Abstract
Preferential killing of transformed cells, while keeping normal cells and organs unharmed, is the main goal of cancer gene therapy. Genetically engineered trackable markers and imaging reporters enable noninvasive monitoring of transduction efficiency and pharmacokinetics of anticancer virotherapeutics. However, none of these reporters can differentiate between infection in the targeted tumors and that in the normal tissue. Thus, we constructed oncolytic measles virus (MV) armed with a human light immunoglobulin chain reporter gene for the treatment of multiple myeloma (MM). Excessive production of monoclonal immunoglobulin is a key characteristic and marker for diagnostics of MM. Once expressed in infected target cells, vector-encoded λ protein recombines with myeloma IgG-κ immunoglobulin creating a unique IgG-κ/λ. A modified immunoassay technique allows precise quantification of converted marker molecules. Only antibody producing cells were able to assemble this chimeric immunoglobulin molecule, whereas other cells secreted only free λ light chain. Human myeloma xenografts inoculated with λ chain expressing MV secreted converted IgG-κ/λ in the plasma of tumor bearing animals and elevated reporter levels correlated with response to the therapy. This is the first report of a gene therapy vector engineered to discriminate between infection in malignant and normal cells by molecular modification of a tumor-specific protein.
Introduction
Oncolytic virotherapy uses replication competent viruses and genetically engineered virus-based vectors in the treatment of human malignancies.1 Oncolytic agents are intended to selectively destroy tumor tissue via direct lysis of transformed cells, inhibition of neovasularization, or induction of anticancer immunity. Combined viro-chemotherapy using transgene-encoded prodrug-convertase enzymes augment antineoplastic potency of virotherapeutics.2 Noninvasive monitoring of reporter gene expression could provide crucial information about viral propagation and distribution of infection in cancer patients. Vector-encoded human thyroidal sodium iodide symporter concentrates radioiodine in transduced cells enabling noninvasive image-guided radiovirotherapy of tumor xenografts.3,4,5,6,7 Herpes simplex virus thymidine kinase can modify radiolabeled nucleotide analogs causing accumulation of the radionuclides at the site of infection. Viral thymidine kinase–transduced tumor lesions can be visualized by positron emission tomography or single photon emission computed tomography.1,8,9,10,11 Preclinical studies use human chorionic gonadotropin or truncated carcinoembryonic antigen to track oncolytic agent in vivo.12 Trackable-marker expression can also provide data about kinetics of neutralization and clearance of viral vectors by host immune mechanisms. However, all previous studies assume that tumor cells are the main targets of infection and source of marker production. The ideal reporter protein would be nonimmunogenic, sensitive to detection by routine laboratory tests, and unique to the cancer cells. This final attribute could be achieved using a vector gene product that modifies or conjugates to tumor-specific marker.
Herein, the feasibility of reporter-based marker conversion is demonstrated for multiple myeloma (MM) virotherapy. MM is a hematological malignancy characterized by clonal expansion of plasma cells, monoclonal protein production, and bone destruction. Anemia, renal insufficiency, and hypercalcemia are common clinical features and criteria for diagnosis.13 Despite the progress in understanding genetics, pathogenesis and treatment, MM is still incurable with reported median survival of 44.8 months for newly diagnosed patients.14 Plasma cells are genetically programmed to synthesize only one kind of immunoglobulin with unique antigen-binding specificity.15 The molecule is tetrameric, composed of two identical heavy and two identical light chains linked covalently. Somatic DNA recombination of immunoglobulin genes initiates at the early pro-B cell stage of B-cell lineage development and results in a fixed rearrangement of one of the gene loci and expression of only one κ- or λ-type light chain.16,17 Monoclonal-myeloma immunoglobulin is produced in ~75%, whereas free-light chain form of the disease is diagnosed in 16% of the cases. Therefore, an ideal tumor-specific reporter can be formed with λ light chain being the reporter when MM cells produce κ immunoglobulin. Conversely, λ immunoglobulin-producing myelomas could be monitored using κ light chains as reporter. Plasma cells transduced with the opposite light chain would secrete immunoglobulin with either two λ, two κ, or one of each, i.e., κ/λ hybrid reporter/marker molecule.
In this study, we report on the construction of a recombinant measles virus (MV) carrying human λ light immunoglobulin chain (MV-λ) as the reporter. MV-λ infection of IgG-κ secreting MM cells converted the myeloma marker to a unique double positive IgG-κ/λ. In vivo administered MV-λ suppressed growth of human MM xenografts in mice. Improved survival of the treated animals correlated with IgG-κ/λ expression level in the serum samples.
Results
Rescue and growth characteristics of MV-λ
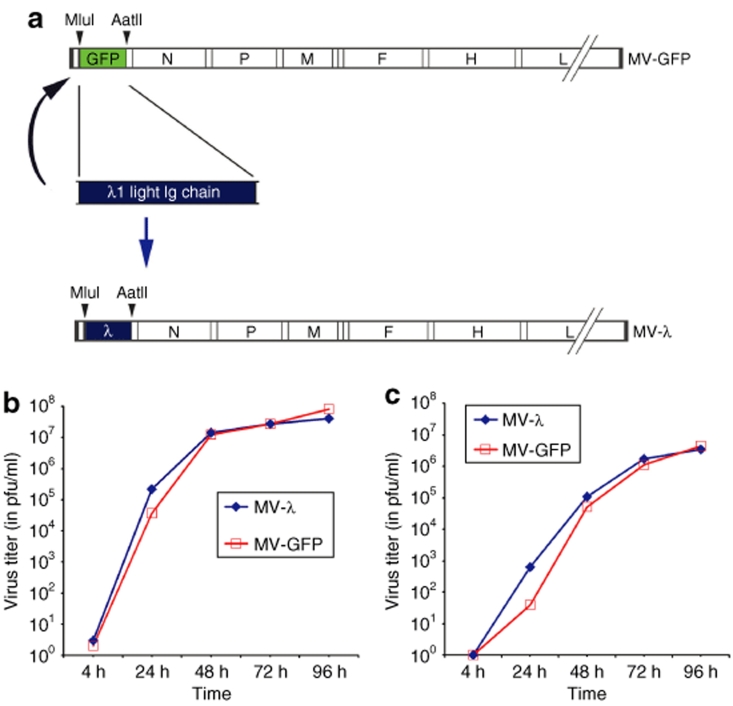
Human λ light chain was successfully cloned in TA-cloning plasmid and its DNA sequence was compared to the available databases of human λ immunoglobulin genes in the National Center for Biotechnology Information using BLAST search (Supplementary Figure S1). The MluI/AatII flanked gene was inserted in the place of green fluorescent protein (GFP) upstream of nucleoprotein (N protein) in MV genome (Figure 1a). Rescued MV-λ expressed human λ light immunoglobulin chain and had a similar growth curve as the GFP-encoding (MV-GFP) control strain (Figure 1b,c). Free virions were detected in the supernants by 24 hours and achieved a maximal titer of 3–4 × 106 plaque-forming units (pfu) per ml after 96 hours. Kinetics of cell-associated virus at 32 °C were also similar between MV-λ and GFP-encoding control MV, indicating that light immunoglobulin protein expression did not significantly affect MV propagation. Quantitative enzyme-linked immunosorbent assay (ELISA) showed production of 4–7 µg/ml of λ chain in the supernatant of infected Vero cells 3 days after inoculation. Large-scale production of second and next passages of MV-λ preparations reached titers in excess of 107 pfu/ml. Kinetics of cell–cell fusion and syncytia size were also similar to control virus (data not shown).
Figure 1.
Schematic representation of MV-GFP and human λ light immunoglobulin chain displaying MV-λ genomes. (a) Human λ chain gene was inserted by exchanging the GFP using a MluI/AatII cleavage site upstream of the N protein. Growth kinetics of MV-λ and control MV-GFP in Vero cells. Titer was determined for both (b) cell-associated and (c) supernatant released virions.
In vitro MM marker conversion
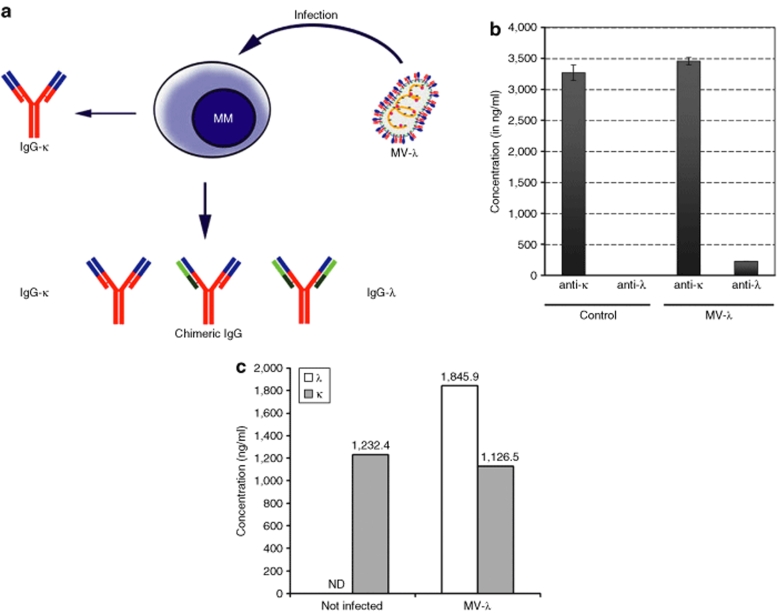
A myeloma cell line secreting monoclonal IgG with κ light chain (KAS-6/1) was inoculated with MV-λ at a multiplicity of infection (MOI) of 0.5. Equal expression of λ chain (MV reporter) and κ chain (from KAS-6/1) in the cells would have yielded randomly assembled IgG-κ/κ, IgG-λ/λ and chimeric IgG-κ/λ molecules (Figure 2a). To determine the recombinant IgG concentration, ELISA plates were coated with anti-λ capturing antibody, and a horse-radish peroxidase (HRPO)–coupled κ-specific antibody was used for detection (Figure 2b). At 72 hours postinoculation of KAS-6/1 with MV-λ at an MOI of 0.5, supernatant concentration IgG-κ/λ was 227.2 ng/ml corresponding to an ~6–7% conversion of the total MM immunoglobulin. The 24-hour expression of λ-light chain by infected KAS-6/1 cells reached ~2 µg/ml, whereas production of κ marker protein was similar for both infected and control cells (Figure 2c). ELISA test confirmed double positive κ/λ marker secretion also in MV-λ infected lymphoblastoid line ARH-77 (Supplementary Figure S2).
Figure 2.
Conversion of MM marker protein after infection with MV-λ. (a) Human λ chain recombined randomly with MM IgG to generate chimeric immunoglobulin carrying light chains of different specificities. (b) Converted κ–λ positive IgG was detected in the supernatant of MV-λ infected but not in control uninfected KAS-6/1 cells after 72 hours of incubation. Enzyme-linked immunosorbent assay (ELISA) plates were coated with κ-chain-specific antibody and anti-κ or anti-λ HRPO-conjugated secondary antibodies were used for detection. Data are shown as mean ± SD. (c) Quantitative ELISA was used to determine daily production of κ and λ immunoglobulin chains in MV-λ-infected and uninfected KAS-6/1 cells. In a separate experiment 106 KAS-6/1 cells were infected with MOI = 1 and resuspended in 1 ml medium. Samples were collected and analyzed after 24-hour incubation.
Immunoblotting was performed to validate the integrity of converted IgG-κ/λ marker. Under nonreducing conditions, KAS-6/1 immunoglobulin appeared as a high MW protein band corresponding to the MW of human IgG (Supplementary Figure S3a). Only KAS-6/1 cells infected with MV-λ secreted MM immunoglobulin with λ-light chains (Supplementary Figure S3b). Purified human IgG corresponding to λ-light chain concentration in the supernatant was immunoprecipitated and used as control. Furthermore, single light chain bands of 22–25 kDa MW were detected on immunoblots under reducing conditions confirming secretion of λ chain converted IgG by MV-λ infected KAS-6/1 cells (Supplementary Figure S3c,d).
Chimeric IgG production after cell carrier transfer of infection
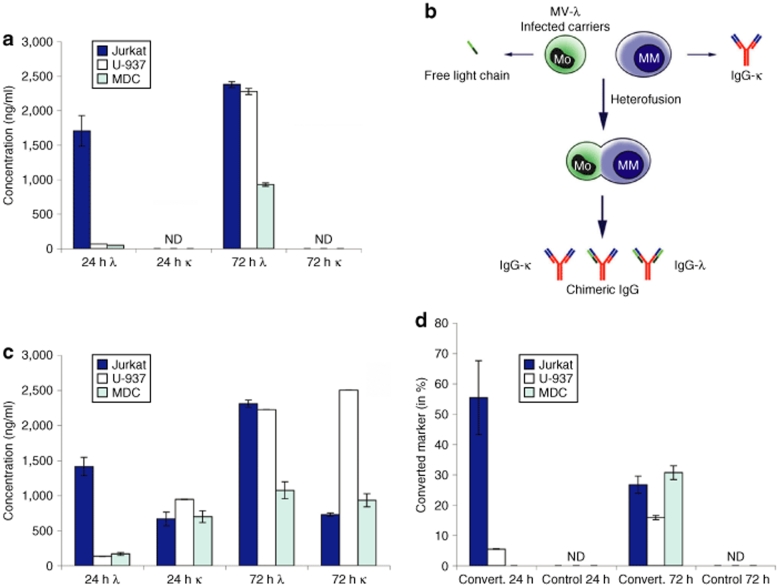
Natural measles infection is spread in human body by cell-associated viremia18 and infected cell carriers have been proposed for delivery of oncolytic MV in cancer patients.19 MV-λ-infected nonmyeloma cells produced only free-light immunoglobulin chains in the supernatant (Figure 3a). Infection of Jurkat T cell line resulted in a peak of λ-chain expression at 24 hours, whereas in the monocytic U-937 line and mature dendritic cells maximum expression was detected on day 3. We hypothesized that cell transfer of measles infection to MM cells can lead to IgG-κ/λ assembly (Figure 3b). All infected carriers delivered MV-λ infection to KAS-6/1 cells and converted marker was detected in the supernatants (Figure 3c). Although Jurkat and U-937 cell lines produced higher level of MV encoded λ-light chain, the highest percentage (>30%) of chimeric κ/λ immunoglobulin expression at 72 hours was observed following cell-mediated transfer of infection with human mature dendritic cells (Figure 3d).
Figure 3.
Converted marker expression by MM cells after cell-mediated transfer of MV-λ infection. (a) MV-λ infected nonplasma cells [mature dendritic cell (MDC), U-937 monocytes, and Jurkat T cells] secreted only free λ chain, while κ was not detectable (ND) in enzyme-linked immunosorbent assay. (b) Cell carrier transfer of MV-λ infection to MM cells resulted in expression of recombinant IgG-κ/λ. (c) Infected carriers mixed with KAS-6/1 cells produced both κ and λ proteins after 24 hours and 72 hours of incubation. (d) MV-λ infection converted myeloma IgG into a double positive IgG-κ/λ, while λ chain was not detected in control uninfected KAS-6/1 cells. Data are from one of three experiments with MDCs collected from different donors.
MV-λ reporter expression in type I interferon-α/β receptor knockout mice
Although mouse cells have not identified in MV receptor, interferon (IFN)-α/β receptor knockout (Ifnarko) facilitates virus spread making the animals permissive for measles infection.20 The animals were inoculated intraperitoneally with 4 × 106 pfu of MV-λ. Control group received equivalent dose of inactivated virus. All serum samples collected on day 2 and 3 from mice injected with MV-λ were positive for human λ protein with concentrations between 150.6 and 256.2 ng/ml (Supplementary Figure S4a). Samples from treated mice were negative on day 1 and 7, indicating that the peak of viral replication was within 2–4 days following infection. All control sera from mice that received heat-inactivated MV-λ were also negative for λ-light chain. Because free λ chain is a small molecule with a short plasma half-life and fast excretion in the kidney, urine samples were also analyzed by λ-specific ELISA. Samples from MV-λ infected mice were positive in low dilutions (up to 1:10) in 2 of 2 mice on day 2 and 2 of 3 tested mice on day 3 (Supplementary Figure S4b). Urine showed negative for excreted λ chain in all control animals injected with inactivated virus.
In vivo MV-λ treatment of MM xenografts
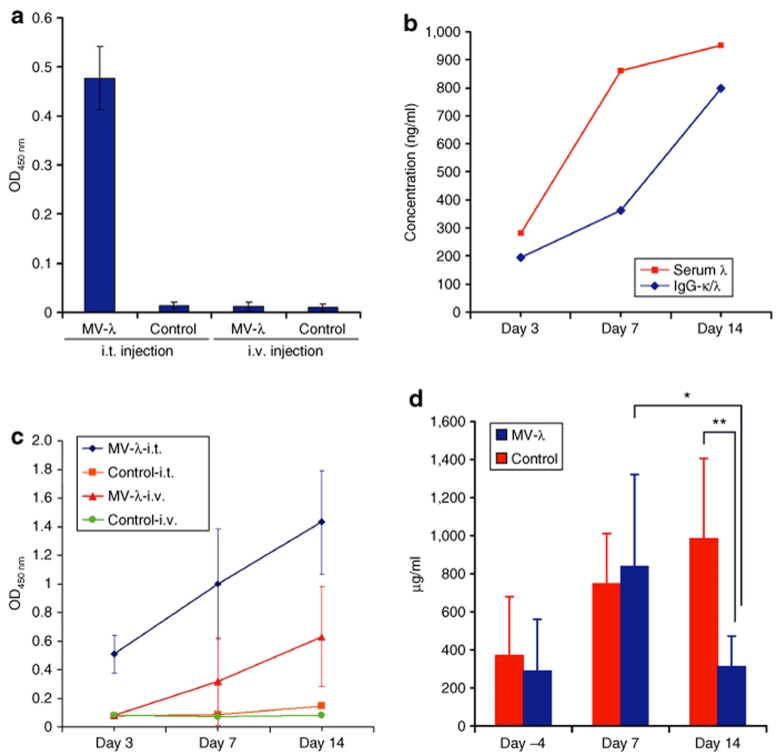
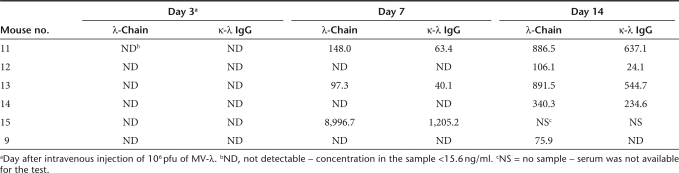
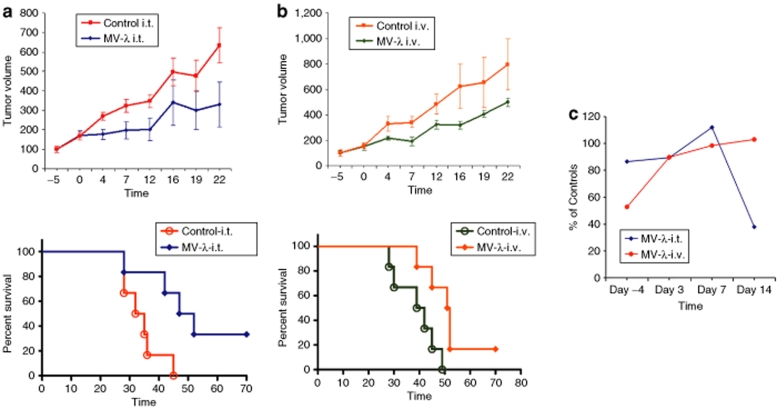
Severe combined immunodeficiency mice were engrafted subcutaneously in KAS-6/1 cells. Visible tumors were observed in all animals on days 14–18 after implantation. Animal groups were assembled based on the tumor volume measurements (Supplementary Table S1). All KAS-6/1 xenografted mice had detectable myeloma immunoglobulin (Supplementary Table S2). Human IgG concentration in the serum showed statistically significant correlation with tumor size (Supplementary Figure S5). The experimental protocol of engraftment and MV-λ treatment of the animals is summarized in Supplementary Figure S6. Mice treated intratumorally with MV-λ had detectable marker conversion by day 3 (Figure 4a). Concentration of chimeric IgG increased gradually over 14 days, paralleling the increasing total λ chain level (Figure 4b). Secretion of λ chain was detected in all mice of MV-λ intravenously treated group with marker conversion in five of them (Table 1). There was no detectable λ protein in control groups injected with inactivated MV-λ. A summary of the ELISA results for double positive IgG-κ/λ expression is presented in Figure 4c. MV-λ maintained significant oncolytic activity in vivo. Both intratumoral and intravenous MV-λ injections inhibited tumor growth and significantly (P values 0.0166 and 0.0161, respectively) prolonged median survival as compared to the controls (Figure 5a,b). Two mice in the intratumorally treated group had almost complete tumor regression (with volumes 138.1 mm3 and 299.2 mm3, respectively) at the end of the experiment. Rapid decrease of serum myeloma IgG corresponded to the xenograft growth inhibition (Figure 5c) and could be explained with the shorter half-life of human immunoglobulins in mice. Free λ-light chain in urine was detected in one intratumorally treated mouse with a peak concentration of 73.4 ng/ml being observed on day 3 after the first therapeutic injection. MV-λ was recovered from infected tumor tissue by Vero cell overlay. All tested tumors from intratumorally treated mice and all except one from the intravenously injected group were positive for virus isolation. Purified IgG or converted IgG-κ/λ from KAS-6/1 MM line were injected in mice and organs were tested for marker deposition on day 2 and 4. All sections from mouse kidney, liver, and lung were negative for human IgG deposits as tested by immunohistochemistry (Supplementary Figure S7 and Supplementary Table S3).
Figure 4.
Expression of converted myeloma marker in vivo. MV-λ infection of KAS-6/1 xenografts in severe combined immunodeficiency mice led to secretion of chimeric IgG-κ/λ in the bloodstream. (a) Converted marker was detected by ELISA (presented as an optical density – OD450 nm) on day 3 after intratumoral treatment of subcutaneously KAS-6/1 tumor with 106 pfu of MV-λ. All other groups including intravenously treated were negative for converted marker on day 3. (b) Total serum λ chain and converted IgG-κ/λ concentration gradually increased during the course of intratumoral MV-λ therapy. In contrast to intratumorally (i.t.) treated group, converted marker was detected later (on day 7) in the sera of intravenously injected mice. (c) Results (in OD450 nm) for all animal groups are compared. Converted IgG-κ/λ expression was demonstrated using anti-λ capture and anti-κ detection antibody. (d) Significant reduction of total myeloma IgG in the serum was observed on day 14 in intratumorally treated mice compared to the level on day 7 (*P < 0.05) and controls on day 14 (**P < 0.01) of the experiment. Data are presented as mean ± SD.
Table 1.
Total λ light chain and κ-λ converted myeloma IgG concentration (ng/ml) in serum samples from MV-λ intravenously treated severe combined immunodeficiency mice bearing subcutaneously KAS-6/1 multiple myeloma xenografts
Figure 5.
MV-λ treatment improved survival of severe combined immunodeficiency (SCID) mice bearing MM xenografts. Both (a) intratumorally (i.t.) and (b) intravenously (i.v.) administered MV-λ inhibited subcutaneously KAS-6/1 tumor growth. The animals were engrafted subcutaneously with 107 exponentially grown KAS-6/1 cells and received three injections of 106 pfu MV-λ when the tumors were 200–400 mm3. Tumor size and survival were monitored 70 days after treatment. Tumor growth progression (mean ± SEM) is presented as % increase of the tumor volume compared to those before (day −5) initiation of the therapy (a and b, top). Kaplan–Meier curves and long-rank test were used for survival data analyses (a and b, bottom). MV-λ oncolytic effect significantly increased survival of the treated animals. Median survival was improved from 33.5 for controls to 49.5 days (P = 0.0166) after initiation of local intratumoral therapy (a, bottom). For systemically injected mice survival improvement was also significant (P = 0.0161) 40.5 and 51.5 days, respectively (b, bottom). (c) MV-λ treatment led to significant (P = 0.009) decrease of serum MM IgG concentration in intratumorally treated mice. Results for both intratumorally and intravenously injected groups are presented as percentage of IgG level in control animals.
Discussion
In this study, we demonstrated that infection of MM cells with an oncolytic MV-encoding λ immunoglobulin gene led to secretion of converted myeloma protein that carried both λ- and κ-type light chain. Heavy γ chains randomly recombined with the κ light chain expressed by KAS-6/1 cells and λ chain from MV-λ to form a complete IgG structure. This chimeric molecule possesses a unique assembly and antigenic determinant profile that cannot be found naturally. Light chains, although part of the immunoglobulin molecule, do not by themselves have antigen-binding properties. In conjunction with the corresponding heavy chains, they form the antigen-binding fragment of the antibodies, but the biological function of the different immunoglobulin isotypes is determined by the heavy chains.15 Reference concentration of λ chain in the serum is reported to be around 4.43 g/l with ratio 2:1 κ/λ.21 Since λ is an abundant self-protein, vector-encoded λ marker is not expected to induce immune response in patients. Furthermore, light chains are not listed as a cause of adverse immune reactions associated with intravenous immunoglobulin therapy in humans.22 Plasma level of λ reporter could be easily quantified by accurate laboratory tests, including recently developed assays for free light immunoglobulins.23 A modified ELISA using capture antibody for one of the two light chains and a second HRPO-conjugated antibody specific for the other light chain allowed detection of chimeric IgG-κ/λ. Immunoblotting analysis demonstrated that virus encoded λ chain recombined with myeloma immunoglobulin and secreted as a tetrameric protein with disulphide linkage between the heavy and light chains. In the event of infection of nonplasma cells, the MV-λ reporter was synthesized and secreted as free light chain. Virus replication and λ protein kinetics in measles infection permissive Ifnarko mice were similar to those reported previously for human carcinoembryonic antigen expressing MV.12,24 Free light chain was found in the serum and urine samples of Ifnarko mice 2–3 days after MV-λ inoculation. These results confirmed that λ marker is expressed in vivo and could be used as indicator for infection of nonplasma cells.
Attenuated MV strains are promising oncolytic agents with potent antineoplastic activity against tumor xenograft in animal models.25,26,27,28,29,30 Oncolytic MV strains are currently tested clinically in the treatment of MM, glioma, and ovarian cancer patients. Previous experiment with MV–sodium iodide symporter and MV–carcinoembryonic antigen, engineered to express human sodium iodide symporter and soluble carcinoembryonic antigen protein, showed correlation between marker expression, viral replication in the tumor tissue, and response to the therapy.4,12 Preclinical work, however, was performed in immunocompromised mice, which are not permissive for measles infection. Viral replication, therefore, is limited to the engrafted human tumor cells. The receptor for the wild-type MV strains is the signaling lymphocyte activation molecule, whereas vaccine strains enter through both signaling lymphocyte activation molecule and CD46 on human cells.18,31 CD46 is ubiquitously expressed on all nucleated cells, and signaling lymphocyte activation molecule is a surface marker for activated lymphocytes and other immune cells including dendritic cells. Immunization with low doses of attenuated MV generates protective humoral and cellular immunity in humans indicating that MV vaccine strains can replicate in normal cells.18 Thus, in humans the source of marker proteins encoded by oncolytic MVs could be both tumor cells and normal tissue.
The marker conversion strategy presented here has one major advantage—the ability to discriminate between infected plasma and nonplasma cells. Recombinant IgG-κ/λ was detected in the serum samples of immunocompromised mice bearing human MM xenografts and treated intravenously or intratumorally with MV-λ. We observed a correlation between the total λ-light chain expression and percentage of the protein incorporated into the modified myeloma IgG. Higher levels of reporter expression in the intratumorally treated group correlated with the antitumor effect. Inhibition of xenograft growth illustrated oncolytic potency of MV-λ and confirmed that λ chain insertion did not affect significantly viral replication in the target cells in vivo. Therefore, the plasma concentration of converted immunoglobulin should correspond to the percentage of infected MM cells in patients. In contrast to IgG, low MW light immunoglobulin chains have a short-half life in the plasma and are rapidly excreted by the kidney. This represents another potential advantage of the marker conversion strategy for monitoring efficiency of oncolytic virus infection in patients. Reporter light chains produced by normal nonplasma cells would undergo rapid elimination, whereas chimeric myeloma protein synthesized by infected MM cells is expected to have pharmacokinetics, half-life and properties specific for the myeloma immunoglobulin isotype. Calculation of the accumulation rate and ratio between converted and nonconverted marker can provide information about dynamics of the infection progress and response to the therapy. Detection of the peak as well as low level of expression of reporters with a short half-life in individual patients can vary depending on the time of sample collection. A reporter, with a longer half-life, however, can facilitate detection of low levels of infection and render the peak of marker expression more accurate and independent of the timing of sample collection. Light chain–encoding vectors could be useful in treatment of ~75% of MM cases characterized by secretion of a complete immunoglobulin molecule.32 Future studies on this marker conversion strategy could broaden its application among MM patients. For example, heavy chain–encoding MV could be used in light chain (Bence-Jones variant) MM observed in ~15–16% of MM patients.32 Similarly to MM, solid tumor markers or tumor expressed enzymes also could be used to convert vector-encoded transgenes to soluble reporters with unique determinants. We currently study the possibility to use tumor-specific proteases, like tissue kallikreins33 to convert vector-encoded proteins to soluble markers. Another approach is to detect tumor-specific glycosylated epitopes expressed on the glycoprotein backbone of oncolytic virus reporter (I. Iankov and E. Galanis, unpublished results).
Recently, cell carrier-based delivery of virotherapeutics has been proposed as a strategy to evade the host response in MM patients.34 Innate and adaptive immune mechanisms are the major problem for systemically administered oncolytic agents. Cell carrier transport to the tumor site can prevent virus neutralization in the plasma. All cells that are target for measles infection in vivo, such as monocytes, lymphocytes, dendritic cells, and endothelial cells,18,35,36 can also be promising candidates for cell-based delivery of oncolytic MVs. Our results proved that marker conversion can differentiate between infection of tumor cells and reporter gene expression by the carriers. Dendritic cells, monocytes, and T cells produced high level of free light chain protein after MV-λ infection. Subsequent transfer of infection to MM cells resulted in assembly of λ chain with myeloma immunoglobulin and secretion of chimeric IgG-λ/κ marker. These data support the concept that converted marker detection could represent direct evidence for successful carrier-based delivery of oncolytic viruses in MM patients.
Application of marker conversion strategy is not limited to monitoring transduction efficiency in tumor cells following gene therapy or virotherapy. B-cell non-Hodgkins lymphomas are another group of hematological malignances characterized with a clonal expansion of cells expressing unique immunoglobulin idiotype. Idiotypic determinants are distinctive feature of antigen-binding antibody fragment formed by the variable domains of heavy and light chain. Variable immunoglobulin regions from lymphoma cells have been cloned and used as patient-specific vaccine against advanced stage follicular B-cell non-Hodgkins lymphoma.37,38,39 Genetic modification of MV-λ allows insertion of single epitopes or immunostimulatory peptides in the variable domain of λ light chain (I. Iankov and E. Galanis, unpublished results). We hypothesize that assembly of the modified λ chain with lymphoma surface immunoglobulin could trigger anti-idiotypic response. In contrast to generation of “individual” vaccines, the vector-based immunoglobulin conversion could work as an universal immunotherapeutic for B-cell non-Hodgkins lymphoma treatment.
In conclusion, this is the first study of a reporter gene encoded by an engineered oncolytic virus that can discriminate between tumor and normal tissue transduction in vivo. Quantification of converted tumor marker enables noninvasive monitoring of the actual oncolytic virus infection in malignant cells and response to virotherapy in cancer patients.
Materials and Methods
Generation of MV expressing human λ light immunoglobulin chain. Total RNA extract from frozen human tonsillar tissue was isolated using RNAeasy kit (Qiagen Sciences, Germantown, MD). Forward primer specific for the leader peptide of λ chain gene and reverse primer specific for the carboxyl terminus of λ chain constant domain were used for amplification by reverse transcription–PCR (Superscript One-step kit; Invitrogen, Carlsbad, CA). The product was cloned in TA-cloning vector (KA-2060, Invitrogen) and analyzed by DNA sequencing. MluI/AatII (New England BioLabs, Ipswich, MA) digested fragment was subcloned into the full-length infectious clone MV Edmonston vaccine strain p(+)MVeGFP plasmid (kindly provided by Cattaneo, Mayo Clinic, Rochester, MN),40 replacing the GFP gene. MV-encoding human λ light chain designed as MV-λ (clone MV-λ131) was rescued by transfection of 293-3-46 cells as described previously.41
Human dendritic cell isolation. Peripheral blood was collected from healthy volunteers after institutional review board approval. Monocytes were isolated from peripheral blood mononuclear population by CD14 selection using CD14 microbeads (Miltenyi Biotec, Auburn, CA). Mature dendritic cells were produced by stimulation of CD14+ cells with cytokines and mitogens as described previously.42,43
Cell culture and virus propagation. African green monkey Vero cells and human lines U-937 (monocytic), ARH-77 (IgG-κ expressing lymphoblastoid cells) and Jurkat (T cell) were grown according to ATCC recommendations (ATCC, Rockville, MD). 293-3-46 cells used for MV rescue was kindly provided by R. Cattaneo (Mayo Clinic, Rochester, MN) and were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, antibiotics, and Geneticin (G-418) as selective factor (Invitrogen). Human MM line KAS-6/1 was kindly provided by D. Jelinek (Mayo Clinic, Rochester, MN) and was grown in RPMI1640 medium supplemented with fetal bovine serum and 1 ng/ml human recombinant interleukin-6 (R&D Systems).
MV-λ and MV-GFP were propagated on Vero cells as described previously.40 Viral stocks were prepared by repeat freezing–thawing and titrated on Vero cells.44 MV titer was calculated in both tissue-culture infectious doses 50% and pfu/ml on day 2–3 of culture.45 For one-step MV growth kinetics, Vero cells were inoculated with MOI of 1 (of MV-λ or control MV-GFP) and were incubated at 32 °C. Both supernatant and cell-associated viral progeny were determined at 4, 24, 48, 72, and 96 hours of infection.
ELISA for human IgG, κ and λ light immunoglobulin chains. Production of IgG-κ by KAS-6/1 myeloma line was verified by IgG and κ ELISA kits according to manufacturer's protocol (Bethyl Laboratories, Montgomery, TX). For detection of λ light chain produced by infected Vero, U-937 and Jurkat cells, cell culture supernatants were collected 48–72 hours after inoculation with MV-λ and were analyzed by λ-specific ELISA kit (Bethyl Laboratories). Concentration of λ protein in the viral stock has also been measured. Kinetics of λ chain production by KAS-6/1 MM cells was determined at 24, 48, and 72 hours after infection with MOI = 0.2–1.0 of MV-λ. For detection of myeloma protein conversion to double light chain positive IgG-κ/λ ELISA plates were coated with goat anti-λ capturing antibody (Bethyl Laboratories). Samples were added to the wells for 1 hour and reaction was developed after incubation with anti-κ HRPO-conjugated secondary antibody (Bethyl Laboratories). Reaction was also run using anti-κ (and also anti-IgG) capture and anti-λ detection variant of the reaction. Serum and urine samples from mice were diluted serially from 1:20–25 and analyzed for IgG, κ, λ chain level and IgG-k/λ conversion as described.
Heterofusion experiments. Dendritic cells, U-937 and Jurkat cell lines were infected with MOI = 1 of MV-λ, washed and mixed 1:1 (5 × 104 cells) with KAS-6/1 cells. Supernatant samples were collected 24 hours and 72 hours later and were analyzed for converted monoclonal immunoglobulin production by ELISA as described above.
Immunoprecipitation, SDS–polyacrylamide gel electrophoresis, and immunoblotting. Supernatants from infected cells were incubated with Protein G PLUS agarose for 2 hours at 4 °C according to the manufacturer protocol (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoprecipitates were washed, mixed with sample buffer and subjected to SDS–polyacrylamide gel electrophoresis on 10 or 12.5% gels under reducing or nonreducing conditions using Criterion electrophoresis system (Bio-Rad, Hercules, CA). For immunoblotting fractionated proteins were blotted on polyvinylidene fluoride membranes (Bio-Rad) using semi-dry transfer system (Bio-Rad). The blots were blocked for 1 hour in phosphate-buffered saline with 0.05% Tween 20 and 5% skim milk. Membranes were washed and incubated for 1 hour at room temperature with human light (λ or κ) immunoglobulin chain–specific goat, HRPO-conjugated antibodies (Bethyl Laboratories) diluted 1:12,500 in phosphate-buffered saline with 0.05% Tween 20 and 5% skim milk. Specific protein bands were visualized using chemiluminescence reagent (Pierce, Rockford, IL).
In vivo studies. All in vivo experiments were approved by the Mayo Foundation Institutional Animal Care and Use Committee. Mice were maintained in the animal facilities of Mayo Clinic, Rochester, Minnesota.
In vivo λ-light chain expression in type I mice. IFN receptor knockout and human CD46 expressing transgenic mice are suitable model to study MV pathogenesis in vivo.20,46,47 Measles permissive Ifnarko mice used in the experiment are CD46 negative substrain of Ifnarko-CD46Ge line.24 Female 6–8 weeks old animals were injected intraperitoneally with 4 × 106 pfu of MV-λ in Opti-MEM medium (Invitrogen). Control group received equivalent dose of heat-inactivated (60 °C for 30 minutes) virus. Serum and urine samples were collected from 2–3 animals per group on day 1, 2, and 3 postinoculation. On day 7, all mice were terminally bled and euthanized. Serum and urine samples were stored frozen at −80 °C and human λ-light immunoglobulin chain concentration was determined using ELISA as described above.
Myeloma immunoglobulin conversion and protective efficacy of MV-λ in MM xenograft model. Five-week-old severe combined immunodeficiency mice (Harlan, Indianapolis, IN) were engrafted subcutaneously using fine needle injection with 107 exponentially grown in the presence of human IL-6 KAS-6/1 cells. Tumors were measured twice per week and volume was calculated according to the formula: V = a2b/2 where “a” is the width and “b” is the length of the tumor. When the tumors reached 200–400 mm3, mice were randomized (six animals per group) according to the tumor size. Serum level of myeloma IgG was measured before treatment. Mice were inoculated intratumorally or intravenously with 106 pfu of MV-λ. The injections were repeated on day 7 and 14 of the therapy. Control groups received an equivalent dose of UV-light inactivated virus. Serum samples from treated and control groups were collected on days 3, 7, and 14 of the experiment. Survival was monitored up to day 70 after the therapy and mice with tumor volumes over 2,000 mm3 were terminally bled and killed. Tumors were harvested and cells were overlaid on Vero monolayers for virus recovery. Serum concentration of human IgG, κ- and λ-light chains as well as converted double positive κ-λ IgG was determined by ELISA as described above. Urine samples were collected on day 1, 2, 3, and 7 after the first therapeutic injection.
Immunohistochemistry for detection of human IgG deposition in mouse organs. Myeloma immunoglobulin from MV-λ infected or noninfected KAS-6/1 cells was purified by affinity chromatography and injected in mice. Immunohistochemistry for human IgG deposits was performed on frozen sections of organs injected and control animals on day 2 and 4 (for details see Supplementary Materials and Methods).
Statistical analyses. The results were analyzed using GraphPad Prism statistical program (GraphPad Software, San Diego, CA).
SUPPLEMENTARY MATERIALFigure S1. DNA and protein sequence of the cloned λ chain. DNA sequence of the human λ immunoglobulin chain cloned and inserted in MV genome – designed MV-lambda (A) and corresponding protein translation of the cloned human λ chain (B).Figure S2. Expression of λ chain by MV-λ-infected ARH-77 cells. Reporter expression after MV-lambda infection of IgG-κ producing human lymphoblastoid cell line ARH-77. Concentration of λ light chain in the supernatants (in ng/ml) from two experiments (exp.1 and exp.2, run in duplicates) after 96-h infection with MOI=0.5 of MV-lambda (A). Control samples were negative for λ protein (not detectable – ND). ELISA for κ/λ conversion using anti-λ coating and anti-κ detection antibodies (B). ELISA results are mean ± SD of absorbance (OD450nm) above the control.Figure S3. Immunoblot analysis of converted myeloma immunoglobin. Immunoblotting of converted IgG assembled and secreted by MV-lambda infected MM cells. Supernatants from MV-lambda-infected or control KAS-6/1 cells diluted to 2 μg/ml and IgG was immunoprecipitated using Protein G agarose. Immunoblots were visualized after incubation with κ (A and C) or λ specific (B and D) HRPO-conjugated antibodies. In A and B two-fold dilutions of immunoprecipitates were run in four lanes under non-reducing SDS-PAGE conditions. Control human serum IgG (co-sIgG) was used in concentration corresponding to λ-light chain level in MV-lambda-infected samples (as measured by λ specific ELISA). Bands of λ chains have similar intensity for converted marker (MV-lambda infected cells) and co-sIgG but are not detectable for non-infected control (B and D). When reaction was normalized to the κ concentration in the samples, a similar amount of protein was detected under reducing conditions (C,D). In C and D: lane 1 – MV-lambda infected cells; lane 2 – sample from uninfected cells; lane 3 – co-sIgG.Figure S4. In vivo expression of human λ light chain in MV-λ-infected Ifnarko mice. Serum concentration of λ protein reached more than 150 ng/ml on day 2 (2 of 2 mice) and day 3 (3 of 3 mice) of infection but was not detectable (ND) on day 7 (A). Free λ chain was excreted and detected in urine samples from the same animals on day 2 and in 2 of 3 mice on day 3 (B).Figure S5. Tumor volume and serum myeloma IgG in KAS-6/1 engrafted mice. Correlation between tumor volume and serum level of MM IgG before the therapy. Spearman test – r=0.5505; p=0.0029.Figure S6. MV-λ treatment of MM xenografts. Schematic representation of in vivo therapy protocol. KAS-6/1 cells were implanted s.c. in the right flank of female SCID mice on day 1. The animals received three therapeutic injections of 106 pfu of MV-lambda. Tumor growth and survival were monitored up to day 70 of the study.Figure S7. Immunohistochemistry analysis for MM marker deposits in mice. IHC for detection of human immunoglobulin deposits in the organs (A) of mice injected with purified KAS-6/1 IgG (m3,4) or converted IgG from MV-lambda infected KAS-6/1 cells (m5,6) compared to the controls (m1,2). IHC was performed on frozen sections from lung, liver and kidney collected 48 h (m1,3,5) and 96 h (m2,4,6) after IgG injection (right columns). Parallel sections were stained with hematoxylin/eosin (left columns). Jurkat and KAS-6/1 cells were used as negative and positive controls of the assay (B) counterstained with hematoxylin (top) or fast nuclear red (bottom). For concentrations of human IgG in the serum of injected animals, see Supplementary table 3.Table S1. Tumor volumes of subcutaneously KAS-6/1 engrafted SCID mice before intratumoral or systemic treatment with MV-λ.Table S2. Tumor volume (in mm3) and serum level of MM IgG (in ng/ml) in SCID mice engrafted subcutaneously KAS-6/1 cells before the intratumoral or intravenous therapy with MV-λ.Table S3. Injection and serum concentrations of KAS-6/1 myeloma IgG in mice analyzed for immunoglobulin deposition in the organs.Materials and Methods.
Supplementary Material
DNA and protein sequence of the cloned λ chain. DNA sequence of the human λ immunoglobulin chain cloned and inserted in MV genome – designed MV-lambda (A) and corresponding protein translation of the cloned human λ chain (B).
Expression of λ chain by MV-λ-infected ARH-77 cells. Reporter expression after MV-lambda infection of IgG-κ producing human lymphoblastoid cell line ARH-77. Concentration of λ light chain in the supernatants (in ng/ml) from two experiments (exp.1 and exp.2, run in duplicates) after 96-h infection with MOI=0.5 of MV-lambda (A). Control samples were negative for λ protein (not detectable – ND). ELISA for κ/λ conversion using anti-λ coating and anti-κ detection antibodies (B). ELISA results are mean ± SD of absorbance (OD450nm) above the control.
Immunoblot analysis of converted myeloma immunoglobin. Immunoblotting of converted IgG assembled and secreted by MV-lambda infected MM cells. Supernatants from MV-lambda-infected or control KAS-6/1 cells diluted to 2 μg/ml and IgG was immunoprecipitated using Protein G agarose. Immunoblots were visualized after incubation with κ (A and C) or λ specific (B and D) HRPO-conjugated antibodies. In A and B two-fold dilutions of immunoprecipitates were run in four lanes under non-reducing SDS-PAGE conditions. Control human serum IgG (co-sIgG) was used in concentration corresponding to λ-light chain level in MV-lambda-infected samples (as measured by λ specific ELISA). Bands of λ chains have similar intensity for converted marker (MV-lambda infected cells) and co-sIgG but are not detectable for non-infected control (B and D). When reaction was normalized to the κ concentration in the samples, a similar amount of protein was detected under reducing conditions (C,D). In C and D: lane 1 – MV-lambda infected cells; lane 2 – sample from uninfected cells; lane 3 – co-sIgG.
In vivo expression of human λ light chain in MV-λ-infected Ifnarko mice. Serum concentration of λ protein reached more than 150 ng/ml on day 2 (2 of 2 mice) and day 3 (3 of 3 mice) of infection but was not detectable (ND) on day 7 (A). Free λ chain was excreted and detected in urine samples from the same animals on day 2 and in 2 of 3 mice on day 3 (B).
Tumor volume and serum myeloma IgG in KAS-6/1 engrafted mice. Correlation between tumor volume and serum level of MM IgG before the therapy. Spearman test – r=0.5505; p=0.0029.
MV-λ treatment of MM xenografts. Schematic representation of in vivo therapy protocol. KAS-6/1 cells were implanted s.c. in the right flank of female SCID mice on day 1. The animals received three therapeutic injections of 106 pfu of MV-lambda. Tumor growth and survival were monitored up to day 70 of the study.
Immunohistochemistry analysis for MM marker deposits in mice. IHC for detection of human immunoglobulin deposits in the organs (A) of mice injected with purified KAS-6/1 IgG (m3,4) or converted IgG from MV-lambda infected KAS-6/1 cells (m5,6) compared to the controls (m1,2). IHC was performed on frozen sections from lung, liver and kidney collected 48 h (m1,3,5) and 96 h (m2,4,6) after IgG injection (right columns). Parallel sections were stained with hematoxylin/eosin (left columns). Jurkat and KAS-6/1 cells were used as negative and positive controls of the assay (B) counterstained with hematoxylin (top) or fast nuclear red (bottom). For concentrations of human IgG in the serum of injected animals, see Supplementary table 3.
Tumor volumes of subcutaneously KAS-6/1 engrafted SCID mice before intratumoral or systemic treatment with MV-λ.
Tumor volume (in mm3) and serum level of MM IgG (in ng/ml) in SCID mice engrafted subcutaneously KAS-6/1 cells before the intratumoral or intravenous therapy with MV-λ.
Injection and serum concentrations of KAS-6/1 myeloma IgG in mice analyzed for immunoglobulin deposition in the organs.
Acknowledgments
We thank our colleagues from Mayo Clinic, Rochester MN: R. Cattaneo for p(+)MV-eGFP plasmid; D. Jelinek for KAS-6/1 MM line, and A. Dogan for the frozen tonsillar tissue. We also thank P. Bulur and P. Ryno for the technical assistance. This work was supported by the Atwater grant, P50CA108961 and Paul Leibson Memorial Fund.
REFERENCES
- Russell SJ., and , Peng KW. Viruses as anticancer drugs. Trends Pharmacol Sci. 2007;28:326–333. doi: 10.1016/j.tips.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerechts G, Springfeld C, Frenzke ME, Lampe J, Johnston PB, Parker WB, et al. Lymphoma chemovirotherapy: CD20-targeted and convertase-armed measles virus can synergize with fludarabine. Cancer Res. 2007;67:10939–10947. doi: 10.1158/0008-5472.CAN-07-1252. [DOI] [PubMed] [Google Scholar]
- Carlson SK, Classic KL, Hadac EM, Bender CE, Kemp BJ, Lowe VJ, et al. In vivo quantitation of intratumoral radioisotope uptake using micro-single photon emission computed tomography/computed tomography. Mol Imaging Biol. 2006;8:324–332. doi: 10.1007/s11307-006-0058-z. [DOI] [PubMed] [Google Scholar]
- Dingli D, Peng KW, Harvey ME, Greipp PR, O'Connor MK, Cattaneo R, et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- Dwyer RM, Schatz SM, Bergert ER, Myers RM, Harvey ME, Classic KL, et al. A preclinical large animal model of adenovirus-mediated expression of the sodium-iodide symporter for radioiodide imaging and therapy of locally recurrent prostate cancer. Mol Ther. 2005;12:835–841. doi: 10.1016/j.ymthe.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Dwyer RM, Bergert ER, O'Connor MK, Gendler SJ., and , Morris JC. Sodium iodide symporter-mediated radioiodide imaging and therapy of ovarian tumor xenografts in mice. Gene Ther. 2006;13:60–66. doi: 10.1038/sj.gt.3302599. [DOI] [PubMed] [Google Scholar]
- Spitzweg C, Baker CH, Bergert ER, O'Connor MK., and , Morris JC. Image-guided radioiodide therapy of medullary thyroid cancer after carcinoembryonic antigen promoter-targeted sodium iodide symporter gene expression. Hum Gene Ther. 2007;18:916–924. doi: 10.1089/hum.2007.081. [DOI] [PubMed] [Google Scholar]
- Bennett JJ, Tjuvajev J, Johnson P, Doubrovin M, Akhurst T, Malholtra S, et al. Positron emission tomography imaging for herpes virus infection: Implications for oncolytic viral treatments of cancer. Nat Med. 2001;7:859–863. doi: 10.1038/89991. [DOI] [PubMed] [Google Scholar]
- Green LA, Yap CS, Nguyen K, Barrio JR, Namavari M, Satyamurthy N, et al. Indirect monitoring of endogenous gene expression by positron emission tomography (PET) imaging of reporter gene expression in transgenic mice. Mol Imaging Biol. 2002;4:71–81. doi: 10.1016/s1095-0397(01)00071-1. [DOI] [PubMed] [Google Scholar]
- Gross S., and , Piwnica-Worms D. Spying on cancer: molecular imaging in vivo with genetically encoded reporters. Cancer Cell. 2005;7:5–15. doi: 10.1016/j.ccr.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Kuruppu D, Dorfman JD., and , Tanabe KK. HSV-1 viral oncolysis and molecular imaging with PET. Curr Cancer Drug Targets. 2007;7:175–180. doi: 10.2174/156800907780058871. [DOI] [PubMed] [Google Scholar]
- Peng KW, Facteau S, Wegman T, O'Kane D., and , Russell SJ. Non-invasive in vivo monitoring of trackable viruses expressing soluble marker peptides. Nat Med. 2002;8:527–531. doi: 10.1038/nm0502-527. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV., and , Kyle RA. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2005;80:1371–1382. doi: 10.4065/80.10.1371. [DOI] [PubMed] [Google Scholar]
- Kumar SK, Rajkumar SV, Dispenzieri A, Lacy HQ, Hayman SR, Bauadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Travers P., and , Walport M.edsAntigen recognition by B-cell and T-cell receptor 2008Garland Science, Taylor & Francis Group: New York and London; 111–142.In: Janeway's Immunobiology [Google Scholar]
- Murphy K, Travers P., and , Walport M.edsThe generation of lymphocyte antigen receptors 2008Garland Science, Taylor & Francis Group: New York and London; 143–179.In: Janeway's Immunobiology [Google Scholar]
- Solomon A. Light chains of immunoglobulins: structural-genetic correlates. Blood. 1986;68:603–610. [PubMed] [Google Scholar]
- Griffin D.Measles virus Fields Virology 2001Lippincott Williams & Wilkins: Philadelphia; 1401–1441.In: Knipe, DM and Howley, PM, (eds) [Google Scholar]
- Iankov ID, Blechacz B, Liu C, Schmeckpeper JD, Tarara JE, Federspiel MJ, et al. Infected cell carriers: a new strategy for systemic delivery of oncolytic measles viruses in cancer virotherapy. Mol Ther. 2007;15:114–122. doi: 10.1038/sj.mt.6300020. [DOI] [PubMed] [Google Scholar]
- Mrkic B, Pavlovic J, Rülicke T, Volpe P, Buchholz CJ, Hourcade D, et al. Measles virus spread and pathogenesis in genetically modified mice. J Virol. 1998;72:7420–7427. doi: 10.1128/jvi.72.9.7420-7427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitta M, Iavarone A, Cappello N, Bergami MR, Fiorucci GC., and , Aguzzi F. Reference values for immunoglobulin kappa and lambda light chains and the kappa/lambda ratio in children's serum. Clin Chem. 1992;38:2454–2457. [PubMed] [Google Scholar]
- Hamrock DJ. Adverse events associated with intravenous immunoglobulin therapy. Int Immunopharmacol. 2006;6:535–542. doi: 10.1016/j.intimp.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Pratt G. The evolving use of serum free light chain assays in haematology. Br J Haematol. 2008;141:413–422. doi: 10.1111/j.1365-2141.2008.07079.x. [DOI] [PubMed] [Google Scholar]
- Iankov ID, Pandey M, Harvey M, Griesmann GE, Federspiel MJ., and , Russell SJ. Immunoglobulin g antibody-mediated enhancement of measles virus infection can bypass the protective antiviral immune response. J Virol. 2006;80:8530–8540. doi: 10.1128/JVI.00593-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen C, Vongpunsawad S, Nakamura T, James CD, Schroeder M, Cattaneo R, et al. Retargeted oncolytic measles strains entering via the EGFRvIII receptor maintain significant antitumor activity against gliomas with increased tumor specificity. Cancer Res. 2006;66:11840–11850. doi: 10.1158/0008-5472.CAN-06-1200. [DOI] [PubMed] [Google Scholar]
- Grote D, Russell SJ, Cornu TI, Cattaneo R, Vile R, Poland GA, et al. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood. 2001;97:3746–3754. doi: 10.1182/blood.v97.12.3746. [DOI] [PubMed] [Google Scholar]
- McDonald CJ, Erlichman C, Ingle JN, Rosales GA, Allen C, Greiner SM, et al. A measles virus vaccine strain derivative as a novel oncolytic agent against breast cancer. Breast Cancer Res Treat. 2006;99:177–184. doi: 10.1007/s10549-006-9200-5. [DOI] [PubMed] [Google Scholar]
- Peng KW, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC., and , Russell SJ. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62:4656–4662. [PubMed] [Google Scholar]
- Peng KW, Ahmann GJ, Pham L, Greipp PR, Cattaneo R., and , Russell SJ. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood. 2001;98:2002–2007. doi: 10.1182/blood.v98.7.2002. [DOI] [PubMed] [Google Scholar]
- Phuong LK, Allen C, Peng KW, Giannini C, Greiner S, TenEyck CJ, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003;63:2462–2469. [PubMed] [Google Scholar]
- Yanagi Y, Takeda M., and , Ohno S.Measles virus: cellular receptors, tropism and pathogenesis J Gen Virol 2006872767–2779.Pt 10 [DOI] [PubMed] [Google Scholar]
- Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- Borgoño CA, Michael IP., and , Diamandis EP. Human tissue kallikreins: physiologic roles and applications in cancer. Mol Cancer Res. 2004;2:257–280. [PubMed] [Google Scholar]
- Munguia A, Ota T, Miest T., and , Russell SJ. Cell carriers to deliver oncolytic viruses to sites of myeloma tumor growth. Gene Ther. 2008;15:797–806. doi: 10.1038/gt.2008.45. [DOI] [PubMed] [Google Scholar]
- Esolen LM, Ward BJ, Moench TR., and , Griffin DE. Infection of monocytes during measles. J Infect Dis. 1993;168:47–52. doi: 10.1093/infdis/168.1.47. [DOI] [PubMed] [Google Scholar]
- Forthal DN, Landucci G, Habis A, Zartarian M, Katz J., and , Tilles JG. Measles virus-specific functional antibody responses and viremia during acute measles. J Infect Dis. 1994;169:1377–1380. doi: 10.1093/infdis/169.6.1377. [DOI] [PubMed] [Google Scholar]
- Barrios Y, Plaza A, Cabrera R, Yáñez R, Suárez E, Fernández MN, et al. Active idiotypic vaccination in a patient with biclonal follicular lymphoma. Cancer Immunol Immunother. 2001;50:87–92. doi: 10.1007/s002620100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios Y, Cabrera R, Yáñez R, Briz M, Plaza A, Forés R, et al. Anti-idiotypic vaccination in the treatment of low-grade B-cell lymphoma. Haematologica. 2002;87:400–407. [PubMed] [Google Scholar]
- Timmerman JM, Czerwinski DK, Davis TA, Hsu FJ, Benike C, Hao ZM, et al. Idiotype-pulsed dendritic cell vaccination for B-cell lymphoma: clinical and immune responses in 35 patients. Blood. 2002;99:1517–1526. doi: 10.1182/blood.v99.5.1517. [DOI] [PubMed] [Google Scholar]
- Duprex WP, McQuaid S, Roscic-Mrkic B, Cattaneo R, McCallister C., and , Rima BK. In vitro and in vivo infection of neural cells by a recombinant measles virus expressing enhanced green fluorescent protein. J Virol. 2000;74:7972–7979. doi: 10.1128/jvi.74.17.7972-7979.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dötsch C, et al. Rescue of measles viruses from cloned DNA. EMBO J. 1995;14:5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz AB, Bulur PA, Knutson GJ, Matasic R., and , Vuk-Pavlovic S. Maturation of human monocyte-derived dendritic cells studied by microarray hybridization. Biochem Biophys Res Commun. 2000;275:731–738. doi: 10.1006/bbrc.2000.3372. [DOI] [PubMed] [Google Scholar]
- Dietz AB, Bulur PA, Emery RL, Winters JL, Epps DE, Zubair AC, et al. A novel source of viable peripheral blood mononuclear cells from leukoreduction system chambers. Transfusion. 2006;46:2083–2089. doi: 10.1111/j.1537-2995.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- Duprex WP, McQuaid S, Hangartner L, Billeter MA., and , Rima BK. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J Virol. 1999;73:9568–9575. doi: 10.1128/jvi.73.11.9568-9575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R, Greiner S, Harvey M, Soeffker D, Frenzke M, Abraham K, et al. Oncolytic activities of approved mumps and measles vaccines for therapy of ovarian cancer. Cancer Gene Ther. 2005;12:593–599. doi: 10.1038/sj.cgt.7700823. [DOI] [PubMed] [Google Scholar]
- Mrkic B, Odermatt B, Klein MA, Billeter MA, Pavlovic J., and , Cattaneo R. Lymphatic dissemination and comparative pathology of recombinant measles viruses in genetically modified mice. J Virol. 2000;74:1364–1372. doi: 10.1128/jvi.74.3.1364-1372.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscic-Mrkic B, Schwendener RA, Odermatt B, Zuniga A, Pavlovic J, Billeter MA, et al. Roles of macrophages in measles virus infection of genetically modified mice. J Virol. 2001;75:3343–3351. doi: 10.1128/JVI.75.7.3343-3351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DNA and protein sequence of the cloned λ chain. DNA sequence of the human λ immunoglobulin chain cloned and inserted in MV genome – designed MV-lambda (A) and corresponding protein translation of the cloned human λ chain (B).
Expression of λ chain by MV-λ-infected ARH-77 cells. Reporter expression after MV-lambda infection of IgG-κ producing human lymphoblastoid cell line ARH-77. Concentration of λ light chain in the supernatants (in ng/ml) from two experiments (exp.1 and exp.2, run in duplicates) after 96-h infection with MOI=0.5 of MV-lambda (A). Control samples were negative for λ protein (not detectable – ND). ELISA for κ/λ conversion using anti-λ coating and anti-κ detection antibodies (B). ELISA results are mean ± SD of absorbance (OD450nm) above the control.
Immunoblot analysis of converted myeloma immunoglobin. Immunoblotting of converted IgG assembled and secreted by MV-lambda infected MM cells. Supernatants from MV-lambda-infected or control KAS-6/1 cells diluted to 2 μg/ml and IgG was immunoprecipitated using Protein G agarose. Immunoblots were visualized after incubation with κ (A and C) or λ specific (B and D) HRPO-conjugated antibodies. In A and B two-fold dilutions of immunoprecipitates were run in four lanes under non-reducing SDS-PAGE conditions. Control human serum IgG (co-sIgG) was used in concentration corresponding to λ-light chain level in MV-lambda-infected samples (as measured by λ specific ELISA). Bands of λ chains have similar intensity for converted marker (MV-lambda infected cells) and co-sIgG but are not detectable for non-infected control (B and D). When reaction was normalized to the κ concentration in the samples, a similar amount of protein was detected under reducing conditions (C,D). In C and D: lane 1 – MV-lambda infected cells; lane 2 – sample from uninfected cells; lane 3 – co-sIgG.
In vivo expression of human λ light chain in MV-λ-infected Ifnarko mice. Serum concentration of λ protein reached more than 150 ng/ml on day 2 (2 of 2 mice) and day 3 (3 of 3 mice) of infection but was not detectable (ND) on day 7 (A). Free λ chain was excreted and detected in urine samples from the same animals on day 2 and in 2 of 3 mice on day 3 (B).
Tumor volume and serum myeloma IgG in KAS-6/1 engrafted mice. Correlation between tumor volume and serum level of MM IgG before the therapy. Spearman test – r=0.5505; p=0.0029.
MV-λ treatment of MM xenografts. Schematic representation of in vivo therapy protocol. KAS-6/1 cells were implanted s.c. in the right flank of female SCID mice on day 1. The animals received three therapeutic injections of 106 pfu of MV-lambda. Tumor growth and survival were monitored up to day 70 of the study.
Immunohistochemistry analysis for MM marker deposits in mice. IHC for detection of human immunoglobulin deposits in the organs (A) of mice injected with purified KAS-6/1 IgG (m3,4) or converted IgG from MV-lambda infected KAS-6/1 cells (m5,6) compared to the controls (m1,2). IHC was performed on frozen sections from lung, liver and kidney collected 48 h (m1,3,5) and 96 h (m2,4,6) after IgG injection (right columns). Parallel sections were stained with hematoxylin/eosin (left columns). Jurkat and KAS-6/1 cells were used as negative and positive controls of the assay (B) counterstained with hematoxylin (top) or fast nuclear red (bottom). For concentrations of human IgG in the serum of injected animals, see Supplementary table 3.
Tumor volumes of subcutaneously KAS-6/1 engrafted SCID mice before intratumoral or systemic treatment with MV-λ.
Tumor volume (in mm3) and serum level of MM IgG (in ng/ml) in SCID mice engrafted subcutaneously KAS-6/1 cells before the intratumoral or intravenous therapy with MV-λ.
Injection and serum concentrations of KAS-6/1 myeloma IgG in mice analyzed for immunoglobulin deposition in the organs.