Abstract
Ornithine transcarbamylase (OTC) deficiency, the most common urea cycle disorder, is associated with severe hyperammonemia accompanied by a high risk of neurological damage and death in patients presenting with the neonatal-onset form. Contemporary therapies, including liver transplantation, remain inadequate with considerable morbidity, justifying vigorous investigation of alternate therapies. Clinical evidence suggests that as little as 3% normal enzyme activity is sufficient to ameliorate the severe neonatal phenotype, making OTC deficiency an ideal model for the development of liver-targeted gene therapy. In this study, we investigated metabolic correction in neonatal and adult male OTC-deficient Spfash mice following adeno-associated virus (AAV)2/8-mediated delivery of the murine OTC complementary DNA under the transcriptional control of a liver-specific promoter. Substantially supraphysiological levels of OTC enzymatic activity were readily achieved in both adult and neonatal mice following a single intraperitoneal (i.p.) injection, with metabolic correction in adults being robust and life-long. In the neonates, however, full metabolic correction was transient, although modest levels of OTC expression persisted into adulthood. Although not directly testable in Spfash mice, these levels were theoretically sufficient to prevent hyperammonemia in a null phenotype. This loss of expression in the neonatal liver is the consequence of hepatocellular proliferation and presents an added challenge to human therapy.
Introduction
The development of safe and effective strategies for liver-targeted gene delivery promises to open a host of therapeutic possibilities. This reflects the complex functions of the liver, which include intermediary metabolism of carbohydrates, lipids, and amino acids, the biosynthesis of plasma proteins and bile acids, and the detoxification of toxic metabolites and xenobiotics.1 Among contemporary vector systems, those based on adeno-associated virus (AAV) show the most immediate promise for liver-targeted gene therapy based on phenotype correction studies in small2,3,4,5,6,7,8,9,10 and large animal models,11,12,13 and initial use in human clinical trials.14,15 Further progress toward successful human therapy requires an increased understanding of host–vector interactions, preferably in disease-specific contexts.
Ornithine transcarbamylase (OTC) deficiency, the most common of the urea cycle disorders, exemplifies the challenge of treating genetic metabolic liver disease by gene therapy and is an ideal model disease. Trace or undetectable enzyme activity results in severe neonatal hyperammonemia associated with a high risk of death, and significant morbidity in those treated early enough to survive the newborn period. Current treatments are inadequate, with liver transplantation the only option for long-term survival. Risk–benefit analysis of experimental intervention is therefore favorable. Moreover, patients with as little as 3% normal OTC activity in the liver have a less severe, more readily managed phenotype,16 suggesting that the gene transfer threshold required for therapeutic benefit is likely to be achievable.
To date, several studies have been performed in OTC-deficient mouse models using adenoviral and more recently recombinant AAV (rAAV) vectors.17,18,19,20 Initial poor results with early generation adenoviral vectors17 have been partially addressed by using more recently developed “gutless” and helper-dependent constructs, which have reduced cytotoxicity, but have not overcome limitations imposed by considerable intrinsic immunogenicity.18,19 In the only study reported to date using rAAV, treatment of adult OTC-deficient mice was investigated using vectors pseudo-serotyped with the type 7, 8, and 9 capsids.20 Promising metabolic correction was achieved, albeit at high vector doses, and declining therapeutic efficacy was observed beyond 250 days. The type 8 capsid proved most effective although the maximal OTC activities achieved were subphysiological.
In this study, the murine OTC (mOTC) complementary DNA was inserted into an AAV2/8 vector system under the transcriptional control of a strong liver-specific promoter. The ability of this vector to correct OTC deficiency was investigated in adult and neonatal Spfash mice, over a range of vector doses (5 × 1010 to 1.5 × 1012 vector genomes (vg) per mouse). The impairment of urea cycle function in these mice is sufficient to cause elevated urinary orotic acid, but not hyperammonemia. Robust and stable correction of urea cycle function, evaluated by measurement of urinary orotic acid levels and the rate of ureagenesis, was achieved in adult mice with supraphysiological levels of enzyme activity up to 17-fold above wild-type values at the highest dose. Highly efficient transduction of the liver in neonatal mice was also demonstrated; however, correction of orotic aciduria was transient and the initial peak in transgene expression declined in the first weeks of life. Nevertheless, OTC activity remained significantly above levels in untreated Spfash mice into adulthood. Although not directly testable in existing OTC-deficient mouse models, which exhibit a mild phenotype, this residual level of OTC activity is likely to be sufficient to prevent hyperammonemia in the context of an OTC null phenotype.
These data confirm the promise of rAAV-mediated gene transfer for the treatment of metabolic liver disease and highlight the added challenge of targeting the developing liver where hepatocellular replication is associated with loss of episomal vector genomes.21,22,23,24
Results
Robust correction of urea cycle function in adult Spfash mice
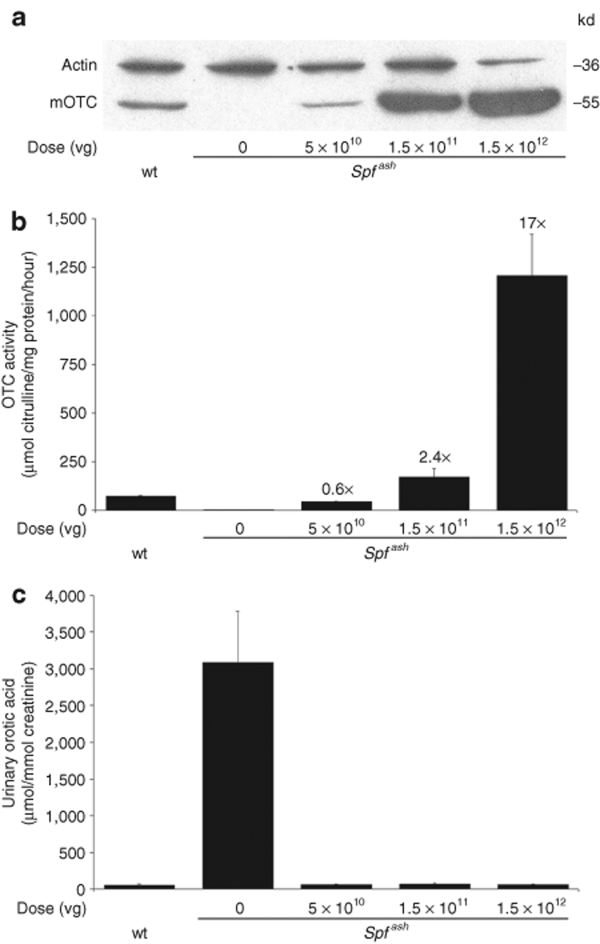
Correction of urea cycle function was investigated in adult male OTC-deficient (Spfash) mice following intraperitoneal (i.p.) injection of an AAV2/8 vector encoding mOTC under the transcriptional control of a liver-specific promoter. In an initial dose-finding experiment, cohorts of mice were injected with vector over a 30-fold dose range. Western blot analysis of liver harvested 2 weeks later revealed surprisingly effective gene transfer with substantially supraphysiological levels of OTC expression achieved at all but the lowest vector dose examined (Figure 1a). Remarkably, at the highest vector dose examined, OTC protein was over-expressed ~40-fold. Corresponding OTC enzymatic activity at the lowest, middle, and highest vector doses were 0.6-, 2.4-, and 17-fold, respectively, above control OTC activity in wild-type mice (Figure 1b). Significantly, orotic aciduria, characteristic of untreated Spfash mice, was normalized at all vector doses tested (Figure 1c).
Figure 1.
Robust correction of OTC deficiency and orotic aciduria in adult Spfash mice. Adult male Spfash mice were injected intraperitoneally with 5 × 1010 (n = 5), 1.5 × 1011 (n = 6), or 1.5 × 1012 (n = 3) vg of rAAV2/8-LSP1-mOTC, and 2 weeks later liver and urine were collected for analysis. Wild-type (n = 7) and untreated Spfash (n = 6) mice were included as positive and negative controls. (a) Western analysis of liver lysates (30 µg protein per lane) from control and vector-treated mice. Actin was included as a loading control. Molecular weight markers are shown (kd). (b) OTC activity in liver lysates from control and vector-treated mice. Fold differences compared to wild-type activity are indicated. (c) Urinary orotic acid normalized against creatinine from control and vector-treated mice. Error bars represent mean ± SEM. OTC, ornithine transcarbamylase; Spfash, OTC-deficient; vg, vector genome; wt, wild-type.
To further confirm correction of urea cycle function, the rate of ureagenesis was measured in vivo 28 days after injection of Spfash mice with the intermediate vector dose. Consistent with the levels of OTC expression achieved, the rate of conversion of stable-isotope (15N)-labeled ammonium chloride to plasma urea was normalized to wild-type levels (Figure 2).
Figure 2.
Normalization of ureagenesis in adult Spfash mice. Adult male Spfash mice were injected intraperitoneally with 1.5 × 1011 vg, and 1 month later vector-treated Spfash (n = 5), control untreated Spfash (n = 7), and wild-type (n = 9) mice were challenged with 4 mmol/kg of 15NH4Cl. The isotopic abundance (atom % excess) of 15N urea in plasma was determined 20 minutes later. Error bars represent mean ± SEM. OTC, ornithine transcarbamylase; Spfash, OTC-deficient; wt, wild-type; vg, vector genome.
Restoration of physiological levels of OTC activity within 7 days of vector delivery
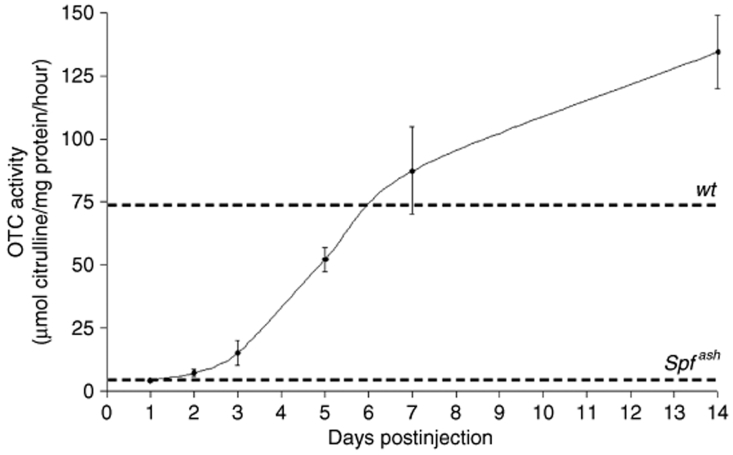
Given the potential clinical benefit of rapid correction of urea cycle function in OTC deficiency, the rate of onset of OTC expression was then examined. Adult male Spfash mice were injected with 1.5 × 1011 vg, the lowest dose to achieve physiological OTC expression levels in initial studies, and OTC activity analyzed at intervals up to 2 weeks. By 2 days, OTC activity was measurably higher than untreated Spfash mice and by 7 days exceeded wild-type activity levels (Figure 3). Between 3 and 7 days the rate of increase in OTC activity was maximal and close to linear, but subsequently slowed appreciably reaching approximately twofold physiological levels by 14 days postinjection.
Figure 3.
Onset of OTC activity following vector delivery. Adult male Spfash mice were injected intraperitoneally with 1.5 × 1011 vg, and OTC enzyme activity determined in liver harvested at 1, 2, 3, 5, 7, and 14 days postinjection (n = 3–5 mice per time-point). Upper and lower horizontal lines represent liver OTC activity levels in wild-type and Spfash, respectively. Error bars represent mean ± SEM. Spfash, OTC-deficient; wt, wild-type; OTC, ornithine transcarbamylase; vg, vector genome.
Life-long correction of orotic aciduria after a single vector dose
Having achieved robust and rapid correction of urea cycle function, the durability of this effect was next examined over an extended time-frame, following delivery of 5 × 1010 or 1.5 × 1011 vg/mouse, using urinary orotic acids levels as a surrogate measure of urea cycle function (Figure 4a). Mice injected with 1.5 × 1011 vg showed normalization of orotic aciduria within 1 week and stable correction for life (18–22 months postinjection). Mice receiving 5 × 1010 vg corrected gradually over a 2–3 week period, maintained wild-type values for 6 months and subsequently showed a small but relatively stable increase in urinary orotic acid content.
Figure 4.
Life-long correction of orotic aciduria and underlying OTC deficiency in adult Spfash mice. Adult male Spfash mice were injected intraperitoneally with vector doses shown to provide short-term correction (Figure 2), and urinary orotic acid levels followed up to 22 months. Liver OTC activity was also examined at 2 weeks, 12, and 18–20 months postinjection. (a) Urinary orotic acid levels in pre- and postinjection urine samples from wild-type (n = 10), untreated Spfash (n = 8), and Spfash mice injected with 5 × 1010 vg (n = 6) or 1.5 × 1011 vg (n = 4). (b) OTC enzyme activity in liver lysates from representative wild-type (n = 1–6), untreated Spfash (n = 1–6), and Spfash mice injected with 5 × 1010 vg (n = 1–5) or 1.5 × 1011 vg (n = 1–6). Error bars in a and b represent mean ± SEM. OTC, ornithine transcarbamylase; Spfash, OTC-deficient; vg, vector genome; wt, wild-type.
Measurement of OTC activity in liver lysates from representative mice in each treatment group at 2 weeks, 12, and 18–20 months postinjection revealed an initial decline in OTC activity between 2 weeks and 12 months, but relatively stable persistence of transgene expression thereafter (Figure 4b). Notably, mice receiving 1.5 × 1011 vg retained supraphysiological levels of OTC activity throughout life despite the early decline in expression. This result is entirely consistent with the life-long correction of orotic aciduria observed at this vector dose. Even in mice that received the lower dose of 5 × 1010 vg, OTC expression levels remained three- to fivefold above those of untreated Spfash mice, which was clearly sufficient to maintain substantially reduced urinary orotic acid levels.
No mice required culling due to the development of liver tumors, although small well-circumscribed tumors were observed in a proportion of mice culled for experimental purposes after 18 months of age. Frequencies were 20% for wild-type mice, 25% for Spfash mice, and 10% for vector-treated Spfash mice, consistent with a background rate of tumor formation with increasing age in the mouse strain used.
Transient correction of orotic aciduria in neonatal OTC-deficient Spfash mice
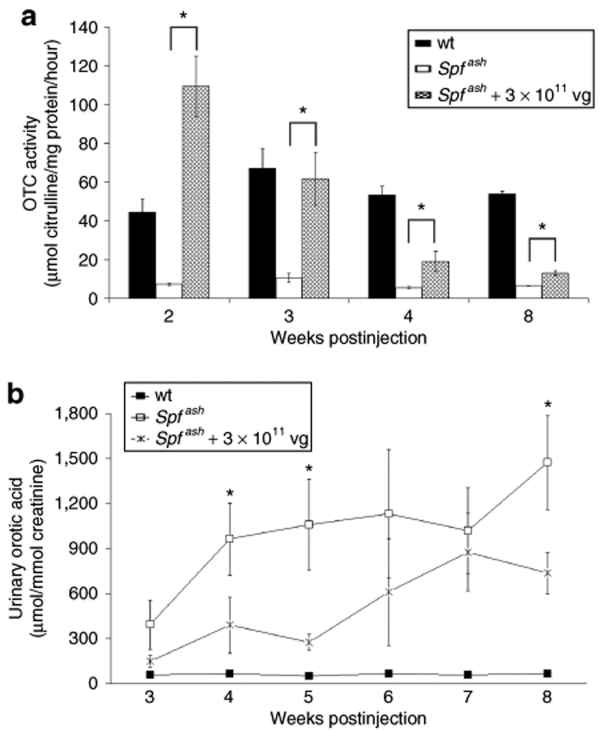
Ultimately, success in treating the severe neonatal form of OTC deficiency will require efficient and persistent gene transfer soon after birth. The efficiency of gene transfer and resultant reduction in orotic aciduria was therefore investigated following vector delivery to male Spfash mice at 1.5 days of age. Supraphysiological levels of enzyme activity were observed 2 weeks postinjection, but declined rapidly through 4 weeks before showing evidence of stabilization at levels approximately twofold above untreated Spfash control values (Figure 5a). Corresponding urinary orotic acid levels, measured from the time of weaning at 3 weeks, were markedly reduced at early time-points, but increased slowly through to early adulthood (Figure 5b), stabilizing at values intermediate between untreated Spfash and wild-type levels.
Figure 5.
Transient correction of OTC activity and orotic aciduria in neonatal Spfash mice. Neonatal male Spfash mice were injected intraperitoneally with 3 × 1011 vg at 1.5 days of age, and liver and urine harvested for analysis at intervals up to 8 weeks of age. (a) OTC enzyme activity in liver 2, 3, 4, and 8 weeks postinjection in wild-type, untreated Spfash, and vector-treated Spfash mice. (b) Corresponding urinary orotic acid levels, normalized against creatinine, from wild-type, untreated Spfash, and vector-treated Spfash mice collected weekly, from weaning at 3 weeks. Error bars in a (n = 3–6) and b (n = 3–19) represent mean ± SEM. The significance of the difference between vector-treated and untreated Spfash mice was calculated by the nonparametric Mann–Whitney U test. Asterisks indicate P ≤ 0.05. OTC, ornithine transcarbamylase; Spfash, OTC-deficient; vg, vector genome; wt, wild-type.
Higher transgene expression in the perivenous region of the hepatic lobule
Urea cycle enzymes are most highly expressed in the periportal region of the hepatic lobule, a phenomenon known as metabolic zonation. The distribution of transgene activity across the hepatic lobule was therefore examined in liver sections using an in situ histochemical staining protocol. Wild-type mice displayed the expected periportal pattern of endogenous OTC expression (Figure 6a), while little or no OTC activity was detected in untreated Spfash mice (Figure 6b). In contrast, liver sections harvested from adult mice 1 month after injection with 1.5 × 1011 vg showed liver-wide OTC transgene expression with the highest levels of OTC activity in hepatocytes located in the perivenous region of the hepatic lobule (Figure 6c), which was definitively identified by counterstaining with an antibody against glutamine synthetase (Figure 6d). Liver sections harvested 2 weeks after neonatal delivery of vector also displayed widespread OTC transgene expression (Figure 6e), but with no obvious bias toward the perivenous regions of the hepatic lobule. By 8 weeks postinjection, there was a significant loss of transgene-positive cells and clusters of high-expressing cells (2–6) were observed (Figure 6f).
Figure 6.
Distribution of OTC transgene expression across the hepatic lobule. OTC enzyme activity was examined in liver sections (5 µm) from wild-type, untreated, and vector-treated Spfash mice using an in situ histochemical staining method. Cells expressing OTC stain brown with an intensity that correlates with the level of enzymatic activity. Sections from (a) wild-type and (b) untreated adult Spfash mice. (c) Liver section from an adult mouse injected (i.p.) with 1.5 × 1011 vg and harvested 1 month later. Note higher intensity OTC staining around the central vein. (d) Counterstaining with an antibody to glutamine synthetase antibody to unequivocally identify the central veins. Liver sections from Spfash mice injected (i.p.) at 1.5 days and harvested (e) 2 and (f) 8 weeks postinjection. Note appearance of clusters of OTC-expressing cells. Bar = 100 µm. i.p., intraperitoneally; OTC, ornithine transcarbamylase; Spfash, OTC-deficient; vg, vector genome; wt, wild-type.
Discussion
In this study, we set out to investigate the therapeutic efficacy and durability of rAAV2/8-mediated liver-targeted gene delivery in adult and neonatal OTC-deficient Spfash mice. In adult mice, a single i.p. injection of vector at a dose of 1.5 × 1011 vg/mouse resulted in rapid and life-long phenotype correction. This was initially accompanied by restoration of liver-wide OTC enzyme activity to between two- and threefold above physiological levels. By 12 months, OTC activity had declined by ~50%, but remained supraphysiological and relatively stable thereafter up to 18–20 months. Remarkably, at the tenfold higher vector dose of 1.5 × 1012 vg/mouse, OTC activity levels 17-fold above physiological were achieved. Even at a threefold lower dose of 5 × 1010 vg/mouse, the resultant OTC activity reached ~60% of physiological levels and was sufficient to completely normalize urinary orotic acid content for at least 6 months and subsequently hold levels close to normal values. Efficient transduction of neonatal Spfash mouse liver was also demonstrated, although correction of orotic aciduria was transient and initial supraphysiological levels of OTC activity rapidly declined in the first 4 weeks of life before stabilizing just above those of untreated mice. Importantly, however, this level of persistence of OTC transgene expression was sufficient to hold urinary orotic acid levels at intermediate values into adulthood.
These results contrast with a previous study examining the efficacy of rAAV2/7, 2/8, and 2/9 vectors in the livers of OTC-deficient Spf and Spfash mice.20 At vector doses of 1 × 1012 vg per mouse, given by direct intraportal injection, OTC enzyme activity was increased to between 19 and 51% of wild-type values with rAAV2/8 and 2/9 vectors being most effective. Urinary orotic acid levels normalized slowly over at least 28 days and remained at wild-type levels for up to 250 days before rising back into the pathological range. We achieved better than equivalent results with a 20-fold lower vector dose (5 × 1010 vg per mouse), and persistently supraphysiological OTC activity with robust life-long correction of orotic aciduria from 7 days post-treatment at a six- to sevenfold lower vector dose (1.5 × 1011 vg per mouse).
The significantly greater therapeutic efficacy of our vector is likely to be the consequence of promoter selection. We used the apolipoprotein E/human α1-antitrypsin enhancer/promoter combination,13,14,25,26,27 as opposed to the human thyroid hormone binding globulin promoter, and also included a woodchuck post-transcriptional regulatory element. Notably, in a previous study exploiting the apolipoprotein E/human α1-antitrypsin enhancer/human promoter combination, we demonstrated a preference for higher levels of transgene expression in the perivenous region of the hepatic lobule.24 This finding has particular significance in the context of this study, as urea cycle function is most active in the periportal regions of the hepatic lobule, a phenomenon known as metabolic zonation.28,29 Thus, more efficient correction of OTC deficiency is likely to be achievable than reported here by incorporation of promoter enhancer elements of equivalent transcriptional activity that express more highly in the periportal zones. Another difference in our study was the use of the i.p. route for vector delivery as opposed to the direct intraportal route used in the earlier study. No direct comparison of these delivery routes has yet been reported, although intuitively the direct intraportal route might be expected to be more efficient, making our current results all the more remarkable.
Although the therapeutic efficacy obtained in adult mice is impressive, our results in neonatal mice raise a number of important questions directly relevant to human therapy. The severe form of OTC deficiency presents in the neonatal period where the durability of gene transfer will be influenced by hepatocellular proliferation occurring as a consequence of liver growth. In a previous study, we observed highly efficient AAV2/8-mediated transduction of the neonatal mouse liver followed by a dramatic drop in the number of transgene-expressing hepatocytes over the first few weeks of life due to loss of episomal vector genomes.24 A basal level of stably transduced hepatocytes (5–8%) remained, however, presumably due to vector integration. Results obtained in this study, including the observation of clusters of OTC-expressing cells in adult mice treated as neonates, are entirely consistent with these observations. Importantly, the residual levels of OTC expression persisting into adulthood were at least twofold above the levels found in untreated Spfash mice (~7% of wild-type) and sufficient to reduce urinary orotic acid levels. In affected male infants, as little as 3% normal OTC activity is sufficient to prevent persistent hyperammonemia and to confer a less severe phenotype.16 It is therefore conceivable, but not directly testable in the Spfash mouse model, that the modest levels of stable OTC expression achieved in this study following neonatal delivery would be adequate to give long-term correction of hyperammonemia. Further studies are required to definitively resolve this question. The need for vector readministration in human infants is likely to depend on whether the proportion of stably integrated rAAV genomes is sufficient to maintain clinically useful phenotype amelioration as episomal vector genomes are progressively lost in concert with liver growth. In any case, because liver growth proceeds at a much slower rate in human infants30 compared to mice,24 near complete loss of episomal vector genomes would be predicted to take several years.
In summary, we have shown that AAV2/8-mediated transgene delivery is an extremely powerful tool for life-long correction of OTC deficiency in the adult mouse, but is less robust in neonatal mice where substantial loss of transgene expression occurs in concert with liver growth. Successful treatment of human neonates with severe OTC deficiency is likely to need to accommodate this challenge, either by vector readministration or by achieving adequately high levels of gene transfer upon initial treatment to ensure that the fraction of vector genomes that undergo integration is sufficient to maintain phenotype correction.
Materials and Methods
Cell culture. Human embryonic kidney 293 cells31 were maintained in Dulbecco's modified Eagle medium (Gibco, Invitrogen, Grand Island, NY) supplemented with 10% (vol/vol) fetal bovine serum (JRH Biosciences, Lenexa, KS) and 1% (wt/vol) L-glutamine (Gibco, Invitrogen), and maintained at 37 °C in a humidified 5% CO2–air atmosphere.
Virus production and purification. The 1,065-base pair coding sequence of mOTC (Genbank accession no. M17030), with optimized Kozak sequence, was inserted downstream of the apolipoprotein E/human α1-antitrypsin enhancer/promoter elements in pLSP1eGFP24 as an EcoRI/EcoRV fragment replacing the eGFP transgene to produce pLSP1-mOTC. This vector construct was used to produce rAAV2/8-LSP1mOTC. The vector was pseudo-serotyped with the AAV8 capsid using p5E18-VD2/8 (courtesy of James M. Wilson, University of Pennsylvania) and packaged by triple transfection of human embryonic kidney 293 cells with pLSP1-mOTC, p5E18-VD2/8, and adenoviral helper plasmid pXX6 (courtesy of Jude Samulski, University of North Carolina) by calcium phosphate/DNA coprecipitation. Vector particles were purified from cell lysate by standard CsCl gradient centrifugation32 and vector genomes were titered by real-time quantitative PCR as previously described33 with modifications.24
Animals. All animal care and experimental procedures were evaluated and approved by the Children's Medical Research Institute and The Children's Hospital at Westmead Animal Care and Ethics Committee. Breeding pairs of Spfash mice (C57BL/6/C3H-F1 background) were obtained from The Jackson Laboratory (Bar Harbor, ME). All injections were administered via the i.p. route at 1–2 days (neonatal) or 8–12 weeks (adult) of age.
Measurement of urinary orotic acid. Urine was collected for orotic acid analysis by placing mice on a wire mesh over Whatman filter paper for a 24-hour period. Urine-soaked filter paper was air-dried and then 10 circular punches (3 mm in diameter) were randomly sampled from each paper. Urine was eluted in 300 µl of water/sample at room temperature for 3 hours, and 50 µl of each sample transferred to a 96-well polypropylene v-base plate (Nunc, Rosklide, Denmark). An internal standard (100 µmol/l stock of stable isotope–labeled 1,3-15N2 orotic acid; Cambridge Isotope laboratories, Andover, MA) was added to each sample (50 µl/well). Samples were analyzed for orotic acid levels using liquid chromatography/tandem mass spectrometry by multiple reaction monitoring on a Quattro Micro (Waters Australia, Rydalmere, Australia) using the transitions 155.1 > 111.1 and 157.1 > 113.1 for native and stable isotope–orotic acid, respectively. Ionization suppression was minimized by a short chromatographic separation using a 150 mm × 2.1 mm C18 column (Grace Davison, Baulkham Hills, Australia) and 50% acetonitrile:water mobile phase. Results were standardized against creatinine levels, measured by the modified Jaffe reaction.
Measurement of ureagenesis. Mice were administered excess nitrogen in the form of stable-isotope (15N)-labeled ammonium chloride, similar to experiments described previously.34 The mice were fasted for 3 hours before receiving 4 mmol/kg of 15NH4Cl (Cambridge Isotope Laboratories, Andover, MA) by i.p. injection. Heparinized blood was collected by tail incision 20 minutes after injection, and the plasma analyzed for the % of [15N] isotope enrichment of urea by gas chromatography/mass spectrometry.35
OTC enzyme activity analyses. OTC enzyme activity was assayed in liver lysate as described previously.36 Distribution of OTC activity across the hepatic lobule was analyzed in frozen liver sections as described.36 Briefly, thin slices of liver were fixed in 4% (wt/vol) paraformaldehyde, cryoprotected in 10–30% (wt/vol) sucrose, and frozen in OCT. Sections (5 µm) were incubated in two changes of a lead nitrate–containing reaction buffer for 10 minutes each, washed with distilled water then reacted with ammonium sulfide solution for 1 minute followed by further washing.
Western blot analysis. Proteins in liver lysates (30 µg per lane) were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis37 through a 4% stacking and 12% separating gel. The proteins were transferred overnight to a nitrocellulose membrane, blocked in 5% (wt/vol) skim milk, and 0.05% (vol/vol) Tween-20 in phosphate-buffered saline, and probed with rabbit antihuman OTC antibody (1/500 dilution; courtesy of Nick Hoogenraad, La Trobe University, Melbourne, Australia) and rabbit anti-actin antibody (1/250 dilution; Sigma Chemical, St Louis, MO). Bound primary antibody was detected with goat anti-rabbit IgG (1/20,000 dilution; BioRad, Hercules, CA) and SuperSignal West Pico Chemiluminescence Substrate (Pierce, Rockford, IL).
Immunohistochemistry. For detection of glutamine synthetase, frozen liver sections (5 µm) were permeabilized in methanol, blocked in phosphate-buffered saline containing 10% (vol/vol) fetal calf serum and 10% (vol/vol) goat serum, and reacted with a rabbit polyclonal anti-glutamine synthetase primary antibody (1/150 dilution; Abcam, Cambridge, UK). Bound primary antibody was detected with an Alexa Fluor 594 goat anti-rabbit IgG secondary antibody (1/1,000 dilution; Invitrogen, Carlsbad, CA) and images captured using an Olympus BX50 fluorescent microscope (Olympus, Center Valley, PA) and the ProgRes CapturePro 2.6 software (Jenoptik Laser, Jena, Germany).
Acknowledgments
We thank Samantha Ginn and Grant Logan for critical reading of the manuscript, and Margot Latham (The Children's Hospital at Westmead) for assistance in manuscript preparation. This work was supported by a grant (423400) from the National Health and Medical Research Council of Australia.
REFERENCES
- Rodes J, Benhamou J-P, Blei AT, Reichen J., and , Rizzetto M. The Textbook of Hepatology: From Basic Science to Clinical Practice. Blackwell Publishing: London; 2007. [Google Scholar]
- Oh HJ, Park ES, Kang S, Jo I., and , Jung SC. Long-term enzymatic and phenotypic correction in the phenylketonuria mouse model by adeno-associated virus vector-mediated gene transfer. Pediatr Res. 2004;56:278–284. doi: 10.1203/01.PDR.0000132837.29067.0E. [DOI] [PubMed] [Google Scholar]
- Mochizuki S, Mizukami H, Ogura T, Kure S, Ichinohe A, Kojima K, et al. Long-term correction of hyperphenylalaninemia by AAV-mediated gene transfer leads to behavioral recovery in phenylketonuria mice. Gene Ther. 2004;11:1081–1086. doi: 10.1038/sj.gt.3302262. [DOI] [PubMed] [Google Scholar]
- Ding Z, Georgiev P., and , Thöny B. Administration-route and gender-independent long-term therapeutic correction of phenylketonuria (PKU) in a mouse model by recombinant adeno-associated virus 8 pseudotyped vector-mediated gene transfer. Gene Ther. 2006;13:587–593. doi: 10.1038/sj.gt.3302684. [DOI] [PubMed] [Google Scholar]
- Lebherz C, Gao G, Louboutin JP, Millar J, Rader D., and , Wilson JM. Gene therapy with novel adeno-associated virus vectors substantially diminishes atherosclerosis in a murine model of familial hypercholesterolemia. J Gene Med. 2004;6:663–672. doi: 10.1002/jgm.554. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Allamarvdasht M, Pan CJ, Sun MS, Mansfield BC, Byrne BJ, et al. Long-term correction of murine glycogen storage disease type Ia by recombinant adeno-associated virus-1-mediated gene transfer. Gene Ther. 2006;13:321–329. doi: 10.1038/sj.gt.3302650. [DOI] [PubMed] [Google Scholar]
- Koeberl DD, Sun BD, Damodaran TV, Brown T, Millington DS, Benjamin DK, et al. Early, sustained efficacy of adeno-associated virus vector-mediated gene therapy in glycogen storage disease type Ia. Gene Ther. 2006;13:1281–1289. doi: 10.1038/sj.gt.3302774. [DOI] [PubMed] [Google Scholar]
- Sun B, Zhang H, Franco LM, Young SP, Schneider A, Bird A, et al. Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol Ther. 2005;11:57–65. doi: 10.1016/j.ymthe.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Seppen J, Bakker C, de Jong B, Kunne C, van den Oever K, Vandenberghe K, et al. Adeno-associated virus vector serotypes mediate sustained correction of bilirubin UDP glucuronosyltransferase deficiency in rats. Mol Ther. 2006;13:1085–1092. doi: 10.1016/j.ymthe.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Kügler S, Hahnewald R, Garrido M., and , Reiss J. Long-term rescue of a lethal inherited disease by adeno-associated virus-mediated gene transfer in a mouse model of molybdenum-cofactor deficiency. Am J Hum Genet. 2007;80:291–297. doi: 10.1086/511281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty RM, Jackson M, Peterson D, Bird A, Brown T, Benjamin DK, et al. Delivery of glucose-6-phosphatase in a canine model for glycogen storage disease, type Ia, with adeno-associated virus (AAV) vectors. Gene Ther. 2002;9:1015–1022. doi: 10.1038/sj.gt.3301728. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y, et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount JD, Herzog RW, Tillson DM, Goodman SA, Robinson N, McCleland ML, et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, Meulenberg J, Hui D, Basner-Tschkarajan E, de Jong A, Pos P, et al. Capsid-specific T cell responses in humans upon intramuscular administration of an AAV-1 vector expressing LPLS447X transgene. Abstract. Hum Gene Ther. 2007;18:991–992. [Google Scholar]
- Tuchman M, Jaleel N, Morizono H, Sheehy L., and , Lynch MG. Mutations and polymorphisms in the human ornithine transcarbamylase gene. Hum Mutat. 2002;19:93–107. doi: 10.1002/humu.10035. [DOI] [PubMed] [Google Scholar]
- Kiwaki K, Kanegae Y, Saito I, Komaki S, Nakamura K, Miyazaki JI, et al. Correction of ornithine transcarbamylase deficiency in adult Spf (ash) mice and in OTC-deficient human hepatocytes with recombinant adenoviruses bearing the CAG promoter. Hum Gene Ther. 1996;7:821–830. doi: 10.1089/hum.1996.7.7-821. [DOI] [PubMed] [Google Scholar]
- Mian A, McCormack WM, Mane V, Kleppe S, Ng P, Finegold M, et al. Long-term correction of ornithine transcarbamylase deficiency by WPRE-mediated overexpression using a helper-dependent adenovirus. Mol Ther. 2004;10:492–499. doi: 10.1016/j.ymthe.2004.05.036. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Clarke C, Mane V, Palmer DJ, Lanpher B, Sun Q, et al. Phenotypic correction of ornithine transcarbamylase deficiency using low dose helper dependent adenoviral vectors. J Gene Med. 2008;10:890–896. doi: 10.1002/jgm.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscioni D, Morizono H, McCarter RJ, Stern A, Cabrera-Luque J, Hoang A, et al. Long-term correction of ammonia metabolism and prolonged survival in ornithine transcarbamylase-deficient mice following liver-directed treatment with adeno-associated viral vectors. Mol Ther. 2006;14:25–33. doi: 10.1016/j.ymthe.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Nakai H, Yant SR, Storm TA, Fuess S, Meuse L., and , Kay MA. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J Virol. 2001;75:6969–6976. doi: 10.1128/JVI.75.15.6969-6976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon TJ, Cossette T, Erger K, Choi YK, Clarke T, Scott-Jorgensen M, et al. Efficient hepatic delivery and expression from a recombinant adeno-associated virus 8 pseudotyped α1-antitrypsin vector. Mol Ther. 2005;12:867–875. doi: 10.1016/j.ymthe.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Grimm D, Pandey K, Nakai H, Storm TA., and , Kay MA. Liver transduction with recombinant adeno-associated virus is primarily restricted by capsid serotype not vector genotype. J Virol. 2006;80:426–439. doi: 10.1128/JVI.80.1.426-439.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham SC, Dane AP, Spinoulas A, Logan GJ., and , Alexander IE. Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Mol Ther. 2008;16:1081–1088. doi: 10.1038/mt.2008.72. [DOI] [PubMed] [Google Scholar]
- Hafenrichter DG, Wu X, Rettinger SD, Kennedy SC, Flye MW., and , Ponder KP. Quantitative evaluation of liver-specific promoters from retroviral vectors after in vivo transduction of hepatocytes. Blood. 1994;84:3394–3404. [PubMed] [Google Scholar]
- Okuyama T, Huber RM, Bowling W, Pearline R, Kennedy SC, Flye MW, et al. Liver-directed gene therapy: a retroviral vector with a complete LTR and the ApoE enhancer-α 1-antitrypsin promoter dramatically increases expression of human α 1-antitrypsin in vivo. Hum Gene Ther. 1996;7:637–645. doi: 10.1089/hum.1996.7.5-637. [DOI] [PubMed] [Google Scholar]
- Miao CH, Ohashi K, Patijn GA, Meuse L, Ye X, Thompson AR, et al. Inclusion of the hepatic locus control region, an intron, and untranslated region increases and stabilizes hepatic factor IX gene expression in vivo but not in vitro. Mol Ther. 2000;1:522–532. doi: 10.1006/mthe.2000.0075. [DOI] [PubMed] [Google Scholar]
- Morris SM. Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105. doi: 10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- Jungermann K., and , Katz N. Functional specialization of different hepatocyte populations. Physiol Rev. 1989;69:708–764. doi: 10.1152/physrev.1989.69.3.708. [DOI] [PubMed] [Google Scholar]
- Stocker JT., and , Dehner LP. Pediatric Pathology. Lippincott, Williams and Wilkins: Philadelphia, PA; 2002. [Google Scholar]
- Graham FL, Smiley J, Russell WC., and , Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Snyder RO, Xiao X., and , Samulski RJ.Dracopoli N, Haines J, Krof B, Moir C, Morton C, Seidman C.Production of recombinant adeno-associated viral vectors Current Protocols in Human Genetics 1996Wiley & Sons: New York, NY; 12.0.1–12.1.24M.et aleds [DOI] [PubMed] [Google Scholar]
- Veldwijk MR, Topaly J, Laufs S, Hengge UR, Wenz F, Zeller WJ, et al. Development and optimization of a real-time quantitative PCR-based method for the titration of AAV-2 vector stocks. Mol Ther. 2002;6:272–278. doi: 10.1006/mthe.2002.0659. [DOI] [PubMed] [Google Scholar]
- Batshaw ML, Robinson MB, Ye X, Pabin C, Daikhin Y, Burton BK, et al. Correction of ureagenesis after gene transfer in an animal model and after liver transplantation in humans with ornithine transcarbamylase deficiency. Pediatr Res. 1999;46:588–593. doi: 10.1203/00006450-199911000-00016. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Jawad A, Wilson J., and , Batshaw M. In vivo nitrogen metabolism in ornithine transcarbamylase deficiency. J Clin Invest. 1996;98:2167–2173. doi: 10.1172/JCI119023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Robinson MB, Batshaw ML, Furth EE, Smith I., and , Wilson JM. Prolonged metabolic correction in adult ornithine transcarbamylase-deficient mice with adenoviral vectors. J Biol Chem. 1996;271:3639–3646. doi: 10.1074/jbc.271.7.3639. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]