Abstract
Stem cell therapy presents an attractive approach to cure cystic fibrosis (CF) lung disease. We set out to investigate the effect of epithelial damage caused by Pseudomonas aeruginosa, a pathogenic bacterium widely occurring in CF, on the engraftment of bone marrow cells in airway epithelium. Intravenous or intratracheal administration of unfractionated green fluorescent protein (GFP+) bone marrow cells in P. aeruginosa-infected mice resulted in none or very few GFP+ cells detected in the lungs of the recipient mice, respectively. Only when GFP+ bone marrow cells were purified to obtain a cell suspension enriched in progenitor cells and injected intratracheally, significant numbers of GFP+ cells were detected. Localization of the donor cells at the level of airway epithelium was confirmed by Y-chromosome fluorescence in situ hybridization (FISH) analysis. All donor-derived Y-chromosome+ cells were found to express cytokeratin (CK). The fractions of GFP+ cells expressing CK were 0.34 and 0.76% for the 105 and 106 colony forming units (cfu) bacterial inoculums, respectively. When scored by Y-chromosome positivity these numbers were 0.60 and 1.12%, respectively. Our results show for the first time that tissue damage inflicted by bacteria like P. aeruginosa facilitates the airway engraftment of heterologous bone marrow–derived stem cells and their epithelial transformation.
Introduction
Adult bone marrow contains a variety of stem cell populations, including multipotent mesenchymal stromal cells,1 hematopoietic stem cells, side population cells, and multipotent adult progenitor cells.2 Several studies have demonstrated the ability of bone marrow–derived hematopoietic stem/progenitor cells to home to the lung and transform into epithelial cells.3,4,5,6,7 Evidence of this recruitment comes from human sex-mismatched lung transplants where tissue biopsies have shown pulmonary epithelial cells from the recipient contributing to the repair of donor lungs, implying that circulating progenitor epithelial cells do contribute to regeneration of the airway epithelium.8,9 This potential plasticity suggests that bone marrow cells could also be used as “cell replacement” therapy for pulmonary diseases, such as cystic fibrosis (CF), a lethal genetic condition characterized by accumulation of a thick mucus layer, which facilitates infection and colonization of the respiratory tract by opportunistic bacteria like Pseudomonas aeruginosa. P. aeruginosa is a Gram-negative pathogen that, after having been acquired from the environment, colonizes the respiratory tract in patients with predisposing conditions, like CF. These bacteria adapt to the CF lung microenvironment, being planktonic at the beginning but as chronic CF lung disease progresses, mucoid, alginate-overproducing strains emerge,10 followed by their organization in biofilms.11 Repeated cycles of infection lead to continuous inflammation, damage to the lung, and ultimately respiratory failure and death.12
Although controversial, some data have suggested the possible involvement of bone marrow–derived stem cells in lung repair following injury.3,13,14,15,16,17,18,19 Different methods have been used to induce marked damage to the respiratory epithelium and evidence has been presented that the extent of lung epithelial cell engraftment from bone marrow–derived cells is dependent on the degree and type of lung damage induced.20,21,22 However, lung injury models employed up till now rely on the use of physical and chemical agents, which might not be applicable to CF patients. For example, it is not unlikely that total body irradiation prior to bone marrow transplantation presents serious risks for CF patients chronically colonized by bacteria. The aim of our work was to establish the feasibility of repopulating damaged respiratory epithelium by isolated bone marrow–derived stem cells. In our model the damage to the airway epithelium was induced by acute infection of the lungs with P. aeruginosa. In this way, we could verify whether the lung damage typical for CF patients is sufficient to facilitate engraftment of bone marrow–derived cells in the lungs.
Results
Acute model of respiratory infection with P. aeruginosa injected into the lung via intratracheal instillation
To determine what quantity of bacteria will produce a marked level of damage to the respiratory epithelium while causing low or null mortality, we injected C57Bl/6 mice in the trachea with different amounts of P. aeruginosa and evaluated survival rates. We have established earlier that of the mice injected with 107 colony forming units (cfu) 25% survived, whereas animals exposed to bacterial doses of 105 and 106 cfu all stayed alive.23 The damage to the respiratory epithelium induced by P. aeruginosa was then evaluated using different methods.
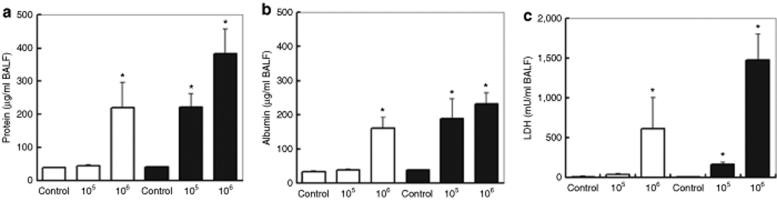
Figure 1a represents total protein concentrations in bronchioalveolar lavage fluid (BALF), an indicator of epithelial integrity. After 1 day of treatment, total protein concentration was increased only in the lungs of mice exposed to 106 cfu of bacteria. No change was observed in the lungs of mice exposed to the lower dose (105 cfu). After a 2-day treatment, total protein concentration further increased in the lungs exposed to 106 cfu. It was also substantially increased in the lungs of the mice exposed to the lower doses, reaching levels similar to those detected for 106 cfu after 1 day.
Figure 1.
Epithelial integrity and vascular permeabilty in the airways of mice infected with Pseudomonas aeruginosa. (a) Total protein, (b) albumin, and (c) lactate dehydrogenase (LDH) levels in bronchioalveolar lavage fluid (BALF) of control and treated animals. C57Bl/6 mice were inoculated with 105 or 106 cfu of P. aeruginosa. The mice were killed 24 (white bars) or 48 hours (black bars) postinstillation and their BALF was recovered. The graphs represent means ± SD. *P < 0.05 (versus control value); n = 5. cfu, colony forming units.
Albumin concentration in BALF, an indicator of vascular permeability, is shown in Figure 1b. Following the short incubation period, increased levels of albumin could only be detected for the inoculum of 106 cfu. However, extending the duration of the experiment up to 48 hours resulted in a significant increase in albumin concentration for both bacterial loads tested. We found significant levels of lactate dehydrogenase (LDH), an indicator of cytotoxicity, only when bacteria were inoculated at a dose of 106 cfu (Figure 1c). Evidently, cell damage already occurred 24 hours after bacterial inoculation and gradually increased further, reaching 1.5 units of LDH per millilitre of BALF after 48 hours.
Histological examination of the lungs of infected mice (48 hours) confirmed damage to the respiratory epithelium induced by P. aeruginosa (Figure 2). Histological evidence of extensive damage was observed on hematoxylin and eosin–stained lung sections from mice that were inoculated with P. aeruginosa at a dose of 106 cfu (Figure 2c). As shown in Figure 2b, bacteria injected at a dose of 105 cfu induced only local damage to the respiratory epithelium. In addition, we observed that in all animals P. aeruginosa-induced damage was limited to one or two lobes, whereas other lobes in the same mouse did not present any signs of injury. This likely reflects the limitation of the dosing volume. Yet, injection of larger volumes was associated with sharply increased mortality.
Figure 2.
Histological assessment of respiratory epithelial damage in the airways following infection with Pseudomonas aeruginosa. (a) Hematoxylin and eosin-stained lung section from control mice and (b,c) hematoxylin and eosin-stained lung sections from P. aeruginosa-infected mice. Bacteria injected at a dose of 105 cfu induced only local damage to the respiratory epithelium (b). Evidence of extensive damage can be observed on lung sections from mice inoculated with P. aeruginosa at a dose of 106 cfu (c). The mice were killed 48 hours postinstillation. Micrographs are representative of lung sections obtained from three animals per group. Staining was performed on 5–10 sections per lobe. Original Magnification ×20. cfu, colony forming units.
Transplantation and localization of donor bone marrow–derived cells
Histological and biochemical analysis of the lungs of mice exposed to P. aeruginosa indicate that our model fulfills requirements needed for transplantation experiments. Therefore, we injected C57Bl/6 mice intratracheally with P. aeruginosa and 2 days later freshly isolated bone marrow cells from green fluorescent protein (GFP+) mice were injected intravenously into the tail vein of the infected animals. The mice were killed 3, 5, 7, or 14 days later and lung sections were screened for GFP+ cells. No GFP+ cells were detectable under those conditions. Therefore, we decided to explore another approach. Instead of injecting the isolated bone marrow cells intravenously, we injected them directly into the trachea. First, C57Bl/6 mice were intratracheally infected with P. aeruginosa. Two days later bone marrow–derived cells, freshly isolated from GFP+ animals, were injected directly into the trachea of the infected animals. The mice were killed 7 or 14 days later and lung sections were screened for GFP+ cells. Although we were able to detect GFP+ cells in the lungs of the animals infected with P. aeruginosa, their number was very small.
In the bone marrow the percentage of stem/progenitor cells oscillates from 0.01 to 0.1%. In an attempt to further improve our system we used a product provided by Stem Cell Technology called EasySep. Briefly, the EasySep system is a negative selection procedure in which unwanted cells are labeled and removed from the suspension, leaving the desired cells unaltered. The kit allows labeling and removing of mouse cells of hematopoietic origin (expressing CD5, CD11B, CD19, CD45R, Ly-6G(GR1), TER119, 7-4). FACS analysis normally confirms that ~20% of lineage minus cells is positive for Sca-1. Thus, a cell suspension can be obtained, which is enriched in progenitor cells. This suspension was injected intratracheally in P. aeruginosa-infected mice. The mice were killed after 6 weeks and the lung sections were screened for GFP+ cells. GFP-expressing cells were readily detected in the lungs of the surviving mice inoculated with P. aeruginosa at a dose of 106 cfu (Figure 3). Only four of the seven mice infected with this large inoculum survived the strenuous double surgery. In general, we observed that in all animals GFP+ cells were limited to one lobe, compatible with our observation that injury was usually also limited to one or two lobes. No GFP+ cells were detected in the lungs of noninfected mice.
Figure 3.
Detection of donor-derived GFP+ cells in mouse lung sections. Recipient C57Bl/6 mice were inoculated with Pseudomonas aeruginosa at a dose of 106 cfu. 100 µl of PBS was instilled into the trachea of control mice. Adult transgenic C57Bl/6 mice constitutively expressing GFP were used as donors. Bone marrow cells isolated from these mice were enriched in progenitor cells by means of the EasySep system. The cell suspension was injected intratracheally in mice infected with P. aeruginosa 2 days earlier. The mice were killed after 6 weeks and the lung sections were screened for GFP+ cells. The presence of donor-derived GFP+ cells was evaluated by immunochemistry with an anti-GFP antibody and using avidin-biotin-peroxidase detection system. GFP-expressing cells were readily detected in the lungs of the surviving mice inoculated with P. aeruginosa at a dose of 106 cfu. (a) Control mice (PBS + GFP) and (b) treated mice (bacteria + GFP). Arrows indicate donor-derived GFP+ cells. Micrographs are representative of lung sections obtained from three animals per group. Staining was performed on 10–15 sections per lobe. Original Magnification ×40. cfu, colony forming units; GFP, green fluorescent protein; PBS, phosphate buffered saline.
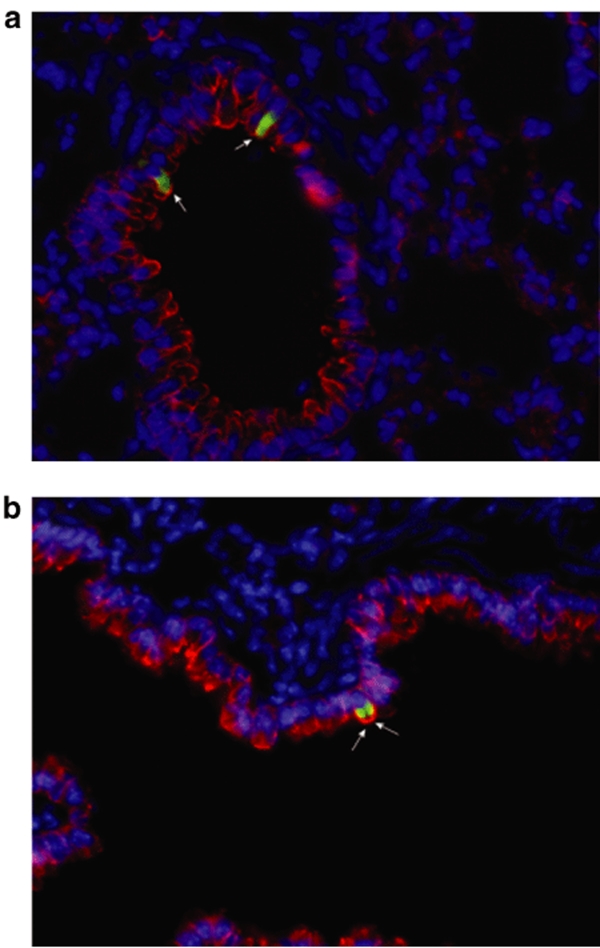
Lung tissue from each transplanted mouse was then examined using a combination of direct fluorescence to visualize GFP-expressing cells and immunofluorescence for pan-cytokeratins (CK) to identify epithelial cells. As shown in Figure 4, donor-derived GFP+ cells were located in bronchial/bronchiolar lining and expressed CKs, indicating that these are cells of an epithelial nature. Moreover, GFP+ cells never stained positively for CD45. CD45 expression was restricted to infiltrating leukocytes (data not shown).
Figure 4.
Donor-derived GFP+ cells are located in the bronchial/bronchiolar lining and express cytokeratins (CKs). (a,b) Presence of donor-derived GFP+/CK+ cells evaluated by direct GFP fluorescence and immunofluorescence in Pseudomonas aeruginosa-infected mice. Recipient C57Bl/6 mice were inoculated with P. aeruginosa at a dose of 106 cfu. Adult transgenic mice C57Bl/6 mice constitutively expressing GFP were used as donors. Bone marrow cells isolated from these mice were enriched in progenitor cells by means of the EasySep system. The cell suspension was injected intratracheally in mice infected with P. aeruginosa 2 days earlier. The mice were killed after 6 weeks and the lung sections were screened for GFP+ cells and CK+ cells. Donor-derived GFP+ cells (green) located in the bronchial lining expressed cytokeratins (red), which indicates that these cells are of an epithelial nature. Nuclei are stained with DAPI. Micrographs are representative of lung sections obtained from three animals per group. Staining was performed on 8–15 sections per lobe. Original magnification ×40. cfu, colony forming units; DAPI, 4′-6-Diamidino-2-phenylindole; GFP, green fluorescent protein.
To further substantiate these observations, bone marrow transplant donors and recipients were sex mismatched so that fluorescence in situ hybridization (FISH) for the Y-chromosome could be used to confirm the presence of donor-derived cells in the lung. To that end, we isolated bone marrow cells from male C57Bl/6 mice and injected them after the EasySep purification step into the trachea of female mice infected with P. aeruginosa at a dose of 106 cfu. Y-chromosome FISH analysis of lung sections showed the presence of donor cells in the lung epithelium of four out of five surviving mice (five out of nine animals survived the double surgery). Figure 5 demonstrates lung sections stained for Y-chromosome FISH and then subjected to immunohistochemistry for the pan-CK epithelial cell marker. Donor-derived Y-chromosome+ cells were found to express CKs (Figure 5a–f). Confocal microscopy analysis confirmed that the Y-chromosome signal was located within nuclei of airway epithelial cells (Figure 5g,h) and in CK-expressing cells (Figure 5i). We did not find any Y-chromosome+ cells expressing the CD45 hematopoietic cell marker. In all animals Y-chromosome+ cells were limited to one lobe.
Figure 5.
Donor-derived Y-chromosome+ cells are embedded in the basal and columnar layers of the respiratory epithelium. Recipient female C57Bl/6 mice were inoculated with Pseudomonas aeruginosa at a dose of 106 cfu. Male C57Bl/6 mice were used as donors. Bone marrow cells isolated from these mice were enriched in progenitor cells using the EasySep system. The cell suspension was injected intratracheally in mice infected with P. aeruginosa 2 days earlier. The mice were killed after 6 weeks and the lung sections were screened for Y-chromosome+ cells and cytokeratin (CK+) cells. (a) Y-chromosome+ cells (green) that are CK+ (red). (b,c) Enlarged micrographs of image a. (d,f) Other examples of Y-chromosome+ cells that express CKs. Arrows indicate donor-derived CK+ cells. (g–i) Images were taken at a confocal laser scanning microscope; g and h show Y-chromosome+ cells, (i) Y-chromosome+ cells (green) that are CK+ (red). Note that donor-derived cells are present in the basal and columnar layers of the respiratory epithelium. Micrographs are representative of lung sections obtained from four animals. Original magnification ×63, except b, c, h, and i (×100). cfu, colony forming units.
Assessment of engraftment levels 6 weeks after intratracheal transplantation
Table 1 presents the frequency of donor-derived epithelial cells 6 weeks after intratracheal transplantation of a cell suspension enriched in progenitor cells by means of EasySep negative selection. As donor-derived cells were never found CD45+, donor-derived epithelial cells were effectively scored by Y-chromosome+/CK+ or by GFP+/CK+ double immunofluorescence. No donor-derived epithelial cells were found in the lungs of control mice, i.e., mice transplanted with bone marrow–derived cells but not infected with P. aeruginosa. The percentages of GFP+ donor–derived cells expressing CK were 0.34 and 0.76 for the inoculum of 105 cfu and 106 cfu, respectively. The numbers were slightly higher when engraftment was assessed by scoring Y-chromosome+/CK+ cells: 0.6 and 1.12%, respectively.
Table 1.
Assessment of engraftment levels 6 weeks after intratracheal transplantation
Discussion
The ultimate goal of CF gene therapy would be to repopulate the respiratory epithelium with cells demonstrating normal cystic fibrosis transmembrane conductance regulator (CFTR) activity. The potential of adult bone marrow cells to home to the airways could provide an attractive therapeutic approach for such repair of pulmonary function. It has been demonstrated that hematopoietic stem cells are able to give rise to lung epithelial cells.3,13,14,21,24 Moreover, it has been shown that lung injury plays a critical role in the engraftment of bone marrow–derived cells into the airway epithelium. Taking into account the course of CF lung disease, usually leading to serious damage caused by infiltrating bacteria, one could hypothesize that this damage in CF patients might be sufficient to facilitate the engraftment of bone marrow cells in the respiratory epithelium. Therefore, in our experimental model we induced some damage to the airway epithelium by infecting mice with the bacterial strain that is usually abundantly present in CF patients, P. aeruginosa. Although the predominant form of P. aeruginosa found in CF patients is mucoid,25 the nonmucoid planktonic form is initially acquired from the environment and colonizes the CF airways, which then predisposes to its adaptation with the secretion of alginate polysaccharides.10 Thus, the acute infection model used in this study mimics the very early phases of host–pathogen interactions.
In our model, controlled damage was achieved by instillation of sublethal doses of P. aeruginosa followed by assessment of different parameters of lung damage. Upon intravenous injection of GFP+ freshly isolated bone marrow–derived cells into P. aeruginosa-infected mice virtually no GFP+ cells were detected in the lungs of the recipient mice. Although initial studies reported high levels of engraftment of bone marrow cells into the lung,3,5 current opinion indicates that only a very small proportion, i.e., <0.01–0.025% of lung epithelial cells, is derived from bone marrow–derived cells.26 Although this could be due to technical problems, it also might be that cells representing whole bone marrow or selected populations thereof encounter intrinsic hurdles in engrafting the lung and transforming into epithelial cells. Our data are difficult to compare with those reported earlier by others, due to differences in the bone marrow population injected intravenously, in the experimental design and in the various techniques to determine treatment efficacy. CD34+ lin−,3,5 plastic-adherent mesenchymal stromal cells,13,16,17,18,19,27 side population cells,14,15 and multipotent adult progenitor cells28 were shown to settle in the lung and give rise to epithelial cells. It must be stressed that in recent work the plasticity of bone marrow cells as well as the engraftment potential of adult-derived stem cells in the lung has been questioned.29
Many of the cited studies have reported engraftment of bone marrow cells into the alveolar region of the lung, whereas our cell target was the bronchial lining of the conducting airways. Krause et al. demonstrated that freshly isolated Sca1+lin− cells were able to achieve lung engraftment as bronchial epithelial cells and type II pneumocytes in irradiated recipient mice.3 The authors observed that engraftment followed two patterns: large-scale repopulation was observed in organs in which X-ray irradiation had caused marked injury (lungs and liver), whereas low-level engraftment was seen in the absence of marked injury (skin and gastrointestinal tract). In later reports by the same group the proportion of bone marrow–derived pneumocytes was <1%.21,24 This discrepancy is explained by the fact that in their more recent papers these authors performed additional analyses to assure the absence of false-positives. MacPherson et al. injected the bone marrow–derived side population cells from ROSA26 mice (constitutively expressing β-galactosidase) into irradiated hosts before polidocanol treatment.14,15 They demonstrated that in mice engrafted with side population cells, donor-derived cells are present in the epithelial lining of the trachea following damage and repair. Donor-derived cells (Y-chromosome+) were found at a frequency of 0.6–1.5%.
Our data demonstrate the presence of significant numbers of GFP+ cells in the lung epithelium, only when the bone marrow cells were first purified to yield a cell suspension enriched in progenitor cells and then injected intratracheally. In control mice, not infected with P. aeruginosa, no GFP+ cells were observed in the lungs. These observations prove that lung injury plays a key role in the engraftment of bone marrow–derived cells into this organ. The requirement of damage for the establishment of pulmonary engraftment was pointed out by several groups. The animal model employed and the type of damage determined at the epithelial level could be other variables. Circulating blood cells contribute to the repair of LPS-,30,31 elastase-,32,33 irradiation-,4,5 naphthalene-,34 or bleomycin-mediated17,18 acute lung injury, thus preventing pathological consequences including emphysema or fibrosis.
The number of donor-derived epithelial cells assessed by scoring Y-chromosome+ cells was slightly higher than when evaluated by direct GFP fluorescence (0.76% versus 1.12% for the inoculum of 106 cfu). This could be the result of inactivation or downregulation of reporter gene in donor cells when they differentiate into specific types of lung epithelial cells (e.g., Clara cells or type I pneumocytes). This appears to apply also to other transgenes. It has been reported that expression of the β-galactosidase transgene in lungs was not evident in cells that could be identified as donor-derived on the basis of Y-chromosome FISH analysis.15 Also FISH analysis for Y-chromosomes is not without limitations. The fraction of cells in the control male section that stains positively for Y-chromosome by FISH analysis never exceeds 90% (our observations and refs. 14,20).
Intratracheal administration of stem cells has been reported earlier.7,34,35,36 The data published by Baber et al.35 show that intratracheal administration of mesenchymal stromal cells reduced pulmonary arterial pressure and pulmonary vascular resistance in rats with monocrotaline-induced pulmonary hypertension. The transplanted cells survived in the lung parenchyma of these animals for at least 21 days and retained smooth muscle and endothelial cell markers. Serikov et al. injected bone marrow–derived mesenchymal-enriched stromal cells intratracheally in sublethally irradiated mice and observed significant engraftment as compared to animals not treated with naphthalene.7 GFP+ cells were present in lung parenchyma and epithelium of conducting airways at 2–6 days after naphthalene treatment, whereas at day 30, GFP+ cell patches in the epithelium were very rare. Finally, Wong et al. reported recently on a plastic-adherent bone marrow population expressing Ccsp, a marker shown to identify endogenous pulmonary progenitor and stem cells, while also expressing both mesenchymal and hematopoietic markers.34,36 Ccsp+ cells preferentially engrafted to naphthalene-damaged airways when delivered transtracheally or intravenously, the former being a more efficient route. Interestingly, our data are in good agreement with studies reporting GFP+ cells only upon intratracheal administration.
As to the relevance of these data for CF, similar (i.e., low) levels of homing to and engraftment of wild-type Cftr bone marrow–derived cells into Cftr−/− mice were observed.37,38 Loi et al. found that administration of plastic-adherent stromal cells to naive nonirradiated mice resulted in the engraftment of donor-derived airway epithelial cells, although in small numbers only (~0.025%).37 The total fraction of chimeric lung epithelial cells exhibiting CFTR expression was only 0.01%. Bruscia et al. transplanted Cftr+/+ GFP+ bone marrow cells into Cftr−/− mice after applying different doses of irradiation.38 Like Loi et al., very low levels of engraftment (0.01–0.1%) were observed in the gut, correlating with very low CFTR mRNA expression. Surprisingly, the bioelectric profile of CF mice transplanted with wild-type bone marrow was significantly improved in both gut and nose compared to animals transplanted with bone marrow from CF mice. Bruscia et al. addressed the question whether the degree of bone marrow to epithelial engraftment would be higher if bone marrow transplantation were performed on 1-day-old mice, when tissues are undergoing rapid growth and remodeling.39 Bone marrow transplantation into newborn mice, regardless of the myeloablative regimen used, did not increase the number of bone marrow–derived epithelial cells over that which occurs after marrow transplantation into lethally irradiated adult mice. Nevertheless, partial restoration of CFTR channel activity in myeloablated newborn CF mice was also evident in this study. These results would imply that a very low level of cell therapy produced an amplified electrophysiological effect. Overall, these data recognize a very limited involvement of bone marrow cells in the appearance of epithelial cells in the lung of CF mice, and for this reason we have not performed such experiments in CF mice. Furthermore, it is well known that CF mice are fragile and they may not survive the double surgery procedure employed in this study.
Our data demonstrate that if bone marrow–derived cells are delivered to the lung by a nonintravenous route, they can acquire the characteristics of epithelial cells. Lung tissue is characterized by slow turnover and rapid repair. It is believed that under normal conditions there are ample resident stem cells in this organ to replace the constant loss of airway epithelial cells. This situation changes at the moment of airway injury. Under those conditions the resident stem cells might not be able to cope with the damage, allowing other stem cells to contribute to the repair process. Krause et al.,3 who first demonstrated that circulating hematopoietic stem cells have the ability to differentiate into cells of the gastrointestinal tract, lung, and skin, suggest that the local environment stimulates a gene expression pattern that causes a morphological change in the phenotype of the cell. More direct evidence comes from the work of MacPherson et al.14 who demonstrated that bone marrow–derived stem cells need specific factors present in the lung to allow them to obtain characteristics of this tissue. The authors demonstrated that despite being unable to contribute to the tracheal epithelium in air/liquid interface cultures, adult bone marrow–derived stem cells can contribute to the tracheal epithelium in vivo. Interestingly, tissue-specific transcription factors appear to be rare and there is increasing evidence suggesting that the same transcription factors present in different ratios induce different patterns of gene expression, thus causing hematopoietic stem cells to differentiate into different types.40,41
Our results demonstrate that intratracheally administered stem cells can differentiate into epithelial cells, particularly in damaged lung tissue. This may provide a promising basis for the development of a stem cell based therapeutic strategy to cure diseases like CF or other pulmonary diseases. As all the studies published up till now demonstrate that engraftment of bone marrow–derived stem cells in the airways is a very inefficient process, studies on the molecular basis governing the homing of circulating stem cells to the lung will have to be performed. The mechanisms determining which circulating stem cells are recruited into injured organs are not fully understood. In a mouse model of sex-mismatched tracheal transplantation, Gomperts et al. demonstrated that a population of oriented progenitor cells expressing the epithelial marker CK5 and the chemokine receptor CXCR4 exists in the bone marrow and that CK5+ circulating progenitor epithelial cells contribute to reepithelialization of the airway and reestablishment of the pseudostratified epithelium.42 Depletion of CXCL12 prevents precursor recruitment and appropriate epithelial repair and favors squamous metaplasia. These findings demonstrate that CK5+CXCR4+ cells play a crucial role in the reepithelialization of tracheal transplants and that the CXCL12/CXCR4 axis is involved in epithelial precursor mobilization and recruitment at sites of injury. The same axis, as well as that based on the CCR2/CCL12 one, is also thought to play an important role in the recruitment and homing of fibrocytes to the lung and in bleocmycin-induced fibrosis.43 HGF induced mobilization of endothelial progenitor cells from bone marrow and alveolar angiogenesis in elastase-induced lung injury.44 Other cytokines and chemokines, such as colony stimulating factors and interleukin-8, can induce mobilization of bone marrow cells (reviewed in ref. 45), but those signals have not been investigated in the context of lung diseases so far.
Because the extent of airway epithelial remodeling with adult marrow stem cells is low and is presently questioned, future research should be focused on studying the potential of different populations of stem cells in repopulating lung tissue.46 Recent data have revealed the potential of cord blood-derived stem cells in this process. However, after systemic administration of human cord blood-derived mesenchymal stromal cells to immunotolerant nonobese diabetic–severe combined immunodeficiency mice, only rarely (0-32-1.51%) cells were found in the airway epithelium that had acquired CK expression.47
In summary, we have validated an acute model of airway infection for studying engraftment of bone marrow cells into the lung, with results similar to other lung injury models. More plastic stem cells should be investigated as source of airway epithelial cells for repairing lung injury or for “cell replacement” therapeutic strategies.
Materials and Methods
Materials. 2,2,2-tribromethanol and tert-amylalcohol (Avertin), ethylenediaminetetraacetic acid (EDTA), and SSC buffer (20×) were purchased from Sigma-Aldrich (St Louis, MO). All reagents were of analytical grade.
Bacterial strain. P. aeruginosa PAO1 bacteria48 were inoculated in Tryptic Soy Broth (Becton Dickinson, Heidelberg, Germany) and incubated overnight at 37 °C. Subsequently, bacteria were grown for 2 hours to reach log phase and diluted appropriately for inoculation.
Animals. Animal studies were conducted according to protocols approved by the San Raffaele Scientific Institute Animal Use Committee and adhered strictly to the Italian Ministry of Health guidelines for the use and care of experimental animals. Mice were housed in pathogen-free barrier facilities. Transgenic C57Bl/6 mice constitutively expressing GFP (C57Bl/6-Tg(ACTB-EGFP)) were purchased from the Jackson Laboratories (Bar Harbor, ME). Wild-type C57Bl/6 mice were purchased from Charles River Laboratories (Calco, Italy). Donor and recipient mice used in transplantation experiments were litter mates.
Bacterial infection of the airways. Bacterial infection of the airways was performed as described earlier.23 Briefly, mice were anesthetized by intraperitoneal injection of a solution of 2.5% Avertin (2,2,2-tribromethanol and tert-amylalcohol) in 0.9% NaCl and administered in a volume of 0.015 ml/g body weight. The trachea was exposed by ventral midline incision and intubated with a sterile, flexible 22-gauge catheter (Becton Dickinson, Heidelberg, Germany) connected with a 1-ml syringe. A 100-µl inoculum of a bacterial suspension of the laboratory strain of P. aeruginosa PAO1 was instilled into the trachea. 100 µl of phosphate buffered saline (PBS) was instilled into the trachea of control mice.
BALF recovery. Mice were killed by cervical dislocation 24 or 48 hours postinstillation. A 22-gauge catheter attached to a 1-ml syringe was inserted into the trachea below the larynx. Ten 0.5-ml aliquots of 0.5 mmol/l EDTA solution in PBS were injected into the lungs, allowed to reside there for 10 seconds, aspired, and pooled. The fluid was centrifuged for 15 minutes at 4 °C and the supernatants were stored at −80 °C for later examination.
Analysis of total protein, albumin and LDH in BALF. Total protein concentration in BALF was quantified using the modified Lowry Method Protein Assay Kit (Sigma-Aldrich) according to the manufacturer's instructions. The procedure was preceded by trichloroacetic acid protein precipitation. Sample absorbance was recorded using a GeneQuant pro spectrophotometer at a wavelength of 600 nm.
The concentration of albumin was assayed using Sigma Diagnostic Albumin Reagent (Sigma-Aldrich), according to the manufacturer's instructions. Albumin, bound to bromocresol green, produces a blue green color with an absorbance maximum at 628 nm.
The concentration of LDH was assayed using the Cytotoxicity Detection Kit (Roche, Mannheim, Germany), according to the manufacturer's instructions. A standard curve was used to quantify LDH levels.
Histology. Lung tissues were fixed in 4% paraformaldehyde and then embedded in paraffin, and 5-µm sections were cut. Every third section was collected for further analysis. Hematoxylin and eosin staining of sections were performed by standard techniques.
Bone marrow cell isolation. Mice were killed by cervical dislocation and the hind legs amputated. Both ends of the femur and tibia were removed and the bone marrow was flushed out with PBS using a needle. Recovered total bone marrow cells were centrifuged, washed, and resuspended in an appropriate medium. The total number of cells was determined with a hemocytometer. On average, ~60 × 106 total bone marrow cells were recovered from a single mouse.
Intravenous injection of bone marrow–derived cells. Two days after P. aeruginosa infection, 6–8-week-old wild-type C57Bl/6 mice received a single tail-vein infusion of 1 × 107 bone marrow–derived cells (in 200 µl) isolated from GFP-transgenic mice.
Intratracheal injection of bone marrow–derived cells. A cell suspension of total bone marrow cells or a cell suspension obtained after an EasySep negative selection procedure was prepared in PBS. The cell suspension was stored on ice until recipient mice were anesthetized. The trachea was exposed by means of a midline incision and intubated with a sterile, flexible 22-gauge catheter (Becton Dickinson) connected to a 1-ml syringe. 100 µl of cell suspension were injected into the trachea. 0.5–1 × 106 cells were injected in each animal. 6–8-week-old heterozygous C57Bl/6 mice constitutively expressing GFP were used as donors and their wild-type littermates were used as recipients. In sex-mismatched experiments, male C57Bl/6 mice were used as donors and the littermate females were used as recipients.
EasySep negative selection. Negative selection of bone marrow progenitor cells by EasySep (EasySep Mouse Hematopoietic Progenitor Enrichment Kit; Stem Cell Technologies, Vancouver, British Columbia, Canada) was performed according to the manufacturer's instructions. Briefly, bone marrow was isolated from the femurs and tibias of 6–8-week-old male C57Bl/6 mice or C57Bl/6 mice constitutively expressing GFP. Recovered total bone marrow cells were centrifuged, washed, and resuspended in an appropriate medium. Subsequently, the antibody cocktail containing biotinylated monoclonal antibodies directed against T cells, B cells, monocytes, granulocytes, and red blood cells was added to isolated bone marrow cells. Then tetrameric antibody complex, which recognizes both dextran and biotin, was added, which was followed by addition of dextran-coated magnetic nanoparticles. Magnetically labeled cells were then separated from unlabeled target cells using the EasySep magnet. Control FACS analysis showed that the lineage minus cells were positive for Sca-1 (20.3 ± 2.4%; n = 3) confirming that bone marrow population was enriched for progenitor cells and deprived of committed cells. Only 5.3 ± 0.5% (n = 3) of the total population of the cells were CD45+.
GFP immunocytochemistry. Lungs from control and treated mice were fixed in 4% paraformaldehyde in PBS, cryoprotected in 30% sucrose and frozen. Immunostaining was done on 5–7 µm cryosections. Incubation of sections with primary antibody (anti-GFP, IgG; Invitrogen, San Giuliano Milanese, Italy) was carried out in PBS/1% bovine serum albumin (BSA)/2% normal goat serum for 1 hour at room temperature. Subsequently, the cryosections were extensively washed and then incubated with biotinylated secondary antibody (antirabbit IgG H+L; Vector Laboratories, Burlingame, CA). The detection was performed using the avidin-biotin-peroxidase detection system (ABC Vectastain Elite kit; Vector Laboratories). Immunochemistry was performed on lung sections taken from all the lobes of three animals per group.
FISH analysis coupled to immunofluorescence. FISH analysis was performed as described by Trotman et al.49 with some modifications. Briefly, paraffin-embedded tissue sections were deparaffinized in xylene, rehydrated in a 100, 95, 70, 50% ethanol series, rinsed three times in water, and then twice in PBS. Subsequently, the slides were immersed in 10 mmol/l sodium citrate for 15 minutes at 96 °C. The slides were removed from the solution, and after 15 minutes of cooling, rinsed in 2× SSC buffer. Lung sections were denatured in 60% formamide/2× SSC for 2 minutes at 70 °C, dehydrated through a cold 70, 95, 100% ethanol series and air-dried.
20 µl of Y-chromosome probe (green-labeled (Star-FISH); Cambio, Cambridge, UK) were applied on the lung sections, covered with a glass coverslip and the edges of the coverslip were sealed with rubber cement. The slides were denatured at 75 °C for 10 minutes. The probe was hybridized to the sections overnight at 37 °C. After the hybridization, the rubber cement was removed, the slides were immersed in 2× SSC and the coverslips were allowed to float off. To remove unbound probe, the slides were washed in 50% formamide/2× SSC for 2 minutes at 42 °C. Subsequently, the slides were washed four times in 2× SSC.
Detection of cell type–specific phenotypic markers, on sections stained for Y-chromosome, was performed using a set of antibodies directed against epithelium and leukocytes. Staining for CK was achieved by incubation with rabbit polyclonal antibody against wide-spectrum CK (DakoCytomation, Carpinteria, CA). Staining for leukocytes was done by incubation with rat antimouse CD45 (Santa Cruz Biotechnology, Santa Cruz, CA). The lung sections were incubated with antibodies overnight at 4 °C in the dark. When the primary antibodies were incubated with lung sections separately, they were detected with goat antirabbit IgG (Alexa568 conjugate; Molecular Probes, Eugene, OR), goat antirat IgG (Alexa568 conjugate), respectively. All the antibodies were diluted 1:500 in 0.5% BSA, 0.1% Triton-X in PBS. For nuclear staining, sections were incubated with 4′-6-Diamidino-2-phenylindole (DAPI) (Invitrogen), for 8 minutes at room temperature. When the primary antibodies were incubated with the lung sections simultaneously, they were detected with goat antirabbit IgG (Alexa568 conjugate) and goat antirat IgG (Alexa647 conjugate; Molecular Probes), respectively. Following the final rinse in water, the slides were mounted (DakoCytomation Fluorescent Mounting Medium; DakoCytomation).
Lung tissues from normal male and female mice were used as controls for the Y-chromosome FISH analysis.
GFP fluorescence detection coupled to immunofluorescence. Lungs from control and treated mice were embedded in paraffin and 3–5 µm sections were cut. Every third section was collected for further analysis. Paraffin-embedded sections were heated to 60 °C. Slides were dewaxed by washing two times in xylene, rehydrated through an ethanol series (100, 90, 70%), and washed in PBS. Lung sections were blocked with 100 µl 5% normal swine serum, 0.5% BSA in PBS for 1 hour in PBS. Detection of cell type–specific phenotypic markers was done using a set of antibodies directed against epithelium and leukocytes. Staining for CK was achieved by incubation with rabbit polyclonal antibody against wide-spectrum CK (DakoCytomation). Staining for leukocytes was done by incubation with rat antimouse CD45 (Santa Cruz Biotechnology). The antibodies were diluted 1:200 in 0.5% BSA, 0.1% Triton-X in PBS and incubated with the samples for 1 hour at room temperature. The primary antibodies were detected with goat antirabbit IgG (Alexa568 conjugate), goat antirat IgG (Alexa568 conjugate), respectively. For nuclear staining, sections were incubated with DAPI for 8 minutes at room temperature. Following the final rinse in water, the slides were mounted (DakoCytomation Fluorescent Mounting Medium). Green fluorescence (GFP) was never found in the red channel (CK) and vice versa.
Assessment of levels of engraftment. This analysis was carried out on five animals per group (uninfected controls, 105 cfu, 106 cfu). Each slide contained two lung tissue sections obtained either for FISH analysis or for GFP fluorescence detection. One of them was incubated with rabbit anticow pan-CK (DakoCytomation) and the other one with rat antimouse CD45 (Santa Cruz Biotechnology), as described in previous sections. The primary antibodies were detected with goat antirabbit IgG (Alexa568 conjugate), goat antirat IgG (Alexa568 conjugate), respectively. As we never observed any donor-derived CD45+ cells, in effect donor-derived epithelial cells were scored by Y-chromosome+/CK+ or by GFP+/CK+ double immunofluorescence. Cell counts were done on 20 (GFP) or 10 (Y-chromosome) 63X fields per lobe. The total number of cells was assessed by counting DAPI-stained nuclei.
Microscopes. Photographs were taken with a Zeiss Axioplan2 microscope equipped with an AxioCam digital camera (Carl Zeiss, Gℰ𝒩𝒯ℐ𝒯𝒴𝒮𝒯𝒜ℛ𝒯ℰ𝒩𝒯ℐ𝒯𝒴ℰ𝒩𝒟ttingen, Germany) or a Nikon C1 confocal laser scanning microscope (Nikon Benelux, Brussels, Belgium).
Statistical analysis. All data are presented as means ± SD. Differences between means were calculated by unpaired Student's t-test. Values of P < 0.05 were considered significant.
Acknowledgments
We thank Joanna Price and Diane Krause (Yale University School of Medicine, New Haven, CT) for helping us with the FISH assay. This study was supported by a grant for cystic fibrosis of the Italian Ministero della Sanità (L. 362/99), and the European Community (Contract #2002-02119).
REFERENCES
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Piro D, Lepore S, Maffione AB., and , Conese M.Bone marrow-derived stem cells: homing and rescue of injury in the lung Hematopoietic Stem Cell Transplantation Research Advances 2008Nova Science Publishers: Hauppauge, NY; 15–43.In: Neumann, KB (ed.) [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Abe S, Boyer C, Liu X, Wen FQ, Kobayashi T, Fang Q, et al. Cells derived from the circulation contribute to the repair of lung injury. Am J Respir Crit Care Med. 2004;170:1158–1163. doi: 10.1164/rccm.200307-908OC. [DOI] [PubMed] [Google Scholar]
- Theise ND, Henegariu O, Grove J, Jagirdar J, Kao PN, Crawford JM, et al. Radiation pneumonitis in mice: a sever injury model for pneumocyte engraftment from bone marrow. Exp Hematol. 2002;30:1333–1338. doi: 10.1016/s0301-472x(02)00931-1. [DOI] [PubMed] [Google Scholar]
- Grove JE, Lutzko C, Priller J, Henegariu O, Theise ND, Kohn DB, et al. Marrow-derived cells as vehicles for delivery of gene therapy to pulmonary epithelium. Am J Respir Cell Mol Biol. 2002;27:645–651. doi: 10.1165/rcmb.2002-0056RC. [DOI] [PubMed] [Google Scholar]
- Serikov VB, Popov B, Mikhailov VM, Gupta N., and , Matthay MA. Evidence of temporary airway epithelial repopulation and rare clonal formation by BM-derived cells following naphthalene injury in mice. Anat Rec (Hoboken) 2007;290:1033–1045. doi: 10.1002/ar.20574. [DOI] [PubMed] [Google Scholar]
- Kleeberger W, Versmold A, Rothämel T, Glöckner S, Bredt M, Haverich A, et al. Increased chimerism of bronchial and alveolar epithelium in human lung allografts undergoing chronic injury. Am J Pathol. 2003;162:1487–1494. doi: 10.1016/S0002-9440(10)64281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer H, Rampling D, Aurora P, Bonnet D, Hart SL., and , Jaffé A. Transbronchial biopsies provide longitudinal evidence for epithelial chimerism in children following sex mismatched lung transplantation. Thorax. 2005;60:60–62. doi: 10.1136/thx.2004.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–325. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ., and , Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- Ratjen F., and , Döring G. Cystic fibrosis. Lancet. 2003;361:681–689. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, et al. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–5188. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- MacPherson H, Keir P, Webb S, Samuel K, Boyle S, Bickmore W, et al. Bone marrow-derived SP cells can contribute to the respiratory tract of mice in vivo J Cell Sci 20051182441–2450.Pt 11 [DOI] [PubMed] [Google Scholar]
- MacPherson H, Keir PA, Edwards CJ, Webb S., and , Dorin JR. Following damage, the majority of bone marrow-derived airway cells express an epithelial marker. Respir Res. 2006;7:145. doi: 10.1186/1465-9921-7-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104:11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, et al. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L131–L141. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- Beckett T, Loi R, Prenovitz R, Poynter M, Goncz KK, Suratt BT, et al. Acute lung injury with endotoxin or NO2 does not enhance development of airway epithelium from bone marrow. Mol Ther. 2005;12:680–686. doi: 10.1016/j.ymthe.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Herzog EL, Van Arnam J, Hu B., and , Krause DS. Threshold of lung injury required for the appearance of marrow-derived lung epithelia. Stem Cells. 2006;24:1986–1992. doi: 10.1634/stemcells.2005-0579. [DOI] [PubMed] [Google Scholar]
- Aliotta JM, Keaney P, Passero M, Dooner MS, Pimentel J, Greer D, et al. Bone marrow production of lung cells: the impact of G-CSF, cardiotoxin, graded doses of irradiation, and subpopulation phenotype. Exp Hematol. 2006;34:230–241. doi: 10.1016/j.exphem.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejman J, Di Gioia S, Bragonzi A., and , Conese M. Pseudomonas aeruginosa infection destroys the barrier function of lung epithelium and enhances polyplex-mediated transfection. Hum Gene Ther. 2007;18:642–652. doi: 10.1089/hum.2006.192. [DOI] [PubMed] [Google Scholar]
- Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS., and , Krause DS. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science. 2004;305:90–93. doi: 10.1126/science.1098925. [DOI] [PubMed] [Google Scholar]
- Doring G, Bellon G., and , Knight R.Immunology of cystic fibrosis Cystic Fibrosis 2000Arnold: London; 109–140.In: Hodson, ME, Geddes, DM (eds.) [Google Scholar]
- Conese M, Copreni E, Piro D., and , Rejman J. Gene and cell therapy for the treatment of cystic fibrosis. Adv Gene Mol Cell Ther. 2007;1:99–119. [Google Scholar]
- Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC., and , Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Kotton DN, Fabian AJ., and , Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33:328–334. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Su X, Popov B, Lee JW, Serikov V., and , Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- Yamada M, Kubo H, Kobayashi S, Ishizawa K, Numasaki M, Ueda S, et al. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol. 2004;172:1266–1272. doi: 10.4049/jimmunol.172.2.1266. [DOI] [PubMed] [Google Scholar]
- Ishizawa K, Kubo H, Yamada M, Kobayashi S, Numasaki M, Ueda S, et al. Bone marrow-derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett. 2004;556:249–252. doi: 10.1016/s0014-5793(03)01399-1. [DOI] [PubMed] [Google Scholar]
- Murakami S, Nagaya N, Itoh T, Iwase T, Fujisato T, Nishioka K, et al. Adrenomedullin regenerates alveoli and vasculature in elastase-induced pulmonary emphysema in mice. Am J Respir Crit Care Med. 2005;172:581–589. doi: 10.1164/rccm.200409-1280OC. [DOI] [PubMed] [Google Scholar]
- Wong AP, Dutly AE, Sacher A, Lee H, Hwang DM, Liu M, et al. Targeted cell replacement with bone marrow cells for airway epithelial regeneration. Am J Physiol Lung Cell Mol Physiol. 2007;293:L740–L752. doi: 10.1152/ajplung.00050.2007. [DOI] [PubMed] [Google Scholar]
- Baber SR, Deng W, Master RG, Bunnell BA, Taylor BK, Murthy SN, et al. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1120–H1128. doi: 10.1152/ajpheart.00173.2006. [DOI] [PubMed] [Google Scholar]
- Wong AP, Keating A, Lu WY, Duchesneau P, Wang X, Sacher A, et al. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J Clin Invest. 2009;119:336–348. doi: 10.1172/JCI36882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi R, Beckett T, Goncz KK, Suratt BT., and , Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult marrow derived cells. Am J Respir Crit Care Med. 2006;173:171–179. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruscia EM, Price JE, Cheng EC, Weiner S, Caputo C, Ferreira EC, et al. Assessment of cystic fibrosis transmembrane conductance regulator (CFTR) activity in CFTR-null mice after bone marrow transplantation. Proc Natl Acad Sci USA. 2006;103:2965–2970. doi: 10.1073/pnas.0510758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruscia EM, Ziegler EC, Price JE, Weiner S, Egan ME., and , Krause DS. Engraftment of donor-derived epithelial cells in multiple organs following bone marrow transplantation into newborn mice. Stem Cells. 2006;24:2299–2308. doi: 10.1634/stemcells.2006-0166. [DOI] [PubMed] [Google Scholar]
- Shivdasani RA., and , Orkin SH. The transcriptional control of hematopoiesis. Blood. 1996;87:4025–4039. [PubMed] [Google Scholar]
- Sieweke MH., and , Graf T. A transcription factor party during blood cell differentiation. Curr Opin Genet Dev. 1998;8:545–551. doi: 10.1016/s0959-437x(98)80009-9. [DOI] [PubMed] [Google Scholar]
- Gomperts BN, Belperio JA, Rao PN, Randell SH, Fishbein MC, Burdick MD, et al. Circulating progenitor epithelial cells traffic via CXCR4/CXCL12 in response to airway injury. J Immunol. 2006;176:1916–1927. doi: 10.4049/jimmunol.176.3.1916. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Gomperts BN., and , Keane MP. The role of CXC chemokines in pulmonary fibrosis. J Clin Invest. 2007;117:549–556. doi: 10.1172/JCI30562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa K, Kubo H, Yamada M, Kobayashi S, Suzuki T, Mizuno S, et al. Hepatocyte growth factor induces angiogenesis in injured lungs through mobilizing endothelial progenitor cells. Biochem Biophys Res Commun. 2004;324:276–280. doi: 10.1016/j.bbrc.2004.09.049. [DOI] [PubMed] [Google Scholar]
- Nervi B, Link DC., and , DiPersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99:690–705. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- Piro D, Rejman J., and , Conese M. Stem cell therapy for cystic fibrosis: current status and future prospects. Exp Rev Resp Med. 2008;2:365–380. doi: 10.1586/17476348.2.3.365. [DOI] [PubMed] [Google Scholar]
- Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, et al. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 2008;177:701–711. doi: 10.1164/rccm.200706-859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, et al. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- Trotman W, Beckett T, Goncz KK, Beatty BG., and , Weiss DJ. Dual Y chromosome painting and in situ cell-specific immunofluorescence staining in lung tissue: an improved method of identifying donor marrow cells in lung following bone marrow transplantation. Histochem Cell Biol. 2004;121:73–79. doi: 10.1007/s00418-003-0598-0. [DOI] [PubMed] [Google Scholar]