Abstract
Adenovirus (Ad) vectors were initially developed for treatment of genetic diseases. Their usefulness for permanent gene replacement was limited by their high immunogenicity, which resulted in rapid elimination of transduced cells through induction of T and B cells to antigens of Ad and the transgene product. The very trait that excluded their use for sustained treatment of genetic diseases made them highly attractive as vaccine carriers. Recently though results showed that Ad vectors based on common human serotypes, such as serotype 5, may not be ideal as vaccine carriers. A recently conducted phase 2b trial, termed STEP trial, with an AdHu5-based vaccine expressing antigens of human immunodeficiency virus 1 (HIV-1) not only showed lack of efficacy in spite of the vaccine's immunogenicity, but also suggested an increased trend for HIV acquisition in individuals that had circulating AdHu5 neutralizing antibodies prior to vaccination. Alternative serotypes from humans or nonhuman primates (NHPs), to which most humans lack pre-existing immunity, have been vectored and may circumvent the problems encountered with the use of AdHu5 vectors in humans. In summary, although Ad vectors have seen their share of setbacks in recent years, they remain viable tools for prevention or treatment of a multitude of diseases.
Introduction
Adenovirus (Ad) vectors were developed to replace genes in inborn errors of metabolism. Enthusiasm toward the use of first-generation Ad vectors in gene replacement therapy diminished because they not only failed to affect sustained gene transfer, but also resulted in significant toxicity and in the death of an individual.1,2,3 Due to their aptitude for inducing potent innate and adoptive immune responses, Ad vectors have been and are being explored as vaccine carriers.4,5 Till recently, replication-defective Ad vectors of the human serotype 5 (AdHu5) were heralded as the most promising vaccine platform for antigens of human immunodeficiency virus (HIV) 1.4 However, they failed to meet expectations and in a large-scale clinical trial, termed STEP trial, not only showed lack of efficacy, but appeared to cause harm by slightly increasing rates of HIV-1 acquisition in individuals with pre-existing neutralizing antibodies to AdHu5.6,7 The underlying mechanisms by which AdHu5 vaccination cause a potentially transient increase in susceptibility to HIV-1 remain unknown. Although the STEP trial was not a success in its ultimate goal to protect against HIV-1, it was a success in its impeccable execution and as such will provide guidance on future vaccine efforts, which at least for HIV-1 are shifting to Ad vectors derived from rare human serotypes8 or from serotypes derived from nonhuman primates (NHPs).9
Here, we briefly review the different applications of Ad vectors and the approaches that are being taken to improve their performance.
Ad Classification, Genetic Organization, and Structure
Ads have been isolated from multiple species including primates, bovines, fowls, reptiles, and frogs. Human Ads have been classified into 51 immunologically distinct serotypes, which are divided into 6 subgroups, i.e., subgroups A–F. Ads commonly used as gene transfer vehicles are human serotypes (AdHu) 4 (E), 5 (C), 26 (D), 35 (B), 41 (F), and 48 (D), and chimpanzee serotypes (AdC) 3, 6, 7, 63, and 68. Most AdC viruses are phylogenetically grouped within human Ads of family E.10 In humans, family E contains only one serotype, i.e., 4, which is cross-neutralized by antibodies to AdC63. Family E viruses thus have evolved into several serotypes in chimpanzees, but into only one serotype in humans. This in turn indicates family E Ads originated in chimpanzees and that AdHu4 may formerly have been an AdC virus that successfully crossed into humans. A uniform nomenclature for chimpanzee-derived Ad vectors referred to here as AdC viruses is not yet available. For clarification, Table 1 shows a list of AdC viruses and their different names.
Table 1.
Nomenclature of adenovirus isolated from chimpanzee
Ads are nonenveloped and simple double-stranded DNA viruses. The Ad genome size is ~30–35 kilobases and contains five segments that encode early gene products, i.e., E1a, E1b, E2a, E2b, E3, and E4, and five segments that encode late gene products, i.e., L1–L5. E1, E2, and E4 gene products have regulatory functions that allow for transcription and translation of the late genes11,12,13 and are indispensable for viral replication. E3 gene products subvert immune responses by affecting antigen presentation, cytokine and apoptosis pathways14,15 and are not needed for viral replication. The Ad capsid is composed of 20 triangular facets of an icosahedron.16 Each icosahedron contains 12 copies of hexon trimers. The fiber proteins, which also form trimers, are inserted at the 12 vertices into the pentameric penton bases. The hexon, the most abundant of the capsid proteins, has a shaft that is highly conserved between different serotypes of Ads, and a tower that contains a number of loops that show variability between different serotypes.10 Most neutralizing antibodies to Ad are directed against the variable hexon loops. The Ad fiber is composed of a thin N-terminal tail, a shaft, and a knob domain. The shaft is composed of variable numbers of β-strand repeats. The numbers of repeats largely determine the length of the shaft, which varies between Ads from different families.17 The knob contains loops that are lettered A–J. The fiber knob loops bind to cellular receptors. The A–B fiber knob loops of most Ad bind to the coxsackie Ad receptor (CAR),18 which after birth is expressed mainly on epithelial cells.19 Other Ads bind to CD46,20 a complement regulatory protein that is expressed on most cells in humans and NHPs, but only in testes and sperm in mice.21 Cell entry is mediated upon binding of a secondary receptor, which in some Ad viruses such as AdHu5, is provided by an Arg-Gly-Asp motif within the penton base that binds to vβ3 or vβ5 integrins.22 The Ad genome encodes several additional minor capsid proteins, termed proteins IIIa, VI, VIII, and IX, which have been reviewed previously.23
Immune Responses to Ads
Natural infections with Ads induce antibodies. Neutralizing antibodies, directed mainly against the hexon loops, are serotype-specific. They affect the efficacy of Ad vector gene transfer by blocking cell transduction.24,25,26 This in turn reduces transgene product expression. Measures of neutralizing antibodies have been used to estimate prevalence rates of Ad infections. Neutralizing antibodies to AdHu5 virus, the basis for the most commonly used Ad-based gene transfer vehicles, are present in about 40–45% of adults in the United States and in up to 90% of humans residing in sub-Saharan Africa.27 The prevalence of neutralizing antibodies to other serotypes also varies.28,29 More recent efforts have focused on developing vectors based on so-called rare serotypes, such as AdHu26, 35, or 48, to which most humans do not have neutralizing antibodies.30,31 Notwithstanding, it should be pointed out that the seroprevalence rates of neutralizing antibodies to different Ad serotypes vary considerably between geographic regions, so the serotypes that may be rare in the United States can be quite common in other regions.29 Neutralizing antibody titers are typically determined in tissue culture by neutralization of vectors expressing a reporter protein. A recent publication suggests that such assays may not faithfully recapitulate neutralization in vivo.32 Non-neutralizing binding antibodies to Ads crossreact among different serotypes (Z.Q. Xiang and H.C.J. Ertl, unpublished results).
T-cell responses to natural infections with Ads have been studied less vigorously. CD4+ and CD8+ T-cell responses have been demonstrated in humans,33,34,35 and as confirmed by studies in rodents, these cells appear to crossreact between multiple serotypes,25 which is to be expected considering the high sequence homology of Ad gene products. Several groups are currently assessing T-cell responses to Ad antigens in human subjects, and most of these data have been presented at conferences but not yet been published. Results indicate that most human adults have circulating Ad-specific T cells. One publication reported the presence of Ad-specific T cells in human gut tissue and also showed that circulating Ad-specific T cells are mainly of the central memory CD4+ T-cell subset, producing predominantly tumor-necrosis factor-α.36 Other groups reported discordant results, and one publication showed that humans have both circulating CD4+ and CD8+ T cells in blood that predominantly produce interferon γ.7 The most sensitive method to measure T-cell responses in outbred populations is based on intracellular cytokine staining with concomitant staining for cell surface markers that identify T-cell subsets and their differentiation status and functions. This assay, which commonly uses antibodies with up to 16 different dyes, is technically difficult, which may in part explain the contradicting results. Also, the outcome of such experiments is influenced by the amount of antigen used for in vitro stimulation of the T cells and this amount varied between the different reports.7,36
Ads induce potent inflammatory responses, in part due to the activity of structural viral proteins. Activation of innate responses appears to involve several pathways, including at least two toll-like receptors, i.e., toll-like receptor 2 and toll-like receptor 9, as well as type 1 interferon.37,38,39 In addition, the Ad DNA is recognized in the cytosol by NALT3, a NOD-like receptor family member, which in turn triggers a pro-inflammatory cytokine response.40 Overall, Ad viruses and vector are recognized by innate sensors through multiple pathways that all result in activation of cytokine and chemokine release, which in turn impose dose-limiting toxicity for the use of Ad vectors in humans.41
Types of Ad Used as Vectors
Early Ad gene transfer vectors were deleted in the E3 domain, a deletion that does not render the virus replication-defective.42 Most currently used vectors are deleted in E1 and E3 (Table 2). The E1 deletion renders the virus replication-defective, whereas the E3 deletion may affect the vectors' interaction with the host's immune system. This effect is expected to be insignificant in E1-deleted Ad vectors due to lack of transcription of the E3 gene product. Nevertheless, E3 deletions are useful to increase the packaging capacity of Ad vectors. Although E3 is not needed for viral replication, E1 is essential and has to be transcomplemented in suitable packaging cell lines in order to allow viral propagation. Two packaging cell lines are commonly used to grow E1-deleted Ad vectors: (i) human embryonic kidney (HEK) 293 cells contain a large segment of the AdHu5 genome including the E1 domain43 and (ii) PerC6 cells contain only the E1 genes of AdHu5 virus.44 Outgrowth of replication-competent Ads is common in HEK 293 cells due to homologous recombination between the E1 flanking regions in the vector and the viral sequences present in HEK 293 cells. PerC6 cells do not allow for homologous recombination and are therefore superior for production of replication-defective AdHu5 vectors—unfortunately, this cell line is not available to academic investigators. Serotypes other than AdHu5 can be grown on HEK 293 or PerC6 cells.44,45 For some serotypes, this requires the replacement of the open reading frame 6 of E4 with that of AdHu5.46 For some applications, only E3 or E1 and E3 deleted vectors are used. To avoid outgrowth of replication-competent adenoviruses on HEK 293 packaging cells, E1 deleted vectors with further deletions in E4 are being tested as vaccine vectors.47 Additional deletions including so-called fully gutted vectors have been explored for gene transfer.48 As a rule, most additional deletions in early genes reduce the immune response to the Ad vectors and prolong transgene product expression.48 Fully gutted Ad vectors, in which all of the viral coding sequences have been removed, have shown reduced toxicity caused by innate immune responses and achieved sustained gene transfer in immunocompetent rodents.49 Nevertheless, even fully gutted Ad vectors can elicit immune responses to the transgene product.50 Due to cross-priming, a process in which antigen degraded by other types of cells is acquired and presented by professional antigen-presenting cells,51 one would expect that large doses of gutted Ad vectors would also elicit T-cell responses to structural proteins of the vector particles, especially in humans with pre-existing immunological memory to Ad viruses.52
Table 2.
Examples of adenovirus vectors used for vaccination
More recent efforts have focused on modifying rather than deleting genes of Ad vectors. Structural gene products of Ad vectors have been modified to improve their performance for a specific application. Modifications of the hexon protein of an AdC vector by a point mutation in the loop that carries the dominant neutralizing antibody epitope was shown to reduce binding of serotype-specific antibodies in vitro, but still resulted in vector neutralization in vivo.32 Replacement of all of the hexon loops of AdHu5 with those of AdHu48 reduced binding of AdHu5 neutralizing antibodies, and allowed for the effective use of this vector as a vaccine carrier in animals with pre-existing neutralizing antibodies to AdHu5.53 Replacement of the fiber knob of a CD46-binding AdHu35 vector with the fiber knob of the CAR-binding AdHu5 virus changes the tropism of the vector and increases transgene product–specific immune responses.54 Reversely, replacing the AdHu5 fiber with that of AdHu3, another CD46-binding virus, broadens the tropism of the chimeric AdHu5/3 vectors to CD46-expressing cells.55 Insertion of an Arg-Gly-Asp sequence, which binds to integrins, into the H–I loops of the fiber knob of a CAR-binding vector also increases the tropism of such vectors.56 Similar effects were seen upon incorporation of a polylysine motif into fiber, which allows binding to heparan sulfate receptors.57
AdHu5 vectors have a high tropism for liver where most vector particles are taken up and destroyed by Kupffer cells.58 A number of modifications have been attempted to reduce the high liver tropism of AdHu5 vectors. Some reported that simultaneous deletions of CAR-binding sequences in fiber and the integrin-binding sequences in penton greatly reduce liver transduction.59,60 However, other groups failed to confirm these results.61 Subsequent studies showed that CAR is not used for liver transduction, but the coagulation factor (F)X binds to the AdHu5 hexon and that this complex is readily taken up by hepatocytes due to a heparin-binding motif in the FX serine protease domain.62,63 Mutation of a putative heparan sulfate proteoglycan—binding motif in the fiber shaft of AdHu5 vector was shown to reduce levels of liver transduction in animals.64
Biomedical Applications for Ad Vectors
Gene transfer by replication-defective Ad vectors has mainly been explored for gene replacement and vaccination.
Gene replacement
Ad vectors were initially developed for gene replacement therapy, where they effected cures in immunocompromised animal models but failed in immunocompetent hosts including humans, as adaptive immune responses to the antigens of Ad, most notably, CD8+ T cells, rapidly eliminated the transduced cells.65 Ad vectors showed significant toxicity in human volunteers.66 A number of second-generation Ad vectors were tested subsequently to circumvent vector elimination by the immune system.67,68 The immune system, which has evolved over the millennia to fight off pathogens such as viruses, cannot be fooled that easily. As detailed above, Ads are ubiquitous viruses that infect all humans. They also persist69 and one would expect that they are periodically reactivated—otherwise, evolution would not have favored that trait. Repeated infection, combined with repeated reactivation of a persisting virus, will not only augment immune responses but also maintain T cells at a fairly active stage (Tables 3 and 4). Avoiding such potent responses even temporarily by the use of immunosuppressants designed to prevent T-cell proliferation rather than effector functions will not be possible without causing irreversible harm to the patients' immune system.
Table 3.
Examples of structural modifications on adenovirus vectors
Table 4.
Examples of nonstructural modifications on adenovirus vectors
Ad vectors for vaccination
Ad vectors have been tested extensively as vaccine carriers. For vaccination, most vectors are deleted in E1 or E1/E3. Initially, most vaccines were based on AdHu5 virus.4,70 In preclinical models, these vectors were shown to induce potent transgene product–specific T- and B-cell responses.71,72 T cells were mainly of the CD8+ T-cell subset, although low CD4+ T-cell responses of the T-helper (Th) cell type 1 were also induced. However, responses were clearly reduced by pre-existing neutralizing antibodies to AdHu5 virus.25,27 In addition, repeated immunizations with AdHu5 vectors given in a prime boost regimen were not overly effective.9 T-cell responses were remarkably sustained and failed to contract after the initial effector phase. This was linked to the vectors' ability to persist in a transcriptionally active form in T cells.73
Expressing conserved antigens of HIV-1 for induction of CD8+ T-cell responses, which are assumed to provide some protection against the virus, early phase clinical trials with an AdHu5 vaccine yielded sufficiently promising results in volunteers at low risk for HIV-1 acquisition to allow for two large phase 2b trials in humans at high risk for HIV-1 acquisition. Two trials, called STEP and Phambili trials, were powered to assess the effect of vaccination on rate of acquisition of HIV-1 and on viral set points. The vaccine expressed several antigens of HIV-1 to induce T cells and was given three times to volunteers that had either no or moderate-to-high titers of neutralizing antibodies to AdHu5. The STEP trial was stopped and unblinded before enrollment had been completed because an interim analysis showed lack of efficacy and even more worrisome trend toward increased HIV-1 acquisition in vaccine recipients. A more detailed analysis of the data gathered before unblinding showed that in the STEP trial, only men had become infected in the vaccine group. Infection rates were slightly higher in vaccinated males with moderate-to-high pre-existing neutralizing antibody titers to AdHu5 than in the corresponding control group.6 The acquisition rate of HIV-1 was identical among men who were seronegative for AdHu5 before the trial. Further analysis has shown that the trend for higher HIV-1 acquisition rates in the vaccinated group mainly affected uncircumcised men with moderate-to-high pre-existing Abs to AdHu5.6 Additional data have been gathered from these cohorts since unblinding of the trials, which suggest that the observed trend of increased HIV-1 acquisition may have been transient and could no longer be confirmed in follow-up studies. Notwithstanding, it has to be stressed that acquisition trends, after the trial was stopped, have to be interpreted with extreme caution, as the unblinding of the trial and the high publicity of the trial result may well have affected the behavior of the vaccine recipients.
An assessment of the vaccine-induced immune responses showed that, as expected, the vaccine stimulated HIV-1–specific CD8+ and CD4+ T-cell responses.7 Responses were slightly lower in individuals with pre-existing neutralizing antibodies to the vaccine carrier. There was no striking difference in the functionality of the T-cell responses in individuals with or without pre-existing neutralizing antibodies to AdHu5, nor was there a clear difference in other T-cell markers used to determine the differentiation status of T cells. There were also no differences upon comparison of preinfection samples from vaccinated cases or noncases. None of the results of these thorough studies could explain the increased acquisition rates, leading to the formulation of several hypotheses. Some argued that the increase in rates of HIV-1 acquisition was unrelated to the vaccine. Others postulated that antibodies to the vaccine carrier affected vector targeting and hence antigen presentation, in a way that favored the induction of a Th2 response to antigens of HIV-1; or that CD4+ T cells to antigens of Ad induced at very high frequencies in Ad seropositive individuals served as target cells for HIV-1 infection. Available data support neither of these hypotheses.
HIV-1 differs from other pathogens by primarily infecting and replicating in activated CD4+ T cells. CD4+ T cells have to be induced by vaccines to promote activation of CD8+ T cells and B cells. Other vaccine platforms, such as protein vaccines or poxvirus vectors that induce CD4+ T cells, were not found to increase HIV-1 acquisition rates in clinical efficacy trials. These vaccines induce T cells that rapidly transition into central memory cells after an initial effector phase, unlike Ad vectors, which stimulate a protracted effector/effector memory phase due to low-level persistence of the viral vector.73 Whether the prolonged effector/effector memory phase was causative for the increased susceptibility to HIV-1 infection remains unknown.
The STEP trial results may have consequences for the use of Ad vectors as vaccine carriers in general. Without a firm understanding of the underlying pathways that led to the trend of increased HIV-1 acquisition in vaccinated Ad seropositive individuals, AdHu5 vectors may no longer be used as vaccine carriers for HIV-1, nor may they be used in postpubescent juveniles or adults as vaccine carriers for other pathogens. Replication-competent Ad vectors74 are being developed as vaccine carriers for HIV-1 antigens, intended for oral delivery in enteric-coated capsules. Immunity to these vectors based on AdHu4 and AdHu7 is common in humans, but oral delivery may circumvent the effect of pre-existing neutralizing antibodies.75 On the other hand, induction of CD4+ T cells within the intestine, the preferred targets of early HIV-1 replication, may pose risks.
Lack of efficacy of the AdHu5 vaccine in the STEP trial in individuals without pre-existing neutralizing antibodies may reflect that T cells cannot protect against HIV-1. Alternatively, the vaccine may not have achieved responses that were of sufficient magnitude and/or quality to increase resistance against HIV-1 infection or spread. The latter is supported by the finding that vaccination of Ad seronegative elite controllers, which in general mount higher CD8+ T-cell responses to some antigens of HIV-1, appeared to provide some protection to this small subgroup, as they developed significantly lower viral loads upon infection compared to nonvaccinated elite controllers. With the caveat that the trial had not been designed for such a subgroup analysis, the results are nevertheless intriguing, as they may suggest that a vaccine to HIV-1 that achieves a more potent T-cell response than the STEP trial vaccine may be efficacious. The repeated use of a viral vector for boosting of insert-specific immune responses is relatively ineffective, and one would expect, as has been shown in preclinical studies, that prime boost regimens combining different serotypes of Ad vectors or Ad vectors with other vaccine platforms, such as poxvirus vectors, would induce higher frequencies of T cells, which may suffice to induce protective immunity.76,77,78,79
Efforts to develop HIV-1 vaccines based on different serotypes of Ad continue. In NHPs, the STEP trial vaccine induced protection against challenge with a pathogenic simian immunodeficiency virus/HIV chimera called SHIV89.6P, which is now considered a “soft” challenge virus, but failed to protect against an infection with simian immunodeficiency virus. Ad vaccine regimens based on the sequential use of different serotypes, which induced more potent T-cell responses in NHPs and provided protection against simian immunodeficiency virus, are now undergoing or scheduled for early stage clinical testing.8
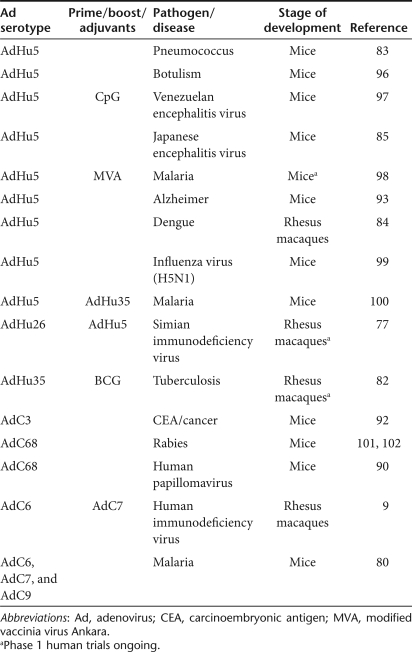
Ad vector vaccines are being developed for pathogens other than HIV-1 as well as for cancer and degenerative diseases. Pathogens targeted by Ad vectors include parasites such as Plasmodium falciparum,80 leishmania,81 bacteria such as Mycobacterium tuberculosis82 or pneumococcus,83 and viruses such as dengue virus,84 Japanese85 or Venezuelan encephalitis virus,86 rabies virus,72 influenza virus,87 or hepatitis viruses.88,89 Tumor antigens that have been tested include oncoproteins of human papillomavirus,90 tumor-associated antigens of melanoma such as MART-191 or carcinoembryonic antigen.92 The amyloid β protein is being tested to reduce the amyloid load in Alzheimer disease.93 Many investigators are still focusing on AdHu5 vectors, although these vectors are expected to perform suboptimally in humans with high titers of AdHu5-specific neutralizing antibodies. Other vaccine efforts are shifting to rare human serotypes or AdC vectors. The immunogenicity of Ad vectors of either serotype is being augmented by coexpression of antigens with immunomodulators such as cytokines94 or inhibitors of immunosuppressive pathways90 or by retargeting through structural modifications of capsid proteins.95
Summary
Although, in our minds, Ad vectors should not be pursued for permanent replacement of genes, their use for vaccination should be explored further. New serotypes to which most humans lack neutralizing antibodies have been vectored and are expected to circumvent problems encountered with pre-existing neutralizing antibodies in humans. The effect of pre-existing memory T cells that crossreact between different serotypes on Ad-mediated gene transfer remains to be investigated in more depth. Advances in our understanding of the basic biology of Ad viruses and the structure of their main capsid antigens now allows for targeted modifications that are expected to further improve the performance of these highly versatile vectors.
Acknowledgments
This work was funded by NIH grant 5U19AI074078 and the Wistar Cancer Center Support grant (NCI-P30 CA 010815). We would like to thank Colin Barth and Christina Cole for help in preparation of the manuscript. We have no financial interests associated with the work presented.
REFERENCES
- Benihoud K, Yeh P., and , Perricaudet M. Adenovirus vectors for gene delivery. Curr Opin Biotechnol. 1999;10:440–447. doi: 10.1016/s0958-1669(99)00007-5. [DOI] [PubMed] [Google Scholar]
- Carmen IH. A death in the laboratory: the politics of the Gelsinger aftermath. Mol Ther. 2001;3:425–428. doi: 10.1006/mthe.2001.0305. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li Q, Ertl HC., and , Wilson JM. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiver JW., and , Emini EA. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med. 2004;55:355–372. doi: 10.1146/annurev.med.55.091902.104344. [DOI] [PubMed] [Google Scholar]
- Tatsis N., and , Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsis N, Lasaro MO, Lin SW, Xiang ZQ, Zhou D, Dimenna L, et al. Adenovirus vector-induced immune responses in nonhuman primates: responses to prime boost regimens. J Immunol. 2009;182:6587–6599. doi: 10.4049/jimmunol.0900317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rux JJ, Kuser PR., and , Burnett RM. Structural and phylogenetic analysis of adenovirus hexons by use of high-resolution x-ray crystallographic, molecular modeling, and sequence-based methods. J Virol. 2003;77:9553–9566. doi: 10.1128/JVI.77.17.9553-9566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins JR. Control of cellular and viral transcription during adenovirus infection. CRC Crit Rev Biochem. 1986;19:307–322. doi: 10.3109/10409238609082543. [DOI] [PubMed] [Google Scholar]
- Pruzan R., and , Flint SJ.Transcription of adenovirus RNA polymerase III genes Curr Top Microbiol Immunol 1995199201–226.Pt 1 [DOI] [PubMed] [Google Scholar]
- Weitzman MD. Functions of the adenovirus E4 proteins and their impact on viral vectors. Front Biosci. 2005;10:1106–1117. doi: 10.2741/1604. [DOI] [PubMed] [Google Scholar]
- Burgert HG, Ruzsics Z, Obermeier S, Hilgendorf A, Windheim M., and , Elsing A. Subversion of host defense mechanisms by adenoviruses. Curr Top Microbiol Immunol. 2002;269:273–318. doi: 10.1007/978-3-642-59421-2_16. [DOI] [PubMed] [Google Scholar]
- Windheim M, Hilgendorf A., and , Burgert HG. Immune evasion by adenovirus E3 proteins: exploitation of intracellular trafficking pathways. Curr Top Microbiol Immunol. 2004;273:29–85. doi: 10.1007/978-3-662-05599-1_2. [DOI] [PubMed] [Google Scholar]
- Rux JJ., and , Burnett RM. Adenovirus structure. Hum Gene Ther. 2004;15:1167–1176. doi: 10.1089/hum.2004.15.1167. [DOI] [PubMed] [Google Scholar]
- Nicklin SA, Wu E, Nemerow GR., and , Baker AH. The influence of adenovirus fiber structure and function on vector development for gene therapy. Mol Ther. 2005;12:384–393. doi: 10.1016/j.ymthe.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Zhang Y., and , Bergelson JM. Adenovirus receptors. J Virol. 2005;79:12125–12131. doi: 10.1128/JVI.79.19.12125-12131.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., and , Pettersson RF. The coxsackie-adenovirus receptor—a new receptor in the immunoglobulin family involved in cell adhesion. Curr Top Microbiol Immunol. 2004;273:87–111. doi: 10.1007/978-3-662-05599-1_3. [DOI] [PubMed] [Google Scholar]
- Gaggar A, Shayakhmetov DM., and , Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9:1408–1412. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- Liszewski MK, Kemper C, Price JD., and , Atkinson JP. Emerging roles and new functions of CD46. Springer Semin Immunopathol. 2005;27:345–358. doi: 10.1007/s00281-005-0002-3. [DOI] [PubMed] [Google Scholar]
- Hidaka C, Milano E, Leopold PL, Bergelson JM, Hackett NR, Finberg RW, et al. CAR-dependent and CAR-independent pathways of adenovirus vector-mediated gene transfer and expression in human fibroblasts. J Clin Invest. 1999;103:579–587. doi: 10.1172/JCI5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellinga J, Van der Heijdt S., and , Hoeben RC.The adenovirus capsid: major progress in minor proteins J Gen Virol 2005861581–1588.Pt 6 [DOI] [PubMed] [Google Scholar]
- Bessis N, GarciaCozar FJ., and , Boissier MC.Immune responses to gene therapy vectors: influence on vector function and effector mechanisms Gene Ther 200411S10–S17.suppl. 1 [DOI] [PubMed] [Google Scholar]
- Fitzgerald JC, Gao GP, Reyes-Sandoval A, Pavlakis GN, Xiang ZQ, Wlazlo AP, et al. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J Immunol. 2003;170:1416–1422. doi: 10.4049/jimmunol.170.3.1416. [DOI] [PubMed] [Google Scholar]
- Nunes FA, Furth EE, Wilson JM., and , Raper SE. Gene transfer into the liver of nonhuman primates with E1-deleted recombinant adenoviral vectors: safety of readministration. Hum Gene Ther. 1999;10:2515–2526. doi: 10.1089/10430349950016852. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Li Y, Cun A, Yang W, Ellenberg S, Switzer WM, et al. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerging Infect Dis. 2006;12:1596–1599. doi: 10.3201/eid1210.060078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostense S, Koudstaal W, Sprangers M, Weverling GJ, Penders G, Helmus N, et al. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS. 2004;18:1213–1216. doi: 10.1097/00002030-200405210-00019. [DOI] [PubMed] [Google Scholar]
- Metzgar D, Osuna M, Yingst S, Rakha M, Earhart K, Elyan D, et al. PCR analysis of egyptian respiratory adenovirus isolates, including identification of species, serotypes, and coinfections. J Clin Microbiol. 2005;43:5743–5752. doi: 10.1128/JCM.43.11.5743-5752.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81:4654–4663. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holterman L, Vogels R, van der Vlugt R, Sieuwerts M, Grimbergen J, Kaspers J, et al. Novel replication-incompetent vector derived from adenovirus type 11 (Ad11) for vaccination and gene therapy: low seroprevalence and non-cross-reactivity with Ad5. J Virol. 2004;78:13207–13215. doi: 10.1128/JVI.78.23.13207-13215.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichla-Gollon SL, Lin SW, Hensley SE, Lasaro MO, Herkenhoff-Haut L, Drinker M, et al. Effect of preexisting immunity on an adenovirus vaccine vector: in vitro neutralization assays fail to predict inhibition by antiviral antibody in vivo. J Virol. 2009;83:5567–5573. doi: 10.1128/JVI.00405-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leen AM, Christin A, Khalil M, Weiss H, Gee AP, Brenner MK, et al. Identification of hexon-specific CD4 and CD8 T-cell epitopes for vaccine and immunotherapy. J Virol. 2008;82:546–554. doi: 10.1128/JVI.01689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive M, Eisenlohr L, Flomenberg N, Hsu S., and , Flomenberg P. The adenovirus capsid protein hexon contains a highly conserved human CD4+ T-cell epitope. Hum Gene Ther. 2002;13:1167–1178. doi: 10.1089/104303402320138952. [DOI] [PubMed] [Google Scholar]
- Onion D, Crompton LJ, Milligan DW, Moss PA, Lee SP., and , Mautner V.The CD4+ T-cell response to adenovirus is focused against conserved residues within the hexon protein J Gen Virol 2007882417–2425.Pt 9 [DOI] [PubMed] [Google Scholar]
- Calcedo R, Vandenberghe LH, Roy S, Somanathan S, Wang L., and , Wilson JM. Host immune responses to chronic adenovirus infections in human and nonhuman primates. J Virol. 2009;83:2623–2631. doi: 10.1128/JVI.02160-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appledorn DM, Patial S, McBride A, Godbehere S, Van Rooijen N, Parameswaran N, et al. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J Immunol. 2008;181:2134–2144. doi: 10.4049/jimmunol.181.3.2134. [DOI] [PubMed] [Google Scholar]
- Hensley SE, Giles-Davis W, McCoy KC, Weninger W., and , Ertl HC. Dendritic cell maturation, but not CD8+ T cell induction, is dependent on type I IFN signaling during vaccination with adenovirus vectors. J Immunol. 2005;175:6032–6041. doi: 10.4049/jimmunol.175.9.6032. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Kawabata K, Koizumi N, Sakurai F, Nakashima K, Sakurai H, et al. Role of MyD88 and TLR9 in the innate immune response elicited by serotype 5 adenoviral vectors. Hum Gene Ther. 2007;18:753–762. doi: 10.1089/hum.2007.016. [DOI] [PubMed] [Google Scholar]
- Muruve DA, Pétrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- Nazir SA., and , Metcalf JP. Innate immune response to adenovirus. J Investig Med. 2005;53:292–304. doi: 10.2310/6650.2005.53605. [DOI] [PubMed] [Google Scholar]
- Ginsberg HS. The life and times of adenoviruses. Adv Virus Res. 1999;54:1–13. doi: 10.1016/s0065-3527(08)60363-2. [DOI] [PubMed] [Google Scholar]
- Kamen A., and , Henry O.Development and optimization of an adenovirus production process J Gene Med 20046S184–S192.suppl. 1 [DOI] [PubMed] [Google Scholar]
- Fallaux FJ, Bout A, van der Velde I, van den Wollenberg DJ, Hehir KM, Keegan J, et al. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum Gene Ther. 1998;9:1909–1917. doi: 10.1089/hum.1998.9.13-1909. [DOI] [PubMed] [Google Scholar]
- Marzio G, Kerkvliet E, Bogaards JA, Koelewijn S, De Groot A, Gijsbers L, et al. A replication-competent adenovirus assay for E1-deleted Ad35 vectors produced in PER.C6 cells. Vaccine. 2007;25:2228–2237. doi: 10.1016/j.vaccine.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Havenga M, Vogels R, Zuijdgeest D, Radosevic K, Mueller S, Sieuwerts M, et al. Novel replication-incompetent adenoviral B-group vectors: high vector stability and yield in PER.C6 cells J Gen Virol 2006872135–2143.Pt 8 [DOI] [PubMed] [Google Scholar]
- Yeh P, Dedieu JF, Orsini C, Vigne E, Denefle P., and , Perricaudet M. Efficient dual transcomplementation of adenovirus E1 and E4 regions from a 293-derived cell line expressing a minimal E4 functional unit. J Virol. 1996;70:559–565. doi: 10.1128/jvi.70.1.559-565.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt A., and , Kay MA. Gutted adenovirus: a rising star on the horizon. Gene Ther. 2005;12:1540–1541. doi: 10.1038/sj.gt.3302597. [DOI] [PubMed] [Google Scholar]
- Toietta G, Mane VP, Norona WS, Finegold MJ, Ng P, McDonagh AF, et al. Lifelong elimination of hyperbilirubinemia in the Gunn rat with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci USA. 2005;102:3930–3935. doi: 10.1073/pnas.0500930102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R, Dudley RW, Liu AB, Petrof BJ, Nalbantoglu J., and , Karpati G. Prolonged dystrophin expression and functional correction of mdx mouse muscle following gene transfer with a helper-dependent (gutted) adenovirus-encoding murine dystrophin. Hum Mol Genet. 2003;12:1287–1299. doi: 10.1093/hmg/ddg141. [DOI] [PubMed] [Google Scholar]
- Prasad SA, Norbury CC, Chen W, Bennink JR., and , Yewdell JW. Cutting edge: recombinant adenoviruses induce CD8 T cell responses to an inserted protein whose expression is limited to nonimmune cells. J Immunol. 2001;166:4809–4812. doi: 10.4049/jimmunol.166.8.4809. [DOI] [PubMed] [Google Scholar]
- Seiler MP, Cerullo V., and , Lee B. Immune response to helper dependent adenoviral mediated liver gene therapy: challenges and prospects. Curr Gene Ther. 2007;7:297–305. doi: 10.2174/156652307782151452. [DOI] [PubMed] [Google Scholar]
- Roberts DM, Nanda A, Havenga MJ, Abbink P, Lynch DM, Ewald BA, et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- Nanda A, Lynch DM, Goudsmit J, Lemckert AA, Ewald BA, Sumida SM, et al. Immunogenicity of recombinant fiber-chimeric adenovirus serotype 35 vector-based vaccines in mice and rhesus monkeys. J Virol. 2005;79:14161–14168. doi: 10.1128/JVI.79.22.14161-14168.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni S, Gaggar A, Di Paolo N, Li ZY, Liu Y, Strauss R, et al. Evaluation of adenovirus vectors containing serotype 35 fibers for tumor targeting. Cancer Gene Ther. 2006;13:1072–1081. doi: 10.1038/sj.cgt.7700981. [DOI] [PubMed] [Google Scholar]
- Wakayama M, Abei M, Kawashima R, Seo E, Fukuda K, Ugai H, et al. E1A, E1B double-restricted adenovirus with RGD-fiber modification exhibits enhanced oncolysis for CAR-deficient biliary cancers. Clin Cancer Res. 2007;13:3043–3050. doi: 10.1158/1078-0432.CCR-06-2103. [DOI] [PubMed] [Google Scholar]
- Ranki T, Kanerva A, Ristimäki A, Hakkarainen T, Särkioja M, Kangasniemi L, et al. A heparan sulfate-targeted conditionally replicative adenovirus, Ad5.pk7-Delta24, for the treatment of advanced breast cancer. Gene Ther. 2007;14:58–67. doi: 10.1038/sj.gt.3302830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JS, Xu Z, Tian J, Stevenson SC., and , Byrnes AP. Interaction of systemically delivered adenovirus vectors with Kupffer cells in mouse liver. Hum Gene Ther. 2008;19:547–554. doi: 10.1089/hum.2008.004. [DOI] [PubMed] [Google Scholar]
- Koizumi N, Kawabata K, Sakurai F, Watanabe Y, Hayakawa T., and , Mizuguchi H. Modified adenoviral vectors ablated for coxsackievirus-adenovirus receptor, αv integrin, and heparan sulfate binding reduce in vivo tissue transduction and toxicity. Hum Gene Ther. 2006;17:264–279. doi: 10.1089/hum.2006.17.264. [DOI] [PubMed] [Google Scholar]
- Koizumi N, Mizuguchi H, Sakurai F, Yamaguchi T, Watanabe Y., and , Hayakawa T. Reduction of natural adenovirus tropism to mouse liver by fiber-shaft exchange in combination with both CAR- and αv integrin-binding ablation. J Virol. 2003;77:13062–13072. doi: 10.1128/JVI.77.24.13062-13072.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayakhmetov DM, Li ZY, Ni S., and , Lieber A. Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J Virol. 2004;78:5368–5381. doi: 10.1128/JVI.78.10.5368-5381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhniy O, Di Paolo NC, Silvestry M, Hofherr SE, Barry MA, Stewart PL, et al. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci USA. 2008;105:5483–5488. doi: 10.1073/pnas.0711757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington SN, McVey JH, Bhella D, Parker AL, Barker K, Atoda H, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Nicol CG, Graham D, Miller WH, White SJ, Smith TA, Nicklin SA, et al. Effect of adenovirus serotype 5 fiber and penton modifications on in vivo tropism in rats. Mol Ther. 2004;10:344–354. doi: 10.1016/j.ymthe.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ertl HC., and , Wilson JM. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Raper SE, Yudkoff M, Chirmule N, Gao GP, Nunes F, Haskal ZJ, et al. A pilot study of in vivo liver-directed gene transfer with an adenoviral vector in partial ornithine transcarbamylase deficiency. Hum Gene Ther. 2002;13:163–175. doi: 10.1089/10430340152712719. [DOI] [PubMed] [Google Scholar]
- Andrews JL, Kadan MJ, Gorziglia MI, Kaleko M., and , Connelly S. Generation and characterization of E1/E2a/E3/E4-deficient adenoviral vectors encoding human factor VIII. Mol Ther. 2001;3:329–336. doi: 10.1006/mthe.2001.0264. [DOI] [PubMed] [Google Scholar]
- Croyle MA, Chirmule N, Zhang Y., and , Wilson JM. “Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J Virol. 2001;75:4792–4801. doi: 10.1128/JVI.75.10.4792-4801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett CT, Erdman D, Xu W., and , Gooding LR. Prevalence and quantitation of species C adenovirus DNA in human mucosal lymphocytes. J Virol. 2002;76:10608–10616. doi: 10.1128/JVI.76.21.10608-10616.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertl HC., and , Xiang Z. Novel vaccine approaches. J Immunol. 1996;156:3579–3582. [PubMed] [Google Scholar]
- He Z, Wlazlo AP, Kowalczyk DW, Cheng J, Xiang ZQ, Giles-Davis W, et al. Viral recombinant vaccines to the E6 and E7 antigens of HPV-16. Virology. 2000;270:146–161. doi: 10.1006/viro.2000.0271. [DOI] [PubMed] [Google Scholar]
- Xiang ZQ, Yang Y, Wilson JM., and , Ertl HC. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology. 1996;219:220–227. doi: 10.1006/viro.1996.0239. [DOI] [PubMed] [Google Scholar]
- Tatsis N, Fitzgerald JC, Reyes-Sandoval A, Harris-McCoy KC, Hensley SE, Zhou D, et al. Adenoviral vectors persist in vivo and maintain activated CD8+ T cells: implications for their use as vaccines. Blood. 2007;110:1916–1923. doi: 10.1182/blood-2007-02-062117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidajat R, Xiao P, Zhou Q, Venzon D, Summers LE, Kalyanaraman VS, et al. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J Virol. 2009;83:791–801. doi: 10.1128/JVI.01672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang ZQ, Gao GP, Reyes-Sandoval A, Li Y, Wilson JM., and , Ertl HC. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J Virol. 2003;77:10780–10789. doi: 10.1128/JVI.77.20.10780-10789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barefoot B, Thornburg NJ, Barouch DH, Yu JS, Sample C, Johnston RE, et al. Comparison of multiple vaccine vectors in a single heterologous prime-boost trial. Vaccine. 2008;26:6108–6118. doi: 10.1016/j.vaccine.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ewald BA, Lynch DM, Denholtz M, Abbink P, Lemckert AA, et al. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J Virol. 2008;82:4844–4852. doi: 10.1128/JVI.02616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AR, Fitzgerald JC, Giles-Davis W, Gao GP, Wilson JM., and , Ertl HC. Induction of CD8+ T cells to an HIV-1 antigen through a prime boost regimen with heterologous E1-deleted adenoviral vaccine carriers. J Immunol. 2003;171:6774–6779. doi: 10.4049/jimmunol.171.12.6774. [DOI] [PubMed] [Google Scholar]
- Tatsis N, Lin SW, Harris-McCoy K, Garber DA, Feinberg MB., and , Ertl HC. Multiple immunizations with adenovirus and MVA vectors improve CD8+ T cell functionality and mucosal homing. Virology. 2007;367:156–167. doi: 10.1016/j.virol.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Sandoval A, Sridhar S, Berthoud T, Moore AC, Harty JT, Gilbert SC, et al. Single-dose immunogenicity and protective efficacy of simian adenoviral vectors against Plasmodium berghei. Eur J Immunol. 2008;38:732–741. doi: 10.1002/eji.200737672. [DOI] [PubMed] [Google Scholar]
- Resende DM, Caetano BC, Dutra MS, Penido ML, Abrantes CF, Verly RM, et al. Epitope mapping and protective immunity elicited by adenovirus expressing the Leishmania amastigote specific A2 antigen: correlation with IFN-γ and cytolytic activity by CD8+ T cells. Vaccine. 2008;26:4585–4593. doi: 10.1016/j.vaccine.2008.05.091. [DOI] [PubMed] [Google Scholar]
- Magalhaes I, Sizemore DR, Ahmed RK, Mueller S, Wehlin L, Scanga C, et al. rBCG induces strong antigen-specific T cell responses in rhesus macaques in a prime-boost setting with an adenovirus 35 tuberculosis vaccine vector. PLoS ONE. 2008;3:e3790. doi: 10.1371/journal.pone.0003790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arévalo MT, Xu Q, Paton JC, Hollingshead SK, Pichichero ME, Briles DE, et al. Mucosal vaccination with a multicomponent adenovirus-vectored vaccine protects against Streptococcus pneumoniae infection in the lung. FEMS Immunol Med Microbiol. 2009;55:346–351. doi: 10.1111/j.1574-695X.2008.00518.x. [DOI] [PubMed] [Google Scholar]
- Raviprakash K, Wang D, Ewing D, Holman DH, Block K, Woraratanadharm J, et al. A tetravalent dengue vaccine based on a complex adenovirus vector provides significant protection in rhesus monkeys against all four serotypes of dengue virus. J Virol. 2008;82:6927–6934. doi: 10.1128/JVI.02724-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appaiahgari MB, Saini M, Rauthan M, Jyoti, and , Vrati S. Immunization with recombinant adenovirus synthesizing the secretory form of Japanese encephalitis virus envelope protein protects adenovirus-exposed mice against lethal encephalitis. Microbes Infect. 2006;8:92–104. doi: 10.1016/j.micinf.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Phillpotts RJ, O'brien L, Appleton RE, Carr S., and , Bennett A. Intranasal immunisation with defective adenovirus serotype 5 expressing the Venezuelan equine encephalitis virus E2 glycoprotein protects against airborne challenge with virulent virus. Vaccine. 2005;23:1615–1623. doi: 10.1016/j.vaccine.2004.06.056. [DOI] [PubMed] [Google Scholar]
- Lo CY, Wu Z, Misplon JA, Price GE, Pappas C, Kong WP, et al. Comparison of vaccines for induction of heterosubtypic immunity to influenza A virus: cold-adapted vaccine versus DNA prime-adenovirus boost strategies. Vaccine. 2008;26:2062–2072. doi: 10.1016/j.vaccine.2008.02.047. [DOI] [PubMed] [Google Scholar]
- Fattori E, Zampaglione I, Arcuri M, Meola A, Ercole BB, Cirillo A, et al. Efficient immunization of rhesus macaques with an HCV candidate vaccine by heterologous priming-boosting with novel adenoviral vectors based on different serotypes. Gene Ther. 2006;13:1088–1096. doi: 10.1038/sj.gt.3302754. [DOI] [PubMed] [Google Scholar]
- Thammanichanond D, Moneer S, Yotnda P, Aitken C, Earnest-Silveira L, Jackson D, et al. Fiber-modified recombinant adenoviral constructs encoding hepatitis C virus proteins induce potent HCV-specific T cell response. Clin Immunol. 2008;128:329–339. doi: 10.1016/j.clim.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Lasaro MO, Tatsis N, Hensley SE, Whitbeck JC, Lin SW, Rux JJ, et al. Targeting of antigen to the herpesvirus entry mediator augments primary adaptive immune responses. Nat Med. 2008;14:205–212. doi: 10.1038/nm1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield LH, Comin-Anduix B, Vujanovic L, Lee Y, Dissette VB, Yang JQ, et al. Adenovirus MART-1-engineered autologous dendritic cell vaccine for metastatic melanoma. J Immunother. 2008;31:294–309. doi: 10.1097/CJI.0b013e31816a8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruzzi D, Dharmapuri S, Cirillo A, Bruni BE, Nicosia A, Cortese R, et al. A novel chimpanzee serotype-based adenoviral vector as delivery tool for cancer vaccines. Vaccine. 2009;27:1293–1300. doi: 10.1016/j.vaccine.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Yao Z, Zhang G, Wang H, Xu J, Yew DT, et al. Vaccination of Alzheimer's model mice with adenovirus vector containing quadrivalent foldable Aβ(1-15) reduces Aβ burden and behavioral impairment without Aβ-specific T cell response. J Neurol Sci. 2008;272:87–98. doi: 10.1016/j.jns.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Bayer W., and , Wildner O. In situ tumor vaccination with adenovirus vectors encoding measles virus fusogenic membrane proteins and cytokines. World J Gastroenterol. 2007;13:3063–3070. doi: 10.3748/wjg.v13.i22.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada N, Saito T, Masunaga Y, Tsukada Y, Nakagawa S, Mizuguchi H, et al. Efficient antigen gene transduction using Arg-Gly-Asp fiber-mutant adenovirus vectors can potentiate antitumor vaccine efficacy and maturation of murine dendritic cells. Cancer Res. 2001;61:7913–7919. [PubMed] [Google Scholar]
- Xu Q, Pichichero ME, Simpson LL, Elias M, Smith LA., and , Zeng M. An adenoviral vector-based mucosal vaccine is effective in protection against botulism. Gene Ther. 2009;16:367–375. doi: 10.1038/gt.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins SD, Williams AJ, O'Brien LM, Laws TR., and , Phillpotts RJ, et al. CpG used as an adjuvant for an adenovirus-based Venezuelan equine encephalitis virus vaccine increases the immune response to the vector, but not to the transgene product. Viral Immunol. 2008;21:451–457. doi: 10.1089/vim.2008.0052. [DOI] [PubMed] [Google Scholar]
- Draper SJ, Moore AC, Goodman AL, Long CA, Holder AA, Gilbert SC, et al. Effective induction of high-titer antibodies by viral vector vaccines. Nat Med. 2008;14:819–821. doi: 10.1038/nm.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelscher MA, Singh N, Garg S, Jayashankar L, Veguilla V, Pandey A, et al. A broadly protective vaccine against globally dispersed clade 1 and clade 2 H5N1 influenza viruses. J Infect Dis. 2008;197:1185–1188. doi: 10.1086/529522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shott JP, McGrath SM, Pau MG, Custers JH, Ophorst O, Demoitié MA, et al. Adenovirus 5 and 35 vectors expressing Plasmodium falciparum circumsporozoite surface protein elicit potent antigen-specific cellular IFN-γ and antibody responses in mice. Vaccine. 2008;26:2818–2823. doi: 10.1016/j.vaccine.2008.03.080. [DOI] [PubMed] [Google Scholar]
- Worgall S, Krause A, Qiu J, Joh J, Hackett NR., and , Crystal RG. Protective immunity to pseudomonas aeruginosa induced with a capsid-modified adenovirus expressing P. aeruginosa OprF. J Virol. 2007;81:13801–13808. doi: 10.1128/JVI.01246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Cun A, Li Y, Xiang Z., and , Ertl HC. A chimpanzee-origin adenovirus vector expressing the rabies virus glycoprotein as an oral vaccine against inhalation infection with rabies virus. Mol Ther. 2006;14:662–672. doi: 10.1016/j.ymthe.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Fisher KD, Stallwood Y, Green NK, Ulbrich K, Mautner V., and , Seymour LW. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther. 2001;8:341–348. doi: 10.1038/sj.gt.3301389. [DOI] [PubMed] [Google Scholar]
- Sekine S, Kataoka K, Fukuyama Y, Adachi Y, Davydova J, Yamamoto M, et al. A novel adenovirus expressing Flt3 ligand enhances mucosal immunity by inducing mature nasopharyngeal-associated lymphoreticular tissue dendritic cell migration. J Immunol. 2008;180:8126–8134. [Google Scholar]
- Moraes MP, de Los Santos T, Koster M, Turecek T, Wang H, Andreyev VG, et al. Enhanced antiviral activity against foot-and-mouth disease virus by a combination of type I and II porcine interferons. J Virol. 2007;81:7124–7135. doi: 10.1128/JVI.02775-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien L, Perkins S, Williams A, Eastaugh L, Phelps A, Wu J, et al. α-interferon as an adenovirus-vectored vaccine adjuvant and antiviral in Venezuelan equine encephalitis virus infection J Gen Virol 200990874–882.Pt 4 [DOI] [PubMed] [Google Scholar]
- Liu XY, Qiu SB, Zou WG, Pei ZF, Gu JF, Luo CX, et al. Effective gene-virotherapy for complete eradication of tumor mediated by the combination of hTRAIL (TNFSF10) and plasminogen k5. Mol Ther. 2005;11:531–541. doi: 10.1016/j.ymthe.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Bristol JA, Zhu M, Ji H, Mina M, Xie Y, Clarke L, et al. In vitro and in vivo activities of an oncolytic adenoviral vector designed to express GM-CSF. Mol Ther. 2003;7:755–764. doi: 10.1016/s1525-0016(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Luo J, Xia Q, Zhang R, Lv C, Zhang W, Wang Y, et al. Treatment of cancer with a novel dual-targeted conditionally replicative adenovirus armed with mda-7/IL-24 gene. Clin Cancer Res. 2008;14:2450–2457. doi: 10.1158/1078-0432.CCR-07-4596. [DOI] [PubMed] [Google Scholar]