Abstract
Despite the promise of proangiogenic gene therapy most clinical trials have failed to show benefit for the primary end point analysis. The NOGA angiogenesis Revascularization Therapy: assessment by RadioNuclide imaging (NORTHERN) trial was a double-blind, placebo-controlled study of intramyocardial vascular endothelial growth factor (VEGF165) gene therapy versus placebo, involving seven sites across Canada, designed to overcome major limitations of previous proangiogenic gene therapy trials. A total of 93 patients with refractory Canadian Cardiovascular Society (CCS) class 3 or 4 anginal symptoms were randomized to receive 2,000 µg of VEGF plasmid DNA or placebo (buffered saline) delivered via the endocardial route using an electroanatomical NOGA guidance catheter. There was no difference between the VEGF-treated and the placebo groups in the primary end point of change in myocardial perfusion from baseline to 3 or 6 months, assessed by single photon emission tomography (SPECT) imaging, although a significant reduction in the ischemic area was seen in both groups. Also, similar improvements in exercise treadmill time and anginal symptoms were seen in the VEGF and the placebo groups at 3 and 6 months, although again there were no differences between these groups. Despite the intramyocardial administration of a high “dose” of plasmid DNA using a percutaneous guidance catheter system, there was no benefit of VEGF gene therapy at 3 or 6 months for any of the end points studied.
Introduction
Despite the improvement in revascularization technologies over the course of the last several decades, a significant proportion of patients are not suitable for percutaneous or surgical procedures. Although the prognosis of patients suffering from refractory angina is surprisingly good,1 their quality of life remains very poor, particularly in those with Canadian Cardiovascular Society (CCS) class 3 and 4 symptoms. Therefore, there has been considerable interest in developing new approaches that can improve coronary perfusion within regions of ischemic myocardium.
The body has a natural ability to improve perfusion to regions of chronic ischemia both by angiogenesis, the sprouting of new vessels from existing ones, and by arteriogenesis, the formation of more mature and larger collateral vessels. Many growth factors that are involved in neovessel formation have been elucidated, and paramount among these is the vascular endothelial growth factor (VEGF).2 VEGF is one of the first factors to be expressed at the onset of ischemia and plays a critical role in the initiation of the angiogenic response.2 VEGF protein and gene delivery have been demonstrated to be highly effective in a variety of preclinical models of chronic ischemia, and early clinical studies of VEGF proangiogenesis therapy appeared promising.3,4 However despite this promise, the results from larger and more rigorously designed clinical trials have been mixed.5,6 No study to date has conclusively shown benefit of VEGF therapy for the induction of angiogenesis based on predetermined primary end point analyses, however, in some trials there appeared to be improvement in post hoc subgroup analysis or in secondary end points.7
Moreover, many of these studies suffered from significant limitations in the strategy for delivery of angiogenic factors or genes to the myocardium that could preclude a positive outcome, independent of the innate ability of VEGF to induce effective collaterization. For example, it is likely that intracoronary (IC) administration of recombinant proteins, as in the VIVA (Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis)8 and FIRST (FGF Initiating RevaScularization Trial)9 trials, would not have resulted in sufficient uptake from the coronary circulation or duration of biological activity to induce a robust angiogenic response.10,11,12 Similarly, the Angiogenic Gene Therapy (AGENT) trials13 relied on IC delivery of an adenoviral vector, which can result in variable and inconsistent transfection of the myocardium.14 Thus, because of these limitations, it is uncertain whether the lack of clear benefit in these studies is indeed indicative of a lack efficacy of proangiogenic factors per se, or merely an inability to achieve adequate levels of the therapeutic agent in the ischemic heart.
In the REVASC trial,15 we had previously shown that direct intramyocardial injection of an adenovirus containing VEGF121 resulted in significant improvement at 6 months in the gene therapy group in time to 1 mm ST-depression of exercise treadmill testing, the predefined primary analysis. However, this was not a definitive trial because the surgical approach required for cardiac access and gene delivery precluded blinding, and thus, it was not possible to exclude the potential for bias completely. Percutaneous intramyocardial injection catheters can achieve local myocardial delivery in a manner which is compatible with a double-blinded randomized trial design.3 The “NOGA angiogenesis Revascularization Therapy: assessment by RadioNuclide imaging” (NORTHERN) trial used an electromagnetic mapping catheter system to deliver plasmid VEGF165 DNA to the left ventricular myocardium in patients with chronic myocardial ischemia who were not suitable for standard revascularization procedures. Also, an optimal “dose” of plasmid DNA was used, equivalent to the effective dose in preclinical studies and substantially greater than that used in an earlier NOGA-based VEGF gene therapy trial.16
Results
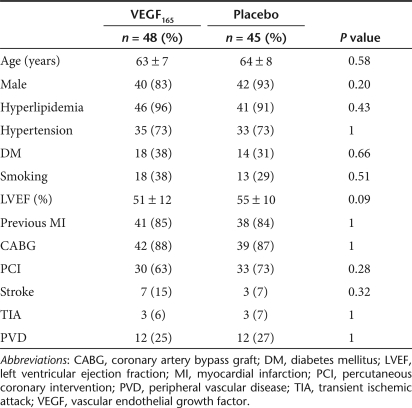
A total of 93 patients were randomized into the VEGF (n = 48) or placebo (n = 45) groups (Figure 1). Table 1 summarizes the main demographic data. There were no significant differences between the treatment and the placebo groups in any of the baseline characteristics, confirming that the randomization strategy was effective. Also, there were no baseline differences between groups 1 and 2 patients (see Supplementary Table S1).
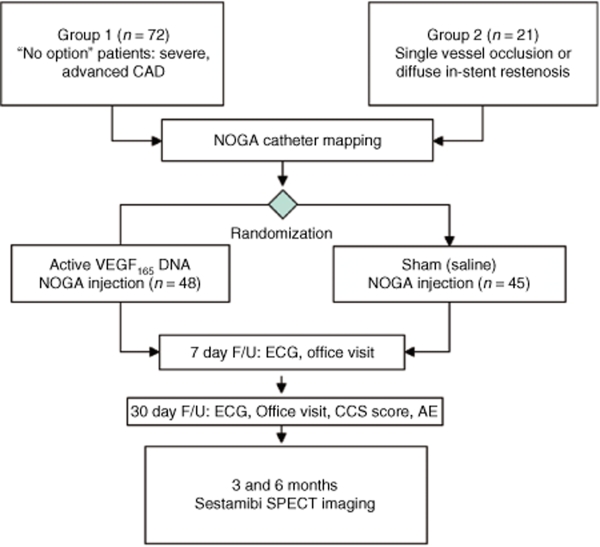
Figure 1.
Flow diagram of the NORTHERN trial. Patients were assigned to group 1—i.e., “no option” patients with advance coronary artery disease (CAD) or group 2—i.e., single vessel occlusion/in stent restenosis based on the coronary anatomy and suitability for standard revascularization procedures. Upon successful NOGA electromechanical mapping, patients were randomized to receive of VEGF plasmid DNA (active treatment) or saline (Sham) divided into 10 intramyocardial injections targeted to the ischemic region under NOGA guidance. Patients were seen at 7 and 30 days for routine follow-up and SPECT sestamibi myocardial perfusion was performed at 3 and 6 months along with exercise treadmill testing and other efficacy and safety assessments. AEs, adverse events; CCS, Canadian Cardiovascular Society; ECG, electrocardiogram; NORTHERN, NOGA angiogenesis Revascularization Therapy: assessment by RadioNuclide imaging; SPECT, single photon emission tomography; VEGF, vascular endothelial growth factor.
Table 1.
Baseline characteristics
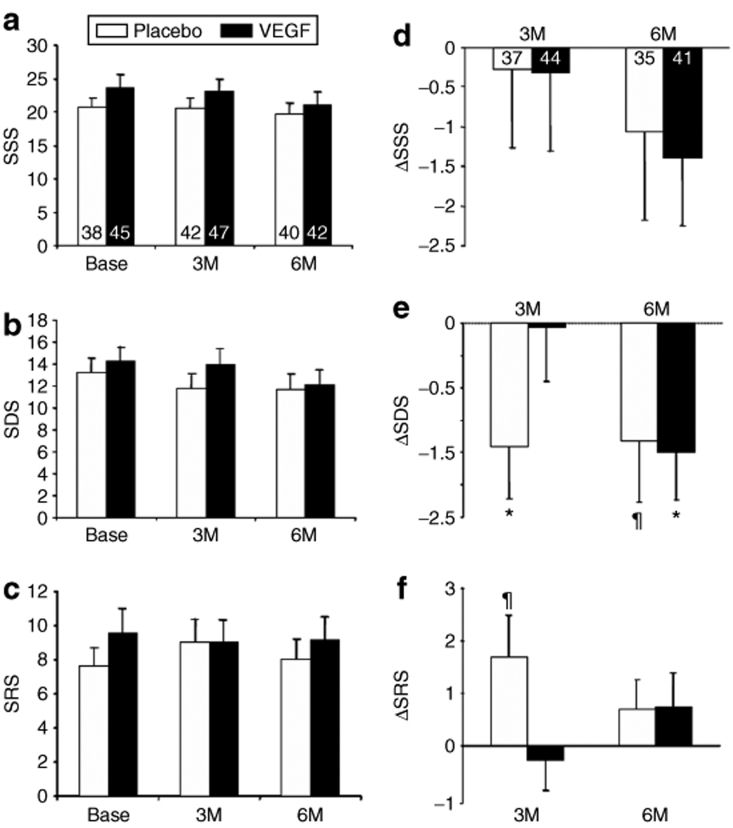
Figure 2 shows myocardial perfusion measured by single photon emission tomography (SPECT) sestamibi imaging at baseline and 3 and 6 months. Summed stress score (panel A), summed defect score (SDS, panel B), and summed reversibility score (panel C) were not different between the treatment and the control groups at any time point, although both groups tended to show a reduction in ischemia score over time. This was confirmed by the predefined primary analysis of the change in score from baseline at either 3 or 6 months (Figure 2d–f). Although again there were no significant differences between the VEGF treatment and the control groups in change in summed stress score, SDS, or summed reversibility score at either 3 or 6 months, there were improvements in perfusion scores within both groups over time which achieved significance for SDS at 3 and 6 months for the placebo and the VEGF groups, respectively (Figure 2e), with trends toward improvement in summed reversibility score at 3 months (Figure 2f) and in SDS at 6 months in the placebo group. Also, there were no differences between myocardial perfusion groups 1 and 2, both of which failed to show evidence of benefit with VEGF gene therapy (see Supplementary Figure S1) This indicates that despite the complete lack of efficacy of VEGF gene therapy over placebo, patients with severe coronary disease and refractory angina showed improvement in myocardial perfusion within the ischemic zone over the study period.
Figure 2.
SPECT sestamibi myocardial perfusion imaging. Summary data for (a) summed stress score (SSS), (b) summed defect score (SDS), and (c) summed reversibility score (SRS) is presented at baseline (Base), 3 and 6 months (M) for patients receiving saline placebo (open bars) or VEGF plasmid DNA (closed bars). Of the 91 patients receiving injections of VEGF or placebo, scans were available in 83 patients at baseline, 89 at 3 months, and 82 at 6 months. The primary analysis, change (Δ) from Base to 3 and 6M is presented in panels d–f. A total of 81 patients (66 in group 1 and 15 in group 2) had complete baseline and 3-month follow-up nuclear perfusion study scores and 76 patients had complete data for the 6-month analysis (61 in group 1 and 15 in group 2). There were no significant differences between the active treatment group (VEGF, closed bars) and the placebo controls (Sham, open bars) in SSS (primary end point analysis), SDS and SRS at either 3 or 6 months. However, there was significant improvement in resting myocardial perfusion within the ischemic zone (SDS) at 6 months in the VEGF group and a trend in placebo group. The placebo group also showed lower SDS at 3 months which was however associated with a trend toward increased SRS. ¶P < 0.1 versus baseline; *P < 0.05 versus baseline. SPECT, single photon emission tomography; VEGF, vascular endothelial growth factor.
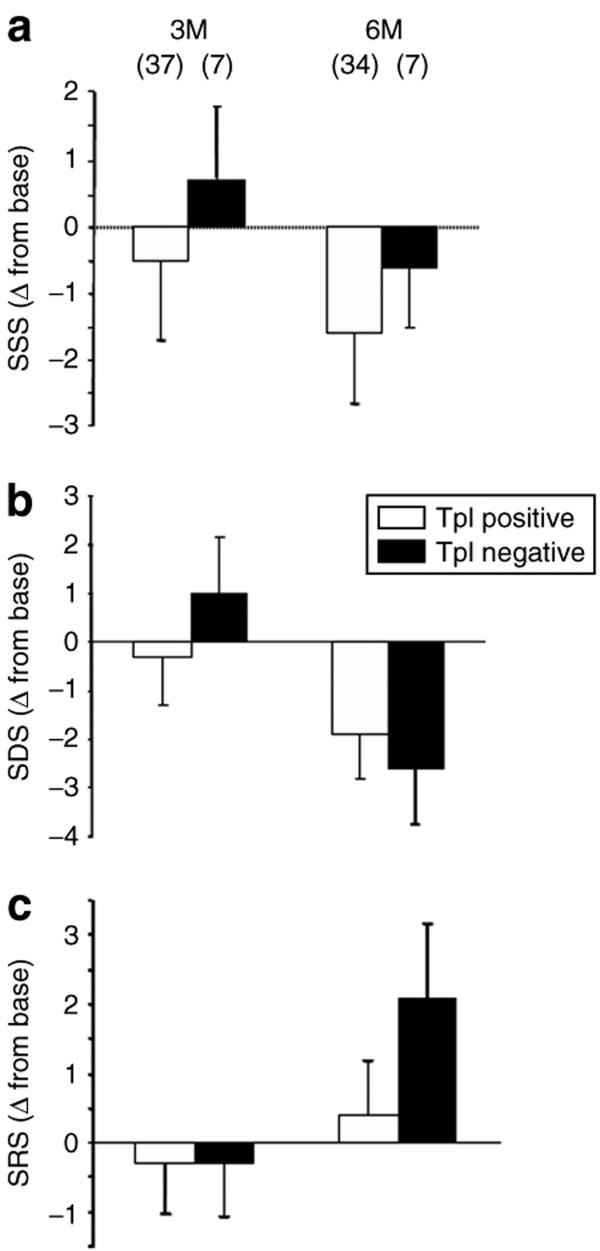
To provide further evidence that VEGF plasmid DNA was indeed delivered into the left ventricular myocardium, we measured troponin (Tp) levels on the morning following delivery. Figure 3 presents the summary myocardial perfusion data for patients receiving VEGF injections divided into two groups based on the presence or absence of elevated Tp. It is important to note that the majority of patients randomized to receive VEGF plasmid DNA had Tp elevation (85%), suggestive of successful delivery. Nonetheless, there were no significant differences qualitatively or quantitatively between the Tp positive and negative groups in change in myocardial perfusion. Moreover, there were no significant relationships between the magnitude of Tp rise and changes in perfusion score or ETT (see Supplementary Figure S3).
Figure 3.
Myocardial perfusion in patients with normal versus elevated levels of troponin I (TpI). Changes in (a) SSS, (b) SDS, and (c) SRS from baseline to 3 and 6 months (M) were not different between VEGF treated patients that exhibited elevated TpI levels (positive, open bars) compared to those that did not (negative, closed bars). SDS, summed defect score; SRS, summed reversibility score; SSS, summed stress score.
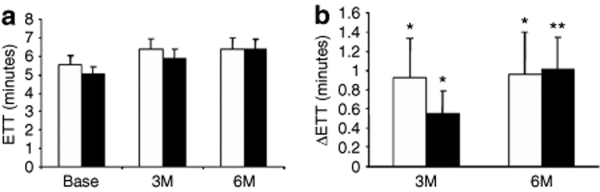
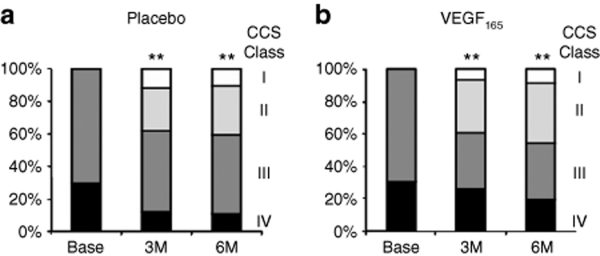
Similarly, there were no differences between the VEGF treatment and placebo groups in total ETT (Figure 4a) or change in ETT from baseline at 3 and 6 months (Figure 4b). Again, there were significant improvements within both groups of almost 1 minute from baseline to 3 and 6 months (Figure 4b). Similarly, there were no differences in change in CCS functional class between patients receiving VEGF or placebo (Figure 5a,b, respectively); however, ~40% of patients in both groups showed improvement by at least one CCS class which was highly significant.
Figure 4.
Exercise treadmill time (ETT). Summary data for ETT is presented in total time in minutes (a) and change from baseline (Base) to 3 and 6 months (M) (b). There were no significant differences between the active treatment group (VEGF, closed bars) and the placebo (open bars) controls; however, both groups showed significant improvements in exercise tolerance at 3 and 6 months (b). *P < 0.05 versus baseline; **P < 0.01 versus baseline. VEGF, vascular endothelial growth factor.
Figure 5.
Proportion of patients in each CCS angina class (I white, II light shading, III dark shading, and IV black) is presented for (a) placebo and (b) VEGF-treated groups at baseline and at 3 and 6 months of follow-up. All patients exhibited class 3 or 4 symptoms at baseline (Base) by protocol, and there was a progressive increase in the proportion of patients within both groups showing improvement ≥1 CCS class which was highly significant; however, there were no significant differences in change in CCS class from baseline to 3 or 6 months between the VEGF and placebo groups. **P < 0.01 versus baseline. CCS, Canadian Cardiovascular Society; VEGF, vascular endothelial growth factor.
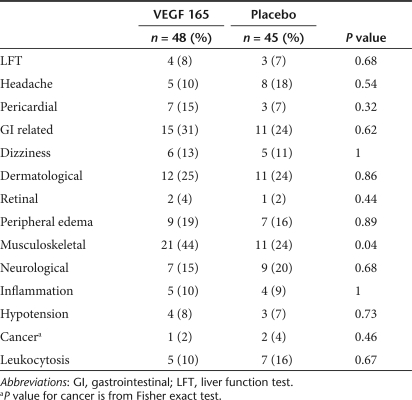
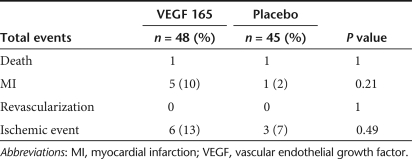
Minor adverse events were well balanced between the VEGF treatment and placebo groups (Table 2), with the possible exception of musculoskeletal complaints which consisted mainly of minor aches and pains. Of note, the incidence of retinal complications was extremely low with both groups having one occurrence of retinal hemorrhage and a single case of minimal diabetic retinopathy in the VEGF group. Also, the incidence of cancer was very low consisting of two cases of basal cell carcinoma in the placebo group and one case in the VEGF group. Serious adverse events appeared to be more frequent in the VEGF treatment group than in the placebo group, with about a threefold greater incidence of the combined end point of death, myocardial infarction or revascularization (Table 3) and a twofold excess of ischemic event requiring hospitalization (defined by the presence of two of the following three criteria: chest pain, dynamic electrocardiogram changes, or enzyme elevation). However, these differences were not significant, possibly due to the overall low number of events. There were only two deaths in the study, equally balanced between the VEGF and placebo groups, and no patients underwent revascularization procedures during the follow-up period.
Table 2.
Adverse events
Table 3.
Serious adverse events
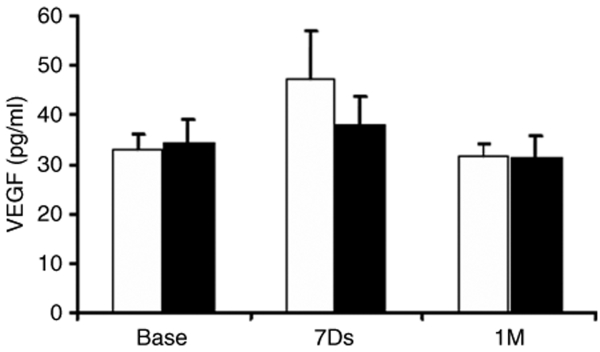
VEGF plasma levels were measured by enzyme-linked immunosorbent assay in a subgroup of 20 patients enrolled at one site (St Michael's Hospital) at baseline, and at 7 days and 1 month after injection of VEGF plasmid or placebo (10 patients in each; Figure 6). The levels of circulating VEGF were neither different at any time point between the two groups, nor was there any significant change over time. Left ventricular ejection fraction, measured by echocardiography, tended to be slightly lower at baseline in the patients randomized to the VEGF treatment (see Supplementary Figure S2, Table 1; P = 0.09), but there was no significant change from baseline to 3 months in either group.
Figure 6.
Plasma VEGF levels measured at baseline (Base), 7 days and 1 month in the VEGF (closed bars) and placebo (open bars) groups in a subset of patient at one clinical center. No significant increases in circulating VEGF levels were seen in either group and there were no differences between groups. VEGF, vascular endothelial growth factor.
Discussion
The results of the randomized, double-blinded NORTHERN trial show no improvement in the primary end point or any of the secondary efficacy analyses in the VEGF-treated compared to the placebo group, despite the delivery of a high dose of plasmid DNA directly to the left ventricular myocardium using a navigational catheter injection system.
Although this is not the first rigorously designed proangiogenesis study to fail to achieve primary end point criteria, the previous trials have not been conclusive, either suggesting benefit in certain subgroups15,16,17 or suffering from important limitations that precluded a definitive interpretation. For example, the FIRST and VIVA trials relied on IC delivery of protein factors.8,9 Although IC injection is a more convenient delivery method, it is uncertain whether sufficient fibroblast growth factor and VEGF protein were taken up by the myocardium, and given the unstable nature of these recombinant proteins, the duration of activity would be sufficient to result in a meaningful angiogenic response within the heart. Indeed, the uptake of proteins by the myocardium has been reported to be extremely low.10,12 Moreover because of systemic effects (i.e., hypotension, proteinuria), there are limitations of total dose of protein factors that can be safely administered by intravascular injection.18,19
An important advantage of a gene transfer is that the vector, once taken up by the target tissue, can result in the continuous production of a potentially therapeutic protein factor over the duration of transgene expression.19 The AGENT trial was the first rigorously designed angiogenesis gene therapy study and used an adenoviral vector to increase the myocardial uptake of the fibroblast growth factor-4 transgene after IC administration.17 The AGENT-3 and -4 trials represent the largest proangiogenesis study to be performed to date.13 Although these trials were stopped prematurely due to futility, a subgroup analysis on the pooled data revealed a significant improvement in ETT in women, suggesting the potential for gender differences in the angiogenic response. However, again it is unclear to what extent this delivery strategy would result in efficient gene transfer to the heart as preclinical studies have shown variable degrees of transfection efficiency with simple IC injection of adenoviral vectors,14 and it has been suggested that strategies to increase coronary endothelial permeability or capillary pressure are required to enhance transfection efficiency. Unfortunately, no reliable indices of local cardiac transgene expression are available to address this concern in the context of a clinical trial, and elevations of circulating levels of VEGF have not been observed even with surgically guided intramyocardial injections of adenoviral vectors,15 which is almost certain to produce substantial levels of transfection of the heart.
The REVASC trial15 was performed specifically to overcome this limitation using a stand-alone thoracotomy to allow surgically guided direct transepicardial intramyocardial injection of an adenoviral vector harboring the VEGF121 transgene. Although this study did suggest a significant increase in time to 1 mm ST-segment depression in the VEGF-treated group compared to control patients, the need for a surgical delivery procedure precluded a blinded design. Thus, despite the attempt to enhance objectivity using a blinded core laboratory to perform the primary analysis, the possibility of a “placebo effect” cannot be excluded. It is of interest to note that the improvement in time to 1 mm ST-segment depression of 1 minute parallels the improvement seen in total ETT in blinded FIRST and VIVA studies, as well as the present trial, in both the treatment and the control groups.
The EUROINJECT trial was the first to use a percutaneous strategy for intramyocardial gene delivery, thus allowing a blinded trial design.16 There was no overall improvement in myocardial perfusion by SPECT imaging; however, this may have been, in part, due to considerable center to center variability in the data, with the best results coming from a small number of high enrolling sites. Nonetheless, benefit was reported for the secondary end point of regional wall motion, suggesting a possible favorable effect of VEGF gene transfer, despite the use of relatively low dose of plasmid DNA. For this reason, we chose to use a nearly fourfold higher dose of plasmid DNA than that of the EUROINJECT-1 trial.16 However, despite using the higher dose of VEGF plasmid DNA, and a similar rigorously designed NOGA guided, percutaneous gene delivery trial, the NORTHERN trial did not support a benefit with VEGF therapy.
Despite the complete lack of any evidence of improvement in perfusion in the VEGF treated versus the placebo groups in the NORTHERN trial, SPECT imaging did reveal a reduction in the ischemic territory (SDS) over the follow-up period which was similar in both groups. This confirms the ability of the primary end point assessment to detect relevant changes in myocardial perfusion in this population. Also, this observation strongly suggests that even patients with extensive coronary disease and various cardiac risk factors were still able to mount a revascularization response, and demonstrate an improvement in collateral vessels in response to chronic ischemia over the study period. Thus, it would appear that the presence of risk factors, which are known to interfere with the angiogenic response, in part, due to reduced nitric oxide bioavailability and impaired downstream signaling in response to angiogenic growth factors,20,21 cannot be the sole reason for lack of efficacy of VEGF therapy in this study. This observation suggests that it is possible that more sophisticated proangiogenic strategies, such as the use of multiple factors, “master switch” genes such as hypoxia-inducible factor-1 and sequential delivery, could succeed in enhancing this endogenous neovascularization response.6,22,23
The NORTHERN trial also confirms the large magnitude of the “placebo” effect which has been demonstrated in other randomized double-blind and placebo-controlled studies of proangiogenic therapies.24 This effect was seen in all secondary end points including functional measures of exercise tolerance and subjective indices of anginal symptoms. As in previous studies, this may have resulted from an expectation on the part of patients or their care givers of benefit engendered by receiving a novel treatment or procedure. However, given the objective evidence of increased myocardial perfusion within ischemic regions by SPECT imaging, it is also possible that to some extent this “placebo effect” may also reflect endogenous neovascularization and collateral vessel formation occurring during the course of the study.
The NORTHERN trial has several limitations which are important to acknowledge, foremost among these being the use of plasmid DNA rather than a viral vector. Although the injection of plasmid DNA can result in minimal gene transfer in many tissues, plasmid vectors have been shown to produce efficient transfection of both skeletal and cardiac muscle,25,26 possibly due to the unique organization of these tissues, characterized by very large myocytes constrained in a well-organized mesh of extracellular matrix material. Cardiac delivery of plasmid DNA has also been shown to be effective in preclinical models27 and was suggested to be potentially beneficial in earlier clinical trials.3,16 To compensate for the lower transfection efficiency, we used as high a dose of plasmid DNA as possible, nearly fourfold higher than that of the EUROINJECT-1 trial.16
Another limitation relates to use of a percutaneous catheter delivery system which, although necessary for the blinded design, makes it difficult to be certain that the DNA product was indeed injected into the myocardium. To overcome this concern, we required the presence of ventricular extrasystoles on needle extension before the injection procedure. Also, we measured Tp levels on the day following transendocardial injection to obtain further evidence to support the success of intramyocardial delivery. Only a small number of patients failed to exhibit Tp elevation, and it is important to note that there was no difference in the outcome in these patients compared to the majority which did exhibit enzyme rise. However, successful gene delivery does not necessarily mean successful myocyte transfection, and in the absence of elevation in circulating levels, this could only be established by tissue analysis. Finally, enrollment for the NORTHERN trial was closed before the predefined target of 100 patients had been reached largely due to increasing difficulty in patient enrollment due to changes in interventional cardiology practice; however, given the complete lack of any tendency toward a benefit in the treatment group in any end point or subgroup examined, it is inconceivable that a small number of additional patients would have changed the results. In fact, the only significant differences in myocardial perfusion favored the placebo group.
It is of potential interest that, although not statistical significant, there appeared to be some excess in major adverse events in the treatment compared to the placebo group. This was particularly the case for myocardial infarction and major ischemic events requiring hospitalization which were twofold to threefold more frequent in the VEGF treatment group. Although such a finding is potentially consistent with an effect of VEGF gene therapy on the stability of atherosclerotic lesions,28,29 there were no measurable increases in circulating levels of VEGF in patients receiving gene injections, a result consistent with that of the REVASC trial which used adenoviral vector,15 and thus, distant vascular effects following intramyocardial VEGF gene delivery would be improbable. Given the very low overall number of major adverse events in our trial, it is not possible to draw any definite conclusions about a deleterious effect of VEGF gene therapy.
Therefore based on the results of the NORTHERN trial, it can be concluded that intramyocardial plasmid VEGF gene therapy is ineffective for improving collateral blood flow into regions of chronic ischemia. However based on endogenous improvement in myocardial perfusion, it is possible that more sophisticated proangiogenic strategies could enhance the native ability for neovascularization that was observed even in patients with severe coronary artery disease.
Materials and Methods
Patient selection. All study patients were required to have Canadian Cardiovascular Class 3 or 4 angina, despite treatment with maximal medical therapy (at least two of the following: long-acting nitrates, β-blockers, or calcium channel blockers, and either aspirin or clopidogrel) for at least 4 weeks, as well as adequate secondary prevention medication. Also, patients had to have a reversible ischemic defect on myocardial stress SPECT imaging or a nonreversible defect having evidence of myocardial viability (echo, or other suggestion of wall motion); left ventricular ejection fraction ≥20%; age ≤75 years; adequate feeder coronary vessels to the territories targeted for injection; left ventricular wall thickness ≥0.9 cm in target region; coronary angiography performed within the past 12 months showing at least one inflow vessel without a significant proximal lesion (i.e., <70%). Patients who were too ill to undergo the procedure or who otherwise might be perceived to be at higher risk for growth factor therapy were excluded. After satisfying the inclusion/exclusion criteria, each patient underwent a comprehensive baseline screening evaluation to ensure that occult malignancy had been excluded.
NOGA catheter mapping and administration of gene therapy. Electroanatomical NOGA mapping was performed in a standardized fashion as described previously,30 within 90 days after the myocardial perfusion studies. Arterial access was obtained through the femoral artery, and 70 IU/kg heparin was administered. A NOGA map of the left ventricle was obtained using a 7F NAVISTAR catheter connected to the NOGA console (Cordis-Biosense Webster, Markham, Ontario, Canada). Areas exhibiting normal voltages (unipolar voltage ≥10 mV) and decreased linear local shortening (linear local shortening ≤8%) on the NOGA map, and geographically concordant with the ischemic territory described by the preprocedural myocardial SPECT imaging, were identified as the target areas and circumscribed using line-drawing software supplied with the system. Immediately after diagnostic NOGA mapping, 10 intramyocardial injections (0.2 ml/injection), at least 1 cm apart, were made over 60 seconds each within the delineated target area with an 8F MYOSTAR (Cordis-Biosense Webster) injection catheter to deliver 2,000 µg VEGF 165 in a solution of 2 ml total volume or an identical volume of placebo (phosphate-buffered saline). Injection criteria to maximize the efficiency of myocardial delivery were as follows: (i) loop stability <2 mm, (ii) catheter tip perpendicular to the myocardial wall confirmed using NOGA and fluoroscopy, (iii) the presence of ventricular ectopy with needle protrusion, (iv) no catheter pushback with needle protrusion under fluoroscopy. Injections were not made into areas with a known wall thickness of ≤9 mm by 2D echocardiography. A 2D echocardiography was performed the morning after the injection procedure, and the patients were subsequently discharged.
Randomization. Treatment was randomly assigned in a 1:1 ratio according to a randomization code prepared by the academic research organization. As shown in Figure 1, patient randomization was stratified into either group 1 (not suitable for standard revascularization therapies) or group 2 (revascularization possible but not deemed advisable) as defined in the Supplementary Materials and Methods. When the investigator was certain that the endocardial map could be completed satisfactorily, the local pharmacist was notified to prepare VEGF165 or matching placebo according to the randomization number provided by the coordinating center. The pharmacist maintained the blind by supplying either VEGF165 or placebo in matching sterile syringes.
Scintigraphic assessment of myocardial perfusion and exercise testing. All patients had a SPECT technetium99m sestamibi study at baseline (within 3 months of the left ventricular mapping and VEGF165 myocardial injection), and repeated at 12 weeks and again at 6 months as described in the Supplementary Materials and Methods. The exercise treadmill test was conducted according to the Asymptomatic Cardiac Ischemia Pilot Treadmill protocol at baseline, 12 weeks, and 6 months as described in the Supplementary Materials and Methods.
VEGF enzyme-linked immunosorbent assay. VEGF enzyme-linked immunosorbent assay was performed using a commercially available enzyme-linked immunosorbent assay as described in the Supplementary Materials and Methods.
Clinical follow-up. Patients were followed for 6 months post mapping and injection procedure. SPECT technetium99m sestamibi studies and exercise treadmill tests were performed at 12 weeks and 6 months. Safety blood work, CCS scores, and electrocardiograms were obtained at 7 days, 12 weeks, and 6 months with quality of life questionnaires (Seattle Angina Questionnaire, SF36) completed at 3 and 6 months, and an echocardiogram at 3 months. Cancer screening, as well as an ophthalmologic exam was repeated at 6 months. Adverse and ischemic events and medication changes were assessed at each study visit. An ischemic event was defined as angina requiring hospitalization with documented evidence of the presence of two of the following criteria: chest pain, new or dynamic electrocardiogram changes reflecting ischemia or cardiac enzyme markers reflecting ischemia. The classification of myocardial infarction was based on the clinical diagnosis made at each center.
Statistical analysis. We calculated that a sample size of 100 subjects would provide 90% power (1-β = 0.90) to detect an improvement of at least 25% in the mean change of stress perfusion score from baseline to 12-week follow-up (see Supplementary Materials and Methods). For continuous variables, the comparisons between the two treatment groups were performed with an independent t-test if they were normally distributed, otherwise the nonparametric Mann–Whitney–Wilcoxon test was performed; the comparisons overtime were performed with paired t-tests for normal variables, although the Wilcoxon signed rank test was used for nonnormal cases. The normality was assessed with Kolgomorov–Smirnof test. Categorical variables were compared using Pearson χ2 test or Fisher exact test if the number of observations per cell was <5. This intent-to-treat analysis was performed using SAS V9.1 (SAS, Cary, NC) according to a prespecified analysis plan. All tests were two-sided and with significant level 0.05.
SUPPLEMENTARY MATERIALFigure S1. Myocardial perfusion in Group 1 and Group 2 patients. 81 patients (66 in group 1 and 15 in group 2) had complete baseline and 3 month follow-up nuclear perfusion studies scores and 76 patients had complete data for the 6 month analysis (61 in group 1 and 15 in group 2). There were no differences between myocardial perfusion groups 1 and 2, both of which failed to show evidence of benefit with VEGF gene therapy. Indeed, the only significant difference between the VEGF treatment and placebo groups favored the control group (SDS, 3 months). Open bars = placebo; closed bars = VEGF plasmid; ¶ =0.05<p<0.1 vs. placebo.Figure S2. Left ventricular ejection fraction (LVEF). There was a trend towards reduced LVEF at baseline in the patients randomized to VEGF plasmid DNA (closed bars) compared with saline (placebo, open bars), however, there was no significant change in either group from baseline to 3 months. ¶ =0.05<p<0.1 vs. placebo.Figure S3. Regression analysis for troponin rise. Linear regression analysis was performed between the fold-increase in troponin (Tp) levels the day following the injection procedure and the change in summed stress score (SSS), summed defect score (SDS), summed reversibility score (SRS), and exercise treadmill time (ETT) from baseline to 3 months post injection. Placebo = red, closed symbol, solid line; VEGF = black, open symbols, dashed line. There were no significant correlations, although a trend towards an relationship between Tp rise and SSS or SDS was observed for VEGF-treated patients. However, this was driven in large part by a reduced perfusion (i.e. higher scores) in patients with low Tp values rather than any greater improvement in perfusion in those with presumed successful gene delivery.Table S1. Baseline characteristics.Materials and Methods.
Supplementary Material
Myocardial perfusion in Group 1 and Group 2 patients. 81 patients (66 in group 1 and 15 in group 2) had complete baseline and 3 month follow-up nuclear perfusion studies scores and 76 patients had complete data for the 6 month analysis (61 in group 1 and 15 in group 2). There were no differences between myocardial perfusion groups 1 and 2, both of which failed to show evidence of benefit with VEGF gene therapy. Indeed, the only significant difference between the VEGF treatment and placebo groups favored the control group (SDS, 3 months). Open bars = placebo; closed bars = VEGF plasmid; ¶ =0.05<p<0.1 vs. placebo.
Left ventricular ejection fraction (LVEF). There was a trend towards reduced LVEF at baseline in the patients randomized to VEGF plasmid DNA (closed bars) compared with saline (placebo, open bars), however, there was no significant change in either group from baseline to 3 months. ¶ =0.05<p<0.1 vs. placebo.
Regression analysis for troponin rise. Linear regression analysis was performed between the fold-increase in troponin (Tp) levels the day following the injection procedure and the change in summed stress score (SSS), summed defect score (SDS), summed reversibility score (SRS), and exercise treadmill time (ETT) from baseline to 3 months post injection. Placebo = red, closed symbol, solid line; VEGF = black, open symbols, dashed line. There were no significant correlations, although a trend towards an relationship between Tp rise and SSS or SDS was observed for VEGF-treated patients. However, this was driven in large part by a reduced perfusion (i.e. higher scores) in patients with low Tp values rather than any greater improvement in perfusion in those with presumed successful gene delivery.
Baseline characteristics.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (No. UCT-51532) and the Heart & Stroke Foundation of Ontario (No. NA-4788), as well as generous support from the St. Michael's Hospital Foundation and the Heart & Stroke/Richard Lewar Centre of Excellence in Cardiovascular research. We are grateful for the tremendous contributions of the Data Safety Monitoring Board members: Salim Yusuf (chair), Jean Rouleau, Robin Roberts, and Aiala Barr. We are also grateful for the contributions by Cordis, Biosense Webster, J & J Medical Products, Canada.
Participating Centers
Edmonton, University of Alberta: Wayne Tymchak, MD; Norma Hogg, RN; Lee Lindholm, RN: Montreal: Serge Doucet, MD; Nathalie St. Jean, RN; Laval University: Eric Larose, MD; Guy Rossignol, M.Sc; Toronto, St. Michael's Hospital: David Latter, MD; Lee Errett, MD; Howard Leong-Poi, MD; Rosemary Dunne, RN, CCRP; Joanna Sloninko, MRT(N); Sunnybrook Health Sciences Centre: Alexander Dick, MD; Lyn Balleza, RN; Inessa Taibert, RN; Mount Sinai Hospital: Alan Barolet, MD; Dianne Locke, RN BScN; Victoria Heart Institute Foundation: Peter Klinke, MD, J. David Hilton, MD; Noreen Lounsbury, RN, BN; Ottawa Health Research Institute: Timothy Ramsay, PhD.
REFERENCES
- Reilly JP, Grise MA, Fortuin FD, Vale PR, Schaer GL, Lopez J.Long-term (2-year) clinical events following transthoracic intramyocardial gene transfer of VEGF-2 in no-option patients J Interv Cardiol 20051827–31.et al [DOI] [PubMed] [Google Scholar]
- Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H.VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells EMBO J 1999183964–3972.et al [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losordo DW, Vale PR, Hendel RC, Milliken CE, Fortuin FD, Cummings N.Phase 1/2 placebo-controlled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia Circulation 20021052012–2018.et al [DOI] [PubMed] [Google Scholar]
- Rosengart TK, Lee LY, Patel SR, Kligfield PD, Okin PM, Hackett NR.Six-month assessment of a phase I trial of angiogenic gene therapy for the treatment of coronary artery disease using direct intramyocardial administration of an adenovirus vector expressing the VEGF121 cDNA Ann Surg 1999230466–470.et al [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N., and , Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- Vincent KA, Jiang C, Boltje I., and , Kelly RA. Gene therapy progress and prospects: therapeutic angiogenesis for ischemic cardiovascular disease. Gene Ther. 2007;14:781–789. doi: 10.1038/sj.gt.3302953. [DOI] [PubMed] [Google Scholar]
- Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ.The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis Circulation 20031071359–1365.et al [DOI] [PubMed] [Google Scholar]
- Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H.Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial Circulation 2002105788–793.et al [DOI] [PubMed] [Google Scholar]
- Filion RJ., and , Popel AS. Intracoronary administration of FGF-2: a computational model of myocardial deposition and retention. Am J Physiol Heart Circ Physiol. 2005;288:H263–H279. doi: 10.1152/ajpheart.00205.2004. [DOI] [PubMed] [Google Scholar]
- Sato K, Laham RJ, Pearlman JD, Novicki D, Sellke FW, Simons M.Efficacy of intracoronary versus intravenous FGF-2 in a pig model of chronic myocardial ischemia Ann Thorac Surg 2000702113–2118.et al [DOI] [PubMed] [Google Scholar]
- Lazarous DF, Shou M, Stiber JA, Dadhania DM, Thirumurti V, Hodge E.Pharmacodynamics of basic fibroblast growth factor: route of administration determines myocardial and systemic distribution Cardiovasc Res 19973678–85.et al [DOI] [PubMed] [Google Scholar]
- Henry TD, Grines CL, Watkins MW, Dib N, Barbeau G, Moreadith R.Effects of Ad5FGF-4 in patients with angina: an analysis of pooled data from the AGENT-3 and AGENT-4 trials J Am Coll Cardiol 2007501038–1046.et al [DOI] [PubMed] [Google Scholar]
- Wright MJ, Wightman LM, Latchman DS., and , Marber MS. In vivo myocardial gene transfer: optimization and evaluation of intracoronary gene delivery in vivo. Gene Ther. 2001;8:1833–1839. doi: 10.1038/sj.gt.3301614. [DOI] [PubMed] [Google Scholar]
- Stewart DJ, Hilton JD, Arnold JM, Gregoire J, Rivard A, Archer SL.Angiogenic gene therapy in patients with nonrevascularizable ischemic heart disease: a phase 2 randomized, controlled trial of AdVEGF(121) (AdVEGF121) versus maximum medical treatment Gene Ther 2006131503–1511.et al [DOI] [PubMed] [Google Scholar]
- Kastrup J, Jorgensen E, Ruck A, Tagil K, Glogar D, Ruzyllo W.Direct intramyocardial plasmid vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris A randomized double-blind placebo-controlled study: the Euroinject One trial J Am Coll Cardiol 200545982–988.et al [DOI] [PubMed] [Google Scholar]
- Grines CL, Watkins MW, Helmer G, Penny W, Brinker J, Marmur JD.Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris Circulation 20021051291–1297.et al [DOI] [PubMed] [Google Scholar]
- Bush MA, Samara E, Whitehouse MJ, Yoshizawa C, Novicki DL, Pike M.Pharmacokinetics and pharmacodynamics of recombinant FGF-2 in a phase I trial in coronary artery disease J Clin Pharmacol 200141378–385.et al [DOI] [PubMed] [Google Scholar]
- Simons M, Bonow RO, Chronos NA, Cohen DJ, Giordano FJ, Hammond HK.Clinical trials in coronary angiogenesis: issues, problems, consensus: an expert panel summary Circulation 200010273–86.et al [DOI] [PubMed] [Google Scholar]
- Fam NP, Verma S, Kutryk M., and , Stewart DJ. Clinician guide to angiogenesis. Circulation. 2003;108:2613–2618. doi: 10.1161/01.CIR.0000102939.04279.75. [DOI] [PubMed] [Google Scholar]
- Kapila V, Sellke FW, Suuronen EJ, Mesana TG., and , Ruel M. Nitric oxide and the angiogenic response: can we improve the results of therapeutic angiogenesis. Expert Opin Investig Drugs. 2005;14:37–44. doi: 10.1517/13543784.14.1.37. [DOI] [PubMed] [Google Scholar]
- Heinl-Green A, Radke PW, Munkonge FM, Frass O, Zhu J, Vincent K.The efficacy of a ‘master switch gene' HIF-1α in a porcine model of chronic myocardial ischaemia Eur Heart J 2005261327–1332.et al [DOI] [PubMed] [Google Scholar]
- Yu J, Lei L, Liang Y, Hinh L, Hickey RP, Huang Y.An engineered VEGF-activating zinc finger protein transcription factor improves blood flow and limb salvage in advanced-age mice FASEB J 200620479–481.et al [DOI] [PubMed] [Google Scholar]
- Rana JS, Mannam A, Donnell-Fink L, Gervino EV, Sellke FW., and , Laham RJ. Longevity of the placebo effect in the therapeutic angiogenesis and laser myocardial revascularization trials in patients with coronary heart disease. Am J Cardiol. 2005;95:1456–1459. doi: 10.1016/j.amjcard.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Majesky MW. A little VEGF goes a long way. Therapeutic angiogenesis by direct injection of vascular endothelial growth factor-encoding plasmid DNA. Circulation. 1996;94:3062–3064. doi: 10.1161/01.cir.94.12.3062. [DOI] [PubMed] [Google Scholar]
- Wolff JA. Naked DNA transport and expression in mammalian cells. Neuromuscul Disord. 1997;7:314–318. doi: 10.1016/s0960-8966(97)00055-2. [DOI] [PubMed] [Google Scholar]
- Lekas M, Kutryk MJ, Latter DA., and , Stewart DJ.Therapeutic neovascularization for ischemic heart disease Can J Cardiol 20042049B–57B.suppl. B [PubMed] [Google Scholar]
- Celletti FL, Hilfiker PR, Ghafouri P., and , Dake MD. Effect of human recombinant vascular endothelial growth factor165 on progression of atherosclerotic plaque. J Am Coll Cardiol. 2001;37:2126–2130. doi: 10.1016/s0735-1097(01)01301-8. [DOI] [PubMed] [Google Scholar]
- Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W., and , Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999;99:1726–1732. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- Kornowski R, Hong MK, Gepstein L, Goldstein S, Ellahham S, Ben-Haim SA.Preliminary animal and clinical experiences using an electromechanical endocardial mapping procedure to distinguish infarcted from healthy myocardium Circulation 1998981116–1124.et al [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Myocardial perfusion in Group 1 and Group 2 patients. 81 patients (66 in group 1 and 15 in group 2) had complete baseline and 3 month follow-up nuclear perfusion studies scores and 76 patients had complete data for the 6 month analysis (61 in group 1 and 15 in group 2). There were no differences between myocardial perfusion groups 1 and 2, both of which failed to show evidence of benefit with VEGF gene therapy. Indeed, the only significant difference between the VEGF treatment and placebo groups favored the control group (SDS, 3 months). Open bars = placebo; closed bars = VEGF plasmid; ¶ =0.05<p<0.1 vs. placebo.
Left ventricular ejection fraction (LVEF). There was a trend towards reduced LVEF at baseline in the patients randomized to VEGF plasmid DNA (closed bars) compared with saline (placebo, open bars), however, there was no significant change in either group from baseline to 3 months. ¶ =0.05<p<0.1 vs. placebo.
Regression analysis for troponin rise. Linear regression analysis was performed between the fold-increase in troponin (Tp) levels the day following the injection procedure and the change in summed stress score (SSS), summed defect score (SDS), summed reversibility score (SRS), and exercise treadmill time (ETT) from baseline to 3 months post injection. Placebo = red, closed symbol, solid line; VEGF = black, open symbols, dashed line. There were no significant correlations, although a trend towards an relationship between Tp rise and SSS or SDS was observed for VEGF-treated patients. However, this was driven in large part by a reduced perfusion (i.e. higher scores) in patients with low Tp values rather than any greater improvement in perfusion in those with presumed successful gene delivery.
Baseline characteristics.