, no significant acceleration of tumor onset in the presence of human γC was observed in either the gCA or gCB transgenic lines (Figure 3a). A slight trend toward acceleration in the gCB line was not sustained as the cohort aged.
Figure 3.
Lack of tumor acceleration by human γC in mice. (a) Tumor free survival in two CD2-hγC transgenic lines gCA (n = 45) and gCB (n = 19), and controls (n = 41) after infection with MoMLV. Animals were on a C57/B16xCBA background where wild-type controls were strain and generation matched littermates. In animals succumbing to neoplastic disease there was no significant difference in the rates of lymphoma development between either transgenic line or the controls (gCA versus control, P = 0.32; gCB versus control, P = 0.51). Neither was there a significant difference between the two transgenic lines (gCA versus gCB, P = 0.15). (b) Tumor free survival of CD2-hγC/p53−/− (n = 17) mice was indistinguishable (P = 0.63) to that observed with p53−/− littermates without the human γC transgene (n = 15).
As a further stringent test of oncogenic potential, the gCB transgenic line was crossed with a variety of lymphoma-prone models. The human γC transgene was first crossed onto a p53−/− background, which collaborates strongly with a range of oncogenes.23,29 From a cohort of 17 transgene positive, p53 null mice (CD2-hγCxp53−/−), 15 developed tumors with an average latency of 178 days. For the transgene negative littermate controls (p53−/−) 14 out of 15 animals succumbed with an average latency 187 days (Figure 3b), showing no significant acceleration of tumor onset by the γC transgene on a strain-matched background. Animals null for the p53 gene succumb to a variety of tumor types of lymphoid and nonlymphoid origin,30 thus a further test of oncogene cooperation can be observed from the tumor spectrum. The presence of the human γC transgene did not alter the tumor spectrum of the p53−/− animals with similar numbers of lymphoma and solid tissue tumors observed irrespective of the transgene status. This contrasts with other oncogene models where a shift in the tumor phenotype to lymphoid tumors is noted.23,29 Previously we have monitored the incidence and latency of lymphoma development in p53 heterozygous animals carrying the Myc transgene.31 The presence of the gCB transgene on this strain-matched background (n = 21) did not increase the rate of tumor development (data not shown) confirming that the CD2-hγC transgene does not predispose to lymphoid malignancy.
The CD2-hγC transgene reconstitutes T and B-cell development on a γC null background
Human patients with γC deficiency lack T cells, and have dysfunctional B cells. By comparison, mice lacking a functional γC gene display thymic hypoplasia and have reduced numbers of peripheral T cells. B cells are also decreased in number and are dysfunctional.32 We wished to test the potential of the CD2-hγC transgene to rescue lymphoid development and function in γC−/− mice, both to confirm its functionality and to explore the potential of this system to model for the clinical setting in SCID-X1 patients where γC expression has been partially restored.
Human γC (gCB) transgenic mice were backcrossed three generations to derive offspring carrying the γC transgene on a γC−/− background. Analysis of the size and cellularity of the thymus in transgenic and control γC−/− mice revealed that the mice positive for the transgene had a significantly higher thymus to body weight ratio than the null animals (Figure 4a) and increased thymic cellularity (Figure 4b) although neither were fully restored to wild-type levels in the reconstituted animals. Western blot analysis confirmed the expression of human γC (Figure 4c). To analyze functionality, we examined the phosphorylation status of STAT5 after mitogenic stimulation. STAT5 proteins A and B are phosphorylated via the Janus kinase protein pathway as a result of activation in response to cytokines including IL-2, 3, 5, and 7 that signal via γC receptor complexes. The phosphorylation status of STAT5 has been used previously as an indicator of restored lymphopoiesis after reconstitution with human γC 33 and confirms here the functionality of CD2-driven human γC (Figure 4c).
Figure 4.
Restoration of γC-null lymphoid compartments was determined by organ weight and cell number at necropsy. The ratio of thymus to body weight (a) and thymic cellularity (b) is given for CD2-hγC reconstituted transgenic mice (where cohort numbers for (a) and (b) respectively are n = 18 & n = 3) compared to the strain matched γC−/− (n = 15 & n = 7) and wild-type (n = 12 & n = 5) controls. *P < 0.05, **P < 0.01. (c) Western blots of thymus extracted from two individual transgenic and control animals showing expression of the transgene only in the CD2-hγC animals. Human γC was functional in the reconstituted animals as confirmed by phosphorylation of STAT5A/B. Loading was controlled with β-actin.
Analysis of cohorts of CD2-hγCx murine γC−/− mice showed that development of mature lymphoid populations was delayed compared to wild-type counterparts. The vestigial thymus of γC−/− mice displays an aberrant population of CD4+ T-cells, and a reduced percentage of double positive CD4/CD8 cells (Figure 5a). At 8 weeks, the transgenic mice showed partial correction of this imbalance, whereas at 16 weeks marker expression was essentially indistinguishable from wild-type. This finding was also reflected in the spleen, where CD8 T-cell populations were not evident until 16 weeks (not shown). B-cell development in the spleen appeared to recover later than thymic T cells. At 8 weeks of age splenic populations were essentially identical to γC−/− mice, but by 16 weeks had recovered in most mice to wild-type levels, as revealed by staining for surface immunoglobulin M and CD45R (Figure 5b). The marked splenomegaly seen in γC−/− mice was also reduced with expression of the transgene (not shown).
Figure 5.
(a) Delayed restoration of T-cell populations in the thymus of γC−/− mice reconstituted with human γC. Examples of CD4/CD8 profiles are given for CD2-hγC+/mγC−/−, γC−/− and strain-matched wild-type (mγC+) at 8 weeks (top) and 16 weeks (bottom) of age. At 16 weeks, the average percentages ± s.d. for CD4SP were 16.6 ± 8.0, 27.0 ± 16.0 and 17.6 ± 6.8, and for CD4/8DP populations 73.7 ± 11.0, 45.9 ± 20.5 and 74.5 ± 8.8 for the wt (n = 5), null (n = 7) and transgenic (n = 6) animals, respectively. (b) Delayed restoration of the B-cell population in the spleens of γC−/− mice reconstituted with human γC. The panels show representative examples of viable splenocytes analyzed for immunoglobulin M and CD45R surface expression in three different hγC+/mγC−/− animals in addition to γC−/− (n = 5 at 8 weeks and n = 7 at 16 weeks) and strain-matched wild-type (mγC+, n = 5 at 8 weeks and n = 7 at 16 weeks) control mice. (c) Western blot analysis of human γC in sorted splenic B cells. Purified CD45R+ cells were isolated from CD2-hγC+/mγC−/− spleens (n = 4) and analyzed for γC expression. As the antibody crossreacts with murine γC, a positive signal was evident in wild-type mouse spleen, but not in the splenocytes of mγC−/− animals. β-Actin is shown to control for loading. The hγC+ control was the lysate from fibroblast cells (PG13) transfected with the γC vector.
The basis of B-cell reconstitution was of interest as the CD2 promoter/LCR elements were expected to direct transgene expression to the T-cell compartment. However, CD2 promoter function has also been detected in B-cells although its activity appears to decline with maturation.26 To test for sustained expression of the transgene we fractionated CD45R+ cells from the spleens of reconstituted mice and carried out western blot analysis. Abundant transgene expression was detected in CD45R+ as well as CD45R− cell populations (Figure 5c).
Transgenic reconstituted mice display ameliorated disease but no spontaneous lymphoma development
Abnormalities displayed by γC−/− mice due to immune dysregulation include colitis 32,34 and this problem was often manifested in our cohort as rectal prolapse, requiring early termination. It was notable that none of the transgenic mice displayed this condition compared with 16% of γC−/− controls (P < 0.05). This disease amelioration was reflected in a marked survival advantage for transgenic mice over their γC−/− littermates. Some aspects of the γC−/− phenotype continued to be manifested in the transgenic mice, albeit with less acute presentation. However, careful pathological examination of 19 such mice sacrificed between 66 and 318 days of age revealed no evidence of neoplastic disease.
γC−/− mice display high susceptibility to MLV-induced lymphoma which is not altered by the CD2-hγC transgene
As a further test of the intrinsic oncogenicity of ectopic γC expression, we examined the effect of the transgene on susceptibility to MLV leukemogenesis on a γC−/− background. A striking observation is that γC−/− mice are unusually susceptible to MLV leukemogenesis with a rate of onset of tumors substantially faster (P < 0.01) than strain matched wild-type controls (Figure 6). The copy number of integrated MLV was similar in both null and wild-type backgrounds (average ~9 copies/tumor) suggesting that this susceptibility is not directly accounted for by an increased virus load. Nevertheless, the rate of onset of tumors in CD2-hγC reconstituted γC−/− mice was unchanged compared to γC−/− littermates, showing that the γC transgene neither accentuated nor diminished the risk of MLV leukemogenesis (Figure 6).
Figure 6.
Lymphoma-free survival of MoMLV infected CD2-hγC+/mγC−/− (n = 40), mγC−/− (n = 11) and strain matched mγC+ mice (n = 20). The presence or absence of the CD2-hγC transgene had no significant effect on lymphoma-free survival (P = 0.33), although mγC−/− were markedly more sensitive to virus-induced lymphoma than wild-type controls (P < 0.001).
Discussion
In this study, we generated new lines of mice ectopically expressing human γC and examined the effects of the transgene on hematopoietic development and susceptibility to tumor development. Although the transgene induced a mild perturbation in thymic development as revealed by an expansion of mature CD8SP cells, we found no evidence of spontaneous tumor development. Moreover, no significant acceleration of tumor onset was seen in mice where we combined the γC transgene with a strong genetic predisposition to lymphoma (Myc transgene or p53 null) or with neonatal infection by a highly lymphomagenic γ-retrovirus. We confirmed that the transgene was expressed and functional, and capable of driving reconstitution of the lymphoid compartment on a γC−/− background. Even in this context, the transgene did not cause a discernible change in predisposition to MLV-induced lymphoma development.
Our results raise further questions with regard to the role of the γC transgene in leukemias arising in children following SCID-X1 gene therapy. Another study suggested that the gene might be potently oncogenic, as lymphoma development was noted in γC−/− mice that had received a lentivirus vector expressing the γC gene.12 As noted elsewhere 35 this was a relatively small study where the role of insertional mutagenesis was not explored in depth. Although our results do not exclude the possibility that γC can contribute to oncogenesis when expressed at higher level or in a different cell compartment, they show that ectopic expression of the human γC gene at levels sufficient to correct the defect on a γC−/− background do not predispose to tumor development. This conclusion is also supported by the findings of a recent study which showed that LMO2, but not γC, could block T-cell development when expressed from a retroviral vector in human CD34 cells.13 Moreover, studies of the leukemia cells from SCID-X1 patients suggested that γC was not expressed at elevated levels.36
The frequent occurrence of vector insertions at LMO2 in four out of five leukemias arising in SCID-X1 gene therapy subjects indicates a remarkably strong selection for activation of this proto-oncogene.36,37 A previous report suggested the possibility that γC and LMO2 might be acting as cooperating oncogenes in this context.11 This suggestion was based on the co-occurrence of retroviral integrations at both loci in a case of MLV-induced lymphoma, despite the rarity of insertions at each locus in a database of >3,000 tumor-derived MLV insertion sites. However, inspection of the current RTCGD database (http://rtcgd.abcc.ncifcrf.gov/) reveals that the three recorded insertions close to the γC gene are actually located within the 5′ end of a neighboring gene, Med12, that encodes a transcriptional co-activator with a specific role in Wnt signaling.38 As this gene appears a plausible target on the basis of proviral location and orientation,27,39 the possibility has to be considered that the proximity of the cluster to γC is coincidental. The hypothesis that Med12 is the significant target for these insertions is also consistent with the observation that γC expression was not upregulated in the index tumor.11
Recent high throughput studies of vector insertion sites in the peripheral blood of healthy SCID-X1 patients monitored following gene therapy revealed a highly biased pattern, reflecting strong selection for a subset of target genes that are likely to positively affect cell growth.2,3 The emerging picture is that, although the human γC gene plays a facilitating role in allowing the target cell population to expand, it is primarily the insertional mutagenic potential of the vector that drives the process of neoplastic transformation along with other genetic changes typical of T-lineage acute lymphoblastic leukemia, including Notch mutations, chromosomal translocations and CDKN2A deletions.36,37
It is interesting to note that in the present study infection of γC−/− mice with MoMLV was found to drive T-cell lymphomagenesis even faster than in strain matched wild-type mice, in spite of the markedly smaller thymus on this background. The basis of this acute sensitivity is of interest with regard to possible parallels with SCID-X1 disease. Lack of antiviral immunity seems unlikely to be a major factor as neonatal mice are unable to mount a significant response to MLV. It is conceivable that tumor immune surveillance is impaired on the γC−/− background and that transgene-driven reconstitution is too slow to have a decisive effect on the nascent tumor cells. It is interesting to compare our observations with those of a recent study which examined the development of leukemias in a mouse SCID-X1 model entailing vector-driven correction of the defect in an Arf null background.40 The study identified a decisive role for vector insertional mutagenesis but also reported an increased susceptibility when γC−/− donor cells were used instead of wild-type controls. The presence in γC−/− mice of an expanded immature T-cell population that is sensitive to transformation was offered as a potential explanation that would also be consistent with our findings.
In conclusion, this study demonstrates that controlled, stable expression at levels sufficient to correct the SCID-X1 deficit can be achieved without contributing to tumor predisposition. If problems arising from insertional mutagenesis can be controlled by improved vector design or delivery methods,41,42 it would appear that correction of this disease by gene therapy is likely to be achievable with an adequate margin of safety. However, the underlying susceptibility to leukemogenesis imposed by the γC−/− background confers a continuing risk specific to SCID-X1 gene therapy that requires further investigation.
Materials and Methods
Generation of CD2‐hγC. The γC coding region was amplified by PCR from the MFG-γC vector (kindly provided by K. Parsley, ICH, London) using the Taq PCR Core kit according to the manufacturer's instructions (Qiagen, West Sussex, UK) Primers (PciMFG: gcaagcgacatgttgaagcc; MluMFG: gatacgcgttcaggtttcaggc) were used for amplification and the resulting product was cloned into pCR2.1 Topo (Invitrogen; Paisley, UK) and the presence of the γC insert confirmed. The γC coding region was digested with Pci1 and MluI and subcloned into the multiple cloning region of the CD2 minigene vector VA hCD2 (ref. 43) (a kind gift of D. Kioussis). The presence of γC inserts was verified by double restriction digestion with KpnI and XbaI and the 5′ and 3′ ends of positive clones sequenced in order to determine the orientation of γC relative to the promoter. The CD2-hγC DNA was prepared for microinjection by digestion with Kpn1 and Xba1, followed by gel purification using the Geneclean Turbo system (BIO 101; Q-BIOgene, Cambridge, UK) according to the manufacturer's instructions.
Transgenic mice. The 14 kb CD2-hγC restriction fragment was microinjected into C57/B16xCBA/Ca F2 fertilized eggs according to standard protocols44 to generate the CD2‐hγC transgenic mice. CD2-MYC-ER transgenic,31 p53 knockout 30 and mice with a null mutation in γC (γC−/−)45 have been described previously. Mice were housed in conventional or individual ventilated cages. Neonates were infected within 24 hours of birth with ~105 infectious units of MoMLV.28 Animals were routinely monitored and sacrificed when showing signs of ill health. To control for mouse strain and generation all survival analyses were carried out with littermate controls, with the exception of the CD2-hγC/Myc/p53+/− experiment which was strain matched (C57/B16xCBA/Ca). All animal work was undertaken in line with the UK Animals (Scientific Procedures) Act of 1986.
Genotyping and detection by PCR. Human γC transgenic mice were identified by Southern blot hybridization or PCR analysis on DNA extracted after tissue biopsy. Southern blotting was carried out using a probe designed to human γC, nucleotides 345-604 (accession number: D11086). 20 µg of DNA was digested with BglII and NcoI. Hybridization was carried out and filters washed with 2 × SSC, 0.1% SDS.
PCR for human γC was carried out in a 50 µl reaction with 20 pmol of primers 5′-CAG CTC TGA GCC CCA GCC T-3′ and 5′-CTT TTG GGG GAA TCT CAC TGA CGA-3′ using Taq PCR core kit (Qiagen). The copy number of the γC transgene was estimated by Southern blot and densitometry, indicating that the gCA and gCB lines carry two and five copies of transgene, respectively. Transcript levels were estimated by real-time PCR.
Cell isolation and immunophenotype analysis. Lymphoid/tumor tissue was disaggregated in RPMI (Invitrogen, Paisley, UK) containing 10% FCS, 2 mmol/l glutamine, 50 µmol/l β-mercaptoethanol, and penicillin/streptomycin by mashing through a 70-µm cell strainer. Ficoll-Paque (Pharmacia, Kent, UK) separation of viable lymphocytes at 3,000 rpm for 10 minutes was used in all cases. B-cell isolation (to ~85% purity) was carried out on splenocytes using magnetic separation techniques with CD45R microbeads as per manufacturers' instructions (Miltenyi Biotech, Surrey, UK).
Cells (~2 × 106) were washed in cold PBS containing 0.1% bovine serum albumin and 0.01% sodium azide and directly labeled with a combination of rat antimouse monoclonal antibodies: Fluorescein (FITC)-conjugated anti-CD8 (Serotec, Oxford, UK); phycoerythrin (PE)-conjugated anti-CD4, FITC-conjugated anti-heat stable antigen, CyChrome-conjugated T-cell receptor β-chain, PE-conjugated anti-human CD132, PE-conjugated anti-CD43, Cy5-conjugated anti-mouse CD3 (BD PharMingen, Oxford, UK), Tri-color anti-CD19, biotin-conjugated anti-immunoglobulin M and PE-conjugated anti-CD45R (Caltag Medsystems, Buckingham, UK), FITC-conjugated anti-DX5 (Miltenyi Biotech, UK). Cells were washed three times in cold PBS/bovine serum albumin/sodium azide and resuspended in 500 µl of PBS/bovine serum albumin/sodium azide. Analysis was carried out on a Beckman Coulter Epics XL using Expo32 software.
Western blot analysis. Whole-cell extracts were prepared by washing cells in cold PBS followed by lysis in buffer (20 mmol/l HEPES, 5 mmol/l EDTA, 10 mmol/l EGTA, 5 mmol/l NaF, 10% glycerol, 1 mmol/l DTT containing 0.4 mol/l KCl, 0.4% Triton X-100, 0.1 µg/ml okadiac acid, and protease inhibitors, 5 µg/ml leupeptin, 5 µg/ml aprotinin, 5 µg/ml pepstatin A, 1 mmol/l benzamidine, 50 µg/ml phenylmethylsulfonyl fluoride). In all, 20 µg of protein extract were resolved on 12% NuPAGE Novex Bis-Tris gels (Invitrogen) and transferred to Hybond-ECL nitrocellulose membranes (Amersham, Buckinghamshire, UK). Membranes were probed with antibodies to γC (no. AF284; R&D Systems, Oxon, UK), phosphorylated STAT5A/B (no. 06-588; Upstate, Milton Keynes, UK), and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). For activation of STAT5A/B cells were stimulated for 48 hours with human IL-2 (0.06 µg/ml; Peprotech, London, UK) and con-A (2.5 µg/ml; Sigma, Dorset, UK).
Histology of tumor tissue. Tissues were fixed in 10% neutral buffered formalin, dehydrated through graded alcohol solutions and embedded in paraffin wax. Histological sections (3–5 mm thick) were stained with haematoxylin and eosin.
Statistical methods. All data are expressed as mean ± s.d. Data, including survival curves, were analyzed for significance using the Student's t-test (two-tailed). Student's t-test was deemed appropriate for survival analysis as the data met normal distribution criteria.
Acknowledgments
We thank the staff of Biological Services and Veterinary Diagnostics at the University of Glasgow and Kate Parsley for provision of the γC constructs (Institute of Child Health, London). We also thank Claire Crossan for technical assistance and Monica Stewart and Linda Hanlon for advice and support. A.J.T. is a Wellcome Trust Senior Clinical Fellow. This research was supported by the UK Department of Health grant number HTH/011/025/009. R.D.H. is supported by the European Union FP6 programme grant LSHB-CT-2006-037377. The authors declare no competing financial interests.
REFERENCES
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- Deichmann A, Hacein-Bey-Abina S, Schmidt M, Garrigue A, Brugman MH, Hu J, et al. Vector integration is nonrandom and clustered and influences the fate of lymphopoiesis in SCID-X1 gene therapy. J Clin Invest. 2007;117:2225–2232. doi: 10.1172/JCI31659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzwaelder K, Howe SJ, Schmidt M, Brugman MH, Deichmann A, Glimm H, et al. Gammaretrovirus-mediated correction of SCID-X1 is associated with skewed vector integration site distribution in vivo. J Clin Invest. 2007;117:2241–2249. doi: 10.1172/JCI31661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- Rabbitts TH, Bucher K, Chung G, Grutz G, Warren A., and , Yamada Y. The effect of chromosomal translocations in acute leukemias: the LMO2 paradigm in transcription and development. Cancer Res. 1999;59:1794s–1798s. [PubMed] [Google Scholar]
- Cornetta K, Morgan RA., and , Anderson WF. Safety issues related to retroviral-mediated gene transfer in humans. Hum Gene Ther. 1991;2:5–14. doi: 10.1089/hum.1991.2.1-5. [DOI] [PubMed] [Google Scholar]
- Belmont JW. Insights into lymphocyte development from X-linked immune deficiencies. Trends Genet. 1995;11:112–116. doi: 10.1016/S0168-9525(00)89012-5. [DOI] [PubMed] [Google Scholar]
- Uribe L., and , Weinberg KI. X-linked SCID and other defects of cytokine pathways. Semin Hematol. 1998;35:299–309. [PubMed] [Google Scholar]
- Mertsching E, Meyer V, Linares J, Lombard-Platet S., and , Ceredig R. Interleukin-7, a non-redundant potent cytokine whose over-expression massively perturbs B-lymphopoiesis. Int Rev Immunol. 1998;16:285–308. doi: 10.3109/08830189809042998. [DOI] [PubMed] [Google Scholar]
- Soudais C, Shiho T, Sharara LI, Guy-Grand D, Taniguchi T, Fischer A, et al. Stable and functional lymphoid reconstitution of common cytokine receptor γ chain deficient mice by retroviral-mediated gene transfer. Blood. 2000;95:3071–3077. [PubMed] [Google Scholar]
- Dave UP, Jenkins NA., and , Copeland NG. Gene therapy insertional mutagenesis insights. Science. 2004;303:333. doi: 10.1126/science.1091667. [DOI] [PubMed] [Google Scholar]
- Woods NB, Bottero V, Schmidt M, von Kalle C., and , Verma IM. Therapeutic gene causing lymphoma. Nature. 2006;440:1123. doi: 10.1038/4401123a. [DOI] [PubMed] [Google Scholar]
- Pike-Overzet K, de Ridder D, Weerkamp F, Baert MR, Verstegen MM, Brugman MH, et al. Ectopic retroviral expression of LMO2, but not IL2R γ, blocks human T-cell development from CD34+cells: implications for leukemogenesis in gene therapy. Leukemia. 2007;21:754–763. doi: 10.1038/sj.leu.2404563. [DOI] [PubMed] [Google Scholar]
- Greaves DR, Wilson FD, Lang G., and , Kioussis D. Human CD2 3′-flanking sequences confer high-level T cell-specific, position-independent gene expression in transgenic mice. Cell. 1989;56:979–986. doi: 10.1016/0092-8674(89)90631-4. [DOI] [PubMed] [Google Scholar]
- Stewart MA, Cameron E, Toth S, Campbell M, McFarlane R, Onions D, et al. Conditional expression and oncogenicity of a human c-myc transgene linked to a human CD2 dominant control region. Int J Cancer. 1993;53:1023–1030. doi: 10.1002/ijc.2910530628. [DOI] [PubMed] [Google Scholar]
- Badiani PA, Kioussis D, Swirsky DM, Lampert TA., and , Weston K. T-cell lymphomas in v-myb transgenic mice. Oncogene. 1996;13:2205–2212. [PubMed] [Google Scholar]
- Karsunky H, Geisen C, Schmidt T, Haas K, Zevnik B, Gau E, et al. Oncogenic potential of cyclin E in T-cell lymphomagenesis in transgenic mice: evidence for cooperation between cyclin E and Ras but not Myc. Oncogene. 1999;18:7816–7824. doi: 10.1038/sj.onc.1203205. [DOI] [PubMed] [Google Scholar]
- Vaillant F, Blyth K, Terry A, Bell M, Cameron E, Neil J, et al. A full length Cbfa1 gene product perturbs T-cell development and promotes lymphomagenesis in synergy with MYC. Oncogene. 1999;18:7124–7134. doi: 10.1038/sj.onc.1203202. [DOI] [PubMed] [Google Scholar]
- Turner SD, Merz H, Yeung D., and , Alexander DR. CD2 promoter regulated nucleophosmin-anaplastic lymphoma kinase in transgenic mice causes B lymphoid malignancy. Anticancer Res. 2006;26:3275–3279. [PubMed] [Google Scholar]
- van Hamburg JP, de Bruijn MJ, Dingjan GM, Beverloo HB, Diepstraten H, Ling KW, et al. Cooperation of Gata3c–Myc and Notch in malignant transforamtion of double postivie thymocytes. Mol Immunol. 2008;45:3085–3095. doi: 10.1016/j.molimm.2008.03.018. [DOI] [PubMed] [Google Scholar]
- Bernard O, Cory S, Gerondakis S, Webb E., and , Adams JM. Sequence of the murine and human cellular myc oncogenes and two modes of myc transcription resulting from chromosome translocation in B lymphoid tumours. EMBO J. 1983;2:2375–2383. doi: 10.1002/j.1460-2075.1983.tb01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lohuizen M, Verbeek S, Krimpenfort P, Domen J, Saris C, Radaszkiewicz T, et al. Predisposition to lymphomagenesis in pim-1 transgenic mice: cooperation with c-myc and N-myc in murine leukemia virus-induced tumors. Cell. 1989;56:673–682. doi: 10.1016/0092-8674(89)90589-8. [DOI] [PubMed] [Google Scholar]
- Blyth K, Terry A, Mackay N, Vaillant F, Cameron E, Neil JC, et al. Runx2: a novel oncogenic effector revealed by in vivo complementation and retroviral tagging. Oncogene. 2001;20:295–302. doi: 10.1038/sj.onc.1204090. [DOI] [PubMed] [Google Scholar]
- Uren AG, Kool J, Berns A., and , van Lohuizen M. Retroviral insertional mutagenesis: past, present and future. Oncogene. 2005;24:7656–7672. doi: 10.1038/sj.onc.1209043. [DOI] [PubMed] [Google Scholar]
- Fan H. Leukemogenesis by Moloney murine leukemia virus: A multistep process. Trends Microbiol. 1997;5:74–82. doi: 10.1016/S0966-842X(96)10076-7. [DOI] [PubMed] [Google Scholar]
- de Boer J, Williams A, Skavdis G, Harker N, Coles M, Tolaini M, et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur J Immunol. 2003;33:314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- Jonkers J., and , Berns A. Retroviral insertional mutagenesis as a strategy to identify cancer genes. Biochim Biophys Acta. 1996;1287:29–57. doi: 10.1016/0304-419x(95)00020-g. [DOI] [PubMed] [Google Scholar]
- Stewart MA, Terry A, O'Hara M, Cameron ER, Onions DE., and , Neil JC. Til-1, a novel insertion locus for Moloney murine leukaemia virus in lymphomas of CD2-myc transgenic mice. J Gen Virol. 1996;77:443–446. doi: 10.1099/0022-1317-77-3-443. [DOI] [PubMed] [Google Scholar]
- Blyth K, Terry A, O'Hara M, Baxter EW, Campbell M, Stewart M, et al. Synergy between a human c-myc transgene and p53 null genotype in murine thymic lymphomas: contrasting effects of homozygous and heterozygous p53 loss. Oncogene. 1995;10:1717–1723. [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Butel JS, et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Blyth K, Stewart M, Bell M, James C, Evan G, Neil JC, et al. Sensitivity to Myc-induced apoptosis is retained in spontaneous and transplanted lymphomas of CD2-mycER mice. Oncogene. 2000;19:773–782. doi: 10.1038/sj.onc.1203321. [DOI] [PubMed] [Google Scholar]
- Disanto JP, Müller W, Guy-grand D, Fischer A., and , Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin-2 receptor γ chain. Proc Natl Acad Sci USA. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuringa JJ, Chung KY, Morrone G., and , Moore MA. Constitutive activation of STAT5A promotes human hematopoietic stem cell self-renewal and erythroid differentiation. J Exp Med. 2004;200:623–635. doi: 10.1084/jem.20041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disanto JP, Guygrand D, Fisher A., and , Tarakhovsky A. Critical role for the common cytokine receptor γ chain in intrathymic and peripheral T cell selection. J Exp Med. 1996;183:1111–1118. doi: 10.1084/jem.183.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasher AJ, Gasper HB, Baum C, Modlich U, Schambach A, Candotti F, et al. Gene therapy: X-SCID transgene leukaemogenicity. Nature. 2006;443:E5–E6. doi: 10.1038/nature05219. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe SJ, Mansour MR, Schwarzwaelder K, Bartholomae C, Hubank M, Kempski H, et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J Clin Invest. 2008;118:3143–3150. doi: 10.1172/JCI35798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Xu X, Hecht A., and , Boyer TG. Mediator is a transducer of Wnt/β-catenin signaling. J Biol Chem. 2006;281:14066–14075. doi: 10.1074/jbc.M602696200. [DOI] [PubMed] [Google Scholar]
- Neil JC., and , Cameron ER. Retroviral insertion sites and cancer: Fountain of all knowledge. Cancer Cell. 2002;2:253–255. doi: 10.1016/s1535-6108(02)00158-7. [DOI] [PubMed] [Google Scholar]
- Shou Y, Ma ZJ, Lu T., and , Sorrentino BP. Unique risk factors for insertional mutagenesis in a mouse model of XSCID gene therapy. Proc Natl Acad Sci USA. 2006;103:11730–11735. doi: 10.1073/pnas.0603635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum C, Kustikova O, Modlich U, Li ZX., and , Fehse B. Mutagenesis and oncogenesis by chromosomal insertion of gene transfer vectors. Hum Gene Ther. 2006;17:253–263. doi: 10.1089/hum.2006.17.253. [DOI] [PubMed] [Google Scholar]
- Zhang F, Thornhill SI, Howe SJ, Ulaganathan M, Scharnbach A, Sinclair J, et al. Lentiviral vectors containing an enhancer-less ubiquitously acting chromatin opening element (UCOE) provide highly reproducible and stable transgene expression in hematopoietic cells. Blood. 2007;110:1448–1457. doi: 10.1182/blood-2006-12-060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhumabekov T, Corbella P, Tolaini M., and , Kioussis D. Improved version of a human CD2 minigene based vector for T cell-specific expression in transgenic mice. J Immunol Methods. 1995;185:133–140. doi: 10.1016/0022-1759(95)00124-s. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Constantini F., and , Lacey E. Manipulating the Mouse Embryo: A Laboratory Manual. 2nd edn. Cold Spring Harbot Laboratory Publications: New York; 1994. [Google Scholar]
- Goldman JP, Blundell MP, Lopes L, Kinnon C, Di Santo JP., and , Thrasher AJ. Enhanced human cell engraftment in mice deficient in RAG2 and the common cytokine receptor γ chain. Br J Haematol. 1998;103:335–342. doi: 10.1046/j.1365-2141.1998.00980.x. [DOI] [PubMed] [Google Scholar]