Abstract
Despite improving survival rates for children with cancer, a subset of patients exist with disease resistant to traditional therapies such as surgery, chemotherapy, and radiation. These patients require newer, targeted treatments used alone or in combination with more traditional approaches. Oncolytic herpes simplex virus (HSV) is one of these newer therapies that offer promise for several difficult to treat pediatric malignancies. The potential benefit of HSV therapy in pediatric solid tumors including brain tumors, neuroblastomas, and sarcomas is reviewed along with the many challenges that need to be addressed prior to moving oncolytic HSV therapy from the laboratory to the beside in the pediatric population.
Introduction
Although pediatric cancer survival rates have improved greatly over the past 30 years, there remains a significant subset of children, ~25%, who succumb to their disease.1,2 Deaths occur when tumors progress in the face of optimal therapies and acquire resistance to current treatment modalities like chemotherapy or radiotherapy, or the toxicities from such therapies become too great. Augmenting the dose of current therapies is likely to increase toxicity without improving survival significantly. Therefore, novel, targeted treatment strategies that evince effective oncolysis are desperately needed to decrease toxicity and, thereby, improve quality of life and survival rates for children with malignancies. This review will focus on the potential benefit of adjunctive oncolytic virotherapy that utilizes genetically engineered herpes simplex viruses, type-1 (HSVs-1).
HSV-1 is a unique virus that offers promise in treating several pediatric cancers including brain tumors, neuroblastomas, and sarcomas. The virus can be employed as oncolytic viral therapy, a direct, targeted attack or via gene therapy in which foreign genes are expressed therapeutically in cancer cells.3 Oncolytic viral therapy relies on virus replication in infected tumor cells that die and release infectious virus. In gene therapy, expression of therapeutic foreign gene products either directly or indirectly leads to cell death. Oncolytic viruses that utilize gene therapy are perceived to be more effective at killing tumors.
Genetically engineered HSV-1 has a number of advantages in the treatment of a subset of pediatric malignancies. HSV-1 is a large (152 kb, 89 genes, multiple open-reading frames), double-stranded DNA, enveloped virus that does not integrate into host DNA. HSV is a neurotropic virus thus making cancers of neural origin, including brain tumors and neuroblastomas, ideal targets.4 However, it is equally able to infect and kill cells from a variety of cancers including sarcomas, melanomas, colon, breast, lung, prostate, and hepatic tumors.5,6,7,8,9,10 Normal cells are spared, whereas tumor cells are targeted by deleting genes critical for viral replication in normal cells but not necessary in tumor cells such as the “neurovirulence” gene, γ134.5.11,12 It has been estimated that up to 30 kb of the HSV genome is nonessential for replication in tumor cells and, therefore, can be replaced with foreign DNA for gene therapy without affecting the virus' ability to infect tumor cells and replicate. From a safety standpoint, antiviral agents in clinical use are readily available in the unlikely event that the mutant HSV produces toxicity to normal tissues.
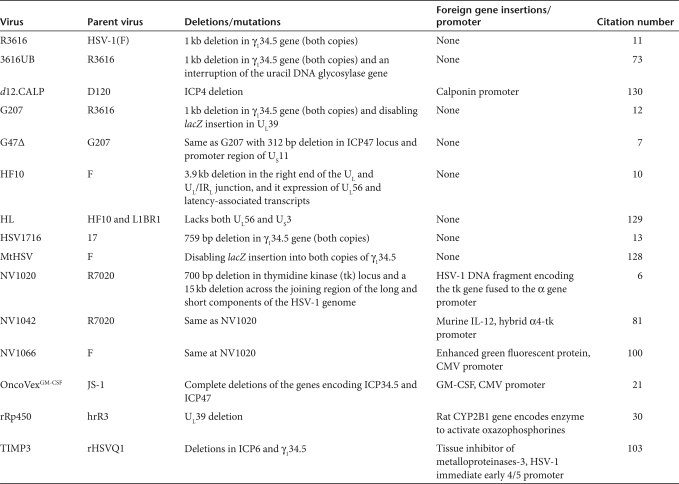
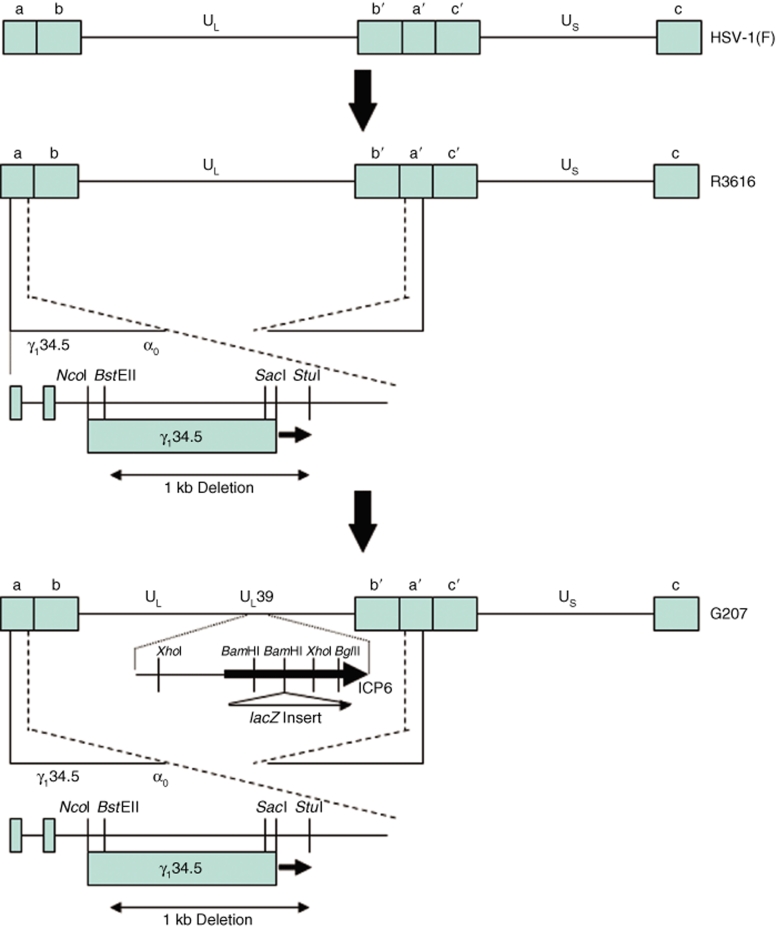
Currently, four different γ134.5-deleted viruses have been used in adult phase I trials (G207, 1716, NV1020, OncoVexGM-CSF). G207 and HSV1716 have been used to treat patients with recurrent glioblastoma multiforme (GBM) (Table 1).12,13 G207 was derived from R3616 that had been created by deleting both copies of γ134.5 gene from the wild-type isolate, HSV-1(F) strain. G207 was created by inserting the lacZ gene encoding β-galactosidase into the UL39 locus, thus disabling the expression of ICP6, the heavy chain for ribonucleotide reductase (Figure 1). HSV1716 was created by deleting both copies of the γ134.5 gene (also known as RL) from the wild-type isolate, strain 17 (Figure 2). HSV-1(F) is a temperature-sensitive isolate, whereas strain 17 is not. These two HSV-1 parents likely have different degrees of virulence, as these γ134.5-deleted variants have different LD50 values for mice.14,15
Table 1.
Summary of engineered HSV discussed in the text
Figure 1.
Schematic representation of the derivation of G207 HSV. Chou et al. deleted both copies of the γ134.5 gene from HSV strain F, a temperature-sensitive clinical isolate (HSV-1(F)) to create recombinant virus R3616.11 Only one of the two deletions is shown for simplicity. This deletion ablated the ability of the virus to overcome interferon resistance in normal cells and made the virus aneurovirulent. Mineta et al.12 modified R3616 by inserting the Escherichia coli β-galactosidase gene (lacZ) to produce a functional deletion of the HSV UL39 gene, which encodes the heavy chain or ribonucleotide reductase (infected cell protein 6—ICP6), as described by Goldstein and Weller.137 This second disabling deletion was performed to ensure added safety for intracerebral human trials.7
Figure 2.
Schematic representation of the derivation of 1716 HSV. In clinical isolate strain 17+ HSV, a 759 bp deletion was produced that extended from the DR1/Ub boundary in the “a” sequence to remove 105 bp on the 5′ end of the RL open reading frame.13 This deletion was produced in both long-repeat regions (terminal repeat-long, inverted repeat-long) of the HSV genome, but only the deletion for the TRL is shown for simplicity. The strain 17+ HSV had a LD50 of <10 PFU, whereas the 1716 mutant was significantly neuroattenuated with a LD50 of 7 × 106 PFU.
In the first trial of G207, a maximum tolerated dose was not reached and no definitive dose-limiting toxicities occurred with stereotactic injection of up to 3 × 109 plaque-forming units (PFUs) of virus directly in up to 5 loci within the enhancing portions of recurrent tumors.16 A phase Ib trial of stereotactic catheter inoculation of G207 followed by resection and reinoculation into the tumor bed of recurrent GBMs likewise confirmed safety.17 Although the trials were only designed to determine safety, response was seen in some patients, and two patients were long-term survivors (>5 years). A third trial utilizing stereotactic injection of G207 into recurrent tumors in five loci followed within 24 hours by a single fraction of irradiation (5 Gy) has finished accrual and will be reported shortly.
Similar results were seen in trials using HSV1716, although the highest dose achieved was 1 × 105 PFU, due to its more aggressive behavior in mice. At this dose, the virus was safe with direct injection into tumors or tumor beds after tumor resection, and resulted in several long-term survivors.18,19,20
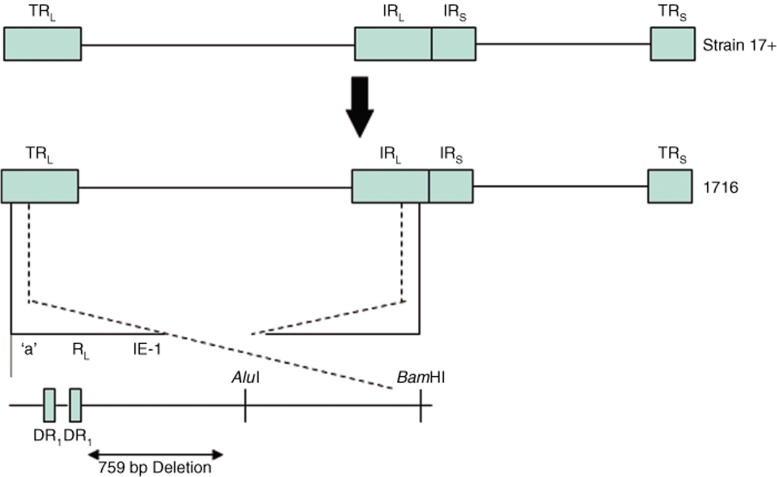
NV1020 is a rederived virus based on the construction of R7020 that was developed for HSV immunization. NV1020 has only one of the γ134.5 loci deleted and contains an insertion of HSV-2 sequences (Figure 3). It is a replication-competent, attenuated-engineered virus, but due to its remaining γ134.5 sequence is believed to have greater potential for neurotoxicity. Nevertheless, NV1020 was infused into the hepatic artery of patients with metastatic colorectal carcinoma to the liver, safely with minimal and self-limited serious adverse events.6 A multi-institutional phase II trial is ongoing. Similarly, a second generation oncolytic HSV expressing granulocyte macrophage colony–stimulation factor (GM-CSF), OncoVexGM-CSF, was safe and well tolerated when injected intratumorally in patients with recurrent cutaneous or subcutaneous deposits of breast, head and neck, gastrointestinal cancers or malignant melanoma.21 Several patients had stable disease without progression.
Figure 3.
Schematic representation of the derivation of R7020 (aka NV1020). This virus was constructed for the purpose of creating a vaccine for HSV-1 and HSV-2 that would be administered by intramuscular injection of living virus.138 HSV-1(F) was initially deleted for thymidine kinase (UL23) and UL24. The internal set of the inverted repeats, containing α0, α4, γ134.5, ORF O, and ORF P, was replaced with a novel construct that contained UL23 fused to the α4 promoter-regulatory region and 3′ to a series of HSV-2 genes (US2, HSV protein kinase, US5, glycoproteins G, D, and I, as well as a portion of E). The terminal inverted repeat remained intact. Thus, this chimeric virus, containing both HSV-1 and HSV-2 sequences, has been extensively tested in both rodent and primate species for safety (LD50 = 2.7 × 106 PFU versus 3.8 × 102 PFU for HSV-1(F)) and genetic stability.138,139
Potential causes for the failure of genetically engineered HSVs to be uniformly curative are legion, but can be broadly grouped into general categories that include, but are not limited to: (i) host antiviral immune responses, (ii) tumor genotype heterogeneity, and (iii) extracellular matrix/tumor microenvironment. Seropositivity to HSV may pose a barrier to repeated administration, however, preclinical and clinical studies suggest that it does not have a deleterious impact on the initial intratumoral administration of HSVs.16,22,23,24,25,26 More concerning is the observation that infiltrating mononuclear inflammatory cells may destroy virally infected cells prematurely, limiting virus production and eventually the scope of the anti-tumor effect.27,28 Strategies that utilize agents (e.g., cyclophosphamide) to block bone marrow–derived generation of inflammatory cells seem to benefit the oncolytic effect.29,30 Likewise, use of agents either exogenously administered (cilengitide, bevacizumab) or re-engineered into the virus (vasculostatin) to inhibit vascular permeability or formation of neovasculature in the tumor could significantly limit the extravasation of inflammatory cells into the tumor bed and permit greater virus production in the tumor cells.31,32
Tumor genotype heterogeneity poses an inherent problem for an oncolytic virus that has been seriously attenuated to make it safe and aneurovirulent. These differences include (i) reduced or complete lack of expression of the key HSV entry molecule, nectin-1 (CD111), or its family members and (ii) a nonpermissive environment for late gene expression and virus replication events. Solutions include (i) re-engineering the virus to express ligands that bind receptors expressed exclusively on tumor cells or (ii) modulating the cellular replication machinery with irradiation or DNA-damaging agents to engage the DNA repair response, which supports virus replication.33,34,35,36,37,38 In some instances, it may be possible to re-engineer the virus to express mutant proteins that activate signaling pathways that would provide a more hospitable virus replication environment.39 Production of a chimeric HSV that contains a gene from human cytomegalovirus, a related β-herpes virus also enhances late gene expression without compromising safety of the virus.40
Finally, to be effective, infectious HSV must disseminate widely throughout the tumor. Extracellular matrix generated by the tumor provides a critical barrier. A potential solution is to re-engineer the virus to express ECM-proteolyzing enzymes to enhance percolation of infectious virus particles through the interstitial spaces of the tumor.41 Many of these immune, genetic and physical barriers to effective virus oncolysis are being resolved almost as rapidly as they are identified. However, it is the lag between developing the next-generation virus and its application in a phase I clinical trial that remains the most serious impediment in the quest for more effective therapies for children with cancer. To date, no pediatric trials employing engineered HSV have been conducted. However, several preclinical studies have shown promise. The potential benefit of HSV therapy in pediatric solid tumors including brain tumors, neuroblastomas, and sarcomas is reviewed in the following section.
Brain Tumors
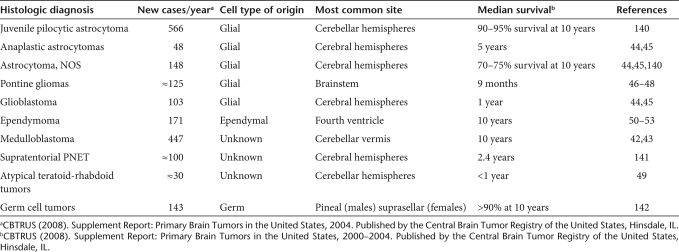
Central nervous system tumors account for ~25% of all childhood malignancies, and brain tumors are the leading cause of cancer-related morbidity and mortality in children (Table 2). Although survival rates for patients with low-grade, localized tumors have improved to >80%, there is a poor outcome subset of children with disseminated medulloblastoma, high-grade gliomas, intrinsic pontine gliomas, atypical teratoid-rhabdoid tumors (AT/RT), and incompletely resected or metastatic ependymomas. Medulloblastomas are the most common malignant pediatric brain tumor (~20% of cases) and an estimated one-third of these are disseminated at diagnosis.42 Progression-free survival in patients with high risk, metastatic medulloblastomas only approaches 50%, despite surgery, radiation, and chemotherapy.43 Similarly, outcomes for children with high-grade gliomas are poor with 5-year event-free survival rates ~20% despite multimodality therapy.44,45 With current chemotherapy and radiotherapy, intrinsic pontine gliomas are still uniformly fatal with a median survival rate of ~9 months.46,47,48 Patients with AT/RT, a recently recognized distinct tumor that tends to occur in children <3 years, have dismal median survival rates of <1 year.49 Ependymomas most often arise in the posterior fossa from the fourth ventricle with brainstem involvement occurring in up to 50% of cases making gross total resection difficult.50,51 Over a quarter of patients are <2 years of age and experience significant morbidity if potentially curative radiation doses are used.52 Outcomes for children with ependymomas, who have a subtotal resection, who are <3 years of age, or who have disseminated disease to the cord are grim with survival rates of <20%.53 Because of poor outcomes, children with disseminated medulloblastomas, high-grade gliomas, intrinsic pontine gliomas, AT/RT, and ependymomas may benefit from a novel, targeted therapy such as mutant oncolytic HSV. Although phase I adult trials have confirmed safety of mutant HSV stereotactic injections in the brain, safety in the developing human brain is of paramount concern.
Table 2.
Incidence of histologically defined intrinsic CNS tumors of childhood
The safety of G207 was confirmed first in CBA/J, BALB/c, and A/J strain mice and then in nonhuman primate studies in New World owl monkeys (Aotus nancymae), as young as 1 year old.54,55 Different strains of mice have widely differing levels of sensitivity to genetically engineered HSV injected into the brain, and this largely follows the patterns of sensitivity defined years ago by injecting wild-type HSVs intraperitoneally.56,57 Owl monkeys were chosen because of their extreme sensitivity to HSV, similar to that of neonatal children. Magnetic resonance imagings obtained between 10 days and 12 months, after initial injection of HSV into the brain, showed no abnormalities that could be attributed to HSV toxicity, which suggested that G207 injection did not result in short- or long-term changes in the brain, other than those induced by the mechanical trauma of injection. Importantly, Radbill et al. found no significant difference in long-term physical development (body weight, brain weight, limb strength), cognitive performance (eight arm radial maze), or exploratory behaviors (open-field maze) between groups of mice that had received a 2 µl intracerebral inoculation of 105 PFU of G207 versus control saline at 4 days of life.58 However, five of seven mice in the G207 group had histological and magnetic resonance imaging evidence of unilateral ventriculomegaly ipsilateral to the site of injection as compared to only one of eight control mice. Free-hand injection was used, which likely resulted in intraventricular delivery of the virus and led the authors to conclude that G207 is unlikely to cause significant neurodevelopmental changes. However, an initial study in children should exclude patients with tumors in or near the ventricles and children under two. Currently, there have been no animal studies examining the safety of engineered HSV on the developing cerebellum or brain stem.
The initial focus in using mutant HSV has been on cerebral tumors because brain stem tumors pose additional patient safety challenges. Damage to the brain stem can affect the body's integrative functions resulting in severe brain injury and death. Additionally, cranial nerves III through XII emerge from the brain stem and are in danger of being injured by local injection trauma, virus infection, or a localized immune response. Gene therapy oncolytic viruses that utilize a local immune response such as those expressing interleukins may not be the best choice on the sensitive brain stem. Studies need to be performed to elucidate whether or not engineered HSV can be safely delivered locally to brain stem tumors.
One way to circumvent potential injury to patients via localized injection is to inject the virus systemically. However, systemic delivery of virus for treatment of brain tumors or metastases to the brain is complicated by the blood-brain barrier (BBB). The BBB consists of brain endothelial cells, which are strongly juxtaposed via high-resistance tight junctions and have minimal pinocytosis, which together results in a physical barrier to drugs and other blood-borne molecules like viruses.59,60 This barrier can be disrupted by osmotic solutions like mannitol or inflammatory mediators such as bradykinin to allow delivery of intracarotid or intra-arterial injected herpes virus to the brain.61,62,63,64 Using intracarotid delivery of an engineered oncolytic herpes vector, G47Δ, after 25% mannitol, Rabkin et al. significantly increased survival of nude mice with metastatic breast cancer in the brain.7 G47Δ is a variant of G207 with the additional deletion of both the gene that blocks TAP protein presentation of antigens on the cell surface and the promoter region of US11. Deletion of that promoter region places the late US11 gene under control of the immediate-early ICP47 promoter; thereby, blocking shutoff of protein synthesis and creating a more oncolytic virus than G207.65 They found extensive virus replication in intracerebral tumors after BBB disruption, and importantly, staining of peripheral organs was minimal. Disrupting the BBB followed by systemic injection of HSV may prove to be a novel approach to treating tumors difficult to completely resect, such as intrinsic pontine gliomas.
As virus safety and route of delivery in pediatric patients continue to be refined, the efficacy of HSV oncolytic therapy in pediatric brain tumors needs further study. We have shown that a pediatric frontal lobe GBM, D456MG, was more sensitive to infection and killing by engineered HSV than six adult glioblastomas in tissue culture.66 Additionally, we found that glioma stem cells, cells believed to be responsible for the inherent resistance of GBMs to traditional therapies, were equally sensitive to killing by HSV as nonstem cell tumor cells. Further studies on pediatric high-grade gliomas are planned.67 Otsuki et al. have suggested that glioma stem cells grown in tumorspheres are more resistant due to intact interferon pathways, and thus have devised a virus to target these cells by placing expression of ICP34.5 under control of the nestin enhancer regulatory element.68 Nestin is a neural stem cell marker and its expression level along with CD133 has recently been shown to correlate with worse clinical outcomes in glioma patients.69 A potential concern is that any virus that targets CD133 or nestin-expressing tumor cells may also be effective in killing normal neural stem cells. Whether this would be detrimental in the (developing) pediatric brain remains to be determined. A potential benefit is suggested by mouse brain tumor model studies by the Holland and Canoll laboratories in which they found that spontaneously occurring gliomas attract normal neural stem/glial progenitor cells that may eventually comprise as much as half of the tumor mass; however it is unknown if this occurs in human brain tumors.70,71
Several human medulloblastoma cell lines have been tested for sensitivity to engineered HSV. Lasner et al. found that D283 cells were sensitive to HSV1716 infection and D283 tumor-bearing mice had a statistically significant increased survival advantage compared to mock-treated tumor-bearing mice.72 DAOY cells were efficiently killed in vitro by a double-mutant engineered HSV, 3616UB, and intratumoral injection of DAOY cells in C.b-17 SCID mice resulted in significant growth arrest and tumor regression suggesting that human medulloblastomas may be sensitive to mutant HSV.73
To date, no studies examining the sensitivity of intrinsic pontine gliomas, AT/RT, or ependymomas have been published. On a related note, Guzman et al. found weak focal expression of nectin-1, an important entry receptor for HSV, in ependymomas, suggesting that ependymomas may be sensitive to infection by HSV.74 Nectin-1 is a cell surface adhesion molecule that is widely expressed in cell lines of different lineages including neuronal cells, and recently has been shown to predict sensitivity to herpes oncolytic therapy in several different types of tumors including thyroid cancer and invasive squamous cell carcinoma.8,75,76 Future studies are needed to further define which brain tumor types are sensitive to engineered HSV, however based on current in vitro and in vivo data, a phase I trial utilizing engineered HSV in children with recurrent supratentorial tumors should begin without delay.
Neuroblastoma
Neuroblastoma is a neural crest-derived neuroendocrine tumor, making it an ideal target for a neurotropic virus like HSV. It is the most common malignancy in infancy and the most common extracranial solid tumor in children accounting for an estimated 8% of all childhood malignancies.77 Approximately 60% of patients older than 1 year and 45% of all patients with neuroblastoma have disseminated disease at diagnosis.78 Patients with stage 4, metastatic disease have 3-year event-free survival rates of 55% despite autologous transplantation and 13-cis-retinonic acid therapy.79 However, longer follow-up confirms that many high-risk patients relapse after transplantation suggesting that further dose augmentation of current therapies is unlikely to be beneficial.80 Therefore, novel therapeutics are needed for high-risk patients, and engineered HSV has shown promise in treating neuroblastoma.
Although local tumor injection may be beneficial for solitary tumors, high-risk neuroblastoma tends to be widely disseminated and would require systemic treatment with virus. Systemic administration of oncolytic HSV has proven safe and efficacious in several mouse models. A murine IL-12-expressing virus, NV1042, was found safe when injected systemically into the tail vein of mice with squamous cell carcinoma pulmonary metastases.81 No animals developed clinical signs attributed to virus administration, and importantly, histological examination of nontumor-bearing areas of lung, brain, liver, spleen, and pancreatic tissue all appeared completely normal without cytopathic effects. Significantly, improved survival was seen in NV1042-treated mice, and the long-term survivors had no evidence of weight loss, poor grooming, neurotoxicity, mucosal ulcerations, or any other visible morbidity. Using the same virus inoculated via the tail vein of transgenic C57BL/6-TRAMP mice with spontaneous primary and metastatic prostate cancer, Varghese et al. found that multiple injections did not seem toxic to the animals.9,82 However, as Lopez has shown, this particular strain of mice is very resistant to HSV toxicity, presumably because of low nectin-1 expression. Infected tumor cells were detected and persisted in the prostates, periaortic lymph nodes, and lungs of the mice but not in healthy organs. Intraperitoneal injection of HF10, a spontaneously occurring, highly attenuated virus, in immunocompetent mice with peritoneal disseminated melanoma was similarly deemed safe, and all mice survived as compared to 100% fatality in control mice, who did not receive virus.10 Successful treatment may also be a function of tumor genotype. Veerapong et al. have demonstrated localization and spread of systemically administered Δγ134.5 HSV in tumors with high levels of expression of activated MAP kinase (MEK).83
From these studies, it would appear that disseminated tumor cells can be killed via systemic injections of mutant HSVs. However, in the human population, 70–90% of adults have significant pre-existing immunity with detectable seropositivity due to subclinical infections with HSV-1. This has the potential to impact systemic therapy negatively, although preclinical studies have not demonstrated this uniformly. Although gene transfer to brain tumors using a HSV-1 vector was decreased in HSV-1 immunized rats, several groups demonstrated that prior immunization with HSV-1 and/or HSV seropositivity in mice did not significantly affect viral oncolysis, particularly, when the virus was given at suitable doses and in proximity to tumor targets.22,23,24,25 Thus, systemic administration of HSV could remain a challenge for adult cancer therapy. The proportion of pediatric cancer patients with HSV premunition, however, is considerably lower and systemic therapy could be more successful.84 The prevalence of HSV-1 seropositivity rises with age: estimated under 20% for children 1–4; 26% by age 6–7 years; 36% at age 12–13 years; and 39% for adolescents age 14–19 years in the United States.85,86 Thus, younger children may be better candidates for systemic therapy.
A number of newly constructed viruses have been tested in a murine neuroblastoma model, Neuro-2a. Neuro-2a cells are syngeneic to A/J strain mice (an HSV-sensitive strain), are poorly immunogenic, and support effective HSV infection, providing a convenient model for testing newly constructed mutant viruses.87,88,89,90,91,92 However, Neuro-2a only contains single-copy MYC-N, which is typical of lower-risk, favorable neuroblastoma.93 MYC-N amplification is an important prognostic factor associated with advanced, rapidly progressive disease and a poor prognosis.94,95 Therefore, Neuro-2a cells are likely not the best model of resistant, difficult to treat neuroblastoma. Nevertheless, several groups have shown sensitivity of Neuro-2a to infection and killing by novel viruses including chimeric viruses, which express the human cytomegalovirus PKR-evasion genes, vectors expressing interleukin 18 and B7-1 immunoglobulin ± interleukin 12, and virus expressing bacterial cytosine deaminase.40,96,97,98,99
Importantly, eight separate human neuroblastoma cell lines, including several MYC-N amplified tumors and tumors established after patients had received standard chemotherapy and radiation, were uniformly sensitive to infection and killing with the attenuated HSV mutant NV1066 in tissue culture.100,101 Two human neuroblastoma cell lines were used for in vivo studies including one MYC-N nonamplified and one MYC-N amplified cell line.102 A single dose of NV1066 injected into flank tumors of nude mice resulted in significant antitumor responses and prolonged survivals of mice as compared to PBS-treated controls for both cell lines tested. Recently, Mahller et al. showed increased cytotoxicity both in vitro and in vivo on human neuroblastoma cell lines, including a MYC-N amplified line, by an engineered HSV that expressed human tissue inhibitor of metalloproteinase-3.103 Increased metalloproteinase expression has been correlated with worse prognosis in pediatric neuroblastomas.104,105,106 Furthermore, they demonstrated that several human neuroblastoma cell lines contain tumor-initiating cells that express neurogenic stem cell markers CD133, ABCG2, and nestin, similar to gliomas.107 When grown in tumorspheres, these tumor-initiating cells were sensitive to a nestin-targeted virus. These promising preclinical studies suggest that HSV therapy may be beneficial for local control of unresectable neuroblastoma tumors, but further studies are needed to establish any potential benefits of oncolytic HSV for metastatic disease.
Sarcomas
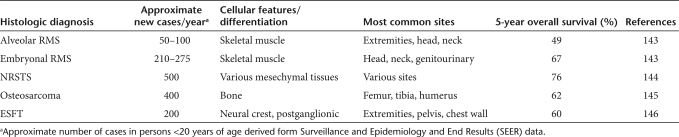
Pediatric sarcomas represent a diverse group of tumors that include rhabdomyosarcoma (RMS), non-RMS soft-tissue sarcomas (NRSTS), osteosarcoma, and Ewing sarcoma family of tumors (ESFT) including Ewing sarcoma and its counterpart primitive neuroectodermal tumor (PNET) (Table 3). RMS is the most common soft-tissue sarcoma in children accounting for ~5% of all pediatric malignancies and half of soft-tissue sarcomas of childhood.108 There are two main histological subtypes of RMS, embryonal (E-RMS) and alveolar (A-RMS). Most A-RMS tumors are found to have the t(2;13) PAX3-FOX01 translocation.109 The majority of E-RMS tumors display loss of heterozygosity (LOH) at the chromosome 11p15.5 locus.110 The A-RMS subtype is more common in older patients and ~15% of A-RMS patients have metastatic disease at diagnosis, which is associated with a 5-year survival rate of <25%.111,112,113 Augmentation of conventional chemotherapy regimens has not significantly improved survival for such patients. Further, surviving patients often suffer from lifelong treatment-related morbidity. Therefore, well-tolerated and effective novel targeted therapies are critically needed for A-RMS tumors. NRSTS represent a heterogeneous group of tumors that collectively account for ~4% of all childhood malignancies.114 Similar to the poor outcomes for patients with high-risk RMS, children with large, high-grade, or unresectable NRSTS have less than a 50% chance of survival, and those with metastatic disease at diagnosis have survival rates under 20%.115,116,117 Osteosarcoma and ESFT are the two most common malignant bone tumors in childhood and adolescence. Osteosarcoma tumors often have alterations of p53 and RB1 tumor suppressor genes, whereas ESFT are unified by the t(11;22) EWS-FLI or similar translocations.118,119,120 Despite dose-intensified chemotherapy, outcomes for patients with recurrent or metastatic osteosarcoma or ESFT are poor with survival rates under 30%.121,122 Recent studies have shown that intensified treatment regimens with more agents or higher dose chemotherapy only increases toxicity and secondary malignancies, without improving survival.123,124 Very few salvage therapies are available for these malignancies, and newer, less toxic, targeted approaches such as mutant HSV, are desperately needed.
Table 3.
Incidence of histologically defined sarcomas of childhood
Children with sarcomas that are localized and fully resected, generally, have favorable outcomes with well-tolerated treatment regimens and would not be candidates for engineered HSV-based therapies. However, children with large, unresectable, metastatic, or relapsed tumors could potentially benefit from such therapy. The proposed approach, like neuroblastoma, would involve local injection of unresectable tumors or systemic delivery of virus for metastatic disease. Several in vitro studies and in vivo intratumoral and intravenous injection studies have demonstrated that mutant HSV is oncolytic for sarcomas.
Initial studies by Bharatan et al. found that mutant HSV one embryonal RMS human cancer line, RD, and two alveolar lines, RhRKM-P4 and RH18, were very sensitive with complete cytopathic effects seen using NV1020 and G207 at low multiplicities of infection (≤0.5 PFU/cell).5 Currier et al. confirmed that immunocompromised mice with a flank human alveolar or embryonal tumor <250 mm3 showed a complete response to a single-intratumoral injection of NV1020. In contrast, only half of those with bulky disease (>250 mm3) had complete regression of their tumor with a single injection.125 Using five fractionated injections in multiple sites, they obtained more widespread intratumoral distribution of virus and improved control of large tumors, suggesting that patients with bulky disease may benefit from oncolytic HSV delivered to multiple sites of a tumor.
Confirming sensitivity of RMS to HSV, Cinatl et al. found marked cytotoxic and replicative effects of G207 in three human embryonal and four human alveolar RMS cell lines in tissue culture.126 Intratumoral injection of G207 in the flanks of mice xenotransplanted with human RMS cell lines resulted in marked tumor regression as well as complete resolution of the tumor in 25% of mice. Intravenous G207 administration in similar mice led to significant tumor-growth inhibition. They found complete tumor regression of alvelolar RMS in five of eight animals who received intravenous G207 combined with vincristine, a drug used as part of frontline therapy for patients with RMS. Similarly, an oncolytic herpes vector rRp450, which expresses a prodrug-converting enzyme for cyclophosphamide, another frontline chemotherapeutic for patients with RMS, prolonged survival in athymic nude mice with flank human alveolar RMS tumors, when the virus was combined with intraperitoneal cyclophosphamide.30 The virus was deemed safe when given both intravenously and intracranially alone or in combination with cyclophosphamide. Cyclophosphamide has also been shown to increased HSV efficacy by suppressing monocyte influx into infected tumors.127 Taken together, these data suggest that mutant HSV alone or combined with current chemotherapy may offer significant therapeutic benefits for children with RMS.
Several different NRSTS appear to be sensitive to oncolytic HSV including fibrosarcoma, angiosarcoma, synovial sarcoma, and leiomyosarcoma. Two groups have shown sensitivity in murine adult-type fibrosarcoma models, S-180 and NfSa Y83. S-180 bearing BALB/c mice received a single-intratumoral injection of mtHSV, an oncolytic double γ134.5 deleted virus, or PBS.128 Significant growth inhibition was seen in mice receiving the virus, and viral replication was limited to tumor cells. Likewise, immunocompetent C3H mice (HSV-resistant) with the highly aggressive sarcoma NfSa Y83, injected into the neck or flank, had marked tumor regression after intratumoral injection with a multimutated HSV, HL.129 Approximately 75% of flank tumors and 50% of neck tumors completely resolved. There are no reports of human fibrosarcoma cell lines being tested. Intratumoral injection of 3616UB into human angiosarcoma xenografts in SCID mice produced significant growth arrest and some tumor regression.73 The herpes vector d12.CALP, which utilizes calponin to drive expression of a major trans- activating factor for HSV viral genes, was tested against human synovial sarcoma and leiomyosarcoma cell lines.130 Calponin is normally expressed in mature smooth muscle cells but has been shown to be aberrantly expressed in a variety of NRSTS and osteosarcoma.131,132,133,134,135,136 Yamamura et al. found that d12.CALP selectively killed calponin-positive human synovial sarcoma, leiomyosarcoma, and osteosarcoma cells.130 Additionally, injection of the virus into human leiomyosarcoma in the flanks of athymic nude mice resulted in cure in four of five mice by day 35, and virus replication was noted in a nontreated tumor distant to the site of intratumoral virus inoculation. Importantly, vascular smooth muscle cells as well as normal cells in the brain, lung, liver, kidney, heart, small intestine, and uterus were not infected by the virus. These studies suggest that NRSTS can be targeted and killed by engineered HSV, while normal cells are unharmed.
There is less data regarding the efficacy of mutant HSV in pediatric bone tumors. The sensitivity of two human osteosarcoma cell lines and four Ewing sarcoma/PNET cell lines to G207 and NV1020 was investigated by Bharatan et al.,5 who found intermediate sensitivity of osteosarcoma cells as compared to RMS. Ewing sarcoma/PNET cell lines were the least sensitive with one cell line, 5838, resistant to both viruses. The resistance mechanism is not yet known as HSV entry and gene transduction occurred in resistant cell lines. Further studies examining sensitivities of osteosarcoma and Ewing sarcoma/PNET cell lines are needed, and resistance mechanism(s) need to be elucidated so that circumvention strategies can be developed.
Conclusion
Despite improving survival rates for children with cancer, subsets of patients exist with disease resistant to traditional therapies such as surgery, chemotherapy, and radiation. These patients require newer, targeted treatments used alone or in combination with more traditional approaches. Oncolytic HSV affords a candidate therapy with promise for several difficult-to-treat pediatric malignancies including certain brain tumors, neuroblastomas, and sarcomas. Despite many encouraging in vitro and in vivo studies, unfortunately, no pediatric trials utilizing engineered HSV have been conducted to date.
Many challenges still exist and need to be addressed in order to maximize the benefit of HSV therapy in pediatric patients. Further studies should be conducted to confirm viral safety in the developing human cerebrum, cerebellum, brain stem, and other developing organs. Virus delivery via direct inoculation needs to be tested and perfected especially in sensitive areas like the brain stem, and continued work on improving systemic delivery is necessary. As newer combined modality viruses for adult cancer therapies are created, they should also be tested against pediatric malignancies, and more effort needs to be made at improving tropism of HSV to individual pediatric tumor tissues. Hopefully, with continued advances in virotherapy, oncolytic HSV therapy will become a viable and effective adjuvant treatment for pediatric patients with traditionally dismal outcomes.
Acknowledgments
This work was supported in part by the Dixon Fellowship (G.K.F.), the Pediatric Brain Tumor Foundation of the US (A.T.R.), and USPHS NCI grants P01 CA071933 (J.M.M., G.Y.G) and P50 CA097247 (J.M.M., G.Y.G). No conflict of interest exists with any of the authors.
REFERENCES
- Gatta G, Capocaccia R, Coleman MP, Ries LA., and , Berrino F. Childhood cancer survival in Europe and the United States. Cancer. 2002;95:1767–1772. doi: 10.1002/cncr.10833. [DOI] [PubMed] [Google Scholar]
- Ries LAG, Melbert D, Krapcho M, Mariotto A, Miller BA, Feuer EJ.SEER Cancer Statistics Review, 1975-2004, National Cancer Institute Bethesda, MD; et aleds < http://seer.cancer.gov/csr/1975_2004/ >, based on November 2006 SEER data submission, posted to the SEER Web site, 2007 [Google Scholar]
- Markert JM, Parker JN, Gillespie GY., and , Whitley RJ. Genetically engineered human herpes simplex virus in the treatment of brain tumours. Herpes. 2001;8:17–22. [PubMed] [Google Scholar]
- Shah AC, Benos D, Gillespie GY., and , Markert JM. Oncolytic viruses: clinical applications as vectors for the treatment of malignant gliomas. J Neurooncol. 2003;65:203–226. doi: 10.1023/b:neon.0000003651.97832.6c. [DOI] [PubMed] [Google Scholar]
- Bharatan NS, Currier MA., and , Cripe TP. Differential susceptibility of pediatric sarcoma cells to oncolysis by conditionally replication-competent herpes simplex viruses. J Pediatr Hematol Oncol. 2002;24:447–453. doi: 10.1097/00043426-200208000-00008. [DOI] [PubMed] [Google Scholar]
- Kemeny N, Brown K, Covey A, Kim T, Bhargava A, Brody L, et al. Phase I, open-label, dose-escalating study of a genetically engineered herpes simplex virus, NV1020, in subjects with metastatic colorectal carcinoma to the liver. Hum Gene Ther. 2006;17:1214–1224. doi: 10.1089/hum.2006.17.1214. [DOI] [PubMed] [Google Scholar]
- Liu R, Martuza RL., and , Rabkin SD. Intracarotid delivery of oncolytic HSV vector G47Delta to metastatic breast cancer in the brain. Gene Ther. 2005;12:647–654. doi: 10.1038/sj.gt.3302445. [DOI] [PubMed] [Google Scholar]
- Yu Z, Chan MK, O-charoenrat P, Eisenberg DP, Shah JP, Singh B, et al. Enhanced nectin-1 expression and herpes oncolytic sensitivity in highly migratory and invasive carcinoma. Clin Cancer Res. 2005;11:4889–4897. doi: 10.1158/1078-0432.CCR-05-0309. [DOI] [PubMed] [Google Scholar]
- Varghese S, Rabkin SD, Nielsen GP, MacGarvey U, Liu R., and , Martuza RL. Systemic therapy of spontaneous prostate cancer in transgenic mice with oncolytic herpes simplex viruses. Cancer Res. 2007;67:9371–9379. doi: 10.1158/0008-5472.CAN-07-0674. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Goshima F, Mori I, Tamada Y, Matsumoto Y., and , Nishiyama Y. Oncolytic virotherapy for malignant melanoma with herpes simplex virus type 1 mutant HF10. J Dermatol Sci. 2008;50:185–196. doi: 10.1016/j.jdermsci.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Chou J, Kern ER, Whitley RJ., and , Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990;250:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- Mineta T, Rabkin SD, Yazaki T, Hunter WD., and , Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- MacLean AR, ul-Fareed M, Robertson L, Harland J., and , Brown SM. Herpes simplex virus type 1 deletion variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene 1 and the ‘a' sequence. J Gen Virol. 1991;72 (Pt 3):631–639. doi: 10.1099/0022-1317-72-3-631. [DOI] [PubMed] [Google Scholar]
- Chambers R, Gillespie GY, Soroceanu L, Andreansky S, Chatterjee S, Chou J, et al. Comparison of genetically engineered herpes simplex viruses for the treatment of brain tumors in a scid mouse model of human malignant glioma. Proc Natl Acad Sci USA. 1995;92:1411–1415. doi: 10.1073/pnas.92.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolovan CA, Sawtell NM., and , Thompson RL. ICP34.5 mutants of herpes simplex virus type 1 strain 17syn+ are attenuated for neurovirulence in mice and for replication in confluent primary mouse embryo cell cultures. J Virol. 1994;68:48–55. doi: 10.1128/jvi.68.1.48-55.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther. 2000;7:867–874. doi: 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- Markert JM, Parker JN, Buchsbaum DJ, Grizzle WE, Gillespie GY., and , Whitley RJ. Oncolytic HSV-1 for the treatment of brain tumours. Herpes. 2006;13:66–71. [PubMed] [Google Scholar]
- Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–866. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- Papanastassiou V, Rampling R, Fraser M, Petty R, Hadley D, Nicoll J, et al. The potential for efficacy of the modified (ICP 34.5(-)) herpes simplex virus HSV1716 following intratumoural injection into human malignant glioma: a proof of principle study. Gene Ther. 2002;9:398–406. doi: 10.1038/sj.gt.3301664. [DOI] [PubMed] [Google Scholar]
- Harrow S, Papanastassiou V, Harland J, Mabbs R, Petty R, Fraser M, et al. HSV1716 injection into the brain adjacent to tumour following surgical resection of high-grade glioma: safety data and long-term survival. Gene Ther. 2004;11:1648–1658. doi: 10.1038/sj.gt.3302289. [DOI] [PubMed] [Google Scholar]
- Hu JC, Coffin RS, Davis CJ, Graham NJ, Groves N, Guest PJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12:6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- Herrlinger U, Kramm CM, Aboody-Guterman KS, Silver JS, Ikeda K, Johnston KM, et al. Pre-existing herpes simplex virus 1 (HSV-1) immunity decreases, but does not abolish, gene transfer to experimental brain tumors by a HSV-1 vector. Gene Ther. 1998;5:809–819. doi: 10.1038/sj.gt.3300643. [DOI] [PubMed] [Google Scholar]
- Chahlavi A, Rabkin S, Todo T, Sundaresan P., and , Martuza R. Effect of prior exposure to herpes simplex virus 1 on viral vector-mediated tumor therapy in immunocompetent mice. Gene Ther. 1999;6:1751–1758. doi: 10.1038/sj.gt.3301003. [DOI] [PubMed] [Google Scholar]
- Delman KA, Bennett JJ, Zager JS, Burt BM, McAuliffe PF, Petrowsky H, et al. Effects of preexisting immunity on the response to herpes simplex-based oncolytic viral therapy. Hum Gene Ther. 2000;11:2465–2472. doi: 10.1089/10430340050207957. [DOI] [PubMed] [Google Scholar]
- Lambright ES, Kang EH, Force S, Lanuti M, Caparrelli D, Kaiser LR, et al. Effect of preexisting anti-herpes immunity on the efficacy of herpes simplex viral therapy in a murine intraperitoneal tumor model. Mol Ther. 2000;2:387–393. doi: 10.1006/mthe.2000.0133. [DOI] [PubMed] [Google Scholar]
- Markert JM, Liechty PG, Wang W, Gaston S, Braz E, Karrasch M, et al. Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther. 2009;17:199–207. doi: 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ., and , Markert JM. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci USA. 2000;97:2208–2213. doi: 10.1073/pnas.040557897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulci G, Dmitrieva N, Gianni D, Fontana EJ, Pan X, Lu Y, et al. Depletion of peripheral macrophages and brain microglia increases brain tumor titers of oncolytic viruses. Cancer Res. 2007;67:9398–9406. doi: 10.1158/0008-5472.CAN-07-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS, Yu J, et al. Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci USA. 2006;103:12873–12878. doi: 10.1073/pnas.0605496103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currier MA, Gillespie RA, Sawtell NM, Mahller YY, Stroup G, Collins MH, et al. Efficacy and safety of the oncolytic herpes simplex virus rRp450 alone and combined with cyclophosphamide. Mol Ther. 2008;16:879–885. doi: 10.1038/mt.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurozumi K, Hardcastle J, Thakur R, Shroll J, Nowicki M, Otsuki A, et al. Oncolytic HSV-1 infection of tumors induces angiogenesis and upregulates CYR61. Mol Ther. 2008;16:1382–1391. doi: 10.1038/mt.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur B, Brat DJ, Devi NS., and , Van Meir EG. Vasculostatin, a proteolytic fragment of brain angiogenesis inhibitor 1, is an antiangiogenic and antitumorigenic factor. Oncogene. 2005;24:3632–3642. doi: 10.1038/sj.onc.1208317. [DOI] [PubMed] [Google Scholar]
- Zhou G., and , Roizman B. Construction and properties of a herpes simplex virus 1 designed to enter cells solely via the IL-13alpha2 receptor. Proc Natl Acad Sci USA. 2006;103:5508–5513. doi: 10.1073/pnas.0601258103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama H, Zhou G., and , Roizman B. Herpes simplex virus 1 recombinant virions exhibiting the amino terminal fragment of urokinase-type plasminogen activator can enter cells via the cognate receptor. Gene Ther. 2006;13:621–629. doi: 10.1038/sj.gt.3302685. [DOI] [PubMed] [Google Scholar]
- Zhou G., and , Roizman B. Characterization of a recombinant herpes simplex virus 1 designed to enter cells via the IL13Ralpha2 receptor of malignant glioma cells. J Virol. 2005;79:5272–5277. doi: 10.1128/JVI.79.9.5272-5277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezhir JJ, Advani SJ, Smith KD, Darga TE, Poon AP, Schmidt H, et al. Ionizing radiation activates late herpes simplex virus 1 promoters via the p38 pathway in tumors treated with oncolytic viruses. Cancer Res. 2005;65:9479–9484. doi: 10.1158/0008-5472.CAN-05-1927. [DOI] [PubMed] [Google Scholar]
- Advani SJ, Mezhir JJ, Roizman B., and , Weichselbaum RR. ReVOLT: radiation-enhanced viral oncolytic therapy. Int J Radiat Oncol Biol Phys. 2006;66:637–646. doi: 10.1016/j.ijrobp.2006.06.034. [DOI] [PubMed] [Google Scholar]
- Aghi M, Rabkin S., and , Martuza RL. Effect of chemotherapy-induced DNA repair on oncolytic herpes simplex viral replication. J Natl Cancer Inst. 2006;98:38–50. doi: 10.1093/jnci/djj003. [DOI] [PubMed] [Google Scholar]
- Smith KD, Mezhir JJ, Bickenbach K, Veerapong J, Charron J, Posner MC, et al. Activated MEK suppresses activation of PKR and enables efficient replication and in vivo oncolysis by Deltagamma(1)34.5 mutants of herpes simplex virus 1. J Virol. 2006;80:1110–1120. doi: 10.1128/JVI.80.3.1110-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AC, Parker JN, Gillespie GY, Lakeman FD, Meleth S, Markert JM, et al. Enhanced antiglioma activity of chimeric HCMV/HSV-1 oncolytic viruses. Gene Ther. 2007;14:1045–1054. doi: 10.1038/sj.gt.3302942. [DOI] [PubMed] [Google Scholar]
- Ganesh S, Gonzalez-Edick M, Gibbons D, Van Roey M., and , Jooss K. Intratumoral coadministration of hyaluronidase enzyme and oncolytic adenoviruses enhances virus potency in metastatic tumor models. Clin Cancer Res. 2008;14:3933–3941. doi: 10.1158/1078-0432.CCR-07-4732. [DOI] [PubMed] [Google Scholar]
- Packer RJ, Rood BR., and , MacDonald TJ. Medulloblastoma: present concepts of stratification into risk groups. Pediatr Neurosurg. 2003;39:60–67. doi: 10.1159/000071316. [DOI] [PubMed] [Google Scholar]
- Crawford JR, MacDonald TJ., and , Packer RJ. Medulloblastoma in childhood: new biological advances. Lancet Neurol. 2007;6:1073–1085. doi: 10.1016/S1474-4422(07)70289-2. [DOI] [PubMed] [Google Scholar]
- Chastagner P, Kalifa C, Doz F, Bouffet E, Gentet JC, Ruchoux MM, et al. Outcome of children treated with preradiation chemotherapy for a high-grade glioma: results of a French Society of Pediatric Oncology (SFOP) Pilot Study. Pediatr Blood Cancer. 2007;49:803–807. doi: 10.1002/pbc.21051. [DOI] [PubMed] [Google Scholar]
- MacDonald TJ, Arenson EB, Ater J, Sposto R, Bevan HE, Bruner J, et al. Phase II study of high-dose chemotherapy before radiation in children with newly diagnosed high-grade astrocytoma: final analysis of Children's Cancer Group Study 9933. Cancer. 2005;104:2862–2871. doi: 10.1002/cncr.21593. [DOI] [PubMed] [Google Scholar]
- Korones DN, Fisher PG, Kretschmar C, Zhou T, Chen Z, Kepner J, et al. Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: a Children's Oncology Group phase II study. Pediatr Blood Cancer. 2008;50:227–230. doi: 10.1002/pbc.21154. [DOI] [PubMed] [Google Scholar]
- Dunkel IJ, Garvin JH Jr, Goldman S, Ettinger LJ, Kaplan AM, Cairo M, et al. High dose chemotherapy with autologous bone marrow rescue for children with diffuse pontine brain stem tumors. Children's Cancer Group. J Neurooncol. 1998;37:67–73. doi: 10.1023/a:1005874508975. [DOI] [PubMed] [Google Scholar]
- Hargrave D, Bartels U., and , Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7:241–248. doi: 10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- Gidwani P, Levy A, Goodrich J, Weidenheim K., and , Kolb EA. Successful outcome with tandem myeloablative chemotherapy and autologous peripheral blood stem cell transplants in a patient with atypical teratoid/rhabdoid tumor of the central nervous system. J Neurooncol. 2008;88:211–215. doi: 10.1007/s11060-008-9553-1. [DOI] [PubMed] [Google Scholar]
- Nazar GB, Hoffman HJ, Becker LE, Jenkin D, Humphreys RP., and , Hendrick EB. Infratentorial ependymomas in childhood: prognostic factors and treatment. J Neurosurg. 1990;72:408–417. doi: 10.3171/jns.1990.72.3.0408. [DOI] [PubMed] [Google Scholar]
- Duffner PK, Horowitz ME, Krischer JP, Friedman HS, Burger PC, Cohen ME, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328:1725–1731. doi: 10.1056/NEJM199306173282401. [DOI] [PubMed] [Google Scholar]
- Pierre-Kahn A, Hirsch JF, Roux FX, Renier D., and , Sainte-Rose C. Intracranial ependymomas in childhood. Survival and functional results of 47 cases. Childs Brain. 1983;10:145–156. doi: 10.1159/000120108. [DOI] [PubMed] [Google Scholar]
- Shu HK, Sall WF, Maity A, Tochner ZA, Janss AJ, Belasco JB, et al. Childhood intracranial ependymoma: twenty-year experience from a single institution. Cancer. 2007;110:432–441. doi: 10.1002/cncr.22782. [DOI] [PubMed] [Google Scholar]
- Sundaresan P, Hunter WD, Martuza RL., and , Rabkin SD. Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation in mice. J Virol. 2000;74:3832–3841. doi: 10.1128/jvi.74.8.3832-3841.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter WD, Martuza RL, Feigenbaum F, Todo T, Mineta T, Yazaki T, et al. Attenuated, replication-competent herpes simplex virus type 1 mutant G207: safety evaluation of intracerebral injection in nonhuman primates. J Virol. 1999;73:6319–6326. doi: 10.1128/jvi.73.8.6319-6326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez C. Resistance to herpes simplex virus - type 1 (HSV-1) Curr Top Microbiol Immunol. 1981;92:15–24. doi: 10.1007/978-3-642-68069-4_2. [DOI] [PubMed] [Google Scholar]
- Lopez C. Resistance to HSV-1 in the mouse is governed by two major, independently segregating, non-H-2 loci. Immunogenetics. 1980;11:87–92. doi: 10.1007/BF01567772. [DOI] [PubMed] [Google Scholar]
- Radbill AE, Reddy AT, Markert JM, Wyss JM, Pike MM, Akella NS, et al. Effects of G207, a conditionally replication-competent oncolytic herpes simplex virus, on the developing mammalian brain. J Neurovirol. 2007;13:118–129. doi: 10.1080/13550280601187177. [DOI] [PubMed] [Google Scholar]
- Neuwelt EA, Pagel MA., and , Dix RD. Delivery of ultraviolet-inactivated 35S-herpesvirus across an osmotically modified blood-brain barrier. J Neurosurg. 1991;74:475–479. doi: 10.3171/jns.1991.74.3.0475. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron. 2002;36:555–558. doi: 10.1016/s0896-6273(02)01054-1. [DOI] [PubMed] [Google Scholar]
- Neuwelt EA, Pagel MA., and , Dix RD. Delivery of ultraviolet-inactivated 35S-herpesvirus across an osmotically modified blood-brain barrier. J Neurosurg. 1991;74:475–479. doi: 10.3171/jns.1991.74.3.0475. [DOI] [PubMed] [Google Scholar]
- Rainov NG, Zimmer C, Chase M, Kramm CM, Chiocca EA, Weissleder R, et al. Selective uptake of viral and monocrystalline particles delivered intra-arterially to experimental brain neoplasms. Hum Gene Ther. 1995;6:1543–1552. doi: 10.1089/hum.1995.6.12-1543. [DOI] [PubMed] [Google Scholar]
- Cloughesy TF., and , Black KL. Pharmacological blood-brain barrier modification for selective drug delivery. J Neurooncol. 1995;26:125–132. doi: 10.1007/BF01060218. [DOI] [PubMed] [Google Scholar]
- Nilaver G, Muldoon LL, Kroll RA, Pagel MA, Breakefield XO, Davidson BL, et al. Delivery of herpesvirus and adenovirus to nude rat intracerebral tumors after osmotic blood-brain barrier disruption. Proc Natl Acad Sci USA. 1995;92:9829–9833. doi: 10.1073/pnas.92.21.9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr I., and , Gluzman Y. A herpesvirus genetic element which affects translation in the absence of the viral GADD34 function. EMBO J. 1996;15:4759–4766. [PMC free article] [PubMed] [Google Scholar]
- Friedman GK, Bharara S, Langford C, Coleman J., and , Gillespie GY. Engineered herpes simpex virus oncolytic therapy kills both tumor cells and glioma progenitor cells in a pediatric high-grade glioma. Poster presentation at the International Symposium of Pediatric Neuro-Oncology Meeting, June 2008. 2008.
- Friedman GK, Bharara S, Langford C, Coleman J., and , Gillespie GY. Heterogeneity of CD111 (nectin-1) expression governs infection of human glioma tumor or progenitor cells by genetically engineered herpes simplex virus. Poster presentation at the Society of Neuro-Oncology Scientific Meeting, November 2007. 2007.
- Otsuki A, Patel A, Kasai K, Suzuki M, Kurozumi K, Chiocca EA, et al. Histone deacetylase inhibitors augment antitumor efficacy of herpes-based oncolytic viruses. Mol Ther. 2008;16:1546–1555. doi: 10.1038/mt.2008.155. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan P, Wang M, Rojiani AM, Tofilon PJ, Chakravarti A, Ang KK, et al. The prognostic value of nestin expression in newly diagnosed glioblastoma: report from the Radiation Therapy Oncology Group. Radiat Oncol. 2008;3:32. doi: 10.1186/1748-717X-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomchenko EI., and , Holland EC. Platelet-derived growth factor-mediated gliomagenesis and brain tumor recruitment. Neurosurg Clin N Am. 2007;18:39–58. doi: 10.1016/j.nec.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Assanah M, Lochhead R, Ogden A, Bruce J, Goldman J., and , Canoll P. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci. 2006;26:6781–6790. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasner TM, Kesari S, Brown SM, Lee VM, Fraser NW., and , Trojanowski JQ. Therapy of a murine model of pediatric brain tumors using a herpes simplex virus type-1 ICP34.5 mutant and demonstration of viral replication within the CNS. J Neuropathol Exp Neurol. 1996;55:1259–1269. doi: 10.1097/00005072-199612000-00010. [DOI] [PubMed] [Google Scholar]
- Pyles RB, Warnick RE, Chalk CL, Szanti BE., and , Parysek LM. A novel multiply-mutated HSV-1 strain for the treatment of human brain tumors. Hum Gene Ther. 1997;8:533–544. doi: 10.1089/hum.1997.8.5-533. [DOI] [PubMed] [Google Scholar]
- Guzman G, Oh S, Shukla D, Engelhard HH., and , Valyi-Nagy T. Expression of entry receptor nectin-1 of herpes simplex virus 1 and/or herpes simplex virus 2 in normal and neoplastic human nervous system tissues. Acta Virol. 2006;50:59–66. [PubMed] [Google Scholar]
- Huang YY, Yu Z, Lin SF, Li S, Fong Y., and , Wong RJ. Nectin-1 is a marker of thyroid cancer sensitivity to herpes oncolytic therapy. J Clin Endocrinol Metab. 2007;92:1965–1970. doi: 10.1210/jc.2007-0040. [DOI] [PubMed] [Google Scholar]
- Yu Z, Adusumilli PS, Eisenberg DP, Darr E, Ghossein RA, Li S, et al. Nectin-1 expression by squamous cell carcinoma is a predictor of herpes oncolytic sensitivity. Mol Ther. 2007;15:103–113. doi: 10.1038/sj.mt.6300009. [DOI] [PubMed] [Google Scholar]
- Gurney JG, Davis S, Severson RK, Fang JY, Ross JA., and , Robison LL. Trends in cancer incidence among children in the U.S. Cancer. 1996;78:532–541. doi: 10.1002/(SICI)1097-0142(19960801)78:3<532::AID-CNCR22>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Friedman GK., and , Castleberry RP.Changing trends of research and treatment in infant neuroblastoma Pediatr Blood Cancer 2007491060–1065.7 Suppl [DOI] [PubMed] [Google Scholar]
- Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- Santana VM, Furman WL, McGregor LM., and , Billups CA. Disease control intervals in high-risk neuroblastoma. Cancer. 2008;112:2796–2801. doi: 10.1002/cncr.23507. [DOI] [PubMed] [Google Scholar]
- Wong RJ, Chan MK, Yu Z, Kim TH, Bhargava A, Stiles BM, et al. Effective intravenous therapy of murine pulmonary metastases with an oncolytic herpes virus expressing interleukin 12 Clin Cancer Res 200410251–259.1 Pt 1 [DOI] [PubMed] [Google Scholar]
- Varghese S, Rabkin SD, Nielsen PG, Wang W., and , Martuza RL. Systemic oncolytic herpes virus therapy of poorly immunogenic prostate cancer metastatic to lung. Clin Cancer Res. 2006;12:2919–2927. doi: 10.1158/1078-0432.CCR-05-1187. [DOI] [PubMed] [Google Scholar]
- Veerapong J, Bickenbach KA, Shao MY, Smith KD, Posner MC, Roizman B, et al. Systemic delivery of (gamma1)34.5-deleted herpes simplex virus-1 selectively targets and treats distant human xenograft tumors that express high MEK activity. Cancer Res. 2007;67:8301–8306. doi: 10.1158/0008-5472.CAN-07-1499. [DOI] [PubMed] [Google Scholar]
- Roizman B, Whitley R., and , Lopez C. The Human Herpesviruses: Biology, Pathogenesis, and Treatment. Raven: New York; 1993. [Google Scholar]
- Xu F, Lee FK, Morrow RA, Sternberg MR, Luther KE, Dubin G, et al. Seroprevalence of herpes simplex virus type 1 in children in the United States. J Pediatr. 2007;151:374–377. doi: 10.1016/j.jpeds.2007.04.065. [DOI] [PubMed] [Google Scholar]
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- Hock RA, Reynolds BD, Tucker-McClung CL., and , Heuer JG. Murine neuroblastoma vaccines produced by retroviral transfer of MHC class II genes. Cancer Gene Ther. 1996;3:314–320. [PubMed] [Google Scholar]
- Rasty S, Poliani PL, Fink DJ., and , Glorioso JC. Deletion of the S component inverted repeat sequence c′ and the nonessential genes U(S)1 through U(S)5 from the herpes simplex virus type 1 genome substantially impairs productive viral infection in cell culture and pathogenesis in the rat central nervous system. J Neurovirol. 1997;3:247–264. doi: 10.3109/13550289709029466. [DOI] [PubMed] [Google Scholar]
- Todo T, Rabkin SD, Sundaresan P, Wu A, Meehan KR, Herscowitz HB, et al. Systemic antitumor immunity in experimental brain tumor therapy using a multimutated, replication-competent herpes simplex virus. Hum Gene Ther. 1999;10:2741–2755. doi: 10.1089/10430349950016483. [DOI] [PubMed] [Google Scholar]
- Todo T, Martuza RL, Rabkin SD., and , Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci USA. 2001;98:6396–6401. doi: 10.1073/pnas.101136398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todo T, Martuza RL, Dallman MJ., and , Rabkin SD. In situ expression of soluble B7-1 in the context of oncolytic herpes simplex virus induces potent antitumor immunity. Cancer Res. 2001;61:153–161. [PubMed] [Google Scholar]
- Orentas RJ, Schauer D, Bin Q., and , Johnson BD. Electrofusion of a weakly immunogenic neuroblastoma with dendritic cells produces a tumor vaccine. Cell Immunol. 2001;213:4–13. doi: 10.1006/cimm.2001.1864. [DOI] [PubMed] [Google Scholar]
- Schwab M, Ellison J, Busch M, Rosenau W, Varmus HE., and , Bishop JM. Enhanced expression of the human gene N-myc consequent to amplification of DNA may contribute to malignant progression of neuroblastoma. Proc Natl Acad Sci USA. 1984;81:4940–4944. doi: 10.1073/pnas.81.15.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur GM, Seeger RC, Schwab M, Varmus HE., and , Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313:1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- Guffey MB, Parker JN, Luckett WS Jr, Gillespie GY, Meleth S, Whitley RJ, et al. Engineered herpes simplex virus expressing bacterial cytosine deaminase for experimental therapy of brain tumors. Cancer Gene Ther. 2007;14:45–56. doi: 10.1038/sj.cgt.7700978. [DOI] [PubMed] [Google Scholar]
- Li H, Dutuor A, Tao L, Fu X., and , Zhang X. Virotherapy with a type 2 herpes simplex virus-derived oncolytic virus induces potent antitumor immunity against neuroblastoma. Clin Cancer Res. 2007;13:316–322. doi: 10.1158/1078-0432.CCR-06-1625. [DOI] [PubMed] [Google Scholar]
- Ino Y, Saeki Y, Fukuhara H., and , Todo T. Triple combination of oncolytic herpes simplex virus-1 vectors armed with interleukin-12, interleukin-18, or soluble B7-1 results in enhanced antitumor efficacy. Clin Cancer Res. 2006;12:643–652. doi: 10.1158/1078-0432.CCR-05-1494. [DOI] [PubMed] [Google Scholar]
- Fukuhara H, Ino Y, Kuroda T, Martuza RL., and , Todo T. Triple gene-deleted oncolytic herpes simplex virus vector double-armed with interleukin 18 and soluble B7-1 constructed by bacterial artificial chromosome-mediated system. Cancer Res. 2005;65:10663–10668. doi: 10.1158/0008-5472.CAN-05-2534. [DOI] [PubMed] [Google Scholar]
- Parikh NS, Currier MA, Mahller YY, Adams LC, Di Pasquale B, Collins MH, et al. Oncolytic herpes simplex virus mutants are more efficacious than wild-type adenovirus Type 5 for the treatment of high-risk neuroblastomas in preclinical models. Pediatr Blood Cancer. 2005;44:469–478. doi: 10.1002/pbc.20268. [DOI] [PubMed] [Google Scholar]
- Keshelava N, Seeger RC, Groshen S., and , Reynolds CP. Drug resistance patterns of human neuroblastoma cell lines derived from patients at different phases of therapy. Cancer Res. 1998;58:5396–5405. [PubMed] [Google Scholar]
- Negroni A, Scarpa S, Romeo A, Ferrari S, Modesti A., and , Raschellà G. Decrease of proliferation rate and induction of differentiation by a MYCN antisense DNA oligomer in a human neuroblastoma cell line. Cell Growth Differ. 1991;2:511–518. [PubMed] [Google Scholar]
- Mahller YY, Vaikunth SS, Ripberger MC, Baird WH, Saeki Y, Cancelas JA, et al. Tissue inhibitor of metalloproteinase-3 via oncolytic herpesvirus inhibits tumor growth and vascular progenitors. Cancer Res. 2008;68:1170–1179. doi: 10.1158/0008-5472.CAN-07-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, Surico G, Vacca A, De Leonardis F, Lastilla G, Montaldo PG, et al. Angiogenesis extent and expression of matrix metalloproteinase-2 and -9 correlate with progression in human neuroblastoma. Life Sci. 2001;68:1161–1168. doi: 10.1016/s0024-3205(00)01030-4. [DOI] [PubMed] [Google Scholar]
- Sakakibara M, Koizumi S, Saikawa Y, Wada H, Ichihara T, Sato H, et al. Membrane-type matrix metalloproteinase-1 expression and activation of gelatinase A as prognostic markers in advanced pediatric neuroblastoma. Cancer. 1999;85:231–239. doi: 10.1002/(sici)1097-0142(19990101)85:1<231::aid-cncr31>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Ara T, Fukuzawa M, Kusafuka T, Komoto Y, Oue T, Inoue M, et al. Immunohistochemical expression of MMP-2, MMP-9, and TIMP-2 in neuroblastoma: association with tumor progression and clinical outcome. J Pediatr Surg. 1998;33:1272–1278. doi: 10.1016/s0022-3468(98)90167-1. [DOI] [PubMed] [Google Scholar]
- Mahller YY, Williams JP, Baird WH, Mitton B, Grossheim J, Saeki Y, et al. Neuroblastoma cell lines contain pluripotent tumor initiating cells that are susceptible to a targeted oncolytic virus. PLoS ONE. 2009;4:e4235. doi: 10.1371/journal.pone.0004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulino AC., and , Okcu MF. Rhabdomyosarcoma. Curr Probl Cancer. 2008;32:7–34. doi: 10.1016/j.currproblcancer.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Xia SJ, Pressey JG., and , Barr FG. Molecular pathogenesis of rhabdomyosarcoma. Cancer Biol Ther. 2002;1:97–104. doi: 10.4161/cbt.51. [DOI] [PubMed] [Google Scholar]
- Visser M, Sijmons C, Bras J, Arceci RJ, Godfried M, Valentijn LJ, et al. Allelotype of pediatric rhabdomyosarcoma. Oncogene. 1997;15:1309–1314. doi: 10.1038/sj.onc.1201302. [DOI] [PubMed] [Google Scholar]
- Breneman JC, Lyden E, Pappo AS, Link MP, Anderson JR, Parham DM, et al. Prognostic factors and clinical outcomes in children and adolescents with metastatic rhabdomyosarcoma—a report from the Intergroup Rhabdomyosarcoma Study IV. J Clin Oncol. 2003;21:78–84. doi: 10.1200/JCO.2003.06.129. [DOI] [PubMed] [Google Scholar]
- Crist W, Gehan EA, Ragab AH, Dickman PS, Donaldson SS, Fryer C, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- Maurer HM, Gehan EA, Beltangady M, Crist W, Dickman PS, Donaldson SS, et al. The Intergroup Rhabdomyosarcoma Study-II. Cancer. 1993;71:1904–1922. doi: 10.1002/1097-0142(19930301)71:5<1904::aid-cncr2820710530>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Linet MS, Ries LA, Smith MA, Tarone RE., and , Devesa SS. Cancer surveillance series: recent trends in childhood cancer incidence and mortality in the United States. J Natl Cancer Inst. 1999;91:1051–1058. doi: 10.1093/jnci/91.12.1051. [DOI] [PubMed] [Google Scholar]
- Spunt SL, Hill DA, Motosue AM, Billups CA, Cain AM, Rao BN, et al. Clinical features and outcome of initially unresected nonmetastatic pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Clin Oncol. 2002;20:3225–3235. doi: 10.1200/JCO.2002.06.066. [DOI] [PubMed] [Google Scholar]
- Spunt SL, Poquette CA, Hurt YS, Cain AM, Rao BN, Merchant TE, et al. Prognostic factors for children and adolescents with surgically resected nonrhabdomyosarcoma soft tissue sarcoma: an analysis of 121 patients treated at St Jude Children's Research Hospital. J Clin Oncol. 1999;17:3697–3705. doi: 10.1200/JCO.1999.17.12.3697. [DOI] [PubMed] [Google Scholar]
- Pratt CB, Maurer HM, Gieser P, Salzberg A, Rao BN, Parham D, et al. Treatment of unresectable or metastatic pediatric soft tissue sarcomas with surgery, irradiation, and chemotherapy: a Pediatric Oncology Group study. Med Pediatr Oncol. 1998;30:201–209. doi: 10.1002/(sici)1096-911x(199804)30:4<201::aid-mpo1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Hansen MF, Koufos A, Gallie BL, Phillips RA, Fodstad O, Brøgger A, et al. Osteosarcoma and retinoblastoma: a shared chromosomal mechanism revealing recessive predisposition. Proc Natl Acad Sci USA. 1985;82:6216–6220. doi: 10.1073/pnas.82.18.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CW, Aslo A, Tsay C, Slamon D, Ishizaki K, Toguchida J, et al. Frequency and structure of p53 rearrangements in human osteosarcoma. Cancer Res. 1990;50:7950–7954. [PubMed] [Google Scholar]
- Delattre O, Zucman J, Melot T, Garau XS, Zucker JM, Lenoir GM, et al. The Ewing family of tumors—a subgroup of small-round-cell tumors defined by specific chimeric transcripts. N Engl J Med. 1994;331:294–299. doi: 10.1056/NEJM199408043310503. [DOI] [PubMed] [Google Scholar]
- Miser JS, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Treatment of metastatic Ewing's sarcoma or primitive neuroectodermal tumor of bone: evaluation of combination ifosfamide and etoposide—a Children's Cancer Group and Pediatric Oncology Group study. J Clin Oncol. 2004;22:2873–2876. doi: 10.1200/JCO.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Meyers PA, Heller G, Healey JH, Huvos A, Applewhite A, Sun M, et al. Osteogenic sarcoma with clinically detectable metastasis at initial presentation. J Clin Oncol. 1993;11:449–453. doi: 10.1200/JCO.1993.11.3.449. [DOI] [PubMed] [Google Scholar]
- Miser JS, Goldsby RE, Chen Z, Krailo MD, Tarbell NJ, Link MP, et al. Treatment of metastatic Ewing sarcoma/primitive neuroectodermal tumor of bone: evaluation of increasing the dose intensity of chemotherapy—a report from the Children's Oncology Group. Pediatr Blood Cancer. 2007;49:894–900. doi: 10.1002/pbc.21233. [DOI] [PubMed] [Google Scholar]
- Bruland OS., and , Pihl A. On the current management of osteosarcoma. A critical evaluation and a proposal for a modified treatment strategy. Eur J Cancer. 1997;33:1725–1731. doi: 10.1016/s0959-8049(97)00252-9. [DOI] [PubMed] [Google Scholar]
- Currier MA, Adams LC, Mahller YY., and , Cripe TP. Widespread intratumoral virus distribution with fractionated injection enables local control of large human rhabdomyosarcoma xenografts by oncolytic herpes simplex viruses. Cancer Gene Ther. 2005;12:407–416. doi: 10.1038/sj.cgt.7700799. [DOI] [PubMed] [Google Scholar]
- Cinatl J Jr, Cinatl J, Michaelis M, Kabickova H, Kotchetkov R, Vogel JU, et al. Potent oncolytic activity of multimutated herpes simplex virus G207 in combination with vincristine against human rhabdomyosarcoma. Cancer Res. 2003;63:1508–1514. [PubMed] [Google Scholar]
- Kurozumi K, Hardcastle J, Thakur R, Yang M, Christoforidis G, Fulci G, et al. Effect of tumor microenvironment modulation on the efficacy of oncolytic virus therapy. J Natl Cancer Inst. 2007;99:1768–1781. doi: 10.1093/jnci/djm229. [DOI] [PubMed] [Google Scholar]
- Lan P, Dong C, Qi Y, Xiao G., and , Xue F. Gene therapy for mice sarcoma with oncolytic herpes simplex virus-1 lacking the apoptosis-inhibiting gene, icp34.5. J Biochem Mol Biol. 2003;36:379–386. doi: 10.5483/bmbrep.2003.36.4.379. [DOI] [PubMed] [Google Scholar]
- Sugiura S, Goshima F, Takakuwa H, Sata T, Nakashima T., and , Nishiyama Y. Treatment of solid sarcomas in immunocompetent mice with novel, oncolytic herpes simplex viruses. Otolaryngol Head Neck Surg. 2004;130:470–478. doi: 10.1016/j.otohns.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Yamamura H, Hashio M, Noguchi M, Sugenoya Y, Osakada M, Hirano N, et al. Identification of the transcriptional regulatory sequences of human calponin promoter and their use in targeting a conditionally replicating herpes vector to malignant human soft tissue and bone tumors. Cancer Res. 2001;61:3969–3977. [PubMed] [Google Scholar]
- Takahashi K., and , Nadal-Ginard B. Molecular cloning and sequence analysis of smooth muscle calponin. J Biol Chem. 1991;266:13284–13288. [PubMed] [Google Scholar]
- Jenson HB, Montalvo EA, McClain KL, Ench Y, Heard P, Christy BA, et al. Characterization of natural Epstein-Barr virus infection and replication in smooth muscle cells from a leiomyosarcoma. J Med Virol. 1999;57:36–46. [PubMed] [Google Scholar]
- Miettinen MM, Sarlomo-Rikala M, Kovatich AJ., and , Lasota J. Calponin and h-caldesmon in soft tissue tumors: consistent h-caldesmon immunoreactivity in gastrointestinal stromal tumors indicates traits of smooth muscle differentiation. Mod Pathol. 1999;12:756–762. [PubMed] [Google Scholar]
- Ono H, Yoshikawa H, Ueda T, Yamamura H, Kudawara I, Manou M, et al. Expression of smooth muscle calponin in synovial sarcoma. Sarcoma. 1997;2:107–113. doi: 10.1080/13577149977730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi A, Nikaido T, Ya-Li Z, Ito K, Orii A., and , Fujii S. Heparin inhibits proliferation of myometrial and leiomyomal smooth muscle cells through the induction of alpha-smooth muscle actin, calponin h1 and p27. Mol Hum Reprod. 1999;5:139–145. doi: 10.1093/molehr/5.2.139. [DOI] [PubMed] [Google Scholar]
- Yamamura H, Yoshikawa H, Tatsuta M, Akedo H., and , Takahashi K. Expression of the smooth muscle calponin gene in human osteosarcoma and its possible association with prognosis. Int J Cancer. 1998;79:245–250. doi: 10.1002/(sici)1097-0215(19980619)79:3<245::aid-ijc6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ., and , Weller SK. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988;62:196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meignier B, Longnecker R., and , Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020: construction and evaluation in rodents. J Infect Dis. 1988;158:602–614. doi: 10.1093/infdis/158.3.602. [DOI] [PubMed] [Google Scholar]
- Meignier B, Martin B, Whitley RJ., and , Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020. II. Studies in immunocompetent and immunosuppressed owl monkeys (Aotus trivirgatus) J Infect Dis. 1990;162:313–321. doi: 10.1093/infdis/162.2.313. [DOI] [PubMed] [Google Scholar]
- Packer RJ. Progress and challenges in childhood brain tumors. J Neurooncol. 2005;75:239–242. doi: 10.1007/s11060-005-6745-9. [DOI] [PubMed] [Google Scholar]
- McNeil DE, Coté TR, Clegg L., and , Rorke LB. Incidence and trends in pediatric malignancies medulloblastoma/primitive neuroectodermal tumor: a SEER update. Surveillance Epidemiology and End Results. Med Pediatr Oncol. 2002;39:190–194. doi: 10.1002/mpo.10121. [DOI] [PubMed] [Google Scholar]
- Echevarría ME, Fangusaro J., and , Goldman S. Pediatric central nervous system germ cell tumors: a review. Oncologist. 2008;13:690–699. doi: 10.1634/theoncologist.2008-0037. [DOI] [PubMed] [Google Scholar]
- Punyko JA, Mertens AC, Baker KS, Ness KK, Robison LL., and , Gurney JG. Long-term survival probabilities for childhood rhabdomyosarcoma. A population-based evaluation. Cancer. 2005;103:1475–1483. doi: 10.1002/cncr.20929. [DOI] [PubMed] [Google Scholar]
- Ferrari A, Casanova M, Collini P, Meazza C, Luksch R, Massimino M, et al. Adult-type soft tissue sarcomas in pediatric-age patients: experience at the Istituto Nazionale Tumori in Milan. J Clin Oncol. 2005;23:4021–4030. doi: 10.1200/JCO.2005.02.053. [DOI] [PubMed] [Google Scholar]
- Foster L, Dall GF, Reid R, Wallace WH., and , Porter DE. Twentieth-century survival from osteosarcoma in childhood. Trends from 1933 to 2004. J Bone Joint Surg Br. 2007;89:1234–1238. doi: 10.1302/0301-620X.89B9.19255. [DOI] [PubMed] [Google Scholar]
- Esiashvili N, Goodman M., and , Marcus RB., Jr Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol. 2008;30:425–430. doi: 10.1097/MPH.0b013e31816e22f3. [DOI] [PubMed] [Google Scholar]