Abstract
Artemin is a neurotrophic factor of the glial cell line–derived neurotrophic factor (GDNF) family of ligands that acts through the GDNF family receptor α3 (GFRα3)/ret receptor found predominantly on sensory and sympathetic neurons. In order to explore the potential utility of artemin to improve functional outcome after spinal cord injury (SCI), we constructed a nonreplicating herpes simplex virus (HSV)-based vector to express artemin (QHArt). We found that QHArt efficiently transfects spinal cord neurons to produce artemin. Transgene-mediated artemin supported the extension of neurites by primary dorsal root ganglion neurons in culture, and allowed those cells to overcome myelin inhibition of neurite extension through activation of protein kinase A (PKA) to phosphorylate cyclic adenosine monophosphate (cAMP) response element binding protein (CREB) and increase expression of arginase I. Intraspinal injection of QHArt immediately after thoracic spinal cord dorsal over hemisection produced a statistically significant improvement in motor recovery over the course of four weeks measured by locomotor rating score.
Introduction
Artemin is one of the glial cell line–derived neurotrophic factor (GDNF) family of growth promoting peptides. A member of the transforming growth factor β superfamily of cystine knot proteins, artemin functions as a homodimer to signal through the ret receptor tyrosine kinase.1 Activation of ret by GDNF family ligands requires binding to the GDNF family receptor α (GFRα) coreceptor, a glycosyl phosphatidylinositol anchored membrane protein that recruits ret to the lipid raft and triggers association of ret with intracellular downstream mediators of GDNF family ligand signaling.2 The GFRα subtype GFRα3 serves as the specific coreceptor for artemin,3 although there is evidence that artemin may also cross-activate the GDNF-specific GFRα1 coreceptor.
Artemin signaling is critical for embryonic survival and migration of sympathetic neuron precursors,4 though later in development these cells downregulate expression of GFRα3 to become dependent on target-derived nerve growth factor. During development GFRα3 is expressed in peripheral nerve and some sensory neurons5,6 and in vitro artemin modulates actin polymeration and formation of lamellopodia through regulation of a number of actin interacting proteins and phosphorylation of Src-kinase.7,8 Systemic delivery of artemin enhances regeneration and improves sensory function following injury to the central or peripheral axon of dorsal root ganglia (DRG) neurons.7,9,10
The failure of axons to regenerate after spinal cord injury (SCI) remains a challenge. Inhibitory proteins in central nervous system (CNS) myelin and the formation of the glial scar after SCI are in part responsible for the failure of central axonal growth to long distances. Exposure of neurons to growth factors prevents myelin inhibition of neurite growth by a cyclic adenosine monophosphate (cAMP) dependent mechanism11 and injection of membrane-permeable analogs of cAMP into DRG promotes regeneration of primary sensory axons across the site of spinal injury,12 and inhibition of phosphodiesterase IV to prevent cAMP hydrolysis enhances growth of central serotonergic axons after SCI.13 Stimulation of neurite growth by db-cAMP is, in part, dependent on transcription activation through the cAMP response element binding protein (CREB)14 that leads to an increase in arginase I and subsequent increase of polyamine synthesis.15
Because of the potent neurotrophic and neuroprotective effects of GDNF family ligands in several different models of injury to the adult nervous system, and the observation that GFRs are widely distributed in the spinal cord, we were interested in exploring whether artemin, acting through the cAMP-CREB-arginase I pathway, might improve recovery following injury to the spinal cord. In order to restrict the distribution of this potent bioactive peptide, we constructed a nonreplicating herpes simplex virus (HSV)-based vector to express artemin, and examined the effect of vector administration in dorsal hemisection model of traumatic thoracic SCI.
Results
Construction of an artemin-expressing HSV vector
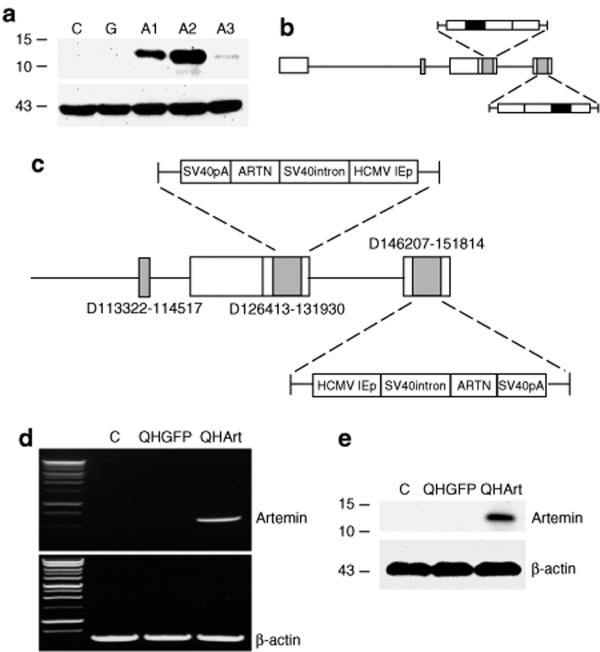
Full length rat artemin was amplified from a cDNA library prepared from total RNA extracted from rat lung, cloned into BamH1-EcoR1 cut HCMV-polyA/SASB3-16 and co-transfected with the nonreplicating HSV recombinant UL41E1G6 on 7b cells. Three clones (designated A1, A2, and A3) selected by identification of clear plaques and purified by limiting dilution were confirmed by PCR followed by DNA sequencing of the insert. Of these recombinants, clone A2 expressed the highest levels of artemin on infection of 293 cells (Figure 1a), was designated QHArt (Figure 1b,c), propagated to high titer (2 × 1011 pfu/ml), and used in the experiments, as described later elsewhere. Control vector QHGFP is identical to QHArt except that the gene for green fluorescent protein (GFP) was placed in the expression cassette in place of the artemin gene.
Figure 1.
Vector construction and characterization. (a) Expression of artemin protein in 293 cells infected with isolates A1, A2, A3. Control (C) and QHGFP (G) infected cells do not express artemin. Lower blot shows β-actin loading control. (b) Herpes simplex virus (HSV) vector schematic. Black rectangles indicate site of artemin (or GFP) insertion in the vector. (c) Schematic indicating specific deletions in the HSV genome and the components of the transgene cassettes inserted into both copies of ICP4. (d) Reverse transcriptase-PCR of artemin from lysate E17 spinal cord neurons infected with QHArt or QHGFP at multiplicity of infection (MOI) of 1. (e) Western blot of artemin in lysate of E17 spinal cord neurons infected with QHArt or QHGFP at MOI of 1. C represents uninfected control. HCMV IEp, human cytomegalovirus immediate-early promoter.
QHArt expresses artemin in primary spinal cord neurons in vitro
Primary spinal cord neurons were cultured from E17 rat embryos, as described in Materials and Methods. These cultures consist of 95% neurons, as determined by β-III tubulin and GFAP immunostaining (data not shown). Infection of spinal cord neuronal cultures for 2 hours with QHArt at a multiplicity of infection of 1, resulted in robust expression of artemin RNA determined by reverse transcriptase (RT)-PCR (Figure 1d) and protein determined by western blot (Figure 1e) of cell lysate, 24 hours after infection.
QHArt-produced artemin supports neurite extension in vitro
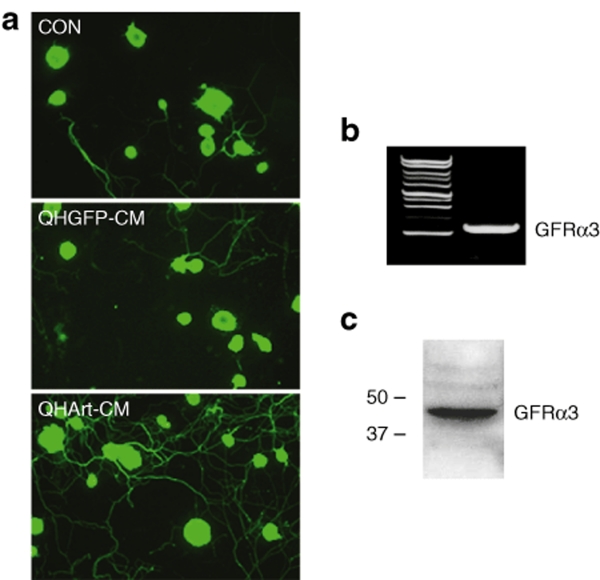
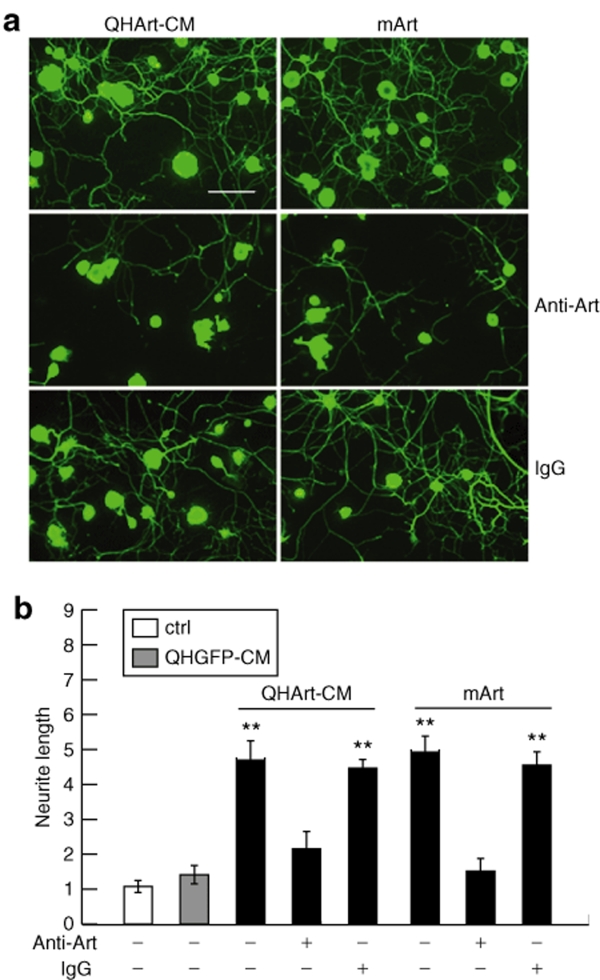
Culture medium containing QHArt-produced artemin was collected 24 hours after infection of primary spinal cord neurons in vitro by QHArt (multiplicity of infection of 1). Exposure of primary P10 DRG neurons to the conditioned medium (CM) resulted in robust extension and elongation of neuritic processes from these cells (Figure 2a). This observed effect is consistent with expression of GFRα3 by the neurons as determined by RT-PCR (Figure 2b) and western blot (Figure 2c). To confirm that the observed effect was mediated by artemin acting through GFRα3, we compared the effect of QHArt-CM with recombinant mouse artermin (mArt, 100 ng/ml). Both QHArt-CM and mArt produced similar effects on neurite extension (Figure 3a,b). To further confirm that the effect of the QHArt-CM was due to artemin in the medium, we determined that both of these effects were blocked by coadministration of a polyclonal antiartemin antibody with QHArt-CM (Figure 3a,b).
Figure 2.
QHArt-CM promotes neurite extension in primary DRG neurons in culture. (a) DRG neurons exposed to QHGFP-CM or QHArt-CM for 20 hours, stained with the Tuj-1 antibody against β-III tubulin. (b) GFRα3 mRNA expression in untreated primary DRG neurons determined by reverse transcriptase-PCR. (c) GFRα3 protein expression in untreated primary DRG neurons determined by western blot. CM, conditioned medium; DRG, dorsal root ganglia; GFRα3, glial cell line–derived neurotrophic factor family receptor α3.
Figure 3.
The QHArt-CM effect is mediated by artemin. (a) Neurite extension promoted by QHArt-CM (left column) and 100 ng/ml recombinant mouse artemin (mArt) (right column) is blocked by antiartemin antibody (20 µg/ml) but not control IgG. Cells immunostained with Tuj-1 antibody. (b) Quantitative analysis of the effect of QHArt-CM, mArt, and the antiartemin antibody on neurite extension Bar = 40 µm. Data are from four separate experiments. Neurite outgrowth expressed as a ratio to control is presented as mean ± SEM; **P < 0.01 compared to control. CM, conditioned medium; IgG, immunoglobulin G.
QHArt effects are mediated through PKA, pCREB, and arginase I
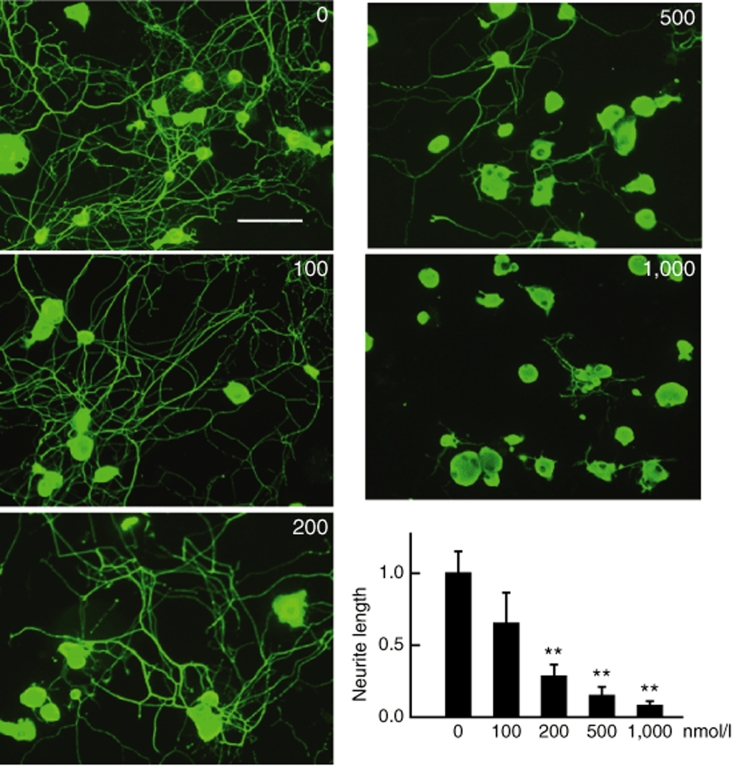
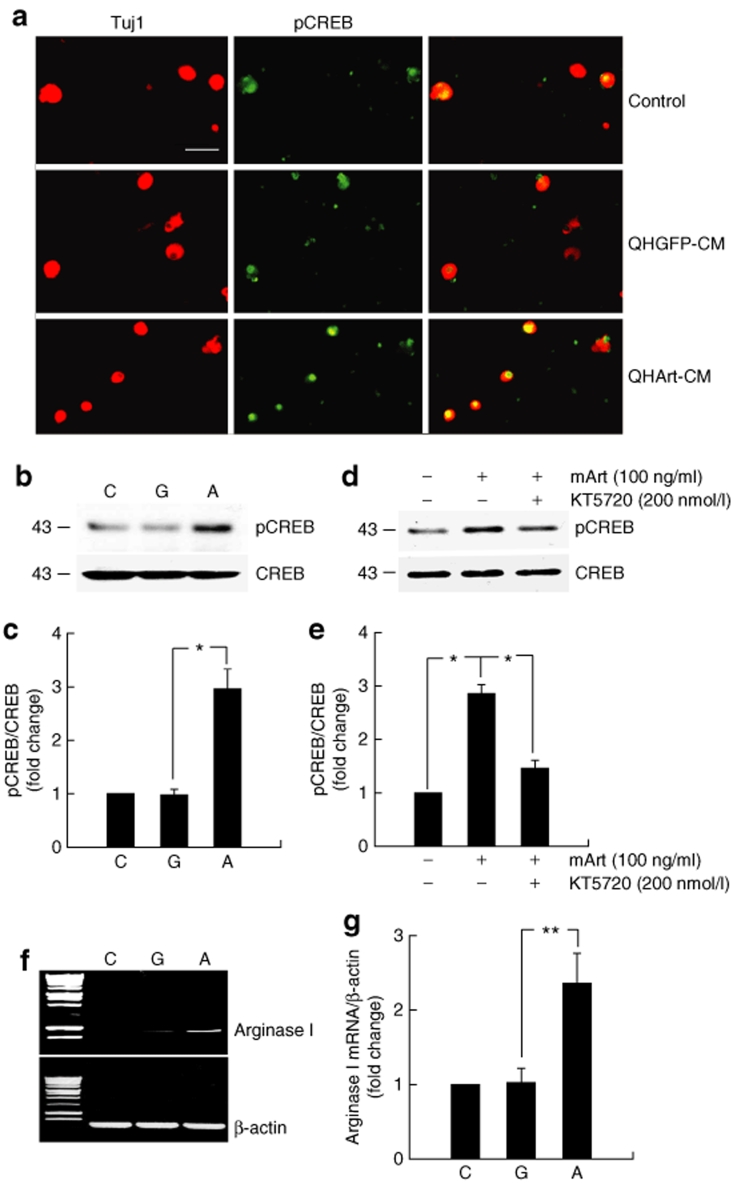
Because previous reports have demonstrated that GDNF activation of GFRα1 produces neurite extension through activation of protein kinase A (PKA),11 we examined whether artemin activation of GFRα3 might act through PKA. We found that the effect of QHArt-CM on neurite extension was blocked in a dose dependent fashion by addition of the PKA inhibitor KT5720 (Figure 4). Because some of the reported effects of PKA activation in the setting of myelin inhibition are mediated through CREB,14 we examined phosphorylation of CREB in cells exposed to QHArt-CM or mArt. Thirty minutes of exposure to QHArt-CM or mArt resulted in a dramatic increase in pCREB in DRG neurons detected by immunocytochemistry (Figure 5a) and western blot (Figure 5b,d). The effect of mArt on phosphorylation of CREB was partially blocked by KT5720. In agreement with other evidence suggesting that other GDNF family ligands act through pCREB to increase expression of arginase I,15 we found that after 2 hours of treatment with QHArt there was a substantial increase in arginase I mRNA in the DRG neurons, as determined by RT-PCR (Figure 5f).
Figure 4.
The QHArt-CM effect on neurite extension is blocked by the protein kinase A inhibitor KT5720. Increasing concentrations of KT5720 added at the time of plating reduced the length of neurites from dorsal root ganglia neurons exposed to QHArt-CM in a dose dependent manner. Cells immunostained with Tuj-1 antibody. Bar = 40 µm. Data represents results of three separate experiments. Neurite outgrowth expressed as a ratio to control is presented as mean ± SEM; **P < 0.01 compared to QHArt-CM alone. CM, conditioned medium.
Figure 5.
Transgene-mediated artemin provokes phosphorylation of cyclic adenosine monophosphate response element binding protein (CREB) and increases expression of arginase I. (a) Immunostaining for pCREB (green) 30 minutes after treatment with QHGFP- or QHArt-CM. Tuj-1 immunostaining shown in red. (b) Western blot of dorsal root ganglia (DRG) cell lysate 30 minutes after exposure to QHGFP or QHArt-CM. (c) Quantitative analysis of western blot shown in b. (d) Cultured DRG neurons were treated with 200 nmol/l KT5720 for 10 minutes, followed by exposure to mouse artemin (mArt) (100 ng/ml) for 30 minutes. The effect of mArt on phosphorylation of CREB was partially blocked by KT5720. (e) Quantitation of western blot shown in d. (f) DRG neurons exposed to QHArt-CM but not QHGFP-CM for 2 hours show increased arginase I mRNA (reverse transcriptase-PCR). (g) Quantitation of western blot shown in f. The graphs present the results of three independent experiments as mean ± SEM; *P < 0.05, **P < 0.01; C = control; G = QHGFP-CM; A = QHArt-CM. Bar = 20 µm. CM, conditioned medium.
Transgene-mediated artemin attenuates myelin inhibition of axonal growth in vitro
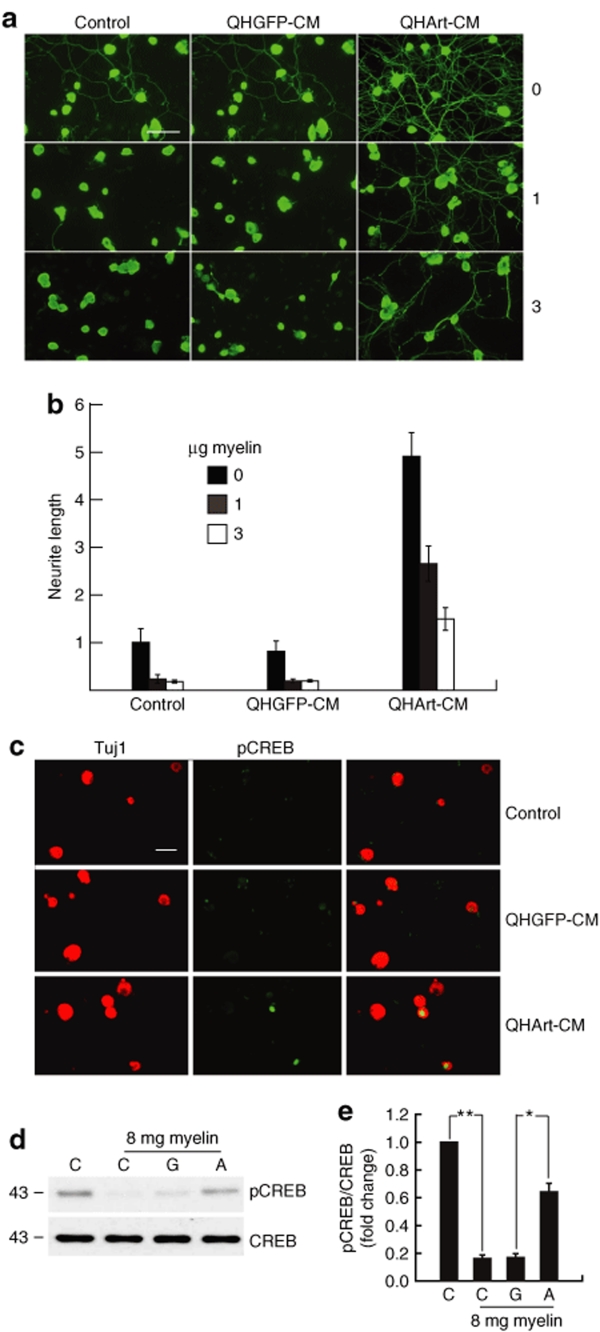
CNS myelin is a major inhibitors of axon elongation after injury in vivo, an effect that can be modeled by the inhibition of neurite extension in vitro. To examine the effect of QHArt-mediated artemin on axonal elongation in the presence of CNS myelin, we cultured primary DRG neurons on wells plated with CNS myelin containing NogoA, MAG, and OMgp proteins. CNS myelin produced a dose-dependent inhibition of neurite extension (Figure 6a,b) that was attenuated by 20-hour exposure to QHArt-CM (Figure 6a,b). In a manner similar to the effect of artemin on DRG neurons cultured in the absence of myelin, QHArt-CM treatment of DRG neurons exposed to myelin resulted in an increase in pCREB in those neurons (Figure 6c,d).
Figure 6.
Transgene-mediated artemin attenuates myelin induced inhibition of axon growth in vitro. (a) Tuj1 immunostaining of DRG neurons plated on 0, 1, or 3 µg central nervous system myelin per well of eight-well chamber slide in the presence of QHGFP-CM or QHArt-CM. Bar = 40 µm. (b) Myelin produces a dose dependent inhibition of neurite extension that is substantially reversed by QHArt-CM. (c) Immunostaining for pCREB (green) of DRG neurons exposed to 1 µg/well myelin. Tuj-1 immunostaining shown in red; Bar = 20 µm. (d) DRG neurons were plated on 24 well plates containing 8.0 µg myelin per well, to which QHGFP-CM or QHArt-CM was added for 30 minutes. Treatment with QHArt-CM attenuated the reduction in CREB phosphorylation caused by exposure to myelin. (e) Quantitation of western blot representing the results of three independent experiments as mean ± SEM; *P < 0.05, **P < 0.01; C = control; G = QHGFP-CM; A = QHArt-CM. CM, conditioned medium; CREB, cyclic adenosine monophosphate response element binding protein; DRG, dorsal root ganglia.
QHArt-mediated artemin expression improves functional recovery after SCI
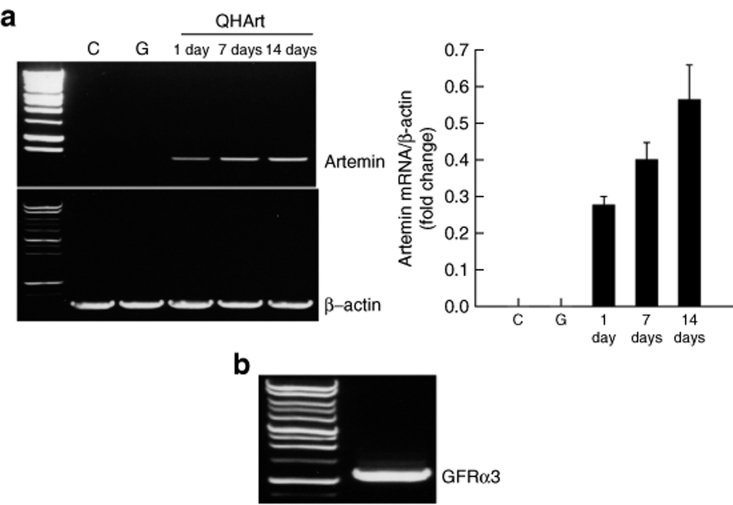
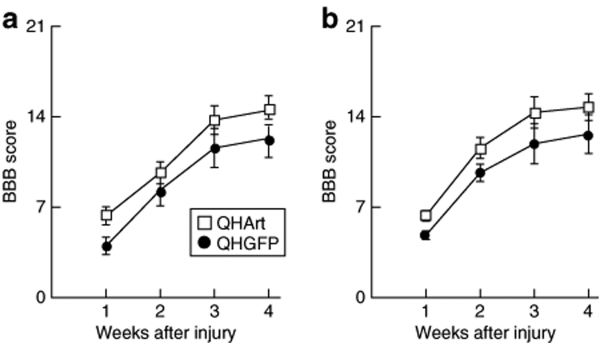
In order to examine the potential of artemin to improve recovery after SCI, we injected 2 µl containing 4 × 108 plaque forming units (pfu) of QHArt directly into the spinal cord at each of the two sites, in the midline 2 mm above and below the lesion at a depth of 1 mm from the surface and at a rate of 1 µl/minute. Transgene-mediated expression of artemin could be detected by RT-PCR beginning as early as 1 day after injection and persisting through 14 days postinjection (Figure 7a). We also determined by RT-PCR that GFRα3 is expressed in the adult spinal cord (Figure 7b). Injection of QHArt into the injured spinal cord 2 hours after dorsal hemisection resulted in a statistically significant improvement in locomotor function bilaterally in the hind limbs assessed by Basso, Beattie, Bresnahan (BBB) locomotor rating scale (Figure 8).
Figure 7.
Intraspinal injection of QHArt produces artemin in vivo. (a) 2 µl of QHArt or QHGFP containing 4 × 108 pfu was injected into thoracic spinal cord. Artemin RNA was detected at 1, 7, and 14 days after injection (QHArt). Control (C); injected with QHGFP (G). (b) GFRα3 RNA is expressed in uninjected thoracic spinal cord (reverse transcriptase-PCR). N = 4 rats per group. GFRα3, glial cell line–derived neurotrophic factor family receptor α3.
Figure 8.
QHArt improves locomotor recovery after spinal cord injury. BBB score for (a) left hind limb and (b) right hind limb over the course of 4 weeks after spinal dorsal over hemisection in rats injected with QHGFP or QHArt. N = 8 animals per group; P < 0.05 by repeated measures ANOVA.
Discussion
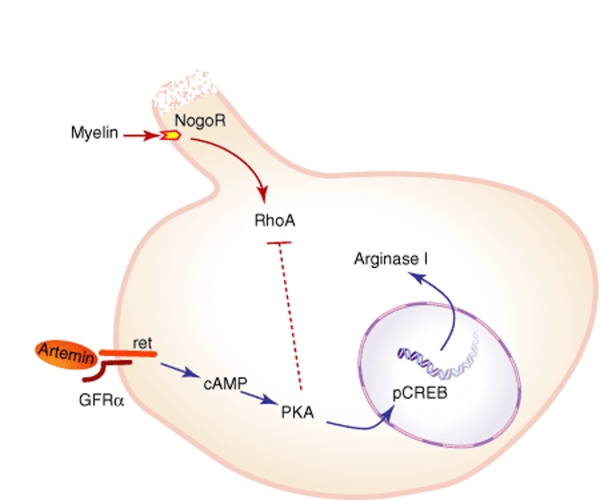
There are four major observations from this study (Figure 9). First, artemin acting through GFRα3/ret activates PKA to phosphorylate pCREB and increases expression of arginase I. Second, artemin supports neurite extension by embryonic DRG neurons in tissue culture conditions. Third, activation of GFRα3/ret activates a PKA-dependent pathway to allow these neurons to overcome the inhibitory effect of CNS myelin on neurite extension. Finally, HSV-mediated expression of artemin in spinal cord, following SCI, improves functional outcome as measured by the BBB score.
Figure 9.
Schematic representation of the artemin pathway in neurons. cAMP, cyclic adenosine monophosphate; CREB, cAMP response element binding protein; GFR α, glial cell line–derived neurotrophic factor family receptor α; PKA, protein kinase A.
GFRα3 is widely expressed in the peripheral nervous system3,16 but does not appear to be critical for survival of sensory neurons during development as DRG appear to be normal in transgenic mice that fail to express either artemin or GFRα3.17 We found that in embryonic DRG neurons under normal culture conditions, application of recombinant artemin protein or CM containing artemin expressed from cells infected with the artemin-expressing vector resulted in the substantial extension of neurites. This result suggests that the formation and maintenance of neurite processes during development may be influenced by artemin.
A critical issue in SCI is the inhibition of axonal extension and regeneration by inhibitory molecules expressed on CNS myelin by oligodendrocytes.18 The full range of intracellular signaling pathways activated by myelin inhibitors including NogoA, MAG, and OMgp to inhibit axonal elongation are beyond the scope of this summary, but several lines of evidence have indicated that this inhibition can be overcome by treatments designed to increase intracellular cAMP levels to activate PKA.19 PKA effect has been shown to be dependent on transcription and to act through CREB to increase expression of genes under the control of the cAMP-response element, including arginase I.14,19 We found in these studies that artemin (either delivered as a recombinant protein or as the transgene-mediated product from CM activates PKA and increases phosphorylation of CREB to lead to an increase in expression of arginase I, and that activation of this pathway allows sensory axons to overcome the inhibitory effect of myelin on process extension in vitro. Arginase I is a key enzyme in the synthesis of a small group of simple aliphatic amines, and these polyamines have been implicated in multiple neuronal processes during development as well as in the protective neuronal response to insult. Polyamines regulate microtubule organization as well as chromatin structure and transcriptional activity, and through these basic cellular mechanisms may promote neurite growth.20
Finally, we found that direct intraspinal injection of the artemin-expressing HSV vector 2 hours after dorsal hemisection injury to the thoracic spinal cord improves functional outcome up to 4 weeks after injury. Our results are in agreement with the enhanced regeneration and restoration of function observed with systemic administration of artemin after dorsal root injury10 and following peripheral nerve injury.7,9 Recovery from SCI is complex and may involve axonal regeneration or sprouting.21,22 In this study, we did not examine the mechanisms underlying the improvement in behavioral outcome, though the degree of improvement over a relatively short time course would suggest that it is not due to long distance regeneration of severed fibers. Nonetheless, there are no available therapies that provide reliable clinical improvement after SCI. Neurotrophic factors are strong candidate molecules for the development of novel therapeutic strategies, but the widespread distribution of receptors for these short-lived highly bioactive pleiotropic peptides within and outside of the nervous system makes it likely that in order to successful exploit the beneficial effects of these peptides for treatment, there will need to be a strategy to achieve local production and release. In this regard, the use of a nonreplicating, nonintegrating vector such as HSV may prove attractive.
Materials and Methods
Vector construction. Two viral vector constructs were employed in this study. QHArt contains rat artemin gene under the control of the human cytomegalovirus immediate-early promoter (HCMV IEp). Control vector QHGFP is identical to QHArt, but contains the GFP gene product in place of artemin. Full-length artemin was amplified from a cDNA library prepared from total RNA extracted from rat lung and cloned into BamHI-EcoRI cut HCMV-polyA/SASB3-16. The HCMV-Art-polyA/SASB3-16 plasmid was co-transfected with the nonreplicating HSV recombinant UL41E1G6 (provided by Joseph Glorioso, University of Pittsburgh) into 7b cells. Three runs of extraction were performed to select clear plaques and the identity of the insert confirmed by PCR followed by DNA sequencing.
Tissue culture and vector infection. DRG were removed from P10 rats and dissociated into single cells by incubation with 0.06% collagenase and 0.03% trypsin (Sigma, St Louis, MO) for 1 hour at 37 °C. According to manufacturer's recommendation (Invitrogen, Grand Island, NY), dissociated neurons were plated on poly-D-lysine (100 µg/ml) and laminin (8 µg/ml) coated plates in defined Neurobasal medium containing B27, Glutamax I, Albumax I, and penicillin/streptomycin. Spinal cord was removed from E17 rats and dissociated into single cells by incubation with 0.25% trypsin-EDTA (Invitrogen) for 15 minutes at 37 °C. Dissociated spinal cord neurons were plated on poly-D-lysine coated six well plates with 2 ml of defined Neurobasal medium (Invitrogen) and treated with FDU/uridine twice during the first week in culture. E17 spinal cord neurons were cultured for 10 days and then infected with either QHArt or QHGFP for 2 hours after which the medium was replaced with fresh medium. The cell lysate obtained 24 hours after infection was used for determination of artemin expression by RT-PCR and western blot. The medium from vector infected spinal cord neurons obtained 24 hours after infection was used as CM for the subsequent experiments.
Neurite outgrowth assay. P10 DRG were plated on poly-D-lysine (100 µg/ml) and laminin (8 µg/ml) coated eight-well chamber slides at 2 × 104 cells per well. CM, mouse artemin recombinant protein (R&D, Minneapolis, MN), goat polyclonal antiartemin antibody (Santa Cruz Biotechnology, Santa Cruz, CA), goat polyclonal IgG (Cappel, Downington, PA), or the PKA inhibitor KT5720 (Calbiochem, Gibbstown, NJ) were added at plating. After culture for an additional 20 hours cells were fixed and immunostained with antibodies against neuron-specific β-III tubulin (Tuj1; Covance, Berkeley, CA). In the myelin study, eight-well chamber slides were precoated with myelin at concentrations of 0, 1, or 3 µg/well. The slides were examined at room temperature using a Nikon E1000 microscope with air objectives and images acquired using a digital camera (Cool Snap ES; Photometrics, Tucson, AZ) and Metamorph software. Digital images were manipulated and arranged using Photoshop 7.0 (Adobe). The length of β-III tubulin immunostained processes was determined using Meta imaging software (Molecular Devices, Sunnyvale, CA). More than 200 randomly selected individual neurons were analyzed per condition in each experiment, and the experiment was repeated 3–6 times.
Myelin isolation. CNS myelin was prepared by sucrose gradient centrifugation according to the protocol described by Norton and Poduslo.23 The protein concentration of the purified myelin preparation was determined using the Pierce BCA assay (BioRad, Hercules, CA). We confirmed, by gel electrophoresis, that the purified myelin contains three of myelin inhibitory molecules, NogoA, MAG, and OMgp (data not shown).
Western blot. Cultured DRG cells, spinal cord, and 293 cells were lysed in lysis buffer containing 50 mmol/l Tris, 10 mmol/l NaCl, 1% NP40, 0.02% sodium azide, and protease inhibitor cocktail (Sigma Aldrich) at pH 7.4. For the detection of pCREB, cultured DRG neurons were lysed in lysis buffer containing 62.5 mmol/l Tris-HCl and 2% SDS, including phosphatase and protease inhibitor cocktail. Cell lysates were sonicated and centrifuged at 15,000g for 10 minutes at 4 °C. Protein concentration in cell lysates was determined using the BCA assay (Pierce Biotechnology, Rockford, IL) and spectrophotometry (AD340; Beckman Coulter, Fullerton, CA). Aliquots containing 20 µg of protein were dissolved in Laemmli buffer and boiled at 95 °C for 5 minutes, and the proteins separated by 12% Tris-Glycine SDS-PAGE (Bio-Rad), transferred to PVDF membranes, blocked, and then incubated with primary antibodies at 4 °C overnight. Primary antibodies included an antibody against artemin (anti-Art, 1:400; Santa Cruz Biotechnology), anti-pCREB (1:1,000; Cell Signaling, Danvers, MA), and anti-CREB (1:1,000; Cell Signaling). Peroxidase-coupled secondary antibodies (Calbiochem) were used for amplification and protein bands visualized using X-OMAT AR film (Kodak, Rochester, NY), after chemiluminescence (DuPont NEN, Boston, MA). The membranes were stripped and reprobed with mouse anti-β-actin (1:2,000; Sigma) as a loading control. The intensity of each band was determined by quantitative chemiluminescence using a PC-based image analysis system (ChemiDoc XRS System; Bio-Rad Laboratories).
Immunochemistry. P10 DRG were plated on poly-D-lysine (100 µg/ml) and laminin (8 µg/ml) coated eight-well chamber slides at 2 × 104 cells per well in the presence or absence of 1 µg of myelin. CM were added at the same time of plating. Thirty minutes after culture, the cells were fixed in 4% paraformaldehyde, washed in PBS, and blocked with 5% normal goat serum with 0.2% Triton X-100 in PBS. The following antibodies were used: anti-pCREB (1:1,000; Cell Signaling) and anti-Tuj1 (1:1,000; Covance). The secondary antibodies utilized were fluorescent antirabbit IgG Alexa Fluor 594 or antimouse IgG Alexa Fluor 488 (1:2,000; Molecular Probes). The fluorescent images were captured using a Nikon Eclipse E1000 microscope.
Experimental animals and surgical procedures. Female Sprague–Dawley rats weighing 200–250 g were used in all experiments. Housing conditions and experimental procedures were approved by the University of Michigan Committee on Use and Care of Animals. Under isoflurane anesthesia, a T6-T7 laminectomy was performed, and the spinal cord was exposed. The dorsal half of the spinal cord was cut with a pair of microscissors to sever the dorsal parts of the corticospinal tracts, and the depth of lesion (~1.8 mm) was assured by passing the sharp part of a number 11 blade across the dorsal half of the cord. 2 µl of QHArt or QHGFP containing 4 × 108 plaque-forming units were injected at two sites (2 mm rostral and caudal of the lesion) into the spinal cord midline at a depth of 1 mm, at a rate of 1 µl/minute, using a Hamilton syringe. The needle was left in place for 1 minute after the injection and then slowly withdrawn. Muscle and fascia were sutured, closed, and the skin closed with autoclips. After surgery, all animals were maintained under preoperative conditions and were eating and drinking within 3 hours after surgery. Locomotor function was examined and recorded using the BBB Locomotor Rating Scale.24
Tissue for RT-PCR. For the determination of expression of artemin in spinal cord in vivo, a T6-T7 laminectomy was performed under isoflurane anesthesia and 2 µl of QHArt or QHGFP containing 4 × 108 plaque-forming units was injected into the spinal cord midline at a depth of 1 mm and at a rate of 1 µl/minute. The Hamilton syringe was left in place for 1 minute after the injection and slowly withdrawn. After surgery, all animals were maintained under preoperative conditions and were eating and drinking within 3 hours after surgery. At 1, 7, or 14 days after injection the animals were sacrificed and spinal cord tissue 1 cm above and below the injection site was collected fresh and processed, as described later in this study. For GFRα3 mRNA detection, tissue from T6-7 spinal cord of normal rats was collected fresh, placed in TRIzol, and processed as described later in this study.
RT-PCR. Total RNA was isolated from P10 DRG, E17 spinal cord neurons, and spinal cord tissue using TRIzol (Invitrogen) isolation reagent, according to the manufacturer's instructions. cDNA prepared from mRNA was amplified using the following primer sets: β-actin-forward (5′-CAG TTC GCC ATG GAT GAC GAT ATC-3′) and β-actin-reverse (5′-CAC GCT CGG TCA GGA TCT TCA TG-3′) for β-actin, artemin-forward (5′-AAC CAG CCT TGT GGC CAA CCC TAG-3′) and artemin-reverse (5′-GGT GTA GTT GGA CAA GTC GTA GG-3′) for artemin, GFRα3-forward (5′-TAG TGA TCC TGC TAC TGG TGC-3′) and GFRα3-reverse (5′-CCA GAG GGT CTG CAG CAG AAT C-3′) for GFRα3, and arginase I-forward (5′-GCT CCA AGC CAA AGC CCA TAG-3′) and arginase I-reverse (5′-GTA GTC AGT CTC TGG CTT ATG-3′) for arginase I. All reactions involved initial denaturation at 94 °C for 5 minutes, followed by 26 cycles for β-actin and arginase I, 32 cycles for Art and GFRα3 (at 94 °C for 30 seconds, 68 °C for 2 minutes and 1 cycle at 68 °C for 8 minutes using a GeneAmp PCR 2700 (Applied Biosystems).
Data analysis. The statistical significance of the difference between treatment groups was determined by ANOVA. Parametric statistics, using the general linear model for repeated measures, were used to identify significant effects of treatment condition on the behavioral measure of BBB score using SPSS 12.0 for Windows (SPSS). Data are expressed as mean ± SEM, with P < 0.05 considered significant.
Acknowledgments
This work was supported by grants from the Department of Veterans Affairs (M.M. and D.J.F.) and the NIH DK44935, NS38850. We acknowledge the excellent technical assistance of Vikram Thakur Singh in production of the vectors used in this study and Shue Liu in tissue culture.
REFERENCES
- Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev. 2001;12:361–373. doi: 10.1016/s1359-6101(01)00012-0. [DOI] [PubMed] [Google Scholar]
- Airaksinen MS., and , Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger KS, et al. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRα3-RET receptor complex. Neuron. 1998;21:1291–1302. doi: 10.1016/s0896-6273(00)80649-2. [DOI] [PubMed] [Google Scholar]
- Nishino J, Mochida K, Ohfuji Y, Shimazaki T, Meno C, Ohishi S, et al. GFRα3, a component of the artemin receptor, is required for migration and survival of the superior cervical ganglion. Neuron. 1999;23:725–736. doi: 10.1016/s0896-6273(01)80031-3. [DOI] [PubMed] [Google Scholar]
- Golden JP, DeMaro JA, Osborne PA, Milbrandt J., and , Johnson EM., Jr Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp Neurol. 1999;158:504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- Bennett DL, Boucher TJ, Armanini MP, Poulsen KT, Michael GJ, Priestley JV, et al. The glial cell line-derived neurotrophic factor family receptor components are differentially regulated within sensory neurons after nerve injury. J Neurosci. 2000;20:427–437. doi: 10.1523/JNEUROSCI.20-01-00427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DL, Boucher TJ, Michael GJ, Popat RJ, Malcangio M, Averill SA, et al. Artemin has potent neurotrophic actions on injured C-fibres. J Peripher Nerv Syst. 2006;11:330–345. doi: 10.1111/j.1529-8027.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- Jeong DG, Park WK., and , Park S. Artemin activates axonal growth via SFK and ERK-dependent signalling pathways in mature dorsal root ganglia neurons. Cell Biochem Funct. 2008;26:210–220. doi: 10.1002/cbf.1436. [DOI] [PubMed] [Google Scholar]
- Gardell LR, Wang R, Ehrenfels C, Ossipov MH, Rossomando AJ, Miller S, et al. Multiple actions of systemic artemin in experimental neuropathy. Nat Med. 2003;9:1383–1389. doi: 10.1038/nm944. [DOI] [PubMed] [Google Scholar]
- Wang R, King T, Ossipov MH, Rossomando AJ, Vanderah TW, Harvey P, et al. Persistent restoration of sensory function by immediate or delayed systemic artemin after dorsal root injury. Nat Neurosci. 2008;11:488–496. doi: 10.1038/nn2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Shen Y, De Bellard M, Tang S., and , Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier-Lavigne M., and , Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- Gao Y, Deng K, Hou J, Bryson JB, Barco A, Nikulina E, et al. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron. 2004;44:609–621. doi: 10.1016/j.neuron.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Cai D, Deng K, Mellado W, Lee J, Ratan RR., and , Filbin MT. Arginase I and polyamines act downstream from cyclic AMP in overcoming inhibition of axonal growth MAG and myelin in vitro. Neuron. 2002;35:711–719. doi: 10.1016/s0896-6273(02)00826-7. [DOI] [PubMed] [Google Scholar]
- Worby CA, Vega QC, Chao HH, Seasholtz AF, Thompson RC., and , Dixon JE. Identification and characterization of GFRα-3, a novel Co-receptor belonging to the glial cell line-derived neurotrophic receptor family. J Biol Chem. 1998;273:3502–3508. doi: 10.1074/jbc.273.6.3502. [DOI] [PubMed] [Google Scholar]
- Honma Y, Araki T, Gianino S, Bruce A, Heuckeroth R, Johnson E, et al. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron. 2002;35:267–282. doi: 10.1016/s0896-6273(02)00774-2. [DOI] [PubMed] [Google Scholar]
- Liu BP, Cafferty WB, Budel SO., and , Strittmatter SM. Extracellular regulators of axonal growth in the adult central nervous system. Philos Trans R Soc Lond B Biol Sci. 2006;361:1593–1610. doi: 10.1098/rstb.2006.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannila SS., and , Filbin MT. The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp Neurol. 2008;209:321–332. doi: 10.1016/j.expneurol.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimchowitsch S., and , Cassel JC. Polyamine and aminoguanidine treatments to promote structural and functional recovery in the adult mammalian brain after injury: a brief literature review and preliminary data about their combined administration. J Physiol Paris. 2006;99:221–231. doi: 10.1016/j.jphysparis.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ., and , McMahon SB. Spinal cord repair strategies: why do they work. Nat Rev Neurosci. 2006;7:644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- Cafferty WB, McGee AW., and , Strittmatter SM. Axonal growth therapeutics: regeneration or sprouting or plasticity. Trends Neurosci. 2008;31:215–220. doi: 10.1016/j.tins.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton WT., and , Poduslo SE. Myelination in rat brain: method of myelin isolation. J Neurochem. 1973;21:749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- Bresnahan JC, Beattie MS, Todd FD., 3rd, and , Noyes DH. A behavioral and anatomical analysis of spinal cord injury produced by a feedback-controlled impaction device. Exp Neurol. 1987;95:548–570. doi: 10.1016/0014-4886(87)90299-8. [DOI] [PubMed] [Google Scholar]