Abstract
The assessment of the risk of germline transmission of vector-coded sequences is critical for clinical translation of gene transfer strategies. We used rabbit models to analyze the risk of germline transmission of adeno-associated viral (AAV) vectors. Intravenous injection of AAV-2 or AAV-8 resulted in liver-mediated, long-term expression of therapeutic levels of human factor IX (hFIX) in a dose-dependent manner. In high-dose cohorts, AAV-8 resulted in twofold higher levels of circulating hFIX and of vector DNA in liver compared to AAV-2. Vector sequences were found in the semen of all rabbits. The kinetics of vector clearance from semen was dose- and time-dependent but serotype-independent. No late recurrence of AAV-8 sequences was found in the semen over several consecutive cycles of spermatogenesis. In a novel rabbit model, AAV-2 or AAV-8 sequences were detected in the semen of vasectomized animals that lack germ cells. Therefore, structures of the genitourinary (GU) tract, as well as the testis, contribute significantly to vector shedding in the semen. Collectively, data from these two models suggest that the risk of inadvertent germline transmission in males by AAV-8 vectors is low, similar to that of AAV-2, and that AAV dissemination to the semen is in part modulated by host-dependent factors.
Introduction
Early-phase clinical studies using recombinant adeno-associated viral (AAV) vectors are encouraging and provide the basis for the treatment of several genetic and acquired diseases. The therapeutic potential of AAV serotype 2 (AAV-2) following local delivery to skeletal muscle or to the subretinal space is attested by the documented long-term local transgene expression and by evident improvement of the disease phenotype, respectively.1,2 However, the use of AAV for the treatment of some diseases will require intravascular delivery of the vector, which imposes additional safety concerns due to systemic vector dissemination. Following hepatic artery delivery of AAV-2 for hemophilia B in humans, we demonstrated that the immune responses to the vector capsid and the risk of germline transmission are critical challenges to the safety of this strategy.3 The characterization of novel AAV vectors derived from alternate serotypes, the development of higher potency vectors derived from modifications in the AAV genomes, and the optimization of transgene expression or function4,5 support the likelihood of achieving efficient liver-directed gene expression by a simple peripheral intravenous injection. Thus, there is a fundamental interest in determining the risk of germline transmission as a key safety issue to support the use of these promising strategies in humans.
In a previous work, we established a rabbit model to assess the risk of germline transmission by AAV-2 vector in males.6,7 We determined that the risk of vector dissemination to the semen was dependent on the route of vector administration; semen tested positive for vector sequences following intravascular delivery but not after intramuscular administration. Following intravascular delivery, vector sequences were transiently detected in semen and disappeared in dose- and time-dependent fashion. The kinetics of vector clearance was faster in the semen fractions enriched for motile sperm than in the total semen fractions. Long-term follow-up spanning hundreds of spermatogenesis cycles in 31 animals showed that there was no recurrence of detectable vector sequences in semen. Infectious vector particles were present only up to day 4 postinjection, and were undetectable thereafter, which limited the risk of transmission of the vector. These data suggest that AAV-2 presents a low risk of germline transmission for humans. Moreover, the data agree with those of adult hemophilia male subjects enrolled in muscle- and liver-directed AAV-2 mediated human factor IX (hFIX) gene transfer trials.3,8
There are two limitations in the previous preclinical experiments assessing germline transmission by AAV-2 vector.7 First, the role of the germ cells in carrying AAV vector sequences into the semen was not clearly defined. Components of semen are contributed by both genital and urinary tracts. Germ cells constitute only 5% of the ejaculate, and several attempts to purify fractions enriched for germ cells from rabbit semen failed. The motile sperm fractions containing mature germ cells ready for fertilization coexist with other cells types, a finding common to semen from both rabbits and humans. Thus, the assessment of the real rates, if any, of exogenous insertion of AAV into germline cells is difficult. Second, the safety of AAV vectors other than serotype 2 cannot be directly inferred from our earlier findings in the rabbit model because alternate serotypes present distinct tissue tropisms that may alter the likelihood of germ-cell transduction.9,10,11,12,13,14
Here, we sought to overcome these limitations by assessing the parameters of vector shedding into the semen in the absence of germ cells using vasectomized rabbits injected with AAV-2 or AAV-8 vectors. This novel rabbit model provides a direct evaluation of the contribution of nongerm cells and components of the genitourinary (GU) tract to AAV distribution in the semen.
Results
Intravenous injection of AAV-2 or AAV-8 results in sustained expression of hFIX in a dose-dependent manner
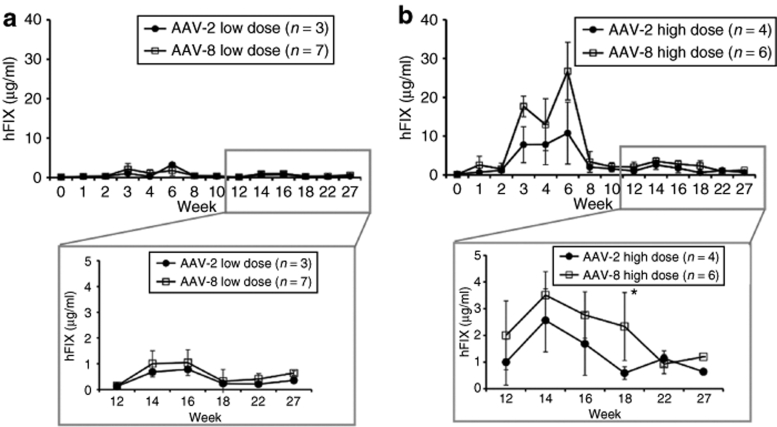
Four groups of rabbits were injected with AAV-2 (n = 8) or AAV-8 (n = 17) vectors expressing hFIX under the control of a liver-specific promoter at two doses: 1 × 1012 vg/kg (low dose) or 1 × 1013 vg/kg (high dose). Injection of AAV vector was uneventful; there was no elevation of liver enzyme levels, which were monitored every week for 2 months after injection (data not shown). Circulating hFIX levels were initially detected at week 1, and reached plateau levels after week 10. In animals without inhibitory antibodies to the transgene, hFIX levels reached a therapeutic range of 6 and 12% (low dose), and 12 and 24% (high dose) of normal levels (5 µg/ml) in the AAV-2 (n = 7) and AAV-8 (n = 13) cohorts, respectively (Figure 1). FIX levels in the AAV-8 injected rabbits were approximately twofold higher than those of AAV-2. However, because of the limited numbers of animals, this difference did not reach statistical significance. These data demonstrate that rabbit hepatocytes are efficiently transduced in vivo by both AAV-2 and AAV-8 vectors.
Figure 1.
Plasma concentration of hFIX in experimental rabbits as a function of time after AAV injection. Rabbits received intravenous injection of AAV-2 or AAV-8 at doses of (a) 1 × 1012 vg/kg (low dose) or (b) 1 × 1013 vg/kg (high dose). *P value (0.0349) was calculated by unpaired t- test for the hFIX mean values at week 18. The numbers of animals per group are indicated.
Immune responses to hFIX are similar between AAV-2 and AAV-8 groups
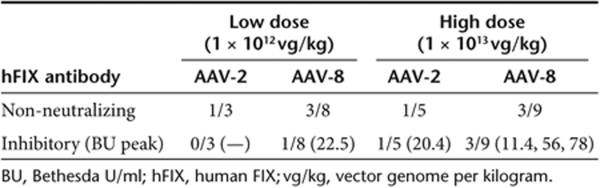
Inhibitory antibodies to hFIX were detected in 12.5% of rabbits (1 of 8 rabbits) injected with AAV-2 and 23.5% of rabbits (4 of 17 rabbits) injected with AAV-8 (P = 0.7, χ2-test) (Table 1) and persisted for the duration of the experiment. The peak inhibitor titers ranged from 20 to 78 Bethesda unit 5 to 12 weeks postvector injection. In all five rabbits with inhibitory antibodies to hFIX, there were no abnormalities in coagulation parameters, suggesting that the inhibitory antibodies observed were specific to the hFIX transgene product and did not inhibit endogenous rabbit FIX.
Table 1.
Summary of antibody formation to hFIX in AAV-injected rabbits
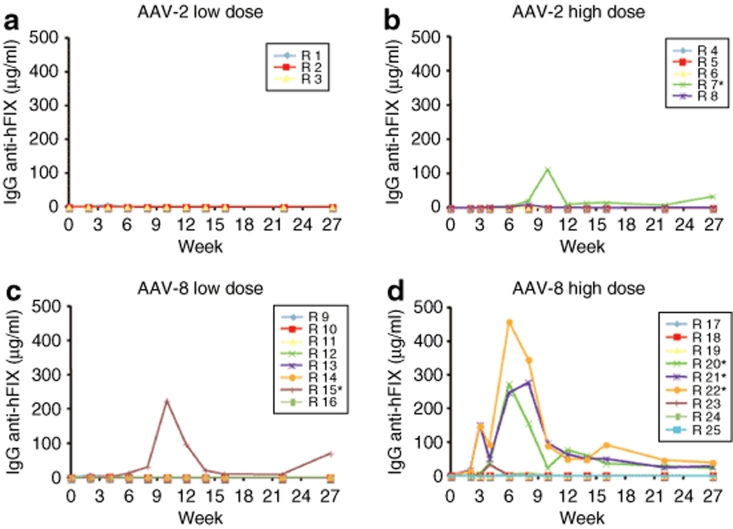
Noninhibitory antibodies [immunoglobin G (IgG)] to hFIX were detected by enzyme-linked immunosorbent assay in 25 (2/8) and 35% (6/17) of the rabbits injected with AAV-2 or AAV-8, respectively. The IgG anti-hFIX levels in these animals were 10–100-fold lower than those of rabbits with inhibitory antibodies (Figure 2). Moreover these antibodies were detected only transiently, starting at week 2, and spontaneously disappeared after week 4 (n = 6) or week 8 (n = 2) postinjection (Figure 2).
Figure 2.
Time course of antibody formation to hFIX. Rabbits were injected with (a,b) AAV-2 or (c,d) AAV-8 at doses of 1 × 1012 vg/kg (low dose) or 1 × 1013 vg/kg (high dose). The data are shown for each individual animal. *Animals that developed inhibitory antibodies against hFIX.
Formation of antibody to vector capsid is more robust for AAV-8 than AAV-2
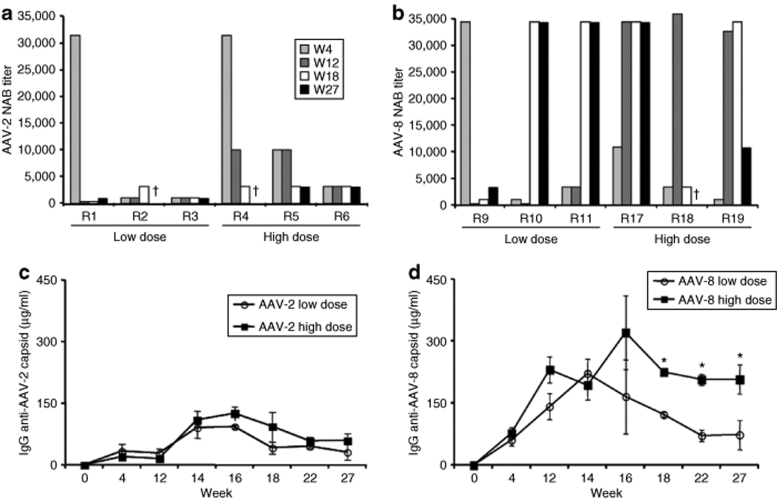
The neutralizing antibody (NAB) titers to AAV capsid proteins were 10-fold higher in rabbits injected with AAV-8 (n = 6) compared to those injected with AAV-2 (n = 6), at samples collected at weeks 4, 12, 18, and 27, the last time point tested (Figure 3a,b). The titers of NAB to AAV-2 for the low- and high-dose cohorts diminished over time. For the AAV-8 groups, the titers of NAB remained at steady levels for the duration of the experiment in three of the six rabbits, whereas in the other animals the NAB titer slowly diminished.
Figure 3.
Antibody formation to AAV capsid. Rabbits were injected with AAV-2 or AAV-8 at doses of 1 × 1012 vg/kg (low dose) or 1 × 1013 vg/kg (high dose). (a,b) Titers of neutralizing antibodies (NABs) to AAV capsid for each animal, measured at weeks 4–27 postvector injection. No samples were obtained from rabbits R2, R4, and R18 at week 27. (c,d) Levels of specific IgG to anti-AAV-2 or anti-AAV-8. *P values <0.05 (unpaired t-test).
We monitored the capsid-specific IgG levels in AAV-2 (n = 8) and AAV-8 (n = 17) injected rabbits. IgG levels start to increase after week 2. Anti-AAV-8 capsid-specific IgG levels were threefold higher compared to those for AAV-2 (Figure 3c,d). Notably the anti-AAV-8 antibodies remained stable for the duration of the experiment (27 weeks) whereas the levels of anti-AAV-2 declined. Dose dependence of IgG levels was observed in the AAV-8 injected rabbits but not for the AAV-2 group.
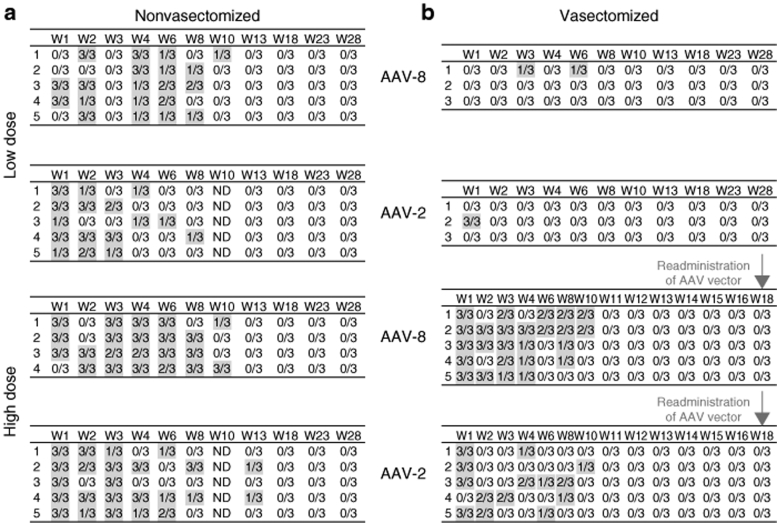
AAV vector DNA is detected in the semen in the absence of germ cells
Early attempts to obtain pure fractions of spermatozoa by repeated semen fractionation were suboptimal, even after using specific reagents developed for rabbit semen.7 The presence of nongerm cells was a common finding that hampered the assessment of the risk of germline transmission by AAV-2 vector. Here, we took an alternative approach by using rabbits in which bilateral surgical vasectomy had been carried out 2 months prior to vector injection. Vasectomy is a surgical sterilization, in which the vas deferens is cut and ligated. A total of 18 adult rabbits underwent the surgical procedure, and analysis of three consecutive semen samples confirmed the absence of germ cells in 16 of 18 animals.
The control groups for the AAV-8 vasectomized rabbits consisted of adult nonvasectomized rabbits (n = 9), including the two animals with unsuccessful vasectomy. The control groups for the vasectomized rabbits injected with AAV-2 consisted of rabbits previously reported, that had been injected with the same AAV-2 vector at identical doses, and had undergone semen testing using a previously reported identical protocol.7
AAV vector DNA sequences were assessed using a PCR assay that enables detection of as few as eight copies per 3 × 105 haploid genomes (i.e., one copy per 37,500 haploid genomes), as previously reported.7 One microgram of semen DNA from nonvasectomized rabbits was used for each PCR. The cellular components of the semen obtained from the vasectomized rabbits consisted of epithelial cells, nucleated uncharacterized cells, and occasional white blood cells. The amounts of DNA recovered from semen samples of vasectomized animals ranged widely (1.5–83 ng/ml), varying between animals and also between serial samples from the same rabbit. Vector sequences were found in semen samples from all vasectomized and nonvasectomized rabbits following high-dose administration of AAV-2 or AAV-8. In the low-dose cohorts of both vector serotypes, the numbers of rabbits testing positive for vector sequences were lower in vasectomized (2/6) compared to nonvasectomized (10/10) animals, independent of the AAV serotype (Figure 4). These data demonstrate that AAV DNA sequences could be found in the semen in the absence of germ cells for both vector serotypes, although the frequency of positive samples was lower in the vasectomized animals.
Figure 4.
Detection of AAV-2 and AAV-8 DNA sequences in the semen of rabbits as a function of time. Numbers of positive PCR assays in triplicate samples of total semen from (a) nonvasectomized, and (b) vasectomized rabbits. Results for each individual animal (numbered from 1 to 5) are shown. W, week following vector injection; ND, not determined.
The kinetics of clearance of AAV sequences in semen is dose- and time-dependent but serotype-independent
We hypothesized that the presence and duration of detectable vector sequences in semen could possibly be influenced by the AAV serotype, the vector dose, and/or by the presence of germ cells (as well as other variables). The kinetics of AAV-2 or AAV-8 DNA clearance in the low-dose groups was faster in the vasectomized rabbits (1 to 6 weeks) compared to nonvasectomized groups (8 to 10 weeks) (Figure 4). However, in the high-dose groups there was no difference in the kinetics of vector clearance from semen with or without germ cells. These data suggest that AAV sequences can be present in the semen in the absence of germ cells for prolonged periods (up to week 10).
In the nonvasectomized rabbits, there was no significant difference in kinetics of clearance between the AAV-2 and AAV-8 groups. Overall, the percentage of samples testing positive for AAV DNA was higher among the high-dose compared to low-dose cohorts. The last positive semen sample was between weeks 8 to 10 for the low-dose and weeks 10 to 13 for the high-dose groups. Together these findings show that the kinetics of AAV clearance is vector dose- and time-dependent but vector-serotype independent, at least for the two serotypes tested.
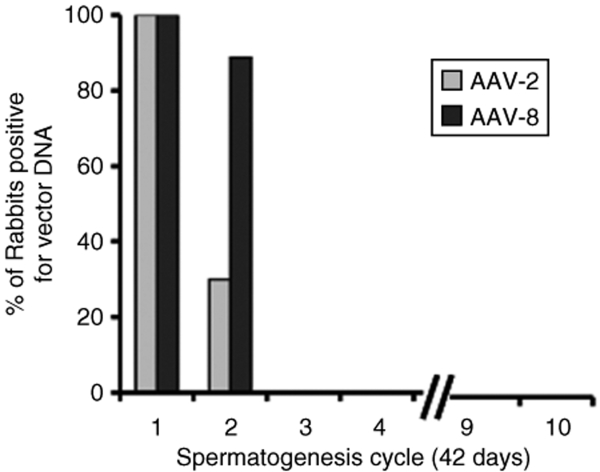
No late recurrence of vector sequences in the semen of AAV-8 injected rabbits
We sought to determine whether AAV-8 DNA sequences in the semen would be present only transiently as documented before for AAV-2 in rabbits and in human subjects.3,7 The follow-up of AAV-8 nonvasectomized rabbits (n = 9) over a 15-month period postinjection revealed no recurrent vector DNA sequences in any of the semen samples tested after obtaining three consecutive negative PCR tests. The long-term follow-up design of this study allowed the assessment of several rabbit spermatogenesis cycles (42 days/cycle). AAV-8 DNA sequences were found only during the first two spermatogenesis cycles, the last positive sample having been collected at day 70 (Figure 5). Previously, we reported that at similar doses of AAV-2, the last positive semen was observed at day 104. Thus, for both AAV-2 and AAV-8 no vector DNA was found in semen after the third spermatogenesis cycle. The analysis of semen for an additional seven sequential spermatogenesis cycles in the AAV-8 injected animals, i.e., a cumulative 63 cycles, revealed no late recurrence of vector DNA. These results were comparable to the earlier data for AAV-2 in rabbits.7
Figure 5.
Long-term follow-up of semen analysis for vector DNA from AAV-injected rabbits as a function of spermatogenesis cycles. Percentage of rabbits that exhibited at least one positive semen sample for AAV vector DNA. Rabbits received AAV-2 (n = 10) or AAV-8 (n = 9) at doses of 1 × 1012 vg/kg (low dose) or 1 × 1013 vg/kg (high dose). The last positive semen samples for AAV-2 injected rabbits were collected at day 56 (low dose) and day 104 (high dose). For the AAV-8 injected rabbits the last semen sample containing vector DNA was from day 70.
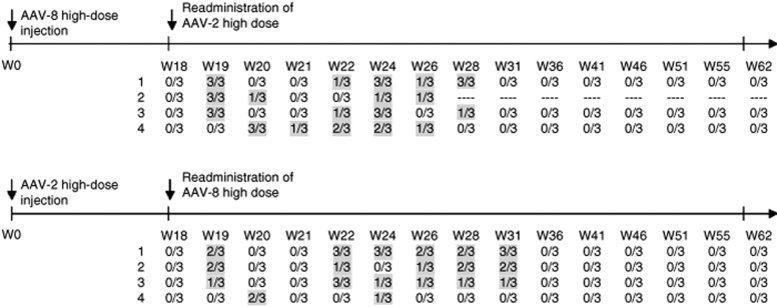
AAV-8 vector shedding in the semen is not prevented by the presence of specific IgG anti-AAV-2 capsid
Humans are natural hosts for AAV-2 and the presence of NAB to the vector capsid is common in the general population.15,16,17 There is evidence that NAB to AAV-2 has the potential to neutralize in vivo transduction with AAV-6 and AAV-8. Therefore, we sought to determine whether the preexisting NAB to AAV-2 in vivo would influence AAV-8 vector shedding in the semen.
Rabbits previously injected with AAV-2 (n = 4) or AAV-8 (n = 4) vectors were readministered at week 18 with AAV-8 and AAV-2, respectively. At the time of vector readministration, the titers of NAB to AAV-2 were 1:3,160 in all animals and to AAV-8 they ranged from 1:1,000 (n = 1) to 1:31,600 (n = 3).
Prior to readministration of vector, semen samples from all animals had tested negative for 7 weeks. After reinjection of AAV, vector sequences reappeared in the semen of all rabbits. The kinetics of vector clearance was indistinguishable from the data obtained in naive rabbits. AAV detection in the semen was transient, and semen samples tested negative after week 10 following the readministration of AAV-2 and after week 13 for those reinjected with AAV-8 (Figure 6). These data suggest that the presence of antibody to AAV-2 or AAV-8 capsid did not prevent vector dissemination to the semen. We also continued to test semen samples from these rabbits for AAV sequences up to 33 weeks after the last sample tested positive for vector DNA. There was no further evidence of AAV-8 or AAV-2 vector sequences in any semen samples tested.
Figure 6.
AAV vector DNA in semen samples following readministration of AAV of alternate serotypes. Rabbits previously injected with AAV-2 (n = 4) or AAV-8 (n = 4) at doses of 1 × 1013 vg/kg were reinjected with AAV-8 and AAV-2, respectively, at week 18. Semen samples were tested in triplicate for vector DNA sequences.
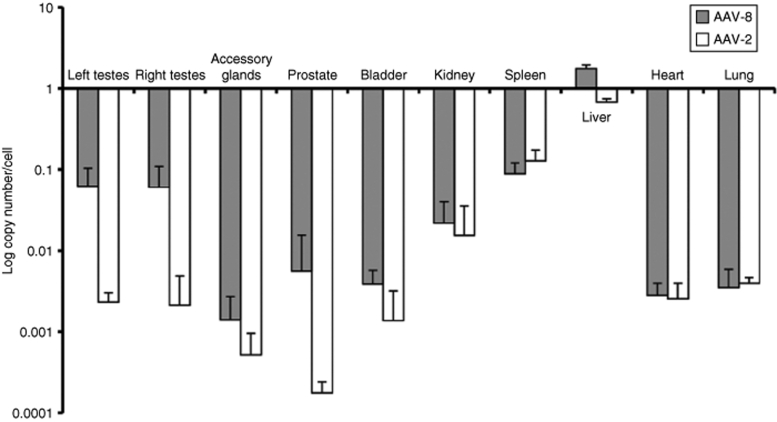
Comparison of biodistribution between AAV-8 and AAV-2 serotypes
Rabbits injected with AAV-8 (n = 4) or AAV-2 (n = 4) at doses of 1 × 1013 vg/kg were sacrificed 15 months following vector administration for biodistribution analysis of vector DNA using real-time quantitative PCR assay. The highest number of vector copies per diploid genome was found in the liver following administration of AAV-8 (1.7 ± 0.2 copy number/cell) and AAV-2 (0.6 ± 0.06 copy number/cell) (Figure 7). The vector DNA content in the tissues harvested from other organs was markedly lower. There were no differences in the vector content of the nontarget tissues from rabbits injected with the two serotypes.
Figure 7.
Biodistribution of AAV–DNA in rabbits 15 months after intravenous injection of AAV vectors. Rabbits received AAV-2 (n = 3) or AAV-8 (n = 4) vectors at doses of 1 × 1013 vg/kg and tissues were harvested at 15 months. Vector DNA content was obtained by real-time quantitative PCR assay. The data are shown as mean values (±SD).
Discussion
In this study, we compared both the safety and efficacy profile in rabbit models of two promising vectors planned or already tested, for human liver-directed gene therapy. The potential risk of foreign DNA insertion into the germ-cell genome and its transmission to subsequent generations, and the potential, through an untoward insertion event, to disrupt the highly ordered sequence of gene expression and repression events that characterize normal embryogenesis, make germ-cell transduction a major safety concern for in vivo gene-based strategies.
The safety of vector-based gene transfer approaches depends, in part, on the degree of vector dissemination to nontarget tissues. Vector dissemination depends on the route of vector administration, tissue tropism, and dose. Intravascular delivery of AAV vector presents a higher risk of germline contamination compared to intramuscular injection, as demonstrated in clinical trials for hemophilia B3,8 and α-1 antitrypsin deficiency.18 These findings were reliably reproduced in a series of preclinical studies in rabbit models.6,7 Viral tissue tropism is determined by the presence of cellular receptors that facilitate viral entry. Because AAV-2 and AAV-8 use distinct cellular receptors, heparan sulfate proteoglycan, αVβ5 integrin19 and human fibroblast growth factor receptor 1 (ref. 20) for AAV-2 and 37/67-kd laminin receptor,10 and other unknown receptors for AAV-8, we hypothesized that the risk of germline transmission may differ between these AAV serotypes. We sought to test whether intravascular injection of two distinct serotypes of AAV in rabbits would provide a useful model for both the assessment of germline transmission and the efficacy of liver-directed gene transfer.
Intravascular injection of AAV-2 or AAV-8 resulted in dose-dependent sustained expression of therapeutic levels of hFIX in rabbits. Overall, the levels of gene expression by AAV vectors, and the immune responses to the transgene and to the vector capsid proteins, were similar in rabbits to those previously reported in nonhuman primates (NHP)21,22 injected with the same vectors via the hepatic artery. Using a dose of 1 × 1013 vg/kg, AAV-8 injected rabbits reached hFIX levels of 24% of normal, approximately twofold higher than the hFIX levels using AAV-2. The relative hFIX levels correlated with vector DNA copy numbers in the liver using the respective vectors. In this model, hFIX expression peaked between 4 and 6 weeks, reaching stable levels after week 10 for both AAV serotypes. These results are consistent with other studies showing a modest transduction advantage using AAV-8 over AAV-2 in large animal models such as dogs23,24 or NHP,12 in contrast to the dramatic advantage of recombinant AAV-8 in mice.14
The use of nonspecies specific transgenes in immunocompetent animals commonly compromises the assessment of long-term efficacy due to immune responses to the transgene. Inhibitor formation is the main complication of clinical treatment of hemophilia, rendering replacement protein therapy ineffective, and associated with increased morbidity and mortality. In this study, the formation of inhibitory antibodies to hFIX occurred in only 20% of animals. Low levels of transient noninhibitory antibodies to hFIX were detected in 30% of the animals and disappeared 4–8 weeks postinjection. These data are comparable to findings in NHP models injected with AAV-hFIX vectors4,22,25 or following infusion of hFIX protein.26 The homology of amino acid sequence between rabbit and hFIX is 79%, and between NHP and hFIX is 89%. The weak immunogenicity of hFIX in rabbits was unexpected because these animals are often used for the generation of antibodies to human proteins, including FIX. Thus, it is possible that AAV-mediated, liver-restricted continuous expression of hFIX at high levels diminished the immunogenicity of the transgene in an immunocompetent large animal model. These findings are in agreement with evidence demonstrating induction of transgene-specific immune tolerance following liver-specific gene expression27 of the transgene from an AAV vector.
Immune responses to AAV capsid proteins were restricted to humoral responses without evidence of cellular cytotoxicity (as evidenced by sustained expression of the transgene and normal levels of liver enzymes). Overall, the NAB titers and IgG formation to AAV-8 capsid proteins were higher and persisted for longer periods when compared to AAV-2. These data recapitulate the time course of the humoral responses to AAV capsid in NHP.21,22 Together our findings suggest that AAV vectors efficiently transduce rabbit hepatocytes in vivo without evoking overwhelming immune responses to the transgene product. These data further support the use of the rabbit model for liver-directed gene transfer by AAV vectors.
The advantages of rabbits over macaques derive from the ability to test long-term efficacy and safety of in vivo transduction of AAV vectors and to perform simultaneously semen analysis in the same cohorts, data lacking in the other large models such as NHP and dogs to date. Moreover, rabbits do not have preexisting immunity to AAV-2 or AAV-8, a limiting factor for the use of NHP as these animals are natural hosts for AAV-8, and can exhibit crossreactivity to AAV-2 (via NAB).
A critical consideration in assessing the risk of germline transmission of viral vectors following in vivo delivery to humans or animal models derives from the difficulties in obtaining semen fractions containing solely germ cells. We addressed this limitation by using vasectomized rabbits, which enables a direct evaluation of the contribution of nongerm cells and components of the GU tract to AAV shedding in the semen. Notably, we have demonstrated that AAV vector sequences reached the semen in the absence of germ cells. The presence of AAV in the semen was vector dose-dependent but serotype-independent. The kinetics of vector clearance was accelerated in the absence of germ cells in the low-dose cohorts when compared to nonvasectomized rabbits. These differences were not found in the high-dose groups. Therefore, these findings provide clear evidence that semen positive for AAV does not necessarily imply transduction of germ cells and the associated risk of germline transmission. There are several pieces of evidence that support this concept. Sperm represents only a minor portion of the ejaculate (5%), whereas seminal fluid and epithelial cells derived from nongonadal GU tract sources provide ~95% of the ejaculate. Thus, it is likely that the contribution of nongerm cells and of anatomical structures of the GU tract contribute significantly to the vector dissemination and kinetics of clearance to the semen. Therefore, age-related anatomical variations, pathological status of seminal vesicles, prostate, bulbourethral, and urethral glands, in addition to testes, all contribute to the vector distribution to the semen. In an early report, we also demonstrate that the higher the frequency of semen collection, the faster the vector clearance from the semen. These findings provide a basis for the clinical variability of the kinetics of AAV clearance in the semen observed in an earlier clinical study in humans in which older men exhibit a prolonged persistence of AAV DNA in the semen when compared to younger subjects.3
The implications of our findings in the vasectomized rabbit model are also relevant to other gene transfer strategies. Intravascular injection of retroviral (Moloney murine leukemia virus) vector in men with hemophilia resulted in a transient detection of provector sequences in the semen in one subject and in the peripheral blood mononuclear cells in all subjects for extended periods.28 Thus, it is possible that the presence of cells other than germ cells were responsible for provector detection in the semen. The low probability of germline transmission by retroviral vectors was also demonstrated in preclinical studies in rabbits.29
The long-term follow-up of rabbits injected with AAV-8, covering 63 cumulative spermatogenesis cycles, showed no late recurrence of vector DNA sequences. Although vector DNA sequences were found in testes after 15 months of injection, the vector copy numbers were present at low levels. Failure to detect recurrence of AAV in the semen argues against the possibility of transduction of an early spermatogenesis precursor exposed to AAV-8 during the hematogenous dissemination to the gonads. Together, these findings demonstrate that the presence of AAV-8 vectors in the gonadal tissue at early and late times postinjection is not associated with a measurable risk of germline transmission.
The presence of high titers of antibodies to AAV capsid prevents gene transfer and transgene expression by intravascular injection of vector to the target organ in large animal models4,21 and in humans.3 We hypothesized that preexisting immunity to AAV-2, present in >30% of humans,15,30 would reduce vector dissemination to nontarget organs. In the rabbit model, we observed a low crossreactivity of AAV-2 capsid-specific IgG to AAV-8 vector. However, in vitro assays underestimate the ability of antibodies to neutralize AAV-2 transduction compared to an in vivo model of passive immunization.31 Here, we showed that vector shedding of AAV-8 to the semen in rabbits with preexisting anti-AAV-2 antibodies was similar to that seen in animals without preexisting NAB to vector capsid. Similar findings were obtained in rabbits with antibody to AAV-8 readministered AAV-2 vectors. Thus, the presence of preexisting immunity to a specific AAV capsid may not prevent dissemination of vector of an alternate serotype to the semen. The limitation in this model resides in the differences between immune responses to recombinant and wild-type AAV antigens, which could result in distinct antibody repertoires.
There is a growing indication of the need for concomitant use of immunomodulatory drugs in some gene transfer settings.22,24 The effects of immunosuppressive drugs on reproductive toxicology are of safety concern for gene transfer if iatrogenic tissue damage and/or dysfunction occurred. Therefore, it is prudent to assess the risk of germline transmission in these clinical studies. In summary, the risk of inadvertent germline transmission following intravascular delivery of AAV-8 vectors is low and similar to the risk of AAV-2 in rabbit models. Therefore, the precautions proposed for AAV-2 clinical studies, i.e., the use of barrier contraception until semen tests negative for vector sequences, and banking of sperm prior to vector injection, are advisable.
Materials and Methods
Production of recombinant AAV serotypes 2 and 8. The transgene cassette was flanked by AAV-2 inverted terminal repeats and was packaged in capsids from either AAV-2 or AAV-8 (ref. 14). Recombinant AAV–hFIX16 vector was produced in an adenovirus-free system in HEK-293 cells using a triple transfection method.32 The plasmid encoding hFIX was under the control of the human α1-antitrypsin promoter and four copies of the apolipoprotein A enhancer, which results in liver-specific transgene expression.33,34 AAV vectors were purified by combined chromatography and cesium chloride density gradient centrifugation,35 and vector titers were determined by quantitative PCR, using hFIX specific primers and probes.36
Intravenous injection of AAV-hFIX vector. Adult male New Zealand white rabbits (3–4 kg) were purchased from Covance Research Products (Denver, PA). Animals underwent bilateral surgical vasectomy (n = 16) and 4 weeks postsurgery were transferred to the animal facility. Nonvasectomized rabbits, or those in which vasectomy failed, were used as control (n = 9). The vector (AAV–hFIX) was administered by a single injection into the marginal ear vein at doses of 1 × 1012 vg/kg or 1 × 1013 vg/kg.
Collection of semen and blood samples. Semen was collected with the aid of an artificial vagina as reported previously.6 For each individual rabbit new collection materials were used. Semen was collected weekly for 2 months, and monthly thereafter, as reported previously.7 Peripheral blood was obtained by marginal ear vein puncture prior to vector injection, and weekly thereafter during long-term follow-up.
Biodistribution studies. Animals were sacrificed 15 months after vector injection, using an intravenous injection of 125 mg/kg of sodium pentobarbital. Testes, prostate, accessory glands, bladder, kidney, spleen, liver, lung, and heart were harvested using fresh sterile instruments for each tissue sample. For quantitative determination of vector genomes in DNA in all tissues, a quantitative PCR assay was performed. A total of 200 ng of genomic DNA extracted from each sample was used for TaqMan real-time PCR (Applied Biosystems, Foster City, CA). The primers (forward: 5′-ttcgatctacaaagttcaccatctataac-3′ and reverse: 5′-aaactggtcccttccacttcag-3′) and the fluorescein aminohexylamidite–labeled probe (5′-aatctctacctccttcatggaagccagca-3′) were designed to detect the AAV-hFIX16 vector sequences. The lowest sensitivity of the quantitative PCR was 25 copies per 1 µg of genomic DNA.
Detection of hFIX levels and anti-hFIX antibodies. hFIX concentration was determined by using an enzyme-linked immunosorbent assay in which a monoclonal antibody to hFIX, clone HIX-1 (Sigma, St Louis, MO), was used as capture antibody at a dilution of 1:800, whereas the detecting antibody is a peroxidase-conjugated polyclonal goat anti-hFIX (Affinity Biologicals, Hamilton, Canada) at a dilution of 1:2,500 (ref. 5). Levels of IgG anti-hFIX antibodies were determined using an enzyme-linked immunosorbent assay, where the plate was coated with purified hFIX (Wyeth, Madison, NJ) at a concentration of 1 ng/µl. The detecting antibody is a peroxidase conjugated polyclonal goat antirabbit IgG (Jackson Immunoresearch, West Grove, PA) at a dilution of 1:3,000.
Bethesda assay. Citrated plasma samples were collected and heat inactivated at 56 °C for 1 hour to eliminate the endogenous rabbit FIX. Serial dilutions of heat-inactivated rabbit plasma were then incubated with human plasma for 2 hours at 37 °C. Residual hFIX clotting activity was determined by one-stage activated partial thromboplastin time and compared with a standard curve. Results are expressed in Bethesda units. One Bethesda unit is the amount of inhibitor that will inactivate 50% of FIX clotting activity in a normal plasma sample.
Detection of anti-AAV capsid antibodies and of NABs to AAV capsid. For detection of anti-AAV capsid antibodies, enzyme-linked immunosorbent assay plates were coated with 5 × 1010 capsid particles/ml AAV-2 empty capsids or with 1011 capsid particles/ml AAV-8 empty capsids. The detecting antibody is a peroxidase-conjugated polyclonal goat antirabbit (Jackson Immunoresearch, West Grove, PA) at a dilution of 1:3,000.
The NAB titer of serum or plasma was determined in vitro in a cell-based assay. 1.7 × 108 or 1.7 × 109 particles of AAV-2 or AAV-8 vector encoding a β-galactosidase reporter gene (AAV–LacZ) were incubated with test serum for 1 hour at 37 °C before addition of the mixture to 2V6.11 cells near confluence in 96-well plates. Control (100%) AAV transduction was defined as the amount of β-galactosidase activity measured in culture 24 hour or 48 hour after transduction with AAV-2 or AAV-8 LacZ vectors, respectively, in the presence of naive mouse serum. A half-log serial dilution of the test serum in naive mouse serum was made to determine the highest dilution of test serum that resulted in 50% or greater inhibition of β-galactosidase expression. Each dilution series was tested in triplicate. Controls that include reference plasma with well-defined AAV-2 or AAV-8 neutralizing titers (internal control) and a negative control (naive mouse serum only) was used to determine the assay background. The titer of NAB was defined as the two dilutions that bracketed the 50% inhibition level, e.g., 1:100 to 1:316.
Vector DNA analysis. DNA from serum samples was isolated with the QIAamp Blood Kit (Qiagen, Chatsworth, CA). The procedure for DNA extraction from semen and testis consisted of overnight incubation with proteinase K before isolating total genomic DNA using the QIAamp Tissue Kit (Qiagen). DNA was resuspended in 50 µl of 10 mmol/l Tris–0.1 mmol/l EDTA. Triplicate PCR assays were carried out using 1 µg of genomic DNA as template per reaction for semen samples from nonvasectomized rabbits. A fragment of 647 bp of the AAV-hFIX16 vector was amplified by PCR, as previously described.7 To determine the sensitivity of the PCR assay, 10-fold serial dilutions of a plasmid encoding AAV-hFIX16 were mixed with 1 µg rabbit semen genomic DNA before the amplification reaction. The PCR products were separated by 2% NuSieve agarose gel electrophoresis and visualized by ethidium bromide staining.
Statistical analysis. Comparison of data between experimental groups was analyzed by unpaired Student's t-test or χ2-test.
Acknowledgments
We thank Aaron Weilerstein (Charles River Laboratories, Horsham, PA) for an excellent technical assistance. We thank Joshua Siner for technical help, and Frederico Xavier, Jonathan Finn, and Junwei Sun for expert assistance. We also thank Patricia Sertich (University of Pennsylvania Veterinary School) for the analysis of semen samples. This work was supported by National Institutes of Health grants R01 HL084220 (V.R.A.) and P01 HL078810 (K.A.H.).
REFERENCES
- Jiang H, Pierce GF, Ozelo MC, de Paula EV, Vargas JA, Smith P, et al. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol Ther. 2006;14:452–455. doi: 10.1016/j.ymthe.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN, et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettrumpf J, Herzog RW, Schlachterman A, Kaufhold A, Stafford DW., and , Arruda VR. Factor IX variants improve gene therapy efficacy for hemophilia B. Blood. 2005;105:2316–2323. doi: 10.1182/blood-2004-08-2990. [DOI] [PubMed] [Google Scholar]
- Arruda VR, Fields PA, Milner R, Wainwright L, De Miguel MP, Donovan PJ, et al. Lack of germline transmission of vector sequences following systemic administration of recombinant AAV-2 vector in males. Mol Ther. 2001;4:586–592. doi: 10.1006/mthe.2001.0491. [DOI] [PubMed] [Google Scholar]
- Schuettrumpf J, Liu JH, Couto LB, Addya K, Leonard DG, Zhen Z, et al. Inadvertent germline transmission of AAV2 vector: findings in a rabbit model correlate with those in a human clinical trial. Mol Ther. 2006;13:1064–1073. doi: 10.1016/j.ymthe.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- Di Pasquale G, Davidson BL, Stein CS, Martins I, Scudiero D, Monks A, et al. Identification of PDGFR as a receptor for AAV-5 transduction. Nat Med. 2003;9:1306–1312. doi: 10.1038/nm929. [DOI] [PubMed] [Google Scholar]
- Akache B, Grimm D, Pandey K, Yant SR, Xu H., and , Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol. 2006;80:9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerford C., and , Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff AM, Gray JT, Ng CY, Zhang Y, Zhou J, Spence Y, et al. Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. Mol Ther. 2005;11:875–888. doi: 10.1016/j.ymthe.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Xiao W, Chirmule N, Berta SC, McCullough B, Gao G., and , Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J., and , Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalenko M, Chen L, van Roey M, Donahue BA, Snyder RO, McArthur JG, et al. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J Virol. 2000;74:1761–1766. doi: 10.1128/jvi.74.4.1761-1766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirmule N, Propert K, Magosin S, Qian Y, Qian R., and , Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- Blacklow NR, Hoggan MD, Sereno MS, Brandt CD, Kim HW, Parrott RH, et al. A seroepidemiologic study of adenovirus-associated virus infection in infants and children. Am J Epidemiol. 1971;94:359–366. doi: 10.1093/oxfordjournals.aje.a121331. [DOI] [PubMed] [Google Scholar]
- Brantly ML, Spencer LT, Humphries M, Conlon TJ, Spencer CT, Poirier A, et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 αl-antitrypsin (AAT) vector in AAT-deficient adults. Hum Gene Ther. 2006;17:1177–1186. doi: 10.1089/hum.2006.17.1177. [DOI] [PubMed] [Google Scholar]
- Summerford C, Bartlett JS., and , Samulski RJ. αVβ5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- Qing K, Mah C, Hansen J, Zhou S, Dwarki V., and , Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA, et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, Edmonson SA, Hui DJ, Sabatino DE, et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110:2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Lillicrap D, Patarroyo-White S, Liu T, Qian X, Scallan CD, et al. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. 2006;108:107–115. doi: 10.1182/blood-2005-12-5115. [DOI] [PubMed] [Google Scholar]
- Wang Z, Kuhr CS, Allen JM, Blankinship M, Gregorevic P, Chamberlain JS, et al. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther. 2007;15:1160–1166. doi: 10.1038/sj.mt.6300161. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Davidoff AM, Hanawa H, Hu Y, Hoffer FA, Nikanorov A, et al. Sustained high-level expression of human factor IX (hFIX) after liver-targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood. 2002;100:1662–1669. doi: 10.1182/blood-2002-02-0589. [DOI] [PubMed] [Google Scholar]
- Lozier JN, Metzger ME, Donahue RE., and , Morgan RA. The rhesus macaque as an animal model for hemophilia B gene therapy. Blood. 1999;93:1875–1881. [PubMed] [Google Scholar]
- Mingozzi F, Liu YL, Dobrzynski E, Kaufhold A, Liu JH, Wang Y, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell JS, Ragni MV, White GC 2nd, Lusher JM, Hillman-Wiseman C, Moon TE, et al. Phase 1 trial of FVIII gene transfer for severe hemophilia A using a retroviral construct administered by peripheral intravenous infusion. Blood. 2003;102:2038–2045. doi: 10.1182/blood-2003-01-0167. [DOI] [PubMed] [Google Scholar]
- Roehl HH, Leibbrandt ME, Greengard JS, Kamantigue E, Glass WG, Giedlin M, et al. Analysis of testes and semen from rabbits treated by intravenous injection with a retroviral vector encoding the human factor VIII gene: no evidence of germ line transduction. Hum Gene Ther. 2000;11:2529–2540. doi: 10.1089/10430340050208000. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- Scallan CD, Jiang H, Liu T, Patarroyo-White S, Sommer JM, Zhou S, et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Elliger S, Elliger C, Podsakoff G, Villarreal L, Kurtzman GJ, et al. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5:938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- Le M, Okuyama T, Cai SR, Kennedy SC, Bowling WM, Flye MW, et al. Therapeutic levels of functional human factor X in rats after retroviral-mediated hepatic gene therapy. Blood. 1997;89:1254–1259. [PubMed] [Google Scholar]
- Hauck B, Chen L., and , Xiao W. Generation and characterization of chimeric recombinant AAV vectors. Mol Ther. 2003;7:419–425. doi: 10.1016/s1525-0016(03)00012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JF, Le T, Prado J, Bahr-Davidson J, Smith PH, Zhen Z, et al. Identification of factors that contribute to recombinant AAV2 particle aggregation and methods to prevent its occurrence during vector purification and formulation. Mol Ther. 2005;12:171–178. doi: 10.1016/j.ymthe.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Sommer JM, Smith PH, Parthasarathy S, Isaacs J, Vijay S, Kieran J, et al. Quantification of adeno-associated virus particles and empty capsids by optical density measurement. Mol Ther. 2003;7:122–128. doi: 10.1016/s1525-0016(02)00019-9. [DOI] [PubMed] [Google Scholar]