Abstract
We report a phase I/II clinical trial in prostate cancer (PCa) using direct intraprostatic injection of a replication defective adenovirus vector (CTL102) encoding bacterial nitroreductase (NTR) in conjunction with systemic prodrug CB1954. One group of patients with localized PCa scheduled for radical prostatectomy received virus alone, prior to surgery, in a dose escalation to establish safety, tolerability, and NTR expression. A second group with local failure following primary treatment received virus plus prodrug to establish safety and tolerability. Based on acceptable safety data and indications of prostate-specific antigen (PSA) responses, an extended cohort received virus at a single dose level plus prodrug. The vector was well tolerated with minimal side effects, had a short half-life in the circulation, and stimulated a robust antibody response. Immunohistochemistry of resected prostate demonstrated NTR staining in tumor and glandular epithelium at all dose levels [5 × 1010–1 × 1012 virus particles (vp)]. A total of 19 patients received virus plus prodrug and 14 of these had a repeat treatment; minimal toxicity was observed and there was preliminary evidence of change in PSA kinetics, with an increase in the time to 10% PSA progression in 6 out of 18 patients at 6 months.
Introduction
Prostate cancer (PCa) is the most common cancer in western men.1 Although local therapy is frequently effective in localized disease, a significant proportion develops recurrence.2,3,4,5 Treatment options for patients with prostate-specific antigen (PSA) recurrence include hormone therapy, prostatectomy, cryotherapy, or radiotherapy. In the last 12 years, 86 PCa gene therapy clinical trials have been registered worldwide,6 of which 14 employed virus directed enzyme-prodrug therapy (VDEPT), the technique reported in this study.
We have previously described results in liver cancer with CTL102, an E1, E3-deleted replication-deficient human adenovirus serotype 5 vector, containing the Escherichia coli nfsB gene under control of the cytomegalovirus immediate early promoter.7,8,9 This gene encodes the enzyme nitroreductase (NTR; E.C.1.6.99.7), which converts the weak monofunctional alkylating agent CB1954 [5-(aziridin-1-yl)-2,4-dinitrobenzamide] to a highly potent bifunctional alkylating agent,10,11,12 producing cell cycle independent DNA interstrand crosslinking,11 particularly important in characteristically slow-growing PCa. The cell-permeable metabolite produced gives a powerful bystander effect13 in a variety of preclinical models, including the PC3 human PCa cell xenograft mouse model.14
Most previous studies of PCa-VDEPT have used an empirical dosing schedule for the virus injection. In this study, we sought to document the effect of virus dose, injection volume, and injection technique on biodistribution of injected CTL102 virus in a manner similar to that previously reported in liver tumors.9 The second stage of the trial was then informed by this distribution data and evaluated the clinical outcomes with combined virus and prodrug in locally recurrent disease. This comprised a standard phase I escalation of virus with fixed prodrug dose, followed by an extended early phase II using the maximum feasible virus dose. The primary clinical endpoint was toxicity. Secondary endpoints were virus and CB1954 pharmacokinetics and immune responses, degree of expression of NTR in the excised prostate (group 1), and outcome reporting of changes in PSA levels and kinetics (groups 2 and 3).
Results
Patients and safety profile
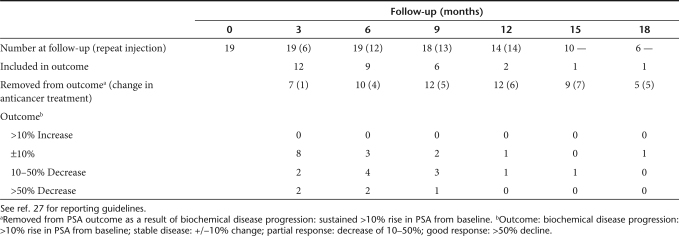
Patient characteristics and toxicity data are presented in Table 1. A total of 20 patients received the CTL102 virus alone (group 1) and 19 patients had virus plus prodrug (groups 2 and 3), of whom 14 received a second cycle of virus plus prodrug for a median of 3 months (range 2–11) after the first cycle. In groups 2 and 3, mean PSA at time of treatment was 12 ng/ml (range 1–52) and median PSA doubling time was 7 months (range 1–53) at study entry. One patient from group 1 had a potential dose limiting toxicity (DLT) (transient grade 3 rise in bilirubin); this was subsequently attributed to a postoperative myocardial infarction. The cohort was expanded, and there were no VDEPT related serious adverse events. The median follow-up was 14.5 months for group 1 (range 1.8–53.0) and 15.1 months for groups 2 and 3 (range 6.1–31.8).
Table 1.
Demographics and toxicity outcomes
Dose escalation to 1 × 1012 virus particles (vp) was completed with only minor transient toxicities: four patients (group 1) and six patients (groups 2 and 3) had grade 2 transient elevation of hepatic transaminases at week 1 following radical prostatectomy or prodrug administration, respectively. One patient suffered grade 3 amnesia 72 hours post-prodrug on his second treatment cycle. No cause was ascertained despite extensive clinical, imaging, and laboratory investigations. One patient had grade 3 pyrexia and a further eight patients had asymptomatic grade 1 pyrexia (=38.5 °C) 4–8 hours after virus injection. Two-thirds of the patients had transient lymphopaenia (1 grade 4, 9 grade 3, 5 grade 2, and 12 grade 1) at day 1 post-virus injection. One patient experienced grade 2 diarrhea after the first injection and was treated with intravenous fluids. This was not observed on the second injection when he was treated at a lower virus dose.
Virus shedding and virus DNA kinetics
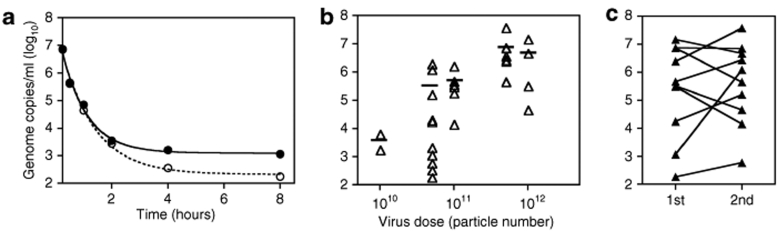
Enzyme-linked immunosorbent assay (ELISA) for adenovirus proteins in plasma, throat swab, urine, and stool found no detectable virus at any dose 24 hours postinjection of CTL102. In contrast, CTL102-specific quantitative PCR detected vector DNA in the blood within 30 minutes following intraprostatic injection in 31/34 treatments tested, declining to undetectable levels by 8–24 hours. An example for one patient (34XX) who received two doses of 5 × 1011 vp is shown in Figure 1a. In two patients (06P and 08P) where no virus DNA was detected, we also observed that the injected fluid had back-tracked down the injection port and that there was no prostate NTR staining. The correlation between dose of CTL102 and level of detectable vector DNA in blood was not statistically significant, although there was indication of an increase in peak concentration with dose (Figure 1b). In some patients who received two treatments there was more virus in the blood after re-treatment, but in others the peak level was the same or lower (Figure 1c). Virus DNA was detected in urine at the first void after virus injection in 13/23 samples tested, and in only five of these at 24 hours. No virus DNA was detected in urine of the two patients whose injection had backtracked.
Figure 1.
CTL102 DNA clearance from peripheral circulation. Virus DNA extracted from whole blood prior to and at intervals after virus injection was analyzed by Taqman quantitative PCR. (a) Example of DNA clearance after injection of 5 × 1011 virus particles (vp); open circles, first treatment; closed circles, re-treatment. (b) Peak DNA concentration immediately following virus injection; bar, mean concentration. (c) Peak DNA concentrations after first and second treatments.
Antibody responses to CTL102
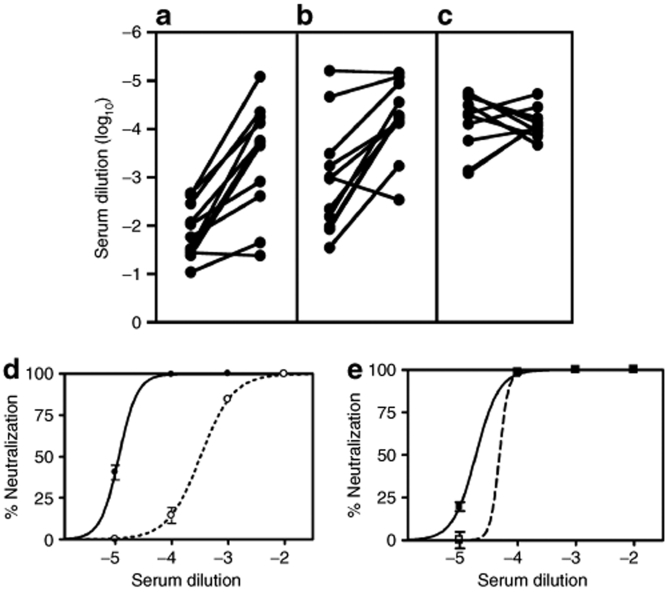
Most patients had detectable antibodies to adenovirus prior to injection of CTL102. An initial rise in antibody titer 7–14 days post-treatment was followed by plateau at 14–32 days in all patients. Anti-NTR antibody assay showed that most patients had a detectable, albeit low, pretreatment response that increased after exposure to CTL102. However, there was no clear correlation with virus dose, preexisting antibody levels, or virus DNA detection in blood. There were no changes in the level of control influenza A antibodies, confirming treatment specificity. The type-specific neutralizing antibody response to adenovirus type 5 showed a trend toward higher titers with increasing dose (not shown) and a maximal response after a single treatment; re-treatment did not result in a further rise in neutralizing titer (Figure 2a–c). Figure 2d,e illustrates a typical response in a patient receiving re-treatment.
Figure 2.
Neutralizing antibody responses to CTL102. Plasma samples were collected pretreatment and at weekly and monthly intervals following treatment. The plasma dilution required to give 50% neutralization was measured, and the pretreatment and peak response titers are shown: (a) group 1, (b) groups 2 and 3 after first treatment, (c) groups 2 and 3 after second treatment. (d,e) Example of titrations for patient (31XX) treated with 1012 virus particles at first treatment (d) and second treatment (e). Pretreatment, dotted line; post-treatment, solid line.
Immunohistochemical analysis of resected prostates
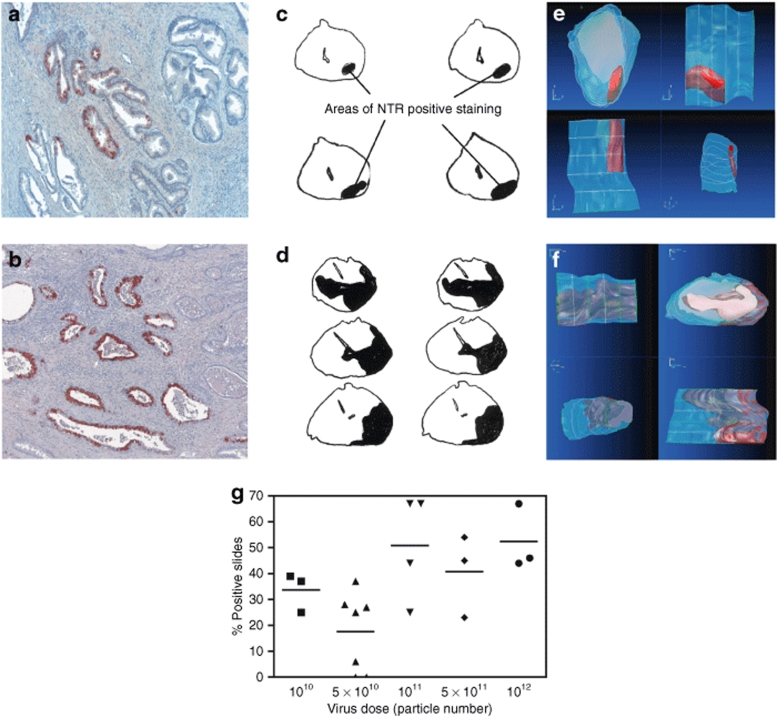
Immunohistochemical staining for NTR in resected tumors detected transgene expression at all dose levels. Staining was predominantly localized to the duct linings in tumor and benign tissue (Figure 3a,b). The initial injection volume of 260 µl produced a relatively small stained volume (Figure 3c,e), and when volumes were subsequently increased, there was an increase in the extent of staining (Figure 3d,f).
Figure 3.
NTR expression. (a,b) Sections of prostate were taken at radical prostatectomy, stained with a polyclonal sheep anti-NTR antibody, and counterstained with Mayer's hematoxylin. Sequential slices at intervals of 100 µm from whole mount sections from a patient treated with (c) 5 × 1010 virus particles in 260 µl; (d) 5 × 1011 virus particles in 1,250 µl. Extent of positive staining across whole mount sections was assessed by eye and outlined. Images of the cross-sections were digitized and reconstructed into 3D volumetric representations (e,f) corresponding to the images in c and d. (g) Quantitation of NTR staining: Slides stained positive with a sheep anti-NTR polyclonal antiserum, as a percentage of the total number of slides. Each symbol represents the value for an individual patient; total slide number varied with the size of the excised prostate (mean 36, range 6–92).
Even with the largest injection volume (1,250 µl), <50% of the prostate stained for NTR, prompting a switch to four injections each of 430 µl for patients of groups 2 and 3. Injection techniques were also modified to improve virus delivery (Table 1), which had a confounding effect on the degree of NTR expression. In two patients (06P and 08P), where a larger bore needle (20 gauge [20G]) was used, virus suspension was observed to backtrack as the needle was withdrawn, and there was no evidence of NTR staining in the resected prostate.
Figure 3g summarizes the extent of NTR expression seen with the differing dose levels; overall the relationship was not statistically significant. However, as noted earlier, a number of changes were made during the trial to injection technique. On the basis of the lack of clear benefit from increased dose in group 1 and manufacturing constraints for the 1 × 1012 dose level, 5 × 1011 was used for group 3.
CB1954 pharmacokinetics
In the majority of patients, the peak concentration of CB1954 in the plasma was detected at the earliest time point (6 minutes) and had cleared by 4–6 hours, with biphasic exponential kinetics. The mean peak concentration was 7.8 µmol/l (SD 3.1, range 2.6–16.8), with an area under the curve of 6.3 µmol/l hours (SD 2.6, range 2.5–13.0); there was no correlation between either parameter and virus dose. The range of values is similar to that reported in our trial of CB1954 alone.15
Analysis of PSA responses
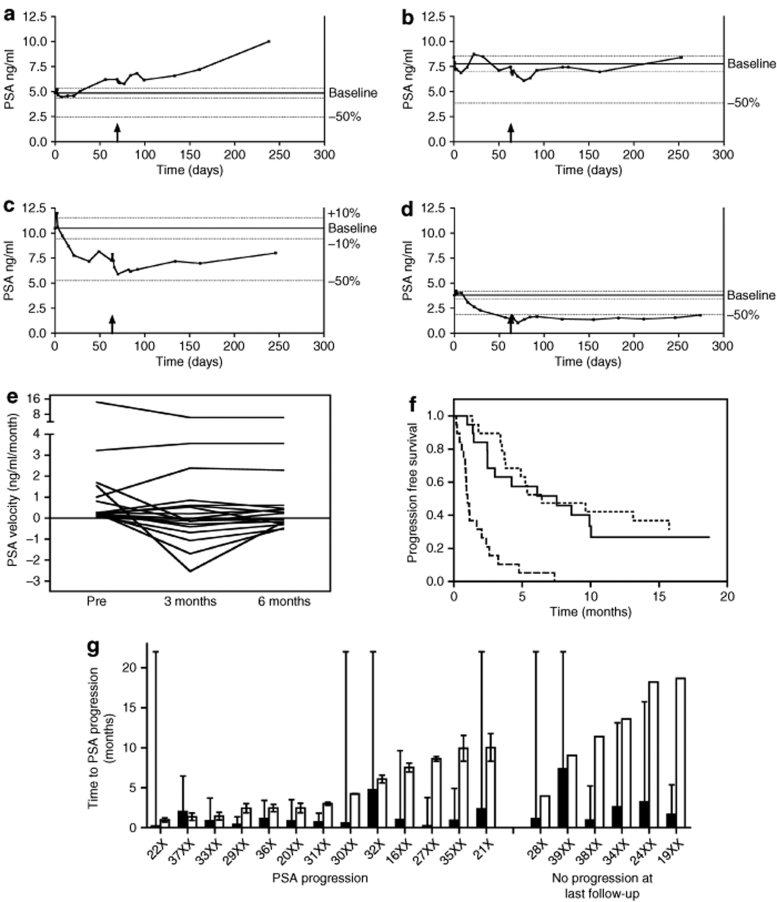
Median follow-up for groups 2 and 3 was 15.1 months (range 6.1–31.8). PSA outcomes are summarized in Table 2 following the recommendations of the PSA Working Group.27 All 19 patients had rising PSA at trial entry, with a median doubling time (PSA-DT) of 7 months. PSA progression was defined conservatively as >10% rise in PSA from baseline (excluding transient rises attributable to prostate injection, biopsy, or other known causes). Typical PSA curves are illustrated in Figure 4a–d. Three patients had rising PSA throughout the therapy (e.g., Figure 4a). PSA levels fell in some patients following treatment, with five patients showing 10–50% decrease, and two showing >50% decrease at 1 month. The mean decline in PSA at nadir after treatment was 23.2% (range 1.2–72.4) and the median time to PSA nadir was 2.4 months (range 1.2–8.1). This was reflected in changes in PSA velocity (Figure 4e), with 11 and 9 patients showing negative velocity (and PSA-DT) at 3 and 6 months, respectively. PSA data for each patient are summarized in Supplementary Table S1.
Table 2.
PSA outcomes: groups 2 and 3
Figure 4.
PSA kinetics. Examples of PSA kinetics in patients classified at 6 months as showing (a) progressive disease (37XX), (b) stable disease (35XX), (c) partial response (34XX), (d) good response (38XX). Upward arrow, re-treatment. (e) PSA velocity at trial entry (baseline), and calculated over months 0–3 (3 months) and 0–6 (6 months). (f) Time to PSA progression: Comparison of observed time to PSA progression with predicted time, calculated using linear least squares regression analysis of pretreatment log PSA values: based on the fitted line (dashed) and the lower 95% confidence limit for the fitted line (dotted). (g) Time to PSA progression for individual patients: Filled bars show the predicted time to PSA progression for each patient (error bar; 95% confidence interval). Open bars (left side of figure) show observed time to PSA progression, taken as the midpoint between the time of PSA measurements before and after progression (error bars); or, (right side of figure), time of last PSA measurement in patients who did not progress on the study.
Figure 4g compares the observed time to PSA progression (TTP) for each patient with a predicted TTP based on regression modeling of their individual PSA kinetics before trial entry (see Materials and Methods). As shown by the error bars (95% confidence interval), there is wide variation in the confidence of these individual predictions; however, there is a clear trend for the actual TTP to be greater than that expected in the absence of treatment. The median TTP was 7.5 months (Figure 4f). The dashed line in Figure 4f shows the predicted TTPs for all the patients (median 0.9 months), the dotted line plots each of the 95% confidence intervals.
Effect of re-treatment
A total of 14 patients received re-treatment and of these five patients remained progression free at end of study (median time 11.4 months). At present, we cannot comment on the effectiveness of re-treatment but can state that it appears safe.
Discussion
This is the first clinical trial of the NTR/CB1954 enzyme/prodrug combination in cancer patients. We saw no DLT up to the maximum dose of 1 × 1012 vp. The only adverse events considered related to treatment were gastrointestinal symptoms, pain at the injection site, asymptomatic low-grade pyrexia, lymphopaenia, and transaminitis; all were transient and did not require additional treatment.
Most prostate gene therapy trials have employed direct intraprostatic injection of virus, with techniques varying from a single injection in 1 ml16 to multiple injections with 3D image guidance.17 These trials included data on viral shedding but not on distribution within the target tissue. Many studies have used gene therapy in a neoadjuvant or combination therapy setting to reduce metastatic potential and thereby improve overall outcome.18,19,20,21,22 Determining clinical efficacy is difficult without a randomized trial design, and a long follow-up is required to demonstrate treatment effect. One animal study used combination therapy to reduce the potential side effects of radiation therapy.20 A clinical trial of an adenovirus vector delivering wild-type p53 in patients scheduled for radical prostatectomy showed expression of adenovirus-encoded protein in tumor tissue at levels that produced clinical effects.23 No studies have been designed to evaluate the effects of different injection techniques on virus distribution.
As reported previously,17 virus DNA was detectable 15–30 minutes postinjection, indicating vector dissemination. Virus could disseminate via the prostatic vasculature, lymphatics, or urothelial route, and indeed there was evidence for viral DNA in the blood and urine, although it should be noted that no virus protein was detected by ELISA assay, indicating the absence of infectious virus. There was no correlation between injection protocol and detection of viral DNA in blood.
Repeat injections of a replication defective adenovirus have been used in neoadjuvant trials without ill effect23,24 and other studies have demonstrated that anti-adenovirus antibodies do not compromise antitumor effects in a suicide gene therapy setting.21,25 CTL102 injection provoked anti-adenovirus antibody responses (Figure 2), which may provide a level of safety against systemic exposure to the virus but may also be an obstacle to repeated administration.
There is no established delivery strategy for intraprostatic gene therapy. One recent study could not demonstrate improved transgene expression with increasing injection numbers with a constant total volume, attributing this to factors such as tumor burden and multicentricity.26 During our study, a number of changes were made to the injection technique: needle diameter (a larger bore needle proved unhelpful as virus leaked along the needle track, with no evidence of virus staining in the resected prostate); injection number (single injections resulted in staining of a small region of the total prostate, leading to a switch to four injections); injection volume (to allow multiple injections a larger total volume was required on practical grounds); injecting surgeon (two hospitals, four operators). It is thus difficult to draw firm conclusions about the effect of dosing regimen on extent of immunostaining. However, we can make recommendations about needle bore (22G): injection volume (larger volumes increase the proportion of prostate stained); injection number (four better than one).
The long clinical course of PCa makes it difficult to use survival or clinical disease progression as endpoints in clinical trials, however PSA is widely regarded as a useful surrogate marker for disease burden and response to treatment. As a phase I/II trial, the primary objective of this study was to determine the safety and tolerability of the treatment, however as is common in such studies, secondary endpoints included evidence of antitumor activity as indicated by change in PSA kinetics. We have adhered to the PSA working party consensus guidelines, in reporting outcomes at different times throughout the follow-up.27 Several patients experienced falls in PSA of >10%, and in two cases of >50% following treatment, implying commensurate reductions in tumor burden; these reductions could be sustained for >6 months (Table 2, Figure 4c,d and Supplementary Table S1). Additional evidence of antitumor activity is provided by analysis of the time to PSA progression; overall the median TTP (10% rise in PSA) was 7.5 months; comparison with the PSA doubling time (i.e., 100% rise) at trial entry (median 7 months) or with the predicted TTP (median 0.9 months; Figure 4f) suggests that the treatment may have delayed PSA progression in at least some of the patients, as also suggested by the patient-specific comparisons (Figure 4g). However, these analyses should be regarded as exploratory, to provide information regarding biological effects of the treatment rather than clinical benefit. A randomized phase III clinical trial would be required to determine statistically whether these observations could translate to a worthwhile level of clinical benefit.
Locally relapsed PCa is a major clinical problem and currently used therapies all have significant morbidity. VDEPT is an attractive alternative as it has shown consistently low toxicity in a range of clinical trials, including this one. This study shows encouraging evidence of PSA responses from a single treatment and also shows that re-treatment was safe. However, the greatest reduction in PSA was only 72%, and only 7/19 patients showed >10% reduction in PSA. We conclude that further development of the system is warranted; improvements in virus delivery (using brachytherapy-style multifocal injection) and repeat treatment more closely scheduled (e.g., monthly to mirror conventional chemotherapy) may produce further antitumor activity and would be unlikely to cause excessive toxicity. Additional modifications to the system, either by increasing the catalytic activity of NTR by targeted mutations or using alternative prodrugs28,29 or the coexpression of immunostimulatory genes such as GM-CSF30 are planned for the future. With further clinical development, we believe VDEPT can become a new therapeutic option for patients with localized PCa.
Materials and Methods
Trial design. The trial was designed with three groups. Group 1 comprised patients with early PCa scheduled for radical prostatectomy. They received intraprostatic CTL102 prior to prostatectomy, in a phase I dose escalation scheme, to establish tolerability and safety of virus alone and to assess virus biodistribution within the prostate. The study design required demonstration of significant NTR expression, assessed by immunohistochemistry of the resected prostate, to trigger the second group. Group 2 comprised patients with rising PSA following primary treatment, and biopsy-confirmed local recurrence with no evidence of spread. They received intraprostatic CTL102 followed 48 hours later by intravenous CB1954 at 24 mg/m2. CB1954 dose was established in an earlier trial.15 Group 2 also followed a standard phase I design; a minimum of three patients treated per CTL102 dose level, with expansion to a maximum of six in the event of DLT. DLT using the NCI Common Toxicity Criteria version 2.0 was defined as: grade 2 renal or neurological toxicity; grade 2 hepatic toxicity lasting >3 weeks; grade 3 mucositis or diarrhea; or grade 4 hematological toxicity lasting >1 week. Group 3 comprised patients who were scheduled to receive the virus/prodrug combination either at the MTD or the maximal feasible dose, as defined in groups 1 and 2. If serum PSA levels were stabilized or reduced at 1 month after treatment, groups 2 and 3 patients were offered a repeat treatment at the same virus dose.
Patient selection. Generic inclusion criteria were life expectancy >3 months; World Health Organisation performance status 0–1; adequate hepatic, renal, and bone marrow function; normal blood clotting; no chemo/radiotherapy within 4 weeks and no concurrent corticosteroid use. Patients were tested for evidence of active adenovirus infection (plasma, throat swab, urine, and stool) in view of the theoretical risk of adenovirus complementation. Follow-up assessments were conducted weekly for 4 weeks, then monthly for 3 months, 3 monthly up to 1 year, and annually thereafter. The study protocol had ethical approval from the UK Gene Therapy Advisory Committee (055) and informed consent was obtained prior to enrollment.
Group-specific inclusion criteria. Group 1: biopsy-proven localized PCa awaiting radical prostatectomy. Groups 2 and 3: biopsy-proven locally recurrent PCa with rising PSA following primary treatment, with no spread on MRI. No change was made to the patient's hormone therapy, if already in place. Patients not on hormone therapy at study entry remained so until deemed clinically necessary.
Virus administration. The construction, manufacture, and characterization of CTL102 have been described.9 The virus stock was free of replication-competent adenovirus contamination and had a particle to infectivity ratio of 20:1. CTL102 was administered by direct intraprostatic injection under transrectal ultrasound guidance in 0.25–1.25 ml isotonic buffer as single or multiple injections using 20G or 22G Chiba or 24G Sprotte needles. Patients who had prior radical prostatectomy (24XX and 31XX) received virus to the prostate bed under transrectal ultrasound guidance, with a 22G Chiba needle.
Patients received prophylactic ciprofloxacin before injection and for 3 days thereafter. The procedure was performed in a purpose-built gene therapy isolation suite and reverse barrier nursing was practised until the absence of virus shedding was confirmed. Virus injection (day 0) was followed by radical prostatectomy at day 2–5 (group 1) or intravenous CB1954 24 mg/m2 at day 2 (groups 2 and 3). Dose escalation (vp) was as follows: 1 × 1010, 5 × 1010, 1 × 1011, 5 × 1011, 1 × 1012. Patients were eligible for re-treatment if they had evidence of disease stabilization or response (by PSA criteria) with no DLT.
Virus shedding and virus DNA kinetics. Virus shedding was assessed by ELISA for the presence of adenovirus proteins prior to injection and at 24 hour intervals. Blood was analyzed for vector DNA by quantitative PCR, as previously described.9 All urine samples up to 24 hours were analyzed by ELISA and PCR.
CB1954 administration and pharmacokinetics. CB1954 was formulated and administered at 24 mg/m2 by intravenous infusion over 5 minutes in 100 ml saline, 48 hours after virus injection. CB1954 pharmacokinetics were assessed as previously described.15,31
Immune responses. Plasma samples were collected pretreatment and at intervals up to 3 months post-treatment, and analyzed by ELISA to quantify total immunoglobulin response against adenovirus, NTR, and influenza A. Neutralizing activity against adenovirus type 5 was tested using an E1/E3-deleted adenovirus type 5 vector expressing β-galactosidase.9,32
Histological assessment of resected prostate specimens. In group 1 whole-mount blocks were prepared according to standard pathology practice. Sections were immunostained using sheep polyclonal antiserum raised against recombinant NTR.9 The proportion of sections demonstrating NTR staining was determined.
PSA kinetics. Serum PSA levels were recorded at each clinic visit, and PSA kinetics determined.27,33 Patients were categorized according to the PSA consensus meeting criteria.27 PSA progression was defined as a confirmed increase in PSA of 10% compared to the measurement taken at the start of therapy (day 0). Censoring was performed if any of the following criteria were met: PSA progression, any initiation/cessation of hormone therapy, any objective disease progression, or addition of any other form of therapy for PCa. In the absence of a control group, the effect of therapy on time to progression was estimated using linear least squares regression analysis of pretreatment PSA values. For each patient, ln (PSA) was modeled as a function of time, using all measurements taken after PSA nadir following primary therapy, and prior to start of the experimental therapy; pointwise 95% confidence limits around the regression line were constructed. This model was then used to predict time of progression for each patient, based on both the fitted line and its lower 95% confidence limit. Time to progression was measured from day 0, with censoring on the date of the last visit. Turnbull's method for interval censored data34 was used to estimate the cumulative probability of PSA progression.
SUPPLEMENTARY MATERIALTable S1. PSA measurements and analysis: groups 2 and 3.
Supplementary Material
PSA measurements and analysis: groups 2 and 3.
Acknowledgments
We thank the following colleagues for their assistance in the preclinical and clinical phases of this study: T. Chen, J. Doran, J. Simpson, V. Parnell, M. Horne, S. Hill, and M. Whitlock. We acknowledge financial support from Innovata plc, the Medical Research Council and Cancer Research, UK.
REFERENCES
- Parkin DM, Bray F, Ferlay J., and , Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- Garnick MB., and , Fair WR. Prostate cancer: emerging concepts. Part I. Ann Intern Med. 1996;125:118–125. doi: 10.7326/0003-4819-125-2-199607150-00008. [DOI] [PubMed] [Google Scholar]
- Moul JW. Prostate specific antigen only progression of prostate cancer. J Urol. 2000;163:1632–1642. [PubMed] [Google Scholar]
- Han M, Partin AW, Pound CR, Epstein JI., and , Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–565. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Journal of Gene Medicine 2006. Gene Therapy Clinical Trials Worldwide: Update 09/2006 < http://www.wiley.co.uk/genmed/clinical/ >.
- Weedon SJ, Green NK, McNeish IA, Gilligan MG, Mautner V, Wrighton CJ, et al. Sensitisation of human carcinoma cells to the prodrug CB1954 by adenovirus vector-mediated expression of E. coli nitroreductase. Int J Cancer. 2000;86:848–854. doi: 10.1002/(sici)1097-0215(20000615)86:6<848::aid-ijc14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Djeha AH, Thomson TA, Leung H, Searle PF, Young LS, Kerr DJ, et al. Combined adenovirus-mediated nitroreductase gene delivery and CB1954 treatment: a well-tolerated therapy for established solid tumors. Mol Ther. 2001;3:233–240. doi: 10.1006/mthe.2000.0250. [DOI] [PubMed] [Google Scholar]
- Palmer DH, Mautner V, Mirza D, Oliff S, Gerritsen W, van der Sijp JR, et al. Virus-directed enzyme prodrug therapy: intratumoral administration of a replication-deficient adenovirus encoding nitroreductase to patients with resectable liver cancer. J Clin Oncol. 2004;22:1546–1552. doi: 10.1200/JCO.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Anlezark GM, Melton RG, Sherwood RF, Coles B, Friedlos F., and , Knox RJ. The bioactivation of 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954)--I. Purification and properties of a nitroreductase enzyme from Escherichia coli--a potential enzyme for antibody-directed enzyme prodrug therapy (ADEPT) Biochem Pharmacol. 1992;44:2289–2295. doi: 10.1016/0006-2952(92)90671-5. [DOI] [PubMed] [Google Scholar]
- Friedlos F, Quinn J, Knox RJ., and , Roberts JJ. The properties of total adducts and interstrand crosslinks in the DNA of cells treated with CB 1954. Exceptional frequency and stability of the crosslink. Biochem Pharmacol. 1992;43:1249–1254. doi: 10.1016/0006-2952(92)90499-9. [DOI] [PubMed] [Google Scholar]
- Grove JI, Searle PF, Weedon SJ, Green NK, McNeish IA., and , Kerr DJ. Virus-directed enzyme prodrug therapy using CB1954. Anticancer Drug Des. 1999;14:461–472. [PubMed] [Google Scholar]
- Bridgewater JA, Knox RJ, Pitts JD, Collins MK., and , Springer CJ. The bystander effect of the nitroreductase/CB1954 enzyme/prodrug system is due to a cell-permeable metabolite. Hum Gene Ther. 1997;8:709–717. doi: 10.1089/hum.1997.8.6-709. [DOI] [PubMed] [Google Scholar]
- Djeha AH, Hulme A, Dexter MT, Mountain A, Young LS, Searle PF, et al. Expression of Escherichia coli B nitroreductase in established human tumor xenografts in mice results in potent antitumoral and bystander effects upon systemic administration of the prodrug CB1954. Cancer Gene Ther. 2000;7:721–731. doi: 10.1038/sj.cgt.7700171. [DOI] [PubMed] [Google Scholar]
- Chung-Faye G, Palmer D, Anderson D, Clark J, Downes M, Baddeley J, et al. Virus-directed, enzyme prodrug therapy with nitroimidazole reductase: a phase I and pharmacokinetic study of its prodrug, CB1954. Clin Cancer Res. 2001;7:2662–2668. [PubMed] [Google Scholar]
- Herman JR, Adler HL, Aguilar-Cordova E, Rojas-Martinez A, Woo S, Timme TL, et al. In situ gene therapy for adenocarcinoma of the prostate: a phase I clinical trial. Hum Gene Ther. 1999;10:1239–1249. doi: 10.1089/10430349950018229. [DOI] [PubMed] [Google Scholar]
- DeWeese TL, van der Poel H, Li S, Mikhak B, Drew R, Goemann M, et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–7472. [PubMed] [Google Scholar]
- Merritt JA, Roth JA., and , Logothetis CJ.Clinical evaluation of adenoviral-mediated p53 gene transfer: review of INGN 201 studies Semin Oncol 200128105–114.5 Suppl 16 [DOI] [PubMed] [Google Scholar]
- Teh BS, Ayala G, Aguilar L, Mai WY, Timme TL, Vlachaki MT, et al. Phase I-II trial evaluating combined intensity-modulated radiotherapy and in situ gene therapy with or without hormonal therapy in treatment of prostate cancer-interim report on PSA response and biopsy data. Int J Radiat Oncol Biol Phys. 2004;58:1520–1529. doi: 10.1016/j.ijrobp.2003.09.083. [DOI] [PubMed] [Google Scholar]
- Dilley J, Reddy S, Ko D, Nguyen N, Rojas G, Working P, et al. Oncolytic adenovirus CG7870 in combination with radiation demonstrates synergistic enhancements of antitumor efficacy without loss of specificity. Cancer Gene Ther. 2005;12:715–722. doi: 10.1038/sj.cgt.7700835. [DOI] [PubMed] [Google Scholar]
- van der Linden RR, Haagmans BL, Mongiat-Artus P, van Doornum GJ, Kraaij R, Kadmon D, et al. Virus specific immune responses after human neoadjuvant adenovirus-mediated suicide gene therapy for prostate cancer. Eur Urol. 2005;48:153–161. doi: 10.1016/j.eururo.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Tetzlaff MT, Teh BS, Timme TL, Fujita T, Satoh T, Tabata K, et al. Expanding the therapeutic index of radiation therapy by combining in situ gene therapy in the treatment of prostate cancer. Technol Cancer Res Treat. 2006;5:23–36. doi: 10.1177/153303460600500104. [DOI] [PubMed] [Google Scholar]
- Pisters LL, Pettaway CA, Troncoso P, McDonnell TJ, Stephens LC, Wood CG, et al. Evidence that transfer of functional p53 protein results in increased apoptosis in prostate cancer. Clin Cancer Res. 2004;10:2587–2593. doi: 10.1158/1078-0432.ccr-03-0388. [DOI] [PubMed] [Google Scholar]
- Shalev M, Kadmon D, Teh BS, Butler EB, Aguilar-Cordova E, Thompson TC, et al. Suicide gene therapy toxicity after multiple and repeat injections in patients with localized prostate cancer. J Urol. 2000;163:1747–1750. [PubMed] [Google Scholar]
- Freytag SO, Khil M, Stricker H, Peabody J, Menon M, DePeralta-Venturina M, et al. Phase I study of replication-competent adenovirus-mediated double suicide gene therapy for the treatment of locally recurrent prostate cancer. Cancer Res. 2002;62:4968–4976. [PubMed] [Google Scholar]
- Ayala G, Satoh T, Li R, Shalev M, Gdor Y, Aguilar-Cordova E, et al. Biological response determinants in HSV-tk + ganciclovir gene therapy for prostate cancer. Mol Ther. 2006;13:716–728. doi: 10.1016/j.ymthe.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Scher HI, Eisenberger M, D'Amico AV, Halabi S, Small EJ, Morris M, et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 2004;22:537–556. doi: 10.1200/JCO.2004.07.099. [DOI] [PubMed] [Google Scholar]
- Grove JI, Lovering AL, Guise C, Race PR, Wrighton CJ, White SA, et al. Generation of Escherichia coli nitroreductase mutants conferring improved cell sensitization to the prodrug CB1954. Cancer Res. 2003;63:5532–5537. [PubMed] [Google Scholar]
- Hu L, Yu C, Jiang Y, Han J, Li Z, Browne P, et al. Nitroaryl phosphoramides as novel prodrugs for E. coli nitroreductase activation in enzyme prodrug therapy. J Med Chem. 2003;46:4818–4821. doi: 10.1021/jm034133h. [DOI] [PubMed] [Google Scholar]
- Green NK, McNeish IA, Doshi R, Searle PF, Kerr DJ., and , Young LS. Immune enhancement of nitroreductase-induced cytotoxicity: studies using a bicistronic adenovirus vector. Int J Cancer. 2003;104:104–112. doi: 10.1002/ijc.10916. [DOI] [PubMed] [Google Scholar]
- Anderson D, Ferry DR, Knox RJ, Andrews SJ, Downes AJ, Kerr DJ, et al. High-performance liquid chromatographic method for sensitive determination of the alkylating agent CB1954 in human plasma. J Chromatogr B Biomed Sci Appl. 1999;731:293–298. doi: 10.1016/s0378-4347(99)00245-5. [DOI] [PubMed] [Google Scholar]
- Stallwood Y, Fisher KD, Gallimore PH., and , Mautner V. Neutralisation of adenovirus infectivity by ascitic fluid from ovarian cancer patients. Gene Ther. 2000;7:637–643. doi: 10.1038/sj.gt.3301152. [DOI] [PubMed] [Google Scholar]
- Cannon GM, Jr, Walsh PC, Partin AW., and , Pound CR.Prostate-specific antigen doubling time in the identification of patients at risk for progression after treatment and biochemical recurrence for prostate cancer Urology 2003622–8.(Suppl 1) [DOI] [PubMed] [Google Scholar]
- Turnbull BW. The empirical distribution function with arbitrarily grouped, censored and truncated data. J R Stat Soc. 1976;38:290–295. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PSA measurements and analysis: groups 2 and 3.