Abstract
Systemically administered vectors must cross the endothelial lining of tumor blood vessels to access cancer cells. Vectors that interact with markers on the lumenal surface of these endothelial cells might have enhanced tumor localization. Here, we generated oncolytic measles viruses (MVs) displaying αvβ3 integrin-binding peptides, cyclic arginine-glycine-aspartate (RGD) or echistatin, on the measles hemagglutinin protein. Both viruses had expanded tropisms, and efficiently entered target cells via binding to integrins, but also retained their native tropisms for CD46 and signaling lymphocyte activation molecule (SLAM). When fluorescently labeled and injected intravascularly into chick chorioallantoic membranes (CAMs), in contrast to unmodified viruses, the integrin-binding viral particles bound to the lumenal surface of the developing chick neovessels and infected the CAM vascular endothelial cells. In a mouse model of VEGF-induced angiogenesis in the ear pinna, the integrin-binding viruses, but not the parental virus, infected cells at sites of new blood vessel formation. When given intravenously to mice bearing tumor xenografts, the integrin-binding virus infected endothelial cells of tumor neovessels in addition to tumor parenchyma. To our knowledge, this is the first report demonstrating that oncolytic MVs can be engineered to target the lumenal endothelial surface of newly formed blood vessels when administered intravenously in living animals.
Introduction
One of the major barriers to successful cancer gene therapy is the inefficiency of gene delivery to sites of tumor growth using currently available vectors.1 It was hoped that replication-competent viruses would overcome this problem by propagating selectively in the tumor, but still this is often not sufficient for tumor eradication.2 There is, therefore, a strong rationale to engineer these vectors to enhance their interactions with tumor blood vessels, and thereby to enhance their delivery to sites of tumor growth.1
Tumor blood vessels are structurally and antigenically distinct from normal quiescent blood vessels. Structurally, tumor vessels are more leaky than normal vessels due to the presence of fenestrae (50–80 nm) and intercellular gaps between tumor endothelial cells (200–900 nm).3 Antigenically, numerous molecular targets have been identified on the lumenal aspect of tumor neovessels, both on the endothelial cell surface and in the exposed extracellular matrix between endothelial cells.4 Building on these observations, several groups have engineered the coat proteins of viral vectors to enhance their interactions with surface markers on proliferating tumor endothelial cells (e.g., αvβ3, α5β1 integrins) or with components of the extracellular matrix exposed in tumor neovessels, (e.g., laminin, fibronectin, collagen) and this has sometimes led to superior antitumor activity.5,6,7 However, perhaps surprisingly, the putative interactions between these engineered viruses and the lumenal surfaces of newly formed blood vessels are seldom demonstrated in vivo, but instead have been inferred from a combination of indirect approaches (e.g., superior antitumor efficacy and ex vivo infection of cultured vascular endothelial cells).
Integrins comprise a family of α- and β-heterodimeric cell surface receptors important for cell proliferation, migration and survival in vitro and in vivo.8 Integrins such as αvβ3, αvβ5, α5β1, and α4β1 are expressed abundantly on actively remodeling vasculature as well as on tumor cells.8 Arginine-glycine-aspartate (RGD) containing peptides can bind to several members of the integrin family (α5β1, α8β1, αvβ1, αvβ3, αvβ5, αvβ6, αvβ8, and αIIbβ3)9 and therefore have the potential to increase viral interactions with a variety of cell types, including tumor cells, endothelial cells and vascular smooth muscle cells, dendritic cells and T lymphocytes.10,11,12 Hence, integrin-binding RGD motifs have been engineered on the surfaces of viral vectors to enhance transduction efficiency, for example, on adenoviral vectors,13,14,15 AAV vectors,16 and oncolytic measles viruses (MVs),17 in all cases with encouraging results. Unfortunately, the superior antitumor activity of these RGD-displaying vectors cannot be definitively attributed to the enhancement of their interactions with vascular endothelial cells because the displayed peptides also enhance their interactions with other cell types, including integrin-expressing tumor cells.17,18,19,20 Moreover, few of these studies have included data to demonstrate that the RGD-displaying vectors or viruses can actually bind neovessels and/or transduce the endothelial lining of neovessels after intravascular administration.15
Live attenuated Edmonston MVs have promising oncolytic activity against a variety of human tumor xenografts models.21,22,23 MV uses its two coat proteins, the attachment hemagglutinin (H) protein and the fusion protein for infection. The natural viral receptors are CD46, which is expressed abundantly on tumor cell surfaces,24 and signaling lymphocyte activation molecule (SLAM), which is expressed on activated immune cells.25 The measles H protein is remarkably flexible and can accommodate insertion of large polypeptides, including single-chain antibodies (scFv), at its extraviral C-terminus.26 Ligands displayed in this way are able to confer new binding specificities and new tropisms to the viral particles on which they are displayed.27 A recombinant MV displaying a mutated form of the integrin-binding snake venom peptide, echistatin was previously reported.17 That virus was able to mediate infection of cultured cells via cell surface αvβ3 integrins, showed superior antitumor activity after intratumoral injection in a subcutaneous myeloma xenograft model, and infected vascular endothelium of chick blood vessels when applied directly on the surface of a 10-day chorioallantoic membrane (CAM). However, for technical reasons, the interaction of this virus with blood vessels walls was not elucidated after intravascular administration. Given the central importance of the angiogenesis targeting paradigm for the cancer gene therapy field, we generated recombinant MVs capable of binding to both αvβ3 and α5β1 integrins and performed a series of high-resolution in vivo studies to elucidate the altered fate of these integrin-binding viruses in newly formed blood vessels in three different animal models; the CAM model, in a VEGF-induced angiogenesis ear pinna model, and human tumor xenografts in mice postintravascular delivery.
Results
Generation and characterization of integrin-targeted MVs
We generated four new recombinant MVs displaying cyclic RGD (a nine amino-acid peptide) or echistatin (a 49 amino-acid polypeptide with an RGD motif) as C-terminal extensions of the measles H protein in viruses expressing either enhanced green fluorescent protein (GFP) (MV-GEcs or MV-GcRGD) or firefly luciferase (MV-LEcs or MV-LcRGD) (Figure 1a). These viruses retain their tropisms for CD46 and SLAM. To confirm correct incorporation of the displayed peptides, immunoblotting using anti-H antibodies was performed. As shown in Figure 1b, the chimeric H proteins of the recombinant viruses had slightly slower mobilities compared to the parental H. Because the displayed peptides are relatively small, reverse transcription-PCR analysis (Figure 1c) and DNA sequencing (data not shown) of viral RNA were also performed to confirm the presence of the additional peptides on the chimeric H transcripts. The recombinant viruses were propagated on Vero producer cells and their one-step growth curves were comparable to that of the parental MV-GFP virus (Figure 1d,e).
Figure 1.
Generation and characterization of the recombinant viruses. (a) Schematic representation of the parental and recombinant measles virus full-length cDNA genomes. Echistatin and cyclic RGD cDNAs were inserted at the COOH-terminal of measles hemagglutinin (H) protein as SfiI–NotI inserts. (b) Immunoblot of the parental and chimeric virions using anti-H and antinucleocapsid (N) antibodies. Equal titers of each virus were loaded. Lane 1, MV-GEcs; Lane 2, MV-GcRGD; Lane 3, MV-GFP. (c) RNA extracted from infected Vero cells were analyzed by RT-PCR to detect the respective H transcripts. (d,e) One-step growth curves of recombinant viruses in Vero producer cells. Cells were infected at MOI of 3.0 TCID50/cell and cell-associated (d) and released virus (e) were harvested and titrated on Vero cells at intervals following infection. cDNA, complementary DNA; MOI, multiplicity of infection; RGD, arginine-glycine-aspartate; RT-PCR, reverse transcription-PCR.
NIH3T3-Ras transformed fibroblasts express mouse αvβ3 and α5β1 integrins but lack the human measles receptors, CD46 and SLAM (Figure 2a). As shown in Figure 2b, NIH3T3-Ras cells were infected by integrin-binding MV-GEcs and MV-GcRGD but not by the parental MV. To confirm the specificity of integrin receptor usage by the recombinant viruses, a competition assay using increasing concentrations of exogenously added soluble echistatin was performed on these cells (Figure 2c). Echistatin binds with high specificity and affinity to αvβ3 and α5β1 integrins.30 Infectivity of MV-GEcs decreased by 90% to baseline levels in the presence of 0.5 µmol/l soluble echistatin. The infectivity of the parental nonintegrin-binding MV-GFP was not affected by the addition of exogenous echistatin.
Figure 2.
Specificity of the integrin-binding viruses. (a) Flow cytometry data on expression of mouse integrins αv, β3, α5β1, human CD46 or human SLAM receptors (filled histogram) on NIH3T3-Ras cells compared with isotype control (empty histogram). (b) NIH3T3-Ras cells were infected with the respective viruses at MOI of 0.5, and photographs were taken 36 hours later. Only the integrin-binding viruses were able to infect and induce syncytia in NIH3T3-Ras cells. In contrast, all viruses were able to infect Vero cells (MOI 0.05). (c) Competition assay. MV-GFP or MV-GEcs (MOI 1.0) were pre-mixed with serially diluted soluble echistatin before inoculation on NIH3T3-Ras cells, and the number of green infected cells was counted at 48 hours postinfection. Experiments were done in triplicates (mean ± SD). MOI, multiplicity of infection; SLAM, signaling lymphocyte activation molecule.
Virus binding and infection of vascular endothelium in developing CAM vessels
Infection of newly formed blood vessels by integrin-binding MVs was evaluated in developing chick CAMs. This model enabled us to perform high-resolution analysis of virus binding and infection of endothelial cells using fluorescence microscopy. We first evaluated if MV displaying RGD peptides could attach to the lumenal surface of neovessels. Both the parental and RGD-displaying viruses were fluorescently labeled with a lipophilic DiO dye to allow direct visualization of the viral particles. These viruses express firefly luciferase reporter genes and thus, any green fluorescence is from the DiO label and not due to GFP expression. The DiO-labeled MVs (2 × 105 TCID50) were injected directly into the large CAM blood vessels at days 7–10 of embryonic development. At 18–24 hours postinjection, whole CAMs from the embryos were dissected out, placed on glass slides and examined using a fluorescence microscope. No green fluorescent virions were seen binding to the blood vessels of CAMs injected intravascularly with the parental MV (Figure 3a). In contrast, numerous small green DiO-labeled particles were seen lining the neovessels of CAMs injected with the RGD-displaying MV (Figure 3a). We estimate the sizes of these fluorescent particles to be ~530 nm when compared against the erythrocytes, which are ~8 µm in diameter. Measles is polymorphic and the diameters of virions range from 350 to 1,000 nm.31,32 Thus these green fluorescent particles are very likely to be measles virions but could also include aggregates of virions. Our method of analysis using fluorescence microscopy on freshly harvested tissues makes it difficult to confirm if the virions are on the lumenal side of the endothelial cells, are within the endothelial cells or have passed beyond the endothelial cell layer through intercellular japs. Future studies electron microscopy will help to further elucidate the precise location of these viruses.
Figure 3.
Binding of DiO-labeled RGD-displaying measles viral particles to CAM neovessels. Seven- to ten-day-old CAMs were injected intravascularly with fluorescently labeled (DiO, green) parental or RGD-displaying measles viruses and examined under a fluorescence microscope 24 hours later at ×200 (a) and ×400 (b) magnification. Photos were taken under blue light and the corresponding phase image was taken under brightfield. Bar = 100 µm. Experiments were repeated at least three times for each virus (n = 20 eggs/virus). White box represents the region magnified in c. (c) Cells on the outside of the CAM micovessels which have taken up fluorescently labeled virions are circled while white arrows point to fluorescently labeled virions in the lumenal wall of blood vessels. Bar = 100 µm. (d) Representative images of cryosections of CAMs previously injected with MV-GEcs and stained with an antibody to measles nucleocapsid protein. Numerous MV positive particles (blue color) were seen in the ectoderm of CAM where microvessels are located. CAM, chorioallantoic membrane; GFP, green fluorescent protein; RGD, arginine-glycine-aspartate.
It is also apparent that in CAMs injected with the MV-cRGD or MV-Ecs, two types of green fluorescent signals were seen. In addition to the small viral particles lining the lumen of the blood vessels (Figure 3b), some cells found on the outside of the blood vessel have green fluorescence throughout their cytoplasm (Figure 3c). Our current hypothesis is that these are cells of the monocytic lineage and that they have phagocytosed the DiO-labeled viral particles that have extravasated through the endothelium through intercellular (leaky) gaps in the endothelial barrier. To confirm that the small fluorescent particles lining the lumen of CAM vessels were MVs, the CAM cryosections were immunostained with antimeasles nucleocapsid (N) protein antibody. As shown in Figure 3d, measles antigen positive (blue color) particles were found in the CAM ectoderm layer where the blood vessels are localized.
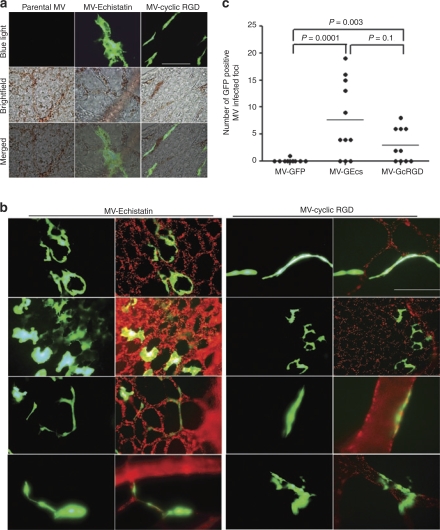
To determine whether the integrin-binding viruses could infect endothelial cells of CAM neovessels, GFP expressing viruses were injected intravascularly into CAMs. Three days later, CAMs were dissected from the embryos and examined under the fluorescence microscope. In contrast to the parental virus, the integrin-binding viruses, MV-GEcs and MV-GcRGD infected endothelial cells in the blood vessels of the developing CAMs (Figure 4a). To facilitate visualization of the CAM vasculature, CAMs were injected intravascularly with Texas-red conjugated Lycopersicon esculentum lectin which binds to lumenal surface of the vascular endothelial cells (Figure 4b). It is apparent in these images that there is good colocalization of green (GFP viral gene expression) and red (endothelial cells) fluorescence signals (Figure 4b), demonstrating infection of endothelial cells lining the lumen of CAM vasculature by the integrin-binding viruses. The proportion of embryos with infected vessels was calculated for each virus and the data are as follows: MV-GFP = 0.1 (n = 10 embryos), MV-Ecs = 0.73 (n = 11), and MV-cRGD = 0.6 (n = 10). There is a significant difference in infection rates between the three viruses (P = 0.0368). Pair-wise comparisons between the three viruses are statistically significant, MV-GFP versus MV-Ecs (P = 0.01), MV-GFP versus MV-cRGD (P = 0.01), and MV-Ecs versus MV-cRGD (P = 0.04).
Figure 4.
RGD-displaying viruses (MV-Echistatin or MV-cyclic RGD) but not the parental virus, infected endothelial cells of developing CAM vasculature. GFP expressing viruses was injected intravascularly into 6–9 day old CAMs. CAMs were harvested 3 days later and examined under brightfield or blue light for GFP fluorescence. (a) Representative pictures of MV infected GFP positive endothelial cells in the CAM neovasculature. Note brown colored erythrocytes in the lumen of microvessels. Bar = 100 µm. (b) CAMs were perfused with Texas-red conjugated tomato lectin (binds to endothelial cells) prior to harvest to highlight the lumen of CAM vasculature (red color). Note GFP positive infected endothelial cells in the CAMs injected intravascularly with MV-Echistatin or MV-cyclic RGD. Bar = 100 µm. (c) The number of GFP positive foci representing infected micovessels was counted at ×100 magnification using the fluorescence microscope in each CAM treated with the respective viruses. — represents the average. Experiments were repeated with at least five batches of eggs (n = 5/virus/batch). The P values are presented. CAM, chorioallantoic membrane; GFP, green fluorescent protein; RGD, arginine-glycine-aspartate.
To quantitate the relative efficiency of neovessel transduction by the viruses, the numbers of GFP positive foci representing infected blood vessels in freshly harvested whole CAMs were counted at ×100 magnification (Figure 4c). The experiment was repeated with at least five batches of eggs where the respective viruses were injected intravascularly into at least five embryos. Mortality of the embryos is high (~50%) due to use of the ex ovo system (embryos living in the Petri dish), coupled with intravascular injection that causes bleeding and trauma to the vessels. Hence, despite using at least 25 eggs per treatment group, only 11 CAMs for MV-GEcs, and 10 CAMs for MV-GFP or MV-GcRGD, survived till the day of analysis (day 3 postvirus injection). For each embryo, the numbers of GFP positive foci were counted and compared. Extent of neovessel infection differs significantly between the three groups (P = 0.0002). Extent of neovessel infection is significantly higher in CAMs injected with the integrin-binding viruses, MV-GEcs (P = 0.0001), or MV-GcRGD (P = 0.0031) compared to those injected with the parental MV-GFP (negative binomial regression model, Figure 4c). There was no significant difference observed between the two RGD-displaying viruses (P = 0.1) despite that echistatin binds to integrins with 1,000-fold higher affinity. MV-GEcs infects about 2.5 times more vessels than MV-GcRGD (95% confidence interval: 0.84–7.7); while MV-GcRGD versus the parent MV-GFP has a 30-fold change (95% confidence interval: 3.2–285), and MV-GEcs versus parent virus has a 76-fold change (95% confidence interval: 8.3–704).
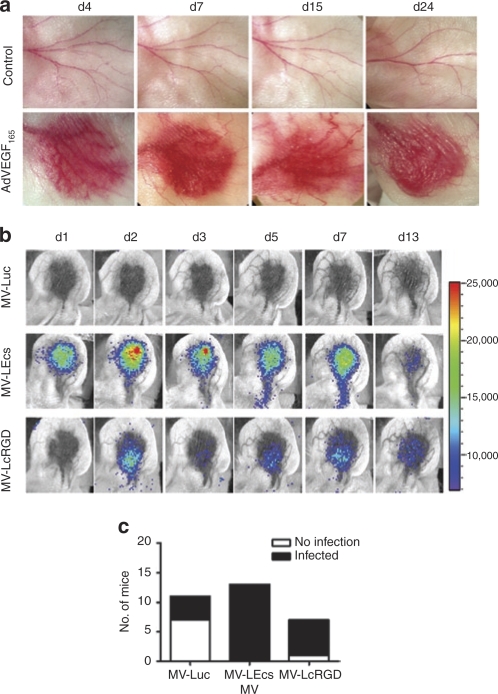
Infection of AdVEGF165 induced neovessel in the ear pinna of nude mice
To determine whether systemically administered RGD-displaying viruses could interact with endothelial cells of neovessels in a living mouse, we adopted a previously described model in which angiogenesis is induced in the ear pinna of nude mice by intradermal injection of 108 vector particles of Ad-hVEGF165. The formation of new blood vessels was apparent by naked eye examination of the injection site from day 4 postvector administration and peaked around day 7 through day 10 (Figure 5a). These mice were given 2 × 106 TCID50 MVs encoding firefly luciferase gene, MV-Luc, MV-LEcs, or MV-LcRGD, intravenously via the tail vein. Viral infection at the target site was monitored regularly using noninvasive bioluminescent imaging for determination of luciferase gene expression. In contrast to the parental virus MV-Luc, which only sporadically resulted in weak luciferase expression at the target site, the RGD-displaying viruses, MV-LEcs and MV-LcRGD frequently did infect the new vessels leading to intense luciferase expression which persisted typically for a week (Figure 5b,c). Of 20 mice that received either MV-LEcs or MV-LcRGD 19 had bioluminescence-detectable luciferase expression in the induced ear whereas only 4 out of 11 mice became weakly luciferase positive after receiving the control parental virus, MV-Luc (P < 0.001, Fisher's Exact test). Again, there is no significant difference between the ability of MV-Ecs and MV-RGD to infect neovessels (P = 0.35). Mice that received MV-LEcs or MV-LcRGD also had higher peak levels of viral expression (i.e., peak photon counts) (mean = 44,846 and 34,857 photons/second/cm2, respectively) at the target site compared to those receiving MV-Luc (mean = 11,818 photons/second/cm2). No bioluminescence signals were ever detected in the control uninjected ears (i.e., not injected with the AdVEGF165 vector) of any of the mice.
Figure 5.
RGD-displaying viruses infects neovessels in the athymic mouse ear pinna angiogenesis model. The right ears of nude mice were injected intradermally with 108 vector particles of Ad-hVEGF165. (a) Formation of new blood vessels were observed daily and photographs taken at indicated day postvector administration. (b) Mice were given 2 × 106 TCID50 measles virus intravenously via the tail vein on day 5 post-Ad-hVEGF165 administration. Firefly luciferase gene expression was monitored by bioluminescent imaging (n ≥ 6 mice per virus group). The number of days postvirus administration is indicated. (c) The numbers of mice with positive bioluminescent signal (filled histogram) in the AdVEGF165 induced ear or no infection (empty histogram) was plotted for each virus. Integrin-binding viruses (MV-Ecs and MV-cRGD) have significantly higher infection frequency compared to parent MV-GFP virus (P < 0.001). RGD, arginine-glycine-aspartate.
Infection of murine tumor blood vessels in tumor xenografts
Severe combined immunodeficiency mice bearing human KAS 6/1 myeloma tumors were injected intravenously via the tail vein with parental MV-GFP or echistatin-displaying MV-GEcs viruses (106 TCID50/100 µl). Tumors were harvested 4 days later for immunohistochemical analysis of MV-N positivity in the tumor neovessels and/or tumor parenchyma. Dual staining using an antimurine CD31 antibody was also performed on the tumor sections to identify endothelial cells of tumor blood vessels. As shown in Figure 6a,b, numerous foci of MV infection (positive N staining, red color) were observed in the tumor parenchyma of mice that received MV-GFP or MV-GEcs. These areas of N positivity were often more or less circular in shape and perivascular, or in close proximity with the CD31 positive tumor vessels (green color). However, in tumors of mice that received MV-GEcs, we observed an additional pattern of MV-N staining that was not observed in MV-GFP treated mice. These “linear” structures, which have the morphological appearance of blood vessels stained positive for MV-N and colocalized very well with CD31 positivity, confirming infection of endothelial cells of tumor blood vessels by the integrin-binding MV (Figure 6c,d).
Figure 6.
Echistatin-displaying measles virus, MV-GEcs, infects endothelial cells of tumor blood vessels postintravenous administration into SCID mice bearing subcutaneous KAS 6/1 human myeloma xenografts. Mice were given 1 x 106 TCID50 of parental MV-GFP virus or MV-GEcs virus. Tumors were harvested 4 days later. Dual staining was performed on the tumor sections using an antibody against measles nucleocapsid (N) protein (red color) and an antibody against murine CD31 (green color). Representative images (×200 magnification) of tumors from different mice are shown. Bar = 20 µm. SCID, severe combined immunodeficiency.
Discussion
Targeting tumor neovessels to enhance systemic delivery of cytotoxic genes remains an important goal in cancer gene therapy. Among the surface markers overexpressed on the endothelial cells, integrins have attracted considerable attention as targets for delivering both therapeutic and diagnostic agents.33 Integrins comprise a family of heterodimeric (α and β chains) cell surface receptors important in cell–cell and cell–substratum interactions.34 The αvβ3 and αvβ5 integrins are highly expressed on activated endothelial cells of tumor blood vessels and on tumor cells,35 and are the most extensively studied integrins for antiangiogenesis strategies and angiogenesis imaging agents.33 However, other integrins, for example, α1β1, α2β1, α5β1 (see below), and α6β4, are also being studied as potential therapeutic targets.36 As such, integrin-binding polypeptides have been displayed on several recombinant viruses and viral gene therapy vectors resulting in enhanced transduction of tumor cells or improved therapy against tumor xenografts, but the question of whether these integrin-binding viruses could interact with endothelial cells of neovessels after intravascular administration in vivo is typically not addressed nor has been the focus of those studies.13,16,17,20,37,38
In this study, we have shown in the well-established CAM in vivo angiogenesis model, that oncolytic MVs displaying integrin-binding peptides (echistatin or cyclic RGD) can localize on endothelial cells lining newly formed blood vessels and can infect endothelial cells of the CAM vasculature. The numbers of virus infected areas were disappointingly low but could potentially be enhanced by injecting more virus into the CAMs. Currently, we could inject only 2 × 105 TCID50 viruses (in 20 µl) intravascularly into the whole embryo as a larger volume of fluid resulted in unacceptably high embryo mortality. Assuming the CAM to have 109 cells (1 g) and because the endothelial cells are a very minor subpopulation within the tissue, probably <0.1% of all cells in the tissue (thus only 1 million cells), then the ratio of virus to cells is quite low at only 0.2. Another parameter that could contribute to low infection is patchy or low integrin expression on the CAM endothelial cells as it is well established that neovascularization is complete by day 10 of embryonic development. With decreasing integrin expression in older CAMs, MV infectivity, which is dependent on receptor density,39 is expected to be low. Using a murine model where the virus was injected intravenously in nude mice that had previously been injected intradermally with AdVEGF165 in the pinna of the ear, the integrin-binding MV-Ecs and MV-cRGD viruses, but not the parental MV-Luc virus, infected and spread in the neovasculature induced at that site. Furthermore, we have demonstrated for the first time that the echistatin-displaying MV can bind to and infect from the lumenal surface of endothelial cells lining tumor blood vessels postintravenous delivery into tumor bearing mice.
Hallak and colleagues previously generated an integrin-binding MV, MV-ERV,17 which displayed a mutant echistatin peptide. Echistatin, a disintegrin isolated from the venom of the saw-scaled viper, Echis carinatus, is a 49 amino-acid peptide with a central RGD motif that binds with high affinity to αvβ3 and α5β1 integrins, both overexpressed on tumor endothelial cells, and to αIIaβ3, which is expressed abundantly on blood platelets.30,40 Amino-acid residue 28 was mutated from Met to Leu in the mutant echistatin displayed by Hallak et al. to reduce its binding affinity for α5β1 integrin without impairing its affinity for αvβ3.40 This original MV-ERV virus was able to infect CAM neovessels when applied directly to the surface of the CAM (it was never injected into CAM blood vessels) and induced more rapid tumor regression compared to the parental MV when injected directly into subcutaneous human multiple myeloma xenografts in severe combined immunodeficiency mice.17 The echistatin-displaying MV generated for this study is different from MV-ERV in that it displays the unmutated parental echistatin peptide and retains intact α5β1 binding capacity. Integrin α5β1, like αvβ3, is also required for tumor angiogenesis.41 Upregulation of integrin α5β1 has been reported both in newly formed blood vessels and on tumor cells.41,42 Integrin α5β1 antagonists are known to inhibit tumor angiogenesis, reduce tumor metastasis and cause tumor regression in animal models.41,43 Taken together, those experiments show that α5β1 integrin like αvβ3 is expressed on tumor blood vessel endothelium and provides a potentially appealing target for vascular targeting.
Cyclic RGD (CDCRGDCFC) is perhaps the most widely exploited integrin-binding peptide and has been used to target the delivery of drugs, proteins, nonviral, and viral vectors or bacteriophages to tumors.5,44,45,46 The cRGD peptide binds to αvβ3 and αvβ5 integrins with 200-fold greater affinity, and to the α5β1 integrin with 50-fold greater affinity than linear RGD (GRGDSP), at least in vitro.47 Pasqualini and colleagues showed that bacteriophages displaying cRGD had 40- to 80-fold greater accumulation in subcutaneous breast cancer xenografts compared to nontargeted phages in vivo.44 The echistatin peptide used in this study has a considerably higher affinity (IC50 = 1.1 nmol/l) for integrins expressed on fibroblasts when compared to short RGD-containing peptides such as acPenRGD, cycloRGDdFV, and GRGDdSP (IC50 = 1,280, 2,380, and 6,730 nmol/l, respectively).40 However, despite this 1,000-fold difference in the αvβ3 binding affinities of cRGD (Kd = 183 nmol/l) and echistatin (Kd = 0.27 nmol/l),30 no statistically significant differences in the behavior of viruses displaying these ligands were observed in vivo. However, although not statistically significant, the MV-Ecs did infect more vessels per CAM and did spread slightly better in the ear pinna angiogenesis mode compared to MV-cRGD. The large difference in receptor binding affinities between the two peptides may have been negated by the multivalent nature of virus binding to its receptor. This possibility is supported by the results of our recent study of a panel of six retargeted MVs displaying anti-HER2/neu single-chain antibodies whose affinities varied between 10‐6 and 10‐11 mol/l. The study revealed that for a given Her-2 positive cell line, there was a threshold affinity at which MV infectivity and intercellular fusion were optimal, and that infectivity did not increase significantly thereafter, even when the affinity was increased ~1,000-fold.39
Oncolytic Edmonston strain MVs are currently being evaluated in three phase I clinical trials at Mayo Clinic where they are being administered intratumorally to patients with glioma, intraperitoneally to patients with ovarian cancer and intravenously to patients with multiple myeloma.21,22,23 As with other oncolytic viruses, efficient delivery of the virus to sites of tumor growth after intravenous administration remains a challenge. Long circulating macromolecules and nanoparticles can preferentially accumulate in tumor xenografts due to a phenomenon called enhanced permeability and retention effect. The enhanced permeability and retention effect in tumors was first described by Matsumura and Maeda;48 tumor blood vessels are structurally different from normal vasculature in that they are leaky with disorganized endothelial cells. Thus, along with poor lymphatic drainage in tumors, long circulating macromolecules diffuse into the tumor parenchyma via the endothelial cell junctions and are often retained in the tumor. In contrast, smaller molecules (<30 kd) are able to diffuse back into the circulation.49,50 This mode of “passive targeting” via enhanced permeability and retention effect requires that the nanoparticles be able to escape sequestration by the reticuloendothelial system of the liver and spleen in order to achieve higher plasma retention times and significant accumulation in the tumors.49,50 Results in Figure 6 indicated that systemically administered parental and integrin-binding MVs can access myeloma tumor xenografts and infect the tumor parenchyma. Dual staining for measles infection (MV-N staining) and murine anti-CD31 endothelial cell markers showed that the areas of MV-N infection are often perivascular for both the parental and echistatin-displaying viruses, suggesting that these viral particles have probably extravasated through the leaky tumor vessels into the tumor parenchyma and initiated an infection in that local environment. A possible strategy to increase plasma retention times and tumor accumulation of viruses is to shield the virions using polyeythlene glycol.49 However, we hypothesize that virus delivery and extravasation at tumor sites might also be enhanced by generating viruses that can interact with the lumenal surface of endothelial cells lining tumor neovessels. In tumors of mice injected intravascularly with the RGD-displaying viruses, MV-N and CD31 staining colocalized in structures that have resemblance to tumor blood vessels, suggesting that the echistatin-displaying virus can recognize its integrin target and can infect CD31 positive endothelial cells of the tumor blood vessels. It is possible that echistatin virus had infected the endothelial cells from the lumenal side, but it could have also extravasated into the tumor through the leaky blood vessel and infected the endothelial cells as integrins are expressed on both the lumenal and basal side of endothelial cells. However, despite that not all tumor blood vessels were infected and the efficiency of target recognition is suboptimal, this study certainly gives us hope that it may be possible to enhance the efficiency of tumor localization of intravenously administered MVs by engineering them to interact more efficiently with tumor neovessels.
In summary, we generated recombinant MVs displaying echistatin or cRGD peptides as C-terminal extensions of the unmutated (i.e., still able to bind to CD46 and SLAM) viral attachment protein. In vivo studies showed that these viruses can localize on and infect the endothelial lining cells of new blood vessels. This is the first study to demonstrate directly that oncolytic MVs can be engineered to target the lumenal endothelial surface of newly formed blood vessels in living animals postintravascular administration.
Materials and Methods
Cell culture. Cell lines were maintained in Dulbecco's Modified Eagle's Medium or RPMI-1640 (Gibco-BRL, Long Island, NY) supplemented with heat inactivated fetal bovine serum at 37 °C in a 5% CO2 humidified incubator. Vero African green monkey kidney cells were maintained in Dulbecco's Modified Eagle's Medium containing 5% fetal bovine serum. NIH3T3-Ras murine hRas-transformed fibroblast cells (a kind gift from Yasuhiro Ikeda, Mayo Clinic) were grown in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum. Human myeloma KAS 6/1 cells were grown in 10% fetal bovine serum-RPMI supplemented with 1 ng/ml recombinant human IL-6 (R&D Systems, Minneapolis, MN).
Generation and characterization of RGD-displaying MVs. Echistatin and cyclic RGD amino-acid sequences were reverse translated to DNA sequences using Vector NTI software (Invitrogen, Carlsbad, CA). The genes with flanking cohesive ends for SfiI and NotI were generated by oligonucleotide synthesis. Complementary oligonucleotides were incubated at 94 °C for 3 minutes, hybridized by gradually decreasing temperatures from 70 °C to 4 °C at 0.1 °C/min. Hybridized DNA fragments were then ligated and inserted in-frame into SfiI/NotI-digested pCGHX-αCD38.28 The genes for the recombinant H glycoproteins were then cloned as PacI/SpeI fragments into the full-length infectious molecular clone of MV expressing GFP or firefly luciferase reporter genes respectively, p(+)MV-eGFP or p(+)MV-Luc. Viruses were rescued as described previously.29
Immunoblot analysis of the H protein. Viral samples (2 × 104 TCID50; virus titer was determined on Vero cells) were directly mixed with an equal volume of sodium dodecyl sulfate loading buffer (130 mmol/l Tris (pH 6.8), 20% glycerol, 10% sodium dodecyl sulfate, 0.02% bromophenol blue, 100 mmol/l dithiothreitol). These samples were denatured for 5 minutes at 95 °C, fractionated on a 7.5% sodium dodecyl sulfate polyacrylamide gel, blotted onto nitrocellulose membrane (Amersham, Piscataway, NJ), and immunoblotted with antimeasles N and H protein as described previously.26
Reverse transcription-PCR. Vero cells were infected with MV, MV-Ecs, or MV-cRGD at multiplicity of infection of 0.02 for 2 hours at 37 °C. When >80% of the cells were in syncytia, cells were lysed and total RNA were extracted with RNeasy mini kit (Qiagen, Valencia, CA). Reverse transcription-PCR was done using Titan one-tube reverse transcription-PCR system (Roche, Indianapolis, IN) according to manufacturer's instructions.
Virus titration. To compare growth characteristics of the recombinant viruses, Vero cells were infected with the viruses at a multiplicity of infection of 3.0 for 2 hours at 37 °C. The inoculum was removed; standard medium was added; and the cells were maintained at 32 °C. At 12, 24, 36, 48, 72, and 96 hours after infection, cells were scraped into 1 ml Opti-MEM (Life Technologies, Rockville, MD), and cell-associated viruses were released by two freeze–thaw cycles. Viral titers were determined by TCID50 titration on Vero cells.
Virus specificity. Expression of integrins on NIH3T3-Ras was determined by flow cytometry. Cells were incubated for 30 minutes on ice with rat antimouse α5β1 (Santa Cruz Biotechnology, Santa Cruz, CA), rat antimouse αv or rabbit anti-β3 antibodies (Chemicon, Temecula, CA), washed twice, and incubated for 30 minutes with 100 µl of 10 µg/ml of Alexa Fluor 488-conjugated goat antirat or antirabbit IgG (Molecular Probes, Eugene, OR). Cells were also screened for expression of human CD46 and SLAM by FACS using phycoerythrin-conjugated anti-CD46 antibody, phycoerythrin-conjugated anti-CD150 antibody or control isotype-matched antibody. All antibodies were purchased from BD Biosciences Pharmingen (San Diego, CA). Cells were infected with viruses (multiplicity of infection = 0.5) for 2 hours at 37 °C, after which the virus inoculum was removed, and the cells were maintained in standard medium for 48 hours. The cells were fixed with 0.5% glutaldehyde, stained with 0.2% crystal violet solution, and photographed. To demonstrate the specificity of receptor usage, soluble echistatin (Sigma, St. Louis, MO) was serially diluted, mixed with recombinant viruses at multiplicity of infection of 1 and were used to infect NIH3T3-Ras cells for 3 hours. Final concentrations of echistatin peptide ranged from 0.5 to 0.0005 µmol/l. Virus inoculum was removed and cells were maintained in medium containing a fusion inhibitory peptide (Bachem, King of Prussia, PA) to prevent syncytia formation. Cells expressing GFP were counted at 48 hours postinfection.
Adenovirus expressing human VEGF165. A seed stock of type 5 adenoviral vector expressing human VEGF165 (Ad-hVEGF165) was obtained from the Gene Vector Laboratory of University of Pittsburgh. The recombinant Ad vectors were propagated in 293 cells, and purified by centrifugation through a cesium chloride gradient. The vector stocks were then dialyzed against 10% glycerol–phosphate buffered saline and frozen at ‐80°C until use. The adenoviral vector particle number (vp) was determined by measuring optical density at 260 nm. Typical vector stock titers were 1012 vp/ml with a 1:100 infectious plaque forming unit:vector particle ratio.
Ear pinna angiogenesis model. All procedures involving animals were reviewed and approved by the Institute Animal Care and Use Committee. Female 4- to 5-week-old NCR athymic mice (Taconic Laboratory, Germantown, NY) were injected in the right ear pinna with Ad-hVEGF165 (108 plaque forming units) using a 28 gauge needle. Mice were observed daily for growth of new blood vessels and were used from day 4 to 6 postvector injection. Mice received 2 × 106 TCID50 MV-Luc, MV-LEcs, or MV-LcRGD intravenously via the tail vein. Mice were imaged using the Xenogen Bioluminescent IVIS 200 Imaging System (Xenogen, Alameda, CA) at regular intervals (1 hour, days 1, 3, 5, 7, and 13) after virus administration. Prior to imaging, mice were injected intraperitoneally with 3 mg/kg of D-luciferin (Xenogen) and images were acquired between 15 and 30 minutes after luciferin injection. Images were analyzed using the Xenogen Living Systems 2.50 software (Xenogen Corp).
Chick CAM assay. Embyronated White Leghorn eggs (Charles River Laboratories, IL) were cleaned with 70% alcohol and incubated at 37 °C with rotation at 60–80% relative humidity. Three days later, the eggs were cracked into deep well Petri dishes and maintained at 37 °C. Six- to ten-day-old embryos were used in the experiments. Viruses (2 × 105 TCID50, 20 µl) were injected intravascularly into a CAM vessel using custom made glass microneedles. In some experiments, CAMs were injected intravascularly with a 1:1 mixture of biotinylated L. esculentum lectin and Texas red-conjugated avidin (Vector Laboratories, Burlingam, CA) at 15 minutes prior to harvest. L. esculentum lectin binds to endothelial cells and helps to highlight the lumen of the CAM blood vessels. CAMs were harvested by dissection from the embryo and washed in phosphate buffered saline. Sections of CAMs were placed on glass slides and examined by fluorescent microscopy under low magnification (×100) and high magnification (×400) using a Nikon Eclipse E400 microscope. Images were captured using a DXM 1200 camera with ACT-1 version 2.2 Software (Nikon, Melville, NY). The numbers of GFP positive virus infected foci were counted. Virus binding studies were performed using fluorescently (DiO) labeled viruses. DiO-labeled viruses were generated by incubating virus infected Vero cells with 28 µmol/l of DiO (Molecular Probes) at 37 °C for 1 hour at 24-hour postinfection, after which the cells were washed three times with phosphate buffered saline to remove excess DiO and maintained in regular medium until viral harvest. Fluorescently labeled viruses were injected intravascularly (20 µl) in a CAM vessel and whole CAMs were harvested 24 hours later and examined using a fluorescence microscope as described above.
Tumor xenograft models. Human KAS 6/1 myeloma cells (5 × 106/100 µl) were implanted as subcutaneous tumors in the right flanks of irradiated (150 cGy) CB17 severe combined immunodeficiency mice (Harlan Sprague Dawley, Indianapolis, IN). When tumors reached 0.5–0.6 cm in diameter, mice were given 1 × 106 TCID50/100 µl of parental MV-GFP or MV-GEcs virus intravenously by the tail vein. At different time points postvirus delivery, mice were killed and tumors were sectioned into halves and frozen in Optimal Temperature Cutting medium.
Immunohistochemistry analysis. Harvested CAMs or tumor xenografts were flash frozen in Optimal Temperature Cutting medium and cryosections (5–8 µm thickness) were made. CAM cyrosections were immunostained for measles N protein using biotinylated antimeasles N antibody (Chemicon) at 5 µg/ml, followed by incubation with avidin–alkaline phosphatase complex (VECTASTAIN ABC Kit; Vector Laboratories) according to manufacturer's protocol. VECTOR Blue AP was used as the alkaline phosphate chromogen substrate (Vector Laboratories). Dual staining for murine endothelial cells lining the tumor blood vessels and measles infected areas were performed using a biotinylated rat antimouse CD31 antibody (6 µg/ml, Chemicon) in conjunction with goat antirat IgG Alexa 488 (2 µg/ml, Invitrogen) and antimeasles N antibody in conjunction with goat antimouse IgG Alexa 555 (2 µg/ml, Invitrogen), respectively.
Statistical analysis. The proportion of CAMs with infected neovessels (infection rate) was compared between the three treatment groups (MV-GFP, MV-GcRGD, and MV-GEcs) via logistic regression. The extent of infection in the CAMs vasculature was determined by counting the number of GFP positive foci representing infected vessels in each CAM. Count data such as this generally follow a Poisson distribution. Parameter estimates from Poisson regression models comparing extent of infection between viruses indicate that there are more zero counts than expected under a Poisson distribution; i.e., the data are overdispersed. This is a common finding in count data. Thus, a negative binomial distribution was assumed for the regression models. This distribution would naturally arise if the expected mean vessel count differs between CAMs. These models were fit using PROC GENMOD in SAS (SAS Institute, Cary, NC). The overall three-group comparison was required to be significant before proceeding to pair-wise comparisons in order to protect against type I error. The ability of the viruses to infect AdVEGF induced blood vessels in the ear pinna of nude mouse was assessed via Fisher's Exact test. A P value <0.05 is considered significant.
Acknowledgments
We are grateful to support from the NIH/NCI (CA118488 and CA129193) and Alliance for Cancer Gene Therapy. We thank Yasuhiro Ikeda (Mayo Clinic, Rochester) for the kind gift of ras-transformed NIH 3T3 cells and Katie Allen Ziegler (Mayo Clinic, Rochester) for help with statistical analysis of the data.
REFERENCES
- Russell SJ., and , Peng KW. Viruses as anticancer drugs. Trends Pharmacol Sci. 2007;28:326–333. doi: 10.1016/j.tips.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SJ. Replicating vectors for cancer therapy: a question of strategy. Semin Cancer Biol. 1994;5:437–443. [PubMed] [Google Scholar]
- Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci USA. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., and , Deisseroth A. Tumor vascular targeting therapy with viral vectors. Blood. 2006;107:3027–3033. doi: 10.1182/blood-2005-10-4114. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Davydova J, Wang M, Siegal GP, Krasnykh V, Vickers SM, et al. Infectivity enhanced, cyclooxygenase-2 promoter-based conditionally replicative adenovirus for pancreatic cancer. Gastroenterology. 2003;125:1203–1218. doi: 10.1016/s0016-5085(03)01196-x. [DOI] [PubMed] [Google Scholar]
- Stevenson M, Hale AB, Hale SJ, Green NK, Black G, Fisher KD, et al. Incorporation of a laminin-derived peptide (SIKVAV) on polymer-modified adenovirus permits tumor-specific targeting via α6-integrins. Cancer Gene Ther. 2007;14:335–345. doi: 10.1038/sj.cgt.7701022. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Chen ZH, Liu L, Whitley M, Wei D, Groshen S, et al. Systemic administration of a matrix-targeted retroviral vector is efficacious for cancer gene therapy in mice. Hum Gene Ther. 2001;12:193–204. doi: 10.1089/104303401750061258. [DOI] [PubMed] [Google Scholar]
- Jin H., and , Varner J. Integrins: roles in cancer development and as treatment targets. Br J Cancer. 2004;90:561–565. doi: 10.1038/sj.bjc.6601576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker GC. Integrins: molecular targets in cancer therapy. Curr Oncol Rep. 2006;8:96–103. doi: 10.1007/s11912-006-0043-3. [DOI] [PubMed] [Google Scholar]
- Hemminki A, Belousova N, Zinn KR, Liu B, Wang M, Chaudhuri TR, et al. An adenovirus with enhanced infectivity mediates molecular chemotherapy of ovarian cancer cells and allows imaging of gene expression. Mol Ther. 2001;4:223–231. doi: 10.1006/mthe.2001.0446. [DOI] [PubMed] [Google Scholar]
- Work LM, Reynolds PN., and , Baker AH. Improved gene delivery to human saphenous vein cells and tissue using a peptide-modified adenoviral vector. Genet Vaccines Ther. 2004;2:14. doi: 10.1186/1479-0556-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krom YD, Gras JC, Frants RR, Havekes LM, van Berkel TJ, Biessen EA, et al. Efficient targeting of adenoviral vectors to integrin positive vascular cells utilizing a CAR-cyclic RGD linker protein. Biochem Biophys Res Commun. 2005;338:847–854. doi: 10.1016/j.bbrc.2005.10.073. [DOI] [PubMed] [Google Scholar]
- Murugesan SR, Akiyama M, Einfeld DA, Wickham TJ., and , King CR. Experimental treatment of ovarian cancers by adenovirus vectors combining receptor targeting and selective expression of tumor necrosis factor. Int J Oncol. 2007;31:813–822. [PubMed] [Google Scholar]
- Wesseling JG, Bosma PJ, Krasnykh V, Kashentseva EA, Blackwell JL, Reynolds PN, et al. Improved gene transfer efficiency to primary and established human pancreatic carcinoma target cells via epidermal growth factor receptor and integrin-targeted adenoviral vectors. Gene Ther. 2001;8:969–976. doi: 10.1038/sj.gt.3301473. [DOI] [PubMed] [Google Scholar]
- Liu Y, Koziol J, Deisseroth A., and , Borgstrom P. Methods for delivery of adenoviral vectors to tumor vasculature. Hum Gene Ther. 2007;18:151–160. doi: 10.1089/hum.2006.135. [DOI] [PubMed] [Google Scholar]
- Shi X, Fang G, Shi W., and , Bartlett JS. Insertional mutagenesis at positions 520 and 584 of adeno-associated virus type 2 (AAV2) capsid gene and generation of AAV2 vectors with eliminated heparin- binding ability and introduced novel tropism. Hum Gene Ther. 2006;17:353–361. doi: 10.1089/hum.2006.17.353. [DOI] [PubMed] [Google Scholar]
- Hallak LK, Merchan JR, Storgard CM, Loftus JC., and , Russell SJ. Targeted measles virus vector displaying echistatin infects endothelial cells via αvβ3 and leads to tumor regression. Cancer Res. 2005;65:5292–5300. doi: 10.1158/0008-5472.CAN-04-2879. [DOI] [PubMed] [Google Scholar]
- Tyler MA, Ulasov IV, Borovjagin A, Sonabend AM, Khramtsov A, Han Y, et al. Enhanced transduction of malignant glioma with a double targeted Ad5/3-RGD fiber-modified adenovirus. Mol Cancer Ther. 2006;5:2408–2416. doi: 10.1158/1535-7163.MCT-06-0187. [DOI] [PubMed] [Google Scholar]
- Bauerschmitz GJ, Kanerva A, Wang M, Herrmann I, Shaw DR, Strong TV, et al. Evaluation of a selectively oncolytic adenovirus for local and systemic treatment of cervical cancer. Int J Cancer. 2004;111:303–309. doi: 10.1002/ijc.20217. [DOI] [PubMed] [Google Scholar]
- Liu Y., and , Deisseroth A. Oncolytic adenoviral vector carrying the cytosine deaminase gene for melanoma gene therapy. Cancer Gene Ther. 2006;13:845–855. doi: 10.1038/sj.cgt.7700962. [DOI] [PubMed] [Google Scholar]
- Peng KW, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC., and , Russell SJ. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62:4656–4662. [PubMed] [Google Scholar]
- Phuong LK, Allen C, Peng KW, Giannini C, Greiner S, TenEyck CJ, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63:2462–2469. [PubMed] [Google Scholar]
- Dingli D, Peng KW, Harvey ME, Greipp PR, O'Connor MK, Cattaneo R, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- Fishelson Z, Donin N, Zell S, Schultz S., and , Kirschfink M. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol. 2003;40:109–123. doi: 10.1016/s0161-5890(03)00112-3. [DOI] [PubMed] [Google Scholar]
- Tatsuo H, Ono N, Tanaka K., and , Yanagi Y. [The cellular receptor for measles virus: SLAM (CDw 150)] Uirusu. 2000;50:289–296. [PubMed] [Google Scholar]
- Nakamura T, Peng KW, Harvey M, Greiner S, Lorimer IA, James CD, et al. Rescue and propagation of fully retargeted oncolytic measles viruses. Nat Biotechnol. 2005;23:209–214. doi: 10.1038/nbt1060. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Nakamura T, Harvey M, Ikeda Y, Oberg A, Figini M, et al. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin Cancer Res. 2006;12:6170–6178. doi: 10.1158/1078-0432.CCR-06-0992. [DOI] [PubMed] [Google Scholar]
- Peng KW, Donovan KA, Schneider U, Cattaneo R, Lust JA., and , Russell SJ. Oncolytic measles viruses displaying a single-chain antibody against CD38, a myeloma cell marker. Blood. 2003;101:2557–2562. doi: 10.1182/blood-2002-07-2195. [DOI] [PubMed] [Google Scholar]
- Nakatsu Y, Takeda M, Kidokoro M, Kohara M., and , Yanagi Y. Rescue system for measles virus from cloned cDNA driven by vaccinia virus Lister vaccine strain. J Virol Methods. 2006;137:152–155. doi: 10.1016/j.jviromet.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Kumar CC, Nie H, Rogers CP, Malkowski M, Maxwell E, Catino JJ, et al. Biochemical characterization of the binding of echistatin to integrin αvβ3 receptor. J Pharmacol Exp Ther. 1997;283:843–853. [PubMed] [Google Scholar]
- Nakai M., and , Imagawa DT. Electron microscopy of measles virus replication. J Virol. 1969;3:187–197. doi: 10.1128/jvi.3.2.187-197.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA., and , Raine CS. Heterogeneity of virus particles in measles virus. J Gen Virol. 1979;45:441–453. doi: 10.1099/0022-1317-45-2-441. [DOI] [PubMed] [Google Scholar]
- Garanger E, Boturyn D., and , Dumy P. Tumor targeting with RGD peptide ligands-design of new molecular conjugates for imaging and therapy of cancers. Anticancer Agents Med Chem. 2007;7:552–558. doi: 10.2174/187152007781668706. [DOI] [PubMed] [Google Scholar]
- Ginsberg MH, Loftus JC., and , Plow EF. Cytoadhesins, integrins, and platelets. Thromb Haemost. 1988;59:1–6. [PubMed] [Google Scholar]
- Eliceiri BP., and , Cheresh DA. The role of αv integrins during angiogenesis: insights into potential mechanisms of action and clinical development. J Clin Invest. 1999;103:1227–1230. doi: 10.1172/JCI6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghisi GC., and , Ruegg C. Vascular integrins in tumor angiogenesis: mediators and therapeutic targets. Endothelium. 2006;13:113–135. doi: 10.1080/10623320600698037. [DOI] [PubMed] [Google Scholar]
- Mathis JM, Stoff-Khalili MA., and , Curiel DT. Oncolytic adenoviruses—selective retargeting to tumor cells. Oncogene. 2005;24:7775–7791. doi: 10.1038/sj.onc.1209044. [DOI] [PubMed] [Google Scholar]
- Zhu ZB, Rivera AA, Makhija SK, Lu B, Wang M, Izumi M, et al. Targeting lung cancer using an infectivity enhanced CXCR4-CRAd. Lung Cancer. 2007;55:145–156. doi: 10.1016/j.lungcan.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Hu C, Nakamura T, Marks JD, Russell SJ., and , Peng KW. Affinity thresholds for membrane fusion triggering by viral glycoproteins. J Virol. 2007;81:13149–13157. doi: 10.1128/JVI.01415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I, Niewiarowski S, Marcinkiewicz C, Calvete JJ, Marcinkiewicz MM., and , McLane MA. Structural requirements of echistatin for the recognition of αvβ3 and α5β1 integrins. J Biol Chem. 1999;274:37809–37814. doi: 10.1074/jbc.274.53.37809. [DOI] [PubMed] [Google Scholar]
- Kim S, Bell K, Mousa SA., and , Varner JA. Regulation of angiogenesis in vivo by ligation of integrin α5β1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons-Wingerter P, Kasman IM, Norberg S, Magnussen A, Zanivan S, Rissone A, et al. Uniform overexpression and rapid accessibility of α5β1 integrin on blood vessels in tumors. Am J Pathol. 2005;167:193–211. doi: 10.1016/s0002-9440(10)62965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeltzing O, Liu W, Reinmuth N, Fan F, Parry GC, Parikh AA, et al. Inhibition of integrin α5β1 function with a small peptide (ATN-161) plus continuous 5-FU infusion reduces colorectal liver metastases and improves survival in mice. Int J Cancer. 2003;104:496–503. doi: 10.1002/ijc.10958. [DOI] [PubMed] [Google Scholar]
- Pasqualini R, Koivunen E., and , Ruoslahti E. αv integrins as receptors for tumor targeting by circulating ligands. Nat Biotechnol. 1997;15:542–546. doi: 10.1038/nbt0697-542. [DOI] [PubMed] [Google Scholar]
- Hood JD, Bednarski M, Frausto R, Guccione S, Reisfeld RA, Xiang R, et al. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296:2404–2407. doi: 10.1126/science.1070200. [DOI] [PubMed] [Google Scholar]
- Stachler MD., and , Bartlett JS. Mosaic vectors comprised of modified AAV1 capsid proteins for efficient vector purification and targeting to vascular endothelial cells. Gene Ther. 2006;13:926–931. doi: 10.1038/sj.gt.3302738. [DOI] [PubMed] [Google Scholar]
- Koivunen E, Wang B., and , Ruoslahti E. Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins. Biotechnology (NY) 1995;13:265–270. doi: 10.1038/nbt0395-265. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., and , Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- Davis ME, Chen ZG., and , Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- Pirollo KF., and , Chang EH. Does a targeting ligand influence nanoparticle tumor localization or uptake. Trends Biotechnol. 2008;26:552–558. doi: 10.1016/j.tibtech.2008.06.007. [DOI] [PubMed] [Google Scholar]