Abstract
Human coagulation factor VIII (fVIII) is inefficiently biosynthesized in vitro and has proven difficult to express at therapeutic levels using available clinical gene-transfer technologies. Recently, we showed that a porcine and certain hybrid human/porcine fVIII transgenes demonstrate up to 100-fold greater expression than human fVIII. In this study, we extend these results to describe the use of a humanized, high-expression, hybrid human/porcine fVIII transgene that is 89% identical to human fVIII and was delivered by lentiviral vectors (LVs) to hematopoietic stem cells for gene therapy of hemophilia A. Recombinant human immunodeficiency virus–based vectors encoding the fVIII chimera efficiently transduced human embryonic kidney (HEK)-293T cells. Cells transduced with hybrid human/porcine fVIII encoding vectors expressed fVIII at levels 6- to 100-fold greater than cells transduced with vectors encoding human fVIII. Transplantation of transduced hematopoietic stem and progenitor cells into hemophilia A mice resulted in long-term fVIII expression at therapeutic levels despite <5% genetically modified blood mononuclear cells. Furthermore, the simian immunodeficiency virus (SIV) -derived vector effectively transduced the human hematopoietic cell lines K562, EU1, U.937, and Jurkat as well as the nonhematopoietic cell lines, HEK-293T and HeLa. All cell lines expressed hybrid human/porcine fVIII, albeit at varying levels with the K562 cells expressing the highest level of the hematopoietic cell lines. From these studies, we conclude that humanized high-expression hybrid fVIII transgenes can be utilized in gene therapy applications for hemophilia A to significantly increase fVIII expression levels compared to what has been previously achieved.
Introduction
Following vessel injury, coagulation factors are activated resulting in a cascade of enzymatic reactions that eventually lead to the formation of a blood clot at the site of injury and restoration of hemostasis. Congenital or acquired deficiencies of these clotting factors can result in life-threatening bleeding episodes. Hemophilia A is defined as the X-linked, recessive hereditary deficiency of blood coagulation factor VIII (fVIII), which occurs at a rate of 1 in 7,500 male births worldwide. Current treatment of hemophilia A involves intravenous infusion of fVIII containing products. Although this form of therapy generally is effective at stopping ongoing bleeding and can be implemented in a prophylactic fashion to prevent bleeding, there are significant deficiencies that warrant the development of improved biopharmaceuticals directed at the treatment of hemophilia A. These deficiencies include (i) the invasiveness of intravenous infusion of fVIII products, (ii) the development of an immune response to human fVIII that prevents future treatment efficacy in 20–30% of patients, and (iii) the limited availability of fVIII products to only 30% of the hemophilia A population worldwide. Therefore, hemophilia A has been targeted by numerous academic and commercial laboratories for the application of gene transfer-based therapies (for review, see ref. 1).
We demonstrated that a B-domain-deleted (BDD) porcine fVIII (pfVIII) transgene is expressed at up to 100-fold greater levels than recombinant human fVIII (hfVIII) in vitro from mammalian cell lines.2 Although the variation in fVIII protein expression correlated with steady-state mRNA levels, BDDpfVIII was expressed at higher levels than BDDhfVIII on a per mRNA basis suggesting a translational or post-translational regulatory differential. In a subsequent study, we constructed human/porcine (HP)-fVIII molecules containing single or combinatorial domain substitutions. Using this strategy, we found that the interspecies-expression differential results from porcine-specific sequences within the A1 and ap-A3 domains.3 Metabolic-labeling experiments with cells stably expressing either hfVIII or the construct containing the porcine A1 and A3 domain substitutions, previously designated HP47 and herein designated HP-fVIII (Figure 1a), revealed that the interspecies-expression differential results from enhanced secretion.
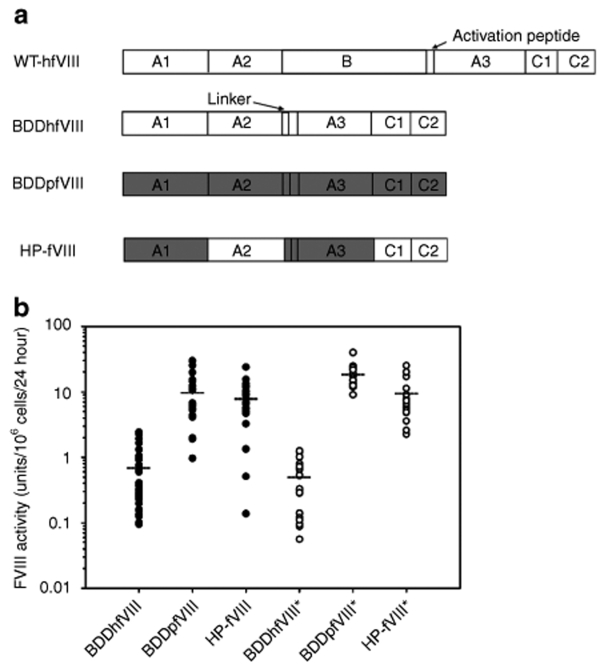
Figure 1.
Heterologous expression of fVIII transgenes. (a) Schematic of fVIII subunits. Full-length human with activation peptide indicated (top), B-domain deleted (BDD) human with the SQ-linker indicated (middle-top), BDD-porcine (middle-bottom), and HP-fVIII (bottom). Dark gray denotes porcine-specific sequences. (b) Human (BDDhfVIII), porcine (BDDpfVIII), and HP-fVIII transgenes were transfected into both BHK-M and Flp-In 293 cells. BHK-M cells were modified using Lipofectamine 2000 and ReNeo plasmid constructs. Flp-In 293 cells were modified by FLP recombinase-mediated site-specific recombination then screened by Southern blot in order to compare only those clones containing a correctly integrated single transgene. FVIII activity was measured from 19 to 33 BHK-M clones (black circles) and 13–22 singly integrated Flp-In clones (white circles) by one-stage coagulation assay. Mean HP-fVIII activity is depicted by a horizontal dotted line.
More recently, we demonstrated the high-expression property of BDDpfVIII using a gene therapy strategy involving transplantation of genetically modified hematopoietic stem cells (HSCs) into hemophilia A mice.4,5,6 In these studies, it was shown that (i) high-expression porcine sequence elements function in vivo to completely correct the fVIII deficiency (100% normal human fVIII levels) in hemophilia A mice following transplantation of genetically modified HSCs even under pretransplantation conditioning protocols that result in engraftment of only 2–10% genetically modified cells, (ii) routinely accepted chemotherapy-based conditioning regimens involving T-cell inhibition can facilitate engraftment of genetically modified cells and prevent immune responses to fVIII, and (iii) HSC transplantation (HSCT) gene therapy incorporating high-expression BDDpfVIII can cure hemophilia A in mice with clinically significant levels of fVIII inhibitors. However, each of these studies were performed using ecotropic-pseudotyped murine stem cell virus (MSCV), which due to tropism restriction is not conducive to use in humans. In this study, we successfully modified the HSCT gene therapy strategy to include the use of VSV-G pseudotyped lentiviruses encoding a humanized version of BDDpfVIII, which retains the high expression properties of BDDpfVIII in transplanted hemophilia A mice and human hematopoietic cell lines.
Results
Comparison of bioengineered fVIII transgenes in heterologous cell culture systems
BDDhfVIII, BDDpfVIII, and HP-fVIII transgenes were each cloned into the ReNeo expression plasmid (described previously in ref. 7) and the pcDNA5/FLP recombination target (FRT) expression plasmid (Invitrogen, Carlsbad, CA) (Figure 1a). A blastp alignment shows that the HP-fVIII (1,467 aa) used in these studies is 89% similar to BDDhfVIII (HSQ:1,457 aa), whereas BDDpfVIII (1,467 aa) is 83% similar to BDDhfVIII, and BDDpfVIII is 93% similar to HP-fVIII at the amino acid level. BHK-M cells were transfected with each ReNeo construct by liposome-mediated transfection and fVIII activity was measured by one-stage coagulation assay (Figure 1b). No significant difference in expression was observed between BDDpfVIII and HP-fVIII. However, the expression was ~13-fold higher than BDDhfVIII expression (P < 0.0001, Mann–Whitney U Test). In an attempt to reduce interclonal variation due to position effect variegation, fVIII expression was compared using the Flp-In system (Invitrogen). This system was designed to create isogenic cell lines using FLP recombinase-mediated site-specific recombination. Flp-In 293 cells were cotransfected with the pcDNA5/FRT/fVIII expression plasmid and plasmid pOG22, the FLP recombinase expression plasmid, to promote site-specific recombination between an FRT site in the expression plasmid and an FRT site engineered into the host cell line. Resulting clones were expanded under hygromycin selection and fVIII activity was measured by one-stage coagulation assay. Clones expressing fVIII then were screened by Southern blot to eliminate any clones with multiple and/or incorrect integrations (data not shown). Only clones with a single correct integration event were used in the comparative analyses. As shown in Figure 1b, fVIII expression was greatest from BDDpfVIII at 19 units/106 cells/24 hour, which was 37-fold higher than BDDhfVIII. HP-fVIII transgene expression was significantly lower at 10 units/106 cells/24 hour (P = 0.0002, Mann–Whitney U test), which was 19-fold higher than BDDhfVIII. This is the first time a significant difference has been observed between BDDpfVIII and HP-fVIII. But, for both porcine fVIII and HP-fVIII, expression was significantly greater than BDDhfVIII (P < 0.0001, Mann–Whitney U test).
Lentiviral transfer and expression of the HP-fVIII transgene in human cell lines
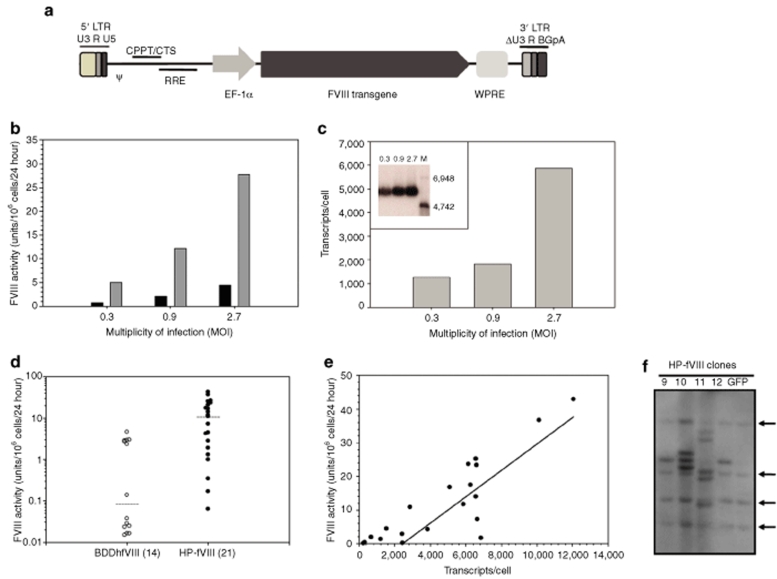
In separate reports, we demonstrated that both γ-retroviral and lentiviral-mediated gene transfer drives high-level expression of BDDpfVIII when compared to BDDhfVIII4,5,6 and (unpublished data). However, there is an inherent risk of immune responses to porcine-specific epitopes that may affect the utility of a strictly porcine fVIII transgene, which can be especially troubling in the context of hemophilia A patients already tolerized to human fVIII. The use of humanized variants such as HP-fVIII, may therefore be more useful for the clinical translation of gene therapy for hemophilia A. To this end, we studied HP-fVIII expression compared to BDDhfVIII utilizing a VSV-G pseudotyped, self-inactivating human immunodeficiency virus (HIV)-1-based lentiviral gene delivery vector system developed by Lentigen (Gaithersburg, MD). Expression was driven by the elongation factor-1 alpha (EF-1α) promoter and the vector included the woodchuck post-transcriptional regulatory element (Figure 2a). Human embryonic kidney (HEK)-293T cells were transduced at various multiplicities of infections (MOIs) and fVIII activity, expression, and integration were analyzed at various times post-transduction. As shown in Figure 2b, an increase in HP-fVIII expression was observed with increasing MOI. At 7-days post-transduction, HP-fVIII expression was 28 units/106 cells/24 hour, which represented a greater than sixfold higher expression level than BDDhfVIII (4.5 units/106 cells/24 hour) at an MOI of 2.7 despite similar transduction levels. Quantitative reverse transcriptase (RT)-PCR analysis performed on total RNA isolated from transduced cells showed an increase in steady-state HP-fVIII mRNA levels similar to that observed for the activity levels (Figure 2c). The increase in HP-fVIII activity compared to BDDhfVIII was not the result of different steady-state mRNA levels, as revealed by quantitative RT-PCR analysis. Consistent with this observation, HP-fVIII activity remains 5- to 13-fold higher than BDDhfVIII when normalized to transcript number at the various MOIs tested (Supplementary Table S1 and Figure S1). Northern blot analysis of total RNA extracted from cells transduced at each MOI demonstrated expression of a single transcript of ~6,300 nucleotides (Figure 2c, inset).
Figure 2.
FVIII expression analysis following lentiviral transduction of HEK-293T cells. (a) HIV-1-based lentiviral vectors were manufactured by Lentigen using the LentiMax Production System. LTR, long terminal repeat; U3, R and U5, promoter/enhancer regions; psi, packaging signal; cPPT, central polypurine tract; RRE, rev response element; eF1-α, elongation factor 1-α promoter; WPRE, woodchuck hepatitis post-transcriptional regulatory element; ΔU3, modified LTR for production of recombinant self-inactivating lentiviral vectors. (b) FVIII expression levels were determined from transduced HEK-293T cells expressing HP-fVIII (shaded bars) or BDDhfVIII (black bars), which was determined by a one-stage clotting assay. Each bar represents the mean activity of two wells. (c) Average transcripts per cell were measured by quantitative PCR from HP-fVIII transduced HEK-293T cells at MOI 0.3, 0.9, and 2.7 (shaded blocks), and northern blot (c, inset) analysis of the same cells indicating fVIII transcripts of approximately 6,300 nucleotides. (M denotes molecular weight marker with sizes as indicated.) (d) HP-fVIII and BDDhfVIII HEK-293T expressing clones were generated by limiting-dilution cloning and fVIII activity levels were measured by one-stage coagulation assay (P < 0.001). Horizontal lines indicate the mean values for each group and the number in parenthesis indicates the number of clones analyzed. (e) The number of HP-fVIII transcripts per cell were determined by quantitative RT-PCR and plotted against fVIII activity from a one-stage clotting assay demonstrating a correlation between transcript number and fVIII activity (r2 = 0.765, P < 0.0001). (f) Southern blot analysis was performed on genomic DNA isolated from clones generated in d and digested with AvrII. The arrows indicate endogenous fVIII DNA fragments.
Individual clones from HEK-293T cell populations transduced with recombinant HIV encoding HP-fVIII were isolated by limiting-serial dilution as described in Materials and Methods. Clones were analyzed for fVIII activity by one-stage coagulation assay, proviral transcription by quantitative real-time RT-PCR analysis, and transgene integration events by Southern blot analysis in order to further characterize HP-fVIII expression when driven by lentiviral gene delivery. HP-fVIII expression levels in the conditioned media ranged from 0.06 to 43 units/106 cells/24 hour (Figure 2d). As observed with the polyclonal transduced cells, median fVIII activity was significantly higher than that of BDDhfVIII (P < 0.0001, Mann–Whitney U Test). Quantitative RT-PCR was performed on total RNA isolated from all clones to determine steady-state mRNA levels. Analysis of transcript expression by linear regression revealed a significant correlation between HP-fVIII activity and transcript levels (Figure 2e, P < 0.0001, two-tailed Student's t-distribution). Integration of proviral transgene DNA was examined by Southern blot analysis performed on all clones following digestion of genomic DNA with AvrII, which cleaves at a single site within the fVIII transgene (Figure 2f). Mean integration number/clone was determined to be 1.9 and 2.8 for the MOIs of 0.9 and 2.7, respectively. These data are consistent with the observation that the polyclonal cell population at 7 days post-transduction contained means of 1.2 ± 0.02 and 2.6 ± 0.15 integrations per cell as determined by competitive PCR analysis. In order to replicate these data in a different human cell line, a similar approach of clonal analysis was performed on HeLa cells transduced with recombinant HIV-1 encoding HP-fVIII. Analysis of 12 clones from the population transduced at an MOI of 3 revealed an average of 3.9 copies/cell with a median fVIII activity of 3.8 units/106 cells/24 hour as determined by Southern blot analysis and one-stage coagulation assay, respectively (data not shown). Likewise, Northern blot analysis revealed a single transcript of ~6,300 nucleotides (data not shown).
HIV-based lentiviral transfer of HP-fVIII into human hematopoietic cell lines
Previously, we demonstrated successful HSCT gene therapy of hemophilia A in a murine model utilizing an MSCV-based oncoretroviral vector delivery system and the BDDpfVIII transgene under multiple transplant conditioning regimens.5,6 These studies demonstrated that high-level expression of BDDpfVIII from hematopoietic cells occurs in vivo in hemophilia A mice. Others have shown expression of BDDhfVIII from lentivirally transduced hematopoietic cell lines supporting the concept that fVIII can be expressed from a variety of different cell types in vitro and in vivo.8,9,10,11,12 In an attempt to further characterize lentiviral-mediated HP-fVIII delivery and expression, we investigated transduction, genetic modification and expression of HP-fVIII from human hematopoietic cell lines. We utilized both simian immunodeficiency virus (SIV)- and HIV-based lentiviral vectors (LVs) to transduce a panel of hematopoietic cell lines including K562 (myeloid–erythroid chronic myelogenous leukemia), EU1 (B-cell acute lymphoblastic leukemia), U.937 (monocytic lymphoma), and Jurkat (acute T-cell lymphoma) cells in comparison to HEK-293T and HeLa cells.
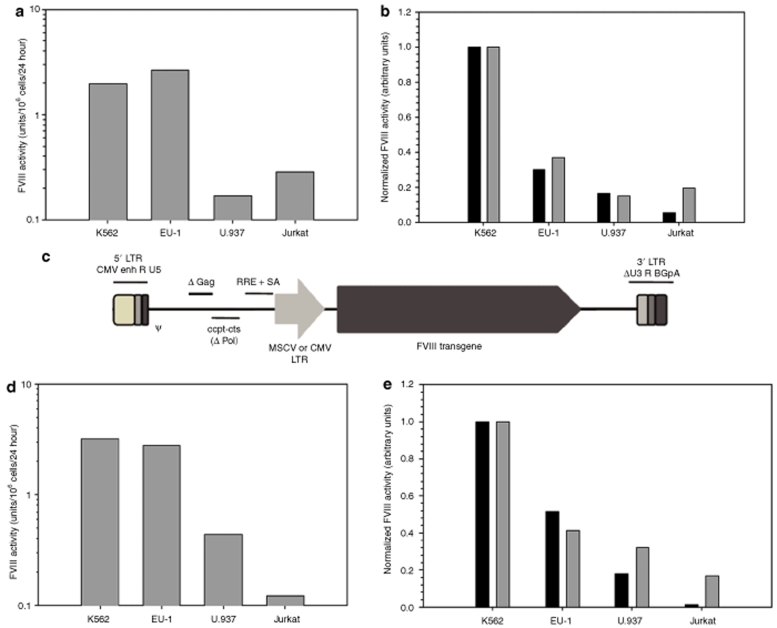
Recombinant viral-based vectors were added to ~100,000 cells at an MOI of 1 using recombinant HIV encoding HP-fVIII. At 7 days post-transduction, transduced cells were analyzed for fVIII activity levels in conditioned media (one-stage coagulation assay), fVIII transcript levels (quantitative real-time RT-PCR), and proviral integrations per cell (quantitative real-time PCR). As shown in Figure 3a, both K562 (2.0 units/106 cells/24 hour) and EU1 (2.6 units/106 cells/24 hour) cells support high-level expression that is comparable to HeLa cells (3.4 units/106 cells/24 hour, data not shown). When normalized to transgene integration events or transcript expression, K562 cells support the greatest level of HP-fVIII expression compared to the other hematopoietic cell lines (Figure 3b). Of note, efficient transduction was observed in Jurkat cells (0.87 copies per cell) when compared to other cell lines despite their observed low-level expression of HP-fVIII.
Figure 3.
FVIII expression from lentivirally transduced human hematopoietic cells lines. Cell lines K562 (myeloid-erythroid CML), EU-1 (B cell ALL), U.937 (monocytic lymphoma) and Jurkat (acute T-cell lymphoma) were transduced with the indicated fVIII transgene and assayed for clotting activity and transcript copy number. (a) FVIII activity was measured by one-stage coagulation assay of conditioned medium from cells transduced at an MOI = 1 with HIV-HP-fVIII. Each bar represents the mean activity of two wells. (b) Clotting activity was normalized to copy number (dark bars) or transcript number (gray bars) as determined by quantitative RT-PCR. (c) A schematic diagram of SIV-based-HP-fVIII, which was used for the transductions presented in d and e. (d) FVIII activity was measured by one-stage coagulation assay of conditioned medium from cells transduced at an MOI = 10 with SIV-HP-fVIII. Each bar represents the mean activity of two wells. (e) Clotting activity was normalized to proviral copy number (dark bars) or transcript number (gray bars) as determined by quantitative RT-PCR.
In preliminary studies, it was observed that the HIV-based LV did not efficiently transduce murine stem cell antigen (sca)-1+ hematopoietic stem and progenitor cells (unpublished data). However, transduction was significantly higher when an SIV-based LV was tested. In an effort to characterize HP-fVIII expression in vivo following HSCT gene therapy, SIV-based LVs were constructed containing either the MSCV-long terminal repeat (LTR) or a cytomegalovirus (CMV) promoter to drive HP-fVIII expression. Initially, we examined the transduction, genetic modification, and expression of HP-fVIII from hematopoietic cell lines transduced with recombinant SIV-MSCV encoding HP-fVIII (Figure 3c). Vectors were added to ~200,000 cells at an MOI of 10 as described under Materials and Methods. FVIII activity was assessed 72 hour post-transduction by a one-stage coagulation assay, and consistent with our previous observations, K562 cells exhibited the highest level of fVIII expression at 3.2 units/106 cells/24 hour (Figure 3d). Normalizing fVIII activity to either transgene integration per cell or transcript expression reveals that K562 cells express the greatest levels of HP-fVIII followed by EU1 > U937 > Jurkat cells (Figure 3e). Again, Jurkat cells were transduced most efficiently (~8.4 transgene copies per cell), but produced the lowest levels of fVIII at 0.12 units/106 cells/24 hour at 72 hour post-transduction. These data demonstrate that high-level HP-fVIII expression is achieved following lentiviral-mediated gene transfer utilizing either HIV or SIV-based lentiviral gene transfer into a variety of human hematopoietic cell lines in vitro.
HSCT lentiviral gene therapy in a murine model of hemophilia A
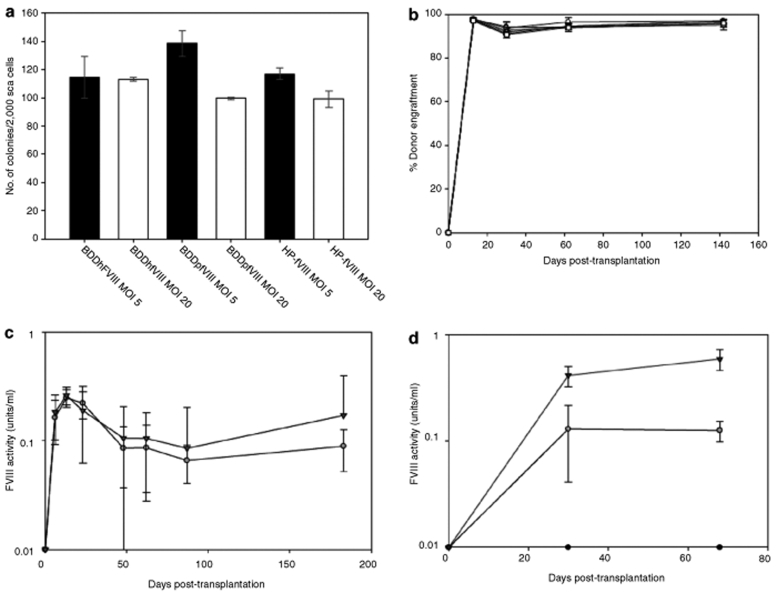
The HSCT gene therapy studies described previously4,5,6 utilized an MSCV-based γ-oncoretroviral vector system. Although clinical trials are still underway using this system, it is speculated that LVs may demonstrate increased safety, and several trials are now being conducted using HIV-based lentivirus systems. We compared expression of HP-fVIII, BDDpfVIII, and BDDhfVIII using SIV-based gene transfer. sca-1+ cells were transduced with SIV-based vectors at an MOI that was predicted, based on eGFP encoding vectors, to yield low transgene copy numbers in mice reconstituted with the genetically modified HSCs. Transduction of sca-1+ cells with control vector encoding eGFP followed by growth in methylcellulose showed that two applications of recombinant SIV at a total MOI of 20 (2 × MOI = 10) resulted in 10–25% genetically modified GFP-positive colonies (data not shown). Therefore, we utilized this protocol to transduce sca-1+ cells under identical conditions with recombinant SIV encoding HP-fVIII, BDDpfVIII, or BDDhfVIII, followed by transplant of the gene-modified cells into lethally irradiated (11 Gy total body irradiation) hemophilia A mice. Before transplantation, transduced sca-1+ were plated in methylcellulose to assess overall toxicity or loss of progenitor phenotype. Although small differences in colony forming units were observed among the groups, little variation was observed even with increasing MOI (Figure 4a). Following transplantation, engraftment and plasma fVIII activity were measured. All mice in each group displayed donor cell engraftment of >90%, as expected for lethal irradiation-based conditioning (Figure 4b). Furthermore, mice that received cells transduced with BDDpfVIII or HP-fVIII contained therapeutically relevant levels (0.02–0.05 units/ml) of circulating plasma fVIII activity (mean activities ranging from 0.07 to 0.3 units/ml) throughout the 175 day follow-up (Figure 4c), while mice transplanted with BDDhfVIII-transduced cells displayed no detectable plasma fVIII activity at any timepoint. Analysis of transgene copy number in peripheral blood revealed <3% (limit of assay detection) genetically modified cells in circulating mononuclear cells in all animals (data not shown). Because the MSCV-LTR promoter has been demonstrated to facilitate oncogenesis in human clinical trials due to insertional mutagenesis, we replaced the MSCV promoter with a CMV promoter, albeit still a strong constitutive promoter, and performed a comparative HSCT-based gene therapy transplantation experiment using SIV-CMV-BDDhfVIII, SIV-CMV-HP-fVIII or SIV-MSCV-HP-fVIII transduced sca-1+ cells. At 30 days post-transplantation, therapeutic levels of fVIII were observed in all mice transplanted with either SIV-CMV-HP-fVIII (n = 6) or SIV-MSCV-HP-fVIII (n = 4) modified cells. FVIII activity was sustained out to 68 days post-transplantation with mean fVIII levels of 0.13 ± 0.03 and 0.49 ± 0.25 (mean ± sample standard deviation) units/ml, respectively. In contrast, no fVIII activity was observed in the CMV-BDDhfVIII cohort (n = 6) at any timepoint (Figure 4d).
Figure 4.
FVIII expression following HSCT lentiviral gene therapy of hemophilia A mice. sca-1+ cells isolated from bone marrow of congenic EGFP-transgenic mice were transduced with recombinant SIV encoding either BDDhfVIII, BDDpfVIII, or HP-fVIII at an MOI of 5 or 20 and 7.5 × 105 cells were transplanted into lethally irradiated (11 Gy total body irradiation) hemophilia A mice. (a) Transduced sca-1+ cells were plated in methylcellulose, cultured for 7 days and colonies were visually counted. (b) Donor cell engraftment was determined by flow cytometry. (c) Plasma fVIII activity levels were determined at several timepoints by chromogenic substrate assay (black triangle, HP-fVIII; gray circles, BDDpfVIII) (Coatest). (d) In vivo expression of BDDhfVIII (black circles) and HP-fVIII was compared using SIV containing either a CMV (open circles) or MSCV-LTR (black triangles) promoter (n = 6, 6, and 4, respectively).
Discussion
A major issue for the clinical translation of gene therapy for hemophilia A has been the subtherapeutic production of the transgene product, coagulation fVIII (for review see ref. 13). In hindsight, this should not have been unexpected due to the low-level production of endogenous human fVIII both naturally, which normally circulates at a concentration of 1 nmol/l, and from heterologous recombinant protein expression systems. There has been some progress enhancing fVIII expression through genetic engineering of the coding sequence (for review see ref. 14). Bioengineering strategies have targeted specific sequences within human fVIII that are thought to be responsible for its generally poor expression, which have been shown to be due to (i) mRNA instability, (ii) interactions with resident endoplasmic reticulum chaperones and (iii) the requirement for carbohydrate-facilitated transport from the endoplasmic reticulum to the Golgi apparatus. In general, these bioengineering efforts have achieved modest increases in fVIII expression that have yet to be translated clinically.15,16
Gene therapy is considered a potential treatment for hemophilia A because (i) even modest increases in fVIII levels (to ≥1% of normal levels) can alleviate spontaneous bleeding episodes, (ii) many different cell types are capable of synthesizing functional fVIII protein meaning virtually any tissue or cell type with access to the bloodstream can be targeted for gene transfer, and (iii) gene therapy should be more economical and less invasive than protein replacement therapy given that it would consist of limited (possibly only one) treatment events. However, poor human fVIII expression levels are at least partially responsible for the insufficient, and often not sustained, fVIII levels observed in studies using recombinant viral gene transfer in animal models of hemophilia A.10,17,18,19,20,21,22,23,24,25 In addition, there have been three phase 1 clinical trials conducted that employed three different gene-transfer strategies (for review, see ref. 13). Although two of the three studies were deemed safe, the lack of sustained plasma fVIII activity reduced enthusiasm for these products and none of these clinical trials progressed beyond phase 1.
It is now well documented that porcine fVIII, specifically a BDD construct (BDDpfVIII), is expressed at significantly higher levels than B-domain deleted human fVIII (BDDhfVIII).2 The increase in protein expression results from increased post-translational secretory transit, which likely occurs in the endoplasmic reticulum through differential interactions with endoplasmic reticulum resident chaperones.3 We previously showed proof-of-principal that the use of the BDDpfVIII transgene dramatically increases total fVIII production in gene-transfer studies incorporating genetically modified HSCs5,6 and, in addition, that gene therapy targeting HSCs can be used to cure hemophilia A complicated by fVIII inhibitors.4
Although BDDpfVIII provides a clear advantage over human fVIII transgenes with respect to protein expression, we postulated that a humanized high expressing transgene would provide additional advantages. For example, patients with hemophilia A typically are treated with recombinant human fVIII products, and some patients develop inhibitors to the recombinant protein. Approximately 30% of hemophilia A patients develop complications due to anti-fVIII inhibitory antibody formation. However, the majority (70%) are tolerant to the infused protein. Therefore, a high-expression product that is engineered to closely resemble the clinically administered products could be more useful to patients that are already tolerized to human fVIII and might gain greater acceptability to patients and physicians. To develop a gene-transfer system using a high-expression humanized construct, we tested several hybrid human/porcine chimeras. FVIII is composed of five distinct domains defined based on internal sequence homology and designated A1, A2, A3, C1, and C2 (ref. 26). Several human/porcine fVIII variants have been constructed by interchanging the various human and porcine domain sequences. These studies identified the sequence elements within the porcine transgene that are necessary and sufficient for high-level expression.3 Specifically, it was shown that introducing the A1 and A3 domains of porcine fVIII into human fVIII created a construct that expressed equally well compared to porcine fVIII. We utilized this information to construct retroviral vectors that encode a hybrid HP-fVIII variant that has 89% identical amino-acid sequence to BDDhfVIII but retains the high-expression capabilities of porcine fVIII. The BDDhfVIII used in these studies is identical in sequence to the clinical hemophilia A product, ReFacto (Wyeth).
In addition to developing and testing high-expression fVIII variants, lentiviral gene-transfer systems also were employed in contrast to our previous studies using oncoretroviral vectors. It has been proposed that lentiviruses (e.g., HIV-1 or SIV-based recombinant vectors) may result in fewer complications due to insertional mutations compared to oncoretroviral vectors because of the tendency of lentiviral-based vectors to integrate within transcriptionally active genes as opposed to oncoretroviruses, which tend to integrate near promoter/enhancer regions. Therefore, we determined whether HP-fVIII can be effectively transferred using LVs incorporating a self-inactivating deletion in the 3′-LTR and an internal EF-1α promoter driving HP-fVIII expression. The purpose of introducing the self-inactivating vectors is to decrease the possibility that expression of cellular genes adjacent to the vector integration site is dysregulated due to viral LTR activity. This study shows that high-expression human/porcine chimeras are transferred efficiently using recombinant LVs.
To test the utility of the HP-fVIII construct, multiple cell lines and gene transfer techniques were used. There was no statistically significant difference between HP-fVIII and BDDpfVIII expression in BHK cells, with both being ~13-fold higher than BDDhfVIII. Single-integrant Flp-In constructs demonstrated that HP-fVIII had a maximal clotting activity ~19-fold higher than human fVIII. However, this was slightly lower than that observed for BDDpfVIII. This is the first system in which this observation has been made and it is possible that this outcome is specific to the Flp-In 293 system. Interestingly, we did not observe a dramatic decrease in the variability of fVIII expression from our clones using the Flip-In system. The basis for the variability observed is not understood, but is consistent with the only other study published using this system.27
With respect to retroviral gene transfer, it was found that HP-fVIII expression increased with increasing MOI, and that the HP-fVIII clotting activity increase correlated to the amount of fVIII RNA transcript. Both HIV- and SIV-LVs were able to transduce human hematopoietic cell lines resulting in stable integration and activity levels as high as 1 unit/106 cells/24 hours. In addition, the high-expression properties of HP-fVIII compared to BDDhfVIII was confirmed in clones isolated from transduced HEK-293T cells. It is unclear why the BDDhfVIII expressing clones displayed a bimodal distribution, but it is possible that some of the integrations were nonfunctional due to position effects, mutation, rearrangement, etc., which could be transgene dependent. These results demonstrate that HP-fVIII retains the high-expression characteristics of BDDpfVIII. The high-expression properties of HP-fVIII translated well to in vivo studies using HSCT gene therapy into a murine model of hemophilia A. Transduction of murine bone marrow sca-1+ cells with HP-fVIII did not affect the growth of hematopoietic progenitor cells, and the transduced cells engrafted normally in hemophilia A mice. Clotting activity was restored in hemophilia A mice transplanted with sca-1+ cells genetically engineered to express HP-fVIII, and the mice display sustained therapeutic levels of fVIII activity. Previous studies using HSC-targeted gene therapy for fVIII delivery showed low level fVIII activity in transplanted animals.10,17,28,29 However, Hawley and colleagues were able to achieve similar fVIII activity levels as that presented in this study, but the expression was achieved by engrafting >20% genetically modified cells.11,12 In this study, therapeutic activity levels were achieved at circulating transgene copy numbers of <5%. Alternatively, LV systems using platelet-specific promoters, which result in fVIII accumulation in platelets rather than systemic expression, also demonstrated correction of the hemophilia A phenotype in mice transplanted with genetically modified HSCs.30,31,32 Because of potential questions regarding the differences in clot formation initiated by circulating fVIII compared to platelet-derived fVIII, the general usefulness of this strategy remains to be tested.33
In this study it was not empirically addressed but because of the long-term engraftment of gene modified-cells and long-term expression of HP-fVIII it is assumed that these mice are tolerant to the fVIII transgene. Because of the aggressive conditioning regimen (i.e., lethal radiation) this was not unexpected. With respect to the immunogenicity of HP-fVIII, it is feasible that reduction of porcine xeno-epitopes in the humanized construct will reduce the incidence of inhibitor formation. This may especially be relevant for patients who have been previously treated with human fVIII and have not formed inhibitors and are presumably tolerant to human fVIII. Conversely, it is possible that the porcine residues incorporated into the HP-fVIII construct, which are required for the high-expression properties of the chimeric protein, render it more immunogenic than human fVIII. However, in the context of HSCT gene therapy, we previously showed that the combination of nonmyelosuppressive conditioning and transient immunosuppression support engraftment of gene-modified cells and long-term expression of the porcine fVIII transgene. Based on our previous and ongoing studies, we think this is a T-cell dependent process and tolerance would occur independent of the fVIII transgene used, e.g., human, porcine, or HP-fVIII. Another issue not addressed in this study is the identification of specific hematopoietic cell lineages expressing fVIII. It is possible that certain cell lineages express fVIII more or less efficiently than others due to differences in the concentrations of proteins necessary for fVIII biosynthesis. However, this is a complex issue to resolve experimentally because it is possible that some cell types may transcribe the fVIII transgene and translate the mRNA, but may not be capable of completeing the post-translational processing and secretory transport of functional fVIII.
These results are consistent with the hypothesis that HP-fVIII contains sequences that confer enhanced fVIII production through more efficient secretion from the cell. Previously, we demonstrated that high-level expression does not rely on cell-type specific factors, but instead appears to be an inherent property of specific protein sequences that functions ubiquitously in all cell types.3 Unlike human fVIII, which is known to contain multiple elements that hinder its production in vitro for recombinant protein products and in vivo for gene transfer-based therapeutics, HP-fVIII has 89% identical amino acid sequence identity to BDDhfVIII, but is still expressed at significantly higher levels, which may be necessary to overcome the expression barrier to gene therapy for hemophilia A. Furthermore, the hybrid construct can be transferred by recombinant LVs to hematopoietic cells, which should facilitate the clinical translation of an HSCT gene therapy-based protocol aimed at curing hemophilia A.
Materials and Methods
Reagents. Dulbecco's Modified Eagle's medium (DMEM), Advanced DMEM/F-12 medium, Aim V medium, RPMI Medium 1640, heat-inactivated fetal bovine serum (FBS), penicillin–streptomycin solution, GlutaMax, and Dulbecco's phosphate-buffered saline were purchased from Invitrogen life technologies. Human fVIII-deficient plasma and normal pooled human plasma (FACT) were purchased from George King Biomedical (Overland Park, KS). Automated activated partial thromboplastin reagent was purchased from BioMérieux (Durham, NC). Clotting times were measured using an ST art Coagulation Instrument (Diagnostica Stago, Asnieres, France). Genomic DNA and total RNA from cultured cells was isolated using QIAGEN DNeasy Blood & Tissue Kit and Rneasy Mini Kit, respectively (Germantown, MD). Taq PCR Master Mix Kit was purchased from QIAGEN. In vitro transcribed fVIII RNA standards were generated using the mMessage mMachine Kit by Ambion (Austin, TX). FVIII RNA quantitation was performed using SYBR GREEN PCR Master Mix, TaqMan Reverse Transcription Reagents, and ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). Oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, IA). Chemicals were purchased from Fisher Scientific (Fair Lawn, NJ) unless otherwise stated. Southern and northern blot analysis was performed using the DIG Nonradioactive Nucleic Acid Labeling and Detection System by Roche (Indianapolis, IN).
Generation of transfected cell lines. BDDhfVIII and BDDpfVIII transgenes were subcloned into the ReNeo and pcDNA5/FRT expression vectors. Generation of HP-fVIII in the ReNeo plasmid has been described previously.3 The pcDNA5/FRT/HP-fVIII construct was created by ligating the NotI/XhoI fragment from ReNeo/HP-fVIII into the pcDNA5/FRT plasmid. ReNeo/fVIII and pcDNA5/FRT/fVIII constructs were transfected into BHK-M and Flp-In 293 cells, respectively, and stable clones were selected by antibiotic resistance. Flp-In 293 clones were screened by Southern blot in order to eliminate any clones with multiple and/or incorrect integrations.
LV production. HIV-based LVs containing fVIII transgenes were manufactured by Lentigen. Briefly, BDDhfVIII and HP-FVIII was subcloned into the LentiMax expression vector. Transgene expression was driven by the human EF-1 α promoter and research grade LV particles were manufactured using the LentiMax Production System (Lentigen, Gaithersburg, MD). LV particles were titered by performing competitive PCR analysis for the fVIII transgene (as described below) on extracted genomic DNA from transduced HEK-293T cells. Lenti-GFP, a LentiMax LV expressing the green fluorescent reporter protein under the control of the EF-1α promoter was used as a negative control.
SIV vector production and murine sca-1+ cell transduction. The SIV vector used in this study was obtained from Arthur Nienhius (St. Jude Children's Hospital, Memphis, TN) and has been described previously.34 BDDhfVIII and HP-fVIII transgenes were cloned into the SIV transfer vector, pCL20cSLFR MSCVGFP, between the BstEII and Not1 restriction sites. A CMV promoter was cloned from the pFRT/hFVIII using Mlu1 digestion onto the pCL20-MSCV-fVIII to generate pCL20-CMV-fVIII. All recombinant viral-based vectors were prepared by transient transfection of 293T cells with the following plasmids: pSIV 2.06 µg, PCAG4 1.25 µg, pVSVG 1.25 µg, PCL expression vector 1.67 µg using 40 µl of lipofectamine 2,000 per plate. One day post-transfection, the media was replaced with fresh DMEM-F12, 10% FBS, 1% penicillin/streptomycin and viral supernatant was collected every 24 hours for 3 days. Pooled viral supernatant was filtered through a 0.22 µmol/l filter and concentrated overnight by sedimentation at 10,000g. The pellet was brought up in StemPro 34 media at 1/100th the initial volume and frozen in 1 ml aliquots. The concentrated vector was titered by transducing HEK-293T cells with increasing vector volumes. At 48–72 hour post-transduction, genomic DNA was isolated and DNA copy number was estimated by quantitative PCR as described previously.5 Titers were determined using the primers designed specifically to amplify the fVIII sequence at a conserved site in the A2 domain (forward: 5′AAA AAC ATA GCC ATT GAT GCT GTG 3′; reverse: 5′ TGG GAC CTT TAC TTT ATG G 3′). sca-1+ cells were isolated using immunomagnetic bead selection from transgenic mice constitutively expressing eGFP+ as described previously.5 Isolated cells were cultured under serum-free conditions as described elsewhere.4,5,6 The cells were counted the day of transduction, resuspended in fresh media supplemented with cytokines and all transductions were done at 1 × 106 million cells/ml at MOIs of 2.5 × 2 (5) or 10 × 2 (20), 12–16 hour apart. Approximately 750,000 cells were transplanted into 11 Gy total body irradiation conditioned congenic hemophilia mice via tail vein injection. For transplanted animals the COATEST (Diapharma, West Chester, OH) was used to determine fVIII expression.
Cell culture, transduction, and clonal selection. Lentiviral transduction of adherent cell lines. HEK-293T were maintained in complete Advanced DMEM/F-12 supplemented with 10% FBS, 1% penicillin–streptomycin solution (100 units/ml each), and 2 mmol/l GlutaMax at 37 °C with 5% CO2. Transductions with lentiviral particles at various MOIs were performed by incubating approximately 100,000 cells/well plated onto collagen-1 coated 24-well plates (BIOCOAT, Becton Dickinson, Franklin Lakes, NJ) with viral-based vectors in a final volume of 500 µl of complete Advanced DMEM/F-12 supplemented with 8 µg/ml of polybrene (Specialty Media). Twenty-four hours post-transduction, vector-containing media was replaced with fresh complete Advanced DMEM/F-12 and transduced cells were expanded for analysis of fVIII activity, integration events, and transcript analysis as described below. Clonal isolation was performed by single-cell cloning by limiting dilution into 96-well plate and visual examination of wells for single cell. After several weeks in culture, single cell clones were expanded for further testing.
Lentiviral transduction of suspension cell lines. Hematopoietic cell lines were maintained in RPMI medium 1640 with L-glutamine supplemented with 10% FBS and 1% penicillin–streptomycin solution (100 units/ml each) at 37 °C with 5% CO2. Transduction of SIV-based lentivirus or LentiMax LV particles was performed by incubating either 100,000 or 200,000 cells/well plated onto RetroNectin-coated 24-well plates with retroviral vector in a final volume of 500 µl of complete RPMI medium 1640 supplemented with 8 µg/ml of polybrene (Specialty Media). Twenty-four hours post-transduction, vector-containing media was replaced by spinning cells and replacing medium with fresh complete RPMI 1640. At various times post-transduction, cells were analyzed for fVIII activity, integration events, and transcript expression as described below.
Measurement of fVIII activity from cell lines. For all in vitro experiments using cell lines, FVIII activity was measured using the activated partial thromboplastin reagent-based one-stage coagulation assay in an ST art Coagulation Instrument (Diagnostica Stago) using human fVIII-deficient plasma as a substrate as previously described.2 All coagulation assays were carried out on cells cultured in low serum-containing Advanced DMEM/F-12 media (2% FBS) or AIM V media for 24 hours before assaying fVIII activity.
Measurement of fVIII transgene copy number. Transgene copy number was determined by either real-time quantitative PCR or competitive PCR analysis of genomic DNA isolated from transduced cells using the following fVIII-specific primers-forward:5′-TGG GAC CTT TAC TTT ATG G-3′ and reverse: 5′-AAA AAC ATA GCC ATT GAT GCT GTG-3′.To determine transgene copy number by competitive PCR, a protocol was developed in which a known amount of fVIII encoding pUA2 plasmid is added to a PCR reaction containing a known amount total genomic DNA harboring an unknown number of proviral integration events. The derived product from the pUA2 plasmid comprises an fVIII product containing a 70-bp deletion that can be distinguished from the integrated fVIII transgene. During the PCR reaction, the pUA2 template competes with the integrated fVIII transgene in the genomic DNA and the point of equivalent amplification determines the number of fVIII transgenes in the unknown sample. The PCR reactions were carried out in 25 µl of total volume containing 1× Taq PCR Master Mix, 2 µmol/l forward and reverse primers, and 100 ng of total DNA. PCR cycling was carried out in a Mastercycler gradient (Eppendorf, Westbury, NY) by performing one cycle at 95 °C for 3 minutes followed by 30 cycles at 95 °C for 1 minute, 58 °C for 1 minute, and 72 °C for 1 minute, and a final extension at 72 °C for 10 minutes. Densitometric quantitation of PCR products (520 bp for fVIII transgene and 450 bp for competitor) was performed using the ImageJ 1.37v Software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD). The percent competitor density as a function of copies per reaction and the point of equivalence was determined by linear regression analysis.
Measurement of fVIII transcript expression. Porcine fVIII RNA standard, used for absolute quantitation of fVIII transcripts by reverse transcription RT-PCR, were generated as described previously.2 PCR reactions were carried out in 25 µl of total volume containing 1× SYBR Green PCR master mix, 300 µmol/l forward and reverse primers, 12.5 units of MultiScribe, 10 units of RNase Inhibitor, and 5 ng of sample RNA. PCR reactions using porcine fVIII RNA standard also included 5 ng of yeast tRNA to mimic RNA environment of sample RNA using serial dilutions (102–106 transcripts/reaction) as template RNA. The oligonucleotide primers used were located in the fVIII sequence encoding the A2 domain located at positions 2,047–2,067 for forward primer (5′-ATGCACAGCATCAATGGCTAT-3′) and 2,194–2,213 for reverse primer (5′-GTGAGTGTGTCTTCATAGAC-3′) of the human fVIII cDNA sequence. Primers recognize both human and porcine fVIII as sequences. One-step real time RT-PCR was performed by incubation at 48 °C for 30 minutes for reverse transcription followed by one cycle at 95 °C for 10 minutes, and 40 amplification cycles of 90 °C for 15 seconds then 60 °C for 1 minute. Postreaction dissociation analysis was performed to confirm single-product amplification by performing a single cycle of 95 °C for 15 seconds, 60 °C for 30 seconds, and 95 °C for 15 seconds. Number of transcripts per cell was determined using an average value of 142.7 transcripts per 5 ng of RNA.
Northern blot analysis. A total of 5 µg of RNA sample were separated, transferred, hybridized, and detected using a 1% agarose/formaldehyde/MOPS gel following the technique outlined in the DIG Application Manual for Filter Hybridization (Roche). Digoxigenin (DIG)-labeled RNA molecular weight marker I (Roche) was used as a marker. Hybridization was performed using a PCR DIG-labeled probe specific to either porcine A1 domain (A1 probe) or human A2 domain (A2 probe) generated using the PCR DIG Probe Synthesis Kit (Roche). The oligonucleotide primers used to generate for A1 and A2 probe were 5′-CGGCAAAGTGAACTCCTCCGTG–3′ and 5′-CTGACGTGAGCCTCCATGCCACCA-3′ (porcine fVIII cDNA sequence) or 5′-ACATTGCTGCTGAAGAGGAGGACT–3′ and 5′-ATGTTGGAGGCTTGGAACTCTGGA–3′ (human fVIII cDNA sequence), respectively. Immunological detection of DIG-labeled probes was performed using the DIG Wash and Block Buffer Set (Roche) using a concentration of anti-DIG-AP of 1:25,000. CDP-Star (Roche) was used for chemiluminescent substrate detection of alkaline phosphatase. Imaging was performed using a Luminescent image analyzer (LAS)-3000 (FUJIFILM) followed by X-ray film (Kodak).
Southern blot analysis. To confirm that pcDNA/FRT clones contain a single integration event, 8 µg of genomic DNA from each stable clone was digested with ScaI and fragments separated on a 1% agarose gel. DNA was transferred by capillary action using 20× SSC transfer buffer to a positively charged nylon membrane and hybridized overnight with a probe complimentary to either the porcine A1 domain or the human A2 domain as described above for northern blot analysis. Stable transfectants with the correct single integration were confirmed by a single band of approximately 12,400 base pairs. Southern blot analysis of HEK-293T HP-fVIII clones was performed using 5–10 µg of AvrII digested genomic DNA as described for pcDNA/FRT clones.
Supplementary Material Figure S1. HP-FVIII cDNA sequence. Table S1. FVIII acvtivity normalized to mRNA transcript copies for transductions at various MOIs.
Supplementary Material
HP-FVIII cDNA sequence.
FVIII acvtivity normalized to mRNA transcript copies for transductions at various MOIs.
Acknowledgments
We thank Arthur Nienhuis (St. Jude University, Memphis, TN) for the SIV vector system. This work was supported by grants from the National Heart, Lung and Blood Institute of the National Institutes of Health to C.B.D. (HL083531), Children's Healthcare of Atlanta, and the Georgia Research Alliance (H.T.S.). C.B.D. and H.T.S. are cofounders and hold equity positions in Expression Therapeutics, LLC, which owns the intellectual property for high expression fVIII technology. K.W.K. and G.D. are employees of Expression Therapeutics, LLC.
REFERENCES
- Murphy SL., and , High KA. Gene therapy for haemophilia. Br J Haematol. 2008;140:479–487. doi: 10.1111/j.1365-2141.2007.06942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering CB, Healey JF, Parker ET, Barrow RT., and , Lollar P. High-level expression of recombinant porcine coagulation factor VIII. J.Biol.Chem. 2002;277:38345–38349. doi: 10.1074/jbc.M206959200. [DOI] [PubMed] [Google Scholar]
- Doering CB, Healey JF, Parker ET, Barrow RT., and , Lollar P. Identification of porcine coagulation factor VIII domains responsible for high level expression via enhanced secretion. J Biol Chem. 2004;279:6546–6552. doi: 10.1074/jbc.M312451200. [DOI] [PubMed] [Google Scholar]
- Doering CB, Gangadharan B, Dukart HZ., and , Spencer HT. Hematopoietic stem cells encoding porcine factor VIII induce pro-coagulant activity in hemophilia A mice with pre-existing factor VIII immunity. Mol Ther. 2007;15:1093–1099. doi: 10.1038/sj.mt.6300146. [DOI] [PubMed] [Google Scholar]
- Gangadharan B, Parker ET, Ide LM, Spencer HT., and , Doering CB. High-level expression of porcine factor VIII from genetically modified bone marrow-derived stem cells. Blood. 2006;107:3859–3864. doi: 10.1182/blood-2005-12-4961. [DOI] [PubMed] [Google Scholar]
- Ide LM, Gangadharan B, Chiang KY, Doering CB., and , Spencer HT. Hematopoietic stem-cell gene therapy of hemophilia A incorporating a porcine factor VIII transgene and nonmyeloablative conditioning regimens. Blood. 2007;110:2855–2863. doi: 10.1182/blood-2007-04-082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow RT, Healey JF, Jacquemin MG, Saint-Remy JM., and , Lollar P. Antigenicity of putative phospholipid membrane-binding residues in factor VIII. Blood. 2001;97:169–174. doi: 10.1182/blood.v97.1.169. [DOI] [PubMed] [Google Scholar]
- Herder C, Tonn T, Oostendrop R, Becker S, Keller U, Peschel C, et al. Sustained expansion and transgene expression of coagulation factor VIII-transduced cord blood-derived endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2003;23:2266–2272. doi: 10.1161/01.ATV.0000100403.78731.9F. [DOI] [PubMed] [Google Scholar]
- Tiede A, Eder M, von Depka M, Battmer K, Luther S, Kiem HP, et al. Recombinant factor VIII expression in hematopoietic cells following lentiviral transduction. Gene Ther. 2003;10:1917–1925. doi: 10.1038/sj.gt.3302093. [DOI] [PubMed] [Google Scholar]
- Kootstra NA, Matsumura R., and , Verma IM. Efficient production of human FVIII in hemophilic mice using lentiviral vectors. Mol Ther. 2003;7::623–631. doi: 10.1016/s1525-0016(03)00073-x. [DOI] [PubMed] [Google Scholar]
- Moayeri M, Hawley TS., and , Hawley RG. Correction of murine hemophilia a by hematopoietic stem cell gene therapy. Mol Ther. 2005;12:1034–1042. doi: 10.1016/j.ymthe.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayeri M, Ramezani A, Morgan RA, Hawley TS., and , Hawley RG. Sustained phenotypic correction of hemophilia a mice following oncoretroviral-mediated expression of a bioengineered human factor VIII gene in long-term hematopoietic repopulating cells. Mol Ther. 2004;10:892–902. doi: 10.1016/j.ymthe.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Chuah MK, Collen D., and , VandenDriessche T. Clinical gene transfer studies for hemophilia A. Semin Thromb Hemost. 2004;30:249–256. doi: 10.1055/s-2004-825638. [DOI] [PubMed] [Google Scholar]
- Pierce GF, Lillicrap D, Pipe SW., and , Vandendriessche T. Gene therapy bioengineered clotting factors and novel technologies for hemophilia treatment. J Thromb Haemost. 2007;5:901–906. doi: 10.1111/j.1538-7836.2007.02410.x. [DOI] [PubMed] [Google Scholar]
- Swaroop M, Moussalli M, Pipe SW., and , Kaufman RJ. Mutagenesis of a potential immunoglobulin-binding protein-binding site enhances secretion of coagulation factor VIII. J Biol Chem. 1997;272:24121–24124. doi: 10.1074/jbc.272.39.24121. [DOI] [PubMed] [Google Scholar]
- Miao HZ, Sirachainan N, Palmer L, Kucab P, Cunningham MA, Kaufman RJ, et al. Bioengineering of coagulation factor VIII for improved secretion. Blood. 2004;103:3412–3419. doi: 10.1182/blood-2003-10-3591. [DOI] [PubMed] [Google Scholar]
- Evans GL., and , Morgan RA. Genetic induction of immune tolerance to human clotting factor VIII in a mouse model for hemophilia A. Proc Natl Acad Sci USA. 1998;95:5734–5739. doi: 10.1073/pnas.95.10.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuah MK, Brems H, Vanslembrouk V, Collen D, Vandendriessche T, Chiang GG, et al. Bone marrow stromal cells as targets for gene therapy of hemophilia A. Hum Gene Ther. 1998;9:353–365. doi: 10.1089/hum.1998.9.3-353. [DOI] [PubMed] [Google Scholar]
- Chuah MK, van Damme A, Zwinneh H, Goovaerts I, Vanslembrouck V, Collen D, et al. Long-term persistence of human bone marrow stromal cells transduced with factor VIII-retroviral vectors and transient production of therapeutic levels of human factor VIII in nonmyeloablated immunodeficient mice. Hum Gene Ther. 2000;11:729–738. doi: 10.1089/10430340050015626. [DOI] [PubMed] [Google Scholar]
- Van Damme A, Chuah MK, Collen D., and , VandenDriessche T. Onco-retroviral and lentiviral vector-based gene therapy for hemophilia: preclinical studies. Semin Thromb Hemost. 2004;30:185–195. doi: 10.1055/s-2004-825632. [DOI] [PubMed] [Google Scholar]
- Thorrez L, VandenDriessche T, Collen D., and , Chuah MK. Preclinical gene therapy studies for hemophilia using adenoviral vectors. Semin Thromb Hemost. 2004;30:173–183. doi: 10.1055/s-2004-825631. [DOI] [PubMed] [Google Scholar]
- Chao H, Mao L, Bruce AT., and , Walsh CE. Sustained expression of human factor VIII in mice using a parvovirus-based vector. Blood. 2000;95:1594–1599. [PubMed] [Google Scholar]
- Scallan CD, Liu T, Parker AE, Patarroyo-White SL, Chen H, Jiang H, et al. Phenotypic correction of a mouse model of hemophilia A using AAV2 vectors encoding the heavy and light chains of FVIII. Blood. 2003;102:3919–3926. doi: 10.1182/blood-2003-01-0222. [DOI] [PubMed] [Google Scholar]
- Scallan CD, Lillicrap D, Jiang H, Qian X, Patarroyo-White SL, Parker AE, et al. Sustained phenotypic correction of canine hemophilia A using an adeno-associated viral vector. Blood. 2003;102:2031–2037. doi: 10.1182/blood-2003-01-0292. [DOI] [PubMed] [Google Scholar]
- Burton M, Nakai H, Colosi P, Cunningham J, Mitchell R., and , Couto L. Coexpression of factor VIII heavy and light chain adeno-associated viral vectors produces biologically active protein. Proc Natl Acad Sci USA. 1999;96:12725–12730. doi: 10.1073/pnas.96.22.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitschier J, Wood WI, Goralka TM, Wion KL, Chen EY, Eaton DH, et al. Characterization of the human factor VIII gene. Nature. 1984;312:326–330. doi: 10.1038/312326a0. [DOI] [PubMed] [Google Scholar]
- Liu W, Xiong Y., and , Gossen M. Stability and homogeneity of transgene expression in isogenic cells. J Mol Med (Berl) 2006;84:57–64. doi: 10.1007/s00109-005-0711-z. [DOI] [PubMed] [Google Scholar]
- Park F, Ohashi K., and , Kay MA. Therapeutic levels of human factor VIII and IX using HIV-1-based lentiviral vectors in mouse liver. Blood. 2000;96:1173–1176. [PubMed] [Google Scholar]
- Kang Y, Xie L, Tran D, Stein CS, Hickey M, Davidson BL., and , McCray PB. Persistent expression of fVIII in vivo following nonprimate lentiviral gene transfer. Blood. 2005;106:1552–1558. doi: 10.1182/blood-2004-11-4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox DA, Shi Q, Nurden P, Haberichter SL, Rosenberg JB, Johnson BD, et al. Induction of megakaryocytes to synthesize and store a releasable pool of human factor VIII. J Thromb Haemost. 2003;12:2477–2489. doi: 10.1111/j.1538-7836.2003.00534.x. [DOI] [PubMed] [Google Scholar]
- Ohmori T, Mimuro J, Takano K, Madoiwa S, Kashiwakura Y, Ishiwata A, et al. Efficient expression of a transgene in platelets using simian immunodeficiency virus-based vector harboring glycoprotein Ibalpha promoter: in vivo model for platelet-targeting gene therapy. FASEB J. 2006;20:1522–1524. doi: 10.1096/fj.05-5161fje. [DOI] [PubMed] [Google Scholar]
- Shi Q, Wilcox DA, Fahs SA, Fang J, Johnson BD, DU LM, et al. Lentivirus-mediated platelet-derived factor VIII gene therapy in murine haemophilia A. J Thromb Haemost. 2007;5:352–361. doi: 10.1111/j.1538-7836.2007.02346.x. [DOI] [PubMed] [Google Scholar]
- Neyman M, Gewirtz J., and , Poncz M. Analysis of the spatial and temporal characteristics of platelet-delivered factor VIII-based clots. Blood. 2008;112:1101–1108. doi: 10.1182/blood-2008-04-152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa H, Hematti P, Keyvanfar K, Metzger ME, Krouse A, Donahue RE, et al. Efficient gene transfer into rhesus repopulating hematopoietic stem cells using a simian immunodeficiency virus-based lentiviral vector system. Blood. 2004;103:4062–4069. doi: 10.1182/blood-2004-01-0045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HP-FVIII cDNA sequence.
FVIII acvtivity normalized to mRNA transcript copies for transductions at various MOIs.